Abstract
There is significant lack of basic hematologic and immunological data in adult sows. Therefore, aim of this study was to provide respective reference intervals. 32 clinically healthy multiparous Large White sows aged 33.5 ± 9.6 months and all of them two months postpartum were included in this study. Mean erythrocyte count was 5.5 ± 0.7 × 106/μl and total leukocyte count was 12.1 ± 2.1 × 103/μl. Proportion of lymphocytes was 44.7 ± 10.2% and of neutrophils 41.6 ± 11.0%. The ratio of naïve T helper (Th) cells to memory Th cells was 1:3.1 and the ratio of Th cells to cytotoxic T cells (CTLs) was 1:4.2. Proportions of regulatory T cells, NK cells, and CD21+ B cells were lower (3.1, 2.6, and 6.0%) than those of memory Th cells ranging from 8.8 to 27.5% depending on the activation status and CTLs with 37.3%. γδ T cells were found at comparably high numbers (19.1%). Flow cytometric measurement of intracellular cytokines in PBMCs revealed marginal levels for IL-1β, IL-2, IL-4, IL-6, IL-10, and IL-12p35, but remarkable levels for TNF-α and IFN-γ. Highest mRNA levels were found for IL-1, IL-10, and TNF-α, with TNF-α showing the least inter-individual variation.
Keywords: Cytokines, Leukocyte phenotypes, Pig
1. Introduction
It is fundamental to basic, applied, and translational clinical veterinary research to have reliable physiologic data of the species of interest. There is nearly a complete lack on actual hematologic values as well as detailed immune system data of adult sows as most biomedical as well as clinical investigations dealing with porcine hematology and immunity focus on juvenile animals (Gerner et al., 2009; Sinkora and Butler, 2009; Sipos et al., 2004, 2010). Published hematologic data of adult sows are scarce and date back more than 20 years (Friendship et al., 1984). The only available recent data set has been provided ten years ago, but is based on a very small number of animals in the respective age group (Thorn, 2000). Also, information regarding the size of the reference population, analytical methods, and statistical processing is missing. Thus a transfer procedure for reference intervals cannot be accomplished. Changes in analytical technology, such as the switch from impedance technology for cell counting to laser-based flow cytometry, as well as the tremendous genetic progress in commercial pig breeds, warrant re-evaluation of published reference intervals. Additionally, immunological research in adult sows has been neglected so far.
2. Materials and methods
2.1. Animals
32 clinically healthy multiparous Large White sows aged 33.5 ± 9.6 months and all of them two months postpartum were included in this study. Animals were group housed and derived from a commercial sow herd (n = 600), which was serologically tested negative for antibodies against Porcine Reproductive and Respiratory Syndrome Virus and Porcine Circovirus Type 2, and with routinely performed vaccinations against Erysipelothrix rhusiopathiae, Porcine Parvovirus, and swine influenca virus H1N1 and H3N2. Blood collections by venipuncture of the jugular vein were approved by the animal trial ethics committees of the University of Veterinary Medicine Vienna and the Austrian Ministry of Science.
2.2. Hematology, flow cytometry
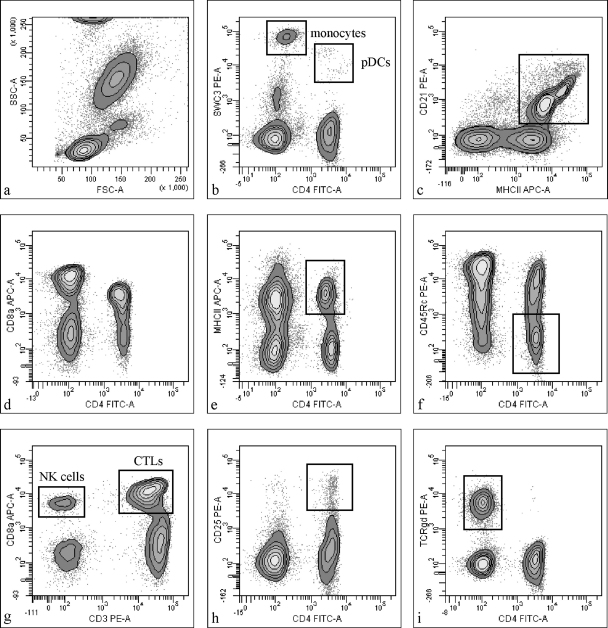
Hematological analyses were performed out of K2-EDTA-blood using an ADVIA®120 with the ADVIA®120 multi-species software version 3 3.1.8.0-MS (Siemens Health Care Diagnostics, Deerfield, IL, USA). FACS analysis was performed to differentiate between PBMC subpopulations (Fig. 1) and to measure intracellular cytokine expressions using a FACSAria® flow cytometer (Becton Dickinson, San Jose, CA, USA). Antibodies targeting surface markers, cytokines, as well as isotype controls are listed in Table 1. Triple staining of surface markers was designed so that primary antibodies formed a combination of mouse immunoglobulin isotypes IgG1, IgG2a, and IgG2b and therefore could be labeled with the same set of secondary antibodies, consisting of anti-IgG1-PE (SouthernBiotech, Birmingham, AL, USA), anti-IgG2a-Alexa Flour 647, and anti-IgG2b-Alexa Fluor 488 (both Molecular Probes, Eugene, OR, USA). Intracellular single cytokine staining of PBMCs was performed as described earlier (Sipos et al., 2005a). After short-time stimulation in the presence of brefeldin-A, ionomycin, and phorbol-12-myristate-13-acetate, cells were fixed, permeabilized, and incubated with the respective anti-porcine cytokine antibodies. Before adding the anti-IgG1 or anti-IgG2b PE-conjugated second-step antibodies (SouthernBiotech), a pre-incubation step with heat-inactivated pig serum was performed. Each experiment included second-step antibody and isotype controls.
Fig. 1.
Contour plots of analysed leukocyte populations and lymphocyte subpopulations. (a) Main PBMC populations in the forward vs. side scatter. (b) Monocytes and pDCs as characterized by their expression of the porcine pan-myeloid marker SWC3 as well as CD4. (c) CD21+ B cells. (d) Typical CD4 and CD8 expression pattern of porcine lymphocytes with a significant proportion of extrathymic CD4+CD8+ double positive cells. (e) Activated Th cells. (f) Memory Th cells. (g) NK cells and CTLs. (h) Tregs. (i) γδ T cells.
Table 1.
Antibody clones used for surface marker and intracellular cytokine analysis.
| Target | Clone no. | Isotype | Reference |
|---|---|---|---|
| Isotype control | NCG01 | mIgG1 | Dianova, Hamburg, Germany |
| Isotype control | NCG2A.01 | mIgG2a | Dianova, Hamburg, Germany |
| Isotype control | NCG2B.01 | mIgG2b | Dianova, Hamburg, Germany |
| SWC3a | 74-22-15A | IgG2b | Lunney (1993) |
| MHC-II | MSA3 | IgG2a | Hammerberg and Schurig (1986) |
| CD3 | PPT3 | IgG1 | Yang et al. (1996) |
| CD4 | 74-12-4 | IgG2b | Pescovitz et al. (1984) |
| CD8α | 11/295/33 | IgG2a | Jonjic and Koszinowski (1984) |
| CD21 | B-ly4 | IgG1 | BD Biosciences Pharmingen, San Jose, CA, USA |
| CD25 | 3B2 | IgG1 | Bailey et al. (1992) |
| TCRγδ | PGBL22A | IgG1 | Davis et al. (1998) |
| CD45RC | 3a56 | IgG1 | Zuckermann et al. (1994a,b) |
| Porcine cytokines | IgG1, IgG2b | R&D Systems, Minneapolis, MN, USA |
2.3. Real-time RT-PCR for cytokine mRNA quantification
Cell preparation and RT-PCR were performed as described earlier (Sipos et al., 2010). For preparation of total RNA out of PBMCs, samples were processed approximately 3 h after blood withdrawal. Blood cells were centrifuged, the pellet was then suspended in 1.2 ml of hemolysis buffer (140 mM NH4Cl, 17 mM Tris, pH 7.2) and incubated at 37 °C for 15 min. Samples were then centrifuged and supernatant was removed. Pelleted white blood cells were resuspended in 1 ml of TriReagent® (Molecular Research Center Inc., Cincinnati, USA) and frozen at −80 °C until analysis. Total RNA was extracted in accordance to the manufacturers’ protocol. Integrity, quantity, and contamination with genomic DNA was analysed on the Agilent BioAnalyser 2100 (Agilent Technologies, Palo Alto, USA) using the RNA6000 Nano LabChip® kit (Agilent). One μg of total RNA was used to synthesize cDNA using Superscript® II RNAse H-reverse transcriptase (200 U/reaction; Invitrogen, Carlsbad, USA) and anchored oligo-dT-primers (3.5 μM final concentration). To check the generation of amplifiable cDNA in the reverse transcription, a conventional PCR step was performed using GAPDH specific primers. The profiling of the expressions of cytokine, inducible NO synthase, and heme oxygenase-1 genes as well as the three housekeeping genes cyclophilin, GAPDH, and β-actin was performed by means of real-time triplex RT-PCR, using TaqMan probes and specific primer pairs on the iCycler iQ® (Bio-Rad, Hercules, USA). Raw data (Ct values) were used as indication for the respective mRNA amount present in the processed samples. In order to visualize the expression ranges of the genes of interest, expression data were calculated using the comparative ΔCt method and an internal standard, and normalized by the mean expression values of the three housekeeping genes. Data were then calculated relative to the median determined within the investigated population.
2.4. Statistics
Descriptive statistics was calculated with PASW-Statistic Software, version 17.0.2.
3. Results and discussion
It is common knowledge that different techniques deliver different results. Also, pig breeds further specialized through intensified breeding efforts during the last decades and this also led to differing hematologic and other physiologic basic data between breeds (Egbunike and Akusu, 1983; Falkenberg et al., 1996). The investigated sows were of the Large White breed, which is established worldwide. When comparing our hematologic data with the ones provided by Thorn (2000), who investigated nine non-pregnant Duroc-Jersey sows older than one year, their data corresponded well with the data of our study. Mean erythrocyte number (±SD and range) of our sows was 5.5 ± 0.7 × 106/μl (R: 4.4–6.8 × 106/μl), mean hemoglobin was 11.8 ± 0.9 g/dl (R: 10.0–13.8 g/dl), and mean hematocrit was 34.4 ± 2.7% (R: 29.6–40.6%). Mean total leukocyte number was 12.1 ± 2.1 × 103/μl (R: 8.1–15.7 × 103/μl), mean percentages of monocytes were 4.5 ± 1.4 (R: 3.0–8.5), of lymphocytes 44.7 ± 10.2 (R: 20.9–74.0%), of neutrophils 41.6 ± 11.0 (R: 8.0–56.9), of eosinophils 6.2 ± 3.4 (R: 2.1–19.1), and of basophils 0.8 ± 0.3 (R: 0.0–1.3). The only differences were the higher MCHC (33.3–35.9 g/dl vs. 30–33 g/dl), the lower WBC count in our animals (compared to 11.6–21.0 × 103 cells/μl), and the lower count for eosinophils (compared to 10% at maximum in the Duroc-Jersey sows).
Proportions of leukocyte populations and lymphocyte subpopulations in our sows are given in Table 2. Total leukocyte number of adult sows is markedly lower than that of growing pigs with 21.9 × 103/μl (Sipos et al., 2005b). This makes sense in that the cellular immune system is not fully mature (Sinkora and Butler, 2009) and at the same time has to encounter a series of until then foreign pathogens. Probably, the juvenile immune system thus tries to compensate this functional immaturity by higher numbers of leukocytes, especially lymphocytes representing over 70% of WBCs (Sipos et al., 2005b) as compared to 44.7% in adult sows. Lymphocytes as part of the adaptive immune system are of special interest in the growing as well as the adult animal. Porcine CD4+ lymphocytes are unique in that they increase their CD8α expression when they become activated and further develop into memory Th cells (Saalmüller et al., 2002). Thus, the percentage of CD4CD8α double positive extrathymic Th cells is also increased in older animals. Consequently, the ratio of naïve Th cells to memory Th cells in 4-week-old piglets of the Large White breed was 1:0.6 (Sipos et al., 2003), whereas the corresponding ratio in the multiparous sows of our study was 1:3.1. Also, the ratio of CTLs increased markedly. In the young pigs of the aforementioned study, a CD4+CD8α− cell to CD4−CD8α+ cell ratio of 1:2.3 was observed, whereas the ratio for adult sows is 1:4.2. Another interesting feature of porcine αβ and probably also γδ T cells is that they express MHC-II molecules not only when being activated but also in the resting state (Gerner et al., 2009).
Table 2.
Leukocyte populations and lymphocyte subpopulations in % of total leukocytes.
| Cell population | Surface markers | Mean | SD | Range |
|---|---|---|---|---|
| CD21+ B cells | CD21+MHC-II+SWC3− | 6.0 | 2.5 | 2.2–12.2 |
| Plasmacytoid DCs | SWC3+CD4+CD3− | 1.6 | 0.9 | 0.4–4.4 |
| Monocytes | SWC3highCD4−CD3− | 7.8 | 4.9 | 1.3–24.7 |
| Naïve Th cells | CD3+CD4+CD8α− | 8.8 | 2.9 | 1.8–17.5 |
| Activated Th cells | CD3+CD4+MHC-II+ | 12.9 | 4.7 | 5.2–22.3 |
| Memory Th cells | CD4+CD8+CD45RC− | 16.8 | 4.6 | 10.2–28.3 |
| Activated/memory Th cells | CD3+CD4+CD8α+ | 27.5 | 6.4 | 13.6–41.9 |
| CTLs | CD3+CD4−CD8αhigh | 37.3 | 7.0 | 23.8–54.8 |
| Tregs | CD4+CD8−CD25+ | 3.1 | 1.5 | 1.0–7.2 |
| γδ T cells | TCRγδ+CD4−CD8− | 19.1 | 7.8 | 8.2–42.4 |
| Cytolytic γδ T cell subpop. | TCRγδ+CD4−CD8αhigh | 9.4 | 4.5 | 3.3–20.1 |
| NK cells | CD3−CD4−CD8α+ | 2.6 | 2.7 | 0.3–10.9 |
In this study, only CD21+ B cells were investigated. There exists also a population of CD21− B cells in pigs, but the exact importance of the presence or absence of this marker is unknown. Probably they represent B memory cells (Sinkora and Butler, 2009). CD79α is a pan B cell marker, thus also recognizing CD21− B cells, but was not applied in this study due to its intracellular localisation. The comparably low percentage of B cells in adult sows might be caused by the presumably higher number of plasma cells in the bone marrow providing antibody clones for the common pathogens the sow's immune system has to fight thus lessening the need for a larger proportion of circulating B cells. We also analysed the presence of pDCs in the peripheral blood of sows. This cell population is specialized in IFN-α production and is characterized by low expression of SWC3 and positivity for CD4 (Álvarez et al., 2008). As expected, this specialized cell population comprises only a minor fraction of leukocytes.
Whereas definite cell populations increase in age, there is a decrease in NK cells and γδ T cells in pigs (Hirt et al., 1990; Gerner et al., 2009). Surprisingly, these adult sows’ cell populations fitted well within the published ranges for juvenile animals. However, we did not perform a longitudinal study and thus cannot exclude a significant age-dependent decline in NK and γδ T cells. It would be interesting to analyse their functional capacity in older animals in future studies.
We investigated cytokine expression out of PBMCs at the protein level by means of intracellular cytokine staining as well as at the mRNA level by means of real-time RT-PCR (Table 3). With regard to cytokine production by porcine PBMCs we again have to rely on published data for juvenile pigs (Sipos et al., 2004, 2005b). In general, PBMCs of young pigs seem to express higher amounts of all so far investigated cytokines at the protein level, with the exception of TNF-α, which is produced by healthy adult sows at a comparable niveau. This makes sense as the cellular immune system has to expand and mature in young animals and most cytokines function as immunocyte-targeting growth and differentiation factors. With regard to mRNA data, there is strong evidence from existing literature that proinflammatory, mostly monocyte-derived cytokine mRNAs are upregulated within a short time-span following blood-withdrawal (Duvigneau et al., 2007). This might be the explanation for the relatively high variation seen in the mRNA expression levels of most cytokines investigated as well as inducible NO synthase mRNA, as the blood samples were collected under field conditions with a time-span of approximately three hours between sampling and the start of total RNA preparation. Although most cytokine mRNAs appear to be less suitable targets to be investigated under these conditions, we consider that TNF-α, heme oxygenase-1, IL-10, and IL-2, all showing acceptable variation, may be used for monitoring mRNA expression even under field conditions.
Table 3.
Expression of cytokines as well as iNOS and HO-1 in PBMCs as measured by flow cytometry and real-time RT-PCR.
| Cytokine | Protein level (% of positively stained cells) |
Variation of normalized mRNA expression calculated relative to group median (=1) |
|||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Positive/total (min. Ct) | Min | Max | Variance | |
| IL-1 | 7.1 | 3.5 | 1.8–19.0 | 32/32 (20.75) | 0.10 | 40.86 | 50.29 |
| IL-2 | 7.9 | 3.9 | 2.1–21.7 | 32/32 (29.6) | 0.03 | 11.81 | 19.98 |
| IL-4 | 7.3 | 2.5 | 4.1–13.7 | 31/32 (36.9) | 0.02 | 49.39 | 77.59 |
| IL-6 | 6.3 | 2.6 | 1.4–11.4 | 28/32 (32.6) | 0.01 | 123.79 | 823.62 |
| IL-10 | 5.5 | 1.9 | 2.3–9.3 | 32/32 (22.6) | 0.23 | 19.64 | 25.86 |
| IL-12p35 | 6.0 | 2.6 | 2.5–13.2 | – | – | – | – |
| TNF-α | 55.6 | 10.4 | 33.2–73.0 | 32/32 (22.6) | 0.15 | 1.38 | 4.8 |
| IFN-γ | 26.1 | 8.5 | 12.1–46.3 | 32/32 (28.6) | 0.16 | 82.82 | 208.41 |
| iNOS | – | – | – | 30/32 (30.7) | 0.0003 | 58.32 | 160.99 |
| HO-1 | – | – | – | 32/32 (29.9) | 0.02 | 1.64 | 4.7 |
One limitation of the study is the limited number of sows derived from only one herd and the cross-sectional study design. Therefore our data should only be regarded as exploratory as already stated in the title. Another limitation is that only sows of one breed have been investigated, so the obtained data may not reflect the reference intervals of other commonly housed sow breeds.
Acknowledgements
This study was supported by a grant from the Austrian Science Fund, Project No.: P20337-B13. We thank Dr. Hanna Worliczek for her excellent technical assistance and Prof. Armin Saalmüller for providing most of the anti-porcine leukocyte surface marker mAbs.
References
- Álvarez B., Revilla C., Doménech N., Pérez C., Martínez P., Alonso F., Ezquerra A., Domínguez J. Expression of toll-like receptor 2 (TLR2) in porcine leukocyte subsets and tissues. Vet. Res. 2008;39:13. doi: 10.1051/vetres:2007051. [DOI] [PubMed] [Google Scholar]
- Bailey M., Stevens K., Bland P.W., Stokes C.R. A monoclonal antibody recognizing an epitope associated with pig interleukin-2 receptors. J. Immunol. Methods. 1992;153:85–91. doi: 10.1016/0022-1759(92)90309-h. [DOI] [PubMed] [Google Scholar]
- Davis W.C., Zuckermann F.A., Hamilton M.J., Barbosa J.I., Saalmüller A., Binns R.M., Licence S.T. Analysis of monoclonal antibodies that recognize γδ T/null cells. Vet. Immunol. Immunopathol. 1998;60:305–316. doi: 10.1016/s0165-2427(97)00107-4. [DOI] [PubMed] [Google Scholar]
- Duvigneau C.J., Sipos W., Hartl R.T., Bayer M., Moldzio M., Stevenson L., Adair B., Gemeiner M. Heparin and EDTA as anticoagulant differentially affect cytokine mRNA level of cultured porcine blood cells. J. Immunol. Methods. 2007;324:38–47. doi: 10.1016/j.jim.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Egbunike G.N., Akusu M.O. Breed and sex influences on porcine haematological picture under hot and humid climatic conditions. Vet. Res. Commun. 1983;6:103–109. doi: 10.1007/BF02214902. [DOI] [PubMed] [Google Scholar]
- Falkenberg H., Micklich D., Matthes H.D., Mohring H. Reference blood values in different breeds of pigs in relation to indoor or outdoor keeping. Arch. Tierzucht. 1996;39:153–168. [Google Scholar]
- Friendship R.M., Lumsden J.H., McMillan I., Wilson M.R. Hematology and biochemistry reference values for Ontario Swine. Can. J. Comp. Med. 1984;48:390–393. [PMC free article] [PubMed] [Google Scholar]
- Gerner W., Käser T., Saalmüller A. Porcine T lymphocytes and NK cells—an update. Dev. Comp. Immunol. 2009;33:310–320. doi: 10.1016/j.dci.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Hammerberg C., Schurig G.G. Characterization of monoclonal antibodies directed against swine leukocytes. Vet. Immunol. Immunopathol. 1986;11:107–121. doi: 10.1016/0165-2427(86)90092-9. [DOI] [PubMed] [Google Scholar]
- Hirt W., Saalmüller A., Reddehase M.J. Distinct g/d T cell receptors define two subsets of circulating porcine CD2−CD4−CD8− T lymphocytes. Eur. J. Immunol. 1990;20:265–269. doi: 10.1002/eji.1830200206. [DOI] [PubMed] [Google Scholar]
- Jonjic S., Koszinowski U.H. Monoclonal antibodies reactive with swine lymphocytes I. Antibodies to membrane structures that define the cytolytic T lymphocyte subset in the swine. J. Immunol. 1984;133:647–652. [PubMed] [Google Scholar]
- Lunney J.K. Characterization of swine leukocyte differentiation antigens. Immunol. Today. 1993;14:147–148. doi: 10.1016/0167-5699(93)90227-C. [DOI] [PubMed] [Google Scholar]
- Pescovitz M.D., Lunney J.K., Sachs D.H. Preparation and characterisation of monoclonal antibodies reactive with porcine PBL. J. Immunol. 1984;133:368–375. [PubMed] [Google Scholar]
- Saalmüller A., Werner T., Fachinger V. T-helper cells from naïve to committed. Vet. Immunol. Immunopathol. 2002;87:137–145. doi: 10.1016/s0165-2427(02)00045-4. [DOI] [PubMed] [Google Scholar]
- Sinkora M., Butler J.E. The ontogeny of the porcine immune system. Dev. Comp. Immunol. 2009;33:273–283. doi: 10.1016/j.dci.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipos W., Duvigneau C., Pietschmann P., Höller K., Hartl R., Wahl K., Steinborn R., Gemeiner M., Schmoll F. Parameters of humoral and cellular immunity following vaccination of pigs with a European modified-life strain of porcine reproductive and respiratory syndrome virus (PRRSV) Viral Immunol. 2003;16:335–346. doi: 10.1089/088282403322396136. [DOI] [PubMed] [Google Scholar]
- Sipos W., Duvigneau C., Willheim M., Schilcher F., Hartl R., Hofbauer G., Exel B., Pietschmann P., Schmoll F. Systemic cytokine profile in feeder pigs suffering from natural postweaning multisystemic wasting syndrome (PMWS) as determined by semiquantitative RT-PCR and flow cytometric intracellular cytokine detection. Vet. Immunol. Immunopathol. 2004;99:63–71. doi: 10.1016/j.vetimm.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Sipos W., Willheim M., Hofbauer G., Pietschmann P. Evaluation of the suitability of monoclonal antibodies applied for flow cytometric intracellular cytokine detection in porcine peripheral blood lymphocytes. J. Vet. Med. A. 2005;52:55–60. doi: 10.1111/j.1439-0442.2005.00689.x. [DOI] [PubMed] [Google Scholar]
- Sipos W., Duvigneau J.C., Pietschmann P., Schilcher F., Hofbauer G., Hartl R.T., Schmoll F. Porcine Dermatitis and Nephropathy Syndrome (PDNS) is associated with a systemic cytokine expression profile indicative of proinflammation and a Th1 bias. Vet. Immunol. Immunopathol. 2005;107:303–313. doi: 10.1016/j.vetimm.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Sipos W., Duvigneau J.C., Sterz F., Weihs W., Krizanac D., Bayegan K., Graf A., Hartl R.T., Janata A., Holzer M., Behringer W. Changes in interleukin-10 mRNA expression are predictive for 9-day survival of pigs in an emergency preservation and resuscitation model. Resuscitation. 2010;81:603–608. doi: 10.1016/j.resuscitation.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Thorn C. Normal hematology of the pig. In: Feldman B.F., Zinkl J.G., Jain N.C., editors. Schalm's Veterinary Hematology. 5th ed. Lippincott Williams & Wilkins; Baltimore, MD, USA: 2000. pp. 1089–1095. [Google Scholar]
- Yang H., Oura C.A., Kirkham P.A., Parkhouse R.M. Preparation of monoclonal anti-porcine CD3 antibodies and preliminary characterization of porcine T lymphocytes. Immunology. 1996;88:577–585. doi: 10.1046/j.1365-2567.1996.d01-682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann F.A., Binns R.M., Husmann R., Yang H., Carr M.M., Kim Y.B., Davis W.C., Misfeldt M., Lunney J.K. Analyses of monoclonal antibodies reactive with porcine CD44 and CD45. Vet. Immunol. Immunopathol. 1994;43:293–305. doi: 10.1016/0165-2427(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Zuckermann F.A., Schabacker D., Binns R.M. Biochemical analysis of molecules reactive with monoclonal antibodies specific for porcine CD45. Vet. Immunol. Immunopathol. 1994;43:307–313. doi: 10.1016/0165-2427(94)90152-x. [DOI] [PubMed] [Google Scholar]