Abstract
Phospholipase A2 (PLA2) is a group of enzymes that hydrolyze the sn-2 position of glycerophospholipids to yield fatty acids and lysophospholipids. Of many PLA2s or related enzymes identified to date, secreted PLA2s (sPLA2s) comprise the largest family that contains 10 catalytically active isozymes. Besides arachidonic acid released from cellular membranes for eicosanoid synthesis, several if not all sPLA2s have recently been implicated in hydrolysis of phospholipids in lipoprotein particles. The sPLA2-processed low-density lipoprotein (LDL) particles contain a large amount of lysophospholipids and exhibit the property of “small-dense” or “modified” LDL, which facilitates foam cell formation from macrophages. Transgenic overexpression of these sPLA2s leads to development of atherosclerosis in mice. More importantly, genetic deletion or pharmacological inhibition of particular sPLA2s significantly attenuates atherosclerosis and aneurysm. In this article, we will give an overview of current understanding of the role of sPLA2s in atherosclerosis, with recent lipidomics data showing the action of a subset of sPLA2s on lipoprotein phospholipids.
Keywords: Lipids, Enzymes, Mass spectrometry
Classification of sPLA2s
The sPLA2 family represents structurally related, disulfide-rich, low molecular weight, lipolytic enzymes with a His-Asp catalytic dyad. sPLA2s occur in a wide variety of vertebrate and invertebrate animals, plants, bacteria, and viruses, and 10 catalytically active sPLA2 isozymes (IB, IIA, IIC, IID, IIE, IIF, III, V, X, and XIIA) are identified in mammals. Of these, sPLA2s belonging to the group I/II/V/X collection are closely related 14–19-kDa proteins of secreted enzymes with a highly conserved Ca2+-binding loop and a His-Asp catalytic site. In addition to these elements, there are six absolutely conserved disulfide bonds and up to two additional unique disulfide bonds, which contribute to the high degree of stability of these enzymes. Group III and group XII sPLA2s share little homology with the I/II/V/X collection of sPLA2s except for the Ca2+-binding loop and the catalytic site, thereby representing the distinct group collections. Unlike intracellular PLA2s, sPLA2s hydrolyze glycerophospholipids only in the presence of millimolar concentrations of Ca2+, suggesting that they primarily act on “extracellular” substrates. Since individual sPLA2s display distinct cellular/tissue distributions and substrate head group/fatty acid specificities, they may play non-redundant, isoform-specific roles in vivo. The latest biochemistry and biology of the sPLA2 family have been detailed in recent reviews [1, 2].
Lipoprotein hydrolysis by sPLA2s: in vitro studies
Lipoprotein modification and atherosclerosis; a general view
A lipoprotein is a biochemical assembly that contains both proteins and lipids whose function is to transport water-insoluble lipids in the water-based bloodstream. Examples include the high-density (HDL) and low-density (LDL) lipoproteins, which enable fats to be carried between the blood and tissues. Since higher levels of LDL particles promote health problems and cardiovascular disease typically known as atherosclerosis, they are often called the bad cholesterol particles, as opposed to HDL particles that are frequently referred to as good cholesterol or healthy cholesterol particles. LDL particles vary in size and density, and studies have shown that high plasma levels of small dense LDL particles rather than larger and less dense LDL particles well correlate with a higher risk for coronary heart disease. The surfaces of LDL and HDL are surrounded by phospholipids, mainly phosphatidylcholine (PC), which, as a matter of fact, serves as a very good “extracellular” target of several if not all sPLA2 isoforms.
It has been believed that a key step of pro-atherogenic small-dense LDL generation is oxidative modification of the polyunsaturated fatty acids (PUFAs) in phospholipids on LDL surface. However, the “oxidation hypothesis of atherosclerosis” [3] still remains inconclusive, as oxidation alone cannot fully explain the accumulation of large amounts of lipids and lysophosphatidylcholine (LPC) in foam cells and fatty streak lesion formation [4]. Current knowledge suggests that sPLA2-mediated modification of lipoproteins plays a role in the development of atherosclerosis [5, 6]. This idea originally arose from the following key observations. Hydrolysis of PC in lipoproteins by sPLA2 produces free fatty acids (typically unsaturated) and LPC, which can trigger vasoactive, chemotactic, and pro-inflammatory actions leading to the acceleration of atherosclerosis. Hydrolysis of LDL by sPLA2 correlates with production of the more atherogenic, small-dense, modified LDL with increased net negative charge, whereas hydrolysis of HDL reduces the capacity of this anti-atherogenic particle to promote cholesterol efflux from lipid-rich foam cells. Modified LDL retained in atherosclerotic lesions contains less PC and more LPC than does circulating LDL, suggesting that arterial LDL undergoes lipolytic modification by certain extracellular PLA2 enzyme(s) at lesion sites. Further, clinical analyses have shown that elevated plasma PLA2 activity (likely sPLA2-IIA) is an independent risk factor for cardiovascular disease [7, 8], and a low content of surface phospholipids often characterizes the small-dense LDL and HDL subclasses [9].
Hydrolysis of lipoprotein-bound phospholipids by sPLA2s can give rise to the two pro-atherogenic and pro-inflammatory lipid products, lysophospholipids and fatty acids. LPC modulates the expression of a number of proteins such as cytokines, chemokines, growth factors, adhesion molecules, inducible nitric oxide synthase and cyclooxygenase-2 [10]. LPC plays an ethiologic role in atherosclerosis, is a major constituent of atherogenic lipoproteins [11], and exhibits pro-inflammatory functions including activation of macrophages as well as induction of chemotactic factors and adhesion molecules in endothelial cells [12]. Lysophosphatidic acid (LPA), an autotaxin-hydrolyzed product of LPC that elicits numerous effects on cells of the cardiovascular system, induces the formation of arterial neointima lesions, a prelude of atherosclerosis, through the PPARγ-dependent mechanism [13]. LPA accumulates in the lipid-rich core of human carotid atherosclerotic plaques [14]. Arachidonate-oxygenated lipid mediators, including prostaglandins (PGs) and leukotrienes, also have diverse effects on atherosclerosis, as evidenced by studies employing knockout mice for their receptors or biosynthetic enzymes. For instance, gene ablation of thromboxane A2 receptor or PGE2 synthase ameliorates, whereas that of PGI2 receptor or PGD2 synthase exacerbates, the experimental atherosclerosis in mice [15–17]. Mice lacking 5- or 12/15-lipoxygenase are also partially protected from the development of atherosclerosis [18, 19]. Thus, increased production of these pro-atherogenic lipid mediators may account, at least in part, for the pro-atherogenic action of sPLA2s. A proposed idea for the mechanistic action of sPLA2s on the development of atheroslcerosis is illustrated in Fig. 1.
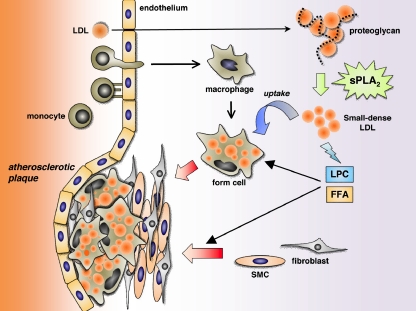
Fig. 1.
A proposed role of sPLA2 in the development of atherosclerosis. In the arterial wall, multiple sPLA2s are present in macrophages and smooth muscle cells as well as in the extracellular matrix. LDL captured by the extracellular matrix proteoglycan is hydrolyzed by proteoglycan-bound (IIA and V) or -unbound (III and X) sPLA2s to be converted to small-dense LDL, which in turn facilitates macrophage foam cell formation and thereby atherosclerosis development. Free fatty acids (FFA) and LPC or their metabolites, released by sPLA2s from LDL, can activate macrophages and smooth muscle cells and promote collagen deposition in the atherosclerotic plaques. Additional effects of sPLA2s within the plaques should also be considered. For details, please see the text
However, a series of initial studies describing the relationship among sPLA2, lipoprotein hydrolysis and atherosclerosis have some concerns that should be interpreted more carefully. First, many studies using sPLA2s from snake or bee venom could be misleading, since the properties of venom sPLA2s are distinct from those of mammalian sPLA2s. Second, even if mammalian sPLA2s were used, their concentrations employed were often very high (>100 nM) that could be out of the physiological level. Third, many investigators had an incorrect recognition that all or most mammalian sPLA2s are induced during inflammation and can exist in the plasma. However, it is only sPLA2-IIA that is strongly induced under pathologic conditions associated with inflammation, tissue injury or infection, and actually there have been no convincing reports that other sPLA2 isoforms are present in the circulation [1, 2]. Fourth, although LPC released by sPLA2s from lipoprotein particles has been proposed to be a critical inducer of atherosclerotic cellular events, LPC already exists in the plasma at a very high level (as much as hundreds μM). Finally, considering the recent concept that atherosclerosis is a mild and chronic inflammation in the arterial wall [20], pro-inflammatory changes, in addition to modification of lipoproteins, in the plaques should be taken into consideration as a causal factor in which sPLA2s might be involved. Nevertheless, the physiological relevance of the potential contribution of sPLA2s to atherosclerosis is recently being elucidated by several elegant studies employing sPLA2 gene-manipulated mice as well as an sPLA2-targeted small molecule inhibitor, as described later.
Application of mass spectrometry in analyzing sPLA2 hydrolysis of lipoprotein-bound phospholipids
In the past five years, several studies have examined the hydrolytic activity of human sPLA2s toward LDL- or HDL-associated phospholipids using mass spectrometry (MS). These approaches have delineated the fundamental differences in lipoprotein hydrolysis by distinct human sPLA2s. Several quantitative analyses have shown that sPLA2-V and -X are 20 ~ 30 times more reactive on PC in HDL and LDL than sPLA2-IB and -IIA [21–23]. Interestingly, sPLA2-X hydrolyzes arachidonate- and linoleate-containing PC species preferentially, group V hydrolyzes oleoyl- and linoleate-PC in preference to arachidonate-PC, and sPLA2-IIA hydrolyzes randomly all diacyl molecular species. The hydrolysis of minor phospholipid species (e.g. phosphatidylinositol and phosphatidylserine) in HDL and LDL by sPLA2-V and -X is low relative to that of PC [24]. As a result, these acidic phospholipids remain at higher levels in both LDL and HDL, thereby increasing the acidity of the modified particles. Although the activity of sPLA2-IIA on lipoproteins is relatively weak (also see below), it can hydrolyze acute phase HDL 2 ~ 3-fold more efficiently than normal HDL, with preferential attack on PC with oxygenated PUFAs [25]. LDL hydrolysis by sPLA2s is also affected by the contents of other lipid components such as sphingomyelin (SM) and neutral lipids, since higher percentages of SM interfere with LDL hydrolysis by sPLA2-IIA and -V [22] and since LDL from patients with type 2 diabetes is more susceptible than that from normal subjects to sPLA2-V hydrolysis [26, 27].
We performed electrospray ionization MS (ESI-MS) to directly compare the hydrolytic activity of six human sPLA2 isoforms, IIA, IIE, IIF, III, V and X, on PC in human LDL and HDL particles (for more details, please see [28]). Both LDL and HDL particles contained three major PC molecular species (C16:0–18:2, C18:0–18:2 and C18:0–20:4) and only trace levels of LPC molecular species (C16:0 and C18:0). When LDL was treated with a low concentration (10 nM) of sPLA2s for 4 h, three sPLA2s, namely III, V and X, robustly increased both LPC species (Fig. 2a). The capacities of these three enzymes to produce LPC and lysophosphatidylethanolamine (LPE) from LDL were nearly comparable (Fig. 2b). Marked increases of LPC species were also observed when HDL was incubated with 10 nM sPLA2-V or -X, whereas the activity of sPLA2-III on HDL-bound PC at this concentration was modest even though significant (Fig. 3a). Notably, all PC species were dramatically reduced by sPLA2-X, linoleate-containing PC species were reduced in preference to arachidonate-containg PC by sPLA2-V, and arachidonate-containing PC was preferentially reduced by sPLA2-III, revealing differences in the potency and fatty acid selectivity of these three sPLA2s on HDL-associated PC. In addition to LPC, LPE was also greatly increased when HDL was treated with sPLA2-V or sPLA2-X, and to a lesser extent with sPLA2-III (Fig. 3b). The ability of sPLA2-IIA and -IIE to hydrolyze PC in LDL and HDL was minimal even at 50 nM, while sPLA2-IIF at this concentration showed significant activity with hydrolysis of arachidonate-PC to produce C16:0-LPC in LDL and to produce both C16:0- and C18:0-LPC in HDL (Fig. 4).
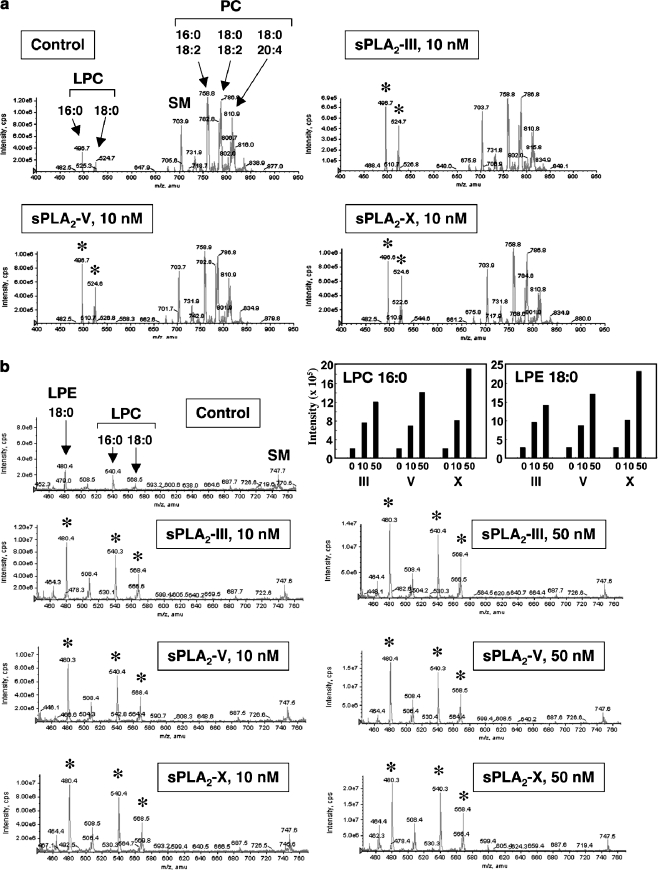
Fig. 2.
Hydrolysis of human LDL by recombinant human sPLA2-III, -V and –X in vitro. After LDL (1 mg/ml) was incubated with or without 10 or 50 nM sPLA2 for 4 h at 37°C, lipids were extracted and applied to ESI-MS (4000Q TRAP; Applied Biosystems), as described previously [74, 75]. Representative results of ESI-MS for choline-containing phospholipids on a positive ion mode (a) and lysophospholipids on a negative ion mode (b) are shown. Major peaks of PC, LPC, LPE and SM molecular species are indicated. Asterisks indicate the peaks that were significantly changed by addition of each sPLA2. Top-right graphs show quantified data of LPC and LPE. For more details, please see [28]
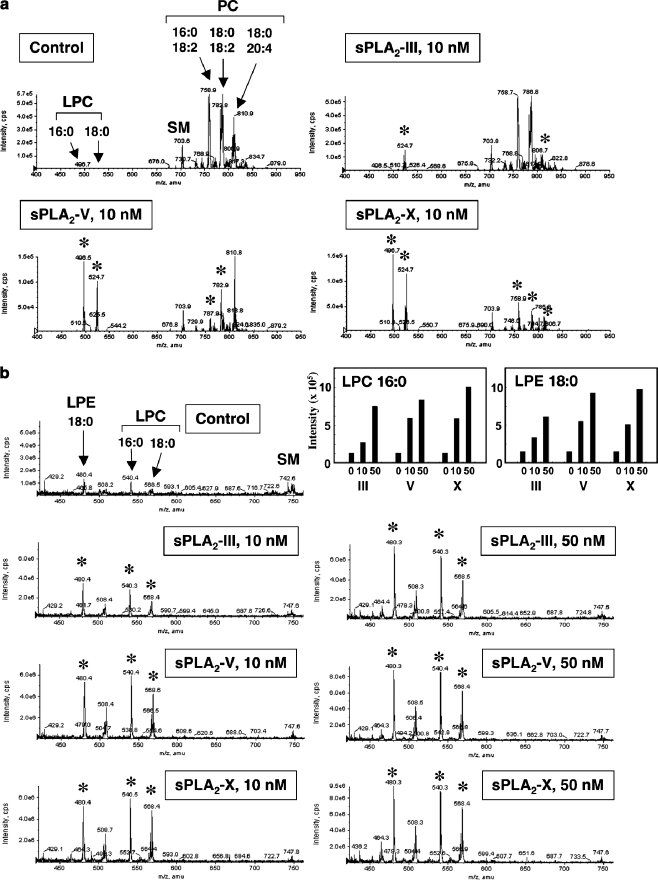
Fig. 3.
Hydrolysis of human HDL by recombinant human sPLA2-III, -V and –X in vitro. After HDL (1 mg/ml) was incubated with or without 10 or 50 nM sPLA2 for 4 h at 37°C, lipids were extracted and applied to ESI-MS. Representative results of ESI-MS for choline-containing phospholipids on a positive ion mode (a) and lysophospholipids on a negative ion mode (b) are shown. Major peaks of PC, LPC, LPE and SM molecular species are indicated. Asterisks indicate the peaks that were significantly changed by addition of each sPLA2. Top-right graphs show quantified data of LPC and LPE. For more details, please see [28]
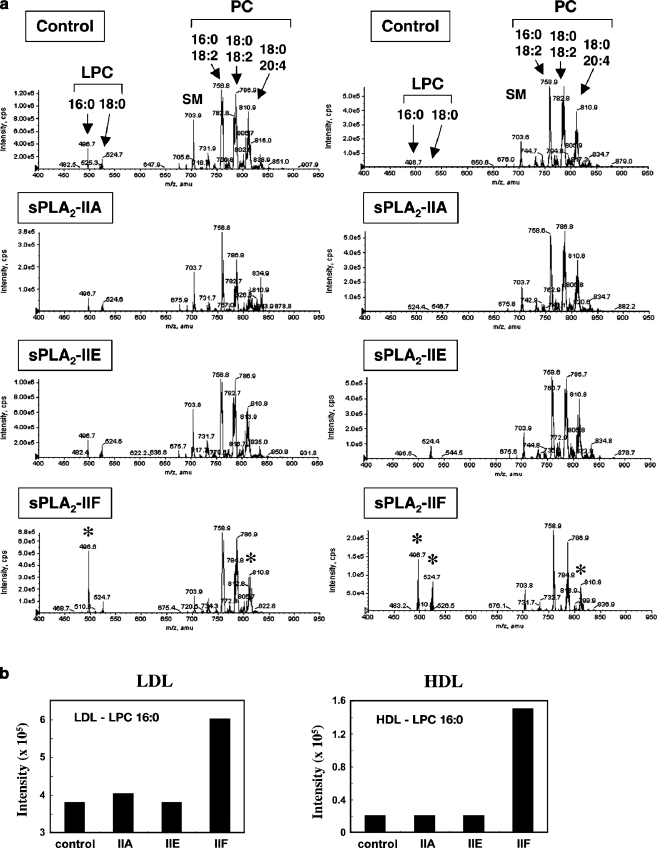
Fig. 4.
Hydrolysis of human LDL and HDL by recombinant human sPLA2-IIA, -IIE and –IIF in vitro. After LDL or HDL (1 mg/ml) was incubated with or without 50 nM sPLA2 for 4 h at 37°C, lipids were extracted and applied to ESI-MS. (a) Representative results of ESI-MS for choline-containing phospholipids on a positive ion mode are shown. Major peaks of PC, LPC, and SM molecular species are indicated on the top. Asterisks indicate the peaks that were significantly changed by addition of each sPLA2. (b) Quantified data of LPC in LDL (left) and HDL (right) after incubation with each sPLA2 are shown. For more details, please see [28]
Taking these results altogether, the rank order of the hydrolytic potency of various human sPLA2s, as evaluated by ESI-MS, is X > V > III > IIF > IIA, IIE for both LDL and HDL. This order appears to roughly correlate with their ability to interact with PC-rich vesicles and with PC-rich cellular plasma membranes [1, 2]. Note that, although sPLA2-IIA did not show a detectable level of activity in our experimental setting (see above), previous studies employing very high concentrations of sPLA2-IIA have shown that it could hydrolyze lipoprotein-bound PC to some extents, particularly oxidized lipoproteins [25, 29, 30]. Since the expression level of sPLA2-IIA is considerably higher than those of other sPLA2s and it is the only sPLA2 isoform detected in the circulation of mammals (except mice) [1, 2], it is still plausible that sPLA2-IIA participates in atherosclerotic lipoprotein hydrolysis in vivo, as discussed below.
Cellular actions of sPLA2-treated LDL
Atherosclerosis, and the resulting coronary heart disease and cerebral stroke, represent one of the most common causes of death in industrial nations. Cholesterol-engorged macrophages and their detritus following cell death comprise a major volume of early fatty streak plaques as well as the most typical advanced lesions of arteries. Unregulated uptake of cholesterol by macrophages results in the accumulation of multiple lipid droplets leading to the aptly named “foam cell” phenotype [31]. Numerous studies have described a variety of foam cell responses that would contribute to the growth and rupture of the vessel wall plaques of atherosclerosis, and the cholesterol-loaded macrophages appear to contribute to the inception of the process, the lethal conclusion in plaque rupture, and the triggering of the occlusive thrombosis [32]. Oxidized LDL, a generally recognized form of modified LDL, is believed to bring about in the sub-endothelial space where circulating anti-oxidant defense are less effective. Mildly oxidized LDL can stimulate the release of chemokines by endothelial cells, increase the adherence and penetration of monocytes, and induce scavenger receptor A (SR-A) and CD36 expression in macrophages [33–35]. Extensively oxidized LDL becomes a ligand for SR-A and other scavenger receptors that contribute to foam cell formation by facilitating uptake of lipoprotein particles.
The sPLA2-hydrolyzed LDL particles, with increased LPC contents and small diameter, can potently promote lipid droplet accumulation in macrophages, a process reminiscent of foam cell formation [28, 36, 37]. Indeed, as does oxidized LDL, sPLA2-modified LDL shows some typical features of pro-atherogenic particles, such as increased affinity for matrix proteoglycans and propensity of aggregation [36, 38]. Association of sPLA2-IIA or -V with matrix proteoglycans increases the hydrolysis of LDL-associated PC [39–41]. Furthermore, treatment with sPLA2-IIA renders LDL more susceptible to oxidative modification and increases its affinity for matrix proteoglycans. Conceivably, the close spatial contact between sPLA2-IIA and LDL on proteoglycans may allow their efficient interaction, and sPLA2-IIA can promote aggregation and fusion of the proteoglycan-bound LDL, leading to progressive deposition of lipids within the extracellular matrices of the arterial intima, a central feature of atherosclerosis [39–41]. Uptake of sPLA2-V-treated LDL by macrophages depends on binding to syndecan 4, a cellular proteoglycan, rather than to the scavenger receptors SR-A and CD36 [41, 42]. LDL lipolysis by sPLA2-V results in production of free fatty acids such as oleic and linoleic acids, which augment TNFα and IL-6 secretion by macrophages [43]. Lipolytic modification of HDL by sPLA2-V or -X reduces its capacity to promote cholesterol efflux from lipid-loaded macrophages, thereby reducing its anti-atherogenic function [44]. The sPLA2-modified LDL can also affect the function of endothelial cells. The pan-sPLA2 inhibitor, indoxam, suppresses LDL modification and associated inflammatory responses (such as NF-κB activation and chemokine production) in TNFα-stimulated human endothelial cells, which express sPLA2-V [45]. Also, LDL modified by sPLA2-X increases the endothelial expression of leukocyte adhesion molecules [46].
Although these studies emphasize the pro-inflammatory (and thereby pro-atherosclerotic) aspects of sPLA2s, the opposite, anti-inflammatory aspect of these enzymes should also be taken into account. Indeed, the biological actions of sPLA2-V, -X and -IID in vivo can often be associated with anti-inflammatory events [47–49], and activation of PPARδ in endothelial cells by snake venom PLA2-released PUFAs can switch on the anti-inflammatory (rather than pro-inflammatory) program [50]. However, it remains unclear whether PUFAs released by mammalian sPLA2(s) from lipoprotein particles would play an anti-inflammatory or anti-atherosclerotic role.
sPLA2s and Atherosclerosis: in vivo studies
Expression of sPLA2s in atherosclerotic plaques
sPLA2-IIA is located in macrophage-rich regions, lipid cores of atheromas, and the extracellular matrix of the diseased intima in association with collagen fibers in human atherosclerotic lesions [51]. sPLA2-V is also enriched in atherosclerotic lesions of humans and experimental animals, in which it is associated with smooth muscle cells and also surrounding foam cells in lipid core areas [52, 53]. A hyperlipidemic high-fat diet up-regulates sPLA2-V expression in the aorta, and apoE −/− x Ldlr −/− mice, in which atherosclerosis develops spontaneously, show elevated aortic sPLA2-V expression [52]. sPLA2-X is also immunohistochemically detected in atherosclerotic lesions in both humans and apoE −/− mice [37, 46]. In humans, sPLA2-X is detected in the intima where it is localized in the majority of foam cells and in phenotypically de-differenciated smooth muscle cells resembling myofibroblasts as well as in the extracellular matrix, but not detectable in T-lymphocytes and in the lesion-free areas. sPLA2-III is focally expressed in advanced lesions of atheroma in human and apoE −/− mice, mainly in macrophages and smooth muscle cells [28]. Other sPLA2s (IID, IIE, IIF) are also detected by immunohistochemistry and in situ hybridization in human atheroslcerotic plaques, with sPLA2-IIF exhibiting the most notable induction in accordance with the developmental process of atherosclerosis [54].
sPLA2-IIA
The principal experimental evidence for the potential role of mammalian sPLA2 in atherosclerosis has arisen from studies employing transgenic mice overexpressing human sPLA2-IIA (PLA2G2A-Tg) [55], beyond the fact that the C57BL/6 mouse strain intrinsically lacks sPLA2-IIA as a result of a natural mutation of its gene [56]. PLA2G2A-Tg mice maintained on high-cholesterol atherogenic diet exhibit increased atherosclerotic lesions [55]. In these mice, sPLA2-IIA is present in atherosclerotic lesions in the aorta, and the plasma level of HDL is lower and that of LDL is slightly higher in PLA2G2A-Tg mice than those in control mice. Of importance, transplantation of bone marrow cells from PLA2G2A-Tg mice into recipient Ldlr −/− mice results in a significant increase in the extent of atherosclerosis in the aortic arch and sinus despite the absence of alteration in lipoprotein composition, suggesting that macrophage-derived sPLA2-IIA can exert a local pro-atherogenic effect with enhancement of collagen deposition by a process independent of systemic lipoprotein metabolism [57]. Thus, even though the hydrolytic action of sPLA2-IIA on PC in LDL and HDL is relatively low, it is still possible that only local modification of lipoproteins by this enzyme within vascular walls is sufficient for development of atherosclerosis.
sPLA2-V
The findings that sPLA2-V can hydrolyze LDL- and HDL-associated PC far more efficiently than does sPLA2-IIA and that the LDL modified by sPLA2-V efficiently induces macrophage foam cell formation, as described above, have led to the idea that this enzyme is more important than sPLA2-IIA for the promotion of atherosclerosis [58]. Importantly, Ldlr −/− mice subjected to retrovirus-mediated gene transfer of Pla2g5 cDNA have increased lesion area in the ascending aortic root with a concomitant elevation of regional collagen deposition, whereas mice transplanted with bone marrow cells from Pla2g5 −/− mice show reduced atherosclerosis in the aortic arch and thoracic aorta [53]. This result clearly indicates that sPLA2-V exerts a pro-atherogenic function in vivo. Surprisingly, however, reduction of atherosclerotic lesion size is not evident in apoE −/− mice reconstituted with Pla2g5 −/− bone marrow cells, probably because the lipoprotein lipid compositions are distinct between the Ldlr −/− and apoE −/− backgrounds [59]. Nevertheless, the collagen content of the plaques is significantly reduced in lesions of apoE −/− mice lacking sPLA2-V. It should be noted, however, that these bone marrow transplantation approaches could assess the role of sPLA2-V expressed only in macrophages or other hematopoietic cells. Hence, the impact of sPLA2-V expressed in non-hematopoietic cells on atherosclerosis still remains unknown. Of note, a recent tagging single nucleotide polymorphism analysis has demonstrated an association of the human PLA2G5, but not PLA2G2A, gene haplotype with the plasma levels of LDL and oxidized LDL in patients with type 2 diabetes [26].
sPLA2-X
sPLA2-X also potently hydrolyzes phospholipids in LDL and HDL in vitro, with an effect even superior to that of sPLA2-V (see above). A recent study has demonstrated that the deficiency of sPLA2-X on the ApoE −/− background significantly reduces the incidence and severity of angiotensin II-induced abdominal aortic aneurysm and atherosclerosis, accompanied by reduction of pro-inflammatory mediators [60]. Moreover, another study using Pla2g10 −/− macrophages has provided an additional view that sPLA2-X negatively regulates cholesterol efflux from macrophages through altering the liver X receptor (LXR)-dependent expression of ABC transporters [61]. These results support the idea that sPLA2-X has a pro-atherogenic role in vivo. In humans, however, non-synonymous polymorphism in the PLA2G10 gene, which leads to a profound change in the expression and activity of sPLA2-X, has no detectable impact on cardiovascular disease risk, whereas another polymorphism located in the 5′-untranslated region is associated with a decreased, rather than increased, risk of recurrent cardiovascular events [62]. Considering that sPLA2-X can also exert an anti-inflammatory function probably through producing anti-inflammatory PUFAs or their metabolites [48], the mechanistic action of sPLA2-X in atherosclerosis could not be simply explained only by alterations in the lipoprotein modification. Further investigation should be needed to elucidate the role of sPLA2-X.
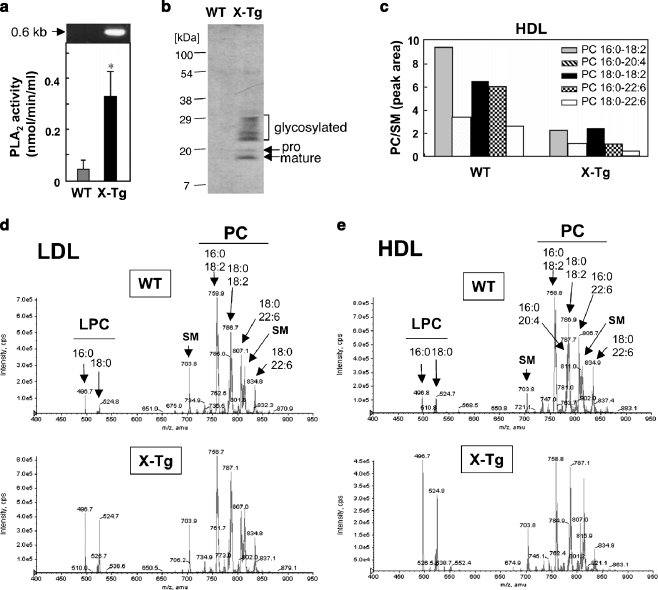
To assess whether sPLA2-X has the capacity to hydrolyze lipoprotein PC in vivo, we examined the lipoprotein profiles in plasma of transgenic mice overexpressing human sPLA2-X (PLA2G10-Tg) [63] in comparison with littermate wild-type (WT) mice. Plasma PLA2 activity, as evaluated by release of [14C]linoleic acid from 1-palmitoyl-2-[14C]linoleoyl-phosphatidylethanolamine, was dramatically elevated in PLA2G10-Tg mice over WT mice (Fig. 5a). sPLA2-X is synthesized as an inactive pro-enzyme, and cleavage of the N-terminal propeptide gives rise to a mature active enzyme, which further undergoes N-glycosylation [64, 65]. Accordingly, sPLA2-X proteins (mature, pro- and glycosylated forms) were detected in the plasma of PLA2G10-Tg mice, as assessed by immunoblotting using anti- sPLA2-X antibody (Fig. 5b). Lipids were extracted from LDL and HDL of these mice and subjected to ESI-MS for phospholipid analysis (Fig. 5c–e). In both LDL and HDL, there were robust increases in C16:0- and C18:0-LPC (Fig. 5d and e), with a concomitant decrease in all PC molecular species (Fig. 5c), in PLA2G10-Tg mice relative to WT mice. These results suggest that sPLA2-X overexpressed in PLA2G10-Tg mice hydrolyzed LDL- and HDL-associated PC robustly in vivo.
Fig. 5.
Altered lipoprotein profiles in PLA2G10-Tg mice. (a and b) PLA2 enzymatic activity was markedly elevated (mean ± S.D., n = 4, *p < 0.05) (a) and sPLA2-X proteins (mature, precursor- and glycosylated forms) were detectable by immunoblotting with anti-sPLA2-X antibody (b) in 8-wk-old PLA2G10-Tg (X-Tg) mice. Top panel in (a) shows the expression of the PLA2G10 transgene in PLA2G10-Tg mice, as assessed by RT-PCR using a primer set that amplified the full-length PLA2G10. (c–e) ESI-MS analysis of choline-containing phospholipids in lipoprotein particles from WT and X-Tg mice. Individual PC molecular species in HDL of PLA2G10-Tg and WT mice were quantified (c). Representative ESI-MS spectra of LDL (d) and HDL (e) particles purified from WT (left) and X-Tg (right) mice are shown. Major peaks of PC, LPC, and SM molecular species are indicated on the top. In both LDL and HDL, peaks of LPC species were markedly increased in X-Tg mice compared with WT mice
It should be noted, however, that endogenous sPLA2-X was undetectable in the plasma of WT mice (Fig. 5b), and that we observed no difference in the lipoprotein composition between Pla2g10 −/− and littermate Pla2g10 +/+ mice under physiological conditions [66]. Therefore, even though the above study employing PLA2G10-Tg mice has pointed that sPLA2-X has the capacity to hydrolyze lipoprotein PC in vivo, its physiological importance remains unclear. Presumably, under certain pathological conditions, the expression level or proteolytic processing of sPLA2-X is increased locally (e.g. in atherosclerotic lesions), where it could contribute to hydrolysis of lipoprotein PC. Indeed, a study using PLA2G10-Tg mice has provided evidence that the proteolytic processing of sPLA2-X is facilitated at inflamed sites [63].
sPLA2-III
sPLA2-III can efficiently hydrolyze PC in LDL and to a lesser extent in HDL (see above). sPLA2-III-modified LDL, like sPLA2-V- or sPLA2-X-treated LDL, facilitates the formation of foam cells from macrophages ex vivo [28]. After intake of an atherogenic diet, aortic atherosclerotic lesions are more severe in mice with transgenic overexpression of human sPLA2-III (PLA2G3-Tg) than in control mice on an apoE −/− background [28]. In these mice, plasma LDL and HDL are significantly hydrolyzed by the enzyme, and peritoneal macrophages readily store lipid droplets in the cytoplasm after exposure to LDL ex vivo. Lipidomics studies demonstrate the elevation of LPC as well as thromboxane A2 and 12-hydoxyeicosaenic acid, which are arachidonate-derived products by activated platelets, in plasma of PLA2G3-Tg mice relative to WT mice. Interestingly, PLA2G3-Tg mice also develop systemic inflammation [67], suggesting that the elevated inflammatory state in the vascular wall may have an additional impact on the promotion of atherosclerosis in these mice. Although these observations suggest a potential functional link between sPLA2-III and atherosclerosis, its pathological relevance awaits further study employing Pla2g3 −/− mice.
Pharmacologic effect of sPLA2 inhibitor on atherosclerosis
Accumulating evidence as mentioned above suggests that sPLA2 may represent a novel target for atherosclerosis and associated cardiovascular diseases. The potent sPLA2 inhibitors, which broadly inhibits sPLA2s in the group I/II/V/X branch at low nM potency in vitro, include the functionalized indole scaffolds, such as indoxam, methyl-indoxam and LY315920, from Eli Lilly and Shionogi [68]. The development of these compounds involves structure-guided improvement of binding capacity starting with a lead compound obtained through high-throughput screening and making use of the X-ray structure of human sPLA2-IIA [69]. Interestingly, A-002 (1-H-indole-3-glyoxamide or varespladib), a lead compound of this pan-sPLA2 inhibitor series, can decrease the area of atherosclerotic lesions dramatically, accompanied by a 1.4-fold increase in HDL, in apoE −/− mice fed a high-fat diet [70–72]. Combinational treatment of animals with pravastatin (a member of the drug class of statins (HMG-CoA reductase inhibitors)) and A-002 decreases the lesion area and plasma cholesterol level even more, suggesting a synergistic effect between the two agents in amelioration of atherosclerosis through decreased levels of systemic inflammation or circulating lipids. A-002 treatment also stabilizes the plaque architecture. Because apoE −/− mice (C57BL/6 background) intrinsically lack sPLA2-IIA due to a natural mutation [56], the anti-atherosclerotic effect of A-002 could be attributable to the inhibition of other sPLA2 isoforms, probably sPLA2-V or –X (note that A-002 does not inhibit sPLA2-III, an atypical sPLA2). Furthermore, a phase II double-blind, randomised, placebo-controlled trial to assess the effects of A-002 in human patients with coronary heart disease has demonstrated that the serum concentration of sPLA2 (most likely sPLA2-IIA), as well as the levels of vascular (oxidized LDL) and general (C-reactive protein) inflammation markers, decreases progressively to nearly an order of magnitude less than the baseline, with no increase in adverse events [73]. A-002 also reduces the concentration of LDL cholesterol and the number of LDL particles, mainly by reducing small-dense LDL. Thus, A-002 shows promising reduction of biomarkers and effects on surrogate endpoints, encouraging further investigation of whether it can reduce cardiovascular disease events without any other off-target toxicity. Although it is uncertain whether A-002 exerts its anti-atherosclerotic effect in humans by inhibiting circulating sPLA2-IIA, plaque-associated sPLA2-V and -X, or both, these animal and early-phase clinical studies nevertheless suggest that sPLA2s could be exciting therapeutic targets for atherosclerosis.
Concluding Remarks
In this article, we have highlighted a current view of the role of sPLA2s in lipoprotein hydrolysis and atherosclerosis. Needless to say, MS-based lipidomics has greatly contributed to expanding our understanding of sPLA2-mediated hydrolysis of lipoprotein phospholipids. sPLA2s have also been implicated in various biological processes, such as asthma, arthritis, cancer, antimicrobial defense and reproduction, among others [1, 2]. However, therapeutic or prophylactic efficacies of the sPLA2 inhibitors should be carefully evaluated, since gene targeting of several sPLA2s have revealed that distinct isoforms often display opposite functions in a given pathology [47]. In this sense, inhibition of multiple sPLA2s altogether could inhibit both offensive and defensive sPLA2 isozymes and thereby cancel the therapeutic effect resulting from the inhibition of the pro-inflammatory one(s), as has been seen for human rheumatoid arthritis in which a pan-sPLA2 inhibitor had no beneficial effect [74]. Thus, using an inhibitor that specifically blocks only the offensive sPLA2 may be a more desirable strategy than using the currently tested pan-sPLA2 inhibitors that block group I/II/V/X sPLA2s altogether. Nonetheless, all the above knowledge, together with application of lipidomics to in vivo systems, should help proper identification of certain PLA2s and their target phospholipids or their metabolites as therapeutic targets or as novel biotherapeutic molecules in various diseases including atherosclerosis.
Acknowledgments
We thank for Drs. R. Taguchi, K. Ikeda (University of Tokyo) and T. Kobayashi (Ochanomizu University) for ESI-MS analysis, and for Dr. Yokoyama (The Tokyo Metropolitan Institute of Medical Science) for English edition. This work was supported by grants-in-aid for Scientific Research and for Young Scientists from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- ESI-MS
Electrospray ionization-mass spectrometry
- HDL
High-density lipoprotein
- LDL
Low-density lipoprotein
- LPA
Lysophosphatidic acid
- LPC
Lysophosphatidylcholine
- LPE
Lysophosphatidylethanolamine
- PC
Phosphatidylcholine
- PG
Prostaglandin
- PUFA
Polyunsaturated fatty acid
- SM
Sphingomyelin
- sPLA2
Secreted phospholipase A2
Footnotes
Published in the special issue Biomedical Mass Spectrometry with Guest Editors Hisao Oka and Mitsutoshi Setou.
References
- 1.Murakami M, Taketomi Y, Girard C, Yamamoto K, Lambeau G. Emerging roles of secreted phospholipase A2 enzymes: lessons from transgenic and knockout mice. Biochimie. 2010;92:561–582. doi: 10.1016/j.biochi.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198901053200122. [DOI] [PubMed] [Google Scholar]
- 4.Quinn MT, Parthasarathy S, Steinberg D. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci USA. 1988;85:2805–2809. doi: 10.1073/pnas.85.8.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webb NR. Secretory phospholipase A2 enzymes in atherogenesis. Curr Opin Lipidol. 2005;16:341–344. doi: 10.1097/01.mol.0000169355.20395.55. [DOI] [PubMed] [Google Scholar]
- 6.Murakami M, Kudo I. New phospholipase A2 isozymes with a potential role in atherosclerosis. Curr Opin Lipidol. 2003;14:431–436. doi: 10.1097/00041433-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Kugiyama K, Ota Y, Takazoe K, Moriyama Y, Kawano H, Miyao Y, Sakamoto T, Soejima H, Ogawa H, Doi H, Sugiyama S, Yasue H. Circulating levels of secretory type II phospholipase A2 predict coronary events in patients with coronary artery disease. Circulation. 1999;100:1280–1284. doi: 10.1161/01.cir.100.12.1280. [DOI] [PubMed] [Google Scholar]
- 8.Mallat Z, Steg PG, Benessiano J, Tanguy ML, Fox KA, Collet JP, Dabbous OH, Henry P, Carruthers KF, Dauphin A, Arguelles CS, Masliah J, Hugel B, Montalescot G, Freyssinet JM, Asselain B, Tedgui A. Circulating secretory phospholipase A2 activity predicts recurrent events in patients with severe acute coronary syndromes. J Am Coll Cardiol. 2005;46:1249–1257. doi: 10.1016/j.jacc.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 9.Hurt-Camejo E, Camejo G, Peilot H, Oorni K, Kovanen P. Phospholipase A2 in vascular disease. Circ Res. 2001;89:298–304. doi: 10.1161/hh1601.095598. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz G, Ruebsaamen K. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis. 2010;208:10–18. doi: 10.1016/j.atherosclerosis.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 11.Kume N, Cybulsky MI, Gimbrone MA., Jr Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest. 1992;90:1138–1144. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murugesan G, Sandhya Rani MR, Gerber CE, Mukhopadhyay C, Ransohoff RM, Chisolm GM, Kottke-Marchant K. Lysophosphatidylcholine regulates human microvascular endothelial cell expression of chemokines. J Mol Cell Cardiol. 2003;35:1375–1384. doi: 10.1016/j.yjmcc.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, Baker DL, Yasuda S, Makarova N, Balazs L, Johnson LR, Marathe GK, McIntyre TM, Xu Y, Prestwich GD, Byun HS, Bittman R, Tigyi G. Lysophosphatidic acid induces neointima formation through PPARγ activation. J Exp Med. 2004;199:763–774. doi: 10.1084/jem.20031619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rother E, Brandl R, Baker DL, Goyal P, Gebhard H, Tigyi G, Siess W. Subtype-selective antagonists of lysophosphatidic Acid receptors inhibit platelet activation triggered by the lipid core of atherosclerotic plaques. Circulation. 2003;108:741–747. doi: 10.1161/01.CIR.0000083715.37658.C4. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi T, Tahara Y, Matsumoto M, Iguchi M, Sano H, Murayama T, Arai H, Oida H, Yurugi-Kobayashi T, Yamashita JK, Katagiri H, Majima M, Yokode M, Kita T, Narumiya S. Roles of thromboxane A2 and prostacyclin in the development of atherosclerosis in apoE-deficient mice. J Clin Invest. 2004;114:784–794. doi: 10.1172/JCI21446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M, Zukas AM, Hui Y, Ricciotti E, Puré E, FitzGerald GA. Deletion of microsomal prostaglandin E synthase-1 augments prostacyclin and retards atherogenesis. Proc Natl Acad Sci USA. 2006;103:14507–14512. doi: 10.1073/pnas.0606586103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ragolia L, Palaia T, Hall CE, Maesaka JK, Eguchi N, Urade Y. Accelerated glucose intolerance, nephropathy, and atherosclerosis in prostaglandin D2 synthase knock-out mice. J Biol Chem. 2005;280:29946–29955. doi: 10.1074/jbc.M502927200. [DOI] [PubMed] [Google Scholar]
- 18.Mehrabian M, Allayee H, Wong J, Shi W, Wang XP, Shaposhnik Z, Funk CD, Lusis AJ. Identification of 5-lipoxygenase as a major gene contributing to atherosclerosis susceptibility in mice. Circ Res. 2002;91:120–126. doi: 10.1161/01.RES.0000028008.99774.7F. [DOI] [PubMed] [Google Scholar]
- 19.Cyrus T, Witztum JL, Rader DJ, Tangirala R, Fazio S, Linton MF, Funk CD. Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in apo E-deficient mice. J Clin Invest. 1999;103:1597–1604. doi: 10.1172/JCI5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 21.Ishimoto Y, Yamada K, Yamamoto S, Ono T, Notoya M, Hanasaki K. Group V and X secretory phospholipase A2s-induced modification of high-density lipoprotein linked to the reduction of its antiatherogenic functions. Biochim Biophys Acta. 2003;1642:129–138. doi: 10.1016/S0167-4889(03)00120-4. [DOI] [PubMed] [Google Scholar]
- 22.Gesquiere L, Cho W, Subbaiah PV. Role of group IIa and group V secretory phospholipases A2 in the metabolism of lipoproteins. Substrate specificities of the enzymes and the regulation of their activities by sphingomyelin. Biochemistry. 2002;41:4911–4920. doi: 10.1021/bi015757x. [DOI] [PubMed] [Google Scholar]
- 23.Pruzanski W, Lambeau L, Lazdunsky M, Cho W, Kopilov J, Kuksis A. Differential hydrolysis of molecular species of lipoprotein phosphatidylcholine by groups IIA, V and X secretory phospholipases A2. Biochim Biophys Acta. 2005;1736:38–50. doi: 10.1016/j.bbalip.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Pruzanski W, Lambeau G, Lazdunski M, Cho W, Kopilov J, Kuksis A. Hydrolysis of minor glycerophospholipids of plasma lipoproteins by human group IIA, V and X secretory phospholipases A2. Biochim Biophys Acta. 2007;1771:5–19. doi: 10.1016/j.bbalip.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Pruzanski W, Stefanski E, de Beer FC, de Beer MC, Vadas P, Ravandi A, Kuksis A. Lipoproteins are substrates for human secretory group IIA phospholipase A2: preferential hydrolysis of acute phase HDL. J Lipid Res. 1998;39:2150–2160. [PubMed] [Google Scholar]
- 26.Wootton PT, Arora NL, Drenos F, Thompson SR, Cooper JA, Stephens JW, Hurel SJ, Hurt-Camejo E, Wiklund O, Humphries SE, Talmud PJ (2007) Tagging SNP haplotype analysis of the secretory PLA2-V gene, PLA2G5, shows strong association with LDL and oxLDL levels, suggesting functional distinction from sPLA2-IIA: results from the UDACS study. Hum Mol Genet 16:1437–1444 [DOI] [PubMed]
- 27.Pettersson C, Fogelstrand L, Rosengren B, Ståhlman S, Hurt-Camejo E, Fagerberg B, Wiklund O. Increased lipolysis by secretory phospholipase A2 group V of lipoproteins in diabetic dyslipidaemia. J Intern Med. 2008;264:155–165. doi: 10.1111/j.1365-2796.2008.01932.x. [DOI] [PubMed] [Google Scholar]
- 28.Sato H, Kato R, Isogai Y, Saka G, Ohtsuki M, Taketomi Y, Yamamoto K, Tsutsumi K, Yamada J, Masuda S, Ishikawa Y, Ishii T, Kobayashi T, Ikeda K, Taguchi R, Hatakeyama S, Hara S, Kudo I, Itabe H, Murakami M. Analyses of group III secreted phospholipase A2 transgenic mice reveal potential participation of this enzyme in plasma lipoprotein modification, macrophage foam cell formation, and atherosclerosis. J Biol Chem. 2008;283:33483–33497. doi: 10.1074/jbc.M804628200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurt-Camejo E, Andersen S, Standal R, Rosengren B, Sartipy P, Stadberg E, Johansen B. Localization of nonpancreatic secretory phospholipase A2 in normal and atherosclerotic arteries. Activity of the isolated enzyme on low-density lipoproteins. Arterioscler Thromb Vasc Biol. 1997;17:300–309. doi: 10.1161/01.atv.17.2.300. [DOI] [PubMed] [Google Scholar]
- 30.Eckey R, Menschikowski M, Lattke P, Jaross W. Minimal oxidation and storage of low density lipoproteins result in an increased susceptibility to phospholipid hydrolysis by phospholipase A2. Atherosclerosis. 1997;132:165–176. doi: 10.1016/S0021-9150(97)00088-9. [DOI] [PubMed] [Google Scholar]
- 31.Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- 32.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990 s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 33.Shiffman D, Mikita T, Tai JT, Wade DP, Porter JG, Seilhamer JJ, Somogyi R, Liang S, Lawn RM. Large scale gene expression analysis of cholesterol-loaded macrophages. J Biol Chem. 2000;275:37324–37332. doi: 10.1074/jbc.M004732200. [DOI] [PubMed] [Google Scholar]
- 34.Cushing SD, Berliner JA, Valente AJ, Territo MC, Navab M, Parhami F, Gerrity R, Schwartz CJ, Fogelman AM. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci USA. 1990;87:5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida H, Quehenberger O, Kondratenko N, Green S, Steinberg D. Minimally oxidized low-density lipoprotein increases expression of scavenger receptor A, CD36, and macrosialin in resident mouse peritoneal macrophages. Arterioscler Thromb Vasc Biol. 1998;18:794–802. doi: 10.1161/01.atv.18.5.794. [DOI] [PubMed] [Google Scholar]
- 36.Wooton-Kee CR, Boyanovsky BB, Nasser MS, de Villiers WJ, Webb NR. Group V sPLA2 hydrolysis of low-density lipoprotein results in spontaneous particle aggregation and promotes macrophage foam cell formation. Arterioscler Thromb Vasc Biol. 2004;24:762–767. doi: 10.1161/01.ATV.0000122363.02961.c1. [DOI] [PubMed] [Google Scholar]
- 37.Hanasaki K, Yamada K, Yamamoto S, Ishimoto Y, Saiga A, Ono T, Ikeda M, Notoya M, Kamitani S, Arita H. Potent modification of low density lipoprotein by group X secretory phospholipase A2 is linked to macrophage foam cell formation. J Biol Chem. 2002;277:29116–29124. doi: 10.1074/jbc.M202867200. [DOI] [PubMed] [Google Scholar]
- 38.Hakala JK, Oörni K, Pentikäinen MO, Hurt-Camejo E, Kovanen PT. Lipolysis of LDL by human secretory phospholipase A2 induces particle fusion and enhances the retention of LDL to human aortic proteoglycans. Arterioscler Thromb Vasc Biol. 2001;21:1053–1058. doi: 10.1161/01.atv.21.6.1053. [DOI] [PubMed] [Google Scholar]
- 39.Sartipy P, Johansen B, Camejo G, Rosengren B, Bondjers G, Hurt-Camejo E. Binding of human phospholipase A2 type II to proteoglycans. Differential effect of glycosaminoglycans on enzyme activity. J Biol Chem. 1996;271:26307–26314. doi: 10.1074/jbc.271.42.26307. [DOI] [PubMed] [Google Scholar]
- 40.Sartipy P, Johansen B, Gâsvik K, Hurt-Camejo E. Molecular basis for the association of group IIA phospholipase A2 and decorin in human atherosclerotic lesions. Circ Res. 2000;86:707–714. doi: 10.1161/01.res.86.6.707. [DOI] [PubMed] [Google Scholar]
- 41.Boyanovsky BB, van der Westhuyzen DR, Webb NR. Group V secretory phospholipase A2-modified low density lipoprotein promotes foam cell formation by a SR-A- and CD36-independent process that involves cellular proteoglycans. J Biol Chem. 2005;280:32746–32752. doi: 10.1074/jbc.M502067200. [DOI] [PubMed] [Google Scholar]
- 42.Sun B, Boyanovsky BB, Connelly MA, Shridas P, van der Westhuyzen DR, Webb NR. Distinct mechanisms for OxLDL uptake and cellular trafficking by class B scavenger receptors CD36 and SR-BI. J Lipid Res. 2007;48:2560–25670. doi: 10.1194/jlr.M700163-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Boyanovsky BB, Li X, Shridas P, Sunkara M, Morris AJ, Webb NR. Bioactive products generated by group V sPLA2 hydrolysis of LDL activate macrophages to secrete pro-inflammatory cytokines. Cytokine. 2010;50:50–57. doi: 10.1016/j.cyto.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishimoto Y, Yamada K, Yamamoto S, Ono T, Notoya M, Hanasaki K (2003) Group V and X secretory phospholipase A2s-induced modification of high-density lipoprotein linked to the reduction of its antiatherogenic functions. Biochim Biophys Acta 1642:129–138 [DOI] [PubMed]
- 45.Sonoki K, Iwase M, Sasaki N, Ohdo S, Higuchi S, Takata Y, Iida M (2008) Secretory PLA2 inhibitor indoxam suppresses LDL modification and TNFα-stimulated human endothelial cells. Br J Pharmacol 153:1399–1408 [DOI] [PMC free article] [PubMed]
- 46.Karabina SA, Brochériou I, Le Naour G, Agrapart M, Durand H, Gelb M, Lambeau G, Ninio E. Atherogenic properties of LDL particles modified by human group X secreted phospholipase A2 on human endothelial cell function. FASEB J. 2006;20:2547–2549. doi: 10.1096/fj.06-6018fje. [DOI] [PubMed] [Google Scholar]
- 47.Boilard E, Lai Y, Larabee K, Balestrieri B, Ghomashchi F, Fujioka D, Gobezie R, Coblyn JS, Weinblatt ME, Massarotti EM, Thornhill TS, Divangahi M, Remold H, Lambeau G, Gelb MH, Arm JP, Lee DM. A novel anti-inflammatory role for secretory phospholipase A2 in immune complex-mediated arthritis. EMBO Mol Med. 2010;2:172–187. doi: 10.1002/emmm.201000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Curfs DM, Ghesquiere SA, Vergouwe MN, van der Made I, Gijbels MJ, Greaves DR, Verbeek JS, Hofker MH, de Winther MP. (2008) Macrophage secretory phospholipase A2 group X enhances anti-inflammatory responses, promotes lipid accumulation, and contributes to aberrant lung pathology. J Biol Chem 283:21640–21648 [DOI] [PubMed]
- 49.von Allmen CE, Schmitz N, Bauer M, Hinton HJ, Kurrer MO, Buser RB, Gwerder M, Muntwiler S, Sparwasser T, Beerli RR, Bachmann MF. Secretory phospholipase A2-IID is an effector molecule of CD4+CD25+ regulatory T cells. Proc Natl Acad Sci USA. 2009;106:11673–11678. doi: 10.1073/pnas.0812569106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Namgaladze D, Morbitzer D, von Knethen A, Brune B. (2010) Phospholipase A2-modified low-density lipoprotein activates macrophage peroxisome proliferator-activated receptors. Arterioscler Thromb Vasc Biol. 2010;30:313–320. doi: 10.1161/ATVBAHA.109.199232. [DOI] [PubMed] [Google Scholar]
- 51.Romano M, Romano E, Bjorkerud S, Hurt-Camejo E. Ultrastructural localization of secretory type II phospholipase A2 in atherosclerotic and nonatherosclerotic regions of human arteries. Arterioscler Thromb Vasc Biol. 1998;18:519–525. doi: 10.1161/01.atv.18.4.519. [DOI] [PubMed] [Google Scholar]
- 52.Rosengren B, Peilot H, Umaerus M, Jönsson-Rylander AC, Mattsson-Hultén L, Hallberg C, Cronet P, Rodriguez-Lee M, Hurt-Camejo E. Secretory phospholipase A2 group V: lesion distribution, activation by arterial proteoglycans, and induction in aorta by a Western diet. Arterioscler Thromb Vasc Biol. 2006;26:1579–1585. doi: 10.1161/01.ATV.0000221231.56617.67. [DOI] [PubMed] [Google Scholar]
- 53.Bostrom MA, Boyanovsky BB, Jordan CT, Wadsworth MP, Taatjes DJ, de Beer FC, Webb NR. Group V secretory phospholipase A2 promotes atherosclerosis: evidence from genetically altered mice. Arterioscler Thromb Vasc Biol. 2007;27:600–606. doi: 10.1161/01.ATV.0000257133.60884.44. [DOI] [PubMed] [Google Scholar]
- 54.Kimura-Matsumoto M, Ishikawa Y, Komiyama K, Tsuruta T, Murakami M, Masuda S, Akasaka Y, Ito K, Ishiguro S, Morita H, Sato S, Ishii T. Expression of secretory phospholipase A2s in human atherosclerosis development. Atherosclerosis. 2008;196:81–91. doi: 10.1016/j.atherosclerosis.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 55.Ivandic B, Castellani LW, Wang XP, Qiao JH, Mehrabian M, Navab M, Fogelman AM, Grass DS, Swanson ME, de Beer MC, de Beer F, Lusis AJ. Role of group II secretory phospholipase A2 in atherosclerosis: 1. Increased atherogenesis and altered lipoproteins in transgenic mice expressing group IIa phospholipase A2. Arterioscler Thromb Vasc Biol. 1999;19:1284–1290. doi: 10.1161/01.atv.19.5.1284. [DOI] [PubMed] [Google Scholar]
- 56.MacPhee M, Chepenik KP, Liddell RA, Nelson KK, Siracusa LD, Buchberg AM. The secretory phospholipase A2 gene is a candidate for the Mom1 locus, a major modifier of ApcMin-induced intestinal neoplasia. Cell. 1995;81:957–966. doi: 10.1016/0092-8674(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 57.Webb NR, Bostrom MA, Szilvassy SJ, van der Westhuyzen DR, Daugherty A, de Beer FC. Macrophage-expressed group IIA secretory phospholipase A2 increases atherosclerotic lesion formation in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:263–268. doi: 10.1161/01.ATV.0000051701.90972.E5. [DOI] [PubMed] [Google Scholar]
- 58.de Beer FC, Webb NR. Inflammation and atherosclerosis: Group IIa and Group V sPLA2 are not redundant. Arterioscler Thromb Vasc Biol. 2006;26:1421–1422. doi: 10.1161/01.ATV.0000227561.89488.9a. [DOI] [PubMed] [Google Scholar]
- 59.Boyanovsky B, Zack M, Forrest K, Webb NR. The capacity of group V sPLA2 to increase atherogenicity of ApoE−/− and LDLR−/− mouse LDL in vitro predicts its atherogenic role in vivo. Arterioscler Thromb Vasc Biol. 2009;29:532–538. doi: 10.1161/ATVBAHA.108.183038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zack M, Boyanovsky BB, Shridas P, Bailey W, Forrest K, Howatt DA, Gelb MH, de Beer FC, Daugherty A, Webb NR. Group X secretory phospholipase A2 augments angiotensin II-induced inflammatory responses and abdominal aortic aneurysm formation in apoE-deficient mice. Atherosclerosis. 2010;214:58–64. doi: 10.1016/j.atherosclerosis.2010.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shridas P, Bailey WM, Gizard F, Oslund RC, Gelb MH, Bruemmer D, Webb NR. Group X secretory phospholipase A2 negatively regulates ABCA1 and ABCG1 expression and cholesterol efflux in macrophages. Arterioscler Thromb Vasc Biol. 2010;30:2014–2021. doi: 10.1161/ATVBAHA.110.210237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gora S, Perret C, Jemel I, Nicaud V, Lambeau G, Cambien F, Ninio E, Blankenberg S, Tiret L, Karabina SA. Molecular and functional characterization of polymorphisms in the secreted phospholipase A2 group X gene: relevance to coronary artery disease. J Mol Med. 2009;87:723–733. doi: 10.1007/s00109-009-0483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohtsuki M, Taketomi Y, Arata S, Masuda S, Ishikawa Y, Ishii T, Takanezawa Y, Aoki J, Arai H, Yamamoto K, Kudo I, Murakami M. Transgenic expression of group V, but not group X, secreted phospholipase A2 in mice leads to neonatal lethality because of lung dysfunction. J Biol Chem. 2006;281:36420–36433. doi: 10.1074/jbc.M607975200. [DOI] [PubMed] [Google Scholar]
- 64.Cupillard L, Koumanov K, Mattei MG, Lazdunski M, Lambeau G. Cloning, chromosomal mapping, and expression of a novel human secretory phospholipase A2. J Biol Chem. 1997;272:15745–15752. doi: 10.1074/jbc.272.25.15745. [DOI] [PubMed] [Google Scholar]
- 65.Masuda S, Murakami M, Takanezawa Y, Aoki J, Arai H, Ishikawa Y, Ishii T, Arioka M, Kudo I. Neuronal expression and neuritogenic action of group X secreted phospholipase A2. J Biol Chem. 2005;280:23203–23214. doi: 10.1074/jbc.M500985200. [DOI] [PubMed] [Google Scholar]
- 66.Sato H, Isogai Y, Masuda S, Taketomi Y, Miki Y, Kamei D, Hara S, Kobayashi T, Ishikawa Y, Ishii T, Ikeda K, Taguchi R, Ishimoto Y, Suzuki N, Yokota Y, Hanasaki K, Yamamoto T, Yamamoto K, Murakami M. (2011) Physiological roles of group X secreted phospholipase A2 in reproduction, gastrointestinal phospholipid digestion, and neuronal function. J Biol Chem 286:11616-11631 [DOI] [PMC free article] [PubMed]
- 67.Sato H, Taketomi Y, Isogai Y, Masuda S, Kobayashi T, Yamamoto K, Murakami M. Group III secreted phospholipase A2 transgenic mice spontaneously develop inflammation. Biochem J. 2009;421:17–27. doi: 10.1042/BJ20082429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dillard RD, Bach NJ, Draheim SE, Berry DR, Carlson DG, Chirgadze NY, Clawson DK, Hartley LW, Johnson LM, Jones ND, McKinney ER, Mihelich ED, Olkowski JL, Schevitz RW, Smith AC, Snyder DW, Sommers CD, Wery JP. Indole inhibitors of human nonpancreatic secretory phospholipase A2. 2. Indole-3-acetamides with additional functionality. J Med Chem. 1996;39:5119–5136. doi: 10.1021/jm960485v. [DOI] [PubMed] [Google Scholar]
- 69.Schevitz RW, Bach NJ, Carlson DG, Chirgadze NY, Clawson DK, Dillard RD, Draheim SE, Hartley LW, Jones ND, Mihelich ED, et al. Structure-based design of the first potent and selective inhibitor of human non-pancreatic secretory phospholipase A2. Nat Struct Biol. 1995;2:458–465. doi: 10.1038/nsb0695-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosenson RS, Hislop C, McConnell D, Elliott M, Stasiv Y, Wang N, Waters DD, Investigators PLASMA. Effects of 1-H-indole-3-glyoxamide (A-002) on concentration of secretory phospholipase A2 (PLASMA study): a phase II double-blind, randomised, placebo-controlled trial. Lancet. 2009;373:649–658. doi: 10.1016/S0140-6736(09)60403-7. [DOI] [PubMed] [Google Scholar]
- 71.Shaposhnik Z, Wang X, Trias J, Fraser H, Lusis AJ. The synergistic inhibition of atherogenesis in apoE−/− mice between pravastatin and the sPLA2 inhibitor varespladib (A-002) J Lipid Res. 2009;50:623–629. doi: 10.1194/jlr.M800361-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fraser H, Hislop C, Christie RM, Rick HL, Reidy CA, Chouinard ML, Eacho PI, Gould KE, Trias J. Varespladib (A-002), a secretory phospholipase A2 inhibitor, reduces atherosclerosis and aneurysm formation in ApoE−/− mice. J Cardiovasc Pharmacol. 2009;53:60–65. doi: 10.1097/FJC.0b013e318195bfbc. [DOI] [PubMed] [Google Scholar]
- 73.Karakas M, Koenig W. Varespladib methyl, an oral phospholipase A2 inhibitor for the potential treatment of coronary artery disease. IDrugs. 2009;12:585–592. [PubMed] [Google Scholar]
- 74.Bradley JD, Dmitrienko AA, Kivitz AJ, Gluck OS, Weaver AL, Wiesenhutter C, Myers SL, Sides GD. A randomized, double-blinded, placebo-controlled clinical trial of LY333013, a selective inhibitor of group II secretory phospholipase A2, in the treatment of rheumatoid arthritis. J Rheumatol. 2005;32:417–423. [PubMed] [Google Scholar]
- 75.Houjou T, Yamatani K, Nakanishi H, Imagawa M, Shimizu T, Taguchi R. Rapid and selective identification of molecular species in phosphatidylcholine and sphingomyelin by conditional neutral loss scanning and MS3. Rapid Commun Mass Spectrum. 2004;18:3123–3130. doi: 10.1002/rcm.1737. [DOI] [PubMed] [Google Scholar]
- 76.Taguchi R, Houjou T, Nakanishi H, Yamazaki T, Ishida M, Imagawa M, Shimizu T. Focused lipidomics by tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;823:26–36. doi: 10.1016/j.jchromb.2005.06.005. [DOI] [PubMed] [Google Scholar]