Abstract
Objective
We investigated the neuroprotective effect of anthocyanin, oxygen radical scavenger extracted from raspberries, after traumatic spinal cord injury (SCI) in rats.
Methods
The animals were divided into two groups : the vehicle-treated group (control group, n=20) received an oral administration of normal saline via stomach intubation immediately after SCI, and the anthocyanin-treated group (AT group, n=20) received 400 mg/kg of cyanidin 3-O-β-glucoside (C3G) in the same way. We compared the neurological functions, superoxide expressions and lesion volumes in two groups.
Results
At 14 days after SCI, the AT group showed significant improvement of the BBB score by 16.7±3.4%, platform hang by 40.0±9.1% and hind foot bar grab by 30.8±8.4% (p<0.05 in all outcomes). The degree of superoxide expression, represented by the ratio of red fluorescence intensity, was significantly lower in the AT group (0.98±0.38) than the control group (1.34±0.24) (p<0.05). The lesion volume in lesion periphery was 32.1±2.4 µL in the control and 24.5±2.3 µL in the AT group, respectively (p<0.05), and the motor neuron cell number of the anterior horn in lesion periphery was 8.3±5.1 cells/HPF in the control and 13.4±6.3 cells/HPF in the AT group, respectively (p<0.05).
Conclusion
Anthocyanin seemed to reduce lesion volume and neuronal loss by its antioxidant effect and these resulted in improved functional recovery.
Keywords: Spinal cord trauma, Anthocyanin, Antioxidants
INTRODUCTION
Traumatic spinal cord injury (SCI) often leads to serious neurological sequelae and medical complications. The secondary damage following SCI is induced by multiple pathophysiological mechanisms, including vascular perturbation, metabolic failure, ionic dysregulation, and celluar excitotoxicity. These mechanisms increase blood-spinal cord barrier permeability, tissue edema, free radical formation, peroxidation of lipid membranes, cytokines release, and inflammation9,11,15). Among these mechanisms, the generation of reactive oxygen species (ROS), such as superoxide, H2O2, and hydroxyl radicals, is one of the main mechanisms in the acute injury of traumatic SCI3,4,26). Furthermore, oxidative injury due to ROS has complex interactions with excitotoxicity, apoptosis, and inflammation2). Therefore, antioxidants are hypothesized to be neuroprotective.
Anthocyanins are natural pigments belonging to the flavonoid family and are present in fruits and vegetables. It is known to have powerful antioxidant effects, inhibit inflammation, and have anticarcinogenic properties and potent cardioprotective effects13,19,28,33,35,37,41). Among anthocyanins, cyanidin 3-O-β-glucoside (C3G) has protective effects as a scavenger of active oxygen species in hepatic ischemia-reperfusion damage and cerebral ischemia models18,31,36). In addition, anthocyanins are taken up by brain endothelial cell lines and can possibly cross the monolayer in blood-brain barrier (BBB) in vitro model40). These results suggest that anthocyanins have potent protective effects in the oxidative stress-mediated disease of central nervous system (CNS). In 2006, two studies were published on pre-/post-treatment of C3G which showed significant improvement of neurological recovery and decrease of infarction volume in cerebral ischemia rat models18,31). However, the effects of anthocyanin have not yet been reported in traumatic SCI. In the present study, we aim to clarify whether treatment with anthocyanin (C3G) can reduce superoxide production, neuron cell damage, lesion volume, and neurological dysfunction in a rat model of traumatic SCI.
MATERIALS AND METHODS
Animal model of traumatic SCI
Forty adult male Sprague-Dawley rats weighing 250-300 g were used. All procedures were performed in accordance with the guidelines for care and use of laboratory animals approved by Chung-Ang University's Institutional Animal Care and Use Committee.
Moderate-grade SCIs were induced in adult rats using the pneumatic impact device (Chung-Ang University Hospital Model 2.0) as previously described38). The contusion grade was controlled by depth of deformation, dwell time and velocity of impact. The initial BBB scores of moderate-grade SCI were 5 to 1038). Anesthesia was induced with inhalation of 2.0% enflurane and total laminectomy of T10 was done with preservation of the dura. Because the diameter of impact tip was 2 mm, the exposed surface of the spinal cord had to be larger than 3 mm in diameter. After total laminectomy, the animal was placed in a prone position and its spine was fixed with spine clamps (Sang Chung Commercial Corporation, Seoul, Korea). The settings for the impact were 0.2 sec dwell time, 2 mm depth of deformation, and 3 m/sec velocity. The target point was the midline dorsal aspect of the spinal cord.
Administration of anthocyanin
The animals were divided into two groups : the vehicle-treated group (control group, n=20) which were received an oral administration of normal saline via stomach intubation immediately after SCI, and the anthocyanin-treated group (AT group, n=20) which were received 400 mg/kg of C3G in the same way. After the SCI, the wound was closed in layers using aseptic technique. Throughout the procedure, body temperature was maintained at 37℃ with a heating pad. The bladder of the injured rats was manually emptied.
Superoxide expression study
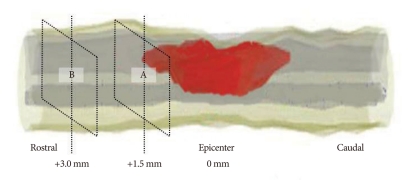
To evaluate the antioxidant effects of anthocyanin during the acute stage of SCI, total twelve rats, six rats from each of the control and AT groups, were sacrificed at 1 hour after SCI by inhalation of 2.0% enflurane. The spinal cords were removed and frozen immediately in liquid nitrogen. The spinal cords were embedded in Tissue-Tek® O.C.T. Compound (Sakur Finetek, Zoeterwoude, Zoeterwoude, Netherland) and cut into 7 µm-thick axial sections. The sections were incubated with dihydroethidium (DHE) (5 mmol/L; Molecular Probes, Inc., Eugene, Ore., USA) in phosphate buffered saline (PBS) for 30 minutes at 37℃ in a humidified chamber protected from light. DHE is oxidized by superoxide to ethidium bromide, which then binds to the DNA in the nucleus and fluoresces red3,16,30). The red fluorescence was detected through a 543-nm long-pass filter, using a laser scanning microscope (LSM510 META; Carl Zeiss, Germany). Two regions of interest (ROIs) were selected at 1.5 mm (A, lesion periphery) and 3 mm (B, control) rostral from the epicenter (Fig. 1). The intensity of red fluorescence was measured using a VH image analyzer and the degrees of superoxide expression were represented by the ratio of red fluorescence in the two ROIs (A value divided by B value).
Fig. 1.
Two regions of interest (ROIs) were selected for each section to evaluate the antioxidant effect of anthocyanin during the acute stage of SCI, The lesion periphery at 1.5 mm (A) and 3.0 mm (B) rostral from the epicenter reflect the lesion and normal area, respectively. The intensity of red fluorescence is measured using a VH image analyzer and the degrees of superoxide expression are represented by the ratio of red fluorescence in the two ROIs (A value divided by B value). SCI : spinal cord injury.
Functional assessments
All rats were examined in an open field environment to assess locomotor function of their hind limbs prior to injury, and at 1, 3, 5, 7, 10 and 14 days post injury using the BBB score, hind foot bar grab, and platform hang5,6,20,29). All tests were performed by two investigators who were blinded to the study groups. The score for each animal was used as the average of the scores evaluating by the two investigators. When the two hind limb scores differed, the worse score was used for data analysis. Motor functions of the hind limbs were assessed by the BBB score. For example, a score of 0 (the lowest score) corresponds to no hind limb motion, while a score of 21 (the highest score) corresponds to normal motion5,6). The hind foot bar grab test was used to test polysynaptic spinal reflexes. A score of 0 (the lowest score) was assigned if the rat did not respond to the bar touching with the hind feet, while a score of 3 (the highest score) was assigned if the rat strongly grabbed the bar and pushed at it29). The platform hang test was used to test the coordinated motor function of the hind limbs. A score of 0 (the lowest score) was assigned if the rat fell, while a score of 4 (the highest score) was assigned if the rat climbed the platform within 5 sec20).
Histopathological studies
To assess histopathology, the remaining twenty-eight rats, fourteen rats from each of the control and AT groups, were sacrificed at 14 days after SCI. The rats were anesthetized by inhalation of 2.0% enflurane and transcardially perfused with 0.1 M PBS followed by 4% buffered paraformaldehyde. The spinal cords were then embedded in paraffin, cut into 5 µm-thick axial sections, and stained with hematoxylin and eosin (H&E) and Luxol fast blue (LFB) to identify motor neuron cell injury, lesion volume, and myelin loss. Motor neuron cells were counted in the anterior horns of 1.5 mm rostral from the epicenter. For each rat, the mean number of neuronal cells was obtained by examining at least three serial axial sections in the anterior horn. In each section, motor neurons were counted in three non-overlapping fields and were averaged into the cell number in HPF (×400) under light microscope. Only complete neuronal cells with a clearly defined cell body and nucleus were counted.
Lesion volume
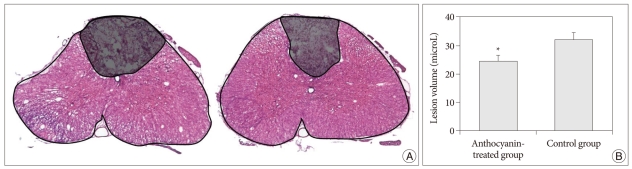
The lesion volume was delineated by its bounding surface, which is defined by a series of closed contours in the H&E stained serial sections (Fig. 2). The software (OPTIMAS 6.5, Optimas, Inc., Bothel, WA, U.S.A.) measured the lesion volume from the two-dimensional images of the axial cord sections. The contours and structures in each spinal cord section were traced to reconstruct the stacked image for volumetric analysis. For each rat, the lesion area was computed using the section from the epicenter, 0.5, 1, 1.5, and 2 mm rostral and 0.5, 1, 1.5, and 2 mm caudal to the epicenter.
Fig. 2.
The lesion volume was delineated by its bounding surface, which can be defined by a series of closed contours in the H&E stained serial sections. We measured the lesion volume from the two-dimensional images of the axial cord sections using the software (OPTIMAS 6.5, Optimas, Inc., Bothel, WA, U.S.A.). A : Left and right panels show the outlines of lesion area from the axial section of the control and AT groups, respectively. B : The histogram shows the lesion volume (mean±standard deviation) in the control and anthocyanin-treated groups. *p<0.05, compared with the control group.
Statistical analysis
Data are expressed as means±standard deviation. Continuous data were compared using student t-test. Non-parametric data were compared using the Mann-Whitney U test. p values of <0.05 were considered statistically significant.
RESULTS
Superoxide concentration after SCI
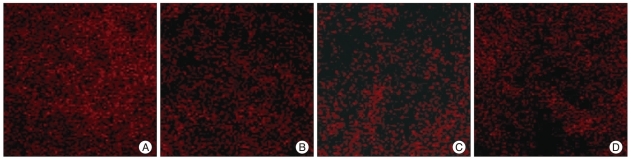
Red fluorescence was higher at the lesion periphery (1.5 mm from the epicenter) at 1 hour after SCI in the control group, but was less prominent in the AT group (Fig. 3). The degrees of red fluorescence of DHE staining, which was calculated as ratios of the intensities at 1.5 (lesion periphery) and 3 mm (control) rostral from the epicenter, were 1.34±0.24 in the control group and 0.98±0.38 in the AT group, respectively. The mean value of the ratio was significantly lower in the AT group (p<0.05). This result indicates that treatment with anthocyanin significantly reduces superoxide concentration in the lesion periphery at 1 hour after SCI.
Fig. 3.
Fluorescence micrographs of the spinal cord stained with dihydroethidium (DHE), superoxide-sensitive dye. In the control group, the fluorescence intensity of the lesion periphery at 1.5 mm (A) is higher than those at 3.0 mm (B) rostral from the epicenter. However, the fluorescence intensity of the lesion periphery at 1.5 mm (C) is less prominent than it at 3.0 mm (D) rostral from the epicenter in the anthocyanin-treated group.
Functional recovery after SCI
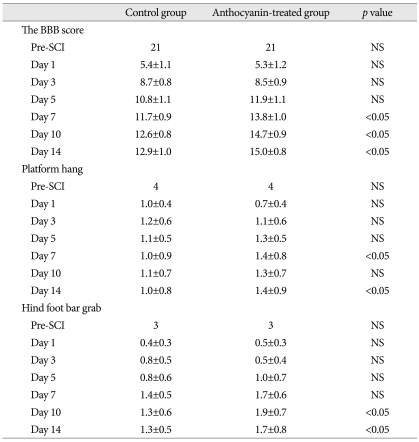
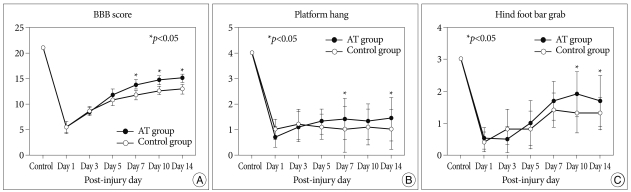
The BBB score, platform hang and hind foot bar grab were used for the evaluation of functional outcomes (Table 1, Fig. 4). Before the injury, all rats showed normal function on the BBB score, platform hang and hind foot bar grab. At day 1 of SCI, the BBB scores of the control and the AT groups were 5.4±1.1 and 5.3±1.2, respectively, a difference that was not significant. However, they improved significantly at days 7, 10 and 14 after SCI (7 days : 11.7±0.9 and 13.8±1.0, 10 days : 12.6±0.8 and 14.7±0.9, 14 days : 12.9±1.0 and 15.0±0.8) (p<0.05 in all data). The platform hang scores in the AT group only at days 7 and 14 were 1.4±0.8 and 1.4±0.9, significantly higher than the scores of 1.0±0.9 (p<0.05) and 1.0±0.8 (p<0.05) in the control group. In the AT group, the hind foot bar grab scores only at days 10 and 14 were 1.9±0.7 and 1.7±0.8, respectively. These scores were significantly higher than the scores of 1.3±0.6 (p<0.05) and 1.3±0.5 (p<0.05) for the control group.
Table 1.
Comparison of functional outcomes between the control and anthocyanin-treated groups after traumatic SCI
All data are presented as the mean±standard deviations. SCI : spinal cord injury. BBB : blood-brain barrier, NS : not significant
Fig. 4.
The time course of functional recovery in the control and anthocyanin-treated groups. A : BBB score. B : Platform hang. C : Hind foot bar grab. BBB : blood-brain barrier.
Lesion volume, motor neuron injury, and myelin loss after SCI
Lesion volumes in the control and the AT groups were 32.1±2.4 µL and 24.5±2.3 µL, respectively (Fig. 2). Anthocyanin significantly reduced the lesion volume by 23.7% in the AT group compared to that of the control group (p< 0.05).
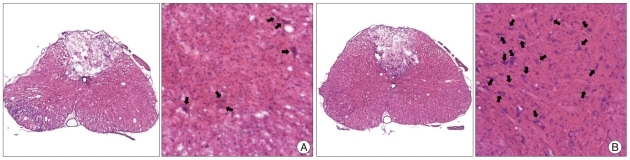
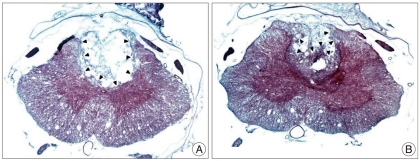
The motor neuron cell number were 8.3±5.1 cells/HPF in the control group and 13.4±6.3 cells/HPF in the AT group, respectively (Fig. 5) (p<0.05). The control group also showed more severe loss of myelin fibers compared with the AT group after SCI (Fig. 6).
Fig. 5.
Axial sections of the spinal cord stained with hematoxylin and eosin (H&E). The dorsal surface is at the top. The left and right panels show the total axial section (magnification, ×40) and the anterior horn area (magnification, ×100) of spinal cord, respectively. There are significant motor neuron cell injury and microcyst formation in the anterior horn area of the control group (A) compared with the anthocyanin-treated group (B). Black arrows indicate intact motor neuron cells.
Fig. 6.
Axial sections of the spinal cord stained with Luxol fast blue (LFB). The control group (A) shows more severe myelin loss than the AT group (B) after SCI. Black arrow heads indicate the contours of preserved myelin fiber in lesion area.
DISCUSSION
Anthocyanins are present in fruits and vegetables, and are especially enriched in raspberries ("bok-bun-ja" in Korean). It provides natural pigmentation and exhibit a wide range of antioxidant and therapeutic benefits7,8,13,19,27,28,33,35,37,41). Among the anthocyanins, C3G has known to have therapeutic effects in hepatic ischemia-reperfusion damage and cerebral ischemia models18,31,36). In addition, it inhibits free radical-induced apoptosis of colon Caco-2 and myocardial cells, and enhances red blood cell resistance to oxidative stress1,12,39). In these studies, the main therapeutic role of C3G is the scavenger of ROS. Furthermore, C3G was taken up by brain endothelial cell lines and could possibly cross the monolayer in blood-brain barrier in vitro model40). From these results, we can speculate that C3G has potent protective effects in secondary injury of the traumatic SCI by ROS3,4,26).
Superoxides are important in oxidative chain reactions, producing highly reactive oxidants21,22,26,32). Direct microdialysis measurements of hydroxyl radicals (one of the most destructive forms of ROS) in injured spinal cords show that hydroxyl radicals are significantly increased at 5 minutes and maximized at 1 hour after SCI3,4,22). Furthermore, dialysate levels of 3, 4-dihydroxylbenzoic acid and oxygen radical productions are correlated with DHE fluorescence intensity3,23,30). Therefore, we sacrificed twelve rats (six from each of the control and AT groups) at 1 hour of SCI and used DHE fluorescence intensity to evaluate the antioxidant effects of C3G during the acute stage of SCI. Hydroxyl radicals are generated from superoxide in the gray matter of the spinal cord through iron-catalyzed Haber-Weiss reactions3,22) and the lesion volume is correlated with the secondary injury due to ROS3,30,34,36), so we checked the motor neuron cell in the anterior horn of lesion periphery and the lesion volume at 14 days of SCI. In the present study, C3G significantly reduced the production of superoxides in lesion peripheries at 1 hour of SCI. In addition, it decreased lesion volume and increased the motor neuron preservation in the anterior horn of lesion peripheries at 14 days of SCI. These results indicate that treatment with C3G significantly improved functional recovery and decreased lesion volume by scavenging ROS production in the acute stage of SCI.
There are two studies on neuroprotective effects of C3G in CNS18,31). Kang and colleagues reported that C3G decreased the infarction volume of the brain by 18% compared with the control group when C3G (10 mg/kg) was administered orally 30 min after the middle cerebral artery (MCA) occlusion. Shin and colleagues reported a more favorable result that the infarction volume was reduced by 29% compared with the control group when 300 mg/kg C3G was administered orally two times : at 24 hours and 30 minutes before MCA occlusion. In two studies, administration time and dosage of C3G were different, resulting in different infarction volume. The results of these studies showed ranges of administration time and dosage of C3G, which showed a neuroprotective effect in CNS injuries. However, 11%, the difference of infarction volume between the two studies, was not higher than that of what we had expected. In 2000, Youdim and colleagues reported that of the oral ingested 100 mg/kg C3G by rats (300 g), less than 0.64 µmol/L was found in the plasma within an hour following supplementation39). In contrast, Miyazawa and colleagues reported higher plasma levels of C3G by same rats (300 mg) even though their supplementation regimen, at approximately 45 mg and 90 mg, was less than that examined in Youdim's study25). These results suggest that differences in environment or condition of rats may have contributed to absorption potency. However, one parallel observation in various studies is that intact C3G rapidly appears in the plasma at 15 minutes after oral administration, with the maximal plasma concentration occurring 1 hour after oral administration of C3G via stomach intubation24,25,39). These results could explain the reasonable outcome in Kang's study despite of low dosage and post-injury administration of C3G. In the present study, 400 mg/kg C3G was administrated via stomach intubation immediately after SCI. Considering SCI could influence the gastrointestinal motility and the rate of C3G absorption in rats, we used 400 mg/kg, higher dosage than previous studies18,31).
In the present study, the AT group showed significant improvement of the BBB score by 16.7% and the decrease of lesion volume by 23.7%, respectively. Those are not good outcomes compared to those of other free radical scavenger studies. Pretreatment of edaravone in SCI rat model improved the motor score by 27.3% and decreased the lesion volume by 36.4% at 7 days of SCI3). Repeated treatment with Neu2000 resulted in a 45.6% decrease in overall lesion volume and approx. 34% improvement in the BBB score at 42 days of SCI34). However, these absolute values could not imply the therapeutic inferiority of C3G, because the study design, such as administration time and method, contusion degree, and outcome measuring time, is different. Further studies with same conditions are needed to compare the therapeutic efficacy of various radical scavengers.
CONCLUSION
C3G has been shown to provide neuroprotective effects10,14,17,18,31), but its role in traumatic SCI was previously unexplored. The results of the present study demonstrate that C3G treatment significantly reduced the synthesis of ROS in the lesion periphery and improves functional outcomes associated with significant decreases in lesion volume and motor neuron injury. To best our knowledge, this study is the first report of C3G as a neuroprotective radical scavenger in traumatic SCI. However, we could not define the administration method, time, and pharmacokinetics for maximal effect, because this study reflected effects for single oral administration of 400 mg/kg C3G immediately after SCI. Further studies are necessary to evaluate the therapeutic time window, the appropriate administration method, and the proper dosage after trauma in order to establish the clinical usefulness of C3G for traumatic SCI.
Acknowledgements
This study was supported by a grant of the Korea Healthcare technology, R&D Project, Ministry of Health & Welfare, Republic of Korea (A091171).
References
- 1.Amorini AM, Lazzarino G, Galvano F, Fazzina G, Tavazzi B, Galvano G. Cyanidin-3-o-beta-glucopyranoside protects myocardium and erythrocytes from oxygen radical-mediated damages. Free Radic Res. 2003;37:453–460. doi: 10.1080/1071576021000055253. [DOI] [PubMed] [Google Scholar]
- 2.Andrabi SA, Sayeed I, Siemen D, Wolf G, Horn TF. Direct inhibition of the mitochondrial permeability transition pore : a possible mechanism responsible for anti-apoptotic effects of melatonin. FASEB J. 2004;18:869–871. doi: 10.1096/fj.03-1031fje. [DOI] [PubMed] [Google Scholar]
- 3.Aoyama T, Hida K, Kuroda S, Seki T, Yano S, Shichinohe H, et al. Edaravone (mci-186) scavenges reactive oxygen species and ameliorates tissue damage in the murine spinal cord injury model. Neurol Med Chir (Tokyo) 2008;48:539–545. doi: 10.2176/nmc.48.539. discussion 545. [DOI] [PubMed] [Google Scholar]
- 4.Bao F, Liu D. Hydroxyl radicals generated in the rat spinal cord at the level produced by impact injury induce cell death by necrosis and apoptosis : protection by a metalloporphyrin. Neuroscience. 2004;126:285–295. doi: 10.1016/j.neuroscience.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 5.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 6.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomo tor outcomes after spinal cord contusion using the nyu weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 7.Beekwilder J, Hall RD, de Vos CH. Identification and dietary relevance of antioxidants from raspberry. Biofactors. 2005;23:197–205. doi: 10.1002/biof.5520230404. [DOI] [PubMed] [Google Scholar]
- 8.Bellido GG, Beta T. Anthocyanin composition and oxygen radical scavenging capacity (ORAC) of milled and pearled purple, black, and common barley. J Agric Food Chem. 2009;57:1022–1028. doi: 10.1021/jf802846x. [DOI] [PubMed] [Google Scholar]
- 9.Blight AR. Miracles and molecules--progress in spinal cord repair. Nat Neurosci. 2002;5(Suppl):1051–1054. doi: 10.1038/nn939. [DOI] [PubMed] [Google Scholar]
- 10.Casadesus G, Shukitt-Hale B, Stellwagen HM, Zhu X, Lee HG, Smith MA, et al. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr Neurosci. 2004;7:309–316. doi: 10.1080/10284150400020482. [DOI] [PubMed] [Google Scholar]
- 11.Cohen DM, Patel CB, Ahobila-Vajjula P, Sundberg LM, Chacko T, Liu SJ, et al. Blood-spinal cord barrier permeability in experimental spinal cord injury : Dynamic contrast-enhanced MRI. NMR Biomed. 2009;22:332–341. doi: 10.1002/nbm.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elisia I, Kitts DD. Anthocyanins inhibit peroxyl radical-induced apoptosis in Caco-2 cells. Mol Cell Biochem. 2008;312:139–145. doi: 10.1007/s11010-008-9729-1. [DOI] [PubMed] [Google Scholar]
- 13.Gabrielska J, Oszmianski J, Komorowska M, Langner M. Anthocyanin extracts with antioxidant and radical scavenging effect. Z Naturforsch C. 1999;54:319–324. doi: 10.1515/znc-1999-5-605. [DOI] [PubMed] [Google Scholar]
- 14.Galli RL, Bielinski DF, Szprengiel A, Shukitt-Hale B, Joseph JA. Blueberry supplemented diet reverses age-related decline in hippocampal HSP70 neuroprotection. Neurobiol Aging. 2006;27:344–350. doi: 10.1016/j.neurobiolaging.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41:369–378. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- 16.Iwai M, Liu HW, Chen R, Ide A, Okamoto S, Hata R, et al. Possible inhibition of focal cerebral ischemia by angiotensin ii type 2 receptor stimulation. Circulation. 2004;110:843–848. doi: 10.1161/01.CIR.0000138848.58269.80. [DOI] [PubMed] [Google Scholar]
- 17.Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, et al. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang TH, Hur JY, Kim HB, Ryu JH, Kim SY. Neuroprotective effects of the cyanidin-3-O-beta-d-glucopyranoside isolated from mulberry fruit against cerebral ischemia. Neurosci Lett. 2006;391:122–126. doi: 10.1016/j.neulet.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 19.Koide T, Hashimoto Y, Kamei H, Kojima T, Hasegawa M, Terabe K. Antitumor effect of anthocyanin fractions extracted from red soybeans and red beans in vitro and in vivo. Cancer Biother Radiopharm. 1997;12:277–280. doi: 10.1089/cbr.1997.12.277. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn PL, Wrathall JR. A mouse model of graded contusive spinal cord injury. J Neurotrauma. 1998;15:125–140. doi: 10.1089/neu.1998.15.125. [DOI] [PubMed] [Google Scholar]
- 21.Kuroda S, Siesjo BK. Reperfusion damage following focal ischemia : pathophysiology and therapeutic windows. Clin Neurosci. 1997;4:199–212. [PubMed] [Google Scholar]
- 22.Liu D, Liu J, Sun D, Wen J. The time course of hydroxyl radical formation following spinal cord injury : the possible role of the iron-catalyzed Haber-Weiss reaction. J Neurotrauma. 2004;21:805–816. doi: 10.1089/0897715041269650. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Liu M, Peterson S, Miyake M, Vallyathan V, Liu KJ. Hydroxyl radical formation is greater in striatal core than in penumbra in a rat model of ischemic stroke. J Neurosci Res. 2003;71:882–888. doi: 10.1002/jnr.10534. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto H, Inaba H, Kishi M, Tominaga S, Hirayama M, Tsuda T. Orally administered delphinidin 3-rutinoside and cyanidin 3-rutinoside are directly absorbed in rats and humans and appear in the blood as the intact forms. J Agric Food Chem. 2001;49:1546–1551. doi: 10.1021/jf001246q. [DOI] [PubMed] [Google Scholar]
- 25.Miyazawa T, Nakagawa K, Kudo M, Muraishi K, Someya K. Direct intestinal absorption of red fruit anthocyanins, cyanidin-3-glucoside and cyanidin-3,5-diglucoside, into rats and humans. J Agric Food Chem. 1999;47:1083–1091. doi: 10.1021/jf9809582. [DOI] [PubMed] [Google Scholar]
- 26.Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury : a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21:754–774. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- 27.Qin Y, Xia M, Ma J, Hao Y, Liu J, Mou H, et al. Anthocyanin supplementation improves serum IDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am J Clin Nutr. 2009;90:485–492. doi: 10.3945/ajcn.2009.27814. [DOI] [PubMed] [Google Scholar]
- 28.Romero I, Teresa Sanchez-Ballesta M, Maldonado R, Isabel Escribano M, Merodio C. Anthocyanin, antioxidant activity and stress-induced gene expression in high CO2-treated table grapes stored at low temperature. J Plant Physiol. 2008;165:522–530. doi: 10.1016/j.jplph.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Seki T, Hida K, Tada M, Koyanagi I, Iwasaki Y. Graded contusion model of the mouse spinal cord using a pneumatic impact device. Neurosurgery. 2002;50:1075–1081. doi: 10.1097/00006123-200205000-00024. discussion 1081-1082. [DOI] [PubMed] [Google Scholar]
- 30.Shichinohe H, Kuroda S, Yasuda H, Ishikawa T, Iwai M, Horiuchi M, et al. Neuroprotective effects of the free radical scavenger edaravone (MCI-186) in mice permanent focal brain ischemia. Brain Res. 2004;1029:200–206. doi: 10.1016/j.brainres.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 31.Shin WH, Park SJ, Kim EJ. Protective effect of anthocyanins in middle cerebral artery occlusion and reperfusion model of cerebral ischemia in rats. Life Sci. 2006;79:130–137. doi: 10.1016/j.lfs.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 32.Siesjö BK, Agardh CD, Bengtsson F. Free radicals and brain damage. Cerebrovasc Brain Metab Rev. 1989;1:165–211. [PubMed] [Google Scholar]
- 33.Spormann TM, Albert FW, Rath T, Dietrich H, Will F, Stockis JP, et al. Anthocyanin/polyphenolic-rich fruit juice reduces oxidative cell damage in an intervention study with patients on hemodialysis. Cancer Epidemiol Biomarkers Prev. 2008;17:3372–3380. doi: 10.1158/1055-9965.EPI-08-0364. [DOI] [PubMed] [Google Scholar]
- 34.Springer JE, Rao RR, Lim HR, Cho SI, Moon GJ, Lee HY, et al. The functional and neuroprotective actions of Neu2000, a dual-acting pharmacological agent, in the treatment of acute spinal cord injury. J Neurotrauma. 2010;27:139–149. doi: 10.1089/neu.2009.0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steed LE, Truong VD. Anthocyanin content, antioxidant activity, and selected physical properties of flowable purple-fleshed sweetpotato purees. J Food Sci. 2008;73:S215–S221. doi: 10.1111/j.1750-3841.2008.00774.x. [DOI] [PubMed] [Google Scholar]
- 36.Tsuda T, Horio F, Kitoh J, Osawa T. Protective effects of dietary cyanidin 3-O-beta-D-glucoside on liver ischemia-reperfusion injury in rats. Arch Biochem Biophys. 1999;368:361–366. doi: 10.1006/abbi.1999.1311. [DOI] [PubMed] [Google Scholar]
- 37.Xu B, Chang SK. Total phenolic, phenolic acid, anthocyanin, flavan-3-ol, and flavonol profiles and antioxidant properties of pinto and black beans (phaseolus vulgaris l.) as affected by thermal processing. J Agric Food Chem. 2009;57:4754–4764. doi: 10.1021/jf900695s. [DOI] [PubMed] [Google Scholar]
- 38.Yeo SJ, Hwang SN, Park SW, Kim YB, Min BK, Kwon JT, et al. Development of a rat model of graded contusive spinal cord injury using a pneumatic impact device. J Korean Med Sci. 2004;19:574–580. doi: 10.3346/jkms.2004.19.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Youdim KA, Shukitt-Hale B, MacKinnon S, Kalt W, Joseph JA. Polyphenolics enhance red blood cell resistance to oxidative stress : In vitro and in vivo. Biochim Biophys Acta. 2000;1523:117–122. doi: 10.1016/s0304-4165(00)00109-4. [DOI] [PubMed] [Google Scholar]
- 40.Youdim KA, Dobbie MS, Kuhnle G, Proteggente AR, Abbott NJ, Rice-Evans C. Interaction between flavonoids and the blood-brain barrier : In vitro studies. J Neurochem. 2003;85:180–192. doi: 10.1046/j.1471-4159.2003.01652.x. [DOI] [PubMed] [Google Scholar]
- 41.Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res. 2007;51:675–683. doi: 10.1002/mnfr.200700002. [DOI] [PubMed] [Google Scholar]