Abstract
Background
Among heart failure (HF) patients, early readmission or death and repeat hospitalizations may be indicators of poor disease management or more severe disease.
Methods and Results
We assessed the association of neighborhood median household income (nINC) and Medicaid status with rehospitalization or death in the Atherosclerosis Risk in Communities cohort study (1987–2004) following an incident HF hospitalization in the context of individual socioeconomic status, and evaluated the relationship for modification by demographic and comorbid factors. We used generalized linear Poisson mixed models to estimate rehospitalization rate ratios and 95% confidence intervals (RR, 95% CI) and Cox regression to estimate hazard ratios (HR, 95% CI) of rehospitalization or death. In models controlling for race/study community, gender, age at HF diagnosis, body mass index, hypertension, educational attainment, alcohol use and smoking, persons with a high burden of comorbidity who were living in low nINC areas at baseline had an elevated hazard of all-cause rehospitalization (1.40, 1.10–1.77), death (1.36, 1.02–1.80), and rehospitalization or death (1.36, 1.08–1.70)—as well as increased rates of hospitalizations—compared to those with a high burden of comorbidity living in high nINC areas. Medicaid recipients with a low level of comorbidity had an increased hazard of all-cause rehospitalization (1.19, 1.05–1.36) and rehospitalization or death (1.21, 1.07–1.37), and a higher rate of repeat hospitalizations compared to non-Medicaid recipients.
Conclusions
Comorbidity burden appears to influence the association between nINC, Medicaid status and rehospitalization and death among HF patients.
Keywords: hospital readmission follow-up studies, socioeconomic position heart failure, mortality, comorbidities heart failure
Hospital discharges for heart failure (HF) increased 157% from 1979 to 20021, and continue to rise2. HF rehospitalizations, which are often preventable3, tend to be higher among older patients, non-whites, and patients with prior hospitalizations and multiple primary care visits4–6. In addition to being recognized as a major cause of serious morbidity7–9, HF mortality is high10,11. From 1980 to 1995, the number of deaths in the US with an underlying cause of HF increased nearly 70%12. HF is a primary or contributory cause of more than 300,000 deaths each year in the US13, and HF mortality rates increase sharply with age.
Among Atherosclerosis Risk in Communities (ARIC) study (1987–2002) cohort members with incident HF, 30-day mortality was 10%, while one- and five-year mortality was 22% and 42%, respectively14. Several studies with a combined endpoint of rehospitalization or mortality report a prevalence of rehospitalization or death of 31–35% at 60 days15, and 81%16 at one year.
A shorter interval of time between initial hospitalization for HF and readmission or death may be an indicator of more severe disease. Chronic conditions such as hypertension, coronary heart disease (CHD), diabetes and obesity are risk factors for the development of HF4, and clinical HF is commonly accompanied by one or more of these factors17. In general, the burden of mortality10,18,19 and rehospitalization20 increases with increasing comorbidity. However, in populations, variations in HF morbidity and mortality are not completely explained by clinical features of the disease21, suggesting the need to explore understudied domains, such as the influence of the socioeconomic context.
Low socioeconomic status is associated with higher HF incidence22–24, rehospitalization and survival25–27. Meanwhile, health insurance status is associated with care-seeking behavior20 and subsequent disease outcomes28. Receipt of Medicaid, in particular, may exert effects on health outcomes which are independent of socioeconomic status29,30, as coverage is determined by having certain diseases and disabilities or an income below the poverty line31. Evidence suggests that social and environmental contexts play an important role in health outcomes32–34, however, research to date has not jointly assessed the effects of neighborhood socioeconomic status and receipt of Medicaid on the risk of rehospitalization or mortality among HF patients in the context of individual socioeconomic factors. Furthermore, no published data are available which address whether the influence of the socioeconomic context differs between patients with and without a high level of comorbidity. We hypothesized that low neighborhood socioeconomic status and receipt of Medicaid, respectively, would lead to earlier readmission or death, and that these factors would impart a larger influence among participants with a higher burden of comorbidity.
Methods
ARIC cohort participants (N=15,792) were enrolled from 1987–1989 from the following four US communities: Forsyth County, North Carolina; Washington County, Maryland; suburbs of Minneapolis, Minnesota and Jackson, Mississippi35. As part of annual follow-up, information regarding inpatient hospital stays is collected from cohort members, and hospitalization data are abstracted from the medical record.
All-cause hospitalizations are identified during annual follow-up or during routine ARIC community surveillance36. For the current study, cardiovascular disease (CVD)-related hospitalizations were further identified from all-cause hospitalizations using International Classification of Diseases, Version 9 (ICD-9) discharge codes 402, 410–414, 427, 428, 430–436 or 518.4; while a HF-related hospitalization was defined as that with an ICD-9 discharge code 42837.
Participants’ addresses obtained at baseline were assigned to the level of the census tract by a vendor with high geocoding accuracy (Mapping Analytics)38. The 1990 US census tract-level neighborhood-level socioeconomic measure selected for study was median household income (nINC). In previous work, the use of the single-variable nINC measure produced results of similar magnitude and precision when compared to a more complex composite index measure of neighborhood SES39. We categorized nINC into community-wide tertiles based upon participants’ place of residence at baseline, during the period 1987–1989: low (<$24,777), medium ($24,777≤–<36,071) and high (≥$36,071).
After excluding 245 participants with prevalent HF at baseline, 1,415 participants had an incident hospitalized HF event through 2004. An additional 70 participants were excluded due to missing data on neighborhood socioeconomic status, and 3 were excluded due to insufficient numbers for analysis because they were not white or black, or were blacks living in Minnesota or Maryland, resulting in a final sample size of 1,342 participants.
Covariates included race/study community, gender, age at incident HF hospitalization and selected socioeconomic, clinical and behavioral characteristics. Educational attainment was assessed at baseline (less than 11 years, high school graduate, and greater than high school), as was health insurance status at the time of the index HF hospitalization (receipt of Medicaid, yes/no). Participants’ body mass index (BMI) was assessed at baseline and classified as normal (<25 kg/m2), overweight (25–<30 kg/m2) or obese (≥30 kg/m2). Hypertensive status at baseline was identified as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or taking hypertensive medication within the previous two weeks. Teaching status of the hospital during the index admission (teaching vs. non-teaching), was based upon whether or not the hospital had an internal medicine residency training program.
We ascertained the prevalence of common underlying conditions at the time of the index HF hospitalization using ICD-9 discharge codes. The Charlson Index, a clinical comorbidity algorithm19, was derived from these data. The Charlson Index is a validated measure used to quantify the burden of comorbidity in several studies of mortality and adverse health outcomes18,19. In its use with HF outcomes, a “modified” Charlson Index excludes chronic HF from the conditions included in the computation of the comorbidity score40. Consistent with previous studies, we defined a high burden of comorbidity as a sum of two or more points on the Charlson Index scale, whereas a low burden of comorbidity was defined with a total of zero to one points.
We used generalized linear Poisson mixed models to estimate all-cause, CVD-related and HF-related rehospitalization rate ratios, comparing the rates of participants from low nINC to high nINC, medium nINC to high nINC and Medicaid recipients to non-Medicaid recipients, along with 95% confidence intervals (RR, 95% CI). This modelling strategy accounted for repeat hospitalizations among patients as well as the clustering of patients within census tracts. Time at risk for rehospitalization was the time elapsed between the incident HF hospitalization admission date and death, loss to follow-up or the end of 2004, whichever came first. We assessed for over-dispersion by consulting the deviance statistic of the Poisson model, and conducted supplementary analyses using negative binomial regression when the deviance statistic exceeded one41.
The product-limit (Kaplan-Meier) method was used to measure time to readmission, death, or readmission or death over the course of follow-up. Multivariate Cox proportional hazard models estimated the risk of death or rehospitalization or death, and rehospitalization alone using death during follow-up as the censoring variable. The model produced survival curves depicting survival free of readmission or death, and the proportional hazards assumption was assessed. All participants were censored at the end of 2004.
Crude nINC-rehospitalization/mortality analyses were conducted, the influence of covariates in a full model were tested, and effect modification (pinteraction<0.05) of the nINC-rehospitalization/mortality relationship was assessed by age, race/study community, gender, hypertension, BMI and comorbidity index score. Analyses were performed by using SAS Version 9.1 (SAS Institute, Inc., Cary, NC).
Results
Among participants with an incident HF hospitalization, 41% lived in low nINC, and one-quarter resided in high nINC, areas at baseline. Approximately half (46%) were female, one-third (33%) were black and the average age at the time of the index event was 67 years. As shown in Table 1, a greater proportion (55%) of participants from low nINC areas had attained 11 or fewer years of education, as compared to participants in medium (35%) and high (19%) nINC areas. Twenty percent of participants living in low nINC areas were Medicaid recipients, in contrast to 3% of those living in medium and high nINC areas (Table 2).
Table 1.
Baseline Characteristics of Participants with Incident Hospitalized Heart Failure, by Medicaid Status and nINC: The ARIC study, 1987–2004.
| Medicaid Recipient | Median Household Income (nINC) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Low | Medium | High | ||||||
| N=135 | N=1,207 | N=553 | N=454 | N=335 | ||||||
| N | % | N | % | N | % | N | % | N | % | |
| Median Household Income (USD), mean | 17,897 | 29,456 | 16,519 | 31,799 | 42,979 | |||||
| Gender | ||||||||||
| Female | 97 | 71.9 | 513 | 42.5 | 309 | 55.9 | 173 | 38.1 | 128 | 38.2 |
| Male | 38 | 28.1 | 694 | 57.5 | 244 | 44.1 | 281 | 61.9 | 207 | 61.8 |
| Race/Study Community | ||||||||||
| Black/Forsyth | 5 | 3.7 | 40 | 3.3 | 26 | 4.7 | 17 | 3.7 | 2 | 0.6 |
| Black/Jackson | 97 | 71.9 | 300 | 24.8 | 369 | 66.7 | 6 | 1.3 | 22 | 6.6 |
| White/Forsyth County | 9 | 6.6 | 264 | 21.9 | 42 | 7.6 | 141 | 31.1 | 90 | 26.9 |
| White/Washington County | 20 | 14.8 | 363 | 30.1 | 103 | 18.6 | 232 | 51.1 | 48 | 14.3 |
| White/Minneapolis | 4 | 3.0 | 240 | 19.9 | 13 | 2.4 | 58 | 12.8 | 173 | 51.6 |
| Hypertensive* | ||||||||||
| Yes | 112 | 66.3 | 598 | 51.0 | 349 | 63.1 | 200 | 44.1 | 161 | 48.1 |
| No | 57 | 33.7 | 564 | 48.1 | 200 | 36.2 | 251 | 55.3 | 170 | 50.8 |
| Missing | - | - | 11 | 0.9 | 4 | 0.7 | 3 | 0.7 | 4 | 1.1 |
| Body Mass Index (BMI)† | ||||||||||
| Obese | 75 | 55.6 | 503 | 41.7 | 273 | 49.4 | 172 | 37.9 | 133 | 39.7 |
| Overweight | 37 | 27.4 | 447 | 37.0 | 186 | 33.6 | 173 | 38.1 | 125 | 37.3 |
| Normal | 23 | 17.0 | 255 | 21.1 | 93 | 16.8 | 109 | 24.0 | 76 | 22.7 |
| Missing | - | - | 2 | 0.2 | 1 | 0.2 | - | - | 1 | 0.3 |
| Current Drinker | ||||||||||
| Yes | 32 | 23.7 | 589 | 48.8 | 168 | 30.4 | 237 | 52.2 | 216 | 64.5 |
| No | 103 | 76.3 | 618 | 51.2 | 385 | 69.6 | 217 | 47.8 | 119 | 35.5 |
| Current Smoker | ||||||||||
| Yes | 56 | 41.5 | 417 | 34.5 | 204 | 36.9 | 161 | 35.5 | 108 | 32.2 |
| No | 79 | 58.5 | 790 | 65.5 | 349 | 63.1 | 293 | 64.5 | 227 | 67.8 |
| Educational Attainment (years) | ||||||||||
| Advanced (17–21) | 12 | 8.9 | 307 | 25.4 | 79 | 14.3 | 106 | 23.4 | 134 | 40.0 |
| Intermediate (12–16) | 26 | 19.3 | 472 | 39.1 | 169 | 30.6 | 190 | 41.9 | 139 | 41.5 |
| Basic (≤11) | 96 | 71.1 | 425 | 35.2 | 302 | 54.6 | 157 | 34.5 | 62 | 18.5 |
| Missing | 1 | 0.7 | 3 | 0.3 | 3 | 0.5 | 1 | 0.2 | - | - |
Systolic blood pressure ≥140mmHg or diastolic blood pressure ≥90mmHg, or blood pressure medication in the last two weeks.
Normal BMI: <25kg/m2; overweight: 25–<30kg/m2; and obese: ≥30kg/m2
Table 2.
Characteristics of Participants During the Index Heart Failure Admission, by Medicaid Status and nINC: The ARIC study, 1987–2004.
| Medicaid Recipient | Median Household Income (nINC) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Low | Medium | High | ||||||
| N=135 | N=1,207 | N=553 | N=454 | N=335 | ||||||
| N | % | N | % | N | % | N | % | N | % | |
| Age, mean (SD) | 67.5 (6.1) | 66.9 (6.9) | 66.0 (6.8) | 67.9 (6.6) | 67.5 (6.9) | |||||
| Medicaid Recipient* | - | - | 111 | 20.1 | 15 | 3.3 | 9 | 2.7 | ||
|
| ||||||||||
| Prevalence of Comorbidities†
| ||||||||||
| Myocardial Infarction | 15 | 11.1 | 155 | 12.8 | 57 | 10.3 | 74 | 16.3 | 39 | 11.6 |
| Peripheral Vascular Disease | 11 | 8.2 | 94 | 7.8 | 38 | 6.9 | 36 | 7.9 | 31 | 9.3 |
| Cerebrovascular Disease | 9 | 6.7 | 38 | 3.2 | 55 | 10.0 | 38 | 8.4 | 28 | 8.4 |
| Dementia | 1 | 0.7 | 7 | 0.6 | 0 | - | 5 | 1.1 | 3 | 0.9 |
| Chronic Pulmonary Disease | 40 | 29.6 | 328 | 27.2 | 124 | 22.4 | 153 | 33.7 | 91 | 27.2 |
| Rheumatologic Disease | 2 | 1.5 | 31 | 2.6 | 11 | 2.0 | 13 | 2.9 | 9 | 2.7 |
| Mild Liver Disease | 0 | - | 11 | 0.9 | 4 | 0.7 | 4 | 0.9 | 3 | 0.9 |
| Moderate or Severe Liver Disease | 0 | - | 6 | 0.5 | 4 | 0.7 | 0 | - | 2 | 0.6 |
| Diabetes Mellitus | 38 | 28.2 | 248 | 20.6 | 137 | 24.7 | 91 | 20.1 | 65 | 19.5 |
| Diabetes with Chronic Complications | 9 | 6.7 | 53 | 4.4 | 20 | 3.6 | 16 | 3.5 | 19 | 5.7 |
| Hemiplegia or Paraplegia | 3 | 2.2 | 17 | 1.4 | 9 | 1.6 | 11 | 2.4 | 0 | - |
| Renal Disease | 4 | 3.0 | 33 | 2.7 | 22 | 4.0 | 8 | 1.8 | 7 | 2.1 |
|
| ||||||||||
| Charlson Comorbidity Index Score‡
| ||||||||||
| ≥ 2 | 37 | 27.4 | 277 | 23.0 | 126 | 22.8 | 118 | 26.0 | 70 | 20.9 |
| <2 | 98 | 72.6 | 930 | 77.0 | 427 | 77.2 | 336 | 74.0 | 265 | 79.1 |
As indicated in medical record
Charlson Index Score Components
Adapted for use with ICD-9 discharge codes
By the end of 2004, 89% of participants with an incident HF hospitalization had been rehospitalized at least once (mean: 3.6; range: 0–47), 47% died, and 91% had been rehospitalized or had died. Figure 1 shows life table trends of rehospitalization, death and rehospitalization or death by person-time elapsed since the incident hospitalized HF event. Of note, the cumulative proportion of persons experiencing rehospitalization or death is quite similar to that of rehospitalization, but not death. At one year, 19% had died, 59% had been rehospitalized, and 62% had been rehospitalized or had died (Figure 1).
Figure 1.
Cumulative proportion of participants with an incident heart failure hospitalization experiencing rehospitalization, death and rehospitalization or death, The ARIC study (1987–2004)
Almost one-quarter of participants had a comorbidity index score of two or greater (Table 2). The most common comorbidities identified at the index hospitalization were chronic pulmonary disease (27%), diabetes (22%) and myocardial infarction (13%). The comorbidity index score modified the nINC-rehospitalization/mortality relationship (p<0.05) in Cox proportional hazards (time-to-event) and Poisson (rate) analyses. Therefore, subsequent results are presented stratified by level of the comorbidity score (≥2 vs. <2).
Time-to-event analyses
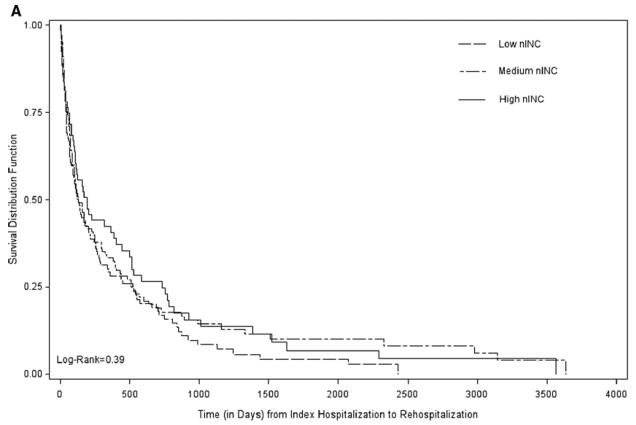
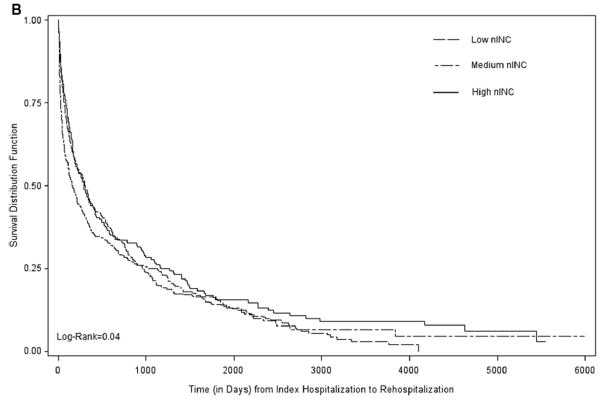
Crude median rehospitalization- and mortality-free survival times, in days, varied by comorbidity index score (high vs. low) among participants in each nINC tertile [low nINC (107 vs. 283), medium nINC (118 vs. 128) and high nINC (161 vs. 229)] as well as by receipt of Medicaid [recipients (60 vs. 168), those not receiving Medicaid (133 vs. 217)]. Figure 2 shows rehospitalization-free survival curves, one for each level of comorbidity burden, stratified by nINC. Among participants with a high burden of comorbidity, those living in high nINC areas experienced the longest rehospitalization-free survival, while those living in low nINC areas experienced the shortest. The observed nINC gradient did not persist among participants with a low burden of comorbidity (Figure 2).
Figure 2.
Survival after the Incident HF Hospitalization: Time to Rehospitalization by Comorbidity Burden and nINC: The ARIC study (1987–2004).
The nINC/Medicaid-rehospitalization/mortality survival relationships (HR, 95% CI) are shown in Table 3. In models controlling for race/study community, gender, age at HF diagnosis, body mass index, hypertension, educational attainment, alcohol use and smoking, persons with a high burden of comorbidity who were living in low nINC areas at baseline had an elevated risk for all-cause rehospitalization (1.40, 1.10–1.77), death (1.36, 1.02–1.80) and rehospitalization or death (1.36, 1.08–1.70) compared to those with a high burden of comorbidity living in high nINC areas. In contrast, participants with a low burden of comorbidity who were living in low nINC areas at baseline did not experience an increased risk for death. Medicaid recipients with a low level of comorbidity had an increased risk of all-cause rehospitalization (1.19, 1.05–1.36) and rehospitalization or death (1.21, 1.07–1.37) compared to non-Medicaid recipients with a low level of comorbidity. Restricting the model to include those in the lowest nINC tertile and combining across comorbidity categories, the risk for all-cause rehospitalization among participants with Medicaid was 1.22 (1.07, 1.38) compared to those without Medicaid. A significantly lower hazard of death was seen among those with a higher burden of comorbidity living in medium nINC areas compared to those living in high nINC areas (0.74, 0.59–0.93).
Table 3.
Hazard Ratios (HR) and 95% Confidence Intervals (95% CI) for all-cause rehospitalization, death, and rehospitalization or death following an Incident Hospitalized Heart Failure Event by nINC, Stratified by Charlson Index Score: The ARIC study, 1987–2004.
| Charlson Index Score ≥2 | Charlson Index Score <2 | |||
|---|---|---|---|---|
| Model 1* | Model 2† | Model 1* | Model 2† | |
| All-cause Rehospitalization
| ||||
| nINC | ||||
| Low | 1.23 (1.00, 1.51) | 1.40 (1.10, 1.77) | 1.13 (1.01, 1.26) | 1.16 (1.04, 1.30) |
| Medium | 1.07 (0.91, 1.27) | 1.14 (0.95, 1.36) | 1.26 (1.15, 1.39) | 1.28 (1.16, 1.41) |
| High | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Medicaid Recipient | ||||
| Yes | 1.18 (0.95, 1.46) | 1.12 (0.89, 1.40) | 1.17 (1.03, 1.32) | 1.19 (1.05, 1.36) |
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
|
| ||||
| Death
| ||||
| nINC | ||||
| Low | 1.34 (1.04, 1.72) | 1.36 (1.02, 1.80) | 1.12 (0.97, 1.30) | 1.09 (0.94, 1.26) |
| Medium | 0.75 (0.61, 0.93) | 0.74 (0.59, 0.93) | 0.91 (0.79, 1.03) | 0.90 (0.78, 1.02) |
| High | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Medicaid Recipient | ||||
| Yes | 0.99 (0.76, 1.30) | 0.95 (0.72, 1.25) | 1.03 (0.87, 1.23) | 0.96 (0.80, 1.14) |
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
|
| ||||
| All-cause Rehospitalization or Death
| ||||
| nINC | ||||
| Low | 1.23 (1.01, 1.50) | 1.36 (1.08, 1.70) | 1.09 (0.98, 1.21) | 1.13 (1.02, 1.26) |
| Medium | 1.00 (0.85, 1.17) | 1.04 (0.87, 1.23) | 1.24 (1.13, 1.36) | 1.27 (1.15, 1.39) |
| High | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Medicaid Recipient | ||||
| Yes | 1.23 (1.00, 1.51) | 1.17 (0.95, 1.45) | 1.17 (1.04, 1.32) | 1.21 (1.07, 1.37) |
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
nINC and Medicaid status plus race/study community, gender and age at index event
Model 1 plus hypertension, body mass index, current smoker, current drinker and educational attainment
Rate analyses
Of 1,342 participants with an incident HF hospitalization, 148 (11%) were not rehospitalized for any cause, while 318 (24%) were not rehospitalized for a CVD-related cause and 590 (44%) were not rehospitalized for HF. All-cause rehospitalization rates per 100 person-years (95% CI) were 71.3 (63.3–80.4) for low nINC, 71.9 (64.5–80.2) for medium nINC, and 54.3 (47.7–61.7) for high nINC.
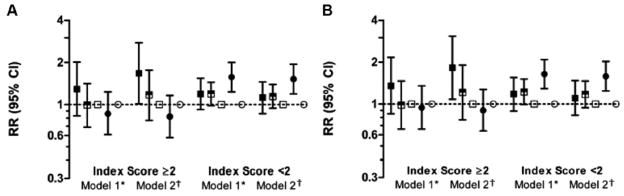
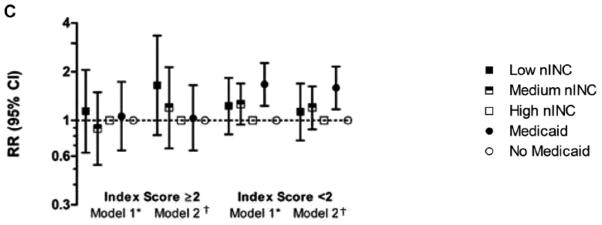
In models controlling for race/study community, gender, age at HF diagnosis, BMI, hypertension, educational attainment, receipt of Medicaid, teaching hospital status, alcohol use and smoking, participants with a higher burden of comorbidity living in low nINC areas had a higher risk of all-cause (1.67, 1.01–2.76) and CVD-related (1.82, 1.08–3.07) – but did not reach statistical significance for HF-related (1.65, 0.81–3.34) – hospitalizations, compared to those with a high burden of comorbidity living in high nINC areas. Participants living in medium nINC areas at baseline did not have an elevated risk compared to participants living in high nINC areas, nor was there an nINC differential among participants with a low burden of comorbidity. Similar results were seen for CVD-related hospitalizations; however, no nINC effect in either strata of comorbidity burden was seen for HF-related hospitalizations, possibly due to relatively few events meeting the criteria for HF-related hospitalizations. Among participants with a low comorbidity burden, Medicaid recipients were at increased risk for all-cause hospitalizations. The observed results persisted for Medicaid recipients with a low comorbidity burden in analyses for CVD- and HF-related hospitalizations (Figure 3).
Figure 3.
Rate Ratios (and 95% CI) for All-cause, CVD- and HF-related Rehospitalizations among Participants with Incident Hospitalized HF: The ARIC study (1987–2004).
*nINC and Medicaid status plus race/study community, gender and age at index event
†Model 1 plus hypertension, body mass index, current smoker, current drinker and educational attainment
In our data, the Poisson models used for estimating rehospitalization rate ratios yielded a deviance statistic of close to four. Thus, over-dispersion was suggested. In response, we fit negative binomial models to the data. As expected, the point estimates of the rate ratios did not change, however, the confidence intervals widened with the application of the negative binomial model, reflecting the effect over-dispersion had on these data. Although the negative binomial estimates were less precise, the analyses accounting for over-dispersion did not change our interpretation of the results.
Discussion
In this study, incident HF hospitalizations were more common among ARIC cohort participants of low and medium nINC compared to those living in high nINC areas at baseline. Further, low nINC participants with an elevated comorbidity index score at the time of the incident hospitalized HF event were rehospitalized at a higher rate than high nINC participants in the same comorbidity category. These findings were consistent with a review concluding that hospital admission rates increase with increased social deprivation42. In addition, participants had an increased hazard of rehospitalization, death and rehospitalization or death if they lived in a low nINC area at baseline and had a higher burden of comorbidity, compared to participants living in high nINC areas at baseline with a similar level of comorbidity.
Patients with limited neighborhood socioeconomic resources may not have adequate social support or access to primary care facilities necessary to manage HF out-of-hospital. Persons living in economically deprived areas may be less likely to have a primary care physician, and thus may seek care in-hospital for conditions commonly managed out-of-hospital. McAlister (2004) reported follow-up rates with primary care physicians were lowest among patients with high neighborhood socioeconomic deprivation23. Fewer primary care visits may be an indication of higher hospital utilization rates among patients of lower nINC. A limitation of our study is that we are unable to take into account out-of-hospital management of HF, as outpatient records were not available for the time period under study. Future investigations in ARIC will, however, attempt to monitor the outpatient events related to HF.
A related limitation of this study is the lack of information regarding HF medication adherence post-discharge. To address this limitation, we assessed whether angiotensin-converting enzyme inhibitors or beta-blockers were given during the hospitalization or at discharge, and controlled for these factors in models containing all potential confounders. Inclusion of the HF medication variables did not appreciably change the estimates (<5%) and did not alter our interpretation of the results.
Medicaid recipients without a high burden of comorbidity tended to have a higher hazard of first rehospitalization, and were rehospitalized more often than participants not receiving Medicaid. It is possible that the Medicaid recipients in this study with greater comorbidity were more likely to seek or be referred to care for symptom managment out-of-hospital and as a result did not require more frequent hospitalizations than non-Medicaid recipients with a high comorbidity burden. Conversely, the Medicaid recipients with fewer comorbidities in this study may not have been as aggressively managed in- or out-of- hospital, leading to a higher hazard of first rehospitalization following the index HF hospitalization. However, these estimates should be interpreted with caution, as the number of Medicaid recipients with a high comorbidity burden in these data was relatively small.
Shorter median times from the index event to readmission among those living in low nINC areas appeared to be a strong influence on the combined rehospitalization/mortality endpoint, as low nINC was not a predictor for HF survival across levels of comorbidity in the ARIC study population. In particular, rehospitalization occurs more often and more quickly among participants living in low nINC areas, especially among those with more comorbidities identified during the incident hospitalized event. In general, patients with more comorbidity may require a greater number of treatments because they are sicker, more susceptible to severe HF, or experience acute exacerbations of the disease. Requiring more medical attention due to a high burden of comorbidity may serve to highlight the limited resources available in low nINC areas, either for adequate self-care43 or out-of-hospital management of disease.
A strength of this study is its inclusion of a racially diverse population of men and women who were free of HF at baseline and followed from 1987 to 2004 in order to capture an incident HF hospitalization, subsequent hospitalizations and fatal events. Longer follow-up more adequately depicts the survival experience and clinical course of HF progression for the majority of HF patients. Blacks living in Jackson, Mississippi constituted the majority of HF patients who both resided in low nINC areas at baseline and were Medicaid recipients. This limitation highlights the difficulty of disentangling race and socioeconomic disadvantage in our society.
The index HF hospitalization was defined as the first mention of a 428 ICD-9 discharge code in the medical record, a technique used in extant studies of HF14. We acknowledge limitations inherent to this method of event identification, such as an inability to distinguish between acute and chronic HF events as well as not being able to determine the etiology of the incident hospitalized event. Although the identification of incident events via ICD-9 discharge codes does not capture outpatient events that may have occurred prior the incident hospitalized event, the distribution of hospitalizations among ARIC participants with incident hospitalized HF were similar to a recently published community-based report which ascertained incident HF cases from both outpatient and inpatient records44.
In the context of increasing hospital discharges for HF and a consistently high rate of mortality from the syndrome, it is critical to identify social and economic neighborhood forces which impact HF rehospitalization or death in the presence of individual socioeconomic, demographic and comorbid factors. Differences by nINC in survival free from readmission or death post-incident HF hospitalization may have important implications for the management and treatment of HF patients45,46. It is likely that nINC in part determines the availability of health care resources in a community, such as the proximity of neighborhood health clinics. Outpatient care is critical to the out-of-hospital monitoring of HF patients, and if less available in low nINC areas, may adversely affect the progression of HF among patients in these communities47. In this study, Medicaid recipients with a low burden of comorbidity were more likely to be admitted to the hospital following an incident hospitalized HF event. Whether these patients are adequately monitored on an outpatient basis remains unclear. Regardless, comorbidity burden appears to modify the association between nINC, Medicaid status and rehospitalization and death among HF patients.
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. The authors thank the staff and participants of the ARIC study for their important contributions.
Sources of Funding
This research was funded in part by NIH, National Heart, Lung and Blood Institute and National Research service award training grant 5-T32-HL007055.
Footnotes
Disclosures
None.
References
- 1.Heart Disease and Stroke Statistics - 2005 Update. Dallas, Texas: American Heart Association; 2005. pp. 1–63. [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics--2010 Update: A Report From the American Heart Association. Circulation. 2010;121:e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 3.MedPAC. Report to the Congress: Promoting Greater Efficiency in Medicare. Medicare Payment Advisory Commission (MedPAC); 2007. [Google Scholar]
- 4.Schocken DD, Benjamin EJ, Fonarow GC, Krumholz HM, Levy D, Mensah GA, Narula J, Shor ES, Young JB, Hong Y. Prevention of Heart Failure. A Scientific Statement From the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544–65. doi: 10.1161/CIRCULATIONAHA.107.188965. [DOI] [PubMed] [Google Scholar]
- 5.Fang J, Mensah GA, Croft JB, Keenan NL. Heart Failure-Related Hospitalization in the U.S., 1979 to 2004. Journal of the American College of Cardiology. 2008;52:428–434. doi: 10.1016/j.jacc.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 6.Inouye SK, Zhang Y, Jones RN, Shi P, Cupples LA, Calderon HN, Marcantonio ER. Risk factors for hospitalization among community-dwelling primary care older patients: development and validation of a predictive model. Med Care. 2008;46:726–731. doi: 10.1097/MLR.0b013e3181649426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowie MR, Fox KF, Wood DA, Metcalfe C, Thompson SG, Coats AJS, Poole-Wilson PA, Sutton GC. Hospitalization of patients with heart failure. A population-based study. Eur Heart J. 2002;23:877–885. doi: 10.1053/euhj.2001.2973. [DOI] [PubMed] [Google Scholar]
- 8.Adams J, KF, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the United States: Rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) American Heart Journal. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Hoyt RE, Bowling LS. Reducing readmissions for congestive heart failure. Am Fam Physician. 2001;63:1593–1598. [PubMed] [Google Scholar]
- 10.Jong P, Vowinckel E, Liu PP, Gong Y, Tu JV. Prognosis and Determinants of Survival in Patients Newly Hospitalized for Heart Failure: A Population-Based Study. Arch Intern Med. 2002;162:1689–1694. doi: 10.1001/archinte.162.15.1689. [DOI] [PubMed] [Google Scholar]
- 11.Nieminen MS, Harjola V-P. Definition and epidemiology of acute heart failure syndromes. Am J Cardiol. 2005;96:5G–10G. doi: 10.1016/j.amjcard.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Haldeman GA, Croft JB, Giles WH, Rashidee A. Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995. American Heart Journal. 1999;137:352–360. doi: 10.1053/hj.1999.v137.95495. [DOI] [PubMed] [Google Scholar]
- 13.Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldmanmd AM, Francis GS, Ganiats TG, Goldstein S, Gregoratos G, Jessup ML, Noble RJ, Packer M, Silver MA, Stevenson LW, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Jacobs AK, Hiratzka LF, Russell RO, Smith SC., Jr ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary a report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee to revise the 1995 guidelines for the evaluation and management of heart failure) Circulation. 2001;104:2996–3007. doi: 10.1161/hc4901.102568. [DOI] [PubMed] [Google Scholar]
- 14.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 15.O’Connor CM, Stough WG, Gallup DS, Hasselblad V, Gheorghiade M. Demographics, clinical characteristics, and outcomes of patients hospitalized for decompensated heart failure: observations from the IMPACT-HF registry. Journal of Cardiac Failure. 2005;11:200–205. doi: 10.1016/j.cardfail.2004.08.160. [DOI] [PubMed] [Google Scholar]
- 16.Zannad F, Briancon S, Juilliere Y, Mertes P-M, Villemot J-P, Alla F, Virion J-M. Incidence, clinical and etiologic features, and outcomes of advanced chronic heart failure: the EPICAL study. Journal of the American College of Cardiology. 1999;33:734–742. doi: 10.1016/s0735-1097(98)00634-2. [DOI] [PubMed] [Google Scholar]
- 17.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J. High Prevalence of Renal Dysfunction and Its Impact on Outcome in 118,465 Patients Hospitalized With Acute Decompensated Heart Failure: A Report From the ADHERE Database. Journal of Cardiac Failure. 2007;13:422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of Clinical Epidemiology. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 20.Philbin EF, DiSalvo TG. Prediction of hospital readmission for heart failure: development of a simple risk score based on administrative data. Journal of the American College of Cardiology. 1999;33:1560–1566. doi: 10.1016/s0735-1097(99)00059-5. [DOI] [PubMed] [Google Scholar]
- 21.Fonarow GC. Epidemiology and risk stratification in acute heart failure. Am Heart J. 2008;155:200–7. doi: 10.1016/j.ahj.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 22.Ingelsson E, Lind L, Arnlov J, Sundstrom J. Socioeconomic factors as predictors of incident heart failure. Journal of Cardiac Failure. 2006;12:540–545. doi: 10.1016/j.cardfail.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 23.McAlister FA, Murphy NF, Simpson CR, Stewart S, MacIntyre K, Kirkpatrick M, Chalmers J, Redpath A, Capewell S, McMurray JJV. Influence of socioeconomic deprivation on the primary care burden and treatment of patients with a diagnosis of heart failure in general practice in Scotland: population based study. BMJ. 2004;328:1110–1113. doi: 10.1136/bmj.38043.414074.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart S, Murphy NF, McMurray JJV, Jhund P, Hart CL, Hole D. Effect of socioeconomic deprivation on the population risk of incident heart failure hospitalisation: An analysis of the Renfrew/Paisley Study. European Journal of Heart Failure. 2006;8:856–863. doi: 10.1016/j.ejheart.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Philbin EF, Dec GW, Jenkins PL, DiSalvo TG. Socioeconomic status as an independent risk factor for hospital readmission for heart failure. The American Journal of Cardiology. 2001;87:1367–1371. doi: 10.1016/s0002-9149(01)01554-5. [DOI] [PubMed] [Google Scholar]
- 26.Rathore SS, Masoudi FA, Wang Y, Curtis JP, Foody JM, Havranek EP, Krumholz HM. Socioeconomic status, treatment, and outcomes among elderly patients hospitalized with heart failure: Findings from the National Heart Failure Project. American Heart Journal. 2006;152:371–378. doi: 10.1016/j.ahj.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen M, Christakis NA. Neighborhood effects on posthospitalization mortality: a population-based cohort study of the elderly in Chicago. Health Services Research. 2005;40:1108–1127. doi: 10.1111/j.1475-6773.2005.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayanian JZ, Kohler BA, Abe T, Epstein AM. The Relation between Health Insurance Coverage and Clinical Outcomes among Women with Breast Cancer. N Engl J Med. 1993;329:326–331. doi: 10.1056/NEJM199307293290507. [DOI] [PubMed] [Google Scholar]
- 29.Foraker RE, Rose KM, McGinn AP, Suchindran CM, Goff DC, Jr, Whitsel EA, Wood JL, Rosamond WD. Neighborhood Income, Health Insurance, and Prehospital Delay for Myocardial Infarction: The Atherosclerosis Risk in Communities Study. Arch Intern Med. 2008;168:1874–1879. doi: 10.1001/archinte.168.17.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross CE, Mirowsky J. Does Medical Insurance Contribute to Socioeconomic Differentials in Health? The Milbank Quarterly. 2000;78:291–321. doi: 10.1111/1468-0009.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenbaum S. Medicaid. N Engl J Med. 2002;346:635–640. doi: 10.1056/NEJM200202213460825. [DOI] [PubMed] [Google Scholar]
- 32.Krieger N, Waterman P, Chen JT, Soobader M-J, Subramanian SV, Carson R. Zip code caveat: bias due to spatiotemporal mismatches between zip codes and US Census-defined geographic areas--the Public Health Disparities Geocoding Project. Am J Public Health. 2002;92:1100–1102. doi: 10.2105/ajph.92.7.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marmot MG. Understanding Social Inequalities in Health. Perspectives in Biology and Medicine. 2003;46:S9–S23. [PubMed] [Google Scholar]
- 34.Diez Roux AV, Borrell LN, Haan M, Jackson SA, Schultz R. Neighbourhood environments and mortality in an elderly cohort: results from the cardiovascular health study. J Epidemiol Community Health. 2004;58:917–923. doi: 10.1136/jech.2003.019596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 36.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA, Investigators TA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: Methods and initial two years’ experience. Journal of Clinical Epidemiology. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 37.E, editor. ARIC. Cohort Event Eligibility Form. 2007. [Google Scholar]
- 38.Whitsel EA, Rose KM, Wood JL, Henley AC, Liao D, Heiss G. Accuracy and repeatability of commercial geocoding. Am J Epidemiol. 2004;160:1023–1029. doi: 10.1093/aje/kwh310. [DOI] [PubMed] [Google Scholar]
- 39.Rose KM, Suchindran CM, Foraker RE, Whitsel EA, Rosamond WD, Heiss G, Wood JL. Neighborhood Disparities in Incident Hospitalized Myocardial Infarction in Four U.S. Communities: The ARIC Surveillance Study. Annals of Epidemiology. 2009;19:867–874. doi: 10.1016/j.annepidem.2009.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Senni M, Santilli G, Parrella P, de Maria R, Alari G, Berzuini C, Scuri M, Filippi A, Migliori M, Minetti B, Ferrazzi P, Gavazzi A. A novel prognostic index to determine the impact of cardiac conditions and co-morbidities on one-year outcome in patients with heart failure. Am J Cardiol. 2006;98:1076–82. doi: 10.1016/j.amjcard.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 41.Rao JNK, Scott AJ. A simple method for analysing overdispersion in clustered Poisson data. Statistics in Medicine. 1999;18:1373–1385. doi: 10.1002/(sici)1097-0258(19990615)18:11<1373::aid-sim133>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 42.Blair AS, Lloyd-Williams F, Mair FS. What do we know about socioeconomic status and congestive heart failure? Journal of Family Practice. 2002;51:169. [PubMed] [Google Scholar]
- 43.Booth GL, Hux JE. Relationship Between Avoidable Hospitalizations for Diabetes Mellitus and Income Level. Arch Intern Med. 2003;163:101–106. doi: 10.1001/archinte.163.1.101. [DOI] [PubMed] [Google Scholar]
- 44.Dunlay SM, Redfield MM, Weston SA, Therneau TM, Hall Long K, Shah ND, Roger VL. Hospitalizations After Heart Failure Diagnosis: A Community Perspective. Journal of the American College of Cardiology. 2009;54:1695–1702. doi: 10.1016/j.jacc.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stjarne MK, Fritzell J, Ponce de Leon A, Hallqvist J. Neighborhood socioeconomic context, individual income and myocardial infarction. Epidemiology. 2006;17:14–23. doi: 10.1097/01.ede.0000187178.51024.a7. [DOI] [PubMed] [Google Scholar]
- 46.Chaix B, Rosvall M, Merlo J. Assessment of the magnitude of geographical variations and socioeconomic contextual effects on ischaemic heart disease mortality: a multilevel survival analysis of a large Swedish cohort. J Epidemiol Community Health. 2007;61:349–355. doi: 10.1136/jech.2006.047597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee G, Carrington M. Tackling heart disease and poverty. Nursing and Health Sciences. 2007;9:290–294. doi: 10.1111/j.1442-2018.2007.00363.x. [DOI] [PubMed] [Google Scholar]