Abstract
Background
Transoral treatment of gastroesophageal reflux disease (GERD) using the EsophyX device enables creation of an esophagogastric fundoplication with potential for better control of reflux than gastrogastric techniques. Efficacy and safety of a rotational/longitudinal esophagogastric transoral incisionless fundoplication (TIF) was evaluated retrospectively using subjective and objective outcomes.
Methods
Thirty-seven consecutive patients on antisecretory medication and with proven gastroesophageal reflux and limited hiatal hernia underwent TIF for persistent GERD symptoms. Five patients were reoperations for failed laparoscopic fundoplication.
Results
Of the 37 treated patients, 57% were female. The median age was 58 (range = 20–81) years and BMI was 25.5 (range = 15.9–36.1) kg/m2. Sixty-eight percent indicated GERD-associated cough, asthma, or aspiration as a primary complaint and 32% complained of heartburn or regurgitation. The TIF procedures created tight wraps of 230°–330° extending 3–4 cm above the Z-line. Two complications occurred: one mediastinal abscess treated laparoscopically and one postoperative bleeding requiring transfusion. At 6 (range = 3–14) months median follow-up TIF resulted in a significant improvement of both atypical and typical symptoms in 64% and 70–80% of patients, respectively, as indicated by the corresponding GERD health-related quality of life (HRQL) and reflux symptom index (RSI) score reduction by 50% or more compared to baseline on proton pump inhibitors (PPIs). No patient reported problems with dysphagia, bloating, or excess flatulence, and 82% were not taking any PPIs. Reflux characteristics were significantly improved and normalized in 61, 89, and 56% of patients in terms of acid exposure, number of refluxates, and DeMeester scores, respectively. TIF was effective in treating GERD in 75% of patients among whom 54% were in a complete “remission” and 21% were “improved.” The remaining 25% were considered failures, and five (13.5%) patients underwent revision.
Conclusion
Rotational/longitudinal esophagogastric fundoplication using the EsophyX device significantly improved symptomatic and objective outcomes in over 70% of patients at median 6-month follow-up. Post-fundoplication side effects were not reported after TIF.
Keywords: Atypical symptoms, Cough, EsophyX, Laryngopharyngeal reflux, Refractory, TIF
Gastroesophageal reflux disease (GERD) is one of the most common clinical disorders observed in developed countries. In the US, nearly 20% of the adult population experiences typical GERD symptoms such as heartburn or acid regurgitation at least once a week, with an annual prevalence reaching 60% [1]. Traditional treatment has been medical (mainly proton pump inhibitors, PPIs), with surgery reserved for patients with incomplete symptom control or intolerance to medical therapy [2]. In recent years, however, there has been a growing number of reports suggesting that approximately 30% (range = 25–40%) of GERD patients do not obtain symptomatic relief (either partially or completely) on standard-dose PPI therapy [3–6]. Medical therapy also appears to have limited effectiveness in 30–50% of GERD patients with atypical laryngopharyngeal reflux (LPR) symptoms who continue to experience hoarseness, sore or burning throat, chronic cough, or excessive throat-clearing despite normalized esophageal acid exposure on aggressive PPI therapy [7, 8]. Studies comparing groups of patients with primarily typical or atypical GERD symptoms have found the same degree of distortion and deterioration of the esophagogastric junction, suggesting a similar pathophysiology [9–11]. This anatomic similarity together with the refractory nature of symptoms establishes surgical fundoplication as appropriate therapy in patients with established GERD and laryngeal symptoms [12, 13].
Antireflux surgery is a well-established surgical procedure in preventing both acid and nonacid reflux and is known to be effective in patients with both typical (heartburn and regurgitation) and atypical GERD symptoms [12–15]. Antireflux surgery recreates a competent gastroesophageal valve and reduces or eliminates reflux. Even though now performed laparoscopically, surgery creates in some patients a super-competent gastroesophageal valve that leads to new side effects of bloating, flatulence, and diarrhea in a small percentage of patients [16]. In addition, traditional surgical techniques were developed to correct severe anatomic defects, including large hiatal hernias, and may be more than needed in patients with less severe anatomic defects that still suffer from GERD. Division of the phrenoesophageal membranes in the performance of laparoscopic fundoplication creates a potential for transthoracic wrap migration, with its attendant morbidity, even in patients whose phrenoesophageal membranes are still relatively intact.
Attempts at transoral fundoplication to treat gastroesophageal reflux have until recently been limited to gastrogastric plications and generated reasonable clinical outcomes [17–20]. The EsophyX device provides the potential to perform a transoral esophagogastric fundoplication. Polypropylene fasteners are placed between the esophageal lumen and the gastric cardia up to 4 cm above the Z-line to create a fundoplication of over 270° in circumference as visualized endoscopically. This newer technique combines rotational and longitudinal plications to create esophagogastric fundoplication and has the potential for improved control of reflux compared to purely longitudinal esophagogastric or gastrogastric plication.
The purpose of this retrospective single-institution, single-surgeon study was to evaluate the efficacy and safety of this esophagogastric transoral incisionless fundoplication (TIF) technique using the EsophyX® device (EndoGastric Solutions, Redmond, WA). Patients who have already had the TIF procedure to treat established GERD were evaluated with appropriate outcome questionnaires along with objective studies including the use of ambulatory pH testing.
Patients and methods
Patients
After obtaining approval from the HCA-HealthOne Institutional Review Board in October 2009, 37 consecutive patients who underwent TIF at our institution between November 2008 and October 2009 were asked to consent to a retrospective evaluation of their clinical course and a follow-up visit at 6 or 12 months for upper GI endoscopy and 48-h telemetry capsule pH testing. One patient was excluded from follow-up analysis due to an early operation for a complication that resulted in takedown of the TIF and creation of a laparoscopic fundoplication (see Safety Outcomes below.)
Patients underwent TIF because they had persistent typical or atypical GERD symptoms on daily antisecretory medication (response <80%), proven gastroesophageal reflux by either 24-h pH/impedance, 48-h pH, or barium swallow testing, and a deteriorated gastroesophageal junction (Hill grade II or III [21]) on endoscopy. Five were reoperations after failed Nissen (n = 4) or Toupet fundoplication.
Preoperative assessment
The preoperative evaluation of TIF patients followed our routine protocol for surgical fundoplication patients [22]. The protocol involved a complete history and physical, symptom severity evaluation using two standardized questionnaires, upper GI endoscopy, and gastroesophageal reflux evaluation by barium swallow, reflux measurement by either 48-h telemetry capsule pH monitoring or 24-h impedance/pH testing, and esophageal manometry. Symptoms were evaluated using the Reflux Symptom Index (RSI) for LPR symptoms [23] and GERD Health-related Quality of Life (GERD-HRQL) for typical GERD symptoms [24].
The TIF procedure was considered an appropriate alternative to laparoscopic fundoplication when the axial height of the hiatal hernia was less than or equal to 2 cm, the transverse dimensions of the hiatal hernia did not exceed 3 cm, and the patient was willing to abide by postoperative recommendations related to diet and physical activity.
TIF technique
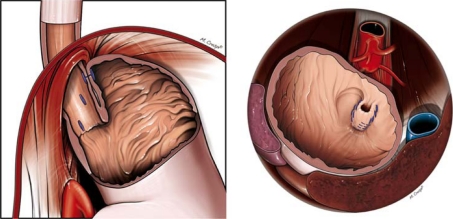
Our rotational/longitudinal esophagogastric TIF technique, including preoperative and postoperative surgical care, has been described in detail previously [25]. The rotational element of the procedure, along with the degree to which the plication is placed above the Z-line, differs from the techniques described initially [17, 19, 20, 26, 27]. Briefly, under general anesthetic, the EsophyX device is introduced over a flexible endoscope into the stomach. With the endoscope retroflexed for visualization, the device’s tissue mold is retroflexed. From the apex of the device a helical retractor is advanced into the Z-line at the lesser curvature. With caudal retraction on the helical retractor, the tissue mold is used to rotate the fundus up and around the esophagus. The tissue mold and helix are locked in place, and polypropylene H-shaped fasteners are deployed above the Z-line so that one leg of the H engages in the esophageal wall and the other in the gastric lumen (Fig. 1). This procedure is repeated at various rotational positions and longitudinal depths until 12–20 fasteners are deployed. This results in an esophagogastric plication that extends above the Z-line for 3–4 cm and circumferentially for more than 270° (Fig. 1).
Fig. 1.
Schematic drawings of the esophagogastric transoral incisionless fundoplication (TIF) technique with a depiction of fastener placement and the plications obtained. Left Fasteners (blue) are placed caudally to the diaphragm but still above the Z-line (left panel). Right Retroflex view of the esophagogastric plications extending circumferentially for more than 270° with external relationship to the aorta, vena cava, liver, and spleen
Postoperative stay was typically 1 day as was duration of IV pain medication. Patients were asked to follow a liquid diet for the first 2 weeks a soft diet for another 2 weeks and to resume a normal diet 4 weeks post procedure. All patients continued on antisecretory medication for 2 weeks after the procedure to minimize the potential for gastric bleeding and then stopped their antisecretory medication. Patients were also asked to refrain from vigorous exercise for 4 weeks.
Follow-up assessment
Patients were seen at 1 week and again at 1 month, 3 months, and then every 6 months postoperatively. Patients unwilling to come to the clinic at 1 or 3 months were followed up by mailed questionnaire and telephone. A complete evaluation was conducted at 6 or 12 months and consisted of symptom evaluation (HRQL, RSI), endoscopy, and a 48-h pH test using a telemetry capsule pH monitoring system (Bravo, Given Imaging, Duluth, GA) after discontinuation of acid-suppressive medication for a minimum of 7 days. Patients experiencing symptom recurrence before the 6-month follow-up underwent a complete objective evaluation with endoscopy and pH testing to determine the reasons for failure and the need for TIF or Nissen revision.
Effectiveness assessment
The primary effectiveness measure was symptom elimination based on RSI and GERD-HRQL score reduction at follow-up compared to baseline on PPIs. The RSI is a nine-item questionnaire developed and validated to measure symptoms associated with laryngopharyngeal reflux (LPR) such as hoarseness, throat-clearing, excess throat mucus, dysphagia, and cough [23]. A visual analog scale (VAS) for each individual item ranges from 0 (no problem) to 5 (severe problem), with a maximum total score of 45 and normality threshold of ≤13. The GERD-HRQL is a validated disease-specific questionnaire measuring the severity of heartburn (six questions), dysphagia (two), bloating (one), and the impact of medication on daily life (one) on the VAS scale from 0 (no symptoms) to 5 (worst symptoms) [24, 28]. Scores of ≤2 to each question are indicative of rare or eliminated symptoms [12]. The last item of the questionnaire evaluates the patient’s general satisfaction with his/her current health condition as “satisfied,” “neutral,” or “dissatisfied.” Total GERD-HRQL scores are calculated by summing the responses to ten questions. The same six questions as those assessing heartburn were used to assess regurgitation scores. A clinically significant improvement was defined in the protocol as a 50% or more reduction in scores at follow-up compared to baseline on PPIs.
Secondary effectiveness measures were normal esophageal acid exposure, PPI discontinuation, hiatal hernia reduction, and Hill grade normalization. Follow-up pH testing was performed using a 48-h telemetry capsule pH monitoring system. Subjects were asked to maintain their normal eating and daily activity patterns as well as record timing of their meals, sleep, and symptoms. Each pH-tracing was examined and considered valid if there was at least 16 h of recording for each 24-h period of monitoring. Esophageal acid exposure corresponded to the percentage of total monitoring time at pH < 4 and was defined as normal if pH ≤ 5.3% while off PPIs [29, 30]. A mean number of reflux episodes of 44 or fewer per 24 h and DeMeester score of ≤14.72 while off PPIs were each considered normal values [31, 32]. Esophageal acid exposure was considered normalized if preoperative values were pathologic. The use of PPIs and other GERD medications was recorded. A complete discontinuation of PPIs was considered clinically significant.
A global assessment of all outcome measures was performed for each patient to determine the effectiveness of TIF in curing GERD [17]. Patients were considered “in remission” if they had no more atypical and typical symptoms, used no PPIs, were satisfied with the condition of their health, and had normalized reflux parameters. Patients were considered “improved” if they had fewer typical or atypical symptoms, required no PPIs, were satisfied with the condition of their health, and had normalized or improved at least one reflux parameter. Patients with “ongoing” GERD showed no alleviation of their typical and atypical symptoms, required daily PPIs, were not satisfied with the condition of their health, and had abnormal pH-metry.
Upper GI endoscopy was performed to assess hiatal hernia length (defined as the distance from the Z-line to diaphragmatic pinch measured in centimeters at retraction of the endoscope without insufflation), reflux esophagitis grade according to the Los Angeles classification [33], and the geometric aspects of the TIF valves such as length, circumference, and Hill grade [17].
The incidences of anticipated and unanticipated serious and nonserious adverse events were recorded and used for safety assessment.
Statistical methods
Data were collected prospectively using a computerized database (Microsoft Access, Microsoft, Redmond, WA). Statistical analysis was performed using Minitab 15 statistical software (Minitab Inc., State College, PA). Continuous variables were summarized as means and standard deviations (SD) or standard errors (SEM), or medians and ranges. Categorical variables were summarized as counts and percentages. P values for changes at follow-up compared to those at baseline were calculated using the Mann–Whitney U and paired t tests. Fisher’s exact test was used to compare frequencies. Values of p < 0.05 were considered significant.
Results
Patient characteristics at baseline
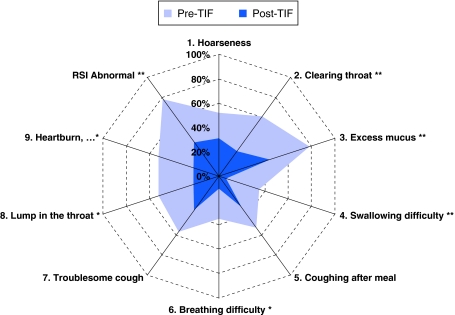
The median age of the 37 treated patients was 58 (range = 20–81) years, 21 (57%) were female, and 35 (95%) had BMI < 35 kg/m2 (Table 1). All patients had a long history of GERD treated with PPI therapy, which was ineffective in controlling symptoms in 92%. The majority of patients (n = 25, 68%) presented with an atypical GERD symptom: chronic cough or chest pain related to cough (n = 15, 41%), asthma (n = 4, 11%), aspiration (n = 2, 5%), hoarseness and vocal cord problems (n = 2, 5%), or chronic sinusitis (n = 2, 5%). All patients with atypical symptoms reported no or partial (<80%) response to PPIs, 88% (15/17) had abnormal RSI scores (Fig. 2), and 40% (10/25) complained also of secondary heartburn or regurgitation, both of which were refractory to PPIs in 70% of the cases. An otolaryngologist or pulmonologist ruled out other diagnoses and confirmed the diagnosis of laryngopharyngeal reflux in all patients with a primary complaint of LPR. Among patients with primarily typical symptoms (n = 11), 60% reported also atypical symptoms as a secondary complaint. Upper abdominal bloating/distention and excess of flatulence were reported by 68% of patients. Twelve patients (33%) had reducible 2-cm-long hiatal hernia, which was accompanied by reflux esophagitis in three (9%) patients (Table 1); the remaining 24 (67%) patients did not have a visible hiatal hernia.
Table 1.
Patient characteristics at the time of esophagogastric transoral incisionless fundoplication (TIF) surgery
| No. patients | 37 |
| Female | 21 (57%) |
| Age (range) (years) | 58 (20–81) |
| ≥65 | 12 (34%) |
| BMI (range) (kg m−2) | 25.5 (15.9–36.1) |
| ≥35 | 2 (5%) |
| Primary GERD symptoms | |
| Typical | 12 (32%) |
| Atypical | 25 (68%) |
| On PPI therapy | 37 (100%) |
| Gastroesophageal reflux | 37 (100%) |
| Hiatal hernia | 25 (68%) |
| Reflux esophagitis | 3 (8%) |
| Barrett’s short segment | 1 (3%) |
Values represent medians (range) or counts (%)
BMI body mass index, PPI proton pump inhibitor
Fig. 2.
Percentage of patients who had abnormal Reflux Symptom Index (RSI) scores before esophagogastric transoral incisionless fundoplication (TIF) while taking PPIs (purple field) compared to that after TIF while not taking PPIs (blue field). Total scores ≥13 with any individual scores >2 (bothersome daily) were indicative of troublesome atypical symptoms. Significant differences indicated by * if p < 0.05 or ** if p ≤ 0.01 (Fisher’s exact test)
Procedure
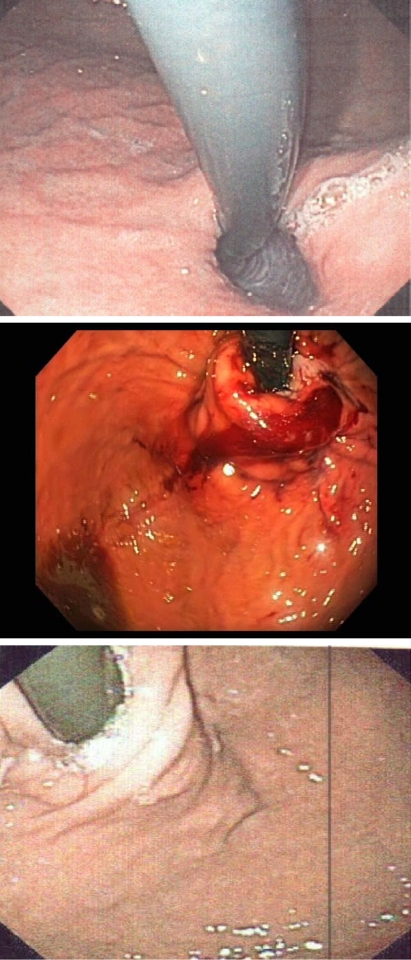
All TIF procedures were completed successfully. Median operating time was 75 min (range = 45–110), and an average of 17 (SD = 3) fasteners were used to create each fundoplication. The TIF procedures reduced all 12 hiatal hernias, and the postoperative endoscopic appearance of the valves was consistent with a posterior partial fundoplication with a mean of 300° (range = 230°–330°) circumference around the esophagus and tight adherence to the endoscope with fasteners placed 3–4 cm above the Z-line (Fig. 3).
Fig. 3.
Retroflex view of the gastroesophageal junction before (top), immediately after (middle), and at 6 months after (bottom) esophagogastric transoral incisionless fundoplication (TIF) surgery
Patients were observed overnight and discharged the following day in 35 cases. One patient required a 3-day stay for pain control. Another patient was hospitalized for pulmonary issues unrelated to the procedure.
Safety outcomes
Two complications directly related to the procedure were observed. One patient presented on postoperative day (POD) 6 with a mediastinal abscess and underwent successful laparoscopic drainage and takedown of the TIF fundoplication, followed by Toupet fundoplication. This patient was not included in the follow-up assessment. Technique modifications related to these complications are described elsewhere [25]. Traumatic dislodgement of the helical screw was the etiology of the bleeding in the second patient in our series. No patient reported side effects of gas-bloat or increased flatulence, and, in fact, bloating was reported less often after the procedure than preoperatively (p < 0.01).
Clinical outcomes
Subjective outcomes
Follow-up assessment was completed for 33 of the 37 treated patients at a median of 6 (range = 3–14) months. Two patients were not able to complete either their questionnaires or endoscopy, one was lost to follow-up, and another one was excluded due to postoperative complication and Toupet revision. Atypical symptoms were significantly improved as indicated by the reduction of mean total RSI scores (mean = 12.9 ± 1.8 SEM, n = 32 vs. 22.8 ± 2.2 pre-TIF on PPIs, n = 23; p = 0.003; Table 2). In 64% (14/22) of patients, RSI scores improved by more than 50% compared to baseline on PPIs, and 52% (11/21) had their scores normalized (Fig. 2, Table 2). Heartburn was also improved significantly as indicated by the reduction of GERD-HRQL scores (mean = 5.0 ± 1.1 SEM, n = 32 vs. 15.3 ± 2.1 pre-TIF on PPIs, n = 21, p = 0.002). GERD-HRQL scores were reduced by more than 50% in 80% of patients and normalized in 50%. Regurgitation scores were also significantly reduced compared to baseline on therapy (mean = 4.3 ± 1.2 SEM, n = 32 vs. 11.2 ± 2.0 pre-TIF on PPIs, n = 21, p = 0.006). In 70% (14/20) of patients the regurgitation score reduction exceeded 50%, and 62% (8/13) of patients had normalized scores. Current health condition after TIF was judged as satisfactory by 66% of patients, dissatisfactory by 22%, and neither satisfactory nor dissatisfactory by 12% compared to 0, 81, and 19% of patients before TIF, respectively (Table 2). Among 33 patients available at follow-up, 82% were completely off all GERD medications.
Table 2.
Reflux Symptom Index (RSI), GERD Health-related Quality of Life (GERD-HRQL), and regurgitation scores before esophagogastric transoral incisionless fundoplication (TIF) surgery while on PPIs and at a median 6 (3–14) months after surgery
| Pre-TIF on PPIs | Post-TIF off PPIs | p value | |
|---|---|---|---|
| RSI scores | |||
| Median (range) | 25 (5–38) n = 23 | 10 (0–39) n = 32 | 0.002 |
| Mean (SEM) | 22.8 (2.2) n = 23 | 12.9 (1.8) n = 32 | 0.003 |
| n (%) abnormala | 20/23 (87%) | 11/32 (34%) | <0.001 |
| n (%) improved by ≥50%b | 14/22 (64%) | ||
| n (%) normalizedc | 11/21 (52%) | ||
| GERD-HRQL scores | |||
| Median (range) | 16 (0–29) n = 21 | 4 (0–27) n = 32 | 0.001 |
| Mean (SEM) | 15.3 (2.1) n = 21 | 5.0 (1.1) n = 32 | 0.002 |
| n (%) abnormala | 17 (81%) | 13 (41%) | <0.001 |
| n (%) improved by ≥50%b | 16/20 (80%) | ||
| n (%) normalizedd | 10/20 (50%) | ||
| Regurgitation scores | |||
| Median (range) | 10 (0–26) n = 21 | 1 (0–25) n = 32 | 0.005 |
| Mean (SEM) | 11.2 (2.0) n = 21 | 4.3 (1.2) n = 32 | 0.006 |
| n (%) abnormala | 11/21 (52%) | 6/32 (19%) | 0.016 |
| n (%) improved by ≥50%b | 14/20 (70%) | ||
| n (%) normalizedd | 8/13 (62%) | ||
| Satisfaction indexe | |||
| n (%) satisfied | 0/23 (0%) | 21/32 (66%) | <0.001 |
| n (%) neutral | 4/23 (19%) | 4/32 (12%) | <0.001 |
| n (%) dissatisfied | 17/23 (81%) | 7/32 (22%) | <0.001 |
p values <0.05 indicate significant differences between medians (Mann–Whitney U test), means (paired t test), and ratios (Fisher’s exact test)
aAbnormal if any individual score >2
bCompared to baseline on PPIs
cNormalized RSI score defined by a total score of ≤13 with each question evaluated as eliminated or rare (score ≤2)
dNormalized if none of the abnormal scores at baseline is >2 at follow-up
eSatisfaction index determined using GERD-HRQL indicates patient satisfaction with current health condition
Objective outcomes
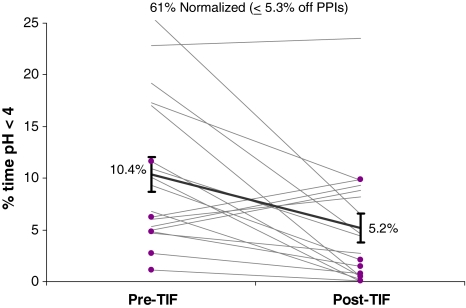
Globally, all reflux characteristics were significantly improved following TIF in the 24 patients who underwent pH testing off PPIs both preoperatively and at follow-up and whose reflux characteristics on PPIs prior TIF were elevated. Mean esophageal acid exposure (% time pH < 4) was significantly (p = 0.003) reduced from 10.4 ± 1.7 to 5.2 ± 1.4% post-TIF (n = 18) and normalized in 61% of patients (Fig. 4). Four patients had an increased (>5% over preoperative value) amount of esophageal acid exposure postoperatively. One of these improved symptomatically and had an intact valve at postoperative endoscopy. Another patient continued to have LPR symptoms and an intact valve at postoperative endoscopy; no further treatment or intervention was performed. Two patients had recurrence of typical GERD symptoms and worsened acid exposure and underwent revision to laparoscopic Nissen (see below).
Fig. 4.
Esophageal acid exposure (% time pH < 4) before and 6 months after esophagogastric transoral incisionless fundoplication (TIF) in patients with abnormal values at baseline either after discontinuation of proton pump inhibitors (PPIs) for 7 days (n = 13) or while on therapy (n = 5, highlighted in purple). Mean acid exposure (black trend line with SEM error bars) was significantly reduced (n = 18, p = 0.003, paired t test)
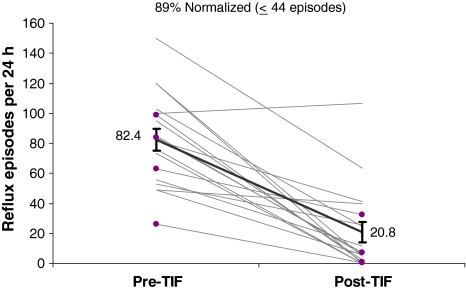
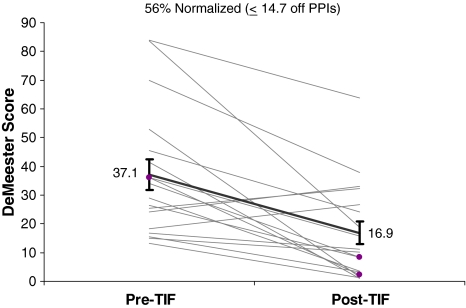
The average number of reflux episodes per 24-h testing period was significantly (p < 0.001) reduced from 82.4 ± 7.2 to 20.8 ± 6.6 post-TIF and normalized in 89% of patients (Fig. 5). DeMeester scores were significantly reduced from 37.1 ± 5.3 to 16.9 ± 4.0 and normalized in 56% of patients (Fig. 6). Most of TIF valves were tight and had Hill grade of I (Table 3). Hiatal hernia was eliminated in 9/9 cases (Table 3). Esophagitis was rarely observed preoperatively as most patients were on antisecretory medication at the time of or until 1 week prior to upper endoscopy.
Fig. 5.
Number of acid reflux episodes (pH < 4) per 24 h before and 6 months after esophagogastric transoral incisionless fundoplication (TIF) in patients with abnormal values at baseline either after discontinuation of proton pump inhibitors (PPIs) for 7 days (n = 14) or while on therapy (n = 4, highlighted in purple). Mean number of episodes (black trend line with SEM bars) was significantly reduced (p < 0.001, paired t test)
Fig. 6.
DeMeester scores before and 6 months after esophagogastric transoral incisionless fundoplication (TIF) in patients with abnormal values at baseline either after discontinuation of proton pump inhibitors (PPIs) for 7 days (n = 16) or while on therapy (n = 2, highlighted in purple). Mean score (black trend line with SEM bars) was significantly reduced (p < 0.001, paired t test)
Table 3.
Endoscopic evaluation before and 6 (3–14) months after esophagogastric transoral incisionless fundoplication (TIF)
| Pre-TIF | 6-mo Post-TIF | |
|---|---|---|
| Hill grade | n = 36 | n = 32 |
| Median size (cm) | 2 (2–3) | 1 (1–3) |
| Grade I | 0 (0%) | 19 (59%) |
| Grade II | 29 (81%) | 9 (28%) |
| Grade III | 7 (19%) | 4 (13%) |
| Grade IV | 0 (0%) | 0 (0%) |
| Reduced to grade II | – | 2 (6%) |
| Normalized to grade I | – | 19 (59%) |
| p value for grade change | <0.001 | |
| Hiatal hernia height | n = 36 | n = 29 |
| None | 24 (67%) | 28/29 (97%) |
| ≥2 cm | 12 (33%) | 1 (3%) |
| Eliminated if ≤1 cm | – | 9/9 (100%) |
| Worsened | – | 1/24 (4%) |
Global assessment
The global assessment of 24 patients who underwent pH testing off PPIs both preoperatively and at follow-up and whose reflux characteristics on PPIs prior TIF were elevated revealed that TIF was effective in treating GERD in 75% of them and among whom 54% were in complete remission and had normal pH studies, and 21% were improved symptomatically and physiologically and required no more PPIs. The remaining 25% of the 24 patients had ongoing GERD and were considered as failures.
Failures and revisions
Among the 37 patients enrolled, 5 (13.5%) underwent revision: two with the TIF procedure within 3 and 6 months following the procedure, respectively, and three with laparoscopic Nissen fundoplication after 4 (n = 1) and 6 (n = 2) months. Laparoscopic revision from a failed TIF to a Nissen fundoplication was relatively straightforward in these three patients. Failures were associated with severe postoperative nausea and retching and severe coughing, and, in one patient (BMI = 34), with a Belsey fat pad that at laparoscopic reoperation was lipomatous and prevented fusion of the fundus to the esophagus. Resolution of symptoms and normalization of acid exposure was observed in four (80%) patients who had had a previous Nissen procedure that was loose but without intrathoracic wrap migration. The one failure was the patient with a prior Toupet fundoplication.
Discussion
The most important goals of any GERD treatment are symptom control, prevention of GERD-related complications, and healing of esophagitis [34]. Although PPIs are very effective in healing esophagitis, a significant proportion of patients continue to be symptomatic. Frequently, these symptoms are laryngopharyngeal in nature. In addition, PPIs do not prevent nonacid reflux, which has been imputed as the cause of GERD-related complications such as asthma, aspiration pneumonia, or cough [35, 36]. Traditional surgical therapy for GERD, whether laparoscopic or open, has been demonstrated to be effective in the treatment of GERD refractory to medical therapy [37]. However, traditional fundoplication creates a super-competent valve, which limits the ability to vent air and to vomit and leads to side effects of dysphagia, bloating, nausea, and meteorism in some patients. The super-competent nature of the valve and concomitant side effects have been a major limiting factor in the acceptance of fundoplication by many patients and gastroenterologists. Consider the following American Gastroenterological Association medical position statement on the management of GERD regarding antireflux surgery [2]: “The potential benefits of antireflux surgery should be weighed against the deleterious effect of new symptoms consequent from surgery, particularly dysphagia, flatulence, an inability to belch, and postsurgery bowel symptoms.” With failures of both current medication regimens and traditional antireflux surgery, there is need for a therapy that would treat medically refractory GERD symptoms without the risks and side effects of traditional surgery. Such a therapy could be acceptable, even if it had a more limited success rate than the current modalities. Because no single-treatment regimen is completely successful, GERD should be considered a chronic condition that requires chronic management and multimodality therapy, much like cardiac disease [38].
With the hope of improving GERD-related quality of life by controlling reflux without creating side effects, various transoral methods of restoring gastroesophageal valve competence have been tried with varying degrees of success [39–42]. Until recently, transoral procedures were limited to gastrogastric plication or attempts to decrease the compliance of the gastroesophageal valve. The EsophyX device enables creation of full-thickness esophagogastric plication transorally and is currently the only device commercially available that does so.
With our technique the device was used to create not merely a longitudinal esophagogastric fundoplication (the so-called TIF 2), but a rotational and longitudinal esophagogastric fundoplication. The phrenoesophageal membranes are left intact and small hiatal hernias are reduced. Based on limited experience, performance of a laparoscopic fundoplication in the case of failure is not significantly more complicated than performance of a primary laparoscopic fundoplication. This retrospective study of our first 37 patients represents the results of our initial learning curve both with the device and with the development of this technique. Despite these potential limitations, our results are significant in terms of symptom improvement and objective reflux control. Perhaps just as important, we have not observed any of the side effects seen with traditional fundoplications.
The utilization of postoperative pH-metry as an end point in this study should be understood in the context of the treatment goals mentioned above, which do not include normalization of acid exposure [43, 44]. Most acid reflux events in GERD patients are asymptomatic [45]. Eighteen to 30% of patients who have met treatment goals of symptom control and healing of esophagitis with PPI therapy have abnormal amounts of esophageal acid exposure [46]. We chose to measure reflux postoperatively with the intent to demonstrate that the TIF procedure produces objectively identifiable changes in the amount of esophageal acid exposure that correlate with symptom improvement. Utilization of a telemetry capsule pH probe was favored over transnasal impedance testing because of patient acceptance, even though it did not provide us with information on nonacid reflux events [47, 48]. In our research protocol we defined pH-metric success by normalization of one or more of the acid reflux characteristics such as acid exposure, number of refluxates, or DeMeester score. By these protocol-specific pH-metric standards, the TIF procedure was successful in up to 89% of patients.
Limitations of this study include its retrospective nature of baseline data collection, with associated incomplete data set for all patients, and the 6-month duration of follow-up. In addition, this study represents our initial learning curve not only with the device but with the evolution of the rotational-longitudinal esophagogastric fundoplication technique. We are currently conducting a prospective study to address these limitations; but we believe this current study’s results to be significant enough to report. This technique should not be considered experimental, a position supported by position statements from the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) [49] and the American Society of General Surgeons (ASGS) [50].
Conclusion
The creation of a radial and longitudinal esophagogastric fundoplication using the EsophyX device significantly improved symptomatic outcomes in 64–80% of patients at a median 6-month follow-up. TIF effectively controlled reflux without patients developing any of the side effects of traditional surgery. Postoperative recovery was rapid and clinical outcomes were encouraging. Proper technique can minimize the risk of complications. Transoral esophagogastric fundoplication has a role in the treatment of GERD.
Acknowledgments
Disclosures
Dr. Bell is on the speaker’s bureau for EndoGastric Solutions and Sandhill Scientific. This study was supported by a small research grant from EndoGastric Solutions for pH and endoscopy testing at follow-up. Ms. Freeman has no conflicts of interest or financial ties to disclose.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Locke GR, III, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., III Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–1456. doi: 10.1016/S0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 2.Kahrilas PJ, Shaheen NJ, Vaezi MF, Hiltz SW, Black E, Modlin IM, Johnson SP, Allen J, Brill JV (2008) American Gastroenterological Association medical position statement on the management of gastroesophageal reflux disease. Gastroenterology 135:1383–1391, 1391.e1–e5 [DOI] [PubMed]
- 3.Carlsson R, Dent J, Watts R, Riley S, Sheikh R, Hatlebakk J, Haug K, de Groot G, van Oudvorst A, Dalvag A, Junghard O, Wiklund I. Gastro-oesophageal reflux disease in primary care: an international study of different treatment strategies with omeprazole. International GORD Study Group. Eur J Gastroenterol Hepatol. 1998;10:119–124. doi: 10.1097/00042737-199802000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Castell DO, Murray JA, Tutuian R, Orlando RC, Arnold R. Review article: the pathophysiology of gastro-oesophageal reflux disease - oesophageal manifestations. Aliment Pharmacol Ther. 2004;20(Suppl 9):14–25. doi: 10.1111/j.1365-2036.2004.02238.x. [DOI] [PubMed] [Google Scholar]
- 5.Fass R. Proton-pump inhibitor therapy in patients with gastro-oesophageal reflux disease: putative mechanisms of failure. Drugs. 2007;67:1521–1530. doi: 10.2165/00003495-200767110-00001. [DOI] [PubMed] [Google Scholar]
- 6.Hunt RH, Yuan Y, Yaghoobi M. GERD: new strategies and new failures. J Clin Gastroenterol. 2007;41(Suppl 2):S72–S80. doi: 10.1097/MCG.0b013e31803238d6. [DOI] [Google Scholar]
- 7.Swoger J, Ponsky J, Hicks DM, Richter JE, Abelson TI, Milstein C, Qadeer MA, Vaezi MF. Surgical fundoplication in laryngopharyngeal reflux unresponsive to aggressive acid suppression: a controlled study. Clin Gastroenterol Hepatol. 2006;4:433–441. doi: 10.1016/j.cgh.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Vaezi MF, Richter JE, Stasney CR, Spiegel JR, Iannuzzi RA, Crawley JA, Hwang C, Sostek MB, Shaker R. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope. 2006;116:254–260. doi: 10.1097/01.mlg.0000192173.00498.ba. [DOI] [PubMed] [Google Scholar]
- 9.Hill LD, Kozarek RA, Kraemer SJ, Aye RW, Mercer CD, Low DE, Pope CE., 2nd The gastroesophageal flap valve: in vitro and in vivo observations. Gastrointest Endosc. 1996;44:541–547. doi: 10.1016/S0016-5107(96)70006-8. [DOI] [PubMed] [Google Scholar]
- 10.Perry KA, Enestvedt CK, Lorenzo CS, Schipper P, Schindler J, Morris CD, Nason K, Luketich JD, Hunter JG, Jobe BA. The integrity of esophagogastric junction anatomy in patients with isolated laryngopharyngeal reflux symptoms. J Gastrointest Surg. 2008;12:1880–1887. doi: 10.1007/s11605-008-0607-7. [DOI] [PubMed] [Google Scholar]
- 11.Seltman AK, Kahrilas PJ, Chang EY, Mori M, Hunter JG, Jobe BA. Endoscopic measurement of cardia circumference as an indicator of GERD. Gastrointest Endosc. 2006;63:22–31. doi: 10.1016/j.gie.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 12.Hunter JG, Trus TL, Branum GD, Waring JP, Wood WC. A physiologic approach to laparoscopic fundoplication for gastroesophageal reflux disease. Ann Surg. 1996;223:673–685. doi: 10.1097/00000658-199606000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sala E, Salminen P, Simberg S, Koskenvuo J, Ovaska J. Laryngopharyngeal reflux disease treated with laparoscopic fundoplication. Dig Dis Sci. 2008;53:2397–2404. doi: 10.1007/s10620-007-0169-7. [DOI] [PubMed] [Google Scholar]
- 14.Walker SJ, Holt S, Sanderson CJ, Stoddard CJ. Comparison of Nissen total and Lind partial transabdominal fundoplication in the treatment of gastro-oesophgeal reflux. Br J Surg. 1992;79:410–414. doi: 10.1002/bjs.1800790512. [DOI] [PubMed] [Google Scholar]
- 15.Westcott CJ, Hopkins MB, Bach K, Postma GN, Belafsky PC, Koufman JA. Fundoplication for laryngopharyngeal reflux disease. J Am Coll Surg. 2004;199:23–30. doi: 10.1016/j.jamcollsurg.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 16.Lundell L. Complications after anti-reflux surgery. Best Pract Res Clin Gastroenterol. 2004;18:935–945. doi: 10.1016/j.bpg.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Cadiere GB, Buset M, Muls V, Rajan A, Rosch T, Eckardt AJ, Weerts J, Bastens B, Costamagna G, Marchese M, Louis H, Mana F, Sermon F, Gawlicka AK, Daniel MA, Deviere J. Antireflux transoral incisionless fundoplication using EsophyX: 12-month results of a prospective multicenter study. World J Surg. 2008;32:1676–1688. doi: 10.1007/s00268-008-9594-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cadiere GB, Van Sante N, Graves JE, Gawlicka AK, Rajan A. Two-year results of a feasibility study on antireflux transoral incisionless fundoplication (TIF) using EsophyX. Surg Endosc. 2009;23:957–964. doi: 10.1007/s00464-009-0384-8. [DOI] [PubMed] [Google Scholar]
- 19.Repici A, Fumagalli U, Malesci A, Barbera R, Gambaro C, Rosati R. Endoluminal fundoplication (ELF) for GERD using EsophyX: a 12-month follow-up in a single-center experience. J Gastrointest Surg. 2010;14:1–6. doi: 10.1007/s11605-009-1077-2. [DOI] [PubMed] [Google Scholar]
- 20.Testoni PA, Corsetti M, Di Pietro S, Castellaneta AG, Vailati C, Masci E, Passaretti S. Effect of transoral incisionless fundoplication on symptoms, PPI use, and pH-impedance refluxes of GERD patients. World J Surg. 2010;34:750–757. doi: 10.1007/s00268-010-0394-7. [DOI] [PubMed] [Google Scholar]
- 21.Hill LD, Kozarek RA. The gastroesophageal flap valve. J Clin Gastroenterol. 1999;28:194–197. doi: 10.1097/00004836-199904000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Bell RC, Hanna P, Mills MR, Bowrey D. Patterns of success and failure with laparoscopic Toupet fundoplication. Surg Endosc. 1999;13:1189–1194. doi: 10.1007/PL00009618. [DOI] [PubMed] [Google Scholar]
- 23.Belafsky PC, Postma GN, Koufman JA (2002) Validity and reliability of the reflux symptom index (RSI). J Voice 16(2):274–279 [DOI] [PubMed]
- 24.Velanovich V. The development of the GERD-HRQL symptom severity instrument. Dis Esophagus. 2007;20:130–134. doi: 10.1111/j.1442-2050.2007.00658.x. [DOI] [PubMed] [Google Scholar]
- 25.Bell RC, Cadiere GB (2010) Transoral esophago-gastric fundoplication: technical and safety considerations. Surg Endosc (accepted) [DOI] [PMC free article] [PubMed]
- 26.Demyttenaere SV, Bergman S, Pham T, Anderson J, Dettorre R, Melvin WS, Mikami DJ. Transoral incisionless fundoplication for gastroesophageal reflux disease in an unselected patient population. Surg Endosc. 2010;24:854–858. doi: 10.1007/s00464-009-0676-z. [DOI] [PubMed] [Google Scholar]
- 27.Jobe BA, O’Rourke RW, McMahon BP, Gravesen F, Lorenzo C, Hunter JG, Bronner M, Kraemer SJ. Transoral endoscopic fundoplication in the treatment of gastroesophageal reflux disease: the anatomic and physiologic basis for reconstruction of the esophagogastric junction using a novel device. Ann Surg. 2008;248:69–76. doi: 10.1097/SLA.0b013e31817c9630. [DOI] [PubMed] [Google Scholar]
- 28.Velanovich V, Vallance SR, Gusz JR, Tapia FV, Harkabus MA. Quality of life scale for gastroesophageal reflux disease. J Am Coll Surg. 1996;183:217–224. [PubMed] [Google Scholar]
- 29.Tseng D, Rizvi AZ, Fennerty MB, Jobe BA, Diggs BS, Sheppard BC, Gross SC, Swanstrom LL, White NB, Aye RW, Hunter JG. Forty-eight-hour pH monitoring increases sensitivity in detecting abnormal esophageal acid exposure. J Gastrointest Surg. 2005;9:1043–1051. doi: 10.1016/j.gassur.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Tutuian R, Castell DO. Diagnostic procedures in GERD: principles and values of esophageal manometry and pH-monitoring. In: Granderath FA, Kamolz T, Pointner R, editors. Gastroesophageal reflux disease: principles of disease, diagnosis, and treatment. New York: Springer-Verlag; 2006. pp. 121–138. [Google Scholar]
- 31.Pandolfino JE, Kwiatek MA. Use and utility of the Bravo pH capsule. J Clin Gastroenterol. 2008;42:571–578. doi: 10.1097/MCG.0b013e31815bb602. [DOI] [PubMed] [Google Scholar]
- 32.Zerbib F, des Varannes SB, Roman S, Pouderoux P, Artigue F, Chaput U, Mion F, Caillol F, Verin E, Bommelaer G, Ducrotte P, Galmiche JP, Sifrim D. Normal values and day-to-day variability of 24-h ambulatory oesophageal impedance-pH monitoring in a Belgian-French cohort of healthy subjects. Aliment Pharmacol Ther. 2005;22:1011–1021. doi: 10.1111/j.1365-2036.2005.02677.x. [DOI] [PubMed] [Google Scholar]
- 33.Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, Tytgat GN, Wallin L. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–180. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2005;100:190–200. doi: 10.1111/j.1572-0241.2005.41217.x. [DOI] [PubMed] [Google Scholar]
- 35.Bredenoord AJ, Dent J. Proton pump inhibitor-therapy refractory gastro-oesophageal reflux disease patients, who are they? Gut. 2007;56:593–594. doi: 10.1136/gut.2006.115287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mainie I, Tutuian R, Agrawal A, Adams D, Castell DO. Combined multichannel intraluminal impedance-pH monitoring to select patients with persistent gastro-oesophageal reflux for laparoscopic Nissen fundoplication. Br J Surg. 2006;93:1483–1487. doi: 10.1002/bjs.5493. [DOI] [PubMed] [Google Scholar]
- 37.Bredenoord AJ, Draaisma WA, Weusten BL, Gooszen HG, Smout AJ. Mechanisms of acid, weakly acidic and gas reflux after anti-reflux surgery. Gut. 2008;57:161–166. doi: 10.1136/gut.2007.133298. [DOI] [PubMed] [Google Scholar]
- 38.Spechler SJ. Surgery for gastroesophageal reflux disease: esophageal impedance to progress? Clin Gastroenterol Hepatol. 2009;7:1264–1265. doi: 10.1016/j.cgh.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jafri SM, Arora G, Triadafilopoulos G. What is left of the endoscopic antireflux devices? Curr Opin Gastroenterol. 2009;25:352–357. doi: 10.1097/MOG.0b013e32832ad8b4. [DOI] [PubMed] [Google Scholar]
- 40.Rothstein RI. Endoscopic therapy of gastroesophageal reflux disease: outcomes of the randomized-controlled trials done to date. J Clin Gastroenterol. 2008;42:594–602. doi: 10.1097/MCG.0b013e31816bcde5. [DOI] [PubMed] [Google Scholar]
- 41.Reavis KM, Melvin WS. Advanced endoscopic technologies. Surg Endosc. 2008;22:1533–1546. doi: 10.1007/s00464-008-9831-1. [DOI] [PubMed] [Google Scholar]
- 42.Pearl JP, Ponsky JL. Natural orifice translumenal endoscopic surgery: a critical review. J Gastrointest Surg. 2008;12:1293–1300. doi: 10.1007/s11605-007-0424-4. [DOI] [PubMed] [Google Scholar]
- 43.Achem SR. Acid inhibition in GERD-how much is enough? Am J Gastroenterol. 2004;99:997–999. doi: 10.1111/j.1572-0241.2004.40316.x. [DOI] [PubMed] [Google Scholar]
- 44.Milkes D, Gerson LB, Triadafilopoulos G. Complete elimination of reflux symptoms does not guarantee normalization of intraesophageal and intragastric pH in patients with gastroesophageal reflux disease (GERD) Am J Gastroenterol. 2004;99:991–996. doi: 10.1111/j.1572-0241.2004.30124.x. [DOI] [PubMed] [Google Scholar]
- 45.Portale G, Peters J, Hsieh CC, Tamhankar A, Arain M, Hagen J, Demeester S, Demeester T. When are reflux episodes symptomatic? Dis Esophagus. 2007;20:47–52. doi: 10.1111/j.1442-2050.2007.00650.x. [DOI] [PubMed] [Google Scholar]
- 46.Grigolon A, Cantu P, Savojardo D, Conte D, Penagini R. Esophageal acid exposure on proton pump inhibitors in unselected asymptomatic gastroesophageal reflux disease patients. J Clin Gastroenterol. 2008;42:969–973. doi: 10.1097/MCG.0b013e31814b8fc2. [DOI] [PubMed] [Google Scholar]
- 47.Sweis R, Fox M, Anggiansah R, Anggiansah A, Basavaraju K, Canavan R, Wong T. Patient acceptance and clinical impact of Bravo monitoring in patients with previous failed catheter-based studies. Aliment Pharmacol Ther. 2009;29:669–676. doi: 10.1111/j.1365-2036.2008.03923.x. [DOI] [PubMed] [Google Scholar]
- 48.von Renteln D, Schmidt D, Riecken B, Caca K. Evaluating outcomes of endoscopic full-thickness plication for gastroesophageal reflux disease (GERD) with impedance monitoring. Surg Endosc. 2010;24:1040–1048. doi: 10.1007/s00464-009-0723-9. [DOI] [PubMed] [Google Scholar]
- 49.SAGES (2009) SAGES Position Statement on Endoluminal Therapies for Gastrointestinal Diseases. Available at www.sages.org/publication/id/ENDOL. Accessed 20 Oct 2010
- 50.ASGS (2009) ASGS Position Statement on Natural Orifice Surgery and Transoral Incisionless Fundoplication. Available at www.theasgs.orgwww.sages.org/publication/id/ENDOL. Accessed 20 Oct 2010