Abstract
The serine/threonine kinase LKB1 is a tumour suppressor that regulates cell growth, polarity, and proliferation in many different cell types. We previously demonstrated that LKB1 controls thymocyte survival via regulation of AMPK activation. In this study, we show that LKB1 was also involved in thymocyte positive selection through regulation of T cell receptor (TCR) signalling. Both Lck-Cre- and CD4-Cre-mediated deletion of LKB1 impaired the generation of mature CD4 and CD8 single positive (SP) thymocytes that might have resulted from the attenuated tyrosine phosphorylation of phospholipase C-γ 1 (PLCγ1) in the absence of LKB1. We found that LKB1 was directly phosphorylated by Lck at tyrosine residues 36, 261, and 365 and predominately interacted with LAT and PLCγ1 following TCR stimulation. Loss of LKB1 led to impaired recruitment of PLCγ1 to the LAT signalosome. Correlatively, LKB1-deficient thymocytes failed to upregulate lineage-specifying factors, and to differentiate into SP thymocytes even if their impaired survival was rescued. These observations indicated that LKB1 is a critical component involved in TCR signalling, and our studies provide novel insights into the mechanisms of LKB1-mediated thymocyte development.
Keywords: LKB1, PLCγ1, positive selection, TCR signalling, thymocyte
Introduction
Positive selection of αβ T cells is a process that drives the development of immature double-positive (DP) precursors into mature CD4 single positive (SP) or CD8 SP thymocytes in the thymus. Only a small proportion of DP cells expressing a functional T cell receptor (TCR) is capable of recognizing self-peptide in the context of major histocompatibility complex (MHC), resulting in progression towards positive selection (Germain, 2002; Starr et al, 2003; Bosselut, 2004; Singer et al, 2008). The early stages of positive selection are associated with the upregulation of TCR, CD5, and CD69 and the degree of upregulation of these respective markers is indicative of the strength of the signal received by the TCR (Bendelac et al, 1992; Swat et al, 1993; Lucas et al, 1994; Azzam et al, 1998; Starr et al, 2003). Positive selection not only rescues short-lived DP thymocytes from programmed cell death by inducing the expression of interleukin 7 receptor (IL-7R) and the anti-apoptotic Bcl-2 protein, but also promotes their differentiation further into functionally distinct helper CD4 or cytotoxic effector CD8 T cells (Linette et al, 1994; Bhatia et al, 1995; Chao and Korsmeyer, 1998; Munitic et al, 2004). In the later stages of positive selection, the expression of heat stable antigen (HSA; mouse CD24) on the cell surface is downregulated (Ramsdell et al, 1991), and newly matured SP thymocytes emigrate from the thymus into peripheral lymphoid tissues (Yin et al, 2006).
As mentioned above, thymocyte selection involves signals triggered following the engagement of the TCR with peptide–MHC complexes present on antigen-presenting cells (APC) (van Meerwijk and Germain, 1994; Hogquist and Bevan, 1996). The formation of the immunological synapse, a supramolecular activation cluster composed of TCRs, adhesion molecules, and intracellular signalling molecules at the T cell-APC interphase, has a critical role in TCR signal transduction and T cell activation (Werlen and Palmer, 2002; Saito and Yokosuka, 2006; Prasad et al, 2009; Fooksman et al, 2010). Following TCR stimulation, CD3 molecules are phosphorylated at immune-receptor tyrosine-based activation motifs (ITAMs) by the Src family tyrosine kinase Lck. Doubly phosphorylated ITAMs then recruit the Syk family kinase Zap70 that also becomes phosphorylated and activated. Activated Zap70 then phosphorylates the adaptor protein LAT, resulting in the formation of a multimolecular signalosome complex (LAT signalosome) consisting of various components (including phospholipase C-γ 1 (PLCγ1), Grb2, Itk, Vav, GADS, and SLP-76) that surround LAT (Werlen and Palmer, 2002; He et al, 2005a; Braiman et al, 2006; Seminario and Bunnell, 2008; Fu et al, 2010). PLCγ1 and other signalling molecules are then activated, leading to Ca2+ mobilization and activation of multiple pathways (including ERK, JNK, and p38 pathways), which eventually activate specific nuclear factors, respectively, that control thymocyte survival, differentiation, and maturation (Winslow et al, 2003; Ashwell, 2006).
The tumour suppressor LKB1 (also known as STK11) is a serine/threonine kinase that phosphorylates and activates AMPK subfamily members in response to energy stress and the LKB1-AMPK kinase cascade has critical roles in controlling cellular polarity, proliferation, and differentiation (Bardeesy et al, 2002; Woods et al, 2003; Spicer and Ashworth, 2004; Alessi et al, 2006; Ji et al, 2007; Mirouse et al, 2007). Recently, we and others found that LKB1 was required for thymic T cell development. Results from both laboratories showed that Lck-Cre-mediated deletion of LKB1 greatly impaired the generation of both CD4 and CD8 T cells (Tamás et al, 2010; Cao et al, 2010). In addition to defects in β selection, the survival of DP thymocytes is greatly shortened, which may have been caused by attenuated AMPK activation and Bcl-XL expression when LKB1 was deleted (Cao et al, 2010).
In this study, we further show that LKB1 is also involved in DP thymocyte positive selection. In the absence of LKB1, positive selection of DP cells did not proceed appropriately as evidenced by reduced surface expression of CD5, CD69, and TCR. In addition, the maturation of both CD4 and CD8 SP thymocytes was severely impaired defined by their inability to downregulate HSA expression. Intriguingly, among the proximal TCR signalling events affected was the reduction in the PLCγ1 phosphorylation levels observed following TCR ligation in the absence of LKB1. We found that LKB1 was directly phosphorylated by Lck at tyrosine residues 36, 261, and 365 and that these residues were critical to TCR signal transduction. In addition, LKB1 preferentially interacted with LAT and PLCγ1 following TCR engagement, whereas loss of LKB1 led to defects in the recruitment of PLCγ1 to the LAT signalosome following stimulation via the TCR. In the absence of LKB1, thymocytes failed to upregulate lineage-specifying factors ThPOK and Runx3. And more, LKB1-deficient thymocytes were unable to differentiate further into CD4 and CD8 SP cells even if their survival was prolonged by ectopic expression of the anti-apoptotic Bcl-XL protein. These studies demonstrated that LKB1 is a critical TCR signalling component and taken together, these observations revealed a novel activity for LKB1 in intrathymic T cell development through the regulation of PLCγ1 activation.
Results
LKB1 deficiency impairs the generation of mature CD4 and CD8 SP thymocyte
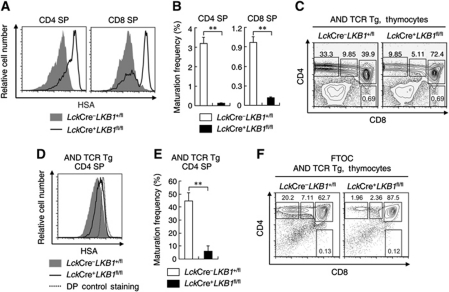
Mice with Lck-Cre-mediated deletion of LKB1 (LckCre+LKB1fl/fl, hereafter referred to as LKB1-deficient mice) had significantly reduced CD4 SP and CD8 SP thymocytes compared with LckCre+LKB1+/fl (heterozygous mutant) and LckCre−LKB1+/fl (non-deletion mutant, hereafter referred to as wild-type) littermates (Cao et al, 2010). In addition, most of these SP cells expressed high levels of an immature thymocyte marker: HSA (mouse CD24) (Figure 1A; Supplementary Figure S1A and B). The strong reduction in HSAlow CD4 SP and CD8 SP thymocyte numbers (compared with DP thymocyte numbers; Figure 1B) suggested that LKB1 is required for SP thymocyte maturation. The impact of LKB1 on thymocyte maturation was further confirmed by introducing the class II-restricted AND TCR transgene into LKB1-deficient mice. The frequencies of AND TCR transgenic CD4 SP cells were significantly decreased in LKB1-deficient cells (Figure 1C) that also failed to downregulate surface HSA expression as observed in the control mice (Figure 1D). Moreover, the maturation frequency of AND TCR transgenic CD4 SP cells from LKB1-deficient mice was significantly lower than those from control mice (Figure 1E) and AND TCR transgenic LKB1-deficient mice failed to develop normal peripheral CD4 T cell populations (Supplementary Figure S2A and B). To further investigate the effect of LKB1 on thymocyte development, fetal thymus organ culture (FTOC) was carried out as previously described (Jones et al, 2000; Anderson and Jenkinson, 2007). As shown in Figure 1F, the CD4 SP population was greatly reduced in AND TCR transgenic mice when LKB1 was ablated.
Figure 1.
LKB1-deficient mice fail to generate mature CD4 and CD8 SP thymocytes. (A) Histograms showing the surface expression of HSA on CD4 and CD8 SP thymocytes from LckCre+LKB1fl/fl mice or littermate controls. (B) Maturation frequencies of CD4 and CD8 SP thymocytes from LckCre+LKB1fl/fl mice or littermate controls were calculated as the number of HSAlow CD4 SP and CD8 SP thymocytes relative to the number of DP thymocytes. Absolute cell numbers of HSAlowCD4 SP, HSAlowCD8 SP, and DP thymocytes were calculated by multiplying total live cell numbers by the fraction of cells in that population. Data are expressed as mean±s.e.m. (n=3) **P<0.01 (Student's t-test). (C) Total thymocytes of AND transgenic LckCre–LKB1+/fl mice or LckCre+LKB1fl/fl mice were analysed for CD4 and CD8 expression by flow cytometry. Numbers next to or within boxes of the respective contour diagrams indicate the percentage of cells examined. (D) Histogram showing surface expression of HSA on AND transgenic LckCre–LKB1+/fl or LckCre+LKB1fl/fl CD4 SP thymocytes. The expression of HSA on AND TCR transgenic LKB1-deficient DP cells was used as control. (E) Maturation frequency of CD4 SP thymocytes from AND transgenic LckCre–LKB1+/fl mice or LckCre+LKB1fl/fl mice was calculated as described above. Data are expressed as mean±s.e.m. (n=4). **P<0.01 (Student's t-test). (F) Fetal thymic lobes were obtained and cultured 6 days for flow cytometric analysis. Harvested thymocytes were stained with anti-CD4, anti-CD8, and propidium iodide for developmental profile analysis. Results are representative of two (F), three (A, B), or four (C, D, E) independent experiments.
Thymocytes fail to undergo normal positive selection in the absence of LKB1
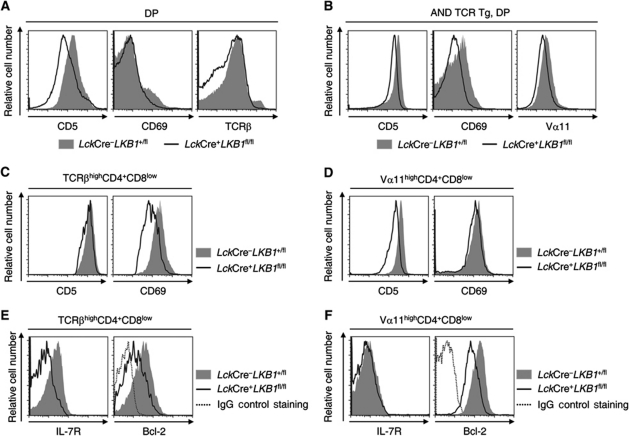
Positive selection of DP thymocytes results from the ligation of MHC–peptide/TCR complexes followed by the upregulation of TCR, CD5, and CD69 (Bendelac et al, 1992; Swat et al, 1993; Lucas et al, 1994; Azzam et al, 1998). Intriguingly, LKB1-deficient mice had significantly fewer TCRhigh, CD5high, and CD69high DP thymocytes (Figure 2A) similar to LKB1-deficient AND TCR transgenic mice (Figure 2B) when compared with littermate controls, respectively. Importantly, the transitional TCRhighCD4+CD8low cells, which include progenitors of both the CD4 and CD8 lineages, did not fully upregulate surface CD69 expression in the absence of LKB1 (Figure 2C). Correlatively, AND TCR transgenic Vα11highCD4+CD8low cells from LKB1-deficient mice failed to express CD5 at a level as that on their controls (Figure 2D). Moreover, LKB1-deficient TCRhighCD4+CD8low and Vα11highCD4+CD8low cells failed to significantly upregulate the expression of either IL-7R or anti-apoptotic Bcl-2 proteins (Figure 2E and F), suggesting that LKB1 was required for them to avoid death by neglect. Taken together, both the initiation and progression of positive selection were severely impacted in the absence of LKB1.
Figure 2.
LKB1-deficient DP thymocytes fail to normally undergo positive selection. Thymocytes from non-TCR transgenic or AND TCR transgenic LckCre+LKB1fl/fl mice and control littermates were stained with antibodies specific for CD4, CD8, and either CD5, CD69, or TCRβ. (A, B) The expression levels of CD5, CD69, and TCRβ (or Vα11) on gated DP thymocytes of the indicated genotypes are shown as histograms. (C, D) The expression levels of surface CD5 or CD69 on gated TCRhighCD4+CD8low or Vα11highCD4+CD8low thymocytes of the indicated genotypes are shown as histograms. (E, F) Thymocytes were stained with antibodies specific for CD4, CD8, TCRβ, and either IL-7R or Bcl-2. The expression levels of surface IL-7R or intracellular Bcl-2 on gated TCRhighCD4+CD8low or Vα11highCD4+CD8low thymocytes of the indicated genotypes are shown as histograms. Results are representative of three (B, D, F) or four (A, C, E) independent experiments.
To further investigate the role of LKB1 in the development of DP thymocytes to SP cells, LKB1fl/fl mice containing the LoxP-flanked gene encoding LKB1 were bred with mice expressing Cre recombinase under the control of the Cd4 promoter (Cd4Cre) to generate Cd4Cre+LKB1fl/fl mice. As expected, attenuated expression of LKB1 mRNA was found at the DP stage but not at the double-negative (DN) stage in Cd4Cre+LKB1fl/fl mice (Supplementary Figure S3A). Cd4Cre+LKB1fl/fl mice failed to generate sufficient CD4 SP and CD8 SP thymocytes compared with littermate controls (Supplementary Figure S3B). Analysis of thymocyte cellularity revealed that the absolute cell numbers of either DP, CD4+CD8low, CD4 SP, or CD8 SP (but not DN cells) were significantly decreased when LKB1 was deleted particularly at the DP stage (Supplementary Figure S3C). The loss of DP thymocytes may be caused by enhanced apoptosis in Cd4Cre+LKB1fl/fl mice (Supplementary Figure S3D), which is consistent with our previous observation that LKB1 was required for the survival of DP thymocytes (Cao et al, 2010). Importantly, neither CD4 SP nor CD8 SP cells from Cd4Cre+LKB1fl/fl mice were observed to have surface HSA downregulation (Supplementary Figure S3E). Moreover, Cd4Cre+LKB1fl/fl mice had lower maturation frequencies of CD4 SP and CD8 SP thymocytes than control animals (Supplementary Figure S3F) and failed to generate normal peripheral CD4 and CD8 T cell populations (Supplementary Figure S3G and H). Consistent with the LKB1-deficient mouse phenotype, Cd4Cre+LKB1fl/fl mice had fewer TCRhigh, CD5high, and CD69high DP thymocytes (Supplementary Figure S3I) and the transitional TCRhighCD4+CD8low cells from Cd4Cre+LKB1fl/fl mice were unable to fully upregulate either CD5, CD69, IL-7R, or Bcl-2 (Supplementary Figure S3J and K), indicating that LKB1 is involved in thymocyte positive selection.
Loss of LKB1 impairs PLCγ1 phosphorylation and calcium flux
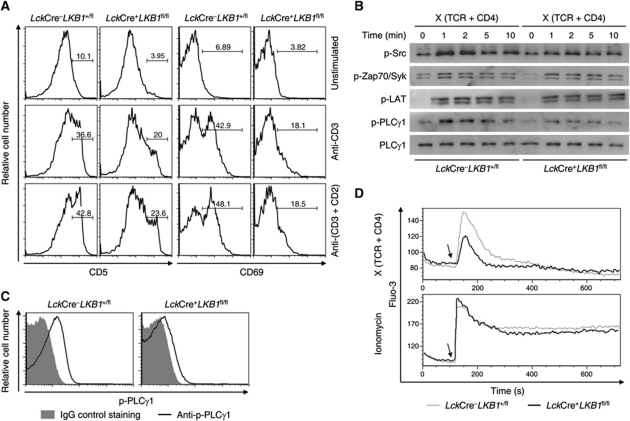
The lack of CD5high and CD69high thymocytes in LckCre+LKB1fl/fl mice provided a hint that LKB1-deficient thymocytes might not be able to receive either appropriate signalling or sufficiently strong signals via their TCRs. To assess the influence of LKB1 on TCR signalling, DP thymocytes were stimulated in vitro with immobilized either anti-CD3 or anti-CD3 and anti-CD2 antibodies as described (Cibotti et al, 1997). TCR stimulated wild-type DP thymocytes presented with significantly upregulated CD5 and CD69 levels compared with LKB1-deficient cells that did not upregulate either CD5 or CD69 (Figure 3A).
Figure 3.
PLCγ1 phosphorylation and calcium flux are impaired in the absence of LKB1. (A) Thymocytes from LckCre+LKB1fl/fl mice or littermate controls were unstimulated or stimulated with plate-bound anti-CD3 (5 μg/ml) or anti-CD3 (5 μg/ml) and anti-CD2 (5 μg/ml) antibodies for 16 h. After collection, cells were stained with surface markers in combination with propidium iodide and gated live cells (over 30%) were analysed for CD5 and CD69 expression. Numbers above the brackets indicate the percentage of CD5high or CD69high cells. (B) Sorted DP thymocytes from LckCre+LKB1fl/fl mice or littermate controls were labelled with biotinylated anti-TCR (10 μg/ml) and anti-CD4 (10 μg/ml) and were then cross-linked with streptavidin for the indicated times or left untreated (0 min). Total lysates from these cells were subjected to SDS–PAGE and analysed with antibodies against the tyrosine phosphorylation form of Src, Zap70, LAT, or PLCγ1, respectively. Expression of total PLCγ1 served as a loading control. (C) Sorted DP thymocytes from LckCre+LKB1fl/fl mice or littermate controls were stimulated as described above and then analysed for intracellular PLCγ1 phosphorylation. (D) Thymocytes harvested from LckCre+LKB1fl/fl mice or littermate controls were loaded with Fluo-3 and surface stained with anti-CD8, biotinylated anti-TCR, and anti-CD4. After cross-linked with streptavidin (top) or stimulated with ionomycin (bottom), gated CD8 positive thymocytes were analysed for Ca2+ mobilization. Arrows indicate the time points when streptavidin or ionomycin were added. Results are representative of three (A–C) or four (D) independent experiments.
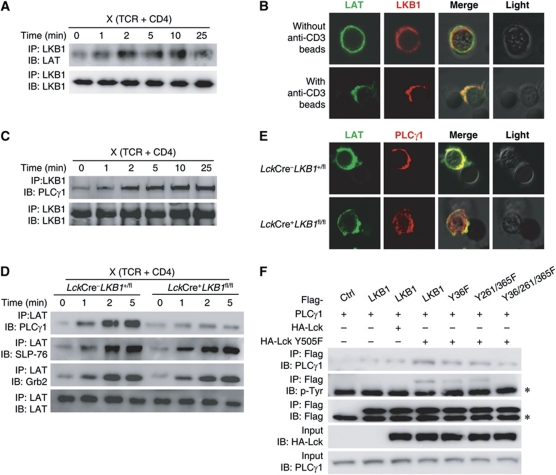
To investigate the role of LKB1 in TCR signal transduction in greater detail, the activation of several critical downstream targets affected by TCR-mediated signalling was screened following TCR stimulation. We first examined whether transient activation components associated with the TCR signal initiation machinery were altered. These results showed that similar phosphorylation levels of Src family kinases and Zap70 were present independent of LKB1 (Figure 3B; Supplementary Figure S4). Further analysis revealed that loss of LKB1 weakened TCR-induced PLCγ1 phosphorylation, but not phosphorylation of the LAT adaptor (Figure 3B; Supplementary Figure S4). Intracellular staining further confirmed that LKB1 was required for complete PLCγ1 phosphorylation following TCR stimulation (Figure 3C). Consistent with the attenuated tyrosine phosphorylation of PLCγ1, the TCR-induced calcium flux was also markedly reduced in LKB1-deficient DP thymocytes compared with DP thymocytes from wild-type mice. The differences were no longer observed when these two thymocyte populations were treated with ionomycin (a calcium ionophore that directly induces intracellular calcium ion mobilization) (Figure 3D). Therefore, loss of LKB1 specifically disrupted PLCγ1 activation and calcium signalling. Correlatively, the expression levels of CD5 were identical between LKB1-deficient and control thymocytes following stimulation with PMA and ionomycin (data not shown).
LKB1 is a substrate for the tyrosine kinase Lck
The above observations suggested that LKB1 was a downstream target associated with TCR signalling and, in fact, LKB1 was tyrosine phosphorylated in DP thymocytes following TCR stimulation (Figure 4A). Since it has been shown that the Src family tyrosine kinase Fyn could phosphorylate LKB1 in adipose tissues (Yamada et al, 2010), we examined whether LKB1 could serve as a downstream target of Lck, the main Src family kinase associated with the TCR signalling pathway. As shown in Supplementary Figure S5 and Figure 4A, LKB1 tyrosine phosphorylation was upregulated following TCR stimulation and this upregulation was markedly diminished in the presence of a widely used Lck-specific inhibitor (Burchat et al, 2000; Treanor et al, 2006). Interestingly, either overexpressed or endogenous LKB1 associated with Lck (Figure 4B and C). Moreover, the constitutive active form of Lck (Lck Y505F), but not the wild-type Lck, was able to phosphorylate LKB1 on tyrosine residues (Figure 4D). In vitro phosphorylation assays further revealed that Lck Y505F directly phosphorylated LKB1 (Figure 4E), demonstrating that LKB1 served as a substrate for the Lck tyrosine kinase and that it was an essential early component associated with TCR signalling.
Figure 4.
LKB1 is tyrosine phosphorylated following TCR signal stimulation. (A) Sorted DP thymocytes were treated with or without the Lck inhibitor (5 μM) and stimulated as described above. LKB1 was subsequently precipitated by anti-LKB1 antibodies. The immunoprecipitated LKB1 protein was analysed for tyrosine phosphorylation levels and precipitated LKB1 served as a loading control. (B) 293T cells were transfected with plasmids encoding LKB1 alone or co-transfected with Lck Y505F and harvested 36 h later. Lysates were precipitated with anti-Flag beads and immunoprecipitated proteins were immunoblotted with the indicated antibodies. The asterisk indicates the IgG heavy chain. (C) Thymocyte lysates were incubated with control IgG or anti-LKB1 antibodies and precipitated with protein A/G-agarose beads. The immunoprecipitated proteins were immunoblotted using the indicated antibodies. (D) 293T cells were transfected with plasmids encoding LKB1 alone or co-transfected with Lck or Lck Y505F and harvested for 36 h. Lysates were precipitated with anti-Flag beads and the immunoprecipitated proteins immunoblotted with the indicated antibodies. The asterisks indicate the IgG heavy chain. (E) His-tagged LKB1 was used for the in vitro kinase assay with His-tagged Lck Y505F. Purified His-tagged LKB1 was incubated with or without purified His-tagged Lck Y505F in Src kinase assay buffer. Tyrosine phosphorylation levels of LKB1 were analysed by immunobloting. All results are representative of three independent experiments.
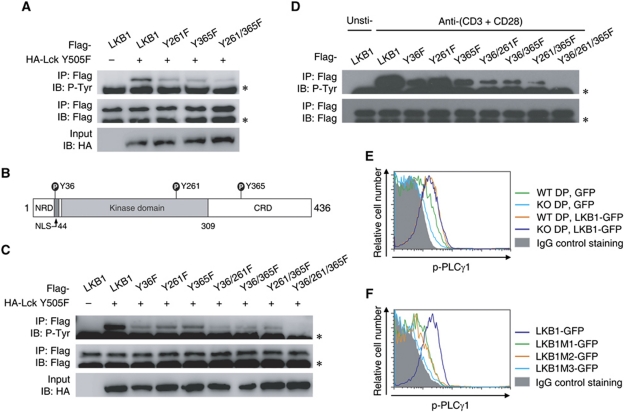
Lck phosphorylates LKB1 at tyrosine residues 36, 261, and 365
Given that Fyn phosphorylates LKB1 on tyrosine residues 261 and 365 (Yamada et al, 2010), we first tried to verify whether these two sites were also phosphorylation sites for Lck. Mutation of either tyrosine 261 or tyrosine 365 attenuated LKB1 phosphorylation levels, suggesting that both were Lck phosphorylation sites (Figure 5A). However, LKB1 phosphorylation was still detected when these two sites were both mutated (Figure 5A), indicating that other candidate phosphorylation sites on LKB1 still existed. Mass spectrometric analysis used to detect LKB1 phosphorylation sites was then performed, and the N-terminal tyrosine 36 was identified as a novel phosphorylation site (Figure 5B; Supplementary Figure S6). A single mutation of tyrosine 36 reduced LKB1 phosphorylation levels, further confirming that tyrosine 36 served as a target for Lck-mediated phosphorylation (Figure 5C). Notably, tyrosine 36 did not seem to be a phosphorylation site for Fyn (Supplementary Figure S7). Only the triple mutant but not any double mutant combinations consisting of tyrosines 36, 261, and 365 abolished LKB1 tyrosine phosphorylation (Figure 5C). These data suggested that LKB1 tyrosine residues 36, 261, and 365 represented the major tyrosine phosphorylation sites for Lck kinase. Importantly, all the single mutants and the double mutants attenuated LKB1 tyrosine phosphorylation following TCR stimulation (Figure 5D). The dramatic decrease in tyrosine phosphorylation of the triple mutant suggested that these residues represented bona fide phosphorylation sites of LKB1 in response to TCR ligation (Figure 5D).
Figure 5.
Identification of tyrosine phosphorylation sites of LKB1. (A) The phosphorylation levels of LKB1 and the respective site-directed mutants were examined as described above. (B) Schematic representation of the phosphorylation sites of the mouse LKB1 protein. (C) The phosphorylation levels of LKB1 mutants were examined as described above. The asterisk indicates the IgG heavy chain. (D) Jurkat cells were electroporated with plasmids expressing Flag-LKB1 or its indicated mutants and stimulated by plate-bound anti-human CD3 (5 μg/ml) and anti-human CD28 (5 μg/ml) for 10 min. Cells were harvested and precipitated with anti-Flag beads, and immunoprecipitated proteins were immunoblotted with the indicated antibodies. The asterisk indicates the IgG heavy chain. (E, F) Thymocytes from LckCre+LKB1fl/fl mice or littermate controls were transfected with plasmids expressing GFP, GFP-tagged LKB1, or GFP-tagged LKB1 mutants (M1, Y36F; M2, Y261/365F; M3, Y36/261/365F), respectively. Cells were cultured for 8 h and then cross-linked for 2 min as described above. The phosphorylation levels of PLCγ1 present in DP cells were detected by intracellular staining with anti-phospho-PLCγ1 or control IgG. All results are representative of three independent experiments.
Before examining the role of these tyrosine sites in the activation of PLCγ1, an in vitro electroporation transfection assay was set up for rescue analysis. As shown in Figure 5E, ectopic expression of GFP-tagged LKB1 (LKB1-GFP) enhanced PLCγ1 phosphorylation in wild-type DP thymocytes following TCR stimulation. Importantly, overexpression of LKB1-GFP but not of GFP efficiently improved PLCγ1 phosphorylation in LKB1-deficient DP thymocytes. The mutant forms of LKB1 were then used for rescue analysis. Both the single mutant (Y36F, LKB1M1-GFP) and the double mutant (Y261/365F, LKB1M2-GFP) partially rescued PLCγ1 phosphorylation in LKB1-deficient thymocytes following TCR stimulation, but neither mutant fully restored PLCγ1 phosphorylation compared with wild-type LKB1. PLCγ1 phosphorylation in the triple mutant (Y36/261/365/F, LKB1M3-GFP) could not be detected (Figure 5F). Taken together, these results demonstrated that these three LKB1 tyrosine residues are indispensable for PLCγ1 activation.
LKB1 promotes PLCγ1 recruitment to the LAT signalosome
Tyrosine phosphorylation of PLCγ1 is mediated by Itk within the LAT signalosome following TCR stimulation (Min et al, 2009). Considering that LKB1 is a bona fide serine/threonine kinase (but not a tyrosine kinase), it is unlikely that LKB1 directly phosphorylates PLCγ1 or its upstream kinases. For this reason, we speculated that the impact of LKB1 on PLCγ1 phosphorylation was to promote the recruitment of PLCγ1 to the LAT signalosome as a scaffold protein. As shown in Figure 6A, LKB1 predominately associated with LAT and colocalized with LAT only after the TCR was engaged (Figure 6B). Further co-immunoprecipitation analysis showed that PLCγ1 associated with LKB1 and that this association increased with prolonged TCR stimulation (Figure 6C). Importantly, TCR-induced association of LAT with PLCγ1, but not with Grb2 or SLP-76, was significantly decreased in the absence of LKB1 (Figure 6D). And the recruitment of PLCγ1 to the LAT signalosome was impaired in the absence of LKB1 (Figure 6E). Intriguingly, the association between LKB1 and PLCγ1 positively correlated with the phosphorylation status of the LKB1 tyrosine residues 36, 261, and 365. The amount of co-immunoprecipitated PLCγ1 was highest when wild-type LKB1 was used, intermediate when LKB1M1 or LKB1M2 were used and lowest in the presence of LKB1M3 (Figure 6F). Together, these observations suggested that TCR engagement leads to the recruitment of LKB1 to the LAT signalosome that in turn enhances the recruitment of PLCγ1 to LAT.
Figure 6.
LKB1 deficiency abrogates the recruitment of PLCγ1 to the LAT signalosome. (A) The interaction between LKB1 and LAT was enhanced following TCR stimulation. Thymocytes were cross-linked as described above and the total cell lysates were precipitated with anti-LKB1 antibody. The immunoprecipitated proteins were immunoblotted using the indicated antibodies. (B) Colocalization of LKB1 with LAT upon TCR stimulation. Sorted T lymphocytes were incubated with anti-CD3-coated Dynabeads, stained with anti-LAT and anti-LKB1 antibodies and imaged using confocal microscopy (magnification, × 630). Images are representative of 99/100 (control) or 90/100 (stimulated) counted cells. (C) The interaction of LKB1 and PLCγ1 was enhanced following TCR stimulation. Thymocytes were cross-linked and the total cell lysates were precipitated with anti-LKB1 as described above. The immunoprecipitated proteins were immunoblotted using the indicated antibodies. (D) Sorted DP thymocytes from LckCre+LKB1fl/fl mice or littermate controls were cross-linked or left untreated as described above and the total cell lysates were precipitated with anti-LAT antibodies. Immunoprecipitated proteins were immunoblotted using the indicated antibodies. (E) The recruitment of PLCγ1 into LAT signalosome upon TCR stimulation. Sorted CD4 SP thymocytes from LckCre+LKB1fl/fl mice or littermate controls were incubated with anti-CD3-coated Dynabeads, stained with anti-LAT and anti-PLCγ1 antibodies, and imaged using confocal microscopy (magnification × 630). Images are representative of 94/100 (wild-type) or 88/100 (LKB1-deficient) counted cells. (F) 293T cells were co-transfected with the indicated plasmids, respectively. Immunoprecipitated proteins were immunoblotted using the indicated antibodies. The asterisk indicates the IgG heavy chain. All results are representative of three independent experiments.
LKB1-deficient thymocytes fail to upregulate lineage-specifying factors
Given that thymocyte positive selection is promoted by TCR-induced gene transcription (Collins et al, 2009) and LKB1-deficient mice failed to generate mature CD4 SP and CD8 SP thymocytes, we finally asked whether LKB1 deletion affects the upregulation of CD4/CD8 lineage-specifying factors, such as ThPOK (which mediates the development of DP thymocytes to the CD4 lineage) (Sun et al, 2005; He et al, 2005b) and Runx3 (which is important for CD8 lineage differentiation) (Sato et al, 2005; Collins et al, 2009). As shown in Figure 7A, the transitional CD4+CD8low cells failed to retain the expression of ThPOK and Runx3 in the absence of LKB1. Sorted CD69low/− DP thymocytes were stimulated with anti-CD3 and anti-CD2 antibodies at various time intervals and real-time RT–PCR analysis was then performed to characterize the transcription status of the above factors. Wild-type thymocytes substantially upregulated the mRNA levels of both ThPOK and Runx3. In contrast, LKB1-deficient thymocytes failed to initiate such a transcriptional programme (Figure 7B). These observations indicated that the triggering of lineage-specific gene transcription depends on LKB1-mediated TCR signals. We thus speculated that LKB1-deficient thymocytes may still be unable to differentiate to the SP stage even if their impaired survival was rescued. To this end, FTOC experiments were performed. The viability of LKB1-deficient thymocytes was enhanced by ectopic expression of the anti-apoptotic Bcl-XL protein (Figure 7C); however, these cells could not differentiate further into CD4 SP and CD8 SP cells (Figure 7D).
Figure 7.
LKB1-deficient DP thymocytes fail to upregulate transcription of lineage-specifying factors. (A) Real-time RT–PCR was used to compare the relative mRNA expression of sorted CD69highCD4+CD8low intermediate thymocytes. Results are presented as mean±s.e.m. (n=4). ***P<0.001 (Student's t-test). (B) Sorted CD69low/− DP thymocytes were left unstimulated (0 h) or stimulated with plate-bound anti-CD3 (2 μg/ml) and anti-CD2 (2 μg/ml) antibodies for indicated time points. Real-time RT–PCR was used to compare the relative mRNA expression of lineage-specifying genes. The percentage of live cells from both LKB1-deficient and wild-type thymocytes was over 87% (4 h), 85% (6 h), and 78% (8 h). Results are presented as mean±s.e.m. (n=4). *P<0.05; **P<0.01; ***P<0.001 (Student's t-test). (C, D) Flow cytometric analysis of Annexin V and propidium iodide (C) or CD4 and CD8 expression (D) on FTOC infected with retroviral constructs. Fetal thymic lobes were infected with retroviruses containing empty retroviral vector or Bcl-XL and then cultured 6 days for flow cytometric analysis. Data are from one representative of three (B, C, D) or four (A) independent experiments.
Discussion
The serine/threonine kinase LKB1 primarily acts as an upstream kinase that phosphorylates threonine residue 172 of the energy sensor AMPK, resulting in an inhibition of cellular energy expenditure, thereby regulating cell survival, growth, differentiation, and proliferation (Woods et al, 2003; Shaw et al, 2004; Alessi et al, 2006; Han et al, 2006; Ji et al, 2007). Similarly, we found that LKB1 controlled the survival of DP thymocytes by regulating AMPK activation and Bcl-XL expression (Cao et al, 2010). Here, we report that by regulating the activation of PLCγ1, LKB1 also controlled thymocyte positive selection. After LKB1 was phosphorylated by the Src family tyrosine kinase Lck at tyrosine residues 36, 261, and 365, activated LKB1 promoted the recruitment of PLCγ1 into the LAT signalosome, thereby regulating TCR signalling and thymocyte positive selection.
LKB1 has been reported to be phosphorylated at several serine (Ser 31, Ser 325, Ser 431, and Ser 404) or threonine (Thr 185, Thr 189, Thr 336, and Thr 366) residues by kinases such as PKA, RSK, or ATM or in some circumstances via auto-phosphorylation (Sapkota et al, 2001, 2002; Alessi et al, 2006). A recent study showed that an Src family tyrosine kinase (Fyn) regulated energy expenditure by phosphorylating LKB1 at tyrosine residues 261 and 365 in adipose tissues, thereby accommodating energy demands via AMPK regulation (Yamada et al, 2010). In this study, we unexpectedly found that the Lck kinase phosphorylated LKB1 at tyrosine residues 36, 261, and 365 and that all of these tyrosine residues were required for optimal TCR signal transduction since mutation of any one of these residues (into phenylalanine) failed to fully activate PLCγ1 following TCR stimulation. Notably, this kind of post-translational modification on LKB1 occurred in response to an external signal, an observation never before reported.
An interesting feature revealed in the present study was that LKB1 had a non-redundant role in the activation of PLCγ1. LKB1 deficiency led to markedly reduced tyrosine phosphorylation of PLCγ1 following TCR stimulation, and consequently, calcium ion mobilization was greatly impaired. However, it is unlikely that LKB1 directly phosphorylated PLCγ1 at Tyr residues and PLCγ1 was unlikely to serve as a substrate for LKB1 (Alessi et al, 2006; Mirouse et al, 2007). It has been reported that PLCγ1 was activated in a two-step manner (Veri et al, 2001). That is, PLCγ1 was first recruited to the LAT signalosome and then phosphorylated by upstream kinases. Moreover, unlike other cells where LKB1 is primarily localized to the nucleus (Smith et al, 1999; Alessi et al, 2006; Yamada et al, 2010), LKB1 localized to the cytoplasm of thymocytes (data not shown). These strongly supported the possibility that LKB1 may serve as a scaffold protein facilitating the recruitment of PLCγ1. In fact, TCR stimulation enhanced the interaction of LKB1 with both PLCγ1 and LAT, and LKB1 deficiency impacted the enhanced recruitment of PLCγ1 to the LAT signalosome. These observations suggested that LKB1 was a critical component associated with TCR signalling pathways. Taken together, we concluded that following TCR stimulation, activated Lck phosphorylated LKB1 at tyrosine residues 36, 261, and 365, thereby promoting the recruitment of PLCγ1 to the LAT signalosome facilitating efficient phosphorylation of PLCγ1 by Itk, an upstream PLCγ1 kinase that also localized within the LAT signalosome.
Given that LKB1 is required for PLCγ1 activation and TCR signal transduction, it is understandable why LKB1-deficient DP thymocytes failed to further differentiate into mature CD4 SP and CD8 SP thymocytes, although other possibilities cannot be excluded, for example metabolic defect of SP thymocytes in the absence of LKB1. LKB1-deficient thymocytes not only failed to fully upregulate TCR-induced transcription of CD5 and CD69 surface molecules, they were also unable to upregulate lineage-specifying factors upon TCR stimulation. One may speculate that the impaired generation of mature SP thymocytes could be the result of decreased thymocyte survival since LKB1/AMPK signalling controls Bcl-XL expression. Interestingly, LKB1-deficient thymocytes were still unable to differentiate further into CD4 and CD8 SP cells even if their survival was restored by ectopic expression of Bcl-XL protein. Thus, it is possible that the failure in the generation of SP cells in LKB1-deficient mice was, at least partially, due to impaired PLCγ1 activation, which is consistent with a recent report that PLCγ1 is required for thymocyte maturation (Fu et al, 2010). Notably, defects in PLCγ1 activation could also be an important reason why β selection was also impaired following LKB1 deletion as observed by us and others (Cao et al, 2010; Tamás et al, 2010). Taken together, LKB1 appeared to execute multiple functions during thymic T cell development by activating distinct pathways that (1) regulated thymocyte survival via LKB1/AMPK signalling pathways (Cao et al, 2010) and (2) controlled thymocyte positive selection via LKB1/PLCγ1/calcium signalling pathways.
Considering the high energy demand associated with TCR activation (Fox et al, 2005), it is probably beneficial to the efficacy of the immune responses that TCR signal transduction pathways lead to the activation of AMPK prior to ATP depletion. Intriguingly, Lck-mediated LKB1 phosphorylation at tyrosine residues 36, 261, and 365 may fulfil this requirement since tyrosine phosphorylation of these three residues efficiently resulted in TCR signal transduction by recruiting PLCγ1, and meanwhile, phosphorylation of tyrosine residues 261 and 365 led to regulation of AMPK activation (Yamada et al, 2010), resulting in a repression of energy-consuming processes and simultaneously enhancing energy-generating processes. Therefore, LKB1 is critical for T lineage cells not only to avoid cell death from energy- or nutrient-related stress, but also to avoid death by energy exhaust following continuous or high intensity TCR engagement.
Materials and methods
Mice
C57BL/6 and AND TCR transgenic mice were maintained in the Shanghai Laboratory Animal Center (Chinese Academy of Sciences). The Lkb1 floxed alleles, the LckCre, and the Cd4Cre mouse strains have been described previously (Bardeesy et al, 2002; Pan et al, 2002; Dong et al, 2008). LckCre or Cd4Cre mice were crossed with LKB1fl/fl mice to generate LckCre+LKB1fl/fl or Cd4Cre+LKB1fl/fl mice. Mice aged 4–8 weeks were used for further analysis. All mice were maintained in a specific pathogen-free (SPF) facility and were genotyped by PCR analysis of genomic DNA. All animal experiments were in compliance with National Institutes of Health Guidelines and were approved by the institutional animal care and use committee of the Shanghai Institutes for Biological Sciences (Chinese Academy of Sciences).
Antibodies and reagents
The following monoclonal antibodies used for cell staining and sorting were purchased from BD PharMingen (San Jose, CA): anti-CD4 (GK1.5 and RM4-4), anti-CD8α (53-6.7), anti-TCRβ (H57-597), anti-TCR Vα11 (RR8-1), anti-CD5 (53-7.3), anti-CD69 (H1.2F3), anti-CD2 (RM2-5), anti-CD3 (145-2C11), anti-HSA (M1/69), anti-IL-7R (SB/199), and anti-Bcl-2 (3F11). Anti-phospho-Zap70 (Y319), anti-LKB1 (M-18, for immunoprecipitation), anti-PLCγ1 (E-12, for immunofluorescence microscopy), and Lck inhibitor (7-cyclopentyl-5-(4-phenoxyphenyl)-7H-pyrrolo [2,3-d] pyrimidin-4-ylamine, sc-204052) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phospho-PLCγ1 (Y783), anti-PLCγ1, anti-phospho-Src (Y319), anti-Grb2, anti-SLP-76, anti-LKB1 (27D10, for immunoblot analysis), and anti-phospho-tyrosine (P-Tyr-100) were purchased from Cell Signaling Technology (Danvers, MA). Anti-phospho-LAT (Y136) was from Abcam (Cambridge, UK). Anti-human CD3 (UCHT1) and anti-human CD28 (CD28.2) were from Biolegend (San Diego, CA).
Cell preparation, staining, and purification
Single-cell thymocyte or lymphocyte suspensions were prepared and surface stained as described (Wang et al, 2008). Cell fluorescence was acquired using a two-laser FACSCalibur (BD Biosciences) flowcytometer and data were analysed with FlowJo software (TreeStar, Inc., Olten, Switzerland). Intracellular staining was carried out as described previously (Liu et al, 2003). Thymocyte subsets were purified by magnetic depletion (Miltenyi Biotec, Bergisch Gladbach, Germany) or by an FACSAria II (BD Biosciences). Cell purity assessed by surface staining and flow cytometry was over 90%.
Cell culture and transfection
The human 293T cell line (American Type Culture Collection, Manassas, VA) was maintained in complete DMEM (Invitrogen, Carlsbad, CA) and transfected by calcium phosphate precipitation. Thymocytes were electroporated as previously described (Bell et al, 2001; Cao et al, 2010). Thymocytes (1 × 107) suspended in 250 μl RPMI 1640 medium mixed with plasmids encoding for GFP, GFP-LKB1, or GFP-LKB1 mutants, respectively, were electroporated using a square wave electroporator (Gene pulser Xcell, Bio-Rad Laboratories, Hercules, CA) using the following settings: pulse length=20 ms, pulses=1 and 4 mm, Gap cuvettes and a voltage=360 V. After culture for 8 h, cells were harvested and used for additional analysis. Jurkat cells were electroporated at 250 V and 950 μF.
Thymocyte stimulation
TCR cross-linking was carried out as previously described (Liu et al, 2003). Briefly, thymocytes were stained with anti-CD4 (RM4-4) and anti-CD8 for FACS sorting. Freshly isolated DP cells were resuspended in RPMI 1640 medium supplemented with 1 mM Na3VO4 (Sigma, St Louis, MO) and surface stained with biotinylated anti-TCR (10 μg/ml) and anti-CD4 (10 μg/ml, GK1.5) on ice for 15 min. Cells were cross-linked with 20 μg/ml streptavidin (Sigma) at various time intervals at 37°C. Samples were subjected to further analysis including TCR stimulation through plate-bound antibodies as described previously by coating tissue culture plates with anti-CD3 or with anti-CD3 and anti-CD2 with indicated concentration in phosphate-buffered saline (PBS) at 4°C overnight (Cibotti et al, 1997). Prepared cells were resuspended at a concentration of 5 × 105/ml in complete RPMI 1640 medium and added to respective wells. Stimulations were performed in a cell culture incubator at 37°C under 5% CO2. PMA (10 ng/ml)+ionomycin (500 ng/ml) stimulation was carried out as previously described.
Calcium flux assay
The calcium flux assay was done as previously described (Liu et al, 2003). Thymocytes were preloaded with Fluo-3 AM (Molecular Probes, Invitrogen) for 30 min at 37°C in the dark and then stained with anti-CD8, biotinylated anti-TCR (10 μg/ml), and anti-CD4 (10 μg/ml) antibodies. Cells were washed and resuspended in HBSS, 2% fetal calf serum (FCS). After the cells were warmed to 37°C, baseline Ca2+ levels were measured for 2 min at quiescence. After addition of streptavidin (5 μg/ml), Ca2+ mobility was measured by Fluo-3 AM fluorescence for 10 min. An FACSAria II was used for data collection.
Real-time RT–PCR
RNA was extracted and quantified as described (Wang et al, 2008). RNA was reverse transcribed to cDNA with SuperScript III First-Strand kit (Invitrogen). The mRNA level of LKB1 was assessed relative to Hprt mRNA level by real-time PCR (Rotor gene 6000; Corbett Life Sciences, Sydney, Australia) with SYBR Green real-time PCR Master Mix (Toyobo Co., Osaka, Japan). The data shown were relative values. Primers used for real-time PCR were as follows: Hprt (5′-CCTGCTGGATTACATTAAAGCACTG-3′; 5′-TTCAACACTTCGAGAGGTCCT-3′); LKB1 (5′-TCAAGGTGGACATCTGGT-3′; 5′-TGGTGGGATAGGTACGAG-3′); ThPOK (5′-CTGCTCGGCTACTGGAAAT-3′; 5′-CTTCTTCGTAGGTCAAGGGAT-3′); Runx3 (5′-GGTTCAACGACCTTCGATTC-3′; 5′-GGTCCATCCACAGTGACCTT-3′).
Immunoprecipitation and immunoblot analysis
For immunoprecipitation, cells were lysed in Triton X-100 lysis buffer (10 mM Tris–HCl, pH 7.4, 150, or 400 mM NaCl, 50 mM NaF, 1 mM EDTA, 10% glycerol, 1% Triton X-100, 2 mM Na orthovanadate, and protease inhibitor cocktail). Supernatants were incubated with the indicated antibodies and then immunoprecipitated using protein A/G sepharose beads (Santa Cruz Biotechnology). Samples were subjected to immunoblot analysis with indicated antibodies.
In vitro phosphorylation assay
In vitro phosphorylation assays were carried out as previously described (Yamada et al, 2010). His-tagged Lck-Y505F mutant and the LKB1 fusion protein were purified, respectively, using Ni-NTA agarose beads (Qiagen, Valencia, CA). His-LKB1 protein (1 μg) and His-Lck-Y505F mutant (1 μg) were incubated in the presence of an Src Mg/ATP cocktail for 1 h at 35°C. Samples were separated on a 10% sodium dodecyl sulphate polyacrylamide gels (SDS–PAGE) and immunobloting was performed with the indicated antibodies as previously described (Wang et al, 2008).
Immunofluorescence microscopy
Immunofluorescence analysis was carried out as previously described (Park et al, 2009). Briefly, sorted CD4 SP thymocytes or Dynabead-purified primary T cells were incubated with Dynabeads (Invitrogen) coated with anti-CD3 at a ratio of 1:1. After the incubation, T cell-conjugated Dynabeads were plated on poly-L-lysine-coated coverslips for 20 min at 37°C. Cells were then fixed with 4% (wt/vol) paraformaldehyde for 15 min at 25°C and permeabilized with permeabilization buffer (0.1% (vol/vol) Triton X-100 and 5% (vol/vol) FCS in PBS, pH 7.5) for 10 min at 25°C. Cells were incubated with anti-LAT and anti-LKB1 (M-18) or anti-PLCγ1 (E-12) at 4°C overnight and then incubated with donkey or goat secondary antibodies conjugated to Alexa Fluor 488 or Cy3, respectively, under similar conditions. Slides were mounted with Poly-Mount (Polysciences, Eppelheim, Germany) after washing. Images were acquired using a TCS SP2 confocal microscope.
Retroviral transduction and FTOC
The Bcl-XL gene was cloned into pMX-IRES-EGFP vector. Retroviral supernatants were collected from cultured medium 60 h after transfection as described previously (Crompton et al, 1996; Hozumi et al, 2000).
Retroviral transduction in FTOC was performed as described (Crompton et al, 1996; Hozumi et al, 2000; Anderson and Jenkinson, 2007). Thymic lobes obtained from E15 murine fetus were incubated in Terasaki wells containing 20 μl medium with retrovirus (over 106 particles per millilitre) expressing GFP or Bcl-XL for 24 h. Lobes were then transferred to nucleopore filers (Millipore) and incubated for 6 days in complete medium containing 20% FBS.
Statistics
All experiments described were performed three or more times. Average values were expressed as mean±s.e.m. The Student's t-test was used for the comparison of two independent groups. For all tests, a P-value <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank X Wu (Fudan University, Shanghai, China) for the LckCre mouse and K Wong (Dana-Farber Cancer Institute, MA) for the LKB1fl/fl mouse. We are grateful to our colleagues B Liu for FTOC analysis, F Liu for animal husbandry, W Bian for cell sorting, and X Wang for real-time PCR analysis. This research was supported in part by the National Natural Science Foundation of China (30872290, 30925031), the Ministry of Science and Technology (2007CB815802, 2007CB914504, 2009ZX10004-105), and Shanghai Municipal Government (09QH1402500).
Author contributions: YC, HLi, and XL designed the experiments. YC, HLi, HLiu, and MZ performed all the experiments. YC, HLi, and XL analysed the data and wrote the manuscript. ZH and HJ provided important tools and reagents. XL coordinated the study and oversaw the research.
Footnotes
The authors declare that they have no conflict of interest.
References
- Alessi DR, Sakamoto K, Bayascas JR (2006) LKB1-dependent signaling pathways. Annu Rev Biochem 75: 137–163 [DOI] [PubMed] [Google Scholar]
- Anderson G, Jenkinson EJ (2007) Investigating central tolerance with reaggregate thymus organ cultures. Methods Mol Biol 380: 185–196 [DOI] [PubMed] [Google Scholar]
- Ashwell JD (2006) The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat Rev Immunol 6: 532–540 [DOI] [PubMed] [Google Scholar]
- Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE (1998) CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med 188: 2301–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeesy N, Sinha M, Hezel AF, Signoretti S, Hathaway NA, Sharpless NE, Loda M, Carrasco DR, DePinho RA (2002) Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature 419: 162–167 [DOI] [PubMed] [Google Scholar]
- Bell MP, Huntoon CJ, Graham D, McKean DJ (2001) The analysis of costimulatory receptor signaling cascades in normal T lymphocytes using in vitro gene transfer and reporter gene analysis. Nat Med 7: 1155–1158 [DOI] [PubMed] [Google Scholar]
- Bendelac A, Matzinger P, Seder RA, Paul WE, Schwartz RH (1992) Activation events during thymic selection. J Exp Med 175: 731–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia SK, Tygrett LT, Grabstein KH, Waldschmidt TJ (1995) The effect of in vivo IL-7 deprivation on T cell maturation. J Exp Med 181: 1399–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosselut R (2004) CD4/CD8-lineage differentiation in the thymus: from nuclear effectors to membrane signals. Nat Rev Immunol 4: 529–540 [DOI] [PubMed] [Google Scholar]
- Braiman A, Barda-Saad M, Sommers CL, Samelson LE (2006) Recruitment and activation of PLC gamma 1 in T cells: a new insight into old domains. EMBO J 25: 774–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchat AF, Calderwood DJ, Hirst GC, Holman NJ, Johnston DN, Munschauer R, Rafferty P, Tometzki GB (2000) Pyrrolo[2,3-d]pyrimidines containing an extended 5-substituent as potent and selective inhibitors of lck II. Bioorg Med Chem Lett 10: 2171–2174 [DOI] [PubMed] [Google Scholar]
- Cao Y, Li H, Liu H, Zheng C, Ji H, Liu X (2010) The serine/threonine kinase LKB1 controls thymocyte survival through regulation of AMPK activation and Bcl-XL expression. Cell Res 20: 99–108 [DOI] [PubMed] [Google Scholar]
- Chao DT, Korsmeyer SJ (1998) Bcl-2 family: regulators of cell death. Annu Rev Immunol 16: 395–419 [DOI] [PubMed] [Google Scholar]
- Cibotti R, Punt JA, Dash KS, Sharrow SO, Singer A (1997) Surface molecules that drive T cell development in vitro in the absence of thymic epithelium and in the absence of lineage-specific signals. Immunity 6: 245–255 [DOI] [PubMed] [Google Scholar]
- Collins A, Littman DR, Taniuchi I (2009) RUNX proteins in transcription factor networks that regulate T-cell lineage choice. Nat Rev Immunol 9: 106–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton T, Gilmour KC, Owen MJ (1996) The MAP kinase pathway controls differentiation from double-negative to double-positive thymocyte. Cell 86: 243–251 [DOI] [PubMed] [Google Scholar]
- Dong X, Li J, Li S, Zhang J, Hua Z (2008) A novel genotyping strategy based on allele-specific inverse PCR for rapid and reliable identification of conditional FADD knockout mice. Mol Biotechnol 38: 129–135 [DOI] [PubMed] [Google Scholar]
- Fooksman DR, Vardhana S, Vasiliver-Shamis G, Liese J, Blair DA, Waite J, Sacristán C, Victora GD, Zanin-Zhorov A, Dustin ML (2010) Functional anatomy of T cell activation and synapse formation. Annu Rev Immunol 28: 79–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CJ, Hammerman PS, Thompson CB (2005) Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol 5: 844–852 [DOI] [PubMed] [Google Scholar]
- Fu G, Chen Y, Yu M, Podd A, Schuman J, He Y, Di L, Yassai M, Haribhai D, North P, Gorski J, Williams C, Wang D, Wen R (2010) Phospholipase C gamma1 is essential for T cell development, activation, and tolerance. J Exp Med 207: 309–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain RN (2002) T-cell development and the CD4-CD8 lineage decision. Nat Rev Immunol 2: 309–322 [DOI] [PubMed] [Google Scholar]
- Han S, Khuri FR, Roman J (2006) Fibronectin stimulates non–small cell lung carcinoma cell growth through activation of Akt/mammalian target of rapamycin/S6 kinase and inactivation of LKB1/AMP-activated protein kinase signal pathways. Cancer Res 66: 315–323 [DOI] [PubMed] [Google Scholar]
- He HT, Lellouch A, Marguet D (2005a) Lipid rafts and the initiation of T cell receptor signaling. Semin Immunol 17: 23–33 [DOI] [PubMed] [Google Scholar]
- He X, He X, Dave VP, Zhang Y, Hua X, Nicolas E, Xu W, Roe BA, Kappes DJ (2005b) The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature 433: 826–833 [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Bevan MJ (1996) The nature of the peptide/MHC ligand involved in positive selection. Semin Immunol 8: 63–68 [DOI] [PubMed] [Google Scholar]
- Hozumi K, Ohtsuka R, Suzuki D, Ando K, Ito M, Nishimura T, Merkenschlager M, Habu S (2000) Establishment of efficient reaggregation culture system for gene transfection into immature T cells by retroviral vectors. Immunol Lett 71: 61–66 [DOI] [PubMed] [Google Scholar]
- Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, Torrice C, Wu MC, Shimamura T, Perera SA, Liang M-C, Cai D, Naumov GN, Bao L, Contreras CM, Li D, Chen L, Krishnamurthy J, Koivunen J, Chirieac LR et al. (2007) LKB1 modulates lung cancer differentiation and metastasis. Nature 448: 807–810 [DOI] [PubMed] [Google Scholar]
- Jones RG, Parsons M, Bonnard M, Chan VS, Yeh WC, Woodgett JR, Ohashi PS (2000) Protein kinase B regulates T lymphocyte survival, nuclear factor kappaB activation, and Bcl-X(L) levels in vivo. J Exp Med 191: 1721–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linette GP, Grusby MJ, Hedrick SM, Hansen TH, Glimcher LH, Korsmeyer SJ (1994) Bcl-2 is upregulated at the CD4+CD8+ stage during positive selection and promotes thymocyte differentiation at several control points. Immunity 1: 197–205 [DOI] [PubMed] [Google Scholar]
- Liu X, Adams A, Wildt KF, Aronow B, Feigenbaum L, Bosselut R (2003) Restricting Zap70 expression to CD4+CD8+ thymocytes reveals a T cell receptor-dependent proofreading mechanism controlling the completion of positive selection. J Exp Med 197: 363–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas B, Vasseur F, Penit C (1994) Production, selection, and maturation of thymocytes with high surface density of TCR. J Immunol 153: 53–62 [PubMed] [Google Scholar]
- Min L, Joseph RE, Fulton DB, Andreotti AH (2009) Itk tyrosine kinase substrate docking is mediated by a nonclassical SH2 domain surface of PLCγ1. Proc Natl Acad Sci USA 106: 21143–21148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirouse V, Swick LL, Kazgan N, Johnston DS, Brenman JE (2007) LKB1 and AMPK maintain epithelial cell polarity under energetic stress. J Cell Biol 177: 387–392 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Munitic I, Williams JA, Yang Y, Dong B, Lucas PJ, El Kassar N, Gress RE, Ashwell JD (2004) Dynamic regulation of IL-7 receptor expression is required for normal thymopoiesis. Blood 104: 4165–4172 [DOI] [PubMed] [Google Scholar]
- Pan L, Hanrahan J, Li J, Hale LP, Zhuang Y (2002) An analysis of T cell intrinsic roles of E2A by conditional gene disruption in the thymus. J Immunol 168: 3923–3932 [DOI] [PubMed] [Google Scholar]
- Park SG, Schulze-Luehrman J, Hayden MS, Hashimoto N, Ogawa W, Kasuga M, Ghosh S (2009) The kinase PDK1 integrates T cell antigen receptor and CD28 coreceptor signaling to induce NF-kappaB and activate T cells. Nat Immunol 10: 158–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A, Zikherman J, Das J, Roose JP, Weiss A, Chakraborty AK (2009) Origin of the sharp boundary that discriminates positive and negative selection of thymocytes. Proc Natl Acad Sci USA 106: 528–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsdell F, Jenkins M, Dinh Q, Fowlkes BJ (1991) The majority of CD4+8− thymocytes are functionally immature. J Immunol 147: 1779–1785 [PubMed] [Google Scholar]
- Saito T, Yokosuka T (2006) Immunological synapse and microclusters: the site for recognition and activation of T cells. Curr Opin Immunol 18: 305–313 [DOI] [PubMed] [Google Scholar]
- Sapkota GP, Boudeau J, Deak M, Kieloch A, Morrice N, Alessi DR (2002) Identification and characterization of four novel phosphorylation sites (Ser31, Ser325, Thr336 and Thr366) on LKB1/STK11, the protein kinase mutated in Peutz-Jeghers cancer syndrome. Biochem J 362: 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota GP, Kieloch A, Lizcano JM, Lain S, Arthur JSC, Williams MR, Morrice N, Deak M, Alessi DR (2001) Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90RSK and cAMP-dependent protein kinase, but not its farnesylation at Cys433, is essential for LKB1 to suppress cell growth. J Biol Chem 276: 19469–19482 [DOI] [PubMed] [Google Scholar]
- Sato T, Ohno S, Hayashi T, Sato C, Kohu K, Satake M, Habu S (2005) Dual functions of Runx proteins for reactivating CD8 and silencing CD4 at the commitment process into CD8 thymocytes. Immunity 22: 317–328 [DOI] [PubMed] [Google Scholar]
- Seminario MC, Bunnell SC (2008) Signal initiation in T-cell receptor microclusters. Immunol Rev 221: 90–106 [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC (2004) The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA 101: 3329–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer A, Adoro S, Park J-H (2008) Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev Immunol 8: 788–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DP, Spicer J, Smith A, Swift S, Ashworth A (1999) The mouse Peutz-Jeghers syndrome gene Lkbl encodes a nuclear protein kinase. Hum Mol Genet 8: 1479–1485 [DOI] [PubMed] [Google Scholar]
- Spicer J, Ashworth A (2004) LKB1 kinase: master and commander of metabolism and polarity. Curr Biol 14: R383–R385 [DOI] [PubMed] [Google Scholar]
- Starr TK, Jameson SC, Hogquist KA (2003) Positive and negative selection of T cells. Annu Rev Immunol 21: 139–176 [DOI] [PubMed] [Google Scholar]
- Sun G, Liu X, Mercado P, Jenkinson SR, Kypriotou M, Feigenbaum L, Galera P, Bosselut R (2005) The zinc finger protein cKrox directs CD4 lineage differentiation during intrathymic T cell positive selection. Nat Immunol 6: 373–381 [DOI] [PubMed] [Google Scholar]
- Swat W, Dessing M, Boehmer HV, Kisielow P (1993) CD69 expression during selection and maturation of CD4+8+ thymocytes. Eur J Immunol 23: 739–746 [DOI] [PubMed] [Google Scholar]
- Tamás P, Macintyre A, Finlay D, Clarke R, Feijoo-Carnero C, Ashworth A, Cantrell D (2010) LKB1 is essential for the proliferation of T-cell progenitors and mature peripheral T cells. Eur J Immunol 40: 242–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treanor B, Lanigan PM, Kumar S, Dunsby C, Munro I, Auksorius E, Culley FJ, Purbhoo MA, Phillips D, Neil MA, Burshtyn DN, French PM, Davis DM (2006) Microclusters of inhibitory killer immunoglobulin-like receptor signaling at natural killer cell immunological synapses. J Cell Biol 174: 153–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meerwijk JP, Germain RN (1994) The different roles of MHC class recognition in thymocyte CD4 versus CD8 lineage commitment and positive selection. Semin Immunol 6: 231–239 [DOI] [PubMed] [Google Scholar]
- Veri M-C, DeBell KE, Seminario M-C, DiBaldassarre A, Reischl I, Rawat R, Graham L, Noviello C, Rellahan BL, Miscia S, Wange RL, Bonvini E (2001) Membrane raft-dependent regulation of phospholipase C gamma-1 activation in T lymphocytes. Mol Cell Biol 21: 6939–6950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Xiao G, Zhang Y, Wen X, Gao X, Okada S, Liu X (2008) Regulation of Tcrb recombination ordering by c-Fos-dependent RAG deposition. Nat Immunol 9: 794–801 [DOI] [PubMed] [Google Scholar]
- Werlen G, Palmer E (2002) The T-cell receptor signalosome: a dynamic structure with expanding complexity. Curr Opin Immunol 14: 299–305 [DOI] [PubMed] [Google Scholar]
- Winslow MM, Neilson JR, Crabtree GR (2003) Calcium signalling in lymphocytes. Curr Opin Immunol 15: 299–307 [DOI] [PubMed] [Google Scholar]
- Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LGD, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D (2003) LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol 13: 2004–2008 [DOI] [PubMed] [Google Scholar]
- Yamada E, Pessin JE, Kurland IJ, Schwartz GJ, Bastie CC (2010) Fyn-dependent regulation of energy expenditure and body weight is mediated by tyrosine phosphorylation of LKB1. Cell Metab 11: 113–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Chtanova T, Ladi E, Robey EA (2006) Thymocyte motility: mutants, movies and migration patterns. Curr Opin Immunol 18: 191–197 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.