Abstract
EMBO J 30 10, 2057–2070 (2011); published online March 25 2011
Neurodegenerative disorders are one among the most debilitating diseases of an ageing population. Understanding the mechanisms of neuronal cell death during pathogenesis of diseases such as Alzheimer's, Parkinson's, Huntington's, and prion diseases is key to addressing the options for treatment and prevention of brain deterioration. One feature of many such diseases is the accumulation of specific misfolded proteins. Often these misfolded proteins take the form of large amyloid fibrils or plaques, but recent observations implicate small soluble oligomers as the primary causes of neuronal dysfunction. How these misfolded proteins trigger cell death pathways is largely unknown, but some reports have suggested mediation by normal cellular prion protein (PrPC). In this issue, Resenberger et al (2011) provide evidence for membrane-anchored PrPC's role in recognizing a variety of β-sheet-rich protein conformers and transducing pro-apoptotic signals.
Among various protein-misfolding diseases, Alzheimer's disease (AD) is characterized by brain plaques composed of the β-amyloid (Aβ) peptide and tau. Huntington's disease is caused by an inherited poly-glutamine expansion in the huntingtin protein, and huntingtin fragments containing these extra residues aggregate and cause neurotoxicity. In Parkinson's disease, alpha synuclein aggregates into intracellular inclusions called Lewy bodies. During prion diseases, PrPC is converted to an abnormal autocatalytic isoform, PrPSc, which can accumulate in diffuse deposits, amyloid fibrils or plaques. Despite accumulating evidence of the importance of protein misfolding and aggregation in these diseases, the precise mechanisms by which these proteins misfold and trigger neurodegeneration remain unclear.
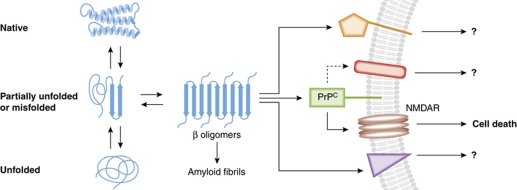
One common feature of these misfolded protein aggregates is their high β-sheet content. PrPC is largely α helical and disordered, but, upon conversion to infectious PrPSc amyloid, becomes predominantly β sheet with bends and turns (Caughey et al, 2009; Smirnovas et al, 2011). Alzheimer's plaques are composed of high β-sheet Aβ fibrils. α-Synuclein exists normally as a natively unfolded protein, but can partially fold into β structures, increasing its propensity for aggregation. The proposed toxic fragment of huntingtin is helical in the native structure, but expanded poly-glutamine sequences can promote the formation of compact β sheet and turn structures. Although large fibrillar and plaque deposits of these misfolded proteins are often the most obvious lesions, the most toxic species appear to be small oligomeric assemblies that may be precursors to larger fibrils (Caughey and Lansbury, 2003) (see Figure 1). The challenge has been not only to define and characterize the toxic oligomeric species but also to determine their neurotoxic mechanisms.
Figure 1.
Schematic of proposed role of PrP in pro-apoptotic signalling during neurodegenerative diseases. Native proteins exist in equilibrium with partially or completely unfolded forms. In some instances, partially misfolded proteins can adopt β structures with the propensity to aggregate and form amyloid fibrils. However, along the pathway to fibril formation, the smaller β oligomers mediate toxic effects. PrPC is one of the potentially multiple cell-surface receptors capable of binding to the toxic oligomers and mediating pro-apoptotic signals in conjunction with other molecules.
Recently, Lauren et al (2009) used Aβ oligomers that were formed in vitro and fractionated by size to probe potential cell-surface binding partners. PrPC showed high affinity and selectivity in its interaction with Aβ oligomers. Moreover, the inhibition of long-term potentiation by Aβ oligomers in hippocampal slices was dependent on PrP. When a familial AD transgenic mouse (APPswe/Psen1ΔE9) was crossed onto a PrP null background, the normal axon degeneration, cell death, and learning impairment found in the AD mice were non-existent (Gimbel et al, 2010). These observations provided the first evidence that PrPC mediates at least some of the neurotoxic effects of Aβ oligomers, a hypothesis that was confirmed by observations that short-term PrP antibody treatment in AD transgenic mice reversed memory impairment and synaptic density loss (Chung et al, 2010).
Although these findings attracted considerable interest, some further studies failed to confirm a role for PrPC in other putative aspects of Alzheimer's pathogenesis. For example, when in vitro Aβ oligomers were injected into either wild-type or PrP null mice, no difference in object recognition memory experiments was seen (Balducci et al, 2010). Furthermore, in another study using a different transgenic AD mouse model (APPKM670/671NL/Psen1L166P), no change in the normal age-dependent deterioration of long-term potentiation was detected as a function of the presence or absence of PrPC (Calella et al, 2010).
Building on observations of a role for PrPC in mediating pro-apoptotic signalling in the presence of PrPSc, Resenberger et al probed the possibility that PrPC might respond similarly to other high β-sheet protein species (β-conformers). They found that cell-surface PrPC bound a variety of β-conformers via its N-terminal domain. Active β-conformers included those associated with prion diseases, AD, and yeast prions. Pro-apoptotic signals, as measured by caspase-3 activation, were only transduced when PrPC was GPI-anchored to the cell surface. Furthermore, PrPC-expressing SH-SY5Y cells co-cultivated alongside an Aβ-secreting Chinese hamster ovary cell line received the pro-apoptotic signal in trans. The toxic effect of the β-conformers appears to involve N-methyl-D-aspartic acid (NMDA) receptors as indicated by blockage with memantine. Collectively, these observations support a role for PrPC in mediating neuronal death induced by β-conformers of a variety of proteins.
One observation that has been consistent among the labs is the remarkable affinity of PrPC for Aβ oligomers from various sources and preparations. Further in vitro studies demonstrated that the very N-terminus of PrP (residues 23–27) is important for recognition of a specific structural conformation of the soluble Aβ oligomers (Chen et al, 2010). However, as also seen in the original paper by the Strittmatter group, there must be other cell surface molecules capable of interacting with Aβ oligomers as only a 50% reduction of Aβ oligomer binding was seen in PrP−/− hippocampal neurons. Thus, alternative binding partners may mediate signalling via other pathways, which might modulate or substitute for PrPC-mediated signalling in some settings. Further complexity likely arises from the fact that a broad array of Aβ assemblies is generated during AD, and some Aβ subpopulations may preferentially bind certain ligands over others. Aβ monomers and oligomers are known to bind other cell-surface receptor signalling molecules, including insulin receptor, nicotinic acetylcholine receptor, and calcium and cation channels (reviewed in Parihar and Brewer, 2010). Although Resenberger et al present evidence for involvement of the NMDA receptor in SH-SY5Y cells (this issue), other pathways may be used in different cell types or circumstances (see Figure 1).
Explaining a role of PrPC becomes even more complicated when considering Huntington's and Parkinson's diseases. In contrast to the situation with AD and prion diseases, the misfolded proteins accumulate primarily in intracellular locations where, under most circumstances, they would not come into contact with GPI-anchored PrPC molecules. This situation may help to explain the observation that, with transgenic mouse models for Huntington's and Parkinson's diseases, the disease phenotypes were not substantially altered by the presence or absence of PrPC (Steele et al, 2009). These results highlight the likelihood that the role(s) of PrPC in protein-misfolding diseases will be variable and context dependent.
Like many reported physiological or pathophysiological roles of PrPC, its mediation of Aβ toxicity remains controversial. Nonetheless, the recent reports from the Strittmatter (Lauren et al, 2009; Chung et al, 2010; Gimbel et al, 2010) and Tatzelt (this issue) groups suggest an intriguing mechanistic link between AD and prion diseases, and identify the PrPC–β conformer interaction as a potential therapeutic target. However, many factors are at play in these complex diseases and further studies will be required to establish the overall importance of PrPC–β conformer interactions and its associated cell death pathway in the pathogenesis of these and other debilitating protein-misfolding diseases.
Footnotes
The authors declare that they have no conflict of interest.
References
- Balducci C, Beeg M, Stravalaci M, Bastone A, Sclip A, Biasini E, Tapella L, Colombo L, Manzoni C, Borsello T, Chiesa R, Gobbi M, Salmona M, Forloni G (2010) Synthetic amyloid-beta oligomers impair long-term memory independently of cellular prion protein. Proc Natl Acad Sci USA 107: 2295–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calella AM, Farinelli M, Nuvolone M, Mirante O, Moos R, Falsig J, Mansuy IM, Aguzzi A (2010) Prion protein and Abeta-related synaptic toxicity impairment. EMBO Mol Med 2: 306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B, Baron GS, Chesebro B, Jeffrey M (2009) Getting a grip on prions: oligomers, amyloids, anchors and pathological membrane interactions. Annu Rev Biochem 78: 177–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B, Lansbury PT (2003) Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci 26: 267–298 [DOI] [PubMed] [Google Scholar]
- Chen S, Yadav SP, Surewicz WK (2010) Interaction between human prion protein and amyloid-beta (Abeta) oligomers: role of N-terminal residues. J Biol Chem 285: 26377–26383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E, Ji Y, Sun Y, Kascsak RJ, Kascsak RB, Mehta PD, Strittmatter SM, Wisniewski T (2010) Anti-PrPC monoclonal antibody infusion as a novel treatment for cognitive deficits in an Alzheimer's disease model mouse. BMC Neurosci 11: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbel DA, Nygaard HB, Coffey EE, Gunther EC, Lauren J, Gimbel ZA, Strittmatter SM (2010) Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J Neurosci 30: 6367–6374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM (2009) Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature 457: 1128–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar MS, Brewer GJ (2010) Amyloid-beta as a modulator of synaptic plasticity. J Alzheimer's Dis 22: 741–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resenberger UK, Harmeier A, Woerner AC, Goodman JL, Müller V, Krishnan R, Vabulas RM, Kretzschmar HA, Lindquist S, Hartl FU, Multhaup G, Winklhofer KF, Tatzelt J (2011) The cellular prion protein mediates neurotoxic signalling of β-sheet-rich conformers independent of prion replication. EMBO J 30: 2057–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnovas V, Baron GS, Offerdahl DK, Raymond GJ, Caughey B, Surewicz W (2011) Structural organization of brain-derived mammalian prions as probed by hydrogen exchange. Nat Struct Mol Biol, Published online 27 March 2011. doi: 10.1038/nsmb.2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele AD, Zhou Z, Jackson WS, Zhu C, Auluck P, Moskowitz MA, Chesselet MF, Lindquist S (2009) Context dependent neuroprotective properties of prion protein (PrP). Prion 3: 240–249 [DOI] [PMC free article] [PubMed] [Google Scholar]