Abstract
Few methods exist to non-invasively study in vivo gastrointestinal motility in animal models of enteric infections. None have been used on mouse pups who often display more severe symptoms during enteric infections than adult mice. This study sought to determine if digital fluoroscopy could be used to evaluate gastrointestinal motility in mouse pups as well as adult mice. Fluoroscopic imaging studies were performed on normal 6-8 week old adult mice and 12 day old pups to develop protocols for evaluating gastric and intestinal wall movements and changes in stomach sizes. These protocols were then applied to evaluate motility in an established rotavirus mouse model. Imaging studies were performed on adult mice at 0, 2, and 4 days post infection and on 12 day old pups at 2 days post infection. Fluoroscopic studies revealed postnatal differences of gastric peristalsis and rates of intestinal contractions between normal mouse pups and adult mice. Studies of the rotavirus mouse model revealed that differences in gastric function occur between rotavirus infected and control mouse pups but no discernible difference occurs between infected and control adult mice. In contrast, there were no detectable differences in rates of intestinal wall movements between control pups with normal stools and infected pups with loose stools. These results demonstrate that fluoroscopy can evaluate in vivo motility in mouse pups and by doing so provide findings that are clinically relevant to the study of enteric infections in young.
Keywords: gastrointestinal motility, fluoroscopy, rotavirus
Introduction
New methods for studying in vivo gastrointestinal motility in small animals are needed to further our understanding of how enteric viruses alter motility in the very young. Prospective studies of young children that are acutely infected are difficult and challenging to perform due to the invasive nature of motility studies, such as manometry, and to the inability to predict the onset of an acute viral gastroenteritis that generally only lasts 48-72 hours. Although animal models of enteric infections exist, prospective studies of in vivo motility still pose significant technical challenges. Most methods require resection of a portion, or the entire gastrointestinal tract, to measure gastrointestinal transit or in vitro contractile activity. In vivo measurements of motility generally involve the use of anesthesia which itself alters motility or prior implantation of sensors, which is difficult in small animals such as mice. Additionally, methods for studying motility in animals are primarily designed for adult animals that often display less severe disease phenotypes than very young animals.1, 2
A common radiological technique, fluoroscopy, has shown promise for non-invasively studying in vivo gastrointestinal motility in small animals.3 Fluoroscopy is used every day in clinics and hospitals to assess the structures and function of gastrointestinal systems in both adults and children and has been used as an aid to evaluate gastrointestinal motility at a single time point in various adult animals, including mice.3, 4 Fluoroscopic imaging allows direct observation of how gastrointestinal contractions move luminal contents through the stomach and small intestines. Since this methodology does not require invasive surgeries or procedures, fluoroscopy has the potential of being performed on the same animal more than once.
The goal of this project was to evaluate whether fluoroscopy is a reliable method to non-invasively evaluate in vivo motility in both adult mice and mouse pups. Imaging studies were performed with a well-established mouse model of rotavirus infections that utilizes both adult and very young mice. As in humans, young mice with acute rotavirus infection appear more ill and develop diarrhea, while adult mice generally do not show any signs of infection.5, 6 Fluoroscopic detection of changes in gastrointestinal motility in mouse pups would verify that fluoroscopy can reliably evaluate in vivo motility in young mice as well as adult mice.
Materials and Methods
Fluoroscopy Equipment and Image Analysis
An OEC-9600 C-arm unit (OEC Medical Systems, Salt Lake City, UT) was utilized to record digital x-ray images at a rate of 15 images per second. The fluoroscopic unit recorded digital black and white 512 × 512 pixel images. The digital x-ray images were recorded onto compact discs for analysis with Amira® visualization software (Visage imaging, Carlsbad, CA).
Analysis of each image was based on assigning each pixel a grayscale value ranging from 0 to 255. Since black equaled 0 and white equaled 255, the pixels that displayed the dark barium contrast within the gastrointestinal lumen possessed low grayscale values. Utilizing the image processing software, AMIRA, pixels were segmented by selecting a threshold value that isolated the pixels of the dark barium contrast that filled the gastrointestinal lumen.
Rates of gastrointestinal wall movements were determined by measuring the average grayscale value for a row of pixels isolated across the gastrointestinal lumen. To calculate the rates of contractions in the stomach, the same number of pixels in a row covering the antrum in the same location were isolated in each frame of the recording (Figure 1A arrow). To calculate the rates of contractions in the small intestine, the same number of pixels in a row covering the intestinal lumen in the same location were isolated in each frame of the recording (Figure 1A arrowhead). The average grayscale value for the isolated rows of pixels was calculated frame by frame. The average grayscale value decreased when dark barium contrast material filled the gastrointestinal lumen. The average grayscale value increased when the lighter gastrointestinal wall tissue displaced the dark contrast from the lumen. The data were graphed as average grayscale values vs. time. The rates of wall movements were subsequently calculated by averaging the number of cycles over 3 separate 30 second interval time periods. Because the wall movements that were analyzed resulted in the displacement of intralumenal contents, these phasic movements were presumed to be the result of smooth muscle contractions.
Figure 1.

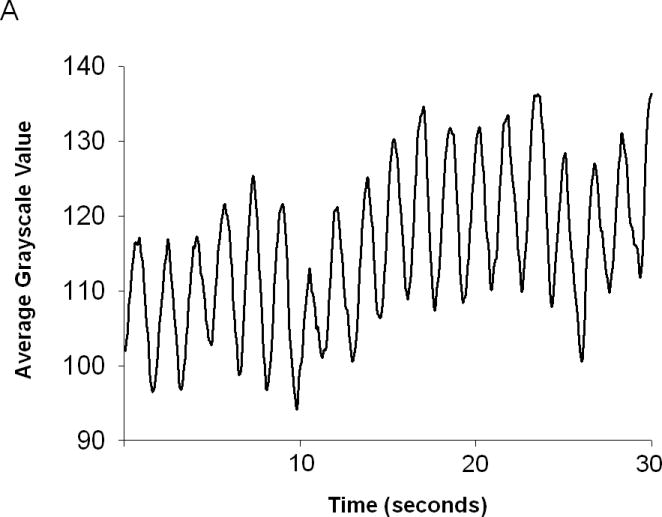
(A.) Fluoroscopic digital x-ray images taken at rate of 15 images per second recorded the movement of barium in live mice (H = head, S = stomach, * = pylorus, I = small intestine). Contractions are seen in the stomach (arrow) and small intestine (arrow head) of an adult mouse as they displace and move the dark barium along the gastrointestinal lumen. (B.) Gastric contractions were determined by isolating a row of pixels (dotted lines in A) across the stomach lumen. Each pixel was assigned a numerical grayscale value quantifying the darkness or lightness of the pixel based upon scale from black (grayscale value = 0) to white (grayscale value = 150). Contractions were graphed by plotting the average grayscale values for a row of pixels isolated within each sequential image. Grayscale values increased as a contraction displaced the dark barium from the row of pixels, and then decreased as barium returned during relaxation.
Initial and final stomach sizes were measured in mouse pups to assess the change in stomach size due to gastric contents emptying into the small intestine. A round metal standard with a radius of 5 millimeters was placed within the field of view to provide a standard scale for measuring the size and areas of objects within the digital images. The number of pixels that comprised the contrast filled stomach and the metal standard were measured. By using the same metal standard for each imaging study, the radius and area (78.5 mm2) of the metal standard projected onto each image was constant. The area of the stomach projected onto an image was calculated and standardized by multiplying the number of stomach pixels by the ratio of area of metal standard to number of metal standard pixels, i.e. stomach area = stomach pixels × (78.5mm2 / metal standard pixels).
Mice
CD-1 female mice were purchased from Charles River Laboratories (Wilmington, Mass.) and maintained according to approved IACUC protocols at Baylor College of Medicine. For the adult imaging studies, 6-8 week old adult female mice were housed in microisolation cages and fed ad libitum until the day of the experiments. For the mouse pup imaging studies, pregnant CD-1 mice were allowed to give birth naturally. At 2 days of life, pups were pooled and randomly redistributed among the dams to create uniform litter sizes in order to alleviate potential bias due to litter origin or litter size. All pups remained with the nursing dams until day 12 of life when the imaging studies were performed.
Imaging of Normal Adult Mice
Adult CD-1 female mice (n=9) were used to establish protocols for evaluating in vivo gastrointestinal motility with fluoroscopy. Modified Broome restraints that were designed by Jan Huizinga at McMaster University were used to keep the adult mice in place during the imaging studies.3, 7 To minimize stress and anxiety that adversely affect gastrointestinal motility, the adult mice were conditioned to the restraints for one-half hour per day for two weeks prior to imaging. On the days of imaging, solid food was removed from the cages for 4 hours prior to imaging to maximize the visualization of contrast material within the stomach. Liquid barium contrast (300 μl) was orally gavaged through a modified plastic neonatal feeding tube (4 Fr) into the stomach prior to placing the mice into the restraints (Figure 1A). A 5 minute recording of fluoroscopic images was initiated once barium contrast began to empty from the stomach through the pylorus into the small intestine.
Imaging of Normal Mouse Pups
Once protocols were established in the adult mice, the protocols were slightly modified to evaluate in vivo gastrointestinal motility in mouse pups. Imaging studies were performed on 12 day old mice (n=9) that were restrained during the imaging with 50 ml conical tubes cut lengthwise down the middle and placed over the pups. Preliminary studies revealed that unconditioned 12 day old pups did not display signs of increased stress when held in place with this minimal restraint. Therefore, successful fluoroscopic imaging of in vivo motility in mouse pups did not require prior conditioning as was required for successful imaging of adult mice. All mouse pups were kept with their nursing dams and littermates until the time of the imaging studies. Liquid barium contrast (150 μl) was orally gavaged through polyethylene tubing (PE-50) into the stomach just prior to placing the pup under the modified 50 ml conical tube. A 5 minute recording of fluoroscopic images was initiated once barium contrast visually began to empty from the stomach through the pylorus and into the small intestine.
Rotavirus inoculation
To determine if fluoroscopy could detect in vivo changes of gastrointestinal motility associated with acute viral gastroenteritis, imaging studies were performed on adult mice and mouse pups that were infected with a murine strain of rotavirus, ECwt, that was produced at Baylor College of Medicine.8 In the adult mouse studies, 6-8 week old CD-1 females were inoculated with either 100 μl of PBS containing gut homogenate with 107 50% infectious doses (ID50) of ECwt or 100 μl of PBS containing normal gut homogenate. In the mouse pup studies, entire mixed litters of 10 day old mouse pups were orally inoculated with either 100 μl of PBS containing gut homogenate with 107 ID50 of ECwt or 100 μl of PBS containing normal gut homogenate. Littermate controls were not possible in our studies because murine rotavirus is highly transmissible between individual animals due to the high titer of virus that is shed in the stool. Therefore, uninfected pups had to be maintained in separate cages from virus-infected pups.
Imaging of Rotavirus Infected Adult Mice
The protocols for imaging that were developed for the normal adult mice were used for two groups of mice, control (n=4) and rotavirus infected (n=5). All mice were imaged just prior to inoculation with inert carrier solution or ECwt at 0 days post inoculation (0 dpi) to get a baseline value of in vivo motility. Imaging studies of both groups were repeated at 2 and 4 days post inoculation (dpi) to investigate if rates of gastrointestinal contractions varied during an acute rotavirus infection.
Imaging of Rotavirus Infected Mouse Pups
The protocols for imaging normal mouse pups were applied to assess changes in gastrointestinal motility associated with acute gastroenteritis in 12 day old mouse pups. Unlike the adult mice studies, imaging was performed only at one time point in the 12 day old pups for two reasons: first, gastrointestinal physiology continues to develop postnatally9; second, repetitive handling and removal of pups from their dams and littermates could add stress that might alter the normal development of gastrointestinal physiology. Imaging pups only at one time point limited variables so that any observed differences in gastrointestinal motility could be associated with acute rotavirus infection. Results were compared between two groups of pups, control (n=9) and rotavirus infected (n=9).
In Vitro Organ Bath Studies
In vitro rates of spontaneous contractions in the proximal small intestine of adult mice and mouse pups were analyzed in organ bath studies of segments of duodenum.10 Whole segments of proximal small intestine, about 7 mm in length, were placed in 15 ml organ baths containing Krebs solution (NaCl 136.9mM, KCl 4.69mM, CaCl2·2H20 2.52mM, MgCl2·2H20 1.52mM,, NaH2PO4·H20 1.81mM, NaHCO3 25.23mM, and C6H12O6 14.98mM) that was maintained at 36° C and gassed with a mixture of 95% O2 – 5% CO2. While one end of the segment was connected to a stationary tissue holder, the other end was connected to a full-bridge force transducer that was coupled to a PowerLab data acquisition system (ADInstruments, Inc., Colorado Springs, CO). Due to the longitudinal orientation of the intestinal segments, the organ bath studies measured changes in force due to spontaneous contractions of the longitudinal smooth muscle. Chart 5 Software included in the PowerLab data package was used to determine the average rates of contractions.
Statistical Analysis
Differences in rates of contraction, areas of stomachs and changes in gastric areas were analyzed using a Wilcoxon rank sum test. A value of p < 0.05 was considered significant. Estimates are presented with mean±SD.
Results
Visual observation of motility in vivo
X-ray imaging via fluoroscopy allowed direct observation of gastrointestinal wall movements and the movement of contrast material from the stomach into the proximal small intestine of both adult mice and mouse pups (Figure 1). Successful recordings in the adult mice required prior conditioning of the mice to the modified Broome restraints that kept them in position during imaging. Unconditioned adult mice appeared agitated, struggled in the restraints, and no peristaltic activity occurred; as evidenced by the lack of contrast material being emptied from their stomachs. Adult mice that were conditioned for one half hour daily for two weeks prior to imaging were much calmer and large slow peristaltic waves were observed in the body of the stomach of these mice soon after barium contrast material was administered (Figure 1A). As peristaltic waves traveled towards the distal end of the stomach, contrast material could be seen crossing the pylorus and entering the proximal small intestine. As the barium filled the lumen of the proximal small intestine, repetitive and coordinated phasic contractions were visualized. The phasic activity both mixed the barium back and forth within a segment of the small intestine and propelled the barium further down the intestinal tract.
Even with prior conditioning, adult mice still moved slightly and changed positions. These slight movements did not inhibit peristaltic activity, but they did alter the orientation of the stomach being projected onto the image, thereby prohibiting quantitative comparisons of changes in stomach sizes in adult mice. Since the stomach was not perfectly round, slight alterations in stomach orientations caused significant increases or decreases in the area projected by the stomach onto subsequent images. For example, if stomachs that initially started in a posterior to anterior view changed from the initial view to a more lateral view the area of the stomach projected onto an image was reduced. Although most stomach projections appeared smaller by the end of an imaging study, investigators could not determine how much the reduction was due to material emptying from the stomach versus a change in the orientation of the stomach.
In comparison to adult mice, mouse pups did not require any prior conditioning for a successful imaging study. Barium contrast quickly emptied from the stomach into the small intestine once the pups were placed on the imaging table. Unconditioned pups appeared even calmer than conditioned adult mice. Unconditioned pups remained still throughout an imaging study while conditioned adult mice changed positions slightly but frequently. The difference in behaviors between the two age groups meant that less preparation time was required for imaging mouse pups versus adult mice.
In addition to the behavioral differences, fluoroscopic imaging revealed differences in gastric activity between young and old mice. Large peristaltic waves were not observed in the stomachs of 12 day old mouse pups as in the 6-8 week old mice. Despite the lack of readily apparent waves of contractions, barium readily emptied from the stomachs of mouse pups soon after administration of the contrast material. As contrast material entered the duodenum, phasic contractions in the small intestines of pups were as easily recognized as phasic contractions in the small intestines of adults. Based upon the similarities and differences observed in the fluoroscopic recordings, image analyses were performed to quantify similar and different aspects of gastrointestinal motility in the two age groups.
Rates of Gastric Contractions in Normal Adult Mice
Because the large peristaltic contractions in the stomach of adult mice were readily observed visually on the recorded images, we first assessed whether measuring changes in grayscale values in fluoroscopic images accurately measured rates of gastric contractions (Figure 1B). Each peak in the graph coincided with the visual observation of a contraction. The average rate of gastric contractions for 9 adult female mice was found to be 4.7±0.68 contractions per minute.
Rates of Intestinal Contractions in Normal Adult Mice
Rates of intestinal smooth muscle contractions were determined based on changes in gray scale values in the same manner as rates of stomach contractions (Figure 2A). Graphs of grayscale averages revealed that the average rate of proximal small intestinal contractions in vivo was 40.4± 2.6 contractions per minute in adult female mice (Figure 2B). Confirmation of this rate of intestinal contractions was attempted by visually counting contractions but while movement of contrast by peristaltic activity was easily seen, visually distinguishing between rapidly occurring contractions of the small intestinal lumen was difficult and rates were not consistent. In lieu of another direct measure to confirm the rates obtained with in vivo imaging, in vitro organ bath studies were performed to obtain rates of spontaneous contractions of intestinal segments and were compared to the rates determined by the in vivo imaging. The rates determined by in vitro organ bath studies were 45.5±5.2 contractions per minute (Figure 2B). The in vitro organ bath and the in vivo imaging results for enteric smooth muscle contractions were similar to each other and to results from previously published imaging studies of mouse gastrointestinal motility.7 Together, these results indicated that analysis of fluoroscopic images was a reliable method to measure in vivo rates of small intestinal smooth muscle contractions.
Figure 2.
(A) Contractions of the proximal small intestine were charted by plotting the average grayscale values for a row of pixels isolated across the intestinal lumen within the fluoroscopic images in the same manner as done for graphs of stomach contractions. (B) In vivo imaging studies and in vitro organ bath studies both detect a significant difference in the rates of intestinal smooth muscle contractions between 8 week old adult mice and 12 day old mouse pups (Wilcoxon rank sum test). Each dot represents individual animal.
Rates of Intestinal Contractions in Normal Mouse Pups
We next sought to determine whether radiological imaging could also reliably measure in vivo rates of contractions in the much smaller mouse pups. Although 12-day-old pups were much smaller than 8-10 week old mice, phasic contractions in the proximal small intestine were still readily seen. Rates of contractions in the proximal small intestine in the pups were as easily determined as those in the adult mice by measuring changes in grayscale values for a row of pixels isolated across the intestinal lumen during contractions. In pups, the average rate of in vivo intestinal contractions was 28.2± 3.1 contractions per minute (Figure 2B). This rate was similar to that determined by in vitro organ bath studies, where the mean rate was 33.7±1.9 contractions per minute (Figure 2B). Interestingly, both methods identified a consistent age-related difference in intestinal contraction rates; the mean rate of intestinal contractions in mouse pups was significantly lower than that determined in adult mice by both in vivo (28.2 vs. 40.4, p<.001) and in vitro (33.7 vs. 45.5, p<.001) methodologies.
Imaging Studies of Rotavirus Infected Adult Mice
We assessed whether in vivo imaging could provide a useful method to screen for changes in rates of gastrointestinal smooth muscle contractions in adult mice during an acute rotavirus infection. Non-invasive imaging allowed rates of contractions in the stomach and small intestine to be studied repeatedly in the same groups of adult mice. We examined time points prior to infection with ECwt on day 0, at the initial onset of virus shedding at 2 dpi, and at peak of rotavirus shedding at 4 dpi. Because the effects of multiple imaging sessions performed on the same mouse were unknown, a vehicle treated control group was imaged at the same time points to address any potential confounding effects of multiple imaging sessions. In control mice, the rates of contraction were similar at 0, 2, and 4 days of recording (Figure 3A). At 0 dpi, there were no significant differences in rates of gastric and small intestinal contractions between the two groups (Figure 3A and B). Similarly, at 2 and 4 dpi, there were no significant differences in the rates of contractions between the rotavirus-infected and control groups (Figure 3A and B) despite the fact that ELISA studies confirmed that the rotavirus inoculated mice were excreting virus on both of these days and that control mice were not (data not shown). Therefore, significant changes in rates of contractions in adult mice did not occur with rotavirus infection or with performing repetitive imaging.
Figure 3.
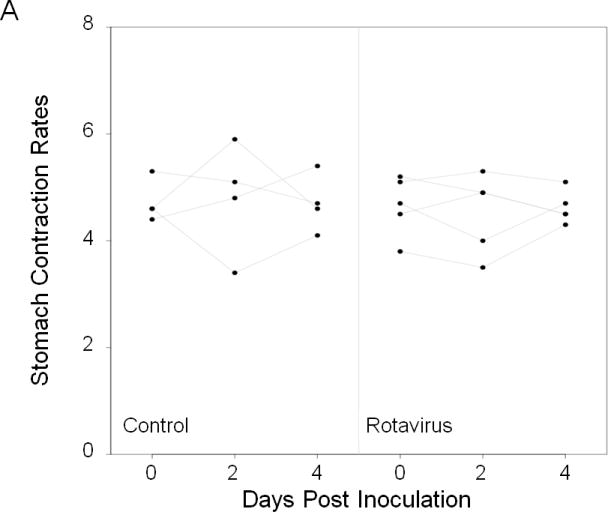
Fluoroscopy was used to evaluate rates of contractions in the stomach and small intestine at three different time points in the same live mice that were inoculated with inert carrier or with a murine strain of rotavirus (ECwt). Imaging studies of 8 week old CD-1 mice were done just prior to inoculation on day 0, and then repeated in the mice on days 2 and 4 post inoculation. No significant difference in the rates of contractions of the stomach (A) and of the small intestine (B) were found during peak infection in mice inoculated with rotavirus (n=5). No significant differences were detected over the 4-5 days of the experiment in the control mice (n=4) or in the infected mice indicating that the rates of contractions were not significantly altered during the onset of rotavirus infection or by repetitive imaging. Dot represents contraction rate of individual mice.
Imaging Studies of Rotavirus Infected Mouse Pups
Qualitative and quantitative assessment of gastric function was performed in control and rotavirus infected mouse pups by observing and measuring differences in the stomach areas in fluoroscopic images. Because pups remained still during the imaging studies, orientation of stomachs projected onto fluoroscopic images remained unchanged during the imaging study. Therefore, any decrease in the size of stomachs in the images was due solely to contents emptying from the stomach.
In control mouse pups, barium readily emptied from the stomach into the proximal small intestine soon after orally gavaging contrast into the stomach. Areas of the stomach projected onto the images decreased over time (Figure 4). Measurement of the areas of the stomachs of the pups on the initial x-ray images immediately following administration of contrast revealed that the mean initial stomach size was 52.2 mm2±6.5mm2. After 5 minutes, the stomachs had reduced in size by an average of 8.5 mm2±3.5mm2.
Figure 4.
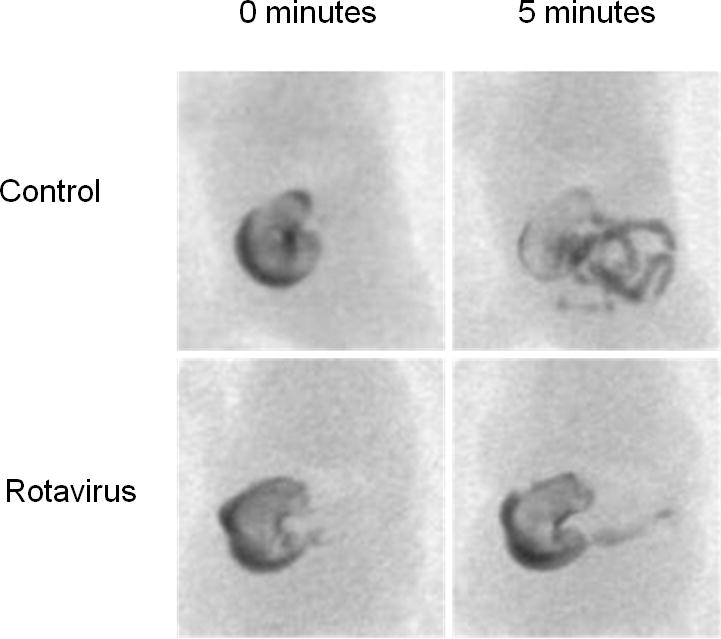
Less contrast material emptied from the stomachs of rotavirus infected pups than of control pups during the 5 minute fluoroscopic studies at 2 dpi. The initial (0 minutes) median stomach size of infected mouse pups was significantly larger than that of the controls. Furthermore, the decrease in stomach size 5 minutes later was significantly less in the infected animals.
In rotavirus infected pups, the initial stomach size, and the stomach size 5 minutes after gavage, appeared larger than in the controls (Figure 4). Quantitative measurements confirmed these impressions. The initial stomach sizes of the infected pups (64.7 mm2±14.5mm2, n= 10) were significantly larger than in the controls (52.2 mm2±6.5mm2, n=8) (p=.009); and over the 5 minute imaging period, the reduction in the size of the stomachs in the infected mice (2.6 mm2±1.6mm2, n=10) was significantly less than in the controls (8.5 mm2±3.5mm2) (p=0.0005). Therefore, fluoroscopic imaging revealed both qualitative and quantitative differences in gastric contents emptying into the small intestine between the control and infected pups.
In addition to altered gastric function, rotavirus infected pups displayed diarrheic stools while control pups displayed normal stools. However, the difference in stool consistency between the two groups was not associated with a difference in the rate of intestinal contractions between infected and control pups (28.2±3.1 vs. 28.2 ±3.1 respectively, n=8), indicating that in this model diarrhea is not associated with increased frequency of contractions of the proximal small intestine.
Discussion
Our results show that digital fluoroscopy provides a reliable method to non-invasively evaluate in vivo motility in both adult mice and mouse pups. In fact, imaging gastrointestinal function in 12 day old mice requires less time and effort to perform than imaging gastrointestinal function in adult mice. Fluoroscopic study of mouse pups also demonstrates that observing in vivo motility in newborn mice can provide information about normal patterns of contractions and how enteric infections may influence these patterns.
Analysis of fluoroscopic images of mice accurately measures the rates of contractile activity in the murine gastrointestinal tract. The rates of gastric and intestinal contractions in adult mice from the present study are consistent with rates of contractions in adult mice from previously published fluoroscopic studies.3, 7 The detection of differences in post natal rates of intestinal contractions by both the in vivo imaging studies and in vitro organ bath studies further confirms the reliability of fluoroscopic evaluation of smooth muscle contractile activity in mouse models.
By accurately assessing rates of in vivo smooth muscle contractions, fluoroscopic imaging studies can be used to evaluate proposed mechanisms that regulate contractile activity. The frequencies of contractions throughout the murine gastrointestinal tract have been associated with the frequencies of electrical slow waves that are generated by the interstitial cells of Cajal within the myenteric plexus (ICC-MY).3, 9, 11-14 The rates of smooth muscle contractions in the adult mice from our fluoroscopic study are similar to published rates of slow wave activity in the stomach and small intestine of adult mice.3, 9, 14 The absence of large peristaltic gastric contractions and the reduced rates of intestinal contractions in mouse pups observed in this study are consistent with reports of immature electrical slow wave activity in newborn mouse pups.9 Although ICC-MY are present in the gastrointestinal tract at birth in mice, both the amplitude and frequency of electrical slow waves in the stomach and the small intestine progressively increase during the first weeks of life of a mouse.9 The association of smooth muscle activity with electrical slow wave activity indicates that fluoroscopy can be used to study ICC-MY development and regulation of gastrointestinal motility in mouse models.
In addition to ICC regulation of smooth muscle contractions, our fluoroscopic results support using fluoroscopy to study other mechanisms that regulate movement of material through the gastrointestinal tract, particularly neuronal regulation of gastric emptying in mouse pups. In adult mice, neuronal and ICC input coordinate the movement of stomach contents across the pylorus into the small intestine.15, 16 Our fluoroscopic evaluation of mouse pups found that contents readily empty from the stomach in the absence of large peristaltic contractions. This fluoroscopic finding suggests that neuronal mechanisms predominantly regulate gastric emptying prior to the full development of the ICC-MY generated slow wave. Further studies are needed to confirm and better understand the regulation of gastric emptying. However, results from the present study verify that real time imaging of mouse pups can provide relevant information about the factors that regulate in vivo motility.
Furthermore, fluoroscopic results from the rotavirus mouse model demonstrate how imaging mouse pups can provide an advantage over imaging adult mice when investigating how enteric infections can change in vivo gastrointestinal motility. Real time imaging revealed delayed gastric emptying in rotavirus infected mouse pups, while no visible change in gastric functioning occurred in rotavirus infected adult mice. The differences in motility with rotavirus infection of the two age groups further confirms the clinical relevance of using a young mouse model for studying rotavirus infection in humans. Infection with rotavirus causes overt gastrointestinal symptoms in both young children and mouse pups while usually causing no visible signs of infection in adult humans or mice.5, 6 Also, impaired gastric function has been reported in children who have been infected with rotavirus.17, 18 In vivo imaging of rotavirus infected mouse pups not only provides evidence that an acute rotavirus infection impairs gastric function in the very young, but also provides a method to further investigate how an infection with rotavirus alters in vivo gastric function in the very young.
Along with the positive finding of delayed gastric emptying in mouse pups, the negative finding of unchanged rates of intestinal contraction during a rotavirus infection also provides valuable information. This negative finding agrees with the clinical report that the average rates of intestinal contractions do not change in children during an acute rotavirus infection.19 The fact that the average rates of contractions remains the same in rotavirus infected mouse pups and adult mice indicates that infection with rotavirus does not alter ICC pacemaker activity. This negative finding also further supports previous evidence that suggests rotavirus associated diarrhea is due primarily to changes in intestinal absorption and secretion and not due to changes in motility.20-22
The finding that contractile activity remained unchanged in the study of rotavirus infected adult mice also provides valuable information about the use of fluoroscopy to study in vivo motility in small animal models. The facts that rates of smooth muscle contractions and gastric function did not change in either the normal or infected adult mouse groups indicates that fluoroscopy can be used repetitively without altering smooth muscle and ICC pacemaker activity. Being able to perform repetitive evaluation in the same animal offers the advantage of conserving and reducing the number of animals needed for experiments and studies of gastrointestinal motility.
Although radiological imaging did not find differences in rates of smooth muscle contractions, other changes in smooth muscle activity could be occurring that cannot be measured with fluoroscopy. A decrease or increase in the force or strength of individual contractions could occur without changing rates of the contractions. Radiological imaging cannot measure the forces generated by intestinal smooth muscle contractions that can be measured in in vitro studies but in vitro studies remove the possibility of studying motility in the same animal more than once.
In summary, fluoroscopy provides a useful tool to observe and evaluate in vivo gastrointestinal motility in mice, especially in young mice. In vivo imaging of mouse pups in this study demonstrates the value of using fluoroscopic studies to detect changes in motility associated with postnatal development and common childhood illnesses that are not detectable by studying only adult mice. Fluoroscopy also provides the advantage of being able to conserve and reduce the number of mice that are needed for in vivo motility studies.
Acknowledgments
We thank David Keeland and the Integrative Biology Core of the Texas Medical Center Digestive Diseases Center for technical assistance and Jan Huizinga of McMaster University who assisted in developing protocols for fluoroscopic study and provided the designs for the modified broom restraints used to image adult mice.
Grants: This work was supported by National Institute of Diabetes and Digestive and Kidney Disease Research Training Grant T32 DK 07664-14, Center Grant P30 DK56338, and Baylor College of Medicine Diabetes and Endocrinology Core (DERC) supported by P30DK079638 (R. G. P.), and National Institute of Allergy and Infectious Disease Grant AI24998 (M.E.C.) and by a Merit Review Grant from the Office of Research and Development, Medical Research Service, Department of Veterans Affairs (M.E.C.)
Works Cited
- 1.Rose J, Franco M, Greenberg H. The immunology of rotavirus infection in the mouse. Adv Virus Res. 1998;51:203–35. doi: 10.1016/s0065-3527(08)60786-1. [DOI] [PubMed] [Google Scholar]
- 2.Estes MK, Morris AP. A viral enterotoxin. A new mechanism of virus-induced pathogenesis. Adv Exp Med Biol. 1999;473:73–82. [PubMed] [Google Scholar]
- 3.Der-Silaphet T, Malysz J, Hagel S, et al. Interstitial cells of cajal direct normal propulsive contractile activity in the mouse small intestine. Gastroenterology. 1998;114:724–36. doi: 10.1016/s0016-5085(98)70586-4. [DOI] [PubMed] [Google Scholar]
- 4.Mashimo H, Kjellin A, Goyal RK. Gastric stasis in neuronal nitric oxide synthase-deficient knockout mice. Gastroenterology. 2000;119:766–73. doi: 10.1053/gast.2000.16509. [DOI] [PubMed] [Google Scholar]
- 5.Burns JW, Krishnaney AA, Vo PT, et al. Analyses of homologous rotavirus infection in the mouse model. Virology. 1995;207:143–53. doi: 10.1006/viro.1995.1060. [DOI] [PubMed] [Google Scholar]
- 6.Starkey WG, Collins J, Wallis TS, et al. Kinetics, tissue specificity and pathological changes in murine rotavirus infection of mice. J Gen Virol. 1986;67(Pt 12):2625–34. doi: 10.1099/0022-1317-67-12-2625. [DOI] [PubMed] [Google Scholar]
- 7.Der T, Bercik P, Donnelly G, et al. Interstitial cells of cajal and inflammation-induced motor dysfunction in the mouse small intestine. Gastroenterology. 2000;119:1590–9. doi: 10.1053/gast.2000.20221. [DOI] [PubMed] [Google Scholar]
- 8.Feng N, Franco MA, Greenberg HB. Murine model of rotavirus infection. Adv Exp Med Biol. 1997;412:233–40. doi: 10.1007/978-1-4899-1828-4_35. [DOI] [PubMed] [Google Scholar]
- 9.Ward SM, Harney SC, Bayguinov JR, et al. Development of electrical rhythmicity in the murine gastrointestinal tract is specifically encoded in the tunica muscularis. J Physiol. 1997;505(Pt 1):241–58. doi: 10.1111/j.1469-7793.1997.241bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao A, Urban JF, Jr, Morimoto M, et al. Contribution of 5-HT2A receptor in nematode infection-induced murine intestinal smooth muscle hypercontractility. Gastroenterology. 2006;131:568–78. doi: 10.1053/j.gastro.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Hennig GW, Spencer NJ, Jokela-Willis S, et al. ICC-MY coordinate smooth muscle electrical and mechanical activity in the murine small intestine. Neurogastroenterol Motil. 22:e138–51. doi: 10.1111/j.1365-2982.2009.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomsen L, Robinson TL, Lee JC, et al. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med. 1998;4:848–51. doi: 10.1038/nm0798-848. [DOI] [PubMed] [Google Scholar]
- 13.Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of cajal from the murine small intestine. J Physiol. 1998;513(Pt 1):203–13. doi: 10.1111/j.1469-7793.1998.203by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beckett EA, McGeough CA, Sanders KM, et al. Pacing of interstitial cells of Cajal in the murine gastric antrum: neurally mediated and direct stimulation. J Physiol. 2003;553:545–59. doi: 10.1113/jphysiol.2003.050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang XY, Lammers WJ, Bercik P, et al. Lack of pyloric interstitial cells of Cajal explains distinct peristaltic motor patterns in stomach and small intestine. Am J Physiol Gastrointest Liver Physiol. 2005;289:G539–49. doi: 10.1152/ajpgi.00046.2005. [DOI] [PubMed] [Google Scholar]
- 16.Sivarao DV, Mashimo H, Goyal RK. Pyloric sphincter dysfunction in nNOS-/- and W/Wv mutant mice: animal models of gastroparesis and duodenogastric reflux. Gastroenterology. 2008;135:1258–66. doi: 10.1053/j.gastro.2008.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bardhan PK, Salam MA, Molla AM. Gastric emptying of liquid in children suffering from acute rotaviral gastroenteritis. Gut. 1992;33:26–9. doi: 10.1136/gut.33.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sigurdsson L, Flores A, Putnam PE, et al. Postviral gastroparesis: presentation, treatment, and outcome. J Pediatr. 1997;131:751–4. doi: 10.1016/s0022-3476(97)70106-9. [DOI] [PubMed] [Google Scholar]
- 19.Bass D, Cordoba E, Dekker C, et al. Intestinal imaging of children with acute rotavirus gastroenteritis. J Pediatr Gastroenterol Nutr. 2004;39:270–4. doi: 10.1097/00005176-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Ball JM, Tian P, Zeng CQ, et al. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. 1996;272:101–4. doi: 10.1126/science.272.5258.101. [DOI] [PubMed] [Google Scholar]
- 21.Halaihel N, Lievin V, Alvarado F, et al. Rotavirus infection impairs intestinal brush-border membrane Na(+)-solute cotransport activities in young rabbits. Am J Physiol Gastrointest Liver Physiol. 2000;279:G587–96. doi: 10.1152/ajpgi.2000.279.3.G587. [DOI] [PubMed] [Google Scholar]
- 22.Tafazoli F, Zeng CQ, Estes MK, et al. NSP4 enterotoxin of rotavirus induces paracellular leakage in polarized epithelial cells. J Virol. 2001;75:1540–6. doi: 10.1128/JVI.75.3.1540-1546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


