Abstract
Four novel ustiloxin D analogues were synthesized focusing on the size of the macrocyclic core, the stereochemistry at the bridgehead ether, and the enantiomer of ustiloxin D. All four were subjected to biological evaluation testing the inhibition of tubulin polymerization. Only 2,2-dimethyl-ustiloxin D retained any activity.
Keywords: Ustiloxin, Tubulin polymerization, Aziridine ring opening, Structure–activity relationships
1. Introduction
The ustiloxins A–F (Fig. 1) are a class of antimitotic natural products isolated from the water extracts of false smut balls, caused by the parasitic fungus Ustilaginoidea virens.1 These compounds universally inhibit the assembly of the α,β-tubulin dimer into microtubules, thus resulting in mitotic arrest of eukaryotic cells. Many antimitotic agents are known, ranging from the vinca alkaloids and taxoids to chalcones,2 but the mechanism of action of the naturally occurring peptides that inhibit tubulin polymerization is still not fully elucidated.3
Figure 1.
Ustiloxins A–F with IC50 values based on inhibition of porcine brain microtubule assembly.
Recent syntheses of ustiloxin D4 and F5 have been published, and the syntheses of eight analogues focusing on structural variations at R2, stereochemistry at C9 and C10, and methylation of the phenol were also reported by our group.6 To complete the SAR analysis we wished to probe the stereochemistry and the substituents at the tertiary aryl-ether bridge, the size of the macrocyclic core, and the enantiomer of ustiloxin D. The four analogues are shown in Figure 2.
Figure 2.
Four ustiloxin D analogues.
2. Synthesis of ustiloxin D analogues
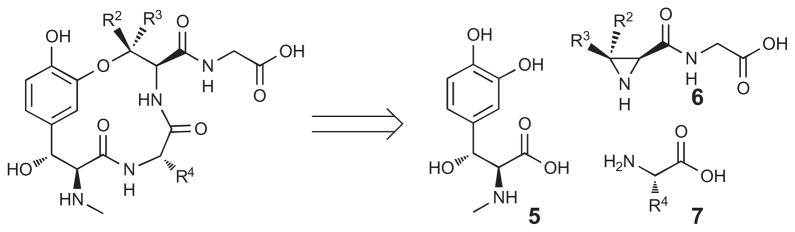
The convergent synthesis of the ustiloxins developed in our laboratory allowed for expeditious synthesis of the four ustiloxin analogues. In a retrosynthetic manner, the ustiloxins can be divided into three constituent parts: β-hydroxytyrosine 5, aziridine 6, and commercially available amino acid 7 (Fig. 3).
Figure 3.
Retrosynthetic analysis of the ustiloxins.
The aziridine 6 and β-hydroxytyrosine moiety 5 can be quickly accessed from D-valine,4e and a (S)-salen-aluminium catalyzed aldol7 reaction, respectively. This convergent route allows for rapid derivation of any of the three components.
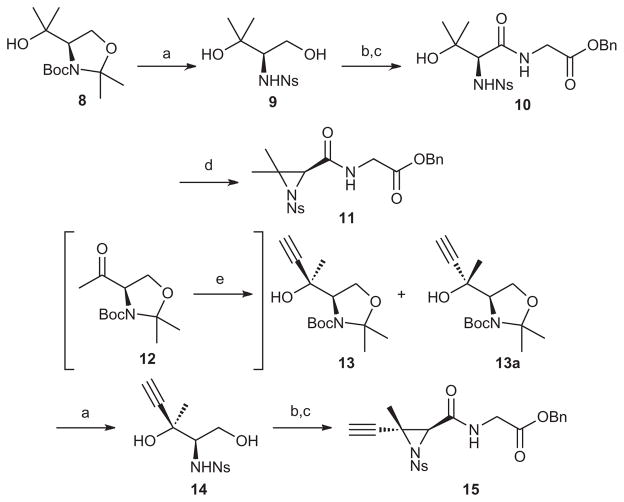
To access 2,2-dimethyl ustiloxin D, oxazolidine 8 was utilized to form the requisite dimethylaziridine 11 (Scheme 1).
Scheme 1.
Synthesis of aziridines 11 and 15. Reagents and conditions: (a) HCl, THF, 0 °C, 1 h, then NsCl, Na2CO3, THF/H2O, rt, 16 h, 58% for 9, 90% for 14; (b) TEMPO, NaClO2, NaClO, Na2HPO4, MeCN, 40 °C, 18 h; (c) H-GlyOBn·HCl, EDCI, HOBt, NaHCO3, DMF, 12 h, rt, 95% for 10, 90% for alkynyl precursor; (d) DIAD, PPh3, CH2Cl2, 18 h, rt, 77% for 11, 68% for 15; (e) ethynylMgBr, THF, rt, 80% ds = 11:1 (13a:13).
Thus, the 2,2-dimethyl aziridine was synthesized by treatment of the tertiary alcohol 8 with aqueous HCl and subsequent ortho-nitrobenzenesulfonamide protection of the amine afforded diol 9. TEMPO oxidation, followed by EDCI-mediated coupling of glycine benzyl ester gave the precursor to aziridine 10, and a Mitsunobu reaction afforded the dimethyl aziridine 11 in good yield. The (2S,3R)-ethynyl aziridine 15 was synthesized by Grignard addition to ketone 12 which resulted in a 11:1 diastereomeric ratio; the minor diastereomer (13) being utilized within this route. The synthesis of 15 was continued supra vida to afford the second aziridine.
The requisite β-hydroxytyrosine moiety (16) was synthesized as previously reported6 and coupled to aziridines 11 and 15 via a regioselective 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) mediated ring opening reaction developed in our laboratory,8 to afford the nosyl protected amines 17 and 18 (Scheme 2).
Scheme 2.
Ring opening of aziridines 11 and 15. Reagents and conditions: (a) TBD, toluene, 0 °C to rt, 3 h.
The synthesis of ent-ustiloxin D followed the previous6 synthesis of ustiloxin D and will not be reported in detail here. An (R)-salen-aluminium catalyzed aldol reaction afforded the β-hydroxytyrosine, L-serine provided the aziridine and D-valine was used to complete the protected linear precursor. The synthesis of 7-N-Gly-ustiloxin D (4) also followed the previous synthesis to give the nosyl protected amine 21 (Scheme 3).
Scheme 3.
Deprotection and coupling of amino acid residues. Reagents and conditions: (a) PhSH, Cs2CO3, DMF, 0 °C, 4 h; (b) N-Boc-Gly, EDCI, HOBt, NaHCO3, DMF, 12 h, rt; (c) N-Boc-Val-Gly, EDCI, HOBt, NaHCO3, DMF, 12 h, 0 °C to rt, 79%.
Removal of the nosyl protecting group on each of the three analogues with benzene thiol proceeded without incident to provide the free amine. EDCI-mediated coupling of tripeptides 17 and 18 with N-Boc-D-valine gave compounds 19 and 20, respectively. Coupling of amine 21 with N-Boc-glycine-valine afforded 22 (Scheme 3).
With all three analogues and the ent-ustiloxin D protected linear precursor in hand (not shown), the synthesis was concluded with a deprotection/macrocyclization/deprotection sequence (Scheme 4).
Scheme 4.
Reagents and conditions: (a) TFA, Et3SiH, CH2Cl2, 0 °C, 4 h; (b) EDCI, HOBt, NaHCO3, DMF, 0 °C to rt, 24 h, 46% for R1 = R2 = Me, 30% for R1=Me R2 = ethyne, 34% for N-Gly macrocycle; (c) Pd/C, H2, THF/H2O, 24 h, 80% for 2, 60% for 3, and 55% for 4.
Treatment of the linear precursors with TFA/Et3SiH afforded the TFA salts, which were subsequently converted into the hydrochloride salts. Subjection of each amino acid salt to EDCI-mediated macrocyclization and concomitant Pd/C catalyzed hydrogenolysis gave ent-ustiloxin D (1), 2,2-dimethyl ustiloxin D (2), (2S)-epi-ustiloxin D (3), and 7-N-Gly-ustiloxin D (4).
3. Bioactivity
The effect of the changes present in the four analogues on the polymerization of purified tubulin was investigated using the IC50 value of ustiloxin D (2.5 μM) as the benchmark. Biological evaluation of ent-ustiloxin D (1) showed no inhibition with an IC50 value >40 μM. Similarly, 7-N-Gly-ustiloxin D (4) showed no inhibition (IC50 >40 μM), suggesting that the naturally occurring ustiloxin D macrocycle size is optimum. (2S)-epi-Ustiloxin D (3) exhibited a very slight inhibitory effect, but overall had an IC50 >40 μM. Interestingly, removal of the stereogenic carbon at the ether bridge afforded 2,2-dimethyl ustiloxin D (2) with an IC50 = 9.2 ± 2 μM.
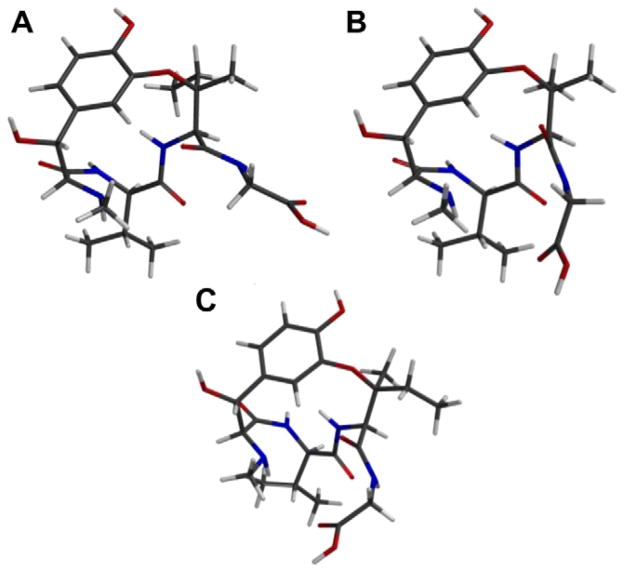
The difference in inhibitory activity of ustiloxin D, (2S)-epi, and the dimethyl species was investigated using molecular modeling (Fig. 4). Comparison of ustiloxin D (A) to 2,2-dimethyl ustiloxin (B) and (2S)-epi-ustiloxin (C) quite clearly shows distortion of the glycine sidechain, progressively pushing it toward the valine residue. The out-of-plane glycine sidechain may lessen the ability of the molecule to bind at this position, but it may also block the binding ability of the valine residue, which was shown to play an important role in the ustiloxins inhibitory effect.6,9
Figure 4.
Spartan®’03 energy minimized molecular models at HF/AM1 level of theory. Ustiloxin D (A), 2,2-dimethyl-ustiloxin D (B), and (2S)-epi-ustiloxin D (C).
4. Conclusions
Novel ustiloxin analogues were synthesized to investigate the effect of changes at the C2 center, the macrocycle size, and the enantiomeric series on tubulin inhibition. The structural modifications produced significant changes in the biological activity suggesting the importance of the valine residue in the binding process.
Acknowledgments
We thank NIH (CA-40081) and NSF (CHE-0951394) for support of this work. Financial support for the departmental instrumentation was provided by NIH (1S10RR23444-1). We also thank Dr. George Furst and Dr. Rakesh Kohli for NMR and MS assistance, respectively.
Footnotes
Dedicated to Professor Harry Wasserman on the occasion of his 90th birthday.
References and notes
- 1.(a) Koiso Y, Li Y, Kobayashi H, Natori M, Hashimoto Y, Iwasaki S, Fujita Y, Sonoda R, Yaegashi H, et al. Tennen Yuki Kagobutsu Toronkai Koen Yoshishu. 1992;34:566. [Google Scholar]; (b) Koiso Y, Natori M, Iwasaki S, Sato S, Sonoda R, Fujita Y, Yaegashi H, Sato Z. Tetrahedron Lett. 1992;33:4157. [Google Scholar]; (c) Koiso Y, Li Y, Iwasaki S, Hanaoka K, Kobayashi T, Sonoda R, Fujita Y, Yaegashi H, Sato Z. J Antibiot. 1994;47:765. doi: 10.7164/antibiotics.47.765. [DOI] [PubMed] [Google Scholar]
- 2.Hamel E. Med Res Rev. 1996;16:207. doi: 10.1002/(SICI)1098-1128(199603)16:2<207::AID-MED4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 3.Hamel E, Covell DG. Curr Med Chem: Anti-Cancer Agents. 2002;2:19. doi: 10.2174/1568011023354263. [DOI] [PubMed] [Google Scholar]
- 4.(a) Cao B. University of Pennsylvania. 2002. [Google Scholar]; (b) Cao B, Park H, Joullié MM. J Am Chem Soc. 2002;124:520. doi: 10.1021/ja017277z. [DOI] [PubMed] [Google Scholar]; (c) Tanaka H, Sawayama AM, Wandless TJ. J Am Chem Soc. 2003;125:6864. doi: 10.1021/ja035429f. [DOI] [PubMed] [Google Scholar]; (d) Sawayama AM, Tanaka H, Wandless TJ. J Org Chem. 2004;69:8810. doi: 10.1021/jo048854f. [DOI] [PubMed] [Google Scholar]; (e) Li P, Evans CD, Joullié MM. Org Lett. 2005;7:5325. doi: 10.1021/ol052287g. [DOI] [PubMed] [Google Scholar]
- 5.Li P, Evans CD, Forbeck EM, Park H, Bai R, Hamel E, Joullié MM. Bioorg Med Chem Lett. 2006;16:4804. doi: 10.1016/j.bmcl.2006.06.071. [DOI] [PubMed] [Google Scholar]
- 6.Li P, Evans CD, Wu Y, Cao C, Hamel E, Joullié MM. J Am Chem Soc. 2008;130:2351. doi: 10.1021/ja710363p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans DA, Janey JM, Magomedov N, Tedrow JS. Angew Chem, Int Ed. 2001;40:1884. [PubMed] [Google Scholar]
- 8.Forbeck EM, Evans CD, Gilleran JA, Li P, Joullié MM. J Am Chem Soc. 2007;129:14463. doi: 10.1021/ja0758077. [DOI] [PubMed] [Google Scholar]
- 9.Cormier A, Marchand M, Ravelli RBG, Knossow M, Gigant B. EMBO Rep. 2008;9:1101. doi: 10.1038/embor.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]