Abstract
Lapatinib is an inhibitor of the tyrosine kinases of human epidermal growth factor receptor type 2 (HER2) and epidermal growth factor receptor type 1, with clinical activity in HER2-positive metastatic breast cancer. We present here a 60 year-old patient with metastatic breast cancer who presented with jaundice and increased serum aminotransferase levels and who had been treated with lapatinib for the previous 14 days. Laboratory tests excluded other causes of acute liver injury. Liver biopsy revealed lesions compatible with drug-induced hepatotoxicity. Bilirubin and liver enzymes returned to normal within three months of lapatinib discontinuation. Lapatinib should be included among the causes of drug-induced hepatitis.
Keywords: Lapatinib, Hepatitis, Hepatotoxicity, Breast cancer, Human epidermal growth factor receptor type 2
INTRODUCTION
Metastatic breast cancer is the leading cause of death from cancer among women worldwide[1]. The overexpression of human epidermal growth factor receptor type 2 (HER2) predisposes patients to a greater risk for disease progression and death than women whose tumors do not overexpress HER2[2]. Therapeutic approaches to block HER2 signaling pathways include both trastuzumab (a recombinant, humanized, monoclonal antibody that binds to the extracellular domain of the HER2) and lapatinib. Lapatinib is an orally administered small molecule that inhibits the tyrosine kinases of HER2 and epidermal growth factor receptor type 1 (EGFR). A number of studies have shown that lapatinib has clinical activity in patients with HER2-positive breast cancer, with a significant reduction in the risk of disease progression[3]. Lapatinib is generally well tolerated and the most common treatment-related adverse events include rash, diarrhea, and nausea[4].
We report here a case of advanced breast cancer treated with lapatinib with drug induced hepatitis. Upon discontinuation of lapatinib, levels of serum aspartate amino transferase (SGOT) and serum alanine amino transferase (SGPT) declined progressively.
CASE REPORT
A 60 year-old woman presented with metastatic breast cancer in the lung. Three years earlier she was diagnosed with invasive ductal adenocarcinoma of the right breast (pT1cN0M0). Immunohistochemistry was positive for estrogen (70% positive cells), progesterone (2% positive cells) receptors and for HER-2. She was treated with lumpectomy and axillary lymph node dissection. Subsequently she received adjuvant chemotherapy (four cycles of epirubicin/cyclophosphamide followed by 4 cycles of paclitaxel) and treatment with the monoclonal antibody trastuzumab for one year. Furthermore, radiotherapy was given to the entire breast as an adjunct to breast conservation treatment and hormonal therapy with the aromatase inhibitor exemestane was started. She remained free of disease for three years.
In terms of periodic reassessment, the patient had a computed tomography (CT) of the chest in January 2010 and was found to have three nodules in the right lung compatible with metastatic sites. A CT of the brain and abdomen showed no abnormalities and a mammogram of both breasts and a bone scan were also normal. According to laboratory data, there were no abnormal values.
The patient commenced therapy with capecitabine (1000 mg/m2 twice daily, day 1-14) and lapatinib (1250 mg/d), while exemestane was discontinued after two and a half years of continuous administration. After ten days, capecitabine was discontinued due to grade 2 diarrhea and the patient continued to receive lapatinib only.
Two weeks later, the patient developed jaundice without any other clinical signs such as asthenia or pruritus and she was evaluated in the Department of Clinical Oncology.
Physical examination was unremarkable except for jaundice and mild hepatomegaly. Laboratory results showed: SGPT 583 U/L units (normal range: 5-45), SGOT 457 U/L (normal range: 5-40), ALP 348 U/L (normal limits < 270), g-glutamyl transpeptidase 213 U/L (normal range: 10-55), total bilirubin 4.1 mg/dL (normal range: 0-1.5), LDH 305 units (normal range: 100-240), total protein 6.4 g/dL (normal range: 6-8.4), albumin 3.7 g/dL (normal range: 3.4-5), white blood cells 7500/mm3, Hb 12.8 g/dL, Hct 38.8%, platelets 118 000/mm3 and international normalized ratio-1.14 (normal range: 0.8-1.2). Other blood chemistry results, including glucose, cholesterol, triglycerides, serum amylase, uric acid, creatinine, BUN, Na, K, Ca as well as urinalysis were normal.
Serology tests for hepatitis A, B, and C viruses were negative. Immune serology was also negative for ANA, anti-DNA, c-ANCA, p-ANCA, antismooth muscle, and antimitochondrial antibodies. Furthermore, thyroid hormone tests, a1-antitrypsin, IgG, IgA, IgM and blood ceruloplasmin levels were within normal limits.
Abdominal ultrasound demonstrated an increased-sized liver with non-homogeneous and diffuse echogenicity, without biliary tract abnormalities. Normal blood flow was seen in the portal vein, hepatic artery, hepatic veins, and inferior vena cava. A new abdominal CT showed hepatomegaly without biliary tract obstruction.
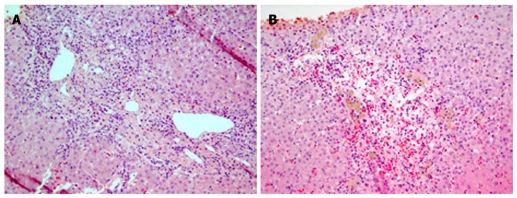
Fifteen days after the clinical evolution, a liver biopsy was performed and showed necrosis of contiguous hepatocytes in portal-to-portal and portal-to-central fashion (bridging necrosis). Foci of severe hemorrhage and hepatocellular dropout around the centrilobular areas were also demonstrated (Figure 1). Eosinophils were observed in more than twelve portal spaces without granulomas.
Figure 1.
Photomicrograph. A: Areas of bridging necrosis (HE x 200); B: Areas of centrilobular hepatocellular dropout, hemorrhage and macrophages (HE x 200).
The patient was not taking other concomitant hepatotoxic medications, herbal or dietary supplements and did not have any underlying liver dysfunction, chronic hepatitis or alcohol use history.
Lapatinib was discontinued and both the patient’s jaundice and liver function tests gradually improved and returned to normal range within 3 mo (Table 1).
Table 1.
Evolution of laboratory tests after lapatinib discontinuation
| SGPT (U/L) | SGOT (U/L) | ALP (U/L) | GGT (U/L) | TBIL (mg/dL) | |
| Baseline (before initiation of capecitabine/lapatinib) | 24 | 21 | 120 | 11 | 0.8 |
| Ten days later (capecitabine discontinuation due to diarrhea) | 30 | 22 | 110 | 20 | 0.7 |
| Two weeks after capecitabine discontinuation (lapatinib discontinuation) | 583 | 457 | 348 | 213 | 4.1 |
| Two weeks after lapatinib discontinuation | 481 | 589 | 310 | 294 | 11.8 |
| 1 mo after lapatinib discontinuation | 260 | 238 | 318 | 160 | 2.7 |
| 2 mo after lapatinib discontinuation | 229 | 180 | 298 | 140 | 2.3 |
| 3 mo after lapatinib discontinuation | 44 | 36 | 246 | 42 | 0.9 |
SGPT: Serum glutamic pyruvic transaminase; SGOT: Serum glutamic oxaloacetic transaminase; ALP: Alcaline phosphatase; GGT: Gamma-glutamyl transpeptidase; TBIL: Total Bilirubin.
DISCUSSION
Lapatinib combined with capecitabine has been approved for the treatment of patients with HER-2 positive metastatic breast cancer improving median time to disease progression[3].
Furthermore, recent studies suggest that single-agent lapatinib has clinical activity with manageable toxicity in HER2-overexpressing breast cancer that progressed on trastuzumab-containing therapy[4,5]. The fact that lapatinib inhibits EGFR signaling may also contribute to its activity in the context of refractory HER2-positive breast cancer.
No significant liver dysfunction has been recorded to lapatinib administration in any daily dose (500-1600 mg) in many phase I and II studies[5,6]. Hence, grade 3 and 4 liver toxicity with elevations in transaminases are uncommon after single agent lapatinib administration and only one out of 37 patients experienced grade 3 elevation of transaminases in a phase II trial in patients with brain metastases from HER-2 positive breast cancer[7].
Lapatinib is predominantly metabolized in the liver via the cytochrome P450 system, by the enzyme P450 3A4 and < 2% of the drug is excreted unchanged in urine.
Concurrent administration of the strong CYP3A4 inhibitor ketoconazole increases the lapatinib area under the curve and prolongs the t1/2. For this reason, strong inhibitors of CYP3A4, including grapefruit juice, should be avoided, as they may increase plasma concentrations of lapatinib and thus lapatinib toxicity[8].
Furthermore, it can be assumed that polymorphisms of the CYP3A4 gene may affect lapatinib disposition[9]. However, pharmacogenomical studies on the same gene did not show any correlation between gene polymorphisms and commonly observed toxicities such as exanthema or diarrhea caused by the use of erlotinib, an epidermal growth factor receptor tyrosine-kinase inhibitor[10].
The patient described in our case developed severe hepatic enzyme disturbances during lapatinib treatment and liver biopsy showed acute drug-induced hepatitis.
The patient did not take any other drug except lapatinib and recovered when the treatment was discontinued. There was no evidence of viral or autoimmune hepatitis or any other cause of hepatitis. Hepatic enzymes normalized rapidly after discontinuation of lapatinib.
The time to onset of jaundice and laboratory test abnormalities as well as the time and course of recovery, while the patient did not receive other medications, led us to support the idea that lapatinib is the most likely cause of hepatic injury. Exemestane appears not to be an important risk factor for the development of hepatitis because this medication was administered for two and a half years, without any clinical symptoms or abnormal laboratory tests.
Based on the fact that capecitabine was stopped only two weeks before the onset of symptoms, it was necessary to assesss the likelihood of the agent being causative. Capecitabine has not been implicated in hepatocellular injury and hepatitis with the specific histological features. Furthermore in order to confirm that liver injury was due to lapatinib, we used the Roussel Uclaf Causality Assesment Method, a scoring system which assigns attribution for drug-induced liver injury[11]. The summed points grouped lapatinib into highly probable category, confirming our suggestion.
Our case demonstrates the potential risk of developing toxic hepatitis during treatment with lapatinib.
To conclude, to our knowledge this is the first case of acute drug-induced hepatitis secondary to lapatinib. Given that lapatinib is now often used in the treatment of HER-2 positive advanced breast cancer, it is mandatory for medical oncologists to be aware of this potential side effect in clinical practice.
Footnotes
Peer reviewer: Mireia Miquel, MD, PhD, Liver Unit, Gastroenterology Service, Parc Taulí s/n, Sabadell, 08201, Spain
S- Editor Sun H L- Editor O’Neill M E- Editor Ma WH
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 4.Blackwell KL, Pegram MD, Tan-Chiu E, Schwartzberg LS, Arbushites MC, Maltzman JD, Forster JK, Rubin SD, Stein SH, Burstein HJ. Single-agent lapatinib for HER2-overexpressing advanced or metastatic breast cancer that progressed on first- or second-line trastuzumab-containing regimens. Ann Oncol. 2009;20:1026–1031. doi: 10.1093/annonc/mdn759. [DOI] [PubMed] [Google Scholar]
- 5.Gomez HL, Doval DC, Chavez MA, Ang PC, Aziz Z, Nag S, Ng C, Franco SX, Chow LW, Arbushites MC, et al. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J Clin Oncol. 2008;26:2999–3005. doi: 10.1200/JCO.2007.14.0590. [DOI] [PubMed] [Google Scholar]
- 6.Burris HA 3rd, Hurwitz HI, Dees EC, Dowlati A, Blackwell KL, O’Neil B, Marcom PK, Ellis MJ, Overmoyer B, Jones SF, et al. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol. 2005;23:5305–5313. doi: 10.1200/JCO.2005.16.584. [DOI] [PubMed] [Google Scholar]
- 7.Lin NU, Carey LA, Liu MC, Younger J, Come SE, Ewend M, Harris GJ, Bullitt E, Van den Abbeele AD, Henson JW, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26:1993–1999. doi: 10.1200/JCO.2007.12.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tevaarwerk AJ, Kolesar JM. Lapatinib: a small-molecule inhibitor of epidermal growth factor receptor and human epidermal growth factor receptor-2 tyrosine kinases used in the treatment of breast cancer. Clin Ther. 2009;31 Pt 2:2332–2348. doi: 10.1016/j.clinthera.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 9.Tan SH, Lee SC, Goh BC, Wong J. Pharmacogenetics in breast cancer therapy. Clin Cancer Res. 2008;14:8027–8041. doi: 10.1158/1078-0432.CCR-08-0993. [DOI] [PubMed] [Google Scholar]
- 10.Rudin CM, Liu W, Desai A, Karrison T, Jiang X, Janisch L, Das S, Ramirez J, Poonkuzhali B, Schuetz E, et al. Pharmacogenomic and pharmacokinetic determinants of erlotinib toxicity. J Clin Oncol. 2008;26:1119–1127. doi: 10.1200/JCO.2007.13.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontana RJ, Seeff LB, Andrade RJ, Björnsson E, Day CP, Serrano J, Hoofnagle JH. Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology. 2010;52:730–742. doi: 10.1002/hep.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]