Abstract
In the current study we tested whether the Dopamine receptor D4 (DRD4) genotype moderates the association of experienced parental problems during childhood (e.g., parental depression, marital discord) with Unresolved loss or trauma during the Adult Attachment Interview. To test the specificity of this moderation the role of the serotonin transporter gene promoter (5-HTTLPR) was also examined. Subjects were 124 adopted adults (mean age 39 years). Participants with the DRD4-7 repeat (7R) allele who experienced parental problems had the highest scores for unresolved loss or trauma whereas participants with DRD4-7R who did not experience parental problems showed the lowest ratings. Among participants without DRD4-7R, the parental problems during childhood did not make a difference. 5-HTTLPR did not moderate the relation between parental problems and unresolved loss or trauma. Our study shows heightened susceptibility to environmental influences for carriers of the DRD4-7R allele, and suggests that the interplay between specific dopamine-related genes and family contexts leads to more or less successful coping with adverse childhood experiences.
Keywords: Unresolved loss or trauma, differential susceptibility, DRD4, GxE, AAI, 5-HTTLPR
Gene-by-environment (GxE) interactions are crucial for understanding the differential susceptibility of vulnerable versus resilient individuals to non-optimal caregiving environments. The last decade has produced some GxE studies in the psychiatric and developmental field (e.g., Fox, Hane, & Pine, 2007; Kaufman et al., 2004), and their number is increasing. At this point one replicated and robust finding has been established, namely the moderating role of the monoamine oxidase A (MAOA) genotype for the impact of childhood maltreatment on the development of antisocial behavior (Kim-Cohen et al., 2006), confirming the results of Caspi et al.’s (2002) groundbreaking study on the interplay between genetic and environmental factors in the cycle of violence. In the current study we examine the moderating role of DRD4 genotype on the impact of experiences of parental problems on subsequent resolution of loss or trauma.
Unresolved state of mind with respect to loss or trauma is evidenced by brief lapses in monitoring of speech during discussion of loss or trauma in the Adult Attachment Interview (AAI, Main, Hesse, & Goldwyn, 2008; Main, Kaplan, & Cassidy, 1985), the gold standard to assess adults’ representations of attachment. Meta-analytically, unresolved state of mind is overrepresented in adult clinical populations in general (Bakermans-Kranenburg & Van IJzendoorn 2009), and more specifically in borderline personality disordered individuals, subjects with experiences of abuse, and suicidal individuals. Severity of unresolved loss or trauma is strongly related to post-traumatic stress symptoms (Harari et al., 2009; Nye et al., 2008; Stovall-McClough, & Cloitre, 2006). In turn, unresolved state of mind – when present in parents - is a robust predictor of infant disorganized attachment (Madigan et al., 2006), a severe type of insecure attachment predicting later child psychopathology, underscoring the clinical significance of the unresolved classification (Hesse & Main, 2006; Lyons-Ruth & Jacobvitz, 2008; Van IJzendoorn, Schuengel, & Bakermans-Kranenburg, 1999).
Interestingly, the association between parental unresolved loss or trauma and infant disorganization may be moderated by the child’s genetic variation at critical monoaminergic loci. One such locus may be the DRD4 exon 3 variable nucleotide repeat (VNTR). At 11p15 locus, humans possess between 2 and 10 copies of a 48 base pair motif in the third cytoplasmic loop of the protein, affecting signal transmission. The 4 repeat motif is the most common allele in individuals of Northern European ancestry while the 7 repeat allele is less frequent. In a study involving mothers selected on the basis of at least one important loss (through death) experience, maternal unresolved loss was associated with infant disorganized attachment, but only for children who carried the less common dopamine D4 receptor 7-repeat (DRD4-7R) allele (Van IJzendoorn & Bakermans-Kranenburg, 2006). The DRD4 receptor gene polymorphism may affect attentional, motivational, and reward mechanisms (Robbins & Everitt, 1999), and the 7R allele has been linked to lower dopamine reception efficiency (Schoots & Van Tol, 2003). DRD4-7R has been associated with pathological impulsive behavior and substance abuse in adults and with Attention Deficit Hyperactivity Disorder (ADHD) in children (Ebstein, 2006; Roussos, Giakoumaki, & Bitsios, 2009; Swanson et al., 2007), and in one study with disorganized attachment in infants (Lakatos et al., 2000), though this finding could not be replicated in other studies (Bakermans-Kranenburg & Van IJzendoorn, 2007; Spangler, Johann, Ronai, & Zimmerman, 2009).
In a second study on the moderating effect of DRD4 genotype, we found that children with the DRD4-7R allele were more vulnerable to their mothers’ insensitive parenting in that they displayed more externalizing behaviors compared to both children with the DRD4-7R allele and sensitive mothers, and children without the DRD4-7R, irrespective of maternal responsiveness (Bakermans-Kranenburg & Van IJzendoorn, 2006). However, children with the DRD4-7R allele in comparison with all other groups showed the lowest levels of externalizing problem behavior when they had sensitive mothers. In a similar vein, Sheese, Voelker, Rothbarth, and Posner (2007), focusing on sensation seeking, found that children with a DRD4-7R allele were more influenced by parenting quality. For these children, lower quality parenting was associated with higher levels of sensation seeking and higher quality parenting with less sensation seeking; whereas children without the DRD4-7R allele were not affected by parenting quality.
Contrasting results were found in a combined sample of American high-risk and Hungarian low-risk families (Gervai et al., 2007), with stronger associations between parenting and infant disorganization for infants without the DRD4 7-R allele than for infants with the DRD4 7-repeat allele. Interestingly, Propper and colleagues found that in European American families correlations between parenting and externalizing behavior were larger for children carrying the DRD4 7-R allele than for those without DRD4 7-R, whereas the reverse was the case for African American children (Propper, Willoughby, Halpern, Carbone, & Cox, 2007).
Recently, Schmidt, Fox, Perez-Edgar, and Hamer (2009) presented an “endophenotypical model” of the influence of the DRD4 gene on infant temperament (depending on frontal EEG asymmetry). Among children with right frontal EEG asymmetry at 9 months, those with DRD4 7-R had significantly more difficulties focusing and sustaining attention at 48 months than those without the DRD4 7-R allele. However, children who exhibited left frontal EEG asymmetry at 9 months and who possessed the DRD4 7-R allele were significantly more soothable at 48 months than other children.
Such outcomes suggest that conceptualizing the DRD4-7R allele exclusively in risk-factor terms is misguided, as this variant – at least for Caucasian children – seems to heighten susceptibility to a wide variety of environments, with supportive contexts possibly promoting positive outcomes, and risky contexts fostering negative outcomes (Belsky, Bakermans-Kranenburg, & Van IJzendoorn, 2007). Indeed, in an intervention experiment aimed at enhancing maternal responsiveness and positive discipline strategies, which can be considered the first experimental GxE test in human development, it was demonstrated that children with the DRD4-7R allele profited most from positive changes in the family environment (Bakermans-Kranenburg et al., 2008a; 2008b). A meta-analytic combination of GxE studies on children under 10 years of age showed that dopamine-related genes may be involved in heightening the susceptibility to the environment, for better and for worse (Bakermans-Kranenburg & Van IJzendoorn, in press).
Not only children but also adults may be differentially susceptible to environmental influences, for better and for worse. The DRD4 and COMT val/met polymorphisms (both implicated in the dopaminergic system) appear to moderate the association between parents’ daily hassles and their responsive parenting. More daily hassles have been associated with less responsive parenting only among parents with a DRD4-7R allele as well as a COMT val allele (Van IJzendoorn, Bakermans-Kranenburg, & Mesman, 2008). At the same time, in the case of fewer daily hassles this group showed higher levels of responsive parenting, supporting the idea that DRD4-7R variant may heighten susceptibility to a wide variety of environments (Ding et al., 2002). Chotai and colleagues found differential effects of season of birth on psychiatric disorders for carriers of the DRD4-7R allele. Birth in February was associated with more depression, but birth in May with less depression, perhaps due to differential susceptibility to seasonal influences during embryonic or perinatal development (Chotai, Serretti, Lattuada, Lorenzi, & Lilli, 2003). Most GxE studies focus however on experiences that took place after the prenatal or perinatal period, and show an emphasis on the negative effects of adverse events; e.g., Dragan and Oniszczenko (2009) documented that among flood survivors in Poland (aged 14-62 years) carriers of the DRD4-7R allele had more intense PTSD symptoms. Reiner and Spangler (2010) noted however that carriers of the DRD4-7R allele with unloving caregiver recollections more often had a secure representation of attachment than their counterparts without the DRD4-7R allele.
In the current study we test whether adopted adults who are carriers of the DRD4-7R allele are more vulnerable to negative childhood parenting experiences such as marital fights, parental depression or alcohol problems. A previous study on a partly overlapping sample (n = 67 overlapping cases) showed a main effect of the serotonin transporter promoter (5-HTTLPR) polymorphism on unresolved loss or trauma as assessed with the AAI (Caspers et al., 2009). Carriers of the short allele of the 5-HTTLPR showed an increased risk for unresolved state of mind. In the current study we examine the additional effect of an adverse childhood context for adoptees with and without the DRD4-7R allele, and we tested for a potential interaction effect of DRD4 and 5-HTTLPR, as for instance documented by Schmidt, Fox, and Hamer (2007) for children and by Armbruster and colleagues (2009) for adults. We hypothesized that the presence of DRD4-7R allele would moderate the association between experienced parental problems and unresolved state of mind. Based on a susceptibility model, we predicted higher scores for unresolved loss or trauma in participants with the DRD4-7R allele who experienced parental problems in their childhood and lower scores in participants with the DRD4-7R allele who did not experience parental problems. Following the steps for testing differential susceptibility as delineated by Belsky et al. (2007), we tested the specificity of the model by replacing the susceptibility factor (5-HTTLPR instead of DRD4) and outcome (depression instead of unresolved loss or trauma).
Method
Procedure
The Iowa Adoption Studies are a longstanding series of 5 adoption studies originating between 1975 and 1989. A follow-up study began in 2000 at which time all previous participants were invited to participate. A total of 772 adoptees (out of 940) agreed to the follow-up. A sibling component was also added in which adoptees and a biologically unrelated sibling completed the same psychiatric interview. In addition, the Adult Attachment Interview was administered to each sibling within an identified sibling pair (total n = 345). A molecular genetic component primarily targeting adopted participants was initiated in 2001. For the current paper, participants with available DRD4 genotyping and Adult Attachment Interviews with at least one reported loss or other traumatic event were selected. In the case of sibling pairs (n = 12) one sibling was excluded, resulting in a total N = 124. The Adult Attachment Interviews were conducted in the home of the adoptee, or a private location if requested (e.g., library). The assessment began with the Adult Attachment Interview followed by a semi-structured psychiatric interview (see Parental Problems section for a description). Questionnaires were mailed ahead of time and collected at the end of the interview. Total time to complete all assessments ranged from three to six hours. All procedures were approved by the University of Iowa Institutional Review Board.
Participants
Participants for this study were enrolled as part of a follow-up study consisting of adoptees separated from their biological parents at birth (see Caspers, Yucuis, Arndt, & Langbehn, 2007 for a complete discussion of sampling and methodology). Original study recruitment of the adoptees was based on problem behaviors of their biological parents at which time roughly half of the biological parents (mother and/or father) had a diagnosis of alcohol problems and/or antisocial behavior problems. The biological parents of the remaining participants had no diagnoses in either parent. At follow-up, a third of the biological mother and/or biological fathers were classified as having alcohol problems or antisocial behavior problems with 40% having both alcohol and antisocial problems in one or both biological parents. The average age at (domestic) adoption was 2.42 months (SD = 6.39) with 71% of the adoptees placed with adoptive parents before 1 month of age and 82% before 3 months of age. Adoptive families were predominantly upper (20%) and middle class (76%). Average adult adoptee household income for the current sample was $40,000 to $49,999 per year. Subjects were predominantly Caucasian (92%). Mean age of participants at the time of interview was 39 years (SD = 7.35), and 53% of the participants were women.
Measures
Unresolved Loss
Adult attachment representations were derived using the Adult Attachment Interview (AAI, Main et al., 1985; 2008), when the participants were on average 39 years old. The AAI is an hour-long, semi-structured interview which assesses an individual’s current state of mind with respect to attachment. Respondents are asked about their childhood attachment experiences with their (adoptive) parents and how they think they were affected by these experiences, as well as about the current relationship with their (adoptive) parents. Other questions concern experiences of loss and trauma (such as the death of family members or experiences of abuse), both during their youth and afterwards. Interviews were audio-recorded, transcribed verbatim, and scored according to the standard AAI classification system (Main et al., 2008).
On top of their main classification as secure, insecure-dismissing, or insecure-preoccupied, individuals are classified as unresolved-disorganized (U) when they show lapses in the monitoring of reasoning or discourse during their discussion of loss or other traumatic events. Scores for unresolved state of mind are assigned using a 9-point rating scale. Unresolved state of mind with respect to loss or trauma is apparent from shifts in discourse and reasoning patterns representing lapses in consciousness, undue influence of overwhelming emotions, or inappropriate interference of memories surrounding the event such that speech is no longer actively being monitored. An individual might use very vivid and sensory detailed speech, or speak about a deceased loved one as though the person was still alive (see Hesse, 2008).
The interviews were anonymously assigned and coded ‘blindly’ by raters who were trained to be reliable to the coding standards of the Berkeley laboratory of Mary Main and Erik Hesse. Roughly fifty percent of all interviews were rated by two coders. If there was disagreement between coders and consensus could not be reached, a third rater was selected. Using all data, inter-rater agreement was 93% for the unresolved/not unresolved classification (κ = .71). Intra-class correlations, computed with exact agreement methods, for unresolved loss or trauma scale was r = .76 and for coherence of transcript was r = .77. Twenty-nine respondents were classified as Unresolved; their mean score on the unresolved loss or trauma scale was 5.73 (SD = 1.44); the mean score for the respondents classified as not unresolved was 2.92 (SD = 1.37).
Parental problems
Consistent with earlier Iowa Adoption Study reports, the adoptees were asked questions about the quality of their adoptive parents’ relationship (e.g., marital quality) and adoptive parent psychological problems while living at home (<18 years of age) using the Semi-Structured Assessment for the Genetics of Alcoholism – II (SSAGA-II; Bucholz, Cadoret, Cloninger, Dinwiddie, Hesselbrock, Nurnberger et al., 1994). The SSAGA-II was administered after the AAI was completed either during the same interview session or by phone at a later date if necessary. The SSAGA-II is an hour long, semi-structured interview that queries lifetime symptoms of DSM-IV psychiatric disorders and specific childhood experiences within the family including parental problems. Adoptees reported on parental marital quality as indicated by marital separation, poor marriage, parents didn’t enjoy one another, parents fought often, and parents hit one another. A single item indicated parent problems with alcohol. Two additional items each probed about adoptive parent treatment for depression and temper problems such as trouble with getting along with others or a violent temper. We counted the number of parental problems (alpha = .68; M = 0.91, SD = 1.40, range 0 – 6).
During initial contact with the adoptee, adoptive parents also reported about the presence of problems (e.g., divorce, marital problems, alcohol problems, and/or mood disorder problems) as part of an hour long interview. Adoptees were at least 18 years of age (M = 24.37, SD = 6.02) at the time of the adoptive parent interview. As with the adoptee, we counted the number of problems reported by the adoptive parent. A different parent interview was used for each of the 5 original studies and exact correspondence was not sought. However, ordered categorical correlation (phi) coefficients allow a rough approximation of traditional correlations. The correlation between the number of problems reported by the adoptive parent and reported by the adoptee was adequate (phi = .53, p < .001).
Depressive Symptoms
The Brief Symptom Instrument (BSI, Derogatis, 1996) is a short form of the SCL-90-R and assesses dimensions of psychological health. Participants rated on a 5-point Likert scale the degree to which they experienced symptoms of depression in the previous seven days. The BSI was administered following administration of the AAI as a measure of concurrent mood disturbance. We derived T-scores for symptoms of depression from published, gender-specific adult non-patient norms. Cronbach’s alpha was good (α = .89).
DRD4 Genotyping
Genetic material was collected following the interview. DNA was obtained either from biomaterial from buccal swabs or from whole blood. Buccal swab DNA was prepared using a QIAmp DNA minikit (Qiagen, INC, Valencia, CA). DNA prepared from whole blood was prepared using cold protein precipitation (Lahiri & Nurnberger, 1991). Polymerase chain reaction (PCR) amplification of the DRD4 exon 3 VNTR was conducted using the primers and conditions as described by Hawi and colleagues (2000). After amplification, the PCR products were diluted with an equal amount of formamide dye. Then, approximately 3 μL of each of the resulting mixture were loaded on a standard 6% polyacrylamide sequencing gel and electrophoresed for 1 to 2 hours. PCR products were visualized using silver staining, the products sized by comparison to internal and external standards, and alleles called by two individuals blind to phenotype. Forty-eight participants (39 %) were carriers of a DRD4-7R allele.
5-HTTLPR Genotyping
Genotyping of the 5-HTTLPR locus was carried out using the primers F- GGCGTTGCCGCYCYGAATGC and R-GAGGGACTGAGCTGGACAACCAC. Vent polymerase was used according to manufacturer’s suggestion (New England Biolabs) and 100 μmol/L 7-deaza guanosine triphosphate (GTP) (Boehringer Mannheim, Indianapolis, IN) was added to aid amplification through this GC-rich region. In addition, 3 μCi of α[35S]-dATP was added to each 10 μl reaction to allow radioactive detection. Cycling parameters were as follows: 98°C × 15 seconds, 68°C × 15 seconds, and 72°C × 45 seconds, with a 7-minute final extension at 72°C. Approximately 3 μL of each of the above polymerase chain reaction (PCR) products were denatured, then loaded on a standard 6% polyacrylamide sequencing gel and electrophoresed for 2 to 3 hours. The gels were exposed to standard X-ray film and the visualized PCR products sized by comparison to an internal sequencing ladder. Genotypic frequencies were ss n = 25, sl n = 54, ll n = 43. For both DRD4 and 5HTTLPR genotypic frequencies were in Hardy-Weinberg equilibrium.
Results
Descriptives
Of the 124 participants, 64 (52%) were classified autonomous, 28 (23%) as dismissing, 2 (2%) as preoccupied, and 30 (24%) unresolved or cannot classify (n = 29 U; n = 1 CC). The distribution differed significantly from expected rates (Bakermans-Kranenburg & Van IJzendoorn, 2009), χ2(3, N = 124) = 17.26, p < .01. Preoccupied classifications were underrepresented in our sample and unresolved classifications were somewhat overrepresented. There was no significant association between attachment classification and sex, χ2(3, N = 124) = 4.32, p = .23, nor between attachment classification and DRD4-7R, χ2(3, N = 124) = 2.77, p = .45, nor between sex and DRD4-7R, χ2(1, N = 124) = 2.83, p = .10. Participants with and without the DRD4-7R allele did not differ in the number of losses or traumatic events they reported during the AAI (see Table 1). DRD4 and 5-HTTLPR genotypes were not associated, χ2(2, N = 122) = 2.50 (p = .29), and 5-HTTLPR was unrelated to attachment classification, sex, and the number of losses or traumatic events they reported during the AAI (.77 < p < .99). All participants reported on at least one loss or trauma (see Procedure).
Table 1.
Descriptives of Participants with and without the DRD4-7R Allele
| Measure | N | DRD4 | t | |||
|---|---|---|---|---|---|---|
| no 7R allele (n=76) | 7R allele (n=48) | |||||
| M | SD | M | SD | |||
| Age | 124 | 38.64 | 6.58 | 39.35 | 8.48 | −0.49 |
| Parental problems | 124 | 0.91 | 1.39 | 0.92 | 1.43 | −0.03 |
| Depression | 121 | 0.43 | 0.60 | 0.33 | 0.61 | 0.89 |
| AAI | ||||||
| Number of losses | 124 | 3.00 | 1.17 | 2.81 | 1.41 | 0.82 |
| Number of traumatic experiences | 124 | 0.32 | 0.64 | 0.33 | 0.52 | −0.16 |
| Unresolved scale score | 124 | 3.22 | 1.98 | 3.23 | 2.00 | −0.02 |
p < .05
Bivariate Associations
Older participants received higher scores on the unresolved loss or trauma scale (r = .23, p < .01), see Table 2. Higher levels of depression were associated with more reported losses or traumatic events reported during the AAI (r = .18, p = .05). The unresolved scale was not related to parental problems, depression, and the number of losses or traumatic experiences. Experiences of parental problems were associated with higher scores on depression (r = .29, p < .01) and with more reported losses or traumatic events (r = .28, p < .01).
Table 2.
Multiple Hierarchical Regression of Age, Depression, Number of Losses/Traumatic Events, DRD4, 5-HTTLPR, and Parental Problems on Unresolved Loss or Trauma
| Age | Depr | Number of losses | 5-HTT | DRD4 | Parental problems | R | R2 | R2Change | Beta1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Criterion | ||||||||||
| Unresolved loss/trauma | .23** | .12 | .14 | −.07 | .00 | .07 | ||||
| 1. Background | .29* | .08 | .08* | |||||||
| Age | -- | −.01 | −.06 | .02 | .05 | −.12 | .26** | |||
| Depression | -- | .18* | .03 | −.08 | .29** | .09 | ||||
| Number of losses/traumatic events (AAI) | -- | .03 | .01 | .28** | .16 | |||||
| 2. Main Effects | .30 | .09 | .01 | |||||||
| 5-HTTLPR | -- | −.07 | −.09 | −.09 | ||||||
| DRD4-7R2 | -- | .00 | −.01 | |||||||
| Parental problems | -- | .07 | ||||||||
| 3. Interaction Effect | .38* | .15 | .05* | |||||||
| DRD4 * 5-HTTLPR | .15 | |||||||||
| DRD4 * Parental problems | .20* | |||||||||
p < .05
p < .01 (N = 124)
Betas derived from the final model.
39 % DRD4 7-repeat
Multivariate Analysis
We conducted a multivariate hierarchical regression on the continuous scale score for unresolved loss or trauma, with age, depression, and the number of losses or traumatic events reported during the AAI as predictors in the first step, DRD4, 5-HTTLPR and thecontinuous score for parental problems in the second step, and the interactions between DRD4 and (centered) 5-HTTLPR and between DRD4 and (centered) parental problems in the last step. The overall regression was significant, F(8, 115) = 2.44, p = .02. Two predictors contributed significantly to the regression equation, age (beta = .26, p < .01), and the interaction between DRD4 and parental problems (beta = .20, p = .03), see Table 2. The interaction between DRD4 and 5-HTTLPR was not significant, (beta = .15, p = .10). Similar results were found when the variable parental problems was dichotomized as absent (57%) or present (43%); F(8, 115) = 2.57, p = .01, significant predictors: age (beta = .27, p < .01), and the interaction between DRD4 and parental problems (beta = .21, p = .02).1
To explore the interaction effect, we computed partial correlations between unresolved loss or trauma scores and parental problems for participants with and without the DRD4-7R allele, controlling for age, depression, and the number of losses and traumatic events. For participants without DRD4-7R the association was not significant, r = −.16 (p = .19), whereas for participants with DRD4-7R the partial correlation was r = .33 (p = .03). These associations were significantly different, Zdiff = 2.58, p = .01. For participants with DRD4-7R the association between experiences of parental problems and unresolved loss was significantly stronger than for participants without DRD4-7R.
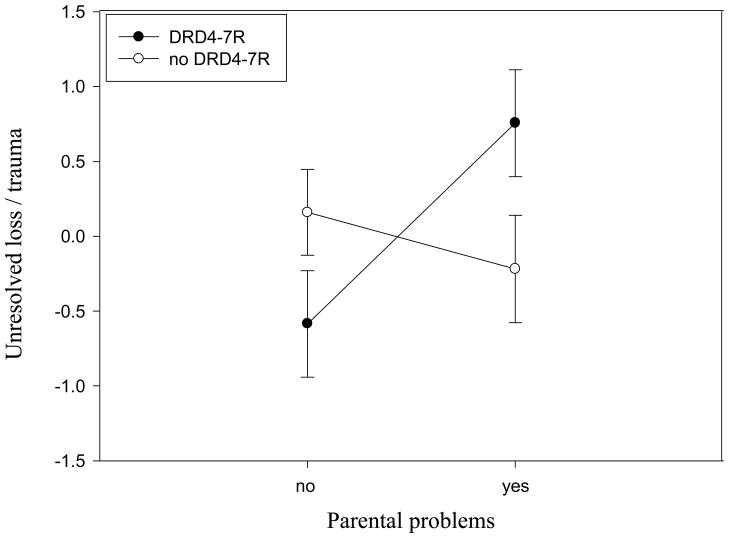
In order to examine whether the results were best explained by a cumulative risk model or a differential susceptibility model (see Belsky et al., 2007), we created four groups: participants with the DRD4-7R allele and parental problems (group 1, n = 21), participants with the DRD4-7R allele without parental problems (group 2, n = 27), participants without DRD4-7R with parental problems (group 3, n = 32), and participants without DRD4-7R allele and without parental problems (group 4, n = 44), see Figure 2. A priori contrasts showed that, controlling for age and depression, among participants without the DRD4-7R allele (groups 3 and 4) the experience of parental problems during childhood did not make a significant difference (t[120] = −0.87, p = .39; Cohen’s d = −0.20), but participants with the DRD4-7R allele (groups 1 and 2) showed significantly higher levels of unresolved loss or trauma when they experienced parental problems during childhood than when they did not experience parental problems (t[120] = 2.45, p = .02; Cohen’s d = 0.71). Moreover, participants with the DRD4-7R allele who experienced parental problems in their childhood (group 1) had significantly higher scores for unresolved loss or trauma than all other participants together (t[120] = 2.15, p = .03; Cohen’s d = 0.52), and participants with the DRD4-7R allele who did not experience parental problems (group 2) had significantly lower scores for unresolved loss or trauma than all other participants (t[120] = 1.98, p = .05; Cohen’s d = 0.43). These results are indicative of differential susceptibility rather than cumulative risk.
In a final step we tested the specificity of the model by replacing the susceptibility factor and outcome, respectively, as prescribed by Belsky et al. (2007) for testing differential susceptibility. Replacing the moderator DRD4 with 5-HTTLPR, we did not find a significant interaction effect of 5-HTTLPR and parental problems (beta = .05, p = .59). The overall regression was not significant. Replacing the outcome (unresolved loss) with depression, the overall model was not significant either, and the interaction between DRD4 and parental problems did not contribute significantly to the prediction of depression (beta = .03, p = .73).
Discussion
Unresolved loss or trauma predisposes to elevated rates of psychopathology. In the current study, we found that carriers of the DRD4-7R allele were more vulnerable to the influence of parental problems such as parental fights or depression during childhood on the development of unresolved loss or trauma in adulthood. Important to the understanding of the underlying processes (Belsky et al., 2007), participants with the DRD4-7R allele did not experience more losses or traumatic events nor were they more likely to have been confronted with parental problems, implying that the results cannot be ascribed to gene-environment correlation. In this sample of adult adoptees genetic transmission of psychological problems is excluded. Moreover, in compliance with our susceptibility hypothesis, when participants with the DRD4-7R allele were not exposed to parental problems they had low scores on unresolved loss or trauma. This pattern of results supports heightened susceptibility rather than increased vulnerability of carriers of the DRD4-7R allele in that they were most affected by unfavorable contexts, but in more favorable contexts showed more positive outcomes than subjects without the 7R allele. The differential susceptibility effect was not replicated for the serotonin-related 5-HTTLPR polymorphism (cf. Belsky & Pluess, 2009; Zimmermann, Mohr, & Spangler, 2009), but was specific for the DRD4-7R allele.
How can this heightened susceptibility be explained in the context of dopamine related genotypes? The effects in the negative direction might be explained by the so-called diathesis-stress model (Clark et al., 1992; Phelps et al., 1998), which predicts negative outcomes when an underlying vulnerability or diathesis is activated by an environmental stressor. Without exposure to stress, the diathesis remains ineffective such that potential negative consequences of the diathesis are not realized. This model can easily be extended in the direction of putative biological or genetic vulnerabilities (Belsky, 1997, 2005; Boyce & Ellis, 2005a,b; Meehl, 1962; Paris, 2000), in our case the dopaminergic system.
As a complementary perspective, we may consider the differential susceptibility model in terms of affecting developmental trajectories. For carriers of the 7R allele, parental problems may have increased their risk of developing attachment disorganization (Cyr et al., 2010; George & Solomon, 2008), which may have in turn hampered resolution of future traumatic events or loss experiences (Hesse & Main, 2006). Thus, early attachment experiences may prevent carriers of the 7R allele to work through loss or other traumatic events not only in childhood but also thereafter. This developmental model is speculative, of course, since we do not have information on participants’ potential attachment disorganization in childhood.
Previous studies show associations between lower dopamine reception efficiency and impulsive behavior and addiction (Ebstein, Benjamin, & Belmaker, 2002). Seeger et al. (2004) suggest that the DRD4-7R allele is associated with a reward deficiency syndrome that may lead to sensation and novelty seeking as well as impulsivity. Recently, Klein et al. (2007) found that low dopaminergic efficiency was associated with decreased sensitivity to negative action consequences. Therefore, as an alternative or additional explanation, we propose that the DRD4-7R genotype may also influence unresolved attachment due to greater susceptibility to the organizing influences (or lack thereof) of early experiences, which when coupled with a greater tolerance for arousal among these individuals, promotes more (or less) organized processing of traumatic events. Specifically, carriers of the 7R allele who were raised in chaotic or insensitive environments may be less motivated to focus on and learn from their disturbed inner feelings. A consequence of such lack of motivation and insight may be an inability to come to terms with major life-events in a coherent manner. Conversely, an environment without the additional strains and stresses of parental problems might facilitate full evaluation of loss or trauma among carriers of the 7R allele. When the environment supports working through traumatic experiences, individuals with the 7R allele might be equally (or even better) equipped than carriers of the shorter DRD4 alleles to openly reflect on their trauma (i.e., re-experience event-related arousal) and learn how to represent these life-events in a more coherent and structured manner (see also Steele & Siever, 2010). This interpretation is speculative since empirical evidence on the bright side of DRD4-7R is scarce (but see Bakermans-Kranenburg & Van IJzendoorn, in press, for meta-analytic evidence); it is however consistent with observed DRD4-7R determined susceptibility to positive changes in family environment (Bakermans-Kranenburg et al., 2008a; 2008b) and the literature demonstrating more effective management of arousal among individuals high on sensation-seeking (Smith, Ptacek, & Smoll, 1992; Solomon, Ginzburg, Neria, & Ohry, 1995).
Davila and Cobb (2003) observed that in attachment research little emphasis has been put on the specific events or conditions in a person’s life that may result in increased coherence and security. In fact, as Belsky (2005) commented, the overwhelming majority of studies on human development seem aimed at exploring the negative consequences of negative environments on vulnerable subjects. Case in point is the pioneering study by Sheese et al. (2007) on the interaction between DRD4 and parenting quality predicting children’s sensation seeking. The authors’ discussion of the results focuses on the association between lower quality parenting and higher levels of sensation seeking, thereby overshadowing the bright side of the findings showing optimal outcomes for children with DRD4-7R experiencing high quality parenting. The distinction between vulnerability and susceptibility might keep us aware of this bright side. Furthermore, future studies may examine the influence of the timing of environmental effects. Epigenetic research in animal models have demonstrated a sensitive period during the first week of life when offspring are most susceptible to the effects of parenting that have a lasting influence on gene expression. The influence of parenting and its interplay with the DRD4 gene in humans may be especially pronounced at certain developmental periods. A recent meta-analysis indicates that in human offspring this type of GxE interplay may be observed from prenatal life throughout early childhood (Bakermans-Kranenburg & Van IJzendoorn, in press), but later effects may be traced as well.
The current study has some strengths and limitations. Parental problems were assessed with retrospective self-reports, which might be biased with current depressive mood or other contaminating influences. We did control for depression, and we found that the adoptees’ report of their parenting experiences converged with their parents’ information about their own parenting, thus providing some validation for the parental problems scale. Of course, marital problems, parental alcohol abuse or depression are not identical with experienced inadequate or insensitive parenting but they elevate the chance of such experiences greatly (Belsky, 1984). It should be noted that in the current sample we did not replicate the previously reported association between the short 5-HTTLPR allele and unresolved loss or trauma (Caspers et al., 2009), which may be due to the fact that (based on the selection criteria for the current study) only roughly half of the sample overlapped. In addition, in the current study methylation was not taken into account, whereas for some genes (e.g., 5HTTLPR) associations between polymorphisms and psychological problems may be significantly altered by environmentally induced methylation patterns (Van IJzendoorn, Caspers, Bakermans-Kranenburg, Beach, & Philibert, 2010). It should be noted that the role of methylation for dopamine-related gene functioning is as yet unclear. Furthermore, a limitation is our sample size. As our main finding is an interaction effect, replication is certainly needed. The interaction effect was predicted a priori, however, and derived from the model of differential susceptibility which also received support from various other GxE studies. The specificity of the effect was demonstrated as the model was not replicated when a different susceptibility factor or outcome was used (Belsky et al., 2007; Caspi & Moffitt, 2006; Rutter, 2006). An important strength of the current study is the careful assessment of unresolved loss or trauma through the gold standard of attachment theory, the Adult Attachment Interview. Furthermore, the adoptive families included in the current sample enabled us to examine the influence of parenting on (currently adult) adoptive children without the potential contaminating effects of genetic kinship. At the same time, some participants may have been biologically susceptible to psychiatric problems related to other genetic variations than those included in the current study. Unresolved classifications were somewhat overrepresented in our sample (24%) compared to the normative sample of North American mothers (18%, Bakermans-Kranenburg & Van IJzendoorn, 2009). This converges with the higher percentage of unresolved classifications in adopted adolescents (17%, Beijersbergen, Bakermans-Kranenburg, Van IJzendoorn, & Juffer, 2008) compared to the normative distribution for adolescents (11% U, Bakermans-Kranenburg & Van IJzendoorn, 2009). Adopted individuals may be more susceptible to experiences of loss given possible feelings of loss associated with being adopted. Although in both studies the adoptees had been placed with their adoptive families very early (10 weeks on average), the awareness of loss of their birth parents and possibly other attachment figures might play a role, even when they do not explicitly remember them.
In sum, our study shows support for heightened susceptibility to environmental influences for carriers of the DRD4-7R allele instead of only increased vulnerability. At the same time, our findings make clear that the impact of traumatic life-events cannot be reduced to a genetic mechanism or to a specific type of parenting. It is the interplay between specific genes and family contexts that leads to more or less successful coping with adverse childhood experiences.
Figure 1.
Scores for unresolved loss and trauma (M, SE, residuals controlled for age and depression) of participants with and without the DRD4 7-repeat allele who did versus did not experience parental problems
Acknowledgments
The first and second authors were supported by research awards from the Netherlands Organization for Scientific Research (MJBK: VIDI grant no. 452-04-306; MHvIJ: NWO SPINOZA prize). Data collection was funded by grants from the United States National Institute of Drug Abuse (RO1 DA05821 and R01 DA015789). We wish to thank the adoptees and their families for their continued participation in this study. We would also like to thank Rebecca Yucuis, Beth Troutman, and Jeanne Frederickson for assistance in coding the Adult Attachment Interviews and Tracy Gunter for her assistance with data management.
Footnotes
Using the unresolved / not unresolved classification as outcome showed analogous results, but the interaction between DRD4 and parental problems fell just short of significance (beta = .17, p = .056).
References
- Armbruster D, Mueller A, Moser DA, Lesch KP, Brocke B, Kirschbaum C. Interaction effect of D4 dopamine receptor gene and serotonin transporter promoter polymorphism on the cortisol stress response. Behavioral Neuroscience. 2009;123:1288–1295. doi: 10.1037/a0017615. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH. Gene-environment interaction of the Dopamine D4 receptor (DRD4) and observed maternal insensitivity predicting externalizing behavior in preschoolers. Developmental Psychobiology. 2006;48:406–409. doi: 10.1002/dev.20152. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH. Genetic vulnerability or differential susceptibility in child development: The case of attachment. Journal of Child Psychology and Psychiatry. 2007;48:1160–1173. doi: 10.1111/j.1469-7610.2007.01801.x. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH. The first 10,000 Adult Attachment Interviews: Distributions of adult attachment representations in clinical and non-clinical groups. Attachment and Human Development. 2009;11:223–263. doi: 10.1080/14616730902814762. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH. Differential susceptibility to rearing environment depending on dopamine-related genes: New evidence and a meta-analysis. Development and Psychopathology. 2011;23 doi: 10.1017/S0954579410000635. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH, Mesman J, Alink LRA, Juffer F. Effects of an attachment-based intervention on daily cortisol moderated by DRD4: A randomized control trial on 1–3-year-olds screened for externalizing behavior. Development & Psychopathology. 2008a;20 doi: 10.1017/S0954579408000382. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH, Pijlman FTA, Mesman J, Juffer F. Differential susceptibility to intervention: Dopamine D4 Receptor Polymorphism (DRD4 VNTR) moderates effects on toddlers’ externalizing behavior in a randomized control trial. Developmental Psychology. 2008b;44:293–300. doi: 10.1037/0012-1649.44.1.293. [DOI] [PubMed] [Google Scholar]
- Belsky J. The determinants of parenting - a process model. Child Development. 1984;55:83–96. doi: 10.1111/j.1467-8624.1984.tb00275.x. [DOI] [PubMed] [Google Scholar]
- Belsky J. Variation in susceptibility to rearing influence: An evolutionary argument. Psychological Inquiry. 1997;8:182–186. [Google Scholar]
- Belsky J. Differential susceptibility to rearing influence: An evolutionary hypothesis and some evidence. In: Ellis B, Bjorklund D, editors. Origins of the social mind: Evolutionary psychology and child development. New York: Guilford; 2005. pp. 139–163. [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, Van IJzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005a;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: II. Empirical explorations of an evolutionary-developmental theory. Development and Psychopathology. 2005b;17:303–328. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability of the SSAGA. Journal of Alcoholism. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Caspers K, Paradiso S, Yucuis R, Troutman B, Arndt S. Association between the Serotonin Transporter Promoter Polymorphism (5-HTTLPR) and Adult Unresolved Attachment. Developmental Psychology. 2009;45(1):64–76. doi: 10.1037/a0014026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers K, Yucuis R, Troutman B, Arndt S, Langbehn D. A sibling adoption study of adult attachment: the influence of shared environment on attachment states of mind. Attachment & Human Development. 2007;9:375–391. doi: 10.1080/14616730701711581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt T, Mill J, Martin J, Craig I, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt T. Gene environment interactions in psychiatry. Nature Reviews Neuroscience. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Chotai J, Serretti A, Lattuada E, Lorenzi C, Lilli R. Gene-environment interaction in psychiatric disorders as indicated by season of birth variations in tryptophan hydroxylase (TPH), serotonin transporter (5-HTTLPR) and dopamine receptor (DRD4) gene polymorphisms. Psychiatry Research. 2003;119:99–111. doi: 10.1016/s0165-1781(03)00112-4. [DOI] [PubMed] [Google Scholar]
- Clark DA, Beck AT, Brown GK. Sociotropy, autonomy, and life event perceptions in dysphoric and nondysphoric individuals. Cognitive Therapy and Research. 1992;16:635–652. [Google Scholar]
- Cyr C, Euser EM, Bakermans Kranenburg MJ, Van IJzendoorn MH. Attachment security and disorganization in maltreating and high-risk families: A series of meta-analyses. Development and Psychopathology. 2010;22:87–108. doi: 10.1017/S0954579409990289. [DOI] [PubMed] [Google Scholar]
- Davila J, Cobb RJ. Predicting change in self-reported and interviewer-assessed adult attachment: Tests of the individual difference and life stress models of attachment change. Personality and Social Psychology Bulletin. 2003;29:859–870. doi: 10.1177/0146167203029007005. [DOI] [PubMed] [Google Scholar]
- Derogatis L. Brief Symptom Instrument. Minneapolis, MN: National Computer Systems; 1996. [Google Scholar]
- Ding YC, Chi HC, Grady DL, Morishima A, Kidd JR, Kidd K, et al. Evidence of positive selection acting at the human dopamine receptor D4 gene locus. PNAS. 2002;99:309–314. doi: 10.1073/pnas.012464099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragan WL, Oniszczenko W. The association between dopamine D4 receptor exon III polymorphism and intensity of PTSD symptoms among flood survivors. Anxiety Stress and Coping. 2009;22:483–495. doi: 10.1080/10615800802419407. [DOI] [PubMed] [Google Scholar]
- Ebstein RP. The molecular genetic architecture of human personality: beyond self-report questionnaires. Molecular Psychiatry. 2006;11:427–445. doi: 10.1038/sj.mp.4001814. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Benjamin J, Belmaker RH. Behavioral genetics, genomics, and personality. In: Plomin R, DeFries JC, Craig IW, McGuffin P, editors. Behavioral genetics in the postgenomic era. Washington, DC: American Psychological Association; 2002. pp. 365–388. [Google Scholar]
- Fox NA, Nichols KE, Henderson HA, Rubin K, Schmidt L, Hamer D, et al. Evidence for a gene-environment interaction in predicting behavioral inhibition in middle childhood. Psychological Science. 2005;16:921–926. doi: 10.1111/j.1467-9280.2005.01637.x. [DOI] [PubMed] [Google Scholar]
- George C, Solomon J. The caregiving system: A behavioral systems approach to parenting. In: Cassidy J, Shaver PR, editors. Handbook of Attachment. Theory, Research, and Clinical Applications. 2. New York: Guilford; 2008. pp. 833–856. [Google Scholar]
- Gervai J, Novak A, Lakatos K, Toth I, Danis I, Ronai Z, et al. Infant genotype may moderate sensitivity to maternal affective communications: Attachment disorganization, quality of care, and the DRD4 polymorphism. Social Neuroscience. 2007;2:307–319. doi: 10.1080/17470910701391893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari D, Bakermans-Kranenburg MJ, De Kloet CS, Geuze E, Vermetten E, Westenberg HGM, Van IJzendoorn MH. Attachment representations in Dutch veterans with and without deployment-related PTSD. Attachment and Human Development. 2009;11:515–536. doi: 10.1080/14616730903282480. [DOI] [PubMed] [Google Scholar]
- Hawi Z, McCarron M, Kirley A, Daly G, Fitzgerald M, Gill M. No association of the dopamine DRD4 receptor (DRD4) gene polymorphism with attention deficit hyperactivity disorder (ADHD) in the Irish population. American Journal of Medical Genetics. 2000;96(3):268–272. doi: 10.1002/1096-8628(20000612)96:3<268::aid-ajmg6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Hesse E. The Adult Attachment Interview: Protocol, method of analysis, and empirical studies. In: Cassidy J, Shaver PR, editors. Handbook of Attachment. Theory, Research, and Clinical Applications. 2. New York: Guilford; 2008. pp. 552–598. [Google Scholar]
- Hesse E, Main M. Frightened, threatening, and dissociative parental behavior in low-risk samples: Description, discussion, and interpretations. Development and Psychopathology. 2006;18:309–343. doi: 10.1017/S0954579406060172. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. Social supports and serotonin transporter gene moderate depression in maltreated children. Proceedings of the National Academy of Sciences, USA. 2004;101:17316–17421. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: new evidence and a meta-analysis. Molecular Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Klein TA, Neumann J, Reuter M, Hennig J, Von Cramon DY, Ullsperger M. Genetically determined differences in learning from errors. Science. 2007;318:1642–1645. doi: 10.1126/science.1145044. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Nurnberger JI. A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Research. 1991;19(19):5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos K, Toth I, Nemoda Z, Ney K, Sasvari-Szekely M, Gervai J. Dopamine D4 receptor (DRD4) gene polymorphism is associated with attachment disorganization in infants. Molecular Psychiatry. 2000;5:633–637. doi: 10.1038/sj.mp.4000773. [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth K, Jacobvitz D. Attachment disorganization: genetic factors, parenting contexts, and developmental transformation from infancy to adulthood. In: Cassidy J, Shaver PR, editors. Handbook of Attachment. Theory, Research, and Clinical Applications. 2. New York: Guilford; 2008. pp. 666–697. [Google Scholar]
- Madigan S, Bakermans-Kranenburg MJ, Van IJzendoorn MH, Moran G, Pederson DR, Benoit D. Unresolved states of mind, anomalous parental behavior, and disorganized attachment: A review and meta-analysis of a transmission gap. Attachment and Human Development. 2006;8:89–111. doi: 10.1080/14616730600774458. [DOI] [PubMed] [Google Scholar]
- Main M, Hesse E, Goldwyn R. Studying differences in language usage in recounting attachment history: An introduction to the AAI. In: Steele H, Steele M, editors. Clinical applications of the Adult Attachment Interview. New York: Guilford; 2008. pp. 31–68. [Google Scholar]
- Main M, Kaplan N, Cassidy J, Bretherton I, Waters E. Security in infancy, childhood and adulthood: A move to the level of representation. Growing points in attachment theory and research. Monographs of the Society for Research in Child Development. 1985;50(1–2):66–106. Serial No. 209. [Google Scholar]
- Meehl PE. Schizotaxia, Schizotypy, Schizophrenia. American Psychologist. 1962;17:827–838. [Google Scholar]
- Nye EC, Katzman J, Bell JB, Kilpatrick J, Brainard M, Haaland KY. Attachment organization in Vietnam combat veterans with posttraumatic stress disorder. Attachment & Human Development. 2008;10(1):41–57. doi: 10.1080/14616730701868613. [DOI] [PubMed] [Google Scholar]
- Paris J. Predispositions, personality traits, and posttraumatic stress disorder. Harvard Review of Psychiatry. 2000;8:175–183. [PubMed] [Google Scholar]
- Phelps JL, Belsky J, Crnic K. Earned security, daily stress, and parenting: A comparison of five alternative models. Development and Psychopathology. 1998;10:21–38. doi: 10.1017/s0954579498001515. [DOI] [PubMed] [Google Scholar]
- Reiner I, Spangler G. Adult attachment and gene polymorphisms of the dopamine D4 receptor and serotonin transporter (5-HTT) Attachment and Human Development. 2010;12:209–229. doi: 10.1080/14616731003759674. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Motivation and reward. In: Zigmond MJ, et al., editors. Fundamental Neuroscience. San Diego: Academic Press; 1999. pp. 1246–1260. [Google Scholar]
- Roussos P, Giakoumaki SG, Bitsios P. Cognitive and emotional processing in high novelty seeking associated with the L-DRD4 genotype. Neuropsychologia. 2009;47:1654–1659. doi: 10.1016/j.neuropsychologia.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Rutter M. Genes and behavior. London: Blackwell; 2006. [Google Scholar]
- Schmidt LA, Fox NA, Perez-Edgar K, Hamer DH. Linking gene, brain, and behavior. DRD4, frontal asymmetry, and temperament. Psychological Science. 2009;20:831–837. doi: 10.1111/j.1467-9280.2009.02374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Hamer DH. Evidence for a gene gene interaction in children’s behavior problems: Association of serotonin transporter short and dopamine receptor D4 long genotypes with internalizing and externalizing behaviors in typically developing 7-year-olds. Development and Psychopathology. 2009;19:1103–1114. doi: 10.1017/S0954579407000569. [DOI] [PubMed] [Google Scholar]
- Schoots O, Van Tol HHM. The human dopamine D4 receptor repeat sequences modulate expression. Pharmacogenomics Journal. 2003;6:343–348. doi: 10.1038/sj.tpj.6500208. [DOI] [PubMed] [Google Scholar]
- Seeger G, Schloss P, Schmidt MH, Rüter-Jungfleisch A, Henn FA. Gene-environment interaction in hyperkinetic conduct disorder (HD + CD) as indicated by season of birth variations in dopamine receptor (DRD4) gene polymorphism. Neuroscience Letters. 2004;366:282–286. doi: 10.1016/j.neulet.2004.05.049. [DOI] [PubMed] [Google Scholar]
- Sheese BE, Voelker PM, Rothbarth MK, Posner MI. Parenting quality interacts with genetic variation in dopamine receptor D4 to influence temperament in early childhood. Development and Psychopathology. 2007;19:1039–1046. doi: 10.1017/S0954579407000521. [DOI] [PubMed] [Google Scholar]
- Smith RE, Ptacek JT, Smoll FL. Sensation Seeking, Stress and Adolescent injuries: A test of stress-buffering, risk-taking, and coping skills hypotheses. Journal of Personality and Social Psychology. 1992;62:1016–1024. doi: 10.1037//0022-3514.62.6.1016. [DOI] [PubMed] [Google Scholar]
- Solomon Z, Ginzburg K, Neria Y, Ohry A. Coping with war captivity: The role of sensation seeking. European Journal of Personality. 1995;9:57–70. [Google Scholar]
- Steele H, Siever L. An attachment perspective on borderline personality disorder: advances in gene-environment considerations. Current Psychiatry Reports. 2010;12:61–67. doi: 10.1007/s11920-009-0091-0. [DOI] [PubMed] [Google Scholar]
- Stovall-McClough KC, Cloitre M. Unresolved attachment, PTSD, and dissociation in women with childhood abuse histories. Journal of Consulting and Clinical Psychology. 2006;74:219–228. doi: 10.1037/0022-006X.74.2.219. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, et al. Etiologic subtypes of attention-deficit/hyperactivity disorder: Brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychology Review. 2007;17:39–59. doi: 10.1007/s11065-007-9019-9. [DOI] [PubMed] [Google Scholar]
- Van IJzendoorn MH, Bakermans-Kranenburg MJ. DRD4 7-repeat polymorphism moderates the association between maternal unresolved loss or trauma and infant disorganization. Attachment & Human Development. 2006;8:291–307. doi: 10.1080/14616730601048159. [DOI] [PubMed] [Google Scholar]
- Van IJzendoorn MH, Bakermans-Kranenburg MJ, Mesman J. Dopamine system genes associated with parenting in the context of daily hassles. Genes, Brain and Behavior. 2008;7:403–410. doi: 10.1111/j.1601-183X.2007.00362.x. [DOI] [PubMed] [Google Scholar]
- Van IJzendoorn MH, Caspers K, Bakermans-Kranenburg MJ, Beach SRH, Philibert R. Methylation matters: Interaction between methylation density and 5HTT genotype predicts unresolved loss or trauma. Biological Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van IJzendoorn MH, Schuengel C, Bakermans-Kranenburg MJ. Disorganized attachment in early childhood: Meta-analysis of precursors, concomitants, and sequelae. Development and Psychopathology. 1999;11:225–249. doi: 10.1017/s0954579499002035. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Mohr C, Spangler G. Genetic and attachment influences on adolescents’ regulation of autonomy and aggressiveness. Journal of Child Psychology and Psychiatry. 2009;50:1339–1347. doi: 10.1111/j.1469-7610.2009.02158.x. [DOI] [PubMed] [Google Scholar]