Abstract
Lymphocytic host response (LHR) in the Risk Model is histologically quantified as the density of lymphocytes at the tumor interface (Brandwein-Gensler in Am J Surg Pathol, 34:676–688, 1; in, Am J Surg Pathol 29:167–178, 2). It is classified as strong, intermediate or weak, and is inversely associated with the risk of decreased time to disease progression. In this study, we test the hypothesis that strong LHR corresponds to a greater degree of adaptive cytotoxic T cell response as compared to moderate LHR. We studied resection specimens of primary oral squamous carcinoma classified as having either strong (n = 16), intermediate (n = 20) or weak (n = 4) LHR. CD20+, CD4+, & CD8+ cells were detected by immunohistochemistry and quantified at 40× with a grid; counting the 10 fields with the most lymphocytes at the tumor interface and within tumors. Mean counts/tumor were analyzed by the 2-sided T-test. Statistically significant differences were observed for interface CD8 cells with respect to strong versus moderate LHR, strong versus weak LHR, and moderate versus weak LHR, and tumor infiltrating CD8 cells with respect to strong versus weak LHR. Statistically significant differences were also observed for interface CD4 cells with respect to strong versus weak LHR, and moderate versus weak LHR. Statistically significant differences in interface B cell counts were seen with respect to strong versus weak LHR, and moderate versus weak LHR. Decreased CD8+ T cells were significantly associated with higher stage at presentation (P = 0.005); a direct, but nonsignificant correlation was seen between decreased CD8+ T cells and decreased survival time. Immune response at the tumor interface correlates with an adaptive T cell response; the degree of cytotoxic CD8+ cells infiltrate can distinguish between strong and intermediate LHR at the interface of oral carcinomas.
Keywords: Squamous cell carcinoma, Oral, Lymphocytic host response, Risk model, Cytotoxic CD8+ T-cells
Introduction
The histological Risk Model significantly correlates with disease progression and survival for patients with primary head and neck squamous cell carcinoma (HNSCC) when adjusted for clinical confounders [1, 2]. The model is based on the histological examination of resection specimens for oral and oropharyngeal squamous carcinomas, with the classification of three histological variables: worst pattern of invasion, perineural invasion and lymphocytic host response (LHR). LHR is assessed at the tumor interface light microscopically, and classified as either strong, intermediate, or limited, based on the presence of lymphoid nodules. Lymphoid nodules are defined as dense collections of lymphocytes directly adjacent to the tumor host interface; at 20× power the lymphocytes comprise at least 50% of the microscopic field adjacent to carcinoma. Tumors with strong LHR are defined as having at least one lymphoid nodule at the tumor interface per each low-power 4× microscopic field. Tumors with lymphoid response below this threshold, but with one or more lymphoid nodules, qualify as having intermediate LHR. Weak LHR was assigned for limited response that lacks any lymphoid nodules. We have demonstrated that strong LHR at the interface is associated with improved outcome [1], which is consistent with the concept that enhanced immune surveillance and adaptive immunity have protective impact for cancer patients [3]. Similarly, light microscopic assessment of tumor-infiltrating lymphocytes (categorized as either brisk, non-brisk, or absent) has been demonstrated to be a valuable prognosticator for patients with stage I/II cutaneous melanoma and vertical growth, as it significantly directly correlates with survival [4]. Galon demonstrated that greater T-cell adaptive immunity, as reflected by lymphocyte populations infiltrating colorectal carcinomas, is significantly associated with decreased recurrence rates [5].
Adaptive immunity is an antigen-specific response mediated by cytotoxic effector cells and antibodies; in contrast, innate immunity is a more generic inflammatory response to infection. CD8+ and CD4+ T-cells in tissue can reflect the potential for adaptive immunity. Cervical carcinomas with greater CD8+ T-cell infiltrates and higher CD8+/CD4+ ratios are less likely to metastasize to lymph nodes [6], whereas metastatic lymph nodes in head and neck carcinomas have significantly decreased CD8+ T-cell levels, supporting the concept that decreased adaptive immunity facilitates cancer metastasis [7]. Our aim here was to assess whether strong LHR corresponds to a greater degree of adaptive cytotoxic T cell response as compared to moderate LHR.
Materials and Methods
Patient Selection
Forty patients with first or second primary squamous cell carcinomas of the oral cavity, including lip, were selected from the archives of the Department of Pathology, Montefiore Medical Center, Bronx, New York based on the availability of resection specimen slides and blocks, and clinical outcome data. Clinicopathologic variables assessed included age, gender, smoking, alcohol use and stage via medical chart review and data from the cancer registry unit at Montefiore Medical Center. This study commenced after approval from the Institutional Review Board.
Assessment of LHR
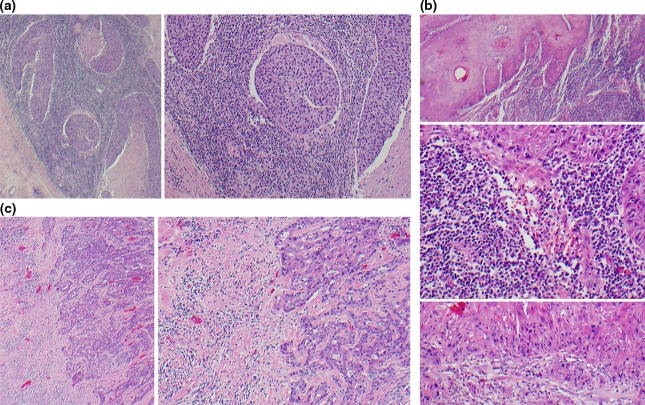
Hematoxylin and eosin stained glass slides were examined for the pattern of LHR and classified as a three-tiered variable: strong, intermediate or weak. A strong LHR was assigned when a continuous and dense rim of lymphoid tissue was present at the tumor advancing edge (interface). The threshold for classifying tumors with strong LHR is the presence of at least one interface lymphoid nodule at each low-power 4× field. Intermediate LHR was assigned when dense, but discontinuous lymphoid infiltrate was observed along the interface. The presence of any lymphoid nodule also qualified a tumor as intermediate LHR. Weak LHR was assigned for limited response that lacked any lymphoid nodules. For each case, the slide with the strongest immune response was selected for study. Strong and intermediate LHR tumors were over-selected for this study, as distinguishing between these two categories by light microscopy may be more difficult than separating either category from limited LHR. Figure 1a, b, and c illustrate examples of strong, intermediate, and weak LHR.
Fig. 1.
Examples of Lymphocytic Host Response (LHR) (a) Strong LHR appears as a dense rim of lymphocytes at the tumor interface. The lymphocytes may extend into the tumor, as is seen here. Left 40× overall magnification. Right 100× overall magnification. (b) A tumor with intermediate LHR has an interrupted lymphoid response at the interface. It is defined as having one or more lymphoid nodules at the interface, with one or more low-power fields lacking lymphoid nodules. Top: Dense interface lymphoid infiltrate is seen in one area of this tumor, 40× overall magnification. Middle: This photomicrograph demonstrates that the lymphocytes comprise at least 50% of the area within a 20× field directly adjacent to the carcinoma interface, 200× overall magnification. Bottom: Other regions of this tumor lacked interface lymphoid nodules, 100× overall magnification. (c) Tumors with weak LHR lack any lymphoid nodules. Left 40× overall magnification. Right 100× overall magnification
Immunohistochemistry, Cell Counting and Analysis
All sections were stained with Vector Monoclonal Mouse Anti-Human CD4 (Clone: 4B12, 1:100) and Dako Monoclonal Mouse Anti-Human CD8 (clone: C8/144B, 1:800) and Dako Monoclonal Mouse Anti-Human CD20 (clone: L26, 1:2000). In brief, 4–6-μm-thick sections were deparaffinized, rehydrated, and blocked with 3% hydrogen peroxide. Antigen was retrieved with Dako Target Retrieval Solution (pH 8 for CD4, pH 9 for CD8, and pH 6 for CD20), blocked in normal goat serum (5%) with bovine serum albumin (1%), and then incubated with primary antibodies (30 min at room temperature), followed by biotinylated secondary antibody (Dako EnVision® horseradish peroxidase labeled polymer conjugated with goat-anti-mouse antibody) for 30 min at room temperature, stained with Ultra-Marque diaminobenzidine reagent (2–4 min) and finally counterstained with hematoxylin (Harris formula, surgipath). Tonsillar tissue served as a positive control for the antibodies. Negative controls, with omission of the primary antibody, were also performed. Areas with the highest density of lymphocytic infiltrate were defined as regions of interest at low power (10× objective). CD4, CD8 and CD20 positive lymphocytes were counted in regions of interest within the tumor and at the tumor-host interface in ten high power microscopic fields (40× objective) using a grid (Olympus WHN10X-1-2).
Results
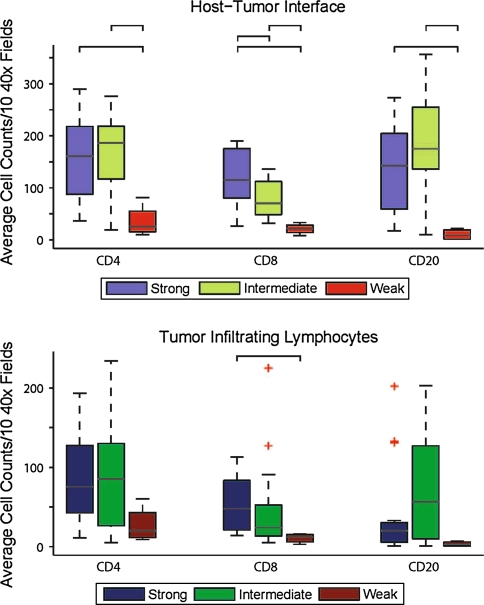
The distribution of tumors with strong, intermediate, and weak LHR was 40, 50 and 10%, respectively (Table 1). Statistically significant differences in interface CD8 cells were seen with respect to strong versus moderate LHR, strong versus weak LHR and moderate versus weak LHR, and CD8 tumor infiltrating lymphocytes (TIL) with respect to strong versus weak LHR (Table 2, Figs. 2, 3). Statistically significant differences between CD4 cells were also seen with respect to strong versus weak LHR, and moderate versus weak LHR, with respect to both TILS and interface lymphocytes. Statistically significant differences in interface CD20 cells were seen with respect to strong versus weak LHR, and moderate versus weak LHR. Based on the p value when the equal variances are assumed, the p for CD20 TIL intermediate versus weak is 0.058, which is not significant.
Table 1.
Clinicopathologic characteristics of patients in this study (n = 40)
| Clinicopathologic characteristic | Proportion |
|---|---|
| Age at diagnosis | |
| Range, mean ± SD | 35–89 years |
| 65.42 ± 13.486 | |
| LHR group | |
| Strong | 16 (40%) |
| Intermediate | 20 (50%) |
| Weak | 4 (10%) |
| Gender | |
| Male | 28 (70%) |
| Female | 12 (30%) |
| Race-ethnicity | |
| White—non Hispanic | 19 (47.5%) |
| White—hispanic | 6 (15%) |
| African American | 5 (12.5%) |
| Other | 1 (2.5%) |
| Unspecified | 9 (22.5%) |
| Tumor site | |
| Lip/oral cavity | 40 (100%) |
| Tumor number | |
| 1st. Primary | 30 (75%) |
| 2nd. Primary | 5 (12.5%) |
| Unspecified | 5 (12.5%) |
| Smoker versus non smoker | 25 (62.5%) |
| Alcohol use | 7 (17.5%) |
| Survival time (month) | |
| Range, mean ± SD | 1–91 months |
| Stage | |
| I | 8 (20%) |
| II | 5 (12.5%) |
| III | 4 (10%) |
| IV | 19 (47.5%) |
| Unspecified | 4 (10%) |
| Treatment modality | |
| Surgical resection | 15 (37.5%) |
| Surgery with other modality | 17 (42.5%) |
| Chemo-radiotherapy | 3 (7.5%) |
| Unspecified | 5 (12.5%) |
Table 2.
Comparison of Interface and Intratumoral Lymphocytes between LHR Groups (* P < 0.05) [Equal variances assumed]
| Cell counts | Host–tumor interface | Tumor infiltrating lymphocytes | ||||||
|---|---|---|---|---|---|---|---|---|
| LHR groups (mean counts) | Sig. (2-tailed) | 95% confidence interval | LHR groups (mean counts) | Sig. (2-tailed) | 95% confidence interval | |||
| Upper | Lower | Upper | Lower | |||||
| CD4 | Strong (163.9) | 0.964 | −53.7 | 51.4 | Strong (90.4) | 0.908 | −42.4 | 47.5 |
| Intermediate (165.1) | Intermediate (87.8) | |||||||
| Strong (163.9) | 0.008* | 37.7 | 219.5 | Strong (90.4) | 0.051 | −0.33 | 126.6 | |
| Weak (35.2) | Weak (27.2) | |||||||
| Intermediate (165.1) | 0.002* | 53.0 | 206.6 | Intermediate (87.8) | 0.092 | −10.8 | 132.0 | |
| Weak (35.2) | Weak (27.2) | |||||||
| CD8 | Strong (121.8) | 0.005* | 13.4 | 71.9 | Strong (54.3) | 0.511 | −20.8 | 41.0 |
| Intermediate (79.1) | Intermediate (44.2) | |||||||
| Strong (121.8) | 0.001* | 44.5 | 156.5 | Strong (54.3) | 0.016* | 8.97 | 78.2 | |
| Weak (21.2) | Weak (10.7) | |||||||
| Intermediate (79.1) | 0.003* | 21.6 | 94.0 | Intermediate (44.2) | 0.233 | −23.1 | 90.1 | |
| Weak (21.2) | Weak (10.7) | |||||||
| CD20 | Strong (140.5) | 0.094 | −111.2 | 9.1 | Strong (41.6) | 0.226 | −66.6 | 16.3 |
| Intermediate (191.6) | Intermediate (66.8) | |||||||
| Strong (140.5) | 0.004* | 46.6 | 214.5 | Strong (41.6) | 0.222 | −25.2 | 101.6 | |
| Weak (10.0) | Weak (3.5) | |||||||
| Intermediate (191.6) | 0.001* | 80.6 | 282.6 | Intermediate (66.8) | 0.058 | −2.3 | 129.0 | |
| Weak (10.0) | Weak (3.5) | |||||||
Fig. 2.
Strong LHR significantly correlates with CD8 cells at the tumor interface. Top: Box plot representing average counts for CD4, CD8, and CD20 cells at the interface for SCC classified as having a strong, intermediate or weak lymphocytic host response (LHR). Bottom: Box plot representing average counts for CD4 CD8, and CD20 tumor infiltrating lymphocytes (TIL) for SCC classified as having a strong, intermediate or weak LHR. Brackets show significant (Student’s t-test, P < 0.05) pair-wise comparisons in T-cell counts between the two LHR groups indicated. Outliers are plotted individually as +. Statistically significant differences were observed for interface CD8 cells with respect to strong versus moderate LHR, strong versus weak LHR, and moderate versus weak LHR, and CD8 TIL with respect to strong versus weak LHR. Statistically significant differences were also observed for interface CD4 cells with respect to strong versus weak LHR, and moderate versus weak LHR. Statistically significant differences in interface CD20 counts were seen with respect to strong versus weak LHR, and moderate versus weak LHR. Based on the p value when the equal variances are assumed, the p for CD20 TIL intermediate versus weak is 0.058, which is not significant
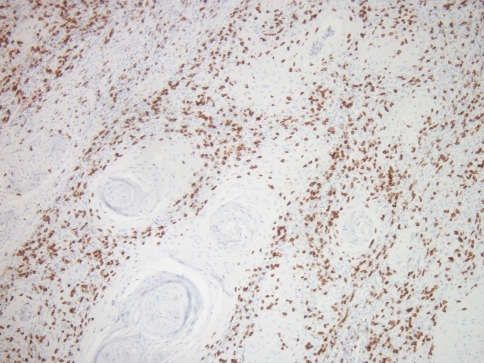
Fig. 3.
CD8 cells at the interface with intra-tumoral extension
Lower CD8+ T cell counts were also significantly associated with higher stage at presentation (P = 0.005). No significant associations were found between CD8+ T cells and patient age, gender, smoking or alcohol exposure. There was a non-significant inverse association between interface CD8+ T cells and decreased overall survival among oral cavity cancer patients after adjusting for nodal status, smoking status and age (adjusted hazards ratio 0.12, 95% CI 0.01–1.1).
Discussion
Here we confirm that lymphocytic host response (LHR), one component of the Risk Model, corresponds to an adaptive T-cell immune response; we also demonstrate that interface CD8+ T cells are significantly increased in tumors classified with strong LHR, as compared to intermediate LHR. LHR is presently evaluated at the tumor interface on hematoxylin and eosin stained slides. To enhance the reproducibility of LHR classification, we adhere to specific light microscopic definitions, based on the recognition of lymphoid nodules [2]. Lymphoid nodules are defined as dense collections of lymphocytes directly adjacent to the tumor host interface. The size and density threshold for these nodules are confirmed at 20× power; the lymphocytes need to comprise at least 50% of the microscopic field adjacent to carcinoma. Tumors with strong LHR are densely rimmed with lymphocytes. There may be gaps in the lymphocyte rimming as long as there is at least one lymphoid nodule at the tumor interface per each low-power 4× microscopic field. Tumors with lymphoid response below this threshold, but with one or more lymphoid nodules qualify as having intermediate LHR. Weak LHR was assigned for limited response that lacks any lymphoid nodules. When assessed by these criteria, the interobserver reproducibility is very good (data not shown).
One precedent for the light microscopic and semiquantitative evaluation of immune response for the purposes of prognostication is the classification of tumor infiltrating lymphocytes (TILs) in melanoma, one of a number of College of American Pathologists mandated variables to be included in surgical pathology reporting of melanoma. In future studies, we will explore whether specific lymphocyte phenotypes at the interface are better predictors of outcome than the current light microscopic LHR measure, and as such should be incorporated into the Risk Model. Our rationale in this study for oversampling tumors with strong and intermediate LHR, is that it can be more difficult to distinguish between these two categories by light microscopy than to separate either category from limited LHR. Our findings justify investigating the predictive performance of interface CD8+ T cells; we expect that examining interface CD8+ T cells will be especially helpful in distinguishing LHR from mucosal-associated B cells.
We find an inverse correlation between interface CD8+ T cells and stage at presentation. We also find a direct correlation between interface CD8+ T cells and improved survival time, albeit not reaching statistical significance; this is consistent with the protective effect of an adaptive immune response to cancer. However, this study design is not powered to observe a significant difference in outcome based on interface CD8+ cells; sample size calculations estimate that approximately 300 patients will be necessary. We adjusted for biological heterogeneity by limiting the study group to a single tumor location (oral cavity and lip, which comprise one staging group in the American Joint Committee on Cancer 7th staging manual). It is unlikely that the inclusion of second primary tumors would impact this study; in our study of 305 patients with 311 carcinomas, we analyzed the data with and without inclusion of subsequent new cancers, and found the results unchanged.
Zancope and colleagues also found that greater interface CD8+ T cells were associated with longer mean survival (58.2 months, 95% CI 44, 73 vs. 31.7 months, 95% CI 17, 47, Kaplan–Meier, log rank; P = 0.09) for patients with oral squamous cell carcinomas [8]. Katou demonstrated differences in the activation states between intratumoral and interface lymphocytes associated with oral squamous cell carcinoma [9]. Intratumoral CD8+ T cells are more likely to co-express PD-1, a marker associated with suppressed function, as compared to interface CD8+ T cells. By contrast interface CD8+ T cells are more likely to express NKG2D and Ki67, reflecting an activated phenotype, as compared to intratumoral CD8+ T cells. They also demonstrated that intratumoral natural killer (NK) cells are more likely to express NKG2A, reflecting suppressed function, as compared to interface NK cells. These researchers recently demonstrated the prognostic significance of T-regulatory cells (T-regs) and CD8+ T cells in a group of 87 patients with primary oral squamous carcinoma [10]. The overall balance between the two opposing functions of cytotoxic CD8+ T cell response versus the T-reg-mediated suppression of CD8+ and dendritic cells is critical to the success of anti-tumor immune surveillance. T-regs (CD4+CD25+Foxp3+) have a suppressive impact on immune response; CCR4+ T-regs (memory-type T-regs) are primed to suppress CD8+ T cell proliferation in the absence of antibody-mediated activation [11]. High numbers of interface CCR+ T-regs, and low ratio of interface CD8+ T-cells/CCR4+ T-regs were significantly associated with decreased overall survival on Kaplan–Meier analysis for these patients with oral cancer [10]. Plotting the ratio of interface CD8+/CCR4+ T-regs demonstrated the greatest separation between the two groups (high interface CD8+ T cells and low CCR4+ T-regs versus low interface CD8+ T cells and high CCR4+ T-regs) with respect to overall survival (P < 0.001), as compared to other variables [10]. However, they demonstrated that interface CD8+ T cells alone are also significantly predictive of overall survival (P = 0.001). This is relevant to the development of a predictive model which is simple to institute and accessible to all surgical pathologists.
In conclusion, we demonstrate evidence of an adaptive immune response to oral squamous carcinomas. Interface CD8+ T cells can significantly distinguish between strong and intermediate LHR as defined by our Risk Model. Thus it will be interesting to compare the predictive performance of LHR to CD8+ T cells with respect to refining the Risk Model.
References
- 1.Brandwein-Gensler M, Smith RV, Wang B, Penner C, Theilken A, Broughel D, Schiff B, Owen RP, Smith J, Sarta C, Hebert T, Nason R, Ramer M, DeLacure M, Hirsch D, Myssiorek D, Heller K, Prystowsky M, Schlecht NF, Negassa A. Validation of the histologic risk model in a new cohort of patients with head and neck squamous cell carcinoma. Am J Surg Pathol. 2010;34:676–688. doi: 10.1097/PAS.0b013e3181d95c37. [DOI] [PubMed] [Google Scholar]
- 2.Brandwein-Gensler M, Teixeira MS, Lewis CM, Lee B, Rolnitzky L, Hille JJ, Genden E, Urken ML, Wang BY. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29:167–178. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 3.Yu P, Fu YX. Tumor-infiltrating T lymphocytes: friends or foes? Lab Invest. 2006;86:231–245. doi: 10.1038/labinvest.3700389. [DOI] [PubMed] [Google Scholar]
- 4.Clemente CG, Mihm MC, Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 6.Piersma SJ, Jordanova ES, Poelgeest MI, Kwappenberg KM, Hulst JM, Drijfhout JW, Melief CJ, Kenter GG, Fleuren GJ, Offringa R, Burg SH. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67:354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- 7.Verastegui E, Morales R, Barrera JL, Müeller A, Guzman B, Meneses A, Alfaro G. Immunological approach in the evaluation of regional lymph nodes of patients with squamous cell carcinoma of the head and neck. Clin Immunol. 2002;102:37–47. doi: 10.1006/clim.2001.5130. [DOI] [PubMed] [Google Scholar]
- 8.Zancope E, Costa NL, Junqueira-Kipnis AP, Valadares MC, Silva TA, Leles CR, Mendonça EF, Batista AC. Differential infiltration of CD8+ and NK cells in lip and oral cavity squamous cell carcinoma. J Oral Pathol Med. 2010;39:162–167. doi: 10.1111/j.1600-0714.2009.00792.x. [DOI] [PubMed] [Google Scholar]
- 9.Katou F, Ohtani H, Watanabe Y, Nakayama T, Yoshie O, Hashimoto K. Differing phenotypes between intraepithelial and stromal lymphocytes in early-stage tongue cancer. Cancer Res. 2007;67:11195–11201. doi: 10.1158/0008-5472.CAN-07-2637. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe Y, Katou F, Ohtani H, Nakayama T, Yoshie O, Hashimoto K. Tumor-infiltrating lymphocytes, particularly the balance between CD8(+) T cells and CCR4(+) regulatory T cells, affect the survival of patients with oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:744–752. doi: 10.1016/j.tripleo.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Baatar D, Olkhanud P, Sumitomo K, Taub D, Gress R, Biragyn A. Human peripheral blood T regulatory cells (Tregs), functionally primed CCR4+ Tregs and unprimed CCR4- Tregs, regulate effector T cells using FasL. J Immunol. 2007;178:4891–4900. doi: 10.4049/jimmunol.178.8.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]