Abstract
Clear cell carcinoma or hyalinizing clear cell carcinoma (CCC) and clear cell odontogenic carcinoma (CCOC) are rare, low-grade and typically indolent malignancies that can be diagnostically challenging. In this study the clinicopathologic, histologic, and immunohistochemical features of 17 CCCs and 12 CCOCs are examined. The differential diagnosis of clear cell malignancies in the head and neck is discussed. The relationship of CCCs and CCOCs to other clear cell tumors on the basis of their immunohistochemical staining patterns is postulated.
Keywords: Clear cell carcinoma, Clear cell odontogenic carcinoma, Immunohistochemistry, Histologic
Background
Clear cell carcinoma or hyalinizing clear cell carcinoma (CCC) is a malignancy that typically arises from the minor salivary glands, especially within the palate or base of the tongue [1]. Rare cases have also been reported in other locations, including the nasopharynx, hypopharynx, subglottic larynx, lacrimal gland, and parotid [2–4]. Although CCC is considered a low-grade malignancy, 25% of patients have been reported to have metastases on presentation and 12% have experienced local recurrence at an average of 26.6 months [1, 3]. Death from disease, however, has been reported in only two cases [1, 3, 5, 6]. Metastases usually involve the regional lymph nodes, and only rarely involve the lung and bones [3, 6].
Clear cell odontogenic carcinoma (CCOC), formerly known as clear cell odontogenic tumor, was first described by Hansen in 1985 [7]. The tumor is generally intraosseous, affecting the mandible most commonly (84%) [8]. Initially thought to be benign, it is now recognized as a locally aggressive tumor, with a 34% local/regional recurrence rate for tumors treated with resection and the capacity to metastasize to distant sites (14%), most frequently the lungs [9–12].
Histologically, CCCs and CCOCs are quite similar. Both tumors are composed of sheets, cords, or nests of monomorphic, plump, polygonal-to-round clear cells with eccentric nuclei, often separated by hyalinized fibrous septa. Pleomorphism can be seen, but mitoses are uncommon. Both tumors are cytokeratin positive, but generally negative for mucicarmine, S-100 protein, smooth muscle actin, calponin, glial fibrillary acidic protein (GFAP), and vimentin. Demonstrating the presence of intracytoplasmic glycogen, both CCCs and CCOCs are PAS positive, diastase-sensitive [1, 3, 5, 13–16].
This considerable histologic overlap, may result, in difficulty in differentiating CCC from CCOC in the maxillary or mandibular region. Adding to this dilemma is the description of a ‘central hyalinizing clear cell carcinoma’ involving the jawbones on the basis of histologic criteria [17]. Finally, the importance of this distinction is not particularly clear at this point. We herein compare the clinicopathologic and immunohistochemical profile of CCC and CCOC in an attempt to resolve this diagnostic challenge.
Materials and Methods
Data Collection
After Institutional Review Board approval (protocol # 06010184), 17 CCC (in 15 patients) and 12 CCOC (in 8 patients) were identified at the University of Pittsburgh Medical Center (UPMC) and the Cleveland Clinic Foundations (CCF) from 1998 to 2010. Hematoxylin and eosin-stained (H&E) slides from all cases were reviewed as well as all available immunohistochemical stains. The antibodies, manufacturers, dilutions, and retrieval methods are listed in Table 1. Clinical parameters (size, sex, location, recurrences, margin status, nodal status, metastases) were recorded when available.
Table 1.
Antibodies used for immunohistochemistry
| Antibody | Company | Clone | Dilution |
|---|---|---|---|
| Cytokeratin 5/6 | Dako, Carpinteria, CA | D5/16 B4 | 1:50 |
| Pankeratin AE1/3 | Dako, Carpinteria, CA | AE1/AE3 | 1:100 |
| P63 | Thermo, Fremont, CA | 4A4 | 1:200 |
| Vimentin | Ventana, Tucson, AZ | V9 | Predilute |
| Smooth Muscle Actin | Cell Marque, Rocklin, CA | IA4 | Predilute |
| Calponin | Dako, Carpinteria, CA | CALP | 1:50 |
| S100 | Dako, Carpinteria, CA | n/a | 1:500 |
Results
The available clinical data for the 17 CCC in 15 patients and 12 CCOC in 8 patients are summarized in Table 2. Briefly, the mean patient age at presentation of CCC was 60.5 (range 32–79 years) with a female-to-male ratio of 1.6:1 (8 females, 5 males). The distribution of CCC by site was as follows: palate-4 (26.7%), base of tongue-2 (13.3%), lip-2(13.3%), tonsil-2 13.3%), anterior floor of mouth-1 (6.7%), parotid-1 (6.7%), buccal mucosa-1 (6.7%), nasal sinus-1 (6.7%) and nasal cavity/ethmoid sinus with extension through the cribiform plate -1 (6.7%). Thus, the majority CCCs affected minor salivary glands. The tumors ranged in size from 0.6 cm to 4.9 cm in greatest dimension. In the 12 cases of CCC where the margin status of the initial resection specimen was known, 58.3% (7/12) had positive margins. One of the patients who initially had positive margins had negative margins on a subsequent excision specimen.
Table 2.
Clinicopathological features
| Patients | Tumors | Size mean (range) cm | Mean age (range) | Sex F:M | Location | Local recurrence | Lymph node metastases | Margin status | |
|---|---|---|---|---|---|---|---|---|---|
| Clear Cell Carcinoma | 15 | 17 | 1.6 (0.6-4.9) | 60.5 (32-79) | 8:5 | Palate-4 (26.7%) base of tongue-2 (13.3%) lip-2 (13.3%) tonsil – 2 (13.3%) | 1 patient presented with a second recurrence at 15 yrs | 2 of 3 positive cases with neck dissection | 7/12 (58.3%) positive |
| Clear Cell Odontogenic Carcinoma | 8 | 12 | 2.7 (1.5-4) | 55.6 (19-76) | 4:4 | Mandible-5 (62.5%) maxilla-3 (37.5%) | 1 patient with a resected mandibular tumor recurred in the buccal mucosa at 4 years | 1 of 3 cases with neck dissection | 7/8 (87.5%) positive |
Lymph node metastases were present in two CCC patients. One consultation case in which the previous histologic specimens were not available for review consisted of a patient with a history of cervical lymph node metastases 15 years prior and subsequent nasopharyngeal tumor. This 66 year old female now had CCC of the nasopharynx with positive lymph nodes and possibly a second recurrence. However, only the recurrent tumor was submitted for consultation, not the involved lymph nodes. Another patient with CCC at the base of the tongue with bilateral positive lymph nodes, ipsilateral level III (2/51) and contralateral level II (2/24), was treated with resection and selective neck dissection. This patient was disease free at 3 years 11 months.
The mean patient age at presentation of CCOC was 55.6 (range 19–76 years) with a female-to-male ratio of 1:1 (4 females, 4 males). The distribution of CCOC by site was as follows: mandible-5 (62.5%), and maxilla-3 (37.5%). The mean size was 2.7 cm in greatest dimension (range 1.5–4 cm). Positive margins were present in 87.5% (7/8) initial CCOC resection specimens. Lymph node metastases were present in one of the three neck dissections performed. A 50 year old female with recurrent mandibular CCOC 4 years status post resection had positive zone I (1/1) and zone II (1/9) ipsilateral lymph nodes. She had a second recurrence in the ipsilateral buccal mucosa 4 years later.
Histologic and Immunohistochemical Features
Each case of CCC and the CCOC was evaluated for the following architectural features: stromal hyalinization, glandular lumina, cysts, squamous metaplasia, perineural invasion, and angiolymphatic invasion. Histologically, all tumors were predominantly composed of round-to-polygonal cells with cytoplasmic clearing arranged in various combinations of solid, nested, and infiltrative patterns of growth. Cells with a granular eosinophillic cytoplasm were seen admixed with the clear cells. At times these nests or islands were separated by hyalinzed fibrous septa. Pleomorphism was occasionally apparent, but mitoses were generally rare. A few tumors had increased mitotic activity. The mean mitotic count per 10 high power fields (hpf, 40 × objective) was 2.4 and 1.9 in CCC and CCOCs, respectively, with ranges of 0–13 and 0–9.
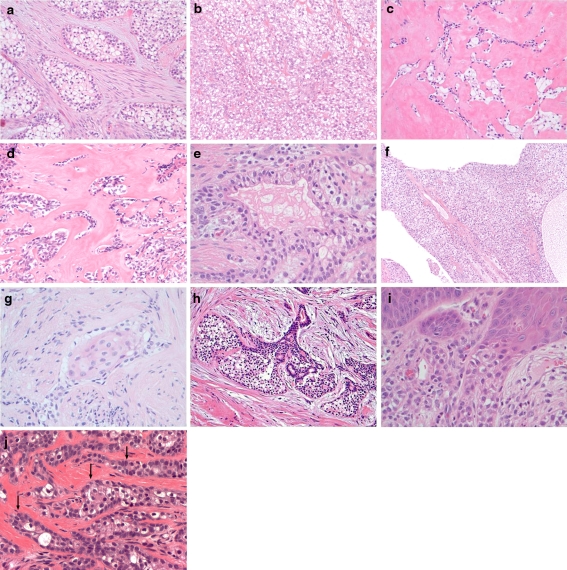
The histologic features are summarized in Table 3 and Fig. 1. Peripheral palisading was present in CCOCs (7/12, 58.3%) but not observed in CCCs. None of the CCOCs show evidence of dentin, or enamel matrix production. There were no other significant morphologic differences between CCCs and CCOCs. Stromal hyalinization was observed in (11/17, 64.7%) and (6/12, 50.0%) of CCCs and CCOCs, respectively. This feature was somewhat more pronounced in CCCs, with 29.4% of CCCs having prominent fields of dense paucicellar hyalinization not present in CCOCs. Gland-like pseudolumina were also seen in (7/17, 41.2%) and (5/12, 41.7%) of CCCs and CCOCs. Squamous metaplasia was seen both CCCs and CCOCs, (3/17, 17.6%) and (3/12, 25.0%). Cyst formation was seen in CCCs (2/17, 11.8%). Both CCCs and the CCOCs exhibited pagetoid surface or periductal involvement in which the tumor was intimately associated with the epithelial structures, (8/17, 47.1%) and (8/12, 66.7%), respectively. Perineural invasion was seen in many of the CCCs and CCOCs, (8/17, 47.1%) and (4/12, 33.3%), respectively but angiolymphatic invasion was less common at (2/17, 11.8%) and (1/12, 8.3%).
Table 3.
Architectural features
| Stromal hyalinization | Glandular pseudolumina | Cysts | Squamous metaplasia | Surface/periducatal involvement | Peripheral palisading | Angiolymphatic invasion | Perineural Invasion | |
|---|---|---|---|---|---|---|---|---|
| Clear Cell Carcinoma | 11/17 (64.7%) | 7/17 (41.2%) | 2/17 (11.8%) | 3/17 (17.6%) | 8/17 (47.1%) | 0/17 (0%) | 2/17 (11.8%) | 8/17 (47.1%) |
| Clear Cell Odontogenic Carcinoma | 6/12 (50.0%) | 5/12 (41.7%) | 0/12 (0%) | 3/12 (25.0%) | 8/12 (66.7%) | 7/12 (58.3%) | 1/12 (8.3%) | 4/12 (33.3%) |
Fig. 1.
Architectural features of a CCC and CCOC, a the classic clear cell nests of CCC are seen within a fibrocellular stroma, 10×; b a solid monotonous sheet of clear cells seen in CCC, 20×; c an infiltrative pattern of CCC is seen with extensive stromal hyalinization, 10×; d extensive stroma hyalinization within a CCOC, 20×; e the presence of glandular pseudolumina within CCC, 40×; f extensive cystic areas within CCC, 10×; g squamous metaplasia within CCOC, 40×; h periductal growth within CCC, 40×; i surface involvement in CCOC, 40×; and j peripheral palisading within CCOC (arrow), 40×
The tumors also showed a similar histochemical and immunohistochemical profile summarized in Table 4 and Fig. 2. All tumors stained were positive for cytokeratin 5/6 (CK 5/6) (9/9 CCCs and 4/4 CCOCs), AE1/3 (9/9 CCCs and 2/2 CCOCs), and P63 (11/11 CCCs and 5/5 CCOCs). Contrary to previous reports in the literature, vimentin staining was also seen in all tested tumors; 6/6 CCCs and 1/1 CCOCs exhibited staining; while, in 4/6 CCCs the staining was only focal or weakly positive. No staining for smooth muscle actin (0/10 and 0/3), calponin (0/11 and 0/3), S-100 protein (0/10 and 0/2), or mucicarmine (0/13 and 0/4) was seen in either the CCCs or CCOC, respectively. The tumors were PAS positive and diastase-sensitive (8/8 CCCs and 5/5 CCOCs).
Table 4.
Immunohistochemical features
| CK 5/6 | AE1/3 | P63 | Vimentin | SMA | Calponin | S100 | PAS | PASD | Mucicarmine | |
|---|---|---|---|---|---|---|---|---|---|---|
| Clear Cell Carcinoma | 9/9 (100%) | 9/9 (100%) | 11/11 (100%) | 6/6 (100%)* | 0/10 (0%) | 0/11 (0%) | 0/10 (0%) | 8/8 (100%) | 0/8 (0%) | 0/13 (0%) |
| Clear Cell Odontogenic Carcinoma | 4/4 (100%) | 2/2 (100%) | 5/5 (100%) | 1/1 (100%) | 0/3 (0%) | 0/3 (0%) | 0/2 (0%) | 5/5 (100%) | 0/5 (0%) | 0/4 (0%) |
* 3 cases focally positive and 1 case weakly positive for vimentin
CK5/6- cytokeratins 5/6, SMA- smooth muscle actin
Fig. 2.
Immunohistochemical staining patterns, a intense CK 5/6 staining is seen within nests of clear cells in CCC, 40×, and b in CCOC, 40×. c P63 staining in CCC, 40×, and d in CCOC, 40×. e no SMA staining is seen in CCC, 40× or f in CCOC, 40×
Discussion
Clear cell lesions in the head and neck evoke a broad differential diagnosis that may encompass a variety of odontogenic, metastatic, and salivary tumors that may be included in the differential diagnosis for CCC or CCOC. Clear cells can be seen in other odontogenic lesions, and the clear cell variant of calcifying epithelial odontogenic tumor (CEOT) may be particularly challenging since it is also composed of nests of polygonal cells with prominent stroma [18]. However, clear cell CEOT is a benign (albeit potentially locally aggressive) neoplasm and will not show perineural or angiolymphatic invasion. Additionally the stromal constituents here will contain amyloid, and the tumors will show concentric ‘Liesegang’ like calcifications that essentially define this tumor. Paradoxically, though benign, CEOT will generally show more nuclear size variation, typically in a random distribution, than CCC or CCOC.
Metastatic lesions such as renal cell carcinoma or balloon cell melanoma might be a consideration. Salivary gland neoplasms with a clear cell appearance would include mucoepidermoid carcinoma (MEC), clear cell myoepithelioma, clear cell myoepithelial carcinoma, clear cell oncocytoma, and clear cell acinic cell carcinoma [19]. Many of these tumors have a morphologically distinct appearance that can be identified with adequate sectioning of the tumor. With immunohistochemical stains, and now in the case of MEC, the identification of the t(11;19)(q21;p13) translocation, essentially all of these other differential diagnostic considerations can be resolved [20, 21].
However, findings indicate that despite a different origin, both CCC and CCOC show such a degree of morphologic and immunophenotypic overlap that distinction is not possible. CCC is a tumor of presumed salivary gland origin while CCOC is thought to arise from the dental lamina. Yet both have similar growth patterns, cytomorphologic features and immunohistochemical profiles.
Initially, CCC was presumed by many to actually to be of a salivary ductal-luminal phenotype. In fact, it was proposed that CCC forms the ‘epithelial’ or ‘ductal’ end of a spectrum that included epithelial-myoepithelial carcinoma (comprised of ductal and myoepithelial components) in the center, and clear cell myoepithelial carcinoma (comprised only of myoepithelial cells) on the opposite end [22, 23]. However, based on our findings and those of others, it is apparent that CCC actually has a distinct phenotype that is more in keeping with a squamous tumor [24, 25]. Our cases had scattered gland-like pseudolumina, but no true ducts, and instead, frequently showed frank keratinization in areas. Furthermore, our cases were positive for CK 5/6 and p63 while negative for SMA, calponin, and S100, which effectively places CCC out of the epithelial myoepithelial carcinoma and clear cell myoepithelial carcinoma spectrum. In fact, recently, Dardick refers to CCC as a glycogen rich variant of squamous cell carcinoma based on ultrastructural findings of squamous differentiation but not glandular or myoepithelial differentiation [24, 25]. Thus, CCC may be justifiably considered a glycogenated low grade squamous cell carcinoma of putative salivary origin.
Odontotogenic lesions such as CCOCs, CEOTs, lateral periodontal cysts, and gingival cysts can have a minor or major clear cell component. One theory is that clear cells in odontogenic tumors originate from the dental lamina. Lateral periodontal cysts and gingival cysts, known to have a clear cell component are also thought to originate from the dental lamina [26]. Possibly clear cells are recapitulating a phase of amelogenesis in which they accumulate glycogen. Eversole proposed that the clear cells were related to the presecretory ameloblast, epithelial in nature, ultrastructurally containing desmosomes, and lacking secretory granules [27]. Electron microscopic examination of CCOCs has revealed organelle poor cells containing lysosomes, mitochondria, tonofilament bundles and desmosomes [27–29] The granules may be a combination of mitochondria and lysosomes. The ultrastructural characteristics of desmosomes and tonofilament bundles hint at squamous differentiation and, as such, it is reasonable to expect a squamous phenotype for CCOC, as confirmed by our study.
The distinction between CCC and CCOC has not been a focus in the majority of literature dedicated to these entities; however, as these are more frequently recognized, this issue will certainly arise more. Brandwein et al. [29] suggested CCOCs have a more lobulated growth pattern with a loose myxoid background. It is interesting to note that Berho and Huvos describe two cases of ‘central’ CCC and distinguish these from CCOC using the presence of hyalinized stroma as indicative of central CCC and a ‘peripheral arrangement of polyhedral cells’ as characteristic of CCOC [17]. In contrast, Ellis favors location as a criterion and suggests that the central osseous destruction seen with CCOC is more supportive of odontogenic origin [19]. In our series the only histologic feature useful in distinguishing CCC from CCOC was the presence of peripheral palisading. Hyalinization was only slightly more frequent and prominent in CCC, making it an unreliable criterion for the distinction. However, almost half of CCOC did not show this palisading; we would be hard pressed to designate all such tumors ‘central CCC’ based on one subtle histologic feature. Thus, our stance is that ‘central CCC’ is thus within the spectrum of CCOC. We would instead then favor the notion that location is the main diagnostic criterion to distinguish CCC from CCOC. Additionally, in validating the common squamous phenotype for both of these tumor types, we demonstrate that these immunostains have no utility in distinguishing CCC from CCOC.
Ultimately, though perhaps taxonomically important, our findings show that the therapeutic implications of distinguishing CCC from CCOC may not be particularly significant. In our series, both had a similar demographic profile aside from site, and a similar incidence of nodal metastasis. Because these tumors tend to be highly infiltrative, both CCC and CCOC have a high frequency of margin positivity, suggesting that a surgical approach should be fairly aggressive to ensure complete excision. A review of the literature also demonstrates similarities in clinical behavior. Both CCCs and CCOCs most commonly present in the fifth decade of life with a female predilection, presenting as a mass, sometimes painful [1, 2, 12, 27]. The immunohistochemical profile is also similar, with both tumors expressing cytokeratins (AE1/3, CK 5/6), P63, vimentin, but are negative for myoepithelial markers (smooth muscle actin, calponin, S100). Recurrence and/or metastatic disease rates are relatively high. A review of 43 reported CCOCs reveals a recurrence rate of 55% [8]. A review of the 55 reported CCCs demonstrates that one-third have metastasized or locally recurred [4]. Both CCCs and CCOCs metastasize most commonly to the regional lymph nodes and less frequently to the lungs.
In summary, CCC and CCOC are malignant locally aggressive tumors with the capacity to metastasize. Based on current observations and immunostains, they are difficult, and in some cases impossible to distinguish morphologically and immunohistochemically despite a different cell of origin. We would suggest that location is the most important distinguishing criterion for these tumors. Our findings support the recent proposal that CCC is a distinct low grade ‘salivary type glycogenated squamous cell carcinoma’ rather than a ductal tumor that is part of the epithelial-myoepithelial carcinoma and clear cell myoepithelial carcinoma spectrum. Fortunately, the importance of this distinction from a therapeutic standpoint is likely minor since both tumors have a similar clinical profile and should be treated by complete excision when possible.
References
- 1.Yang S, Zhang J, Xinming C, et al. Clear cell carcinoma, not otherwise specified, of salivary glands: a clinicopathologic study of 4 cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:712–720. doi: 10.1016/j.tripleo.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 2.Milchgrub S, Gnepp DR, Vuitch F, et al. Hyalinizing clear cell carcinoma of salivary gland. Am J Surg Pathol. 1994;18:74–82. doi: 10.1097/00000478-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Solar AA, Schmidt BL, Jordan RC, et al. Hyalinizing clear cell carcinoma: case series and comprehensive review of the literature. Cancer. 2009;115:75–83. doi: 10.1002/cncr.23974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Sullivan-Mejia E, Massey H, Faquin W, et al. Hyalinizing Clear Cell Carcinoma: Report of Eight Cases and Review of Literature. Head and Neck Pathol. 2009;3:179–185. doi: 10.1007/s12105-009-0124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li TJ, Yu SF, Gao Y, et al. Clear cell odontogenic carcinoma: a clinicopathologic and immunocytochemical study of 5 cases. Arch Pathol Lab Med. 2001;125:1566–1571. doi: 10.5858/2001-125-1566-CCOC. [DOI] [PubMed] [Google Scholar]
- 6.O’Regan E, Shandilya M, Gnepp DR, et al. Hyalinizing clear cell carcinoma of salivary gland: an aggressive variant. Oral Oncol. 2004;40:348–352. doi: 10.1016/j.oraloncology.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Hansen LS, Eversole LR, Green TL, et al. Clear cell odontogenic tumor–a new histologic variant with aggressive potential. Head Neck Surg. 1985;8:115–123. doi: 10.1002/hed.2890080208. [DOI] [PubMed] [Google Scholar]
- 8.Ebert CS, Jr, Dubin MG, Hart CF, et al. Clear cell odontogenic carcinoma: a comprehensive analysis of treatment strategies. Head Neck. 2005;27:536–542. doi: 10.1002/hed.20181. [DOI] [PubMed] [Google Scholar]
- 9.Kumar M, Fasanmade A, Barrett AW, et al. Metastasising clear cell odontogenic carcinoma: a case report and review of the literature. Oral Oncol. 2003;39:190–194. doi: 10.1016/S1368-8375(02)00012-X. [DOI] [PubMed] [Google Scholar]
- 10.Aguiar MC, Gomez RS, Silva EC, et al. Clear-cell ameloblastoma (clear-cell odontogenic carcinoma): report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:79–83. doi: 10.1016/S1079-2104(96)80153-3. [DOI] [PubMed] [Google Scholar]
- 11.Piattelli A, Sesenna E, Trisi P. Clear cell odontogenic carcinoma. Report of a case with lymph node and pulmonary metastases. Eur J Cancer Part B, Oral Oncol. 1994;30B:278–280. doi: 10.1016/0964-1955(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 12.Chera BS, Villaret DB, Orlando CA, et al. Clear cell odontogenic carcinoma of the maxilla: a case report and literature review. Am J Otolaryngol. 2008;29:284–290. doi: 10.1016/j.amjoto.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki H, Yamauchi G, Hashimoto K, et al. Clear cell carcinoma of the mandibular gingiva ‘minor salivary gland’: a case report with immunohistochemical study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:e36–e40. doi: 10.1016/j.tripleo.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 14.Xavier FC, Rodini CO, Ramalho LM, et al. Clear cell odontogenic carcinoma: case report with immunohistochemical findings adding support to the challenging diagnosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:403–410. doi: 10.1016/j.tripleo.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Ruhin-Poncet B, Ghoul-Mazgar S, Hotton D, et al. Msx and dlx homeogene expression in epithelial odontogenic tumors. J Histochem Cytochem. 2009;57:69–78. doi: 10.1369/jhc.2008.951707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumamoto H, Yoshida M, Ooya K, et al. Immunohistochemical detection of hepatocyte growth factor, transforming growth factor-beta and their receptors in epithelial odontogenic tumors. J Oral Pathol Med. 2002;31:539–548. doi: 10.1034/j.1600-0714.2002.00121.x. [DOI] [PubMed] [Google Scholar]
- 17.Berho M, Huvos AG. Central hyalinizing clear cell carcinoma of the mandible and the maxilla a clinicopathologic study of two cases with an analysis of the literature. Hum Pathol. 1999;30:101–105. doi: 10.1016/S0046-8177(99)90308-8. [DOI] [PubMed] [Google Scholar]
- 18.Mesquita RA, Lotufo MA, Sugaya NN, et al. Peripheral clear cell variant of calcifying epithelial odontogenic tumor: Report of a case and immunohistochemical investigation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:198–204. doi: 10.1067/moe.2003.63. [DOI] [PubMed] [Google Scholar]
- 19.Ellis GL. Clear cell neoplasms in salivary glands: clearly a diagnostic challenge. Ann Diagn Pathol. 1998;2:61–78. doi: 10.1016/S1092-9134(98)80035-X. [DOI] [PubMed] [Google Scholar]
- 20.Tonon G, Modi S, Wu L, et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet. 2003;33:208–213. doi: 10.1038/ng1083. [DOI] [PubMed] [Google Scholar]
- 21.Khan H, Loya A, Azhar R, et al. Central mucoepidermoid carcinoma, a case report with molecular analysis of the TORC1/MAML2 gene fusion. Head Neck Pathol. 2010;4:261–264. doi: 10.1007/s12105-010-0191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seethala RR, Barnes EL, Hunt JL, et al. Epithelial-myoepithelial carcinoma: a review of the clinicopathologic spectrum and immunophenotypic characteristics in 61 tumors of the salivary glands and upper aerodigestive tract. Am J Surg Pathol. 2007;31:44–57. doi: 10.1097/01.pas.0000213314.74423.d8. [DOI] [PubMed] [Google Scholar]
- 23.Wang B, Brandwein M, Gordon R, et al. Primary salivary clear cell tumors–a diagnostic approach: a clinicopathologic and immunohistochemical study of 20 patients with clear cell carcinoma, clear cell myoepithelial carcinoma, and epithelial-myoepithelial carcinoma. Arch Pathol Lab Med. 2002;126:676–685. doi: 10.5858/2002-126-0676-PSCCTA. [DOI] [PubMed] [Google Scholar]
- 24.Dardick I, Leong I. Clear cell carcinoma: review of its histomorphogenesis and classification as a squamous cell lesion. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:399–405. doi: 10.1016/j.tripleo.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 25.Dardick I. Salivary gland tumor pathology. New York: Igaku-Shoin Medical Publishers; 1996. pp. 237–240. [Google Scholar]
- 26.Wysocki GP, Brannon RB, Gardner DG, et al. Histogenesis of the lateral periodontal cyst and the gingival cyst of the adult. Oral Surg Oral Med Oral Pathol. 1980;50:327–334. doi: 10.1016/0030-4220(80)90417-X. [DOI] [PubMed] [Google Scholar]
- 27.Eversole LR, Belton CM, Hansen LS. Clear cell odontogenic tumor: histochemical and ultrastructural features. J Oral Pathol. 1985;14:603–614. doi: 10.1111/j.1600-0714.1985.tb00537.x. [DOI] [PubMed] [Google Scholar]
- 28.Fan J, Kubota E, Imamura H, et al. Clear cell odontogenic carcinoma. A case report with massive invasion of neighboring organs and lymph node metastasis. Oral Surg Oral Med Oral Pathol. 1992;74:768–775. doi: 10.1016/0030-4220(92)90406-G. [DOI] [PubMed] [Google Scholar]
- 29.Brandwein M, Said-Al-Naief N, Gordon R, et al. Clear cell odontogenic carcinoma: report of a case and analysis of the literature. Arch Otolaryngol Head Neck Surg. 2002;128:1089–1095. doi: 10.1001/archotol.128.9.1089. [DOI] [PubMed] [Google Scholar]