Abstract
Cardiac fibroblasts play a critical role in maintenance of normal cardiac function. They are indispensable for damage control and tissue remodeling on myocardial injury and principal mediators of pathological cardiac remodeling and fibrosis. Despite their manyfold functions, cardiac fibroblasts remain poorly characterized in molecular terms. Evidence is evolving that cardiac fibroblasts are a heterogeneous population and likely derive from various distinct tissue niches in health and disease. Here, we review our emerging understanding of where cardiac fibroblasts come from, as well as how we can possibly use this knowledge to develop novel therapies for cardiac fibrosis.
Keywords: fibroblasts, fibrosis, fate mapping
Physiological cardiac function and chronic heart failure are controlled by complex interactions of the myocytes, extracellular matrix (ECM), and nonmyocyte cellular components, including the cardiac fibroblasts.1,2 With their various functions, cardiac fibroblasts are at the interface of multiple interactions, playing an integral part in maintaining homeostasis in the heart.3,4 Under physiological conditions, cardiac fibroblasts contribute to the structural, mechanical, biochemical, and electric properties of the heart.1 They maintain ECM homeostasis,5,6 which is critical because the ECM provides a scaffold for all cardiac cell types,1,7 distributes mechanical forces throughout the cardiac tissue, and also electrically separates the atria and the ventricles to facilitate proper cardiac contraction.8 By secreting various growth factors9,10 and via direct cell–cell interactions, fibroblasts directly impact cardiomyocyte function.11 They are indispensable for angiogenesis and vascular homeostasis in the heart.12 Furthermore, fibroblasts support electric properties of the heart, because they provide insulating layers between bundles of cardiomyocytes, allowing for the orchestrated sequential contraction of cardiomyocytes in the heart.13 In this regard, fibroblasts have been suggested to be important for mechanoelectric transduction in the heart.14,15
Importance of fibroblasts in cardiac pathology is even more obvious, because fibroblasts are considered the predominant source of the extracellular matrix, the hallmark of fibrosis1,2,6,16 Most cardiac diseases are associated with an increase of fibrosis in the heart.17 Fibrosis of the cardiac tissue has significant consequences on cardiac function.1 Increased ECM synthesis and decreased degradation result in increased mechanical stiffness and diastolic dysfunction.18 Moreover, increased ECM deposition between layers of cardiomyocytes may disrupt their electric coupling, leading to impaired cardiac contraction.19 Furthermore, inflammation and fibrosis in the perivascular areas may decrease the flow of oxygen and nutrients and increase the pathological remodeling response.20 In summary, there are numerous reasons why we need to understand the functional role of cardiac fibroblasts in health and disease.
Challenges of Studying Fibroblasts in the Heart
Our understanding of how to define cardiac fibroblasts (and fibroblasts in general) is in flux. Traditionally, cardiac fibroblasts were defined by their morphological appearance, namely by their branched cytoplasm surrounding an elliptical, speckled nucleus that typically has 1 or 2 nucleoli.1,2,21 However, study of cardiac fibroblasts has been challenging, and they remain poorly characterized in molecular terms.1 One reason for this is the relative lack of serviceable fibroblast markers, because the known markers either are not specific for fibroblasts or detect only subpopulations of fibroblasts. Vimentin, for example, has been extensively used to label cardiac fibroblasts.22 However, although antibodies to Vimentin label fibroblasts with great sensitivity (at this stage, it is safe to assume that all fibroblasts are Vimentin-positive), they also label various other cell types, including endothelial cells.23–25 In fact, Vimentin was first described as an endothelial cell marker.26 Fibroblast-specific protein (FSP)1 is another fibroblast marker, which was identified in a differential expression screen comparing kidney fibroblasts and kidney epithelial cells.27 Although FSP1, also known as S100A4, is also expressed by metastatic cancer cells, existing evidence suggest that it is specific for cardiac fibroblasts in the heart.28 However, FSP1 antibodies detect only a subset of cardiac fibroblasts, and FSP1+ fibroblasts are rare in the normal heart.28 In the healthy postnatal heart, valvular fibroblasts (also termed valvular interstitial cells) express α-smooth muscle actin (α-SMA), but not interstitial fibroblasts of the myocardium.29 In cardiac fibrosis, myocardial fibroblasts become α-SMA+ (then termed myofibroblasts), which is considered a sign of fibroblast activation.6 Because α-SMA is also expressed by vascular smooth muscle cells and pericytes, both in close proximity to fibroblasts, these cells can be falsely identified as fibroblasts when immunofluorescence techniques with insufficient resolution are used. Discoidin domain receptor (DDR)2, a collagen receptor, has been used to identify and sort cardiac fibroblasts.23,30 However, DDR2 is also expressed by lymphocytic lineages and identifies only subsets of fibroblasts. Periostin, a matricellular protein is specifically expressed by fibroblasts, which originate developmentally from epicardial derived progenitor cells.6 However, because it is an extracellular protein, it has limited utility to detect fibroblasts by means of antibody labeling.6 The issue of identifying fibroblasts is becoming further complicated because evidence is emerging that cardiac fibroblasts are not a homogenous cell type but a highly heterogeneous population.16,28 At the present time, it seems best to identify fibroblasts by combination of several fibroblast markers in context of their location and physiological stage.
Traditionally, fibroblasts are classified as either inactive or activated fibroblasts, based on their morphological appearance (activated fibroblasts are considered to be larger with a prominent Golgi apparatus).6 Activated fibroblasts are believed to produce more collagen and, hence, are considered the main culprits in fibrotic diseases.6 Cardiac fibroblasts are heterogeneous with regard to their phenotype, specific functions, and cellular origins. For example, subpopulations of fibroblasts can be detected based on their expression of FSP1; others, based on expression of α-SMA; and few fibroblasts express both.28 Most fibroblasts synthesize collagen, albeit not at all times. Only a subpopulation of cardiac fibroblasts is involved in electromechanical coupling with cardiomyocytes. Developmentally, resident cardiac fibroblasts have distinct cellular origins. During heart disease, fibroblasts can also originate from endothelial cells or they can be recruited from the bone marrow.1,16 It is not yet known whether different fibroblast origins contribute to phenotypic and functional fibroblast heterogeneity in the heart. A major obstacle in addressing this matter is that fibroblasts do not carry known imprints of their origins. For example, in a human cardiac biopsy, one cannot tell which fibroblast came from where. Study of distinct fibroblast populations of different origins only became possible with emergence of so-called fate-mapping strategies, which are discussed below.
Strategies for Fate Mapping of Cardiac Fibroblasts
Our knowledge that cardiac fibroblasts in the heart have distinct cellular origins is derived from fate-mapping studies. Fate-mapping techniques were originally established to study cell lineages during embryonic development. A “fate map” in its original sense is a representation of the developmental history of each cell in the adult body, just like a family tree or pedigree analysis. Because of the technical difficulties in identifying specific cells within a given tissue over prolonged periods of time, techniques were developed that allowed for retrospective analysis of the origins of an individual cell.
The basic principle of these techniques is that a cell gets an imprint (“tag”) that remains unchanged irrespective of its subsequent fate. Techniques of fate mapping include injection of dyes into cells, creation of chimeric tissues of different species, detection of specific gene sequences (like the Y chromosome in the case of sex-mismatched transplantation experiments or mutations in the case of cancer cells) or the use of genetic reporters (Figure 1A).31
Figure 1. Fate mapping.

A, Schematic displaying the principles of fate mapping using the example of EndMT. Basic principle of fate mapping is that specific cells (in this example, endothelial cells) are irreversibly labeled, in most cases, using dyes, DNA vectors, or transgenic reporters. If a labeled cell migrates from its original site, it will take its tag with it (in this example, the blue fibroblast leaving the endothelium). Because the label stays intact, tissue analysis at later time points allows for reconstruction of cellular ancestries, independent of their present phenotype or location (in this example, the blue label allows for identification of their endothelial origin of select fibroblasts). B, Picture summarizing the Cre/lox system, which is often used for fate mapping in mice using Tie1-Cre;R26-STOP-LacZ double-transgenic mice as example. In these mice, the Cre-recombinase is expressed under the endothelial cell–specific promoter Tie1 (top transgene schematic). R26R-STOP-lacZ mice carry a reporter transgene (in this case, LacZ, which encodes for bacterial β-galactosidase, allowing for distinction from mouse cells), which is controlled by a ubiquitous promoter that is active in most cell types (in this case, the R26 Rosa promoter). Because a STOP cassette (flanked by 2 loxP sites) is inserted between promoter and LacZ gene, the reporter gene is inactive (middle transgene schematic). When Tie-Cre mice are crossed to R26R-STOP-lacZ mice, Cre-recombinase (under control of endothelial cell–specific Tie1 promoter) removes the STOP cassette selectively in endothelial cells. The ubiquitous R26 promoter now constitutively drives LacZ expression, irrespective of the later fate of the endothelial cells, allowing for identification of endothelial origin.
The first fate maps date back to 1929, when Walter Vogt applied vital dyes to regions of the amphibian embryo, allowing him to track which embryonic regions developed into which adult tissues. Carbocyanine dyes (or DNA vectors), which can be injected into specific cells, are still being used to track the fate of cells in embryos. The principle is that these dyes do not diffuse out of the cell once injected and that they are passed on to the progeny of the cell. Although these techniques have high utility in amphibian or even avian embryos, their obvious problem in mammals is that the embryo is not easily accessible, because it develops inside the mother. For this reason, transgenic reporter systems have been developed to study cell fates in mice.
Transgenic reporters either use a reporter gene (such as β-galactosidase or GFP), which is expressed directly under control of a cell-specific promoter (and hence it is dependent on constant activity of this promoter) or use double transgenes, which are independent of the later phenotype of the tagged cell.31
A commonly used approach of genetic fate mapping requires 2 transgenes in mice to mark and visualize individual cells over time (Figure 1B). One transgene expresses a site-specific recombinase, whereas the other is a reporter gene that is designed to permanently and heritably express a marker following site-specific recombination. Two different recombinases are predominantly used for fate mapping: Cre-recombinase (derived from bacterial phage), which acts on loxP sites32,33; and Flp (derived from Saccharomyces cerevisiae), which acts on frt sites.34,35 To mark individual cells, transgenic mice have been generated that express Cre/Flp recombinases under control of cell type–specific promoters (for example Tie1 or VE-cadherin promoters to label endothelial cells).
The second transgene consists of 3 parts, a ubiquitous promoter (which is active in all cells irrespective of their origin), a so-called STOP cassette (which prevents expression of the downstream coding sequence as long as it is intact) flanked by 2 loxP sites (or frt sites) and a reporter gene (a unique protein).36 The concept is that in every cell, in which the Cre-recombinase is expressed once, the Cre-recombinase removes the floxed STOP cassette and the reporter gene is now constitutively expressed in those cells. Ubiquitous promoters that are commonly used are the R26R and actin reporter alleles,37,38 which on removal of the STOP cassette, control cell-specific expression of reporter genes including lacZ, luciferase,38 various fluorescent protein (such as enhanced GFP,38 RFP,39 or YFP40) or alkaline phosphatase.41 Using such an approach, various cellular constituents of the heart can be tagged for fate-mapping studies (Table 1). Transgenic mice for tagging cardiomyocytes include MHC-Cre42,43 or Nkx2.5-Cre mice44–46; mice for tagging endothelial cells include Tie1-Cre,28,47 Tie2-Cre,48–50 or VE-cad-herin–Cre mice.51 To tag fibroblasts, FSP1-Cre52 or collagen 1 α2–Cre mice53 have been used.
Table 1. Available Cre Mice With Cre Expression Under Cell-Specific Promoters to Trace Specific Cell Compartments of the Heart.
Issues Associated With Fate-Mapping Studies
Recombinase-based fate mapping has become an important strategy for defining progenitor–descendant relationships in both normal mammalian development and disease models. However, interpretation of such experiments is complex. Recent data demonstrate that susceptibility of the Cre-dependent reporter to recombination significantly influences Cre-based fate-mapping results.54 Although Cre is expressed in temporally and spatially graded patterns, activation of a Cre-dependent reporter is a binary readout in which cells surpassing a Cre exposure threshold become activated.54 Reporters that are more susceptible to recombination reveal a broader fate map that includes progenitors with lower level or transient Cre expression, whereas less sensitive reporters reveal a more restricted fate map that corresponds to progenitors with higher level or duration of Cre expression.54 An important implication is that lack of Cre reporter activation must be interpreted carefully, because this does not exclude Cre expression at levels below the threshold required for reporter recombination. Another issue that needs to be considered is that on removal of the STOP cassette, reporter gene expression is dependent on the efficacy of the promoter that is being used. Although their activity is considered “ubiquitous,” activity of most commonly used promoters such as the Rosa promoter and actin promoter are not equal in all cell types. Furthermore, these promoters can be silenced by epigenetic modifications including methylation. This means that even when the STOP cassette is being removed, inadequate promoter activity can cause false-negative data interpretation. Finally, detection of the reporter gene can be challenging. Commonly used bacterial β-galactosidase can be detected by means of its enzymatic activity. However, this system is susceptible to yield false-positive data because of endogenous β-galactosidase activity in tissues.55 This issue is further complicated in tissues such intestine, where resident bacteria have β-galactosidase activity.56 Fate-mapping studies often require double labeling for cell lineage markers in addition to the reporter gene (for example, to demonstrate fibroblast identity of the cell). Because combination of enzymatic β-galactosidase activity (blue precipitate) with immunolabeling techniques can be challenging to visualize, immunodetection of β-galactosidase is becoming more often used than enzymatic detection. Other reporter genes include fluorescent proteins such as GFP and RFP. Challenges of these reporter systems include low sensitivity attributable to low transgene expression and false-positive data because proteins leak out of cells within tissue sections. For this reason, tissues are often treated with sucrose.46 Sensitivity can be increased with use of anti-GFP antibodies.57 In summary, because of various potential pitfalls of transgenic systems, both false-positive and false-negative results need to be considered. Ideally, visualization of reporter gene activity should be confirmed by proving removal of the STOP cassette at the molecular level (ie, by PCR).
Similarly, vital dyes come with various pitfalls, mostly because of the need of direct cell manipulation, which complicates its use. Additionally, dyes over time can leak out of cells and on cell division, intracellular cells become diluted, decreasing sensitivity.
Although use of transgenes to fate map cells is the current state-of-the-art, there remains room for improvement. Ideally, one would like to view fibroblasts from different origins enter the cardiac connective tissue in real time. Unfortunately, this is not yet feasible with existing technologies in adult mammals. Ongoing attempts to refine additional fate-mapping strategies like use of heterotypic cell transplantation or use of dyes should be continued to further substantiate our knowledge of fibroblast heterogeneity in the heart.
Fibroblasts of Endothelial Origin
Evidence that endothelial cells can contribute to accumulation of fibroblasts in cardiac fibrosis stems from a study in which cardiac fibrosis was induced in Tie1-Cre;R26Rosa-lox-STOP-lox-LacZ double-transgenic mice (Table 2).28 In these mice, the Cre-recombinase (which is expressed under control of the Tie1 promoter) is expressed in endothelial cells and removes the STOP cassette, causing constitutive expression of the β-galactosidase. In Tie1cre;R26Rosa-lox-STOP-lox-LacZ mice, β-galactosidase–positive cells (which also express fibroblast markers) are present in the fibrotic areas (but not in nonfibrotic hearts), which means that these cells must be derived from endothelial cells via an endothelial–mesenchymal transition (EndMT).28 Using this approach in a mouse model of pressure overload, ≈75% of all α-SMA–positive fibroblasts are EndMT-derived, and 15% of all FSP1-positive fibroblasts are of endothelial origin.28 In total, 27% to 35% of all (α-SMA– or FSP1-positive) fibroblasts are of endothelial origin (Figure 2).28
Table 2. Origins of Fibroblast in the Heart.
| Source and Method of Lineage Tracing | Model Studied | Reference |
|---|---|---|
| Embryonic development | ||
| Epicardium | ||
| Wt1CreERT2/+; Rosa26fsLz mice | Mouse embryo | 8 |
| Wt1CreERT2/+; Rosa26mTmG mice | ||
| Quail–Chick chimera | Chicken embryo | 75–77 |
| Endocardium | ||
| Tie2-Cre;RosaSTOPLacZ mice | Mouse embryo | 78 |
| Cardiac fibrosis | ||
| Endothelial cells | ||
| Tie1-Cre;RosaSTOPLacZ mice | Aortic banding | 28 |
| Bone marrow | ||
| Sex-mismatched bone marrow transplantation | Aortic banding | 28 |
| GFP+ bone marrow transplantation | Myocardial infarction | 63 |
| GFP+ bone marrow transplantation | Autoimmune myocarditis | 79 |
The table summarizes the known cellular sources of cardiac fibroblasts and the methodology of fate mapping to prove the respective origins of cardiac fibroblasts.
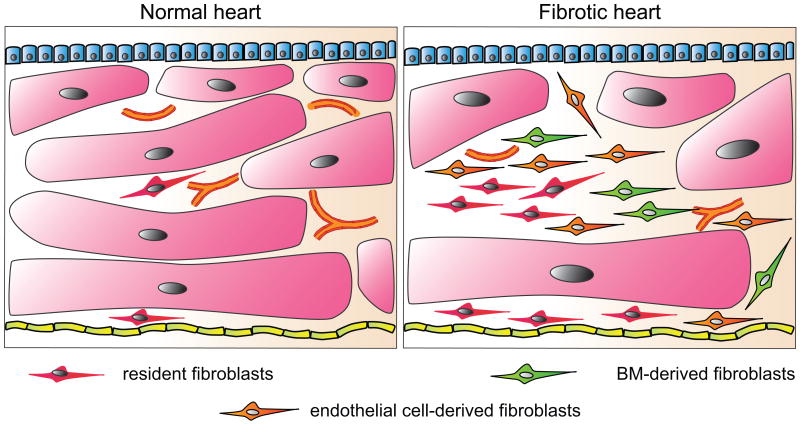
Figure 2. Fibroblasts of different origins in the heart.
Schematic demonstrates the difference in abundance and origins of fibroblasts between a normal and fibrotic heart. Whereas the normal heart contains resident fibroblasts, the number of fibroblasts increases in the fibrotic heart, with different cell sources, such as endothelial cells and bone marrow cells, contributing to the overall pool of fibroblasts. The number of cardiomyocytes and the microvascular density in contrast is reduced. Although it is conceivable that cells from the epicardium or the endocardium could contribute to the pool of fibroblasts in fibrotic hearts, this has not been demonstrated to date.
Does this mean that in fibrotic hearts one-third of fibroblasts derive from the endothelial cells? Interpretation of these data comes with several caveats. First, it is not yet known what percentage of all fibroblasts express α-SMA or FSP1 in the heart (this means the percentage contribution of EndMT to fibroblasts could possibly be less or more than one-third). Second, in the adult, the ROSA promoter is prone to unspecific silencing caused by changes in methylation and chromatin organization. This is reflected by the fact that not all endothelial cells are tagged in these mice. Furthermore, it is conceivable that a complex event like EndMT is associated with dramatic changes of methylation patterns and chromatin organization. Recent data also demonstrate that susceptibility of the Cre-dependent reporter to recombination significantly influences Cre-based fate-mapping results and that the R26Rosa-loxSTOP-lox-LacZ is less efficiently recombined by Cre-recombinase as compared with other reporter genes.54 This means that the number of EndMT-derived fibroblasts may even be higher than one-third. EndMT-derived fibroblasts contribute to cardiac fibrosis by producing collagen28 but also by releasing endothelin-1 into the inflammatory microenvironment.58
Fibroblasts From the Epicardium
Using quail–chicken chimeras, Gittenberger-de Groot and colleagues showed that epicardium-derived cells are present in the annulus fibrosis and express periostin and collagen III.59,60 In vitro studies suggested that epithelial cells from the epicardium have the ability to undergo an epithelial–mesenchymal transition (EMT).61 Fate-mapping studies using the Cre-LoxP approach provided evidence that epicardium-derived cells contribute to accumulation of fibroblasts in the annulus fibrosis by undergoing EMT (Table 1).62 Until now, however, there have been no studies yet to elucidate the role of epicardium derived fibroblasts in cardiac fibrosis.
Fibroblasts From the Bone Marrow
Cardiac fibroblasts not only derive locally, but they can also originate from the bone marrow (Figure 2). In sex-mismatched bone marrow transplantation experiments, in which female mice are transplanted with bone marrow from male donor mice and then subjected to aortic banding, ≈13% of FSP1+ fibroblasts and 21% of α-SMA+ fibroblasts are derived from the bone marrow in fibrotic lesions, as determined by presence of Y chromosome (Table 2).28 Similarly, bone marrow–derived cells contribute to fibroblast accumulation in a mouse model of myocardial infarction.63 The identity of bone marrow–derived cells, which contribute to fibroblasts within the fibrotic tissue, are not yet entirely understood. Because in experiments in which mice are transplanted with bone marrow from Tie1-Cre;R26-STOP-LacZ mice, β-galactosidase–expressing fibroblasts can only be detected sporadically, it is unlikely that circulating endothelial cells home into the injured tissue and then contribute to the fibroblast pool by undergoing EndMT.28
A more likely source is fibrocytes, a distinct population of leukocytes with characteristics of fibroblasts. Fibrocytes can be detected in the blood by their coexpression of myeloid markers CD45R, CD13, CD34, and CD11b and extracellular matrix proteins such as collagen types I and III.64 Circulating fibrocytes were first described in a model of wound repair where they preceded fibroblasts entering the wound from adjacent skin.65 Demonstrating bone marrow origin of fibroblasts in cardiac tissue is straightforward in bone marrow transplantation experiments, in which female mice receive bone marrow from male donor mice (allowing for detecting of the Y chromosome) or transplantation of wild-type mice with bone marrow from transgenic reporter mice (allowing for identification of the reporter in cardiac tissues).28
Monocytes have also been suggested as potential source of fibroblasts in cardiac fibrosis.66 In the model of myocardial infarction, invading fibroblast-like cells in the infarcted cardiac tissue were found to coexpress monocytic (CD45, CD11b) and myofibroblast (S100A4, α-SMA) markers.66 Moreover, inhibition of monocyte recruitment diminishes both the number of cardiac fibroblasts and myocardial remodeling following myocardial infarction.67 Alternatively, these results could also suggest that monocytes are important for the recruitment of cardiac fibroblasts during remodeling.
Resident Fibroblasts
The traditional view is that fibroblasts are direct derivates of embryonic stromal cells and that activated fibroblasts in fibrotic hearts are generated by proliferation and activation of the resident fibroblasts (Figure 2). Neither of these views is based on fate-mapping studies. The concept that cardiac fibroblasts in the adult heart are derivates from embryonic stromal cells is based on the observation that fibroblasts in the adult heart populate the same compartment as stromal cells in the embryonic heart. The concept that fibroblast accumulation is caused by proliferation and activation of the resident fibroblasts is based on the observation that fibroblasts can be induced to proliferate and acquire an activated phenotype by profibrotic stimuli such as transforming growth factor (TGF)-β in culture. Estimates of contribution to resident fibroblast accumulation in fibrosis range from all fibroblasts to the leftover fibroblasts that were detected to be neither of endothelial nor of bone marrow origin. Even though the traditional concept of resident fibroblast accumulation has not yet been disproved, it needs to be verified by cell fate-mapping studies.
Pericytes
Pericytes are mesenchymal cells that envelop endothelial cells in nonmuscular microvessels and capillaries. Because pericytes are α-SMA–positive, it has been postulated that pericytes could contribute to the pool of collagen-producing myofibroblasts in cardiac fibrosis.68 For this to occur, pericytes must detach from the vessel walls, migrate, and acquire a fibroblast-like phenotype to act as collagen-producing cell.68 Based on ultrastructural analysis, it has been estimated that ≈10% of contractile cells in myocardial infarction scars are pericytes.69 Although this concept is appealing, it has not yet been validated with fate-mapping techniques.
Fate Mapping in Human Tissues
The major drawback of such fate-mapping studies, based on transgenes, is that it is applicable exclusively in mice and thus does not directly answer questions about the origin of fibroblasts in humans. The only way to trace the origin of a cell in human hearts is to study patients who either have had a sex-mismatched bone marrow transplantation or a sex-mismatched heart transplantation. Although such studies have been performed to look at possible bone marrow origin of cardiomyocytes, the question of bone marrow–derived fibroblasts has not yet been addressed in these studies.70
Stable cell fate tags such as endothelial-specific methylation or endothelial cell–specific viral infections that would theoretically allow for cell fate mapping have not yet been identified. The field of cellular transitions will further evolve as novel techniques are developed in the future.
In summary, the fate map of cardiac fibroblasts is still incomplete. This stems from imperfect tools to detect fibroblasts on one side, but also from the pitfalls, which come with existing fate-mapping technologies. Furthermore, most studies rely on single techniques and systems, making it difficult to compare studies. Cells within the heart have not yet been followed in real time, and combining detection of transgenes or dyes with high resolution electron microscopy could be improved to get better insights into fibroblast ancestry in the heart. One general issue of fate mapping fibroblasts is that numbers of frequencies of different fibroblast populations often do not match up among studies. One possible explanation is that certain factors favor one event over the other, but our knowledge of factors that stimulate expansion of select fibroblast populations in the heart is still scarce.
Why Does the Heart Recruit Fibroblasts of Different Ancestries?
As we are identifying different sources of fibroblasts in the heart, we can only speculate about the biological significance of this. The fact that fibroblast recruitment via EndMT or from the bone marrow is limited to disease suggests a specific role of these cells in tissue repair. Fibroblast recruitment via EndMT, fibrocytes, or EMT is conceptually a highly efficient way to rapidly recruit fibroblasts to the site of injury as opposed to recruitment of fibroblasts from more distant sites of the tissue (which would encompass migration, activation, and proliferation of these cells). The advantageous economy of fibroblast recruitment by EMT, for example, is even more obvious at sites with more pronounced surfaces (like skin), where EMT is an essential process to seal lesions.60 To date, we have no insights regarding whether different fibroblast ancestries also contribute to functional heterogeneity of cardiac fibroblasts.
What Are the Benefits of Understanding the Origin of Fibroblasts?
Fibroblasts are often seen as the main culprits in cardiac fibrosis, and the potential to simply limit their proliferation or their derivation from other cells is an attractive therapeutic option. The latter approach (inhibition of fibroblast formation from other cellular sources such as endothelial cells via EndMT) appears even more attractive because EndMT and bone marrow–derived fibroblasts within the myocardium are exclusively associated with pathological conditions, but not present in the normal heart. In this regard, treatment with recombinant bone morphogenic protein-7, which inhibits EndMT and ameliorates experimental cardiac fibrosis in mice but does not affect tissue homeostasis of the healthy heart.
Studying mechanisms of EndMT and EMT as sources for fibroblast formation in the adult heart may also provide a unique opportunity to develop novel therapeutic strategies to combat developmental defects in the heart. Both EndMT and EMT are necessary events during embryonic development of the heart. For example, the EndMT of the atrioventricular endocardium is responsible for the development of the endocardial cushion, which later become the septa and valves of the heart (Table 1).71 Defects of EndMT are thought to be associated with a variety of congenital heart diseases. The EMT of epithelial cells from the epicardium is necessary for the formation of the annulus fibrosis, ring structures of dense connective tissue in the heart that separate and electrically insulate the atria from the ventricles, allowing the timed sequential beating of these structures that is necessary for efficient heart function (Table 1).8 Disturbance in this EMT program leads to abnormal annulus fibrosis formation, which could possibly represent the anatomic substrate for abnormal electric impulse conduction, as seen in the Wolff–Parkinson–White syndrome.60,72 Although we believe that studying the origins of fibroblasts in the adult may provide insights into congenital heart disease, it is also important to consider that inhibiting these mechanisms can potentially impact physiological heart structures.
Outlook
Fate-mapping studies have provided evidence that cells with a fibroblast-like appearance can derive from various sources, including endothelial cells, epithelial cells, and bone marrow, and existing knowledge suggests that all of these fibroblasts are profibrogenic in the heart. What is not known yet is how all of these mechanisms also contribute to the remodeling and repair following myocardial infarction. Insights into distinct contribution of select fibroblast populations to physiological and pathological scarring in the heart may provide novel therapeutic targets in the future. Furthermore, understanding of commonalities of EMT and EndMT in adult disease and embryonic development of the heart may provide insights to address EndMT-dependent developmental defects in the future. In this regard, we have just scratched the surface of fibroblast biology in heart disease.
Acknowledgments
Sources of Funding: E.M.Z. is supported by American Heart Association Scientist Development Grant SDG0735602T and NIH Mentored Clinical Scientist Development Award 1K08 CA129204. R.K. is supported by NIH grants DK 061688, AA 013913, DK 55001, CA 125550, and CA 151925 and research funds from the Division of Matrix Biology at the Beth Israel Deaconess Medical Center.
Non-standard Abbreviations and Acronyms
- CD
cluster of differentiation
- DDR
discoidin domain receptor
- ECM
extracellular matrix
- EMT
epithelial-mesenchymal transition
- EndMT
endothelial-mesenchymal transition
- Flp
Flippase
- frt
Flippase recognition target
- FSP
fibroblast-specific protein
- GFP
green fluorescent protein
- lacZ
gene encoding for bacterial β-galactosidase
- loxP
locus of crossover in P1
- MHC
myosin heavy chain
- Nkx2.5
NK2 transcription factor related, locus 5
- RFP
red fluorescent protein
- SMA
smooth muscle actin
- TGF
transforming growth factor
- Tie
tyrosine kinase with immunoglobulin-like and epidermal growth factor-like domains
- VE
vascular endothelial
- YFP
yellow fluorescent protein
Footnotes
This manuscript was sent to Elizabeth McNally, Consulting Editor, for review by expert referees, editorial decision, and final disposition.
Disclosures: None.
References
- 1.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105:1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsuruda T, Imamura T, Hatakeyama K, Asada Y, Kitamura K. Stromal cell biology–a way to understand the evolution of cardiovascular diseases. Circ J. 2010;74:1042–1050. doi: 10.1253/circj.cj-10-0024. [DOI] [PubMed] [Google Scholar]
- 3.van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010;7:30–37. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- 4.Kakkar R, Lee RT. Intramyocardial fibroblast myocyte communication. Circ Res. 106:47–57. doi: 10.1161/CIRCRESAHA.109.207456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brilla CG, Reams GP, Maisch B, Weber KT. Renin-angiotensin system and myocardial fibrosis in hypertension: regulation of the myocardial collagen matrix. Eur Heart J. 1993;14(Suppl J):57–61. [PubMed] [Google Scholar]
- 6.Weber KT. Monitoring tissue repair and fibrosis from a distance. Circulation. 1997;96:2488–2492. [PubMed] [Google Scholar]
- 7.Baxter SC, Morales MO, Goldsmith EC. Adaptive changes in cardiac fibroblast morphology and collagen organization as a result of mechanical environment. Cell Biochem Biophys. 2008;51:33–44. doi: 10.1007/s12013-008-9013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou B, von Gise A, Ma Q, Hu YW, Pu WT. Genetic fate mapping demonstrates contribution of epicardium-derived cells to the annulus fibrosis of the mammalian heart. Dev Biol. 2010;338:251–261. doi: 10.1016/j.ydbio.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long CS. The role of interleukin-1 in the failing heart. Heart Fail Rev. 2001;6:81–94. doi: 10.1023/a:1011428824771. [DOI] [PubMed] [Google Scholar]
- 10.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 1996;27:1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 11.Baudino TA, McFadden A, Fix C, Hastings J, Price R, Borg TK. Cell patterning: interaction of cardiac myocytes and fibroblasts in three-dimensional culture. Microsc Microanal. 2008;14:117–125. doi: 10.1017/S1431927608080021. [DOI] [PubMed] [Google Scholar]
- 12.Murakami M, Simons M. Fibroblast growth factor regulation of neovascularization. Curr Opin Hematol. 2008;15:215–220. doi: 10.1097/MOH.0b013e3282f97d98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, Chen B, Lilly B. Fibroblasts potentiate blood vessel formation partially through secreted factor TIMP-1. Angiogenesis. 2008;11:223–234. doi: 10.1007/s10456-008-9102-8. [DOI] [PubMed] [Google Scholar]
- 14.Banerjee I, Yekkala K, Borg TK, Baudino TA. Dynamic interactions between myocytes, fibroblasts, and extracellular matrix. Ann N Y Acad Sci. 2006;1080:76–84. doi: 10.1196/annals.1380.007. [DOI] [PubMed] [Google Scholar]
- 15.Chilton L, Giles WR, Smith GL. Evidence of intercellular coupling between co-cultured adult rabbit ventricular myocytes and myofibroblasts. J Physiol. 2007;583(Pt 1):225–236. doi: 10.1113/jphysiol.2007.135038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol. 2010;225:631–637. doi: 10.1002/jcp.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boudoulas KD, Hatzopoulos AK. Cardiac repair and regeneration: the Rubik's cube of cell therapy for heart disease. Dis Model Mech. 2009;2:344–358. doi: 10.1242/dmm.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaturvedi RR, Herron T, Simmons R, Shore D, Kumar P, Sethia B, Chua F, Vassiliadis E, Kentish JC. Passive stiffness of myocardium from congenital heart disease and implications for diastole. Circulation. 2010;121:979–988. doi: 10.1161/CIRCULATIONAHA.109.850677. [DOI] [PubMed] [Google Scholar]
- 19.Spach MS, Boineau JP. Microfibrosis produces electrical load variations due to loss of side-to-side cell connections: a major mechanism of structural heart disease arrhythmias. Pacing Clin Electrophysiol. 1997;20(2 Pt 2):397–413. doi: 10.1111/j.1540-8159.1997.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 20.Kai H, Mori T, Tokuda K, Takayama N, Tahara N, Takemiya K, Kudo H, Sugi Y, Fukui D, Yasukawa H, Kuwahara F, Imaizumi T. Pressure overload-induced transient oxidative stress mediates perivascular inflammation and cardiac fibrosis through angiotensin II. Hypertens Res. 2006;29:711–718. doi: 10.1291/hypres.29.711. [DOI] [PubMed] [Google Scholar]
- 21.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 22.Wilke A, Schonian U, Herzum M, Hengstenberg C, Hufnagel G, Brilla CG, Maisch B. The extracellular matrix and cytoskeleton of the myocardium in cardiac inflammatory reaction. Herz. 1995;20:95–108. [PubMed] [Google Scholar]
- 23.Goldsmith EC, Hoffman A, Morales MO, Potts JD, Price RL, McFadden A, Rice M, Borg TK. Organization of fibroblasts in the heart. Dev Dyn. 2004;230:787–794. doi: 10.1002/dvdy.20095. [DOI] [PubMed] [Google Scholar]
- 24.Ausma J, Cleutjens J, Thone F, Flameng W, Ramaekers F, Borgers M. Chronic hibernating myocardium: interstitial changes. Mol Cell Biochem. 1995;147:35–42. doi: 10.1007/BF00944781. [DOI] [PubMed] [Google Scholar]
- 25.Kjorell U, Thornell LE, Lehto VP, Virtanen I, Whalen RG. A comparative analysis of intermediate filament proteins in bovine heart Purkinje fibres and gastric smooth muscle. Eur J Cell Biol. 1987;44:68–78. [PubMed] [Google Scholar]
- 26.Franke WW, Schmid E, Osborn M, Weber K. Intermediate-sized filaments of human endothelial cells. J Cell Biol. 1979;81:570–580. doi: 10.1083/jcb.81.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 29.Snider P, Standley KN, Wang J, Azhar M, Doetschman T, Conway SJ. Origin of cardiac fibroblasts and the role of periostin. Circ Res. 2009;105:934–947. doi: 10.1161/CIRCRESAHA.109.201400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 31.Joyner AL, Zervas M. Genetic inducible fate mapping in mouse: establishing genetic lineages and defining genetic neuroanatomy in the nervous system. Dev Dyn. 2006;235:2376–2385. doi: 10.1002/dvdy.20884. [DOI] [PubMed] [Google Scholar]
- 32.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–1405. [PubMed] [Google Scholar]
- 33.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 34.Dymecki SM, Tomasiewicz H. Using Flp-recombinase to characterize expansion of Wnt1-expressing neural progenitors in the mouse. Dev Biol. 1998;201:57–65. doi: 10.1006/dbio.1998.8971. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- 36.Sauer B, Henderson N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc Natl Acad Sci U S A. 1988;85:5166–5170. doi: 10.1073/pnas.85.14.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soriano SG, Coxon A, Wang YF, Frosch MP, Lipton SA, Hickey PR, Mayadas TN. Mice deficient in Mac-1 (CD11b/CD18) are less susceptible to cerebral ischemia/reperfusion injury. Stroke. 1999;30:134–139. doi: 10.1161/01.str.30.1.134. [DOI] [PubMed] [Google Scholar]
- 38.DiLella AG, Hope DA, Chen H, Trumbauer M, Schwartz RJ, Smith RG. Utility of firefly luciferase as a reporter gene for promoter activity in transgenic mice. Nucleic Acids Res. 1988;16:4159. doi: 10.1093/nar/16.9.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luche H, Weber O, Nageswara Rao T, Blum C, Fehling HJ. Faithful activation of an extra-bright red fluorescent protein in “knock-in” Cre-reporter mice ideally suited for lineage tracing studies. Eur J Immunol. 2007;37:43–53. doi: 10.1002/eji.200636745. [DOI] [PubMed] [Google Scholar]
- 40.Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Awatramani R, Soriano P, Mai JJ, Dymecki S. An Flp indicator mouse expressing alkaline phosphatase from the ROSA26 locus. Nat Genet. 2001;29:257–259. doi: 10.1038/ng1101-257. [DOI] [PubMed] [Google Scholar]
- 42.Reinecke H, Minami E, Poppa V, Murry CE. Evidence for fusion between cardiac and skeletal muscle cells. Circ Res. 2004;94:e56–e60. doi: 10.1161/01.RES.0000125294.04612.81. [DOI] [PubMed] [Google Scholar]
- 43.Parlakian A, Tuil D, Hamard G, Tavernier G, Hentzen D, Concordet JP, Paulin D, Li Z, Daegelen D. Targeted inactivation of serum response factor in the developing heart results in myocardial defects and embryonic lethality. Mol Cell Biol. 2004;24:5281–5289. doi: 10.1128/MCB.24.12.5281-5289.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, Arteaga CL, Moses HL. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abdelhafiz AH, Tan E, El Nahas M. The epidemic challenge of chronic kidney disease in older patients. Postgrad Med. 2008;120:87–94. doi: 10.3810/pgm.2008.11.1943. [DOI] [PubMed] [Google Scholar]
- 46.Zeisberg EM, Ma Q, Juraszek AL, Moses K, Schwartz RJ, Izumo S, Pu WT. Morphogenesis of the right ventricle requires myocardial expression of Gata4. J Clin Invest. 2005;115:1522–1531. doi: 10.1172/JCI23769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gustafsson E, Brakebusch C, Hietanen K, Fassler R. Tie-1-directed expression of Cre recombinase in endothelial cells of embryoid bodies and transgenic mice. J Cell Sci. 2001;114:671–676. doi: 10.1242/jcs.114.4.671. [DOI] [PubMed] [Google Scholar]
- 48.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 49.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 50.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibro-blasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759–767. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- 52.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Denton CP, Khan K, Hoyles RK, Shiwen X, Leoni P, Chen Y, Eastwood M, Abraham DJ. Inducible lineage-specific deletion of TbetaRII in fibroblasts defines a pivotal regulatory role during adult skin wound healing. J Invest Dermatol. 2009;129:194–204. doi: 10.1038/jid.2008.171. [DOI] [PubMed] [Google Scholar]
- 54.Ma Q, Zhou B, Pu WT. Reassessment of Isl1 and Nkx2-5 cardiac fate maps using a Gata4-based reporter of Cre activity. Dev Biol. 2008;323:98–104. doi: 10.1016/j.ydbio.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005;115:1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flier SN, Tanjore H, Kokkotou EG, Sugimoto H, Zeisberg M, Kalluri R. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem. 285:20202–20212. doi: 10.1074/jbc.M110.102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.LeBleu VS, Kalluri R. Stem cell-based therapy for glomerular diseases: an evolving concept. J Am Soc Nephrol. 2008;19:1621–1623. doi: 10.1681/ASN.2008070735. [DOI] [PubMed] [Google Scholar]
- 58.Widyantoro B, Emoto N, Nakayama K, Anggrahini DW, Adiarto S, Iwasa N, Yagi K, Miyagawa K, Rikitake Y, Suzuki T, Kisanuki YY, Yanagisawa M, Hirata K. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation. 2010;121:2407–2418. doi: 10.1161/CIRCULATIONAHA.110.938217. [DOI] [PubMed] [Google Scholar]
- 59.Gittenberger-de Groot AC, Vrancken Peeters MP, Mentink MM, Gourdie RG, Poelmann RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ Res. 1998;82:1043–1052. doi: 10.1161/01.res.82.10.1043. [DOI] [PubMed] [Google Scholar]
- 60.Kolditz DP, Wijffels MC, Blom NA, van der Laarse A, Hahurij ND, Lie-Venema H, Markwald RR, Poelmann RE, Schalij MJ, Gittenberger-de Groot AC. Epicardium-derived cells in development of annulus fibrosis and persistence of accessory pathways. Circulation. 2008;117:1508–1517. doi: 10.1161/CIRCULATIONAHA.107.726315. [DOI] [PubMed] [Google Scholar]
- 61.van Tuyn J, Atsma DE, Winter EM, van der Velde-van Dijke I, Pijnappels DA, Bax NA, Knaan-Shanzer S, Gittenberger-de Groot AC, Poelmann RE, van der Laarse A, van der Wall EE, Schalij MJ, de Vries AA. Epicardial cells of human adults can undergo an epithelial-to-mesenchymal transition and obtain characteristics of smooth muscle cells in vitro. Stem Cells. 2007;25:271–278. doi: 10.1634/stemcells.2006-0366. [DOI] [PubMed] [Google Scholar]
- 62.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Amerongen MJ, Bou-Gharios G, Popa E, van Ark J, Petersen AH, van Dam GM, van Luyn MJ, Harmsen MC. Bone marrow-derived myo-fibroblasts contribute functionally to scar formation after myocardial infarction. J Pathol. 2008;214:377–386. doi: 10.1002/path.2281. [DOI] [PubMed] [Google Scholar]
- 64.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 65.Strieter RM, Keeley EC, Burdick MD, Mehrad B. The role of circulating mesenchymal progenitor cells, fibrocytes, in promoting pulmonary fibrosis. Trans Am Climatol Clin Assoc. 2009;120:49–59. [PMC free article] [PubMed] [Google Scholar]
- 66.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci U S A. 2006;103:18284–18289. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJ. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol. 2007;170:818–829. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diaz-Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martin-Vasallo P, Diaz-Flores L., Jr Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 69.Vracko R, Thorning D. Contractile cells in rat myocardial scar tissue. Lab Invest. 1991;65:214–227. [PubMed] [Google Scholar]
- 70.Hocht-Zeisberg E, Kahnert H, Guan K, Wulf G, Hemmerlein B, Schlott T, Tenderich G, Korfer R, Raute-Kreinsen U, Hasenfuss G. Cellular repopulation of myocardial infarction in patients with sex-mismatched heart transplantation. Eur Heart J. 2004;25:749–758. doi: 10.1016/j.ehj.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 71.Eisenberg LM, Markwald RR. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ Res. 1995;77:1–6. doi: 10.1161/01.res.77.1.1. [DOI] [PubMed] [Google Scholar]
- 72.Kolditz DP, Wijffels MC, Blom NA, van der Laarse A, Markwald RR, Schalij MJ, Gittenberger-de Groot AC. Persistence of functional atrio-ventricular accessory pathways in postseptated embryonic avian hearts: implications for morphogenesis and functional maturation of the cardiac conduction system. Circulation. 2007;115:17–26. doi: 10.1161/CIRCULATIONAHA.106.658807. [DOI] [PubMed] [Google Scholar]
- 73.Moses KA, DeMayo F, Braun RM, Reecy JL, Schwartz RJ. Embryonic expression of an Nkx2-5/Cre gene using ROSA26 reporter mice. Genesis. 2001;31:176–180. doi: 10.1002/gene.10022. [DOI] [PubMed] [Google Scholar]
- 74.Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, Thompson JC, Caserta E, Wang H, Chong JL, Naidu S, Wei G, Sharma SM, Stephens JA, Fernandez SA, Gurcan MN, Weinstein MB, Barsky SH, Yee L, Rosol TJ, Stromberg PC, Robinson ML, Pepin F, Hallett M, Park M, Ostrowski MC, Leone G. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461:1084–1091. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dettman RW, Denetclaw W, Jr, Ordahl CP, Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev Biol. 1998;193:169–181. doi: 10.1006/dbio.1997.8801. [DOI] [PubMed] [Google Scholar]
- 76.Manner J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat Rec. 1999;255:212–226. doi: 10.1002/(sici)1097-0185(19990601)255:2<212::aid-ar11>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 77.Vrancken Peeters MP, Gittenberger-de Groot AC, Mentink MM, Poelmann RE. Smooth muscle cells and fibroblasts of the coronary arteries derive from epithelial-mesenchymal transformation of the epicardium. Anat Embryol (Berl) 1999;199:367–378. doi: 10.1007/s004290050235. [DOI] [PubMed] [Google Scholar]
- 78.Rivera-Feliciano J, Lee KH, Kong SW, Rajagopal S, Ma Q, Springer Z, Izumo S, Tabin CJ, Pu WT. Development of heart valves requires Gata4 expression in endothelial-derived cells. Development. 2006;133:3607–3618. doi: 10.1242/dev.02519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kania G, Blyszczuk P, Stein S, Valaperti A, Germano D, Dirnhofer S, Hunziker L, Matter CM, Eriksson U. Heart-infiltrating prominin-1+/CD133+ progenitor cells represent the cellular source of transforming growth factor beta-mediated cardiac fibrosis in experimental autoimmune myocarditis. Circ Res. 2009;105:462–470. doi: 10.1161/CIRCRESAHA.109.196287. [DOI] [PubMed] [Google Scholar]