Abstract
Purpose
To examine both inter-visit and intra-visit reproducibility of a magnetic resonance (MR) arterial spin labeling (ASL) perfusion technique in native and transplanted kidneys over a broad range of renal function.
Materials and Methods
Renal perfusion exams were performed at 1.5 T in a total of 24 subjects: 10 with native and 14 with transplanted kidneys. Using a flow-sensitive alternating inversion recovery (FAIR) ASL scheme, thirty-two control/tag pairs were acquired and processed using a single-compartment model. Two FAIR-ASL MR exams were performed at least 24 hours apart on all the subjects to assess inter-visit reproducibility. ASL perfusion measurements were also repeated back-to-back within one scanning session in 8 native subjects and in 12 transplant subjects to assess intra-visit reproducibility. Intra-class correlation (ICC) and coefficients of variation (CV) were calculated as metrics of reproducibility.
Results
Intra-visit ICC's ranged from 0.96-0.98 while CV's ranged from 4.8%-6.0%. Inter-visit measurements demonstrated slightly more variation with ICC's from 0.89-0.94 and CV's from 7.6%-13.1%. Medullary perfusion demonstrated greater variability compared to cortical blood flow: intra-visit ICC's from 0.72-0.78 and CV's from 16.7%-26.7%, inter-visit ICC's from 0.13-0.63 and CV's from 19.8%-37%.
Conclusion
This study indicates that a FAIR-ASL perfusion technique is reproducible in the cortex of native and transplanted kidneys over a broad range in renal function. In contrast, perfusion measurements in the medulla demonstrated moderate to poor reproducibility for intra-visit and inter-visit measures respectively.
Keywords: reproducibility, arterial spin labeling, kidney, perfusion, transplant
INTRODUCTION
Pathological conditions commonly arise following renal transplantation, yet assessing functional changes using clinical parameters, such as blood creatinine levels, remains insensitive and identifying the specific pathology currently requires an invasive biopsy. Frequently, transplant patients receive multiple biopsies as longitudinal assessment is necessary to allow early characterization of dysfunction and to help guide treatment decisions. However, complications of biopsy, such as pain, bleeding, infection, and potential graft loss must be considered when utilizing the technique for the evaluation of transplanted kidneys. This has motivated research in developing non-invasive imaging tests to both detect functional changes and characterize renal disease.
Non-invasive magnetic resonance (MR) kidney perfusion measurements may prove helpful in detecting a change in function and identifying pathology. These techniques have shown promise in native and transplanted kidneys, and have also correlated with histology in transplanted rat kidneys (1, 2). In addition, human studies indicate that gadolinium-based, contrast-enhanced MR perfusion may help differentiate cyclosporine toxicity, acute rejection and acute tubular necrosis following transplantation (3-6). Although contrast-based perfusion techniques have provided some of these encouraging results, non-contrast MR perfusion techniques, such as arterial spin labeling (ASL), may be preferable in patients with renal failure (7) and for longitudinal assessment of transplant patients.
ASL techniques selectively label endogenous, inflowing blood to have an opposite magnetization compared to the tissue of interest. Tissue perfusion can then be determined by subtracting the labeled image (tag) and a non-labeled image (control). ASL perfusion has been used extensively in the brain (8-12), and recently has been applied to the kidneys (13-20). Many of these renal studies used a specific Flow-Sensitive Alternating Inversion Recovery (FAIR) ASL method that was first demonstrated in the kidneys by Martirosian et al (17). FAIR-ASL has been utilized to grade renal artery stenosis in native kidneys (16) and to assess transplanted kidneys undergoing an acute deterioration in function (18). Also in the recent literature, Ritt et al validated FAIR-ASL for the measurement of renal perfusion before and after a pharmacological intervention and found good correlation with the gold standard, para-aminohippuric acid plasma clearance (21).
ASL perfusion may be clinically useful but only if the methods are proven reproducible over time. It is important to understand the physiologic variability of blood flow before we can apply ASL to assessing disease. The purpose of this study is to examine both inter-visit and intra-visit reproducibility of renal perfusion measurements using the FAIR-ASL technique in both native and transplant kidney subjects over a broad range in renal function.
MATERIALS AND METHODS
Subjects
This HIPPA-compliant study was approved by our institutional human subjects review committee and written informed consent was obtained from all subjects. Subjects with normal kidney function were recruited from a pool of healthy volunteers who expressed interest in participating in MRI research studies. Kidney transplant recipients and subjects with chronic kidney disease (CKD) were recruited by referring nephrologists, in a consecutive order, when they presented to their routine clinic appointments if they met the study's inclusion criteria. Subjects were included in the study if they were adults (>18 yrs old), MRI compatible, and clinically stable between visits. Subjects were considered stable if their serum creatinine levels varied ≤ 0.3 mg/dL between visits and no events changed their clinical status during the interim. Subjects were included in the study regardless of their underlying disease process. Serum creatinine levels and estimated glomerular filtration rate (eGFR) were used to ensure we recruited subjects with a wide range of renal function.
Between February 2008 and February 2009, MR renal perfusion exams were performed on a total of 24 subjects and a total of 34 kidneys, including ten native subjects (six men, four women; mean age ± standard deviation (SD), 55.2 ± 13.3 years; age range, 33-79 years) and fourteen transplant subjects (eleven men, three women; mean age ± SD, 47.1 ± 13.5 years; age range, 21-66 years). Serum creatinine was measured just prior to the MRI examination and eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) formula (22). Subjects refrained from fluids for four hours before MR imaging.
Reproducibility Studies
Two FAIR-ASL MR exams were performed at least 24 hours apart on all the subjects to assess inter-visit reproducibility. These two exams are referred to as visit 1 and visit 2 exams in the text and figures. The MR exams were performed at the same time of the day on both visits. In order to examine same day or intra-visit reproducibility as well, ASL perfusion measurements were repeated within the same day in eight native subjects (during one of the visits) and in twelve transplant subjects (during each of the visits). The second ASL acquisition was performed immediately following the first acquisition while the subject remained in the scanner. The exams performed on the same day are referred to as scan 1 and scan 2 in the text and figures.
Scan Protocol
Scans were performed on a 1.5 T MR scanner (Signa HDx, GE Healthcare, Milwaukee, WI, USA) with an eight-element phased array cardiac coil (GE Healthcare, Milwaukee, WI, USA). The MR scanner was equipped with a dual speed gradient system. Peak strength and slew rates were 23 mT/m and 77 mT/m/ms for the whole body gradients and 40 mT/m and 150 mT/m/ms for the zoom gradients. ASL images were acquired using a FAIR-balanced steady state free procession (b-SSFP) acquisition scheme (17) with a 20 ms hyperbolic secant adiabatic inversion pulse and the following readout parameters: TR/TE/flip = 4.6/2.3ms/70°, BW = 83.33 kHz, FOV = 34-36 cm, and 128 x 128 matrix. In a few cases, larger subjects required an increase of the FOV above 36 cm, negating the use of zoom gradients and lengthening the TR/TE up to 5.8/2.9 ms. The 8 mm imaging slice, carefully chosen not to include the feeding vessels, was central to a 20 mm slice selective inversion thickness. For native kidneys the oblique-coronal orientation allowed for proper placement posterior to the renal arteries, however in the transplanted kidney, the oblique-sagittal orientation was needed in eight of the fourteen subjects to avoid major feeding arteries. Slice prescriptions were chosen to maximize the cross-sectional area of the kidney for both visit 1 and 2.
The inversion pulse was respiratory triggered using a breathing belt post-exhalation, and following an inversion time (TI) of 1.2 sec, a centric phase encoded balanced-SSFP image was acquired. The subjects were coached, prior to scanning, to breathe after completion of the image readout. Respiratory rates were 12 breaths/minute or lower to allow sufficient magnetization recovery between inversions (≥ 5 seconds between breaths). Control and tag images were alternated until 64 (32 pairs) total images were acquired. Proton density images (to measure M0) were obtained with a NEX = 4 using the b-SSFP readout with no inversion preceding it. Scanning was completed in 6-9 minutes, depending on the respiratory rate.
Segmentation and Processing
Perfusion data were analyzed using custom scripts written in MATLAB (version 7.5, The MathWorks Inc., Cambridge, MA, USA). Rectangular regions of interest (“ROIs”) were drawn around each kidney and were registered independently through the image series by using automated rigid registration based on normalized mutual information (NMI). The mean of the registered tag images, MT, was then subtracted from the mean of the registered control images, MC, to obtain a difference image, ΔM, of the kidney. After manually segmenting out the kidney using the T1-weighted MC image, threshold techniques were used to differentiate cortex from medulla. For a limited number of cases, the coil sensitivity varied across the kidney or segmental vessels were visible necessitating segmentation.
Perfusion was determined based on a one compartment model:
| [1] |
where f ≡ perfusion, λ ≡ partition coefficient = 80 ml/100g (14), α ≡ inversion efficiency = 1.0, TI ≡ inversion time = 1.2 s, and T1 ≡ longitudinal relaxation time = 966 ms for cortex and 1,410 ms for medulla (23). Each pixel's measured ΔM and M0 were used to generate a perfusion map of the entire kidney. The M0 images were shared when processing intra-visit exams in which case the ASL scans were performed back-to-back. Flow values from all pixels in a specific tissue (e.g. cortex) were averaged together to provide mean perfusion for each kidney.
Statistical Analysis
For all subjects, intra-visit and inter-visit perfusion measurements were plotted and a best-fit regression line was computed from the data. The Pearson product-moment correlation coefficient (r) was determined for these related measurements. Reproducibility was also assessed using intra-class correlation (ICC) and coefficients of variation (CV). The ICC was determined based on a two way random effect model using Equation [2] below where n = the number of subjects, k = the number of raters (which was two in this study because ASL data was measured at two distinct time points), MSS = the mean square subjects, MSR = the mean square raters, and MSE = the mean square error (24). Ninety-five percent confidence intervals for the ICC were estimated using the bias accelerated and corrected (BCa) bootstrap method with 2000 iterations (25). The CV was calculated using Equation [3] below where n = the total number of subjects, meas1 = the first measurement, meas2 = the second measurement, and Mean = the mean of all measurements across all subjects (26, 27).
| [2] |
| [3] |
RESULTS
Demographics of the subjects in this study, including mean perfusion values and the number of days between visits, are displayed in Table 1. Across the twenty-four subjects (34 kidneys), cortical perfusion was successfully measured during both visits for all but one native kidney. Neither cortical nor medullary perfusion was measured for this one exception which occurred during the first visit, when intra-exam motion was too severe for the image alignment technique used in this study. Medullary perfusion was also unobtainable during the second visit for one native subject with CKD (eGFR=19 ml/min/1.73m2) because the medulla was not clearly visible.
Table 1.
Subject demographics, perfusion, and the days between visits for the ten subjects with native kidneys and fourteen subjects with transplanted kidneys.
| Native Kidneys | Transplanted Kidneys | |
|---|---|---|
| Males/Females | 6 / 4 | 11 / 3 |
| Age | 55 ± 13 (33-79) | 47 ± 14 (21-66) |
| Creatinine§ | 1.6 ± 0.9 (0.7-3.5) | 1.8 ± 1.0 (1-3.9) |
| eGFR‡ | 55 ± 26 (19-88) | 49 ± 18 (18-74) |
| Cortical Perfusion† | 334 ± 123 (81-456) | 273 ± 82 (125-409) |
| Medullary Perfusion† | 72 ± 29 (33-121) | 35 ± 15 (11-70) |
| Days Between Visits | 74 ± 83 (1-215) | 19 ± 21 (2-66) |
Values given as the mean ± standard deviation (range)
Creatinine given in milligrams per deciliter
eGFR cited in milliliters per minute per 1.73 meters2
Perfusion listed in units of ml/min/100g
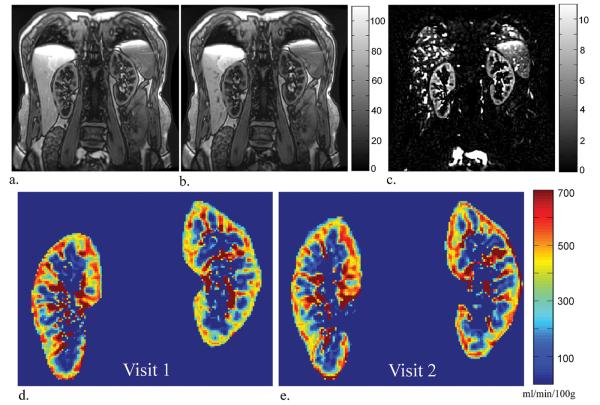
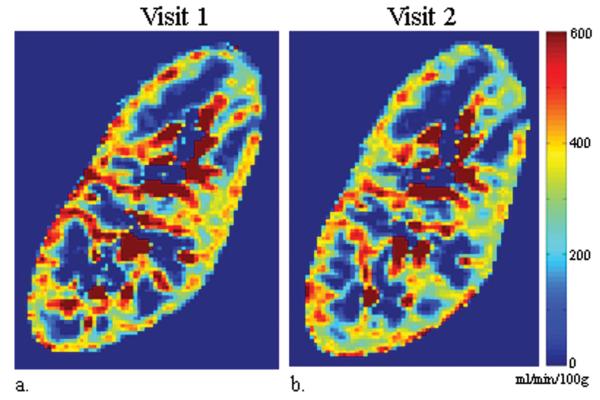
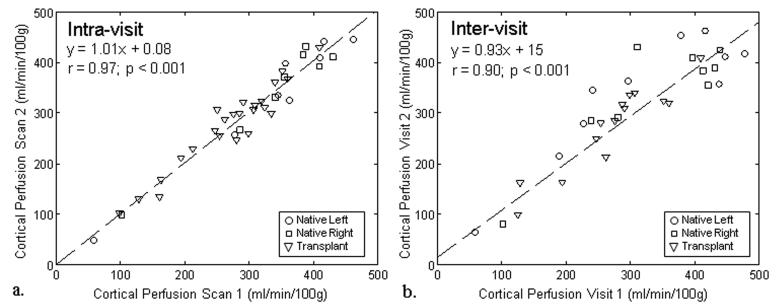
ASL tag, control, and perfusion weighted difference images for one subject with native kidneys are displayed in Figure 1 along with the perfusion maps from visit 1 and 2. ASL perfusion maps processed from exams performed on separate visits are also displayed for one subject with transplanted kidneys (Figure 2). Qualitatively, the color perfusion maps appear to be reproducible between visit 1 and visit 2 for both native and transplanted kidneys. The qualitative assessment is supported by the high ICC and low CV for both the intra-visit (ICC: 0.96-0.98; CV: 4.8-6.0%) and inter-visit (ICC: 0.89-0.94; CV: 7.6-13.1%) cortical perfusion data in all subjects (Table 2). When exams were repeated on the same day, both native and transplant kidneys demonstrated similar reproducibility in cortical perfusion. When exams were performed on separate days, transplanted kidneys showed the best correlation (ICC = 0.94) and lowest variability (CV = 7.6%) in cortical blood flow. Linear regression (Figure 3) also reveals strong agreement between cortical measurements from exams repeated on the same day (r = 0.97, p < 0.001; y = 1.01x + 0.08) and on different days (r = 0.90, p < 0.001; y = 0.93x + 15).
Figure 1.
Tag (a), control (b), and perfusion weighted difference (c) images acquired in a subject with healthy native kidneys (eGFR = 80 ml/min/1.73m2) during visit 1 along with the resulting perfusion map (d). For comparison purposes, this subject's visit 2 perfusion map is displayed on the right (e). From visit 1 to visit 2, the right kidney's mean cortical perfusion decreased from 440 to 425 ml/min/100g while the left kidney cortical perfusion increased from 380 to 453 ml/min/100g. The mean medullary perfusion decreased from 50 to 47 ml/min/100g in the right kidney and increased from 43 to 87 ml/min/100g in the left kidney.
Figure 2.
ASL perfusion maps (sagittal) processed from MR exams performed on visit 1 (a) and visit 2 (b) for the same transplanted kidney (eGFR = 60 ml/min/1.73m2). From visit 1 to visit 2, the kidney's mean cortical perfusion decreased from 352 to 324 ml/min/100g while the medullary perfusion increased from 11 to 27 ml/min/100g.
Table 2.
Intra-visit and Inter-visit Reproducibility of Cortical Perfusion
| Kidney | N | Scan 1 Perfusion* Mean (±SD) |
Scan 2 Perfusion* Mean (±SD) |
Measures of reproducibility |
|
|---|---|---|---|---|---|
| ICC (95% CI**) | CV(%) | ||||
| Intra-visit (same day) | |||||
|
| |||||
| Native Right | 8 | 337 (±105) | 340 (±111) | 0.98 (0.71, 0.99) | 4.8 |
| Native Left | 8 | 337 (±125) | 332 (±132) | 0.98 (0.83, 1.0) | 5.2 |
| Transplant | 24† | 269 (±77) | 275 (±81) | 0.96 (0.89, 0.98) | 6.0 |
|
| |||||
| Inter-visit (different days) | |||||
|
| |||||
| Native Right | 9‡ | 337 (±115) | 339 (±111) | 0.89 (0.23, 0.98) | 11.0 |
| Native Left | 10 | 318 (±137) | 336 (±123) | 0.89 (0.65, 0.97) | 13.1 |
| Transplant | 14 | 271 (±81) | 272 (±85) | 0.94 (0.86, 0.98) | 7.6 |
Perfusion listed in units of ml/min/100g
95% Confidence Intervals
ASL scans were repeated on the same day in twelve transplant subjects for visit 1 and visit 2.
Perfusion was not measured in one of the 10 native, right kidneys because the intra-exam motion was too severe for the image alignment technique used in this study.
Figure 3.
Cortical perfusion measured from FAIR-ASL exams performed on the same day (scan 1 and scan 2) demonstrated excellent correlation (r = 0.97; p < 0.001) between scans for the native and transplanted kidneys (a). For exams performed on different days (visit 1 and visit 2) cortical perfusion measurements again exhibited excellent correlation (r = 0.90; p < 0.001) in native and transplanted kidneys (b). Absolute agreement was strong for the intra-visit (a) and inter-visit (b) data as indicated by the regression lines with slopes near unity and y-intercepts near zero.
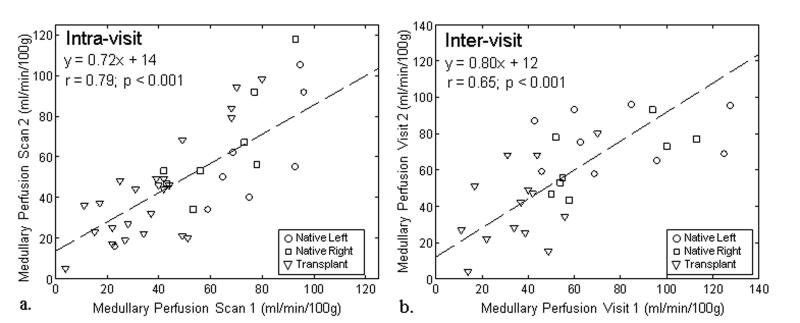
The medullary perfusion measurements demonstrated less agreement. The intra-visit data (ICC: 0.72-0.78; CV: 16.7-26.7%) showed modest reproducibility, while the reproducibility of the inter-visit (ICC: 0.13-0.68; CV: 19.8-37.0%) medullary blood flow data was poor (Table 3). Although variable in both native and transplanted kidneys, medullary perfusion measures repeated on the same day demonstrated moderate correlation (r = 0.79, p < 0.001) while measures repeated on separate days yielded relatively low correlation (r = 0.65, p < 0.001).
Table 3.
Intra-visit and Inter-visit Reproducibility of Medullary Perfusion
| Kidney | N | Scan 1 Perfusion* Mean (±SD) |
Scan 2 Perfusion* Mean (±SD) |
Measures of reproducibility |
|
|---|---|---|---|---|---|
| ICC (95% CI**) | CV(%) | ||||
| Intra-visit | |||||
|
| |||||
| Native Right | 8 | 64 (±19) | 65 (±27) | 0.78 (0.49, 0.87) | 16.7 |
| Native Left | 8 | 72 (±24) | 57 (±30) | 0.72 (0.33, 0.96) | 23.8 |
| Transplant | 24§ | 38 (±20) | 43 (±25) | 0.77 (0.55, 0.88) | 26.7 |
|
| |||||
| Inter-visit | |||||
|
| |||||
| Native Right | 8†‡ | 72 (±26) | 65 (±18) | 0.63 (0.25, 0.90) | 19.8 |
| Native Left | 9‡ | 79 (±32) | 77 (±16) | 0.13 (−0.39, 0.60) | 28.1 |
| Transplant | 14 | 36 (±17) | 40 (±22) | 0.46 (−0.1, 0.83) | 37.0 |
Perfusion listed in units of ml/min/100g
95% Confidence Intervals
ASL scans were repeated on the same day in twelve transplant subjects for visit 1 and again for visit 2.
Perfusion was not measured in one of the 10 native, right kidneys because the intra-exam motion was too severe for the image alignment technique used in this study.
Medullary perfusion could not be measured during visit 2 in one native subject (eGFR=19 ml/min/1.73m2) because the medulla was not clearly visible.
DISCUSSION
Many published studies indicate that MR perfusion imaging can be used in the assessment of kidney function, yet renal perfusion studies to date have offered only a preliminary evaluation of reproducibility (15, 18, 20, 28). ASL perfusion techniques will only be useful in the assessment of kidney function if they are reproducible and robust to motion and disease. In addition, large swings due to physiologic variability from minute to minute or day to day would diminish the usefulness of MRI to diagnose changes in blood flow and ischemia. Our data show that no significant physiological variations are present in cortical blood flow MRI measurements acquired minutes and days apart in patients whose renal function remained clinically stable between visits. Cortical perfusion measurements were very reproducible between scans performed on the same day (intra-visit) and between scans performed on separate days (inter-visit).
Both intra-day and inter-day medullary perfusion measurements appear much less reproducible with ICC values as low as 0.13 and CV values as high as 37% for exams performed during different visits. Medullary perfusion has demonstrated greater variation than cortical perfusion in the literature as well (28). It is well known that cortical and medullary blood flow are under separate controlling mechanisms. The cortex is controlled mainly by the sympathetic nervous system and reacts to increases in total renal blood flow. The medulla is also under sympathetic control, however the local hormonal control of the blood vessels can override the sympathetic stimulations (29-31). In the medulla, the production of vasoactive substances regulates its blood flow, e.g. nitric oxide (32, 33), to stringently control the medullary flow and maintain the balance between medullary oxygen needs and tubular function (29, 34, 35). This balance is adjusted on a minute by minute basis and fluctuates based on the tubular load. Therefore, the lack of reproducibility observed in the medulla may be physiological which would limit its use as a sole clinical parameter. However, it may be useful if paired with cortical perfusion or other functional MRI measures such as blood oxygen level dependent (BOLD) MRI.
Increased variability in medullary measurements could also be due to lower signal-to-noise (SNR) conditions as compared to the cortex. ASL signal is directly proportional to blood flow, and medullary perfusion is much lower than cortical perfusion. Additionally, about 85-90% of the inflowing blood first passes through the cortex before entering the medulla. Imaging at a delay time of 1.2 seconds captures only the blood spins that bypassed the cortex and flowed directly into the medulla. For this reason, imaging at later delay times would likely improve medullary perfusion assessment, although it would not be optimal for measurement in the cortex. Further work is necessary to determine whether greater variability in medullary perfusion measurements is due to physiologic regulatory mechanisms or unfavorable SNR conditions.
The quantitative perfusion measurements in this study agree with other renal ASL studies for native (14-16, 20) and transplant subjects (18). The variation in cortical perfusion observed between visit 1 and 2 is also consistent with preliminary results in the contrast-enhanced and ASL renal perfusion literature (15, 18, 20, 28) and could provide guidance for differentiating normal and abnormal perfusion variation during longitudinal assessment. Some of the variation observed in the inter-visit results may be due to slight variation in slice prescription through the kidney, although care was taken to prescribe a similar slice during both visits. Below the threshold of 12 breaths/minute, respiratory rates were allowed to vary which could have occurred over the course of a scan as well as between visits. Although recovery will nearly be complete, there would be different amounts of time for recovery between respiratory triggered inversions, contributing to variability in the perfusion measurements as well. The exams were performed at the same time of day on both visit 1 and visit 2 to limit potential changes in renal blood flow due to diurnal variations.
Several limitations apply to the present study. Although all subjects refrained from fluids for four hours prior to MR imaging, this study did not regulate dietary intake of patients and the protein content of their diet, which may have caused some variation in renal perfusion between visit 1 and visit 2 measurements (36). In clinical practice, however, it is challenging to regulate protein intake for all patients so it may be helpful to estimate normal perfusion variability including protein-related variation. Another limitation and possible source of variation stems from the assumed values of T1 and inversion efficiency, α, in Eq. [1] used to calculate perfusion. In fact, the cortical T1 assumed in this study may have introduced a bias towards higher perfusion for lower functioning kidneys because cortical T1 can increase with renal insufficiency (37). However, renal function was stable for the subjects in this study so large changes in T1 between visit 1 and 2 would not be expected. Two limitations apply to the medullary perfusion measures as well. First, lower blood flow in the medulla causes the measured signal to be much closer to the noise floor. Second, the 1.2 second delay time used in this study limited the delivery of tagged blood to the medulla. Most of the blood that reaches the medulla first passes through the cortex, so this ASL technique likely only measured the 10-15% of renal artery blood that bypassed the cortex and flowed directly into the medulla.
In addition to demonstrating reproducibility, establishing correlation with a gold standard is also an important step in the future application of FAIR-ASL to clinical practice. In a separate swine study, we compared this FAIR-ASL technique to fluorescent microsphere measurements of cortical perfusion and found very good correlation (r = 0.81), providing validation of this technology for imaging of relative renal perfusion (38). Moreover, other groups have independently validated the FAIR-ASL technique in human subjects using para-aminohippuric acid plasma clearance as the gold standard (21).
Alone ASL can provide useful information on the function of both native and transplanted kidneys, however coupled to other functional MR techniques, such as BOLD, it has the potential to provide insight into oxygen delivery and utilization. Using BOLD MR imaging, investigators have demonstrated an increase in oxygen bioavailability in the medullary regions of transplanted kidneys undergoing acute rejection versus those with normal function, however the underlying cause of these findings was not able to be determined in these initial studies (39, 40). BOLD MRI can only assess the relative concentration of deoxyhemoglobin in the capillaries, but cannot determine whether changes in the deoxyhemoglobin concentration are due to changes in blood flow or changes in intra-cellular oxygen utilization. Later studies using contrast-enhanced MR perfusion techniques, found a decrease in medullary perfusion in transplanted kidneys undergoing acute rejection, compared to allografts with normal function, thereby suggesting the underlying mechanisms for the increase in oxygen bioavailability was more likely due to a decrease in intra-cellular oxygen utilization (5, 6).
In conclusion, a FAIR-ASL protocol offers a non-contrast alternative to gadolinium-based perfusion techniques and is especially appealing in the transplant setting, where longitudinal assessment is imperative. This study indicates that the FAIR-ASL perfusion technique is reproducible in the cortex of native and transplanted kidneys over a broad range in renal function. Documenting the reproducibility and natural variation in renal cortical perfusion hopefully sets the stage for future studies using the FAIR-ASL technique to determine thresholds which are clinically significant indicators of the onset and progression of chronic kidney disease.
Figure 4.
Medullary perfusion measured from FAIR-ASL exams performed on the same day (scan 1 and scan 2) demonstrated moderate correlation (r = 0.79; p < 0.001) between scans for the native and transplanted kidneys (a). For exams performed on different days (visit 1 and visit 2) medullary perfusion measurements demonstrated lower correlation (r = 0.65; p < 0.001) in native and transplanted kidneys (b). Absolute agreement was much lower compared to the cortical perfusion in both the intra-visit data (a) and the inter-visit data (b) which each demonstrated slopes less than one.
Acknowledgments
The authors thank Zhifei Wen, PhD, for help with ASL sequence development and processing, Garima Agrawal for volunteer recruitment and coordination, our research technologists, Kelli Hellenbrand and Sara Pladziewicz, for performing the volunteer scans, and GE Healthcare for their research support.
Funding:
This research was supported by the National Institute of Health (NIH grants R01 DK 073680, R21 DK070243).
References
- 1.Beckmann N, Joergensen J, Bruttel K, Rudin M, Schuurman HJ. Magnetic resonance imaging for the evaluation of rejection of a kidney allograft in the rat. Transpl Int. 1996;9:175–183. doi: 10.1007/BF00335383. [DOI] [PubMed] [Google Scholar]
- 2.Wang JJ, Hendrich KS, Jackson EK, Ildstad ST, Williams DS, Ho C. Perfusion quantitation in transplanted rat kidney by MRI with arterial spin labeling. Kidney Int. 1998;53:1783–1791. doi: 10.1046/j.1523-1755.1998.00945.x. [DOI] [PubMed] [Google Scholar]
- 3.Szolar DH, Preidler K, Ebner F, et al. Functional magnetic resonance imaging of human renal allografts during the post-transplant period: preliminary observations. Magn Reson Imaging. 1997;15:727–735. doi: 10.1016/s0730-725x(97)00088-x. [DOI] [PubMed] [Google Scholar]
- 4.Sharma RK, Gupta RK, Poptani H, Pandey CM, Gujral RB, Bhandari M. The magnetic resonance renogram in renal transplant evaluation using dynamic contrast-enhanced MR imaging. Transplantation. 1995;59:1405–1409. doi: 10.1097/00007890-199505270-00008. [DOI] [PubMed] [Google Scholar]
- 5.Sadowski EA, Djamali A, Wentland AL, et al. Blood oxygen level-dependent and perfusion magnetic resonance imaging: detecting differences in oxygen bioavailability and blood flow in transplanted kidneys. Magn Reson Imaging. 28:56–64. doi: 10.1016/j.mri.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wentland AL, Sadowski EA, Djamali A, Grist TM, Becker BN, Fain SB. Quantitative MR measures of intrarenal perfusion in the assessment of transplanted kidneys: initial experience. Acad Radiol. 2009;16:1077–1085. doi: 10.1016/j.acra.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242:647–649. doi: 10.1148/radiol.2423061640. [DOI] [PubMed] [Google Scholar]
- 8.Alsop DC, Detre JA. Multisection cerebral blood flow MR imaging with continuous arterial spin labeling. Radiology. 1998;208:410–416. doi: 10.1148/radiology.208.2.9680569. [DOI] [PubMed] [Google Scholar]
- 9.Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23:37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- 10.Edelman RR, Siewert B, Darby DG, et al. Qualitative mapping of cerebral blood flow and functional localization with echo-planar MR imaging and signal targeting with alternating radio frequency. Radiology. 1994;192:513–520. doi: 10.1148/radiology.192.2.8029425. [DOI] [PubMed] [Google Scholar]
- 11.Kim SG. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn Reson Med. 1995;34:293–301. doi: 10.1002/mrm.1910340303. [DOI] [PubMed] [Google Scholar]
- 12.Wong EC, Buxton RB, Frank LR. Implementation of quantitative perfusion imaging techniques for functional brain mapping using pulsed arterial spin labeling. NMR Biomed. 1997;10:237–249. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<237::aid-nbm475>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 13.Michaely HJ, Schoenberg SO, Ittrich C, Dikow R, Bock M, Guenther M. Renal disease: value of functional magnetic resonance imaging with flow and perfusion measurements. Invest Radiol. 2004;39:698–705. doi: 10.1097/00004424-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Roberts DA, Detre JA, Bolinger L, et al. Renal perfusion in humans: MR imaging with spin tagging of arterial water. Radiology. 1995;196:281–286. doi: 10.1148/radiology.196.1.7784582. [DOI] [PubMed] [Google Scholar]
- 15.Robson PM, Madhuranthakam AJ, Dai W, Pedrosa I, Rofsky NM, Alsop DC. Strategies for reducing respiratory motion artifacts in renal perfusion imaging with arterial spin labeling. Magn Reson Med. 2009;61:1374–1387. doi: 10.1002/mrm.21960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenchel M, Martirosian P, Langanke J, et al. Perfusion MR imaging with FAIR true FISP spin labeling in patients with and without renal artery stenosis: initial experience. Radiology. 2006;238:1013–1021. doi: 10.1148/radiol.2382041623. [DOI] [PubMed] [Google Scholar]
- 17.Martirosian P, Klose U, Mader I, Schick F. FAIR true-FISP perfusion imaging of the kidneys. Magn Reson Med. 2004;51:353–361. doi: 10.1002/mrm.10709. [DOI] [PubMed] [Google Scholar]
- 18.Lanzman RS, Wittsack HJ, Martirosian P, et al. Quantification of renal allograft perfusion using arterial spin labeling MRI: initial results. Eur Radiol. 2010;20:1485–1491. doi: 10.1007/s00330-009-1675-0. [DOI] [PubMed] [Google Scholar]
- 19.Artz NS, Sadowski EA, Wentland AL, et al. Arterial Spin Labeling MRI for Assessment of Perfusion in Native and Transplanted Kidneys. Magn Reson Imaging. doi: 10.1016/j.mri.2010.07.018. 2010; Sept (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karger N, Biederer J, Lusse S, et al. Quantitation of renal perfusion using arterial spin labeling with FAIR-UFLARE. Magn Reson Imaging. 2000;18:641–647. doi: 10.1016/s0730-725x(00)00155-7. [DOI] [PubMed] [Google Scholar]
- 21.Ritt M, Janka R, Schneider MP, et al. Measurement of kidney perfusion by magnetic resonance imaging: comparison of MRI with arterial spin labeling to para-aminohippuric acid plasma clearance in male subjects with metabolic syndrome. Nephrol Dial Transplant. 2010;25:1126–1133. doi: 10.1093/ndt/gfp639. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 23.de Bazelaire CM, Duhamel GD, Rofsky NM, Alsop DC. MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology. 2004;230:652–659. doi: 10.1148/radiol.2303021331. [DOI] [PubMed] [Google Scholar]
- 24.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychological Methods. 1996;1:30–46. [Google Scholar]
- 25.Davison AC, H DV. Bootstrap Methods and their Applications. Cambridge University Press; Cambridge: 1997. [Google Scholar]
- 26.Bland JM, Altman DG. Measurement error. Bmj. 1996;313:744. doi: 10.1136/bmj.313.7059.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strouthidis NG, White ET, Owen VM, Ho TA, Hammond CJ, Garway-Heath DF. Factors affecting the test-retest variability of Heidelberg retina tomograph and Heidelberg retina tomograph II measurements. Br J Ophthalmol. 2005;89:1427–1432. doi: 10.1136/bjo.2005.067298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lerman LO, Flickinger AL, Sheedy PF, 2nd, Turner ST. Reproducibility of human kidney perfusion and volume determinations with electron beam computed tomography. Invest Radiol. 1996;31:204–210. doi: 10.1097/00004424-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Just A, Arendshorst WJ. Dynamics and contribution of mechanisms mediating renal blood flow autoregulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R619–631. doi: 10.1152/ajpregu.00766.2002. [DOI] [PubMed] [Google Scholar]
- 30.Kompanowska-Jezierska E, Walkowska A, Johns EJ, Sadowski J. Early effects of renal denervation in the anaesthetised rat: natriuresis and increased cortical blood flow. J Physiol. 2001;531:527–534. doi: 10.1111/j.1469-7793.2001.0527i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leonard BL, Malpas SC, Denton KM, Madden AC, Evans RG. Differential control of intrarenal blood flow during reflex increases in sympathetic nerve activity. Am J Physiol Regul Integr Comp Physiol. 2001;280:R62–68. doi: 10.1152/ajpregu.2001.280.1.R62. [DOI] [PubMed] [Google Scholar]
- 32.Norman JT, Stidwill R, Singer M, Fine LG. Angiotensin II blockade augments renal cortical microvascular pO2 indicating a novel, potentially renoprotective action. Nephron Physiol. 2003;94:p39–46. doi: 10.1159/000071289. [DOI] [PubMed] [Google Scholar]
- 33.Pallone TL, Zhang Z, Rhinehart K. Physiology of the renal medullary microcirculation. Am J Physiol Renal Physiol. 2003;284:F253–266. doi: 10.1152/ajprenal.00304.2002. [DOI] [PubMed] [Google Scholar]
- 34.Brezis M, Agmon Y, Epstein FH. Determinants of intrarenal oxygenation. I. Effects of diuretics. Am J Physiol. 1994;267:F1059–1062. doi: 10.1152/ajprenal.1994.267.6.F1059. [DOI] [PubMed] [Google Scholar]
- 35.Brezis M, Heyman SN, Epstein FH. Determinants of intrarenal oxygenation. II. Hemodynamic effects. Am J Physiol. 1994;267:F1063–1068. doi: 10.1152/ajprenal.1994.267.6.F1063. [DOI] [PubMed] [Google Scholar]
- 36.Avasthi PS, Greene ER, Voyles WF. Noninvasive Doppler assessment of human postprandial renal blood flow and cardiac output. Am J Physiol. 1987;252:F1167–1174. doi: 10.1152/ajprenal.1987.252.6.F1167. [DOI] [PubMed] [Google Scholar]
- 37.Lee VS, Kaur M, Bokacheva L, et al. What causes diminished corticomedullary differentiation in renal insufficiency? J Magn Reson Imaging. 2007;25:790–795. doi: 10.1002/jmri.20878. [DOI] [PubMed] [Google Scholar]
- 38.Artz NS, Wentland AL, Sadowski EA, et al. Comparing kidney perfusion using non-contrast arterial spin labeling MRI and microsphere methods in an interventional swine model. Invest Radiol. doi: 10.1097/RLI.0b013e3181f5e101. 2010; Nov (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han F, Xiao W, Xu Y, et al. The significance of BOLD MRI in differentiation between renal transplant rejection and acute tubular necrosis. Nephrol Dial Transplant. 2008;23:2666–2672. doi: 10.1093/ndt/gfn064. [DOI] [PubMed] [Google Scholar]
- 40.Sadowski EA, Fain SB, Alford SK, et al. Assessment of acute renal transplant rejection with blood oxygen level-dependent MR imaging: initial experience. Radiology. 2005;236:911–919. doi: 10.1148/radiol.2363041080. [DOI] [PubMed] [Google Scholar]