Abstract
Objective
In the field of spinal surgery, a few laboratory results or clinical cases about robotic spinal surgery have been reported. In vivo trials and development of related surgical instruments for spinal surgery are required before its clinical application. We investigated the use of the da Vinci® Surgical System in spinal surgery at the craniovertebral junction in a human cadaver to demonstrate the efficacy and pitfalls of robotic surgery.
Methods
Dissection of pharyngeal wall to the exposure of C1 and odontoid process was performed with full robotic procedure. Although assistance of another surgeon was necessary for drilling and removal of odontoid process due to the lack of appropriate end-effectors, successful robotic procedures for dural sutures and exposing spinal cord proved its safety and dexterity.
Results
Robot-assisted odontoidectomy was successfully performed in a human cadaver using the da Vinci® Surgical System with few robotic arm collisions and minimal soft tissue damages. Da Vinci® Surgical System manifested more dexterous movement than human hands in the deep and narrow oral cavity. Furthermore, sutures with robotic procedure in the oral cavity demonstrated the advantage over conventional procedure.
Conclusion
Presenting cadaveric study proved the probability of robot-assisted transoral approach. However, the development of robotic instruments specific to spinal surgery must first precede its clinical application.
Keywords: Cervical, Robotics, Odontoid process, Craniovertebral junction, Transoral surgery
INTRODUCTION
The use of robots has become a reality in many surgical fields, and its popularity is quickly increasing. Much of this popularity stems from the desire to provide patients with the least invasive surgery options available since minimally invasive surgery is associated with fewer complications, shorter hospital stays, and improved cosmetic results. Robotic surgery using the da Vinci® Surgical System (Intuitive Surgical, Sunnyvale, CA, USA) is widely used in urological, gynecological, and general surgery, but, until now, its use has been limited in spinal surgery. Because da Vinci® Surgical System at present is developed for laparoscopic procedures, most neurosurgeons are not familiar with this robotic surgical system1,2,12,23). Furthermore, applicable specific instruments to spinal surgery have not been developed yet. Even though there are many remaining steps for clinical use, we took advantage of this innovative instrument to investigate the use of robot-assisted surgery in performing spinal surgery at the craniovertebral junction because the da Vinci® Surgical System offers three-dimensional visualization, tremor filtration, and an increased freedom of instrument movement within a limited space1,11,12,23).
Transoral surgery is defined as a procedure carried out through the mouth to gain access to the midline, clivus, and craniovertebral junction4,5). A transoral robotic approach already has been utilized for laryngopharyngeal lesions by many head and neck surgeons8,15,16,19-21). A team at the University of Pennsylvania has been able to perform several laryngeal and pharyngeal surgical procedures on a cadaver and removed one-third of the posterior oral tongue and excise a parapharyngeal space neoplasm18,19,24,25).
Despite the vast number of successful applications already reported using this technique, there exists only one case report and cadaveric study demonstrating the feasibility of using a robotic surgical system in the craniovertebral junction13,14). We tried to demonstrate the convenience and safety of the da Vinci® Surgical System for performing transoral procedure. Moreover, it was aimed to find out the advantages of robotic surgery comparing with conventional procedure.
MATERIALS AND METHODS
A 70-year-old female cadaver was employed for the procedure. The cadaver was placed supine with her neck extended, allowing manipulation via a transoral approach. The da Vinci® Surgical System (Intuitive Surgical, Sunnyvale, CA, USA) was used for assistance throughout the procedure.
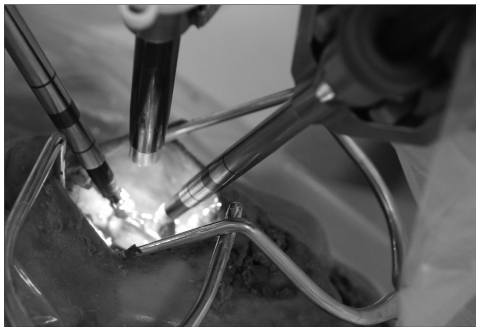
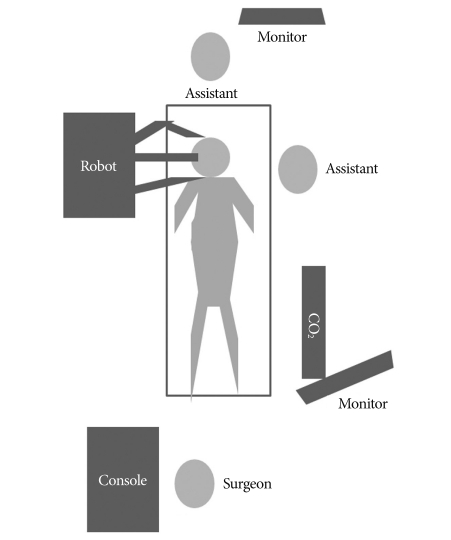
The mouth was opened with a self-retractor and sutured followed by retraction of the swollen tongue to the left side of the oral cavity with a 3-0 silk suture. The da Vinci® Surgical System was introduced at the right side of the cadaveric head (Fig. 1). Common cranial placement for transoral robotic surgery was not applied because of the limited place, but sufficient working space was obtained after maximal mouth opening and neck extension. One arm for 12-mm endoscope and two 5-mm working arms were used. A Maryland bipolar forceps was attached to the left arm and spatula-type monopolar electrocautery was attached to the right arm by an assistant. Unlike a real operation, the soft palate and uvula were divided like two leaflets and then retracted with 3-0 silk sutures by the surgeon at the console (Fig. 2).
Fig. 1.
The da Vinci® Surgical System is introduced at the right side of the cadaver and the robotic arms are inserted through the mouth.
Fig. 2.
Schematic of set up.
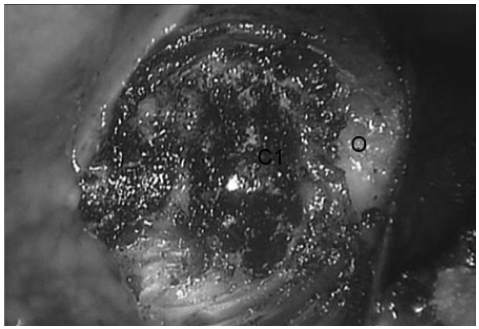
Posterior pharyngeal mucosa was exposed and a vertical incision was made. The mucosal wall was divided and dissected with monopolar electrocautery. The C1 anterior arch and vertebral body of C2 were identified after anterior longitudinal ligament dissection with monopolar electrocautery (Fig. 3).
Fig. 3.
C1 anterior arch and odontoid process are identified after the removal of anterior longitudinal ligament.
Because no commercially-used drill or mongering Endowrist® instrument exists, we stopped the robotic procedure and de-docked the da Vinci® Surgical System. Drilling of the C1 anterior arch and odontoid process was performed with the Midas Rex electric drill by assistant surgeon.
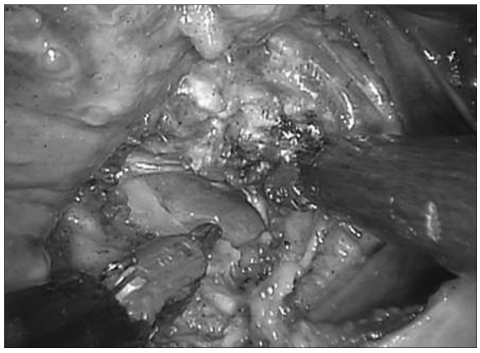
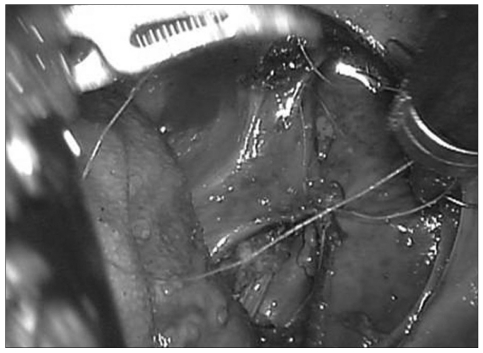
The dural membrane was identified after removal of the apical and cruciate ligaments. In order to evaluate the safety of robot-assisted surgery in patients with an intradural lesion, dural incision and exposure of the spinal cord was achieved by surgeon at the console (Fig. 3). The discolored spinal cord was successfully exposed without significant contact or damage. After the right Endowrist® was exchanged with needle driver, the dural membrane was re-approximated with 4-0 vicryl suture. Although the authors tried continuous watertight sutures, re-approximation did not require more than 30 minutes. Pharyngeal wall was re-approximated with interrupted manner with 3-0 vicryl sutures (Fig. 4).
Fig. 4.
Discolored spinal cord is exposed after incision of the dural membrane.
RESULTS
Initial set-up time was 20 minutes and total operating time was 3 hours. Collisions between the robotic arms occurred 5 times. However, it took only a few minutes to reset the struggling arms each time. Dissection and exposure of bony structures took 40 minutes and drilling and removal of C1 arch and odontoid process took 30 minutes. Unproductive 30 minutes for de-docking robot, changing instruments or resetting arms was spent. However, no major mechanical problem occurred, and time loss was minimal during this procedure. Given intradural manipulation is not required usually in spinal surgery, it seems not a time-consuming technique.
Perioperatively, intraoral soft tissues and lips and teeth were inspected for surveying possible damage caused by accidental injury by the robotic arms. Only a few mucosal injuries of the pharyngeal wall and small lacerations of lips were noticed, and no serious tissue damage or fractures of the teeth, mandible, or hard palate were observed during the procedure. Incision of the dural membrane and exposure of the spinal cord also was performed to demonstrate the safety of robotic surgery in procedures requiring access to critical neural structures, such as the brain and spinal cord. Although lacking haptic function is another disadvantage of da Vinci® Surgical System, we could experience a visual feedback when we handled the Endowrist® around spinal cord. Tactile insensitivity was compensated with excellent visual cues. There was no notable injury to spinal cord during the procedure.
Robotic arms showed many advantages over human hands in the deep oral cavity. Given cerebrospinal fluid leakage is a relatively frequent complication of a transoral procedure, watertight closure of dural membrane is a one of the significant points for good outcome. Although the ventral side of dural membrane around craniovertebral junction is thinner than the dorsal side, we completed re-approximation of dural membrane successfully.
DISCUSSION
In the early 1990s, transoral surgical procedures were commonplace in neurosurgery, used for lesions extending from the sella turcica to the top of the fifth cervical vertebrae4). Today, however, its popularity has waned somewhat, mainly stemming from the many potential difficulties and complications it poses3).
Transoral surgery in the field of spine surgery has been used mostly for atlantoaxial lesions such as rheumatoid arthritis, spinal tumors and other inflammatory or infectious abnormalities. Severe cord compression due to the pannus in patients with rheumatoid arthritis sometimes demands direct decompression of the pannus and odontoidectomy rather than posterior decompression and fixation10,17).
On the basis of our preclinical development, we hypothesized that robotic transoral surgery for craniovertebral junction lesions might have advantages over the conventional transoral surgical method. These potential advantages include more unrestricted movement in the narrow and deep oral cavity, and, as such, tracheostomy may not be necessary13). Furthermore, robot-assisted surgery provides improved optics with tremor filtration, three dimensional visualization, and greater freedom for instrument manipulation1,6,11,18,19,24). Although more comparative study with standard transoral procedure is required to prove the benefits of robotic transoral approach in complication rate or technical comfort, robotic transoral procedure might play a considerable role in anatomically difficult cases such as restricted mouth opening or highly placed odontoid process behind nasopharyx.
In our experiment, we demonstrated the feasibility of using a surgical robot to perform odontoidectomy in a cadaver. The procedures included pharyngeal wall splitting and dissection, C1 arch drilling and dens resection, and dural opening and closure. Given that we had previously experienced some limitations using the da Vinci® Surgical System in spinal surgery, we also recognized the necessity of instruments for spinal bone work in this trial11,26). Although Ponnusamy et al.22) reported a successful experiment with a prototype instrument, potential damage to neural structures due to the rebounds of drilling and insensible trauma during manipulation should be considered sufficiently before development of spinal surgery kits.
Even though this innovative robotic surgical instrument is technically based on laparoscopic surgery, the da Vinci® Surgical System has much potential in the spinal and neurosurgical fields. It seems possible to apply this robotic system to all endoscopic neurosurgical procedures. Of course, the size and numbers of the ports and the requirement of proper Endowrist® instruments are mechanical problems to overcome.
These challenges notwithstanding, one advantage of the system is its three-dimensional and highly magnified visual cues. Conventional endoscopes used in the neurosurgical field provide only two dimensional views. A major advantage of the da Vinci® Surgical System is that, with substantial training and clinical experience, the system can help the surgeon more accurately localize and dissect in spite of the tactile insensitivity. Endoscopic neurosurgery for skull base and craniovertebral junction cases has been performed for several decades7,9,11), however, there exists mechanical limitations such as insufficient dexterity of working instrument and 2D vision.
We performed a transoral approach for odontoidectomy in a human cadaveric model to demonstrate the feasibility of robotic-assisted surgery in the craniovertebral junction. Dissection of the posterior pharyngeal wall was safer and easier than the conventional transoral approach due to a wide and clear surgical field. Robotic surgery also showed probability of managing intradural lesions, while greater protection of the spinal cord remains an important challenge. If robot systems are equipped with instruments specific for bone work, robot-assisted transoral approach to the craniovertebral junction and skull base seems possible in the clinic in the near future.
CONCLUSION
Our experiment shows the potential use of robotic systems in transoral surgery for lesions of the craniovertebral junction. Although further refinement of instruments is necessary, a robot-assisted transoral procedure for spinal surgery may have some merits that make its near future use in the clinic a reality within reach.
Fig. 5.
Pharyngeal wall is re-approximated with interrupted sutures.
Acknowledgements
This work was supported by the 2009 National Agenda Project (NAP), funded by the Korea Research Council of Fundamental Science & Technology (P-09-JC-LU63-C01).
References
- 1.Ballantyne GH. Robotic surgery, telerobotic surgery, telepresence, and telementoring. Review of early clinical results. Surg Endosc. 2002;16:1389–1402. doi: 10.1007/s00464-001-8283-7. [DOI] [PubMed] [Google Scholar]
- 2.Binder J, Kramer W. Robotically-assisted laparoscopic radical prostatectomy. BJU Int. 2001;87:408–410. doi: 10.1046/j.1464-410x.2001.00115.x. [DOI] [PubMed] [Google Scholar]
- 3.Crockard HA. The transoral approach to the base of the brain and upper cervical cord. Ann R Coll Surg Engl. 1985;67:321–325. [PMC free article] [PubMed] [Google Scholar]
- 4.Crockard HA. Transoral surgery : some lessons learned. Br J Neurosurg. 1995;9:283–294. doi: 10.1080/02688699550041304. [DOI] [PubMed] [Google Scholar]
- 5.Crockard HA, Calder I, Ransford AO. One-stage transoral decompression and posterior fixation in rheumatoid atlanto-axial subluxation. J Bone Joint Surg Br. 1990;72:682–685. doi: 10.1302/0301-620X.72B4.2380227. [DOI] [PubMed] [Google Scholar]
- 6.Hockstein NG, O'Malley BW, Jr, Weinstein GS. Assessment of intraoperative safety in transoral robotic surgery. Laryngoscope. 2006;116:165–168. doi: 10.1097/01.mlg.0000199899.00479.75. [DOI] [PubMed] [Google Scholar]
- 7.Husain M, Rastogi M, Ojha BK, Chandra A, Jha DK. Endoscopic transoral surgery for craniovertebral junction anomalies. J Neurosurg Spine. 2006;5:367–373. doi: 10.3171/spi.2006.5.4.367. [DOI] [PubMed] [Google Scholar]
- 8.Iseli TA, Kulbersh BD, Iseli CE, Carroll WR, Rosenthal EL, Magnuson JS. Functional outcomes after transoral robotic surgery for head and neck cancer. Otolaryngol Head Neck Surg. 2009;141:166–171. doi: 10.1016/j.otohns.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL. Expanded endonasal approach : the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus. 2005;19:E3. [PubMed] [Google Scholar]
- 10.Kerschbaumer F, Kandziora F, Klein C, Mittlmeier T, Starker M. Transoral decompression, anterior plate fixation, and posterior wire fusion for irreducible atlantoaxial kyphosis in rheumatoid arthritis. Spine (Phila Pa 1976) 2000;25:2708–2715. doi: 10.1097/00007632-200010150-00029. [DOI] [PubMed] [Google Scholar]
- 11.Kim MJ, Ha Y, Yang MS, Yoon DH, Kim KN, Kim H, et al. Robot-assisted anterior lumbar interbody fusion (ALIF) using retroperitoneal approach. Acta Neurochir (Wien) 2010;152:675–679. doi: 10.1007/s00701-009-0568-y. [DOI] [PubMed] [Google Scholar]
- 12.Lanfranco AR, Castellanos AE, Desai JP, Meyers WC. Robotic surgery : a current perspective. Ann Surg. 2004;239:14–21. doi: 10.1097/01.sla.0000103020.19595.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JY, Lega B, Bhowmick D, Newman JG, O'Malley BW, Jr, Weinstein GS, et al. Da vinci robot-assisted transoral odontoidectomy for basilar invagination. ORL J Otorhinolaryngol Relat Spec. 2010;72:91–95. doi: 10.1159/000278256. [DOI] [PubMed] [Google Scholar]
- 14.Lee JY, O'Malley BW, Newman JG, Weinstein GS, Lega B, Diaz J, et al. Transoral robotic surgery of craniocervical junction and atlantoaxial spine : a cadaveric study. J Neurosurg Spine. 2010;12:13–18. doi: 10.3171/2009.7.SPINE08928. [DOI] [PubMed] [Google Scholar]
- 15.Moore EJ, Olsen KD, Kasperbauer JL. Transoral robotic surgery for oropharyngeal squamous cell carcinoma : a prospective study of feasibility and functional outcomes. Laryngoscope. 2009;119:2156–2164. doi: 10.1002/lary.20647. [DOI] [PubMed] [Google Scholar]
- 16.Mukhija VK, Sung CK, Desai SC, Wanna G, Genden EM. Transoral robotic assisted free flap reconstruction. Otolaryngol Head Neck Surg. 2009;140:124–125. doi: 10.1016/j.otohns.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 17.Mummaneni PV, Haid RW. Transoral odontoidectomy. Neurosurgery. 2005;56:1045–1050. discussion 1045-1050. [PubMed] [Google Scholar]
- 18.O'Malley BW, Jr, Weinstein GS. Robotic skull base surgery : preclinical investigations to human clinical application. Arch Otolaryngol Head Neck Surg. 2007;133:1215–1219. doi: 10.1001/archotol.133.12.1215. [DOI] [PubMed] [Google Scholar]
- 19.O'Malley BW, Jr, Weinstein GS, Snyder W, Hockstein NG. Transoral robotic surgery (TORS) for base of tongue neoplasms. Laryngoscope. 2006;116:1465–1472. doi: 10.1097/01.mlg.0000227184.90514.1a. [DOI] [PubMed] [Google Scholar]
- 20.Ozer E, Waltonen J. Transoral robotic nasopharyngectomy : a novel approach for nasopharyngeal lesions. Laryngoscope. 2008;118:1613–1616. doi: 10.1097/MLG.0b013e3181792490. [DOI] [PubMed] [Google Scholar]
- 21.Park YM, Lee WJ, Lee JG, Lee WS, Choi EC, Chung SM, et al. Transoral robotic surgery (TORS) in laryngeal and hypopharyngeal cancer. J Laparoendosc Adv Surg Tech A. 2009;19:361–368. doi: 10.1089/lap.2008.0320. [DOI] [PubMed] [Google Scholar]
- 22.Ponnusamy K, Chewning S, Mohr C. Robotic approaches to the posterior spine. Spine (Phila Pa 1976) 2009;34:2104–2109. doi: 10.1097/BRS.0b013e3181b20212. [DOI] [PubMed] [Google Scholar]
- 23.Satava RM. Robotic surgery : from past to future--a personal journey. Surg Clin North Am. 2003;83:1491–1500. xii. doi: 10.1016/S0039-6109(03)00168-3. [DOI] [PubMed] [Google Scholar]
- 24.Weinstein GS, O'Malley BW, Jr, Desai SC, Quon H. Transoral robotic surgery : does the ends justify the means? Curr Opin Otolaryngol Head Neck Surg. 2009;17:126–131. doi: 10.1097/MOO.0b013e32832924f5. [DOI] [PubMed] [Google Scholar]
- 25.Weinstein GS, O'Malley BW, Jr, Snyder W, Hockstein NG. Transoral robotic surgery : supraglottic partial laryngectomy. Ann Otol Rhinol Laryngol. 2007;116:19–23. doi: 10.1177/000348940711600104. [DOI] [PubMed] [Google Scholar]
- 26.Yang MS, Yoon DH, Kim KN, Kim H, Yang JW, Yi S, et al. Robot-assisted anterior lumbar interbody fusion in a swine model in vivo test of the da vinci surgical-assisted spinal surgery system. Spine (Phila Pa 1976) 2011;36:E139–E143. doi: 10.1097/BRS.0b013e3181d40ba3. [DOI] [PubMed] [Google Scholar]