Abstract
Recent clinical studies indicate neurobehavioral disturbances in type-2 diabetics. However, there is paucity of preclinical research to support this concept. The validity of db/db mouse as an animal model to study type-2 diabetes and related complications is known. The present study was designed to investigate comprehensively the db/db mouse behavior as preclinical evidence of type-2 diabetes related major neurobehavioral complications. We tested juvenile (5–6 weeks) and adult (10–11 weeks) db/db mice for behavioral depression in forced swim test (FST), psychosis-like symptoms using pre-pulse inhibition (PPI) test, anxiety behavior employing elevated plus maze (EPM) test, locomotor behavior and thigmotaxis using open field test and working memory deficits in Y-maze test. Both juvenile and adult group db/db mice displayed behavioral despair with increased immobility time in FST. There was an age-dependent progression of psychosis-like symptoms with disrupted PPI in adult db/db mice. In the EPM test, db/db mice were less anxious as observed by increased percent open arms time and entries. They were also hypolocomotive as evident by a decrease in their basic and fine movements. There was no impairment of working memory in the Y-maze test in db/db mice. This is the first report of depression, psychosis-like symptoms and anxiolytic behavior of db/db mouse strain. It is tempting to speculate that this mouse strain can serve as useful preclinical model to study type-2 diabetes related neurobehavioral complications.
Keywords: Diabetic mice, Depression, Psychosis, Anxiety, Motor behavior, Memory
1. Introduction
Comorbid neurobehavioral disturbances in the type-2 diabetic population are emerging problems that warrant immediate research attention. The major concerns of comorbid neurobehavioral deficits in the diabetic population are a dramatic rise in mortality rate and substantial impact on the medical cost to the patient. Although comorbidity of neurobehavioral disturbances and type-2 diabetes in clinical practice is evident, it has not been studied comprehensively in preclinical setting. Significant research emphasis is given to the macrovascular and microvascular complications of diabetes [1–5]. Recent data also advocate the central nervous system (CNS) as a crucial target for diabetic complications [6]. For instance, the probability of major depression in diabetic patients is approximately double that of those without diabetes [7]. Further, among diabetic patients, those with comorbid depression are at greater risk of mortality and have twice the probability of cardiovascular risk factors like smoking, obesity and sedentary lifestyle [8]. To date, the majority of preclinical investigations studying interrelationships between diabetes and the CNS focused in principal on type-1 diabetes [9]. There are a few clinical reports that showed an association between diabetes and CNS complications that are most prominent in elderly type-2 diabetic patients [10]. There is a need to study CNS complications in type-2 diabetes animal models.
The diabetic mouse strain db/db harbors an autosomal recessive point mutation in gene encoding for the long isoform (LRb) of leptin receptor [11,12]. It was first witnessed by Hummel and colleagues in C57BL/KsJ strain at Jackson Laboratory [13]. Phenotypes of db/db mice mimics clinical type-2 diabetic conditions such as obesity, hyperglycemia, hyperinsulinemia, hyperphagia, polydypsia, polyurea, impotency, pancreatic β-cell hyperplasia and hypertrophy [14]. At early ages, they serve as a good model of type-2 diabetes, characterized by hyperinsulinemia, obesity and progressive hyperglycemia [15]. Hyperinsulinemia occurs as early as ten days of age, peaks at 3 months of age and then gradually declines to near normal values in the mature adult [15]. Blood glucose levels are slightly elevated by 6 weeks and reaches values of ~315 mg/dL, followed by ~550 mg/dL by 8 weeks [4] and as high as 600 mg/dL by 16 weeks of age [16]. Hitherto, this mouse strain is extensively used as a murine model for evaluating mechanisms of diabetes and to study the cardiovascular, renal and ocular complications of diabetes [17–19]. However, there is a paucity of research evidence that characterized this mouse strain for its neurobehavioral deficits [20–28]. Li and colleagues reported impaired spatial memory function and impairment of long-term potentiation (LTP) of memory in db/db mice using Morris water maze test [22]. A conditioned taste aversion (CTA) learning test in the same strain by Ohta and co-workers suggested no impairment in acquisition but extinction of CTA learned behavior compared to age-matched lean littermates [23]. Moreover, changes in the sleep-wake regulation pattern, locomotor activity [21], nociception [26], feeding behavior and body temperature regulation [20,25] are reported in the db/db mouse strain.
Interestingly, there is widespread distribution of long isoform of leptin receptors (LRb) in key brain regions [29]. Some of these regions, e.g. hypothalamic arcuate nucleus, paraventricular hypothalamic nucleus, dorsomedial hypothalamic nucleus and brainstem nucleus of the solitary tract modulate satiety. On the other hand, the mesolimbic dopamine system's ventral tegmental area and substantia nigra with projections to striatum and amygdala also express leptin receptors [30].While the nigrostiratal system is involved in locomotor activity, the amygdala is believed to be involved in threat identification and assignment of emotional value to new neutral stimuli. Brain regions modulating depressive symptoms e.g. serotonergic raphe nuclei [31] and anxiety behavior, e.g. amygdala were reported to express leptin receptors [32]. In addition, the hippocampus, an anatomical site that governs cognitive functions and spatial learning, express LRb receptor isoform. Regions of the cerebral cortex that are involved in regulation of mood and emotions showed presence of leptin receptors. Several other regions with less understood LRb function also express this particular receptor isoform [33]. Pharmacological and pathological manipulation of endogenous plasma and brain leptin concentration is reported in many neuropsychiatric disorders and after treatment with several drug classes that can affect brain functions [34–37]. Lower brain weights, reduced cortical volume and decrease in total glial and neuronal protein expressions were reported in leptin deficient ob/ob mice and similar effects on protein content and brain weights were observed in db/db mice [38]. Recently, Stranahan and colleagues reported diabetes-induced detrimental effects on hippocampal neuronal dendrite morphology and their recovery post-calorie restriction as well as after increased energy expenditure in db/db mice [28]. Interestingly, leptin can also alter neuronal dendrite morphology [39]. Increase in the concentration of gray matter in the anterior cingulate gyrus was reported in genetically leptin-deficient patients when subjected to leptin treatment [40]. Neonatal and maternal leptin treatments are reported to prevent the appearance of adverse metabolic phenotypes [41]. Intrigued with these reports, the present study evaluated db/db mouse strain for presence of neurobehavioral deficits employing paradigms for behavioral depression, psychosis-like symptoms, anxiety, alterations in motor behavior, thigmotaxis and impairments in working memory.
2. Materials and methods
Male db/db mice (4 week old) with background strain C57BL/KsJ (BKS-Cg-Dock7m +/+ Leprdb/J) and their age-matched non-diabetic lean control mice were purchased from Jackson Laboratories, Bar Harbor, ME, USA. All the mice were singly housed in plastic cages with wooden shavings in a temperature controlled room (22–23 °C) with 12:12 h light : dark cycle (lights off from 1700). Principles of laboratory animal care followed and all experimental protocols were approved by the Wright State University Animal Care and Use Committee.
2.1. Metabolic parameters
Standard pellet diet and tap water were provided ad libitum. To assess the metabolic status as an index of progression of diabetes, body weights, food consumption and water intake of juvenile (5–6 weeks) and adult (10–11 weeks) db/db and lean control mice were monitored weekly. Blood glucose (fed), plasma insulin and plasma glucose concentrations and body fat composition were also measured to verify development of diabetes in db/db mice.
2.1.1. Whole-body fat and lean mass measurements
The EchoMRI whole body composition analyzer (Houston, TX, USA) was used to determine fat and lean body mass of lean control and db/db mice [42]. The EchoMRI is a QNMR instrument that offers most precise and rapid measurement (1–2 min) of whole-body composition parameters like total body fat, lean mass, body fluids and total body water in live mice without sedation and anesthesia. Briefly, EchoMRI instrument was calibrated followed by live mouse placement into a plastic cylinder (inside diameter: 4.7 cm; thickness: 0.15 cm). A plastic plunger was used to restrain mouse movements except to turn back and about 4 cm vertical movements. Post completion of measurement (1–2 min), the mice were returned to their respective home cages. Fat and lean mass were calculated as percent of total mass.
2.1.2. Blood glucose, plasma insulin and plasma glucose measurements
For blood glucose measurements, blood samples were taken from a cut made on the tip of the tail and glucose was determined using an Accu-Check Advantage Blood Glucose Monitor (Roche Diagnostic Corporation, Indianapolis, IN) [Lean control (n = 18–20), db/db (n = 12–13)] to confirm development of diabetes in db/db mice compared to lean controls. Plasma glucose as well as insulin concentrations were also measured in 10 week old db/db mice and their age-matched lean controls that were not fasted. Mice were decapitated, and trunk blood was collected in ice-chilled heparinized tubes. Plasma was immediately separated and stored at −80 °C. The plasma samples were analyzed at Mouse Metabolic Phenotyping Center (Cincinnati, OH) for insulin and glucose according to the manufacturer's specifications (Millipore, St. Charles, MO).
2.2. Behavioral tests
After 1 week acclimatization, mice were subjected to behavioral testing. At two different age points (juvenile: 5–6 weeks and adult: 10–11 weeks) separate groups of mice (db/db, n = 7–10; lean controls, n = 8–10) were subjected to 1) forced swim test, 2) pre-pulse inhibition test, 3) elevated plus maze test, 4) open field test and 5) Y-maze test. All the tests were conducted between the hours of 0900–1400 to minimize circadian influences on mouse behavior.
2.2.1. Forced swim test
As described previously [43] mice were placed in transparent glass cylinders (diameter, 10 cm; height, 25 cm) filled up to 10 cm with water (23–25 °C) for 6 min test and scored for duration of immobility [lean control, n = 8 (juvenile) and 10 (adult); db/db, n = 8 (juvenile) and 7 (adult)]. A mouse was considered as immobile when floating motionless or making only those movements necessary to keep its head above the water. The db/db and lean control mice were tested simultaneously in separate cylinders with opaque white plexiglass sheet placed between them so that mice cannot see each other. During test sessions, mice were video recorded to score immobility time. After the swim test, mice were dried with a towel and returned to the home cage placed on a thermal blanket heated to 37 °C.
2.2.2. Pre-pulse inhibition test
As shown in our previous report [44], mice were tested in automated startle chambers (SM100 Startle Monitor System Version 6.12; Hamilton Kinder, Poway, CA) for pre-pulse inhibition (PPI) [lean control, n = 10 (juvenile and adult); db/db, n = 10 (juvenile) and 7 (adult)]. There were five types of white noise burst stimulus trials: pre-pulse (70 dB), pulse (85 dB and 100 dB) and pre-pulse prior to pulse (70 dB + 85 dB and 70 dB + 100 dB) with background noise (60 dB). Each trial type was presented 10 times. Stimuli were presented in random order to avoid order effects and habituation. The inter-trial intervals were varied from 9 to 16 s. Mice were in holders restricting rearing behavior and placed on pressure-sensor plates transforming movements of the body (jerks) into an analog signal through an interface.
2.2.3. Elevated plus maze test
This is a behavioral paradigm that takes advantage of the conflict behavior of rodents between exploration of a novel area and aversion to open and elevated spaces [45,46]. The maze is made up of opaque black Plexiglas with opposite facing two open (14 × 2 inches) and two enclosed arms (14 × 2 × 6 inches) connected by a central platform (2 × 2 inches). The whole maze is raised 30 inches above the floor. Mice were tested on the plus maze in a room with low, indirect incandescent red lighting and very low noise levels. On the day of testing, the mouse was placed at the center of the maze with head facing an open arm and allowed to explore for 5 min [lean control, n = 8 (juvenile) and 10 (adult); db/db, n = 7 (juvenile and adult)]. The number of entries, time spent and distance traveled in each arm were recorded with the help of automated elevated plus maze system (EPM) (Hamilton Kinder, Version 3.11; Poway, CA). The software was configured to register entry when all four paws of the animal were placed on the arm. The maze was wiped clean with 70% ethanol solution and dried after testing each mouse. Increase in time spent and frequency of open arms entries relative to control mice were considered as indicators of anxiolytic behavior.
2.2.4. Open field test
An automated open field system with microprocessor and infrared photo-beams (Hamilton Kinder, Motor Monitor Version 3.11; Poway, CA) was used to evaluate locomotor and thigmotactic behavior of mice [44]. The open field consisted of 16 × 16 in (40.6 × 40.6 cm) plexiglass square. For analysis, the chamber was divided into central (8 × 8 in) and peripheral (4 in wide) zones. Each mouse was placed in the center of the open field arena and allowed to explore it for 10 min [lean control, n = 10 (juvenile and adult); db/db, n = 10 (juvenile) and 7 (adult)]. During 10 min test session, the variables of locomotor activity, basic movements (quantified by IR beam interruptions due to larger mouse body movements in the open field), fine movements (defined by IR beam interruptions due to fine movements such as head-twitching, grooming etc) and percent time spent in periphery and central zones were recorded using Motor Monitor software. Open field arena was cleaned with 70% ethanol solution and let dry after testing each mouse.
2.2.5. Y-maze test
The Y-maze measures mouse's functional working memory status and exploit the innate tendency to explore novel areas [47]. The apparatus for Y-maze test was made of 3 acrylic plastic arms (arm dimensions: 3.5 cm × 20 cm) at 120 degrees to each other. During test, individual mice were placed on the intersection of 3 arms of the maze and were video recorded for 8 min to score for number and sequence of arm entries [lean control, n = 10 (juvenile and adult); db/db, n = 7 (juvenile and adult)]. An arm entry was registered when all four paws of mouse were within any of 3 arms. Entries into 3 different arms in succession (e.g. ABC or BCA or CBA or CAB etc) were defined as alternations. Percent Y-maze scores were calculated using the formula:
2.3. Data analysis
The results are presented as group means ± S.E.M. and analyzed using Graphpad Prism 5 software. Data were analyzed by 2-way ANOVA test. The two variables for 2-way ANOVA test were ‘strain’ (db/db versus lean control) and ‘age’ of mice (juvenile versus adult). Bonferroni test was used for post-hoc comparisons. Unpaired Student t-test was used to analyze differences in body weight, food intake, water intake, % fat mass, % lean mass, % body water, blood glucose and plasma insulin concentrations. Value of p≤0.05 was considered significant.
3. Results
3.1. Metabolic parameters
As shown in Table 1, the blood glucose concentrations of db/db mice were significantly higher compared to age-matched lean controls (p<0.05, unpaired t-test). There was an age-dependent increase in the blood glucose concentration of db/db mice (juvenile: 230.38 ± 13.25 mg/dL versus adult: 583.20 ± 35.86 mg/dL). However, blood glucose concentrations of lean control mice remained fairly constant (p>0.05). Food intake, water intake, plasma insulin and % fat mass of db/db mice were significantly increased compared to age-matched lean controls (p<0.05, unpaired t-test). However, percent lean mass and percent body water of db/db mice were significantly low compared to age-matched lean controls (p<0.05, unpaired t-test).
Table 1.
Metabolic parameters of lean control and db/db mice.
| Mouse strain | Lean control | db/db | Lean control | db/db |
|---|---|---|---|---|
| Age (weeks) | 5 | 5 | 10 | 10 |
| Group size (n) | 18–20 | 12–13 | 18–20 | 12–13 |
| Body weight (g) | 19.63 ± 0.28 | 24.14 ± 0.6* | 25.18 ± 0.56 | 35.97 ± 1.2* |
| Blood glucose (mg/dL) | 145.25 ± 4.7 | 230.38 ± 13.25* | 140.32 ± 8.81 | 583.20 ± 35.86* |
| Plasma insulin (ng/ml) | ND | ND | 1.27 ± 0.21 | 4.61 ± 1.52* |
| Food intake (g/day/mouse) | 4.17 ± 0.13 | 7.43 ± 0.43* | 3.85 ± 0.11 | 6.74 ± 0.37* |
| Water intake (g/day/mouse) | 7.05 ± 0.13 | 15.66 ± 1.69* | 9.76 ± 0.89 | 20.15 ± 2.39* |
| % Fat mass | ND | ND | 17.94 ± 1.01 | 58.92 ± 0.75* |
| % Lean mass | ND | ND | 70.43 ± 1.01 | 42.78 ± 1.40* |
| % Body water | ND | ND | 59.62 ± 1.00 | 35.85 ± 1.16* |
ND, not determined.
p<0.05 versus age-matched lean controls, unpaired t-test.
3.2. Behavioral tests
3.2.1. Depression-like behavior of db/db mice
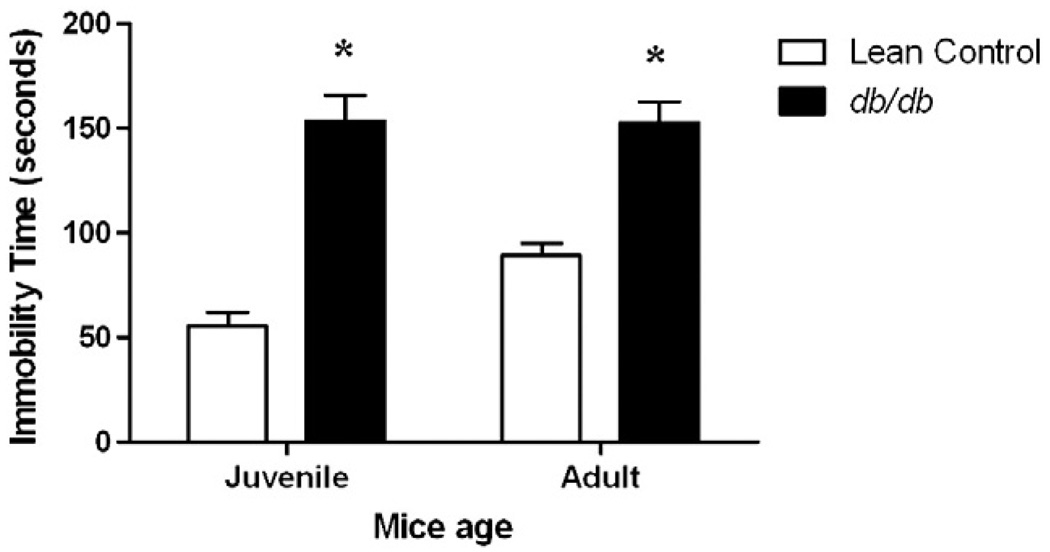
As shown in Fig. 1, juvenile as well as adult db/db mice were immobile for significantly more time than age-matched lean controls in the 6 min forced swim test [factor ‘strain’ F (1, 32) = 83.22, p<0.001]. There was no age-dependent significant change in duration of immobility in lean control as well as db/db mice (p>0.05).
Fig. 1.
Depression-like behavior of db/db mice. Separate groups of juvenile (5–6 weeks) and adult (10–11 weeks) db/db mice and their age-matched lean controls were tested for duration of immobility in forced swim test. Each mouse was forced to swim in water cylinder and scored for duration of immobility. Each bar represents means ± S.E.M. of data from 7–10 mice per group. *p<0.05 vs. age-matched lean control mice.
3.2.2. Psychosis-like behavior of db/db mice (PPI)
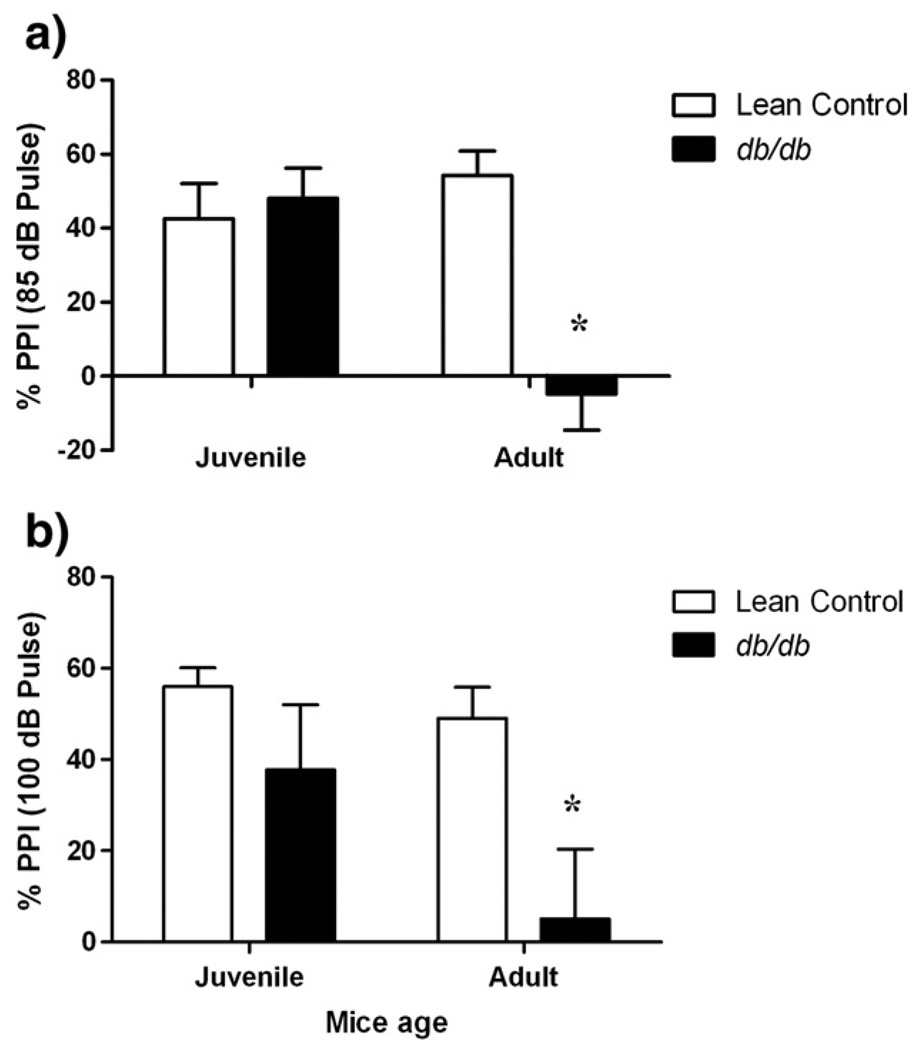
Normal PPI was observed in juvenile db/db mice at both intensities of startle stimuli (Fig. 2; p>0.05). However, percent PPI in adult db/db mice was significantly lowered with respect to age-matched lean controls with (70 dB + 85 dB) [factor ‘strain’ F (1, 36) = 9.705, p<0.001] and (70 dB + 100 dB) stimuli [factor ‘strain’ F (1, 36) = 8.575, p<0.05]. Further, there was an age-dependent decrease in PPI response of db/db mice with (70 dB + 85 dB) startle stimuli [factor ‘age’ F (1, 36) = 3.474, p<0.05] but not with (70 dB + 100 dB) stimuli (p>0.05). 3 out of 10 juvenile db/db mice also showed disruption of PPI for 70 dB + 100 dB (pre-pulse + pulse) startle stimuli, however, no change in PPI response of remaining 7 juvenile db/db mice of the group nullified this difference when compared with age-matched lean control group.
Fig. 2.
Psychosis-like behavior of db/db mice. Different groups of juvenile (5–6 weeks) and adult (10–11 weeks) db/db mice and their age-matched lean controls were tested for PPI behavior. Each mouse was subjected to 5 types of trials and each trial was presented 10 times as pulse alone (85 dB or 100 dB white noise), pre-pulse alone (70 dB) and pre-pulse + pulse (70 dB + 85 dB or 70 dB + 100 dB) in randomized fashion (total trials: 5 × 10 = 50) with background noise (60 dB) in startle chambers and their startle responses to these stimuli (in Newtons; Max[N]) were recorded using pressure-sensitive plates. Each bar represents means ± S.E.M. of data from 7–10 mice per group. *p<0.05 vs. age-matched lean control mice.
3.2.3. Anxiolytic behavior of db/db mice (EPM)
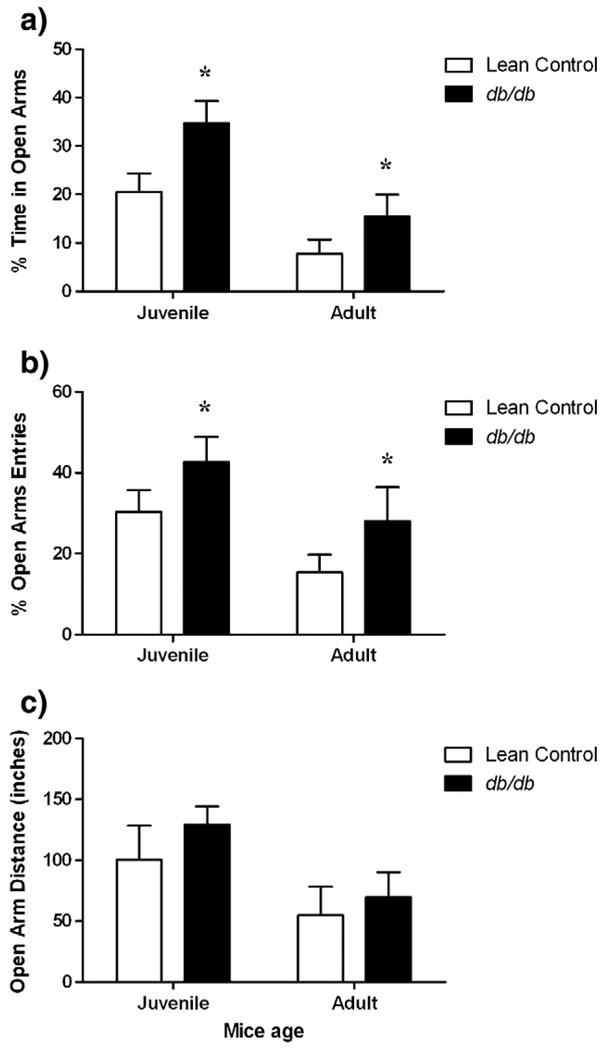
In the elevated plus maze test, there was a significant increase in percent time spent in open arms (Fig. 3a) [factor ‘strain’ F (1, 31) = 7.785, p<0.05, factor ‘age’ F (1, 31) = 16.73, p<0.05)] and percent entries into open arms (Fig. 3b) [factor ‘strain’ F (1, 31) = 4.337, p<0.05, factor ‘age’ F (1, 31) = 6.066, p<0.05)] in juvenile and adult db/db mice as compared to age-matched lean controls. Moreover, db/db mice traveled about equivalent distances on open arms compared to age-matched lean controls (Fig. 3c) (p>0.05).
Fig. 3.
Anxiolytic behavior of db/db mice showing a) Percent time spent in open arms b) Percent open arms entries and c) Open arms distance traveled (inches) on elevated plus maze. Different groups of juvenile (5–6 weeks) and adult (10–11 weeks) db/db mice and their age-matched lean littermates were subjected to 5 min elevated plus maze test. Each bar represents means ± S.E.M. of data from 7–10 mice per group. *p<0.05 vs. age-matched lean control mice.
3.2.4. Hypo-locomotive and thigmotaxis behavior of db/db mice
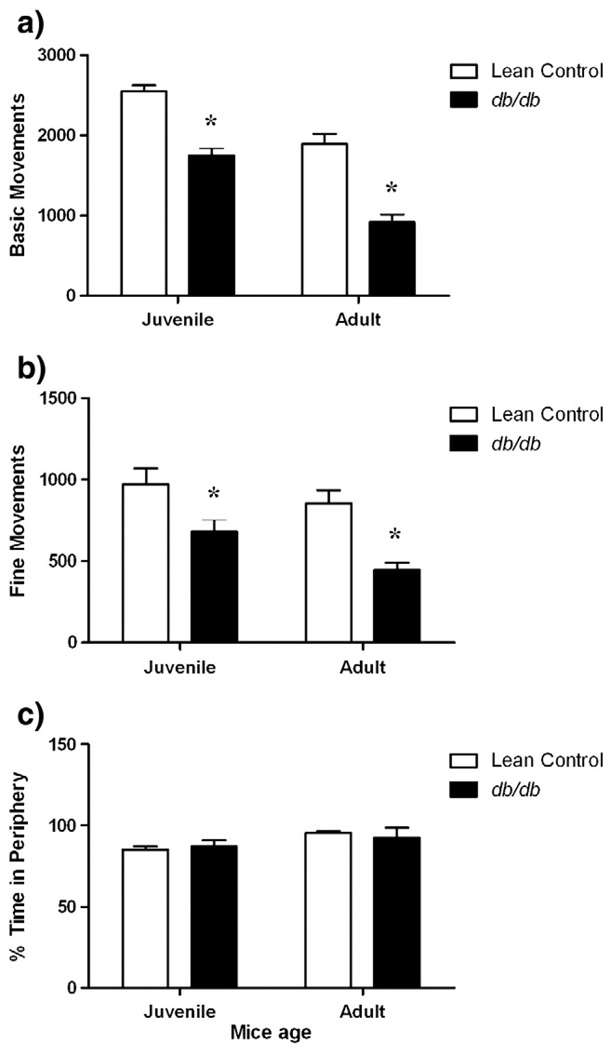
Both juvenile and adult db/db mice had significantly less activity counts for (a) basic movements and (b) fine movements compared to their age-matched lean controls in the open field test (Fig. 4a and b). Both strains also underwent a decrease in these measures over time. 2-way ANOVA showed a significant effect on basic movements [factor ‘strain’ F (1, 35) = 74.17, p<0.001; factor ‘age’ F (1, 35) = 52.94, p<0.001] and fine movements [factor ‘strain’ F (1, 35) = 17.94, p<0.05; factor ‘age’ F (1, 35) = 4.497, p<0.01] of db/db mice compared to age-matched lean controls. During the 10 min test session, db/db and age-matched lean control mice spent significantly more time in the peripheral than the central zone indicative of thigmotaxis (p<0.05). However, there was no difference in percent time spent in periphery between db/db mice and age-matched lean controls (p>0.05) (Fig. 4c).
Fig. 4.
Hypo-locomotive and thigmotaxis behavior of db/db mice in open field test showing a) Basic movements b) Fine movements and c) Percent time spent in periphery. Different groups of juvenile (5–6 weeks) and adult (10–11 weeks) db/db mice and their age-matched lean controls were subjected to 10 min open field test. Each bar represents means ± S.E.M. of data from 7–10 mice per group. *p<0.05 vs. age-matched lean control mice.
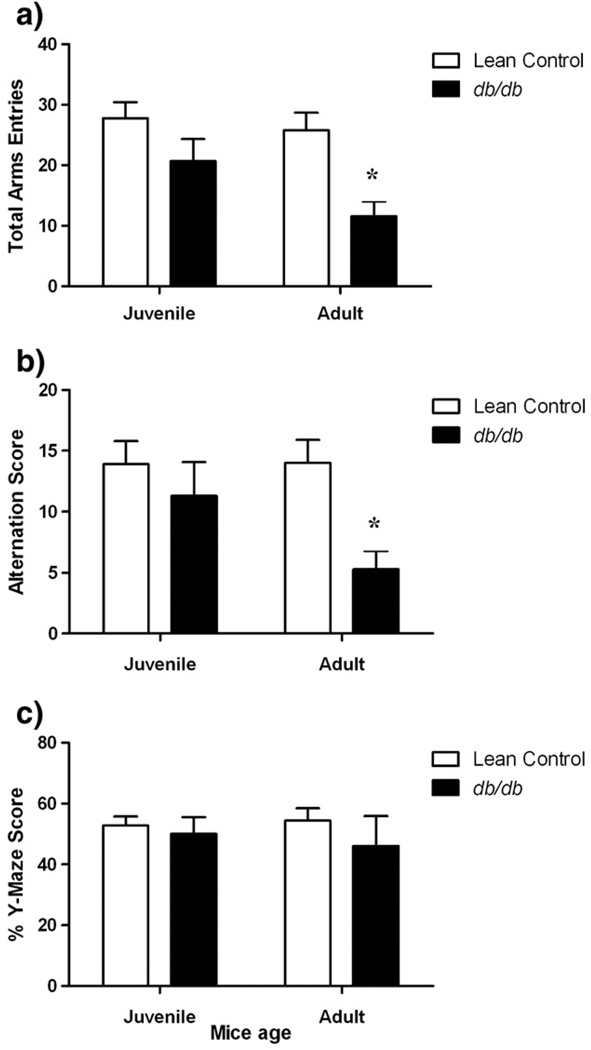
3.2.5. Working memory test in db/db mice
Percent Y-maze scores of juvenile as well as adult db/db mice were not statistically different from age-matched control littermates (p>0.05; Fig. 5). There was a significant decrease in total number of Y-maze arms entries [factor ‘strain’ F (1, 33) = 12.91, p<0.01] and alternation score due to decreased activity [factor ‘strain’ F (1, 33) = 7.469, p<0.05] of adult db/db mice compared to age-matched control mice that led to no change in their percent Y-maze scores (p>0.05).
Fig. 5.
Working memory test in db/db mice. Different groups of juvenile (5–6 weeks) and adult (10–11 weeks) db/db mice and their age-matched lean controls were subjected to 8 min Y-maze test and scored for a) total arm entries, b) alternations and c) percent Y-maze scores. Each bar represents means ± S.E.M. of data from 7–10 mice per group. *p<0.05 vs. age-matched lean control mice.
4. Discussion
The present study is the first report of behavioral depression, age-related advancement of psychosis-like symptoms and anxiolytic behavior in db/db mice. We also observed locomotor deficits in this mouse strain but there were no changes in working memory performance of db/db mice compared to lean controls. The neuropsychological consequences of diabetes are studied with reasonable detail in the clinical setting. However, there is still a scarcity of preclinical research supporting correlations between type-2 diabetes and such consequences. The current study attempted to provide experimental evidence for interrelationship between type-2 diabetes and neuropsychological deficits using db/db mice.
Depression is the major co-occurring psychological disorder with diabetes [48]. We evaluated juvenile and adult db/db mice for behavioral depression using the forced swim test (Fig. 1). Both age-groups of db/db mice showed significant increases in the duration of immobility compared to age-matched lean control mice suggesting the occurrence of depression-like symptoms in db/db mice. While the obesity of the adults may have impaired swimming ability, the swimming deficits in non-obese juveniles suggest that central nervous system mechanisms related to depression are the cause. Additionally, poor thermoregulation in db/db mice can also confound immobility duration in FST. However, Trayhurn had reported similar diurnal rhythm in body temperature of db/db mice to control mice at 23 °C [49]. We used similar temperature range (23–25 °C) for FST. In contrast to rats that generally require two-trial FST, one-trial is adequate to produce consistent immobility scores using mice in FST [50–52].
The probability of depression in diabetic patients is approximately double in comparison to those without diabetes [7]. Behavioral despair in diabetic patients can lead to apathy towards self-care regimens such as regular physical exercise, dietary habits, cessation of smoking and towards medication for diabetes [48]. Diabetic patients with comorbid depression are reported to have double the probability of cardiac risk factors like smoking, obesity, sedentary lifestyle etc [8]. Recently Hirano and colleagues reported a decrease in circulating plasma leptin concentrations and depression-like symptoms in streptozotocin-induced diabetic mice [53]. Interestingly, treatment of these diabetic mice with leptin reversed the depression-like behavior in the tail suspension test (TST), a model for depression. In view of the mutation in leptin receptors (LRb) in db/db mice and an antidepressant-like effect of leptin in a mouse model for diabetes, behavioral depression observed in db/db mice may be the outcome of impaired leptin signaling. Leptin treatment restores preference for sucrose consumption in rodents subjected to chronic stress and reduced preference for sucrose is analogous to anhedonia, a hallmark feature of depression [37,54]. Further, systemic leptin treatment is reported to lower immobility in FST and TST in rodents, an indicator of antidepressant-like effect of leptin that was devoid of stimulation of motor behavior. Thus, leptin can serve as a potential neurobiological substrate for the treatment of depression [37]. Several research groups reported expression of leptin receptors in serotonergic raphe nuclei [31] and dopaminergic ventral tegmental area and substantia nigra [30]. Collin and co-workers reported a fall in serotonin transporter mRNA expression in raphe nuclei of functional leptin deficient ob/ob mice [55]. Leptin increases production of serotonin and its biotransformation product, 5-HIAA in the forebrain [56]. Thus, it is plausible that leptin may interact with monoaminergic neurons involved in the pathophysiology of depression and related disorders by modulating their firing pattern and downstream signaling mechanisms in a manner that may lead to depression in humans and to the behavioral deficits in db/db mice.
We used PPI of startle to evaluate age-dependent progression of psychosis-like symptoms in db/db mice (Fig. 2). While juvenile db/db mice did not differ from age-matched lean controls in terms of percent PPI scores when subjected to a series of randomly presented pre-pulse plus pulse (70 dB + 85 dB and 70 dB + 100 dB) startle stimuli trials, adult db/db mice experienced significant disruption of PPI behavior with respect to age-matched lean controls. Such disruption of PPI behavior in adult but not in juvenile db/db mice signifies age-dependent progression of psychosis-like behavior in this murine model for type-2 diabetes. Three out of 10 juvenile db/db mice also showed psychosis-like behavior, which may indicate that this is the age at which abnormalities begin to develop. PPI behavior is a natural hardwired trait of the normal, non-psychotic subjects. Disruption of PPI is the hallmark symptom to confirm psychosis-like symptoms and it is rare to ever have a disruption in a normal animal.
Comorbid type-2 diabetes is reported to impair physical as well as mental health status of adult schizophrenic patients compared to non-diabetic schizophrenics [57]. Emergence of new-onset diabetes was reported in schizophrenic patients on initiation of atypical antipsychotic medications [58–60]. Hyperglycemia and insulin resistance was also reported in mice that were on chronic antipsychotic treatment [61]. Current clinically proven medications for psychosis may not prove a good strategy to treat co-occurring psychotic symptoms in diabetic population that may further complicate existing diabetic phenotypes. Recently van Nimwegen and co-workers showed that antipsychotic naive schizophrenics also experience hepatic insulin resistance compared to non-schizophrenic controls. Such increased propensity towards insulin resistance in schizophrenics may be the outcome of metabolic abnormalities [62]. These findings hints toward bidirectional link between diabetes and schizophrenia.
We observed db/db mice to be less anxious than age-matched lean controls in the elevated plus maze test (Fig. 3). Juvenile as well as adult db/db mice spent significantly more time on the open arms compared to age matched lean controls. db/db mice also showed a significant increase in percent open arms entries compared to age-matched lean controls. Both age-group db/db mice traveled equal distance on open arms compared to age-matched lean controls suggesting that the observed increase in time spent into open arms by db/db mice is not affected by their hypo-locomotive behavior. Though db/db mice have impaired leptin signaling, previous literature about leptin's role in anxiety behavior is ambiguous. Asakawa and co-workers reported that leptin treatment can ameliorate anxiety-like behavior of ob/ob mice [34]. On the other hand, Buyse and colleagues reported decreased open arms exploration in elevated plus maze test with intraperitoneal leptin treatment in diet-restricted rats [63]. In contrast, Suomalainen and Mannisto reported that higher doses of leptin (10 and 20 mg/kg) failed to affect anxiety behavior in ad libitum fed mice [64]. Also, in spontaneously diabetic INS2Akita mouse, Asakawa and colleagues reported anxiety-like behavior using the elevated plus maze test [65]. Labad and colleagues recently have shown that depression but not anxiety is associated with type-2 diabetes [66]. Results of anxiety and depression screening of db/db mice are consistent with this clinical report showing the presence of depression but not anxiety. Contrary to this, high anxiety scores [67] and increased prevalence of generalized anxiety disorder was reported in type-2 diabetic patients relative to non-diabetic adults [68]. Clinical studies evaluating anxiety levels in type-2 diabetic patients led to conflicting outcomes. Exploring the biological mechanisms that govern anxiolytic behavior of db/db mice may help to understand why there are differences in some but not all neuropsychological deficits with type-2 diabetes.
In the open field test, db/db mice undergo a decrease in basic movements (quantified by IR beam interruptions due to more body movements in the open field) and fine movements (IR beam interruptions due to fine movements such as head-twitching, grooming etc) compared to age-matched lean controls. These results are in agreement of our previous research report [4] and others [21,28]. Laposky and colleagues used 3–4 months (or 12–16 weeks) adult db/db mice and studied total activity counts, while the present study investigated locomotor behavior of much younger (5–6 weeks) db/db mice. Similar changes in locomotor behavior of juvenile db/db mice were reported by Hesse and co-workers [69]. We evaluated db/db mice for basic movements due to whole body movements as well as fine movements resulting from head-twitching, grooming etc. Hypo-locomotion is the hallmark feature of several psychiatric disorders like Parkinson's disease, Huntington chorea, antipsychotic-induced pseudoparkinsonism etc. While some have argued that decreased locomotor activity in the open field test may account for increased duration of immobility in FST, others have observed no correlation between locomotor activity and performance of mice in FST [55,70]. Increased immobility in FST as well as decrease in locomotor activity was reported in ob/ob mouse strain [55]. Further, clinical studies indicated comorbidity of depression with hypolocomotive disorders such as Parkinsonism [71]. If a decrease in locomotor activity is causing increased immobility time in forced swim test, it should also reduce the distance traveled on open arms in the elevated plus maze. However, there was no reduction in the db/db mice compared to age-matched lean controls. We also believe that open field test is not a behavioral model to test depression-like symptoms unless animals are olfactory bulbectomized. It is most appropriately used to reflect deficits in motor behavior and to test partial anxiety than depression of mice with intact olfactory bulbs. Thus, depressive-symptoms can also be observed in hypo-locomotive individuals. Use of alternative models such as learned helplessness, sucrose preference test or TST that mimics depression-like symptoms may further strengthen presence of depressive symptoms in this mouse strain. Unfortunately sucrose consumption test requires an extensive amount of time before screening for depression-like behavior [72] which may preclude evaluation of age-related changes in behavior. Previous reports suggest that TST is not the ideal test for mice with C57 genetic background (like db/db mice) that have a tendency to climb on the tail [73]. Further, TST, like FST, can also depend on motor activity [74]. Like lean controls, db/db mice also exhibited thigmotaxis behavior—a tendency of animals to stay in proximity of support while traveling. This is indicative of a normal response to the low stress environment of an open arena. Stranahan and colleagues also showed similar tendency of db/db mice to spend less time in the center arena. However, they also have reported significant difference in percent time in center arena between db/db mice and control mice [28].
Cognitive deficits are the hallmark features of obese and diabetic rodents [22] and are also prevalent in type-2 diabetic patients [10]. Diabetic obese rodents have impaired spatial memory task performance in Morris water maze test [22]. We tested db/db mice for their working memory rather than spatial memory performance. Both age-group db/db mice did not show signs of impairment of working memory in Y-maze test compared to age-matched lean control mice (Fig. 5). While the frontal cortex is the anatomical site for working memory, the hippocampus's role as the regulator for spatial memory functions is well documented [75,76]. Recently, Stranahan and colleagues reported diabetes-induced detrimental effects on hippocampal neurons, impaired recovery from hippocampal neuronal defects post-calorie restriction and increased energy expenditure in insulin-resistant and leptin-receptor mutated db/db mice [28]. Further, leptin can alter neuronal dendrite morphology [39]. Elevations in corticosterone levels [27] as well as reduced hippocampal brain-derived neurotrophic factor (BDNF) levels [28] are suggested as crucial mechanisms for diabetes-related cognitive deficits. Ohta and colleagues using conditioned taste aversion (CTA) learning test showed that impaired downstream signaling due to the mutation in leptin receptors in db/db mice had no effect on acquisition of CTA learning but promoted faster extinction [23]. Yamamoto suggested the parabrachial nucleus, amygdala, insular cortex, supramammillary nucleus, nucleus accumbens, and ventral pallidum as possible anatomical sites for CTA learning in rats [77]. Li and colleagues reported compromised spatial memory performance in the Morris water maze test [22]. db/db mice may have intact working memory as evident from Y-maze test results but poor spatial memory performance and faster extinction of CTA points to anatomical specificity in memory deficits.
Apart from leptin and insulin resistance [78], hormones like ghrelin [79] and neurotransmitters e.g. norpinephrine [2,80] could be other dominant mechanisms that may govern type-2 diabetes-linked CNS dysfunctions. Evidence suggests that leptin can restore normal glucose levels [81] and improve insulin-sensitivity of target organs [82,83]. In contrast, long-term hyperglycemia lowers leptin levels [84]. Thus insulin and leptin may have similar mechanisms and maintain close temporal synchronism. Although mutation in leptin receptors is not observed clinically, db/db mice could be the important experimental tool to investigate for the possible involvement of leptin in diabetes related insulin resistance and comorbid complications. Recently, Schwartz and Bahn reviewed possible application of altered proteins and metabolites identified in schizophrenic brain cerebrospinal fluid [85]. Investigations for such schizophrenia-specific biomarkers in type-2 diabetic brains may unravel mechanisms that led to age-dependent advancement of psychosis-like behavior in db/db mice. In view of resistance to insulin (severe hyperinsulinemia) and leptin (mutation in leptin receptor LRb isoform), pharmacological manipulation of db/db mice with exogenous insulin or leptin to correct observed behavioral depression and psychosis-like symptoms do not seem to be a convincing rationale. However, our future studies will address the effect of insulin-sensitizers such as rosiglitazone and metformin on neurobehavioral deficits in db/db mice. Our future research will also report marked biochemical differences in dopamine and its major metabolites in db/db mice brain compared to lean control mice (Sharma et al., unpublished data). Our preliminary results suggest a significant relationship between progression of type-2 diabetes, neurobehavioral deficits and dopamine turnover pattern in key brain regions, particularly frontal cortex that regulates PPI or psychosis-like behavior (Sharma et al., unpublished data).
In conclusion, this is the first study to report behavioral depression, psychosis-like symptoms and anxiolytic behavior in db/db mice strain. Also, db/db mice were hypo-locomotive; exhibited normal thigmotaxis and no impairment of working memory. Thus, db/db mice showed the presence of select group of neuropsychological deficits. The db/db mice could be used as a model to study type-2 diabetes-induced depression and psychosis. Investigations to find biochemical and neurobiological markers governing these deficits may help to improve our understanding about interrelationships between diabetes and comorbid CNS disorders.
Acknowledgements
The authors acknowledge the financial support of the AHA SDG 0735112N and the NIH R01 HL093567 (K.M.E.).
References
- 1.Arora RR, Bulgarelli RJ, Ghosh-Dastidar S, Colombo J. Autonomic mechanisms and therapeutic implications of postural diabetic cardiovascular abnormalities. J Diabetes Sci Technol. 2008;2:645–657. doi: 10.1177/193229680800200416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garris DR. Reproductive tract and pancreatic norepinephrine levels in pre- and overt-diabetic C57BL/KsJ mice: Relationship to body weight, blood glucose, serum insulin, and reproductive dysfunction. Proc Soc Exp Biol Med. 1988;189:79–83. doi: 10.3181/00379727-189-42783. [DOI] [PubMed] [Google Scholar]
- 3.Su W, Guo Z, Randall DC, Cassis L, Brown DR, Gong MC. Hypertension and disrupted blood pressure circadian rhythm in type 2 diabetic db/db mice. Am J Physiol Heart Circ Physiol. 2008;295:H1634–H1641. doi: 10.1152/ajpheart.00257.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senador D, Kanakamedala K, Irigoyen MC, Morris M, Elased KM. Cardiovascular and autonomic phenotype of db/db diabetic mice. Exp Physiol. 2009;94:648–658. doi: 10.1113/expphysiol.2008.046474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim AK, Nikolic-Paterson DJ, Ma FY, Ozols E, Thomas MC, Flavell RA, et al. Role of MKK3-p38 MAPK signalling in the development of type 2 diabetes and renal injury in obese db/db mice. Diabetologia. 2009;52:347–358. doi: 10.1007/s00125-008-1215-5. [DOI] [PubMed] [Google Scholar]
- 6.Laron Z. Insulin and the brain. Arch Physiol Biochem. 2009;115:112–116. doi: 10.1080/13813450902949012. [DOI] [PubMed] [Google Scholar]
- 7.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diab Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 8.Katon WJ, Lin EH, Russo J, Von KM, Ciechanowski P, Simon G, et al. Cardiac risk factors in patients with diabetes mellitus and major depression. J Gen Intern Med. 2004;19:1192–1199. doi: 10.1111/j.1525-1497.2004.30405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beauquis J, Homo-Delarche F, Revsin Y, De Nicola AF, Saravia F. Brain alterations in autoimmune and pharmacological models of diabetes mellitus: Focus on hypothalamic-pituitary-adrenocortical axis disturbances. Neuroimmunomodulation. 2008;15:61–67. doi: 10.1159/000135625. [DOI] [PubMed] [Google Scholar]
- 10.Gispen WH, Biessels GJ. Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci. 2000;23:542–549. doi: 10.1016/s0166-2236(00)01656-8. [DOI] [PubMed] [Google Scholar]
- 11.Coleman DL. Lessons from studies with genetic forms of diabetes in the mouse. Metabolism. 1983;32:162–164. doi: 10.1016/s0026-0495(83)80031-6. [DOI] [PubMed] [Google Scholar]
- 12.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 13.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153:1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 14.Chua SC, Jr, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271:994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- 15.Coleman DL, Hummel KP. Hyperinsulinemia in pre-weaning diabetes (db) mice. Diabetologia. 1974;10 Suppl:607–610. doi: 10.1007/BF01221993. [DOI] [PubMed] [Google Scholar]
- 16.Lee SM, Bressler R. Prevention of diabetic nephropathy by diet control in the db/db mouse. Diabetes. 1981;30:106–111. doi: 10.2337/diab.30.2.106. [DOI] [PubMed] [Google Scholar]
- 17.Allen TJ, Cooper ME, Lan HY. Use of genetic mouse models in the study of diabetic nephropathy. Curr Diab Rep. 2004;4:435–440. doi: 10.1007/s11892-004-0053-1. [DOI] [PubMed] [Google Scholar]
- 18.Clements RS, Jr, Robison WG, Jr, Cohen MP. Anti-glycated albumin therapy ameliorates early retinal microvascular pathology in db/db mice. J Diabetes Complications. 1998;12:28–33. doi: 10.1016/s1056-8727(97)00051-2. [DOI] [PubMed] [Google Scholar]
- 19.Pereira L, Matthes J, Schuster I, Valdivia HH, Herzig S, Richard S, et al. Mechanisms of [Ca2+]i transient decrease in cardiomyopathy of db/db type 2 diabetic mice. Diabetes. 2006;55:608–615. doi: 10.2337/diabetes.55.03.06.db05-1284. [DOI] [PubMed] [Google Scholar]
- 20.Faggioni R, Fuller J, Moser A, Feingold KR, Grunfeld C. LPS-induced anorexia in leptin-deficient (ob/ob) and leptin receptor-deficient (db/db) mice. Am J Physiol. 1997;273:R181–R186. doi: 10.1152/ajpregu.1997.273.1.R181. [DOI] [PubMed] [Google Scholar]
- 21.Laposky AD, Bradley MA, Williams DL, Bass J, Turek FW. Sleep-wake regulation is altered in leptin-resistant (db/db) genetically obese and diabetic mice. Am J Physiol Regul Integr Comp Physiol. 2008;295:R2059–R2066. doi: 10.1152/ajpregu.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113:607–615. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 23.Ohta R, Shigemura N, Sasamoto K, Koyano K, Ninomiya Y. Conditioned taste aversion learning in leptin-receptor-deficient db/db mice. Neurobiol Learn Mem. 2003;80:105–112. doi: 10.1016/s1074-7427(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 24.Oomura Y, Aou S, Fukunaga K. Prandial increase of leptin in the brain activates spatial learning and memory. Pathophysiology. 2009 doi: 10.1016/j.pathophys.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Pelleymounter MA, Lorden JF. Feeding, activity, and body temperature following 6-hydroxydopamine lesions in diabetes (db/db) mice. Behav Neurosci. 1983;97:810–821. doi: 10.1037//0735-7044.97.5.810. [DOI] [PubMed] [Google Scholar]
- 26.Wright DE, Johnson MS, Arnett MG, Smittkamp SE, Ryals JM. Selective changes in nocifensive behavior despite normal cutaneous axon innervation in leptin receptor-null mutant (db/db) mice. J Peripher Nerv Syst. 2007;12:250–261. doi: 10.1111/j.1529-8027.2007.00144.x. [DOI] [PubMed] [Google Scholar]
- 27.Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, et al. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19:951–961. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leinninger GM, Myers MG., Jr LRb signals act within a distributed network of leptin-responsive neurones to mediate leptin action. Acta Physiol (Oxf) 2008;192:49–59. doi: 10.1111/j.1748-1716.2007.01784.x. [DOI] [PubMed] [Google Scholar]
- 30.Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 2003;964:107–115. doi: 10.1016/s0006-8993(02)04087-8. [DOI] [PubMed] [Google Scholar]
- 31.Finn PD, Cunningham MJ, Rickard DG, Clifton DK, Steiner RA. Serotonergic neurons are targets for leptin in the monkey. J Clin Endocrinol Metab. 2001;86:422–426. doi: 10.1210/jcem.86.1.7128. [DOI] [PubMed] [Google Scholar]
- 32.Han Z, Yan JQ, Luo GG, Liu Y, Wang YL. Leptin receptor expression in the basolateral nucleus of amygdala of conditioned taste aversion rats. World J Gastroenterol. 2003;9:1034–1037. doi: 10.3748/wjg.v9.i5.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morash B, Li A, Murphy PR, Wilkinson M, Ur E. Leptin gene expression in the brain and pituitary gland. Endocrinology. 1999;140:5995–5998. doi: 10.1210/endo.140.12.7288. [DOI] [PubMed] [Google Scholar]
- 34.Asakawa A, Inui A, Inui T, Katsuura G, Fujino MA, Kasuga M. Leptin treatment ameliorates anxiety in ob/ob obese mice. J Diabetes Complications. 2003;17:105–107. doi: 10.1016/s1056-8727(02)00185-x. [DOI] [PubMed] [Google Scholar]
- 35.Baptista T, Beaulieu S. Leptin and antipsychotic drugs. Br J Psychiatry. 2001;179:560–561. [PubMed] [Google Scholar]
- 36.Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides. 2006;27:1420–1425. doi: 10.1016/j.peptides.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Lu XY, Kim CS, Frazer A, Zhang W. Leptin: A potential novel antidepressant. Proc Natl Acad Sci USA. 2006;103:1593–1598. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahima RS, Bjorbaek C, Osei S, Flier JS. Regulation of neuronal and glial proteins by leptin: Implications for brain development. Endocrinology. 1999;140:2755–2762. doi: 10.1210/endo.140.6.6774. [DOI] [PubMed] [Google Scholar]
- 39.O'Malley D, MacDonald N, Mizielinska S, Connolly CN, Irving AJ, Harvey J. Leptin promotes rapid dynamic changes in hippocampal dendritic morphology. Mol Cell Neurosci. 2007;35:559–572. doi: 10.1016/j.mcn.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matochik JA, London ED, Yildiz BO, Ozata M, Caglayan S, DePaoli AM, et al. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. J Clin Endocrinol Metab. 2005;90:2851–2854. doi: 10.1210/jc.2004-1979. [DOI] [PubMed] [Google Scholar]
- 41.Stocker CJ, Cawthorne MA. The influence of leptin on early life programming of obesity. Trends Biotechnol. 2008;26:545–551. doi: 10.1016/j.tibtech.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Taicher GZ, Tinsley FC, Reiderman A, Heiman ML. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Anal Bioanal Chem. 2003;377:990–1002. doi: 10.1007/s00216-003-2224-3. [DOI] [PubMed] [Google Scholar]
- 43.Porsolt RD, Brossard G, Hautbois C, Roux S. Rodent models of depression: Forced swimming and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci. 2001;Chapter 8 doi: 10.1002/0471142301.ns0810as14. Unit. [DOI] [PubMed] [Google Scholar]
- 44.Mach M, Grubbs RD, Price WA, Nagaoka M, Dubovicky M, Lucot JB. Delayed behavioral and endocrine effects of sarin and stress exposure in mice. J Appl Toxicol. 2008;28:132–139. doi: 10.1002/jat.1258. [DOI] [PubMed] [Google Scholar]
- 45.Hata T, Nishikawa H, Itoh E, Funakami Y. Anxiety-like behavior in elevated plus-maze tests in repeatedly cold-stressed mice. Jpn J Pharmacol. 2001;85:189–196. doi: 10.1254/jjp.85.189. [DOI] [PubMed] [Google Scholar]
- 46.Pellow S, Chopin P, File SE, Briley M. Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 47.Ma MX, Chen YM, He J, Zeng T, Wang JH. Effects of morphine and its withdrawal on Y-maze spatial recognition memory in mice. Neuroscience. 2007;147:1059–1065. doi: 10.1016/j.neuroscience.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 48.Lin EH, Katon W, Von Korff M, Rutter C, Simon GE, Oliver M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diab Care. 2004;27:2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- 49.Trayhurn P. Thermoregulation in the diabetic-obese (db/db) mouse. The role of non-shivering thermogenesis in energy balance. Pflugers Arch. 1979;380:227–232. doi: 10.1007/BF00582901. [DOI] [PubMed] [Google Scholar]
- 50.Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology (Berl) 1988;94:147–160. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- 51.Cryan JF, Mombereau C. In search of a depressed mouse: Utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- 52.Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- 53.Hirano S, Miyata S, Kamei J. Antidepressant-like effect of leptin in streptozotocin-induced diabetic mice. Pharmacol Biochem Behav. 2007;86:27–31. doi: 10.1016/j.pbb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Katz RJ. Animal model of depression: Pharmacological sensitivity of a hedonic deficit. Pharmacol Biochem Behav. 1982;16:965–968. doi: 10.1016/0091-3057(82)90053-3. [DOI] [PubMed] [Google Scholar]
- 55.Collin M, Hakansson-Ovesjo ML, Misane I, Ogren SO, Meister B. Decreased 5-HT transporter mRNA in neurons of the dorsal raphe nucleus and behavioral depression in the obese leptin-deficient ob/ob mouse. Brain Res Mol Brain Res. 2000;81:51–61. doi: 10.1016/s0169-328x(00)00167-4. [DOI] [PubMed] [Google Scholar]
- 56.Calapai G, Corica F, Corsonello A, Sautebin L, Di Rosa M, Campo GM, et al. Leptin increases serotonin turnover by inhibition of brain nitric oxide synthesis. J Clin Invest. 1999;104:975–982. doi: 10.1172/JCI5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dickerson F, Brown CH, Fang L, Goldberg RW, Kreyenbuhl J, Wohlheiter K, et al. Quality of life in individuals with serious mental illness and type 2 diabetes. Psychosomatics. 2008;49:109–114. doi: 10.1176/appi.psy.49.2.109. [DOI] [PubMed] [Google Scholar]
- 58.Lambert BL, Cunningham FE, Miller DR, Dalack GW, Hur K. Diabetes risk associated with use of olanzapine, quetiapine, and risperidone in veterans health administration patients with schizophrenia. Am J Epidemiol. 2006;164:672–681. doi: 10.1093/aje/kwj289. [DOI] [PubMed] [Google Scholar]
- 59.Ramaswamy K, Masand PS, Nasrallah HA. Do certain atypical antipsychotics increase the risk of diabetes? A critical review of 17 pharmacoepidemiologic studies. Ann Clin Psychiatry. 2006;18:183–194. doi: 10.1080/10401230600801234. [DOI] [PubMed] [Google Scholar]
- 60.van Winkel R, De Hert M, Wampers M, Van Eyck D, Hanssens L, Scheen A, et al. Major changes in glucose metabolism, including new-onset diabetes, within 3 months after initiation of or switch to atypical antipsychotic medication in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2008;69:472–479. doi: 10.4088/jcp.v69n0320. [DOI] [PubMed] [Google Scholar]
- 61.Dwyer DS, Donohoe D. Induction of hyperglycemia in mice with atypical antipsychotic drugs that inhibit glucose uptake. Pharmacol Biochem Behav. 2003;75:255–260. doi: 10.1016/s0091-3057(03)00079-0. [DOI] [PubMed] [Google Scholar]
- 62.van Nimwegen LJ, Storosum JG, Blumer RM, Allick G, Venema HW, de Haan L, et al. Hepatic insulin resistance in antipsychotic naive schizophrenic patients: Stable isotope studies of glucose metabolism. J Clin Endocrinol Metab. 2008;93:572–577. doi: 10.1210/jc.2007-1167. [DOI] [PubMed] [Google Scholar]
- 63.Buyse M, Bado A, Dauge V. Leptin decreases feeding and exploratory behaviour via interactions with CCK1 receptors in the rat. Neuropharmacology. 2001;40:818–825. doi: 10.1016/s0028-3908(01)00010-7. [DOI] [PubMed] [Google Scholar]
- 64.Suomalainen M, Mannisto PT. Lack of effect of leptin on the behaviour of mice predicting the level of anxiety and depression. Pharmacol Toxicol. 1998;83:139–142. doi: 10.1111/j.1600-0773.1998.tb01458.x. [DOI] [PubMed] [Google Scholar]
- 65.Asakawa A, Toyoshima M, Inoue K, Koizumi A. Ins2Akita mice exhibit hyperphagia and anxiety behavior via the melanocortin system. Int J Mol Med. 2007;19:649–652. [PubMed] [Google Scholar]
- 66.Labad J, Price JF, Strachan MW, Fowkes FG, Ding J, Deary IJ, et al. Symptoms of depression but not anxiety are associated with central obesity and cardiovascular disease in people with type 2 diabetes: The Edinburgh Type 2 Diabetes Study. Diabetologia. 2010;53:467–471. doi: 10.1007/s00125-009-1628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Collins MM, Corcoran P, Perry IJ. Anxiety and depression symptoms in patients with diabetes. Diabet Med. 2009;26:153–161. doi: 10.1111/j.1464-5491.2008.02648.x. [DOI] [PubMed] [Google Scholar]
- 68.Fisher L, Skaff MM, Mullan JT, Arean P, Glasgow R, Masharani U. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with Type 2 diabetes. Diabet Med. 2008;25:1096–1101. doi: 10.1111/j.1464-5491.2008.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hesse D, Dunn M, Heldmaier G, Klingenspor M, Rozman J. Behavioural mechanisms affecting energy regulation in mice prone or resistant to diet-induced obesity. Physiol Behav. 2009 doi: 10.1016/j.physbeh.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 70.Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, et al. Behavioral phenotypes of inbred mouse strains: Implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 71.Papapetropoulos S, Ellul J, Argyriou AA, Chroni E, Lekka NP. The effect of depression on motor function and disease severity of Parkinson's disease. Clin Neurol Neurosurg. 2006;108:465–469. doi: 10.1016/j.clineuro.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 72.Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29:2007–2017. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- 73.Mayorga AJ, Lucki I. Limitations on the use of the C57BL/6 mouse in the tail suspension test. Psychopharmacology (Berl) 2001;155:110–112. doi: 10.1007/s002130100687. [DOI] [PubMed] [Google Scholar]
- 74.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 75.Bohlen und HO, Zacher C, Gass P, Unsicker K. Age-related alterations in hippocampal spines and deficiencies in spatial memory in mice. J Neurosci Res. 2006;83:525–531. doi: 10.1002/jnr.20759. [DOI] [PubMed] [Google Scholar]
- 76.Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV. An area specialized for spatial working memory in human frontal cortex. Science. 1998;279:1347–1351. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- 77.Yamamoto T. Brain regions responsible for the expression of conditioned taste aversion in rats. Chem Senses. 2007;32:105–109. doi: 10.1093/chemse/bjj045. [DOI] [PubMed] [Google Scholar]
- 78.de la Monte SM, Longato L, Tong M, Wands JR. Insulin resistance and neurodegeneration: Roles of obesity, type 2 diabetes mellitus and non-alcoholic steatohepatitis. Curr Opin Investig Drugs. 2009;10:1049–1060. [PMC free article] [PubMed] [Google Scholar]
- 79.Sun Y, Asnicar M, Smith RG. Central and peripheral roles of ghrelin on glucose homeostasis. Neuroendocrinology. 2007;86:215–228. doi: 10.1159/000109094. [DOI] [PubMed] [Google Scholar]
- 80.Garris DR. Developmental and regional changes in brain norepinephrine levels in diabetic C57BL/KsJ mice: Effects of estradiol and progesterone. Brain Res Dev Brain Res. 1995;89:314–319. doi: 10.1016/0165-3806(95)00121-s. [DOI] [PubMed] [Google Scholar]
- 81.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 82.Levin N, Nelson C, Gurney A, Vandlen R, de Sauvage F. Decreased food intake does not completely account for adiposity reduction after ob protein infusion. Proc Natl Acad Sci USA. 1996;93:1726–1730. doi: 10.1073/pnas.93.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin CY, Higginbotham DA, Judd RL, White BD. Central leptin increases insulin sensitivity in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2002;282:E1084–E1091. doi: 10.1152/ajpendo.00489.2001. [DOI] [PubMed] [Google Scholar]
- 84.Moriya M, Okumura T, Takahashi N, Yamagata K, Motomura W, Kohgo Y. An inverse correlation between serum leptin levels and hemoglobin A1c in patients with non-insulin dependent diabetes mellitus. Diabetes Res Clin Pract. 1999;43:187–191. doi: 10.1016/s0168-8227(99)00013-3. [DOI] [PubMed] [Google Scholar]
- 85.Schwarz E, Bahn S. Cerebrospinal fluid: Identification of diagnostic markers for schizophrenia. Expert Rev Mol Diagn. 2008;8:209–216. doi: 10.1586/14737159.8.2.209. [DOI] [PubMed] [Google Scholar]