Abstract
Background and Objectives
Inappropriately high left ventricular mass (iLVM) is known to be related to cardiovascular prognosis. A non-dipper pattern has a greater mean left ventricular (LV) mass than the dipper pattern in hypertensive patients. However, the appropriateness of LV mass in dipper or non-dipper patterns has not been adequately investigated. The aim of this study was to define the relationship between nocturnal dipping and the appropriateness of LV mass.
Subjects and Methods
Using the ambulatory blood pressure monitoring (ABPM) database, the data of 361 patients who underwent ABPM and echocardiography was analyzed retrospectively. Appropriateness of LV mass was calculated as observed/predicted ratio of LV mass (OPR) using a Korean-specified equation. Nocturnal dipping was expressed as percent fall in systolic blood pressure (BP) during the night compared to the day.
Results
Daytime, nighttime and 24 hours BP in hypertensive patients was 140.4±14.8 mmHg, 143.7±15.2 mmHg and 129.4±20.0 mmHg, respectively. OPR was 106.3±19.9% and nocturnal dipping was 10.2±10.9 mmHg. In a multiple linear regression model, 24 hours systolic BP (β=0.097, p=0.043) and nocturnal dipping (β=-0.098, p=0.046) were independent determinants of OPR as well as age (β=0.130, p=0.025) and body mass index (BMI) (β=0.363, p<0.001). Odds ratio of the non-dipper pattern was 2.134 for iLVM (p=0.021) and 3.694 for obesity (p<0.001; BMI >25 kg/m2).
Conclusion
The non-dipper pattern is independently associated with iLVM in hypertensive patients as well as obesity.
Keywords: Hypertension; Hypertrophy, left ventricular; Blood pressure monitoring, ambulatory
Introduction
Left ventricular hypertrophy (LVH) is a strong, independent predictor of cardiovascular morbidity and mortality in hypertensive patients.1) Left ventricular (LV) mass is influenced by hemodynamic factors, such as high blood pressure (BP) and stroke work, as well as numerous demographic and non-hemodynamic factors.2) However, a number of patients exhibit levels of LV mass that exceed the need to sustain cardiac workload, a condition that has been defined as inappropriately high left ventricular mass (iLVM).3)
iLVM is related to a worse cardiovascular prognosis than appropriate LV mass (aLVM) in patients with or without LVH.2) The iLVM may be explained by several mechanisms. Firstly, the presence of a higher BP load in the ascending aorta cannot be explained by brachial BP derived resting stroke work.3) Secondly, imbalance between growth-promoting factors and growth inhibitory factors has been suggested.4-6) Thirdly, BP variability throughout the day might influence increased LV mass.7) In addition, genetic factors may be responsible for exaggerated or overcompensating hypertrophy to a given pressure load.8) Nonetheless, the underlying pathogenic mechanism is not fully understood.9)
In addition to these mechanisms, the presence of a hidden higher BP load out of the clinic or during sleep which cannot be explained by office BP deserves attention, since non-dipper or reverse white coat effects are common clinical problems. Hypertensive patients with non-dipper patterns in nocturnal BP have heavier LV mass than those with dipper patterns. Patients with non-dipper patterns are also at a higher risk of cardiac and extracardiac morbidity.10),11) Regarding poor prognosis in non-dipper patterns however, it is unclear whether there are other mechanisms such as growth or genetic factors that simply hide BP overload during sleep. Likewise, regarding the heavier LV mass in non-dipper patterns, it is also unclear whether heavier LV mass is due to simply hidden BP overload during sleep or due to other mechanisms related to the non-dipper pattern.
To better understand the mechanisms of iLVM, we hypothesized that nocturnal dipping is one of the factors determining inappropriateness of LV mass in hypertensive patients.
Subjects and Methods
Study population and study design
In a retrospective cross-sectional design, data from 361 patients visiting the Cardiology Center at Hanyang University Hospital, Seoul, Korea from January 1st, 2006 to June 30th, 2010 were analyzed. Among 1,297 patients who underwent ambulatory blood pressure monitoring (ABPM) and echocardiography within 1 week interval for the evaluation of BP before or after anti-hypertensive medication, 361 patient datasets were acquired by applying exclusion criteria. The clinical data were incomplete and as follows; age <45 years, patients with previous history of stroke, acute myocardial infarction, patients with chronic renal insufficiency (serum creatinine >1.4 mg/dL in female, >1.5 mg/dL in male), any regional wall motion abnormalities, grade II or more valvular regurgitation, any valvular stenosis, M-mode interrogation angle >10 degrees and cardiomyopathy based on the echocardiographic findings. Height, weight, clinical BP and heart rate were measured during the study period before ABPM and echocardiography were performed. The study protocol was approved by the Institutional Review Board (IRB) of Hanyang University Medical Center at Seoul. An informed consent process from each patient regarding the process of the examination and use of the data was made exempt by the IRB.
Blood pressure determination
Clinic BP was measured using a mercury sphygmomano-meter as the average of at least 3 measurements by a physician or qualified nurse. ABPM was recorded during a routine day by a TM-2430 device (A&D, Saitama, Japan). The device was applied to the non-dominant arm and was applied for 24 hours. BP was measured every 15 minutes during the daytime and every 30 minutes at nighttime, which was between 10 PM and 6 AM. The patients were instructed to maintain their usual activities during the monitoring process and to stay calm when sensing the cuff inflation. Sleep and wake time were recorded individually according to the patient's self-reported data.
Nocturnal BP was defined by the narrow fixed interval method from midnight to 5 AM as nocturnal systolic/diastolic mean BP. Daytime BP was defined by mean systolic/diastolic BP between 8 AM and 9 PM. Twenty-four hour BP was defin-ed by the following equation, (nocturnal BP×actual sleep in-terval+daytime BP×awake interval)/24. Nocturnal systolic BP (NSBP) fall (mmHg) was defined as the absolute decrease in NSBP compared to daytime systolic BP. Nocturnal dipping (%) was defined by percent decrease in NSBP compared to daytime systolic BP. When nocturnal dipping was less than 10%, it was defined as a non-dipper pattern. The definition of hyper-tension was 24-hour systolic BP ≥125 mmHg or on antihypertensive medication excluding the white-coat hypertension. According to this definition, 309 of the patients were hyperten-sive out of the total 361 patients.
Echocardiography
Two dimensional and guided M-mode echocardiograms were performed on each subject by a single sonographer with a commercially available machine (iE33; Philips medical system, Andover, MA, USA) using a 1-5 MHz transducer. M-mode tracings were recorded on strip-chart paper at 50 mm/s. Measurements of interventricular septal thickness, posterior wall thickness, and LV dimensions were performed at or just below the mitral valve tips, by the leading edge-to-leading edge method, according to the American Society of Echocardiography recommendations.12) LV mass was calculated by the following equation, {1.04×(IVSd+LVDd+PWTd)3-LVDd3}×0.8+0.6,13) where IVSd is diastolic interventricular septum, LVDd is diastolic left ventricular dimension, and PWTd is dia-stolic posterior wall thickness. We adopted the LV mass index by the height to highlight the effect of weight and set the cut-off value {mean+ 2 standard deviation (SD)} for LVH as 54 g/m2.7 for both genders. LV end-systolic, end-diastolic, and stroke volumes were calculated with the use of Teichholz's method.
Appropriateness of left ventricular mass
Appropriateness of LV mass (aLVM) was expressed as the observed/predicted ratio of the LV mass (OPR). The predicted LV mass was calculated as previously described.14) Briefly, the LV mass was predicted by the following equation acquired in subjects with normal body weight and BP: 54.9+7.62×height (m2.7)+0.6×stroke work (g-m/beat)-13.2×gender (male=1, fe-male=2) (constant=54.9±14.7 g, adjusted R2=0.576, SEE=21.67, p=0.001). Gender effect was adjusted by the equation of appropriateness of LV mass. Stroke work refers to the work done by the ventricle to eject stroke volume into the aorta as a single-beat index of cardiac workload. It was calculated by the equation, stroke volume×(MEP-EDP), where MEP is mean ejection pressure and EDP is LV end-diastolic pressure.15) In our study, using a coefficient representing the relationship between systolic BP and (MEP-EDP), stroke work is calculated as stroke volume×systolic BP×0.0144.16) The cut-off value between the iLVM and aLVM groups was 130% or higher for both genders.14)
Statistical analysis
Data were expressed as frequencies and percentages for qualitative variables and as the mean±SD for quantitative variables. Differences in study variables between hypertensive and normotensive patients were assessed with the Pearson χ2 for qualitative variables and the Student's t-test was performed. Pearson's correlation and Spearman's correlation coefficients were calculated. Multiple regression analyses were used to identify significant determinants for OPR or iLVM. Anti-hypertensive drug treatment was included in the multiple logistic regression analysis.
ANCOVA was used to adjust the result of comparing subjects with inappropriately high or adequate LV mass for age, body mass index (BMI), 24 hours BP. Odds ratio of independent variables were calculated by multiple logistic regression analysis. The values of 2-tailed p<0.05 were considered statistically significant. Data were analyzed using the Statistical Package for the Social Sciences (SPSS) 17.0 software (SPSS Inc., Chicago, IL, USA).
Results
General characteristics of the subjects
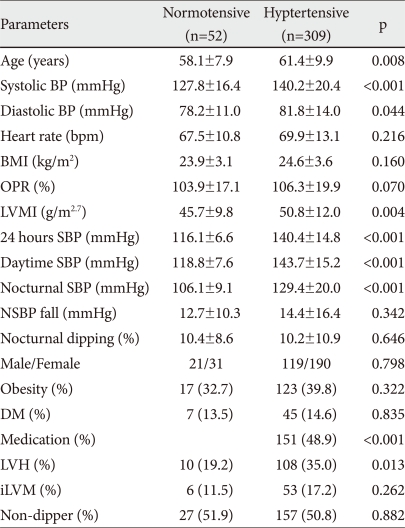
General characteristics of the normotensive subjects versus hypertensive patients are listed in Table 1. Among the 361 patients of the study population, 52 (14.4%), classified as normotensive, were white-coat hypertension patients and 309 (85.6%) were classified as hypertensive patients. Among the 309 hypertensive patients, 151 (48.9%) were on antihypertensive treatment and 45 (14.6%) had diabetes mellitus (DM).
Table 1.
Clinical characteristics of normotensive and hypertensive subjects
Data are reported as mean±SD. BP: blood pressure, BMI: body mass index, OPR: observed/predicted ratio of LV mass, LVMI: left ventricular mass index, SBP: systolic BP, LVH (LVMI ≥54.0 g/m2.7): left ventricular hypertrophy, NSBP: Nocturnal SBP, DM: diabetes mellitus, Medication: Antihypertensive medication, iLVM: inappropriate high LV mass, Obesity: BMI >25 kg/m2
In a comparison of general characteristics between hyperten-sive patients and normotensive subjects, the average age of the hypertensive patients was older (58.1±7.9 vs. 51.5±14.4 years, p=0.02). Clinical BP, 24 hours ambulatory BP and BMI were higher in hypertensive patients.
Correlations between observed/predicted ratio and 24 hours ambulatory blood pressure profile
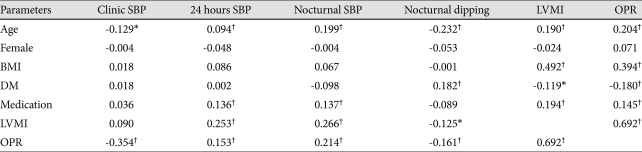
Among general parameters, age, BMI, DM and antihypertensive medication showed a correlation with OPR in hypertensive patients. Twenty four hours systolic BP was positively associated with OPR, in contrast, clinical systolic BP was not, which suggested the white-coat effect. Nocturnal dipping showed an inverse relationship with OPR suggesting a protective relationship (Table 2).
Table 2.
Pearson and Spearman correlation test between OPR and 24 hours ambulatory blood pressure profile in patients (n=361)
*p<0.05, †p<0.001. SBP: systolic blood pressure, BMI: body mass index, OPR: observed/predicted ratio of LV mass, DM: diabetes mellitus, LVMI: left ventricular mass index, Medication: Antihypertensive medication
Multiple linear regression analysis for inappropriate increased LV mass (observed/predicted ratio)
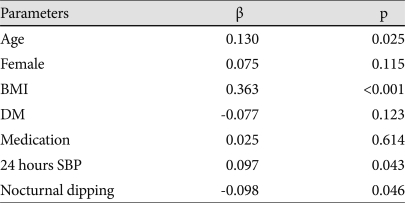
In a multiple linear regression analysis for OPR of LV mass in all patients, age (β=0.130, p=0.025), BMI (β=0.363, p<0.001), 24 hours systolic BP (β=0.097, p=0.043) and nocturnal dipping (β=-0.098, p=0.046) were statistically significant parameters (Table 3). Gender was not statistically significant (p=0.115).
Table 3.
Multiple linear regression for OPR of LV mass in all patients (n=361)
Dependent variable: observed/predicted ratio of LV mass. SBP: systolic blood pressure, BMI: body mass index, DM: diabetes mellitus, Medication: Antihypertensive medication, OPR: observed/predicted ratio, LV: left ventricular
Multiple logistic regression analysis for inappropriately high left ventricular mass
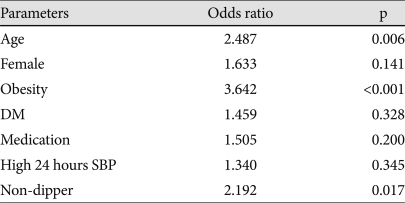
In all patients, odds ratio by the multiple logistic regression analysis for iLVM were 3.694 {95% confidence interval (CI): 1.963-6.758, p<0.001} for obesity, and 2.192 (95% CI: 1.151-4.177, p=0.017) for the non-dipper pattern (Table 4). However, high 24 hours systolic BP was not statistically significant for iLVM.
Table 4.
Multiple logistic regression for iLVM in all patients (n=361)
Dependent variable: inappropriately high left ventricular mass. SBP: systolic blood pressure, BMI: body mass index, OPR: observed/predicted ratio of LV mass, DM: diabetes mellitus, HTN: hypertension, iLVM: inappropriately high left ventricular mass
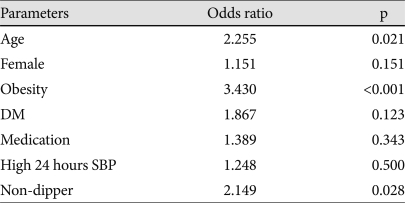
In hypertensive patients, the odds ratio by the multiple logistic regression analysis for iLVM were 2.255 (95% CI: 1.132-4.493, p=0.021) for age, 3.430 (95% CI: 1.781-6.604, p<0.001) for obesity, and 2.149 (95% CI: 1.087-4.249, p=0.028) for the non-dipper pattern (Table 5).
Table 5.
Multiple logistic regression for iLVM in hypertensive patients (n=309)
Dependent variable: inappropriately high left ventricular mass. SBP: systolic blood pressure, OPR: observed/predicted ratio of LV mass, DM: diabetes mellitus, iLVM: inappropriately high left ventricular mass
Comparsion between appropriate left ventricular mass and inappropriately high left ventricular mass group
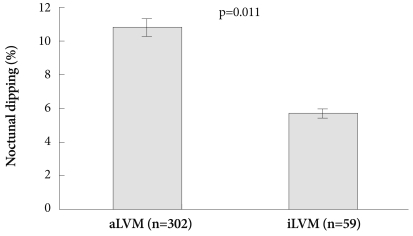
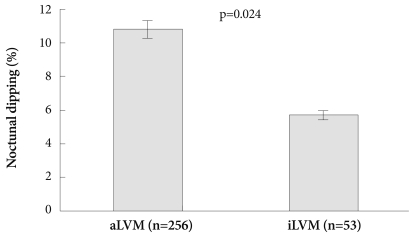
When adjusted by age, BMI and 24 hours ssystolic BP, nocturnal dipping (10.8±10.6 vs. 5.7±9.9, p=0.011) was significantly different between the aLVM and iLVM group in all patients (Fig. 1). In the hypertensive group, nocturnal dipping (10.7±11.0 vs. 5.7±10.0, p=0.024) was significantly different between the aLVM and iLVM group (Table 6) (Fig. 2)
Fig. 1.
Adjusted Nocturnal dipping by age, BMI and 24 hours systo-lic BP between aLVM (n=302) vs. iLVM (n=59) patients. In all pati-ents, nocturnal dipping (10.8±10.6 vs. 5.7±9.9, p=0.011) was signi-ficantly different in the iLVM group vs. the aLVM group. SBP: sys-tolic blood pressure, BMI: body mass index, aLVM: appropriate left ventricular mass, iLVM: inappropriately high left ventricular mass.
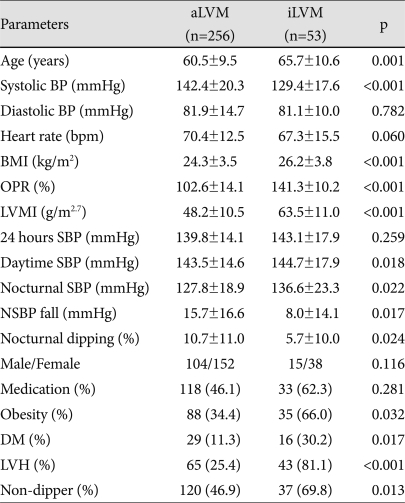
Table 6.
Comparison between aLVM and iLVM in hypertensive patients
Data are reported as mean±SD and adjusted by age, BMI, 24 hours SBP. BP: blood pressure, BMI: body mass index, OPR: observed/predicted ratio of LV mass, LVMI: left ventricular mass index, SBP: systolic BP, LVH (LVMI ≥54.0 g/m2.7): left ventricular hypertrophy, Medication: Antihypertensive medication, NSBP: Nocturnal SBP, DM: diabetes mellitus, aLVM: appropriate LV mass, iLVM: inappropriate high LV mass, Obesity: BMI >25 kg/m2
Fig. 2.
Adjusted nocturnal dipping by age, BMI and 24 hours systolic BP in hypertensive aLVM (n=256) vs. iLVM (n=53) patients. In the hypertensive patients, nocturnal dipping (10.7±11.0 vs. 5.7±10.0, p=0.024) was significantly different in iLVM vs. aLVM groups. SBP: sys-tolic blood pressure, BMI: body mass index, aLVM: appropriate left ventricular mass, iLVM: inappropriately high left ventricular mass.
Discussion
This study was performed for a further understanding of the mechanism of iLVM. Nocturnal dipping as a factor related to OPR was the main interest. The comparison between the dipper and non-dipper group is out of the scope of this study. The main result of our study is that nocturnal dipping is an independent factor determining OPR in essential hypertensive patients. Nocturnal dipping is significantly lower in the iLVM group in hypertensive patients.
Prediction of LV mass routinely preclude gender difference, body size, and the effect of stroke work because these factors are included in the prediction equation derived from subjects with normal body weight and normal BP.16) If it is measured in patients with a white-coat effect, predicted LV mass is overestimated so that OPR tends to be underestimated or low. On the contrary, OPR in patients with masked clinic BP tends to be higher.
Besides measurement issues, it is well known that combination of high BP and obesity biologically has a synergistic effect on the LVH.17) This suggests that ambulatory BP provides a stronger relationship with LV mass increase than that observed in normotensive patients without obesity. Firstly, a stronger association between iLVM and BP might results from accurate BP measurement by ABPM mainly excluding the white-coat and reverse white-coat effect. Secondly, the intrinsic relationship between BP and OPR may be stronger in essential hypertension than in normotensive subjects. Th-irdly, the synergistic effect of obesity and BP may amplify the relationship between BP and OPR.
A previous study showed that iLVM was associated with higher ambulatory BP in hypertensive patients.18) In this study, patients with iLVM had lower 24 hours systolic BP suggesting that the white-coat effect was exposed by ABPM. This st-udy revealed that 24 hours systolic BP and OPR had a weak independent relationship (β=0.097, p=0.043) in the multiple linear regression model, including age, BMI, and nocturnal dipping. Also, the 24 hours systolic BP was not an independent factor determining iLVM in multiple logistic regression analysis. This finding suggests that white-coat or reverse white-coat effects were not high enough for the ABPM to expose the measurement problem. To further understand this issue, OPR defined by the equation using ambulatory BP will be helpful. In addition, a direct study of the relationship between white-coat or reverse white-coat effects and iLVM seems to be necessary.
This study showed that obesity is an important determinant of iLVM in hypertension patients. Using multiple regression analysis, obesity was the strongest determinant of OPR or iLVM. Obesity seems to play a major role to induce inappropriately or excessively high LV mass, which is beyond the proportional increase to BP elevation. There have been numerous studies for the mechanisms of obesity related to LV hypertrophy.19)
In this study, effect of age itself on OPR or iLVM is just com-parable to the 24 hours systolic BP effects or nocturnal dipping. It is also much less than the obesity factor. If ageing itself could induce LVH, bypassing the mechanisms of obesity or systolic BP as well as partly including arterial stiffness, then this would clarify some of the controversy that surrounds this phenomena. This finding is consistent with the previous study that the effect of ageing itself is not known to be significant in subjects with normal BP and BMI.16) The result of this study suggests that the main mechanisms of ageing in regard to inappropriateness of LV mass are mainly composed of obesity, systolic BP, and potentially a non-dipper pattern.
Regarding the mechanisms of nocturnal dipping contributing to OPR, it is less likely that decreased nocturnal dipping contributes to higher OPR as a hidden nocturnal hemodynamic overload because it is independent of 24 hours ambulatory systolic BP. In this study, nocturnal dipping was significantly different between iLVM and aLVM group in all patients and in hypertensive patients. These findings suggest that nocturnal dipping may influence the appropriateness of LV mass by its own non-hemodynamic or intrinsic effect.
It has been a controversy if the main mechanism of non-dipper as a poor prognostic marker for cardiovascular events is related to the nocturnal hypertension or its intrinsic effect. A previous study showed that non-dippers have autonomic dysfunction through the night and that non-dipper patterns were more commonly found among individuals with renal diseases, DM, sleep apnea and secondary hypertension.20) These findings suggest that non-hemodynamic factors are important in manipulating nocturnal dipping. Therefore, the non-dipper pattern itself might be an explanation to the results of this study. However, the reproducibility of the classification of dipper and non-dipper patterns based on single ABPM is not reliable enough to be widely accepted.21)
In this study, anti-hypertensive drug therapy was not an independent factor determining iLVM. The effects of anti-hypertensive drugs such as the lowering of BP and regression of LVH in proportion have been widely reported.22) However, the effect of anti-hypertensive drug classes on nocturnal BP has not been established. Timing of treatment is reported to have an influence on nocturnal BP fall in one study.23) On the other hand, a recent large-scale registry study on Spanish subjects showed that nocturnal dipping patterns were not associated with the timing of treatment.24) It is not clear whether anti-hypertensive drugs have an influence on the relationship between nocturnal BP and target organ damage. Moreover, clinical consequences associated with the treatment of nocturnal BP have not yet been studied. To identify the beyond BP effect or the effect of nocturnal BP on OPR or iLVM according to the classes of anti-hypertensive drugs, further studies using a larger number of subjects are needed.
There are limitations in our study. First, the details of antihypertensive treatment were not surveyed in our study due to small sample size and incomplete data of antihypertensive treatment. Therefore, we could not adequately assess the specific influence of antihypertensive treatment on the nocturnal BP and appropriateness of LV mass. Second, the patients with clinical cardiovascular diseases were excluded in this study. Non-dipper patterns are dominant among patients with renal disease and severe cardiovascular disease which may also have an effect on nocturnal BP patterns and LV mass.20) Our results cannot be applied to these complicated patients.
In conclusion, nocturnal dipping of the circadian rhythm of BP in addition to age, BMI, and 24 hours systolic BP determine OPR in essential hypertension patients. This supports that nocturnal dipping has an intrinsic or non-hemodynamic role on inappropriate LV hypertrophy which is in-dependent of 24 hours BP.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–352. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- 2.Celentano A, Palmieri V, Esposito ND, et al. Inappropriate left ventricular mass in normotensive and hypertensive patients. Am J Cardiol. 2001;87:361–363. doi: 10.1016/s0002-9149(00)01379-5. [DOI] [PubMed] [Google Scholar]
- 3.de Simone G, Verdecchia P, Pede S, Gorini M, Maggioni AP. Prognosis of inappropriate left ventricular mass in hypertension: the MAVI study. Hypertension. 2002;40:470–476. doi: 10.1161/01.hyp.0000034740.99323.8a. [DOI] [PubMed] [Google Scholar]
- 4.Ikeda M, Kohno M, Yasunari K, et al. Natriuretic peptide family as a novel antimigration factor of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1997;17:731–736. doi: 10.1161/01.atv.17.4.731. [DOI] [PubMed] [Google Scholar]
- 5.Inoue M, Kanda T, Arai M, et al. Impaired expression of brain natriuretic peptide gene in diabetic rats with myocardial infarction. Exp Clin Endocrinol Diabetes. 1998;106:484–488. doi: 10.1055/s-0029-1212021. [DOI] [PubMed] [Google Scholar]
- 6.Chen HH, Burnett JC. Natriuretic peptides in the pathophysiology of congestive heart failure. Curr Cardiol Rep. 2000;2:198–205. doi: 10.1007/s11886-000-0069-3. [DOI] [PubMed] [Google Scholar]
- 7.Mancia G, Zanchetti A, Agabiti-Rosei E, et al. Ambulatory blood pressure is superior to clinic blood pressure in predicting treatment-induced regression of left ventricular hypertrophy. SAMPLE study group. Study on ambulatory monitoring of blood pressure and lisinopril evaluation. Circulation. 1997;95:1464–1470. doi: 10.1161/01.cir.95.6.1464. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda T, Matsuda K, Itoh H, et al. Plasma levels of brain and atrial na-triuretic peptides elevate in proportion to left ventricular end-systolic wall stress in patients with aortic stenosis. Am Heart J. 1997;133:307–314. doi: 10.1016/s0002-8703(97)70225-4. [DOI] [PubMed] [Google Scholar]
- 9.Devereux RB, Roman MJ, de Simone G, et al. Relations of left ventricular mass to demographic and hemodynamic variables in American Indians: the Strong Heart study. Circulation. 1997;96:1416–1423. doi: 10.1161/01.cir.96.5.1416. [DOI] [PubMed] [Google Scholar]
- 10.Rim SJ. Blood pressure variation and cardiovascular risks. Korean Circ J. 2008;38:131–134. [Google Scholar]
- 11.O'Brien E, Asmar R, Beilin L, et al. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens. 2005;23:697–701. doi: 10.1097/01.hjh.0000163132.84890.c4. [DOI] [PubMed] [Google Scholar]
- 12.Lim YH, Lee JU, Kim KS, et al. Association between inappropriateness of left ventricular mass and left ventricular diastolic dysfunction: a study using the tissue doppler parameter, e/e'. Korean Circ J. 2009;39:138–144. doi: 10.4070/kcj.2009.39.4.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 14.Shin J, Kim KS, Kim SG, et al. Influences of body size and cardiac workload on the left ventricular mass in healthy Korean adults with normal body weight and blood pressure. Korean Circ J. 2005;35:335–340. [Google Scholar]
- 15.Glower DD, Spratt JA, Snow ND, et al. Linearity of the Frank-Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation. 1985;71:994–1009. doi: 10.1161/01.cir.71.5.994. [DOI] [PubMed] [Google Scholar]
- 16.de Simone G, Devereux RB, Kimball TR, et al. Interaction between body size and cardiac workload: influence on left ventricular mass dur-ing body growth and adulthood. Hypertension. 1998;31:1077–1082. doi: 10.1161/01.hyp.31.5.1077. [DOI] [PubMed] [Google Scholar]
- 17.de Simone G, Devereux RB, Roman MJ, Alderman MH, Laragh JH. Relation of obesity and gender to left ventricular hypertrophy in normotensive and hypertensive adults. Hypertension. 1994;23:600–606. doi: 10.1161/01.hyp.23.5.600. [DOI] [PubMed] [Google Scholar]
- 18.Palmieri V, de Simone G, Roman MJ, Schwartz JE, Pickering TG, Devereux RB. Ambulatory blood pressure and metabolic abnormalities in hypertensive subjects with inappropriately high left ventricular mass. Hypertension. 1999;34:1032–1040. doi: 10.1161/01.hyp.34.5.1032. [DOI] [PubMed] [Google Scholar]
- 19.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 20.Routledge F, McFetridge-Durdle J, Dean C. Night-time blood pres sure patterns and target organ damage: a review. Can J Cardiol. 2007;23:132–138. doi: 10.1016/s0828-282x(07)70733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parati G, Staessen JA. Day-night blood pressure variations: mechanisms, reproducibility and clinical relevance. J Hypertens. 2007;25:2377–2380. doi: 10.1097/HJH.0b013e3282f2d116. [DOI] [PubMed] [Google Scholar]
- 22.Krauser DG, Devereux RB. Ventricular hypertrophy and hypertension: prognostic elements and implications for management. Herz. 2006;31:305–316. doi: 10.1007/s00059-006-2819-5. [DOI] [PubMed] [Google Scholar]
- 23.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Effects of time of antihypertensive treatment on ambulatory blood pressure and clinical characteristics of subjects with resistant hypertension. Am J Hypertens. 2010;23:432–439. doi: 10.1038/ajh.2009.260. [DOI] [PubMed] [Google Scholar]
- 24.de la Sierra A, Redon J, Banegas JR, et al. Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension. 2009;53:466–472. doi: 10.1161/HYPERTENSIONAHA.108.124008. [DOI] [PubMed] [Google Scholar]