Abstract
We have recently shown that IgG1 directed against antigens thought to be involved in the pathogenesis of rheumatoid arthritis harbor different glycan moieties on their Fc-tail, as compared with total sera IgG1. Given the crucial roles of Fc-linked N-glycans for the structure and biological activity of IgG, Fc-glycosylation of antibodies is receiving considerable interest. However, so far little is known about the signals and factors that could influence the composition of these carbohydrate structures on secreted IgG produced by B lymphocytes. Here we show that both “environmental” factors, such as all-trans retinoic acid (a natural metabolite of vitamin A), as well as factors stimulating the innate immune system (i.e. CpG oligodeoxynucleotide, a ligand for toll-like receptor 9) or coming from the adaptive immune system (i.e. interleukin-21, a T-cell derived cytokine) can modulate IgG1 Fc-glycosylation. These factors affect Fc-glycan profiles in different ways. CpG oligodeoxynucleotide and interleukin-21 increase Fc-linked galactosylation and reduce bisecting N-acetylglucosamine levels, whereas all-trans retinoic acid significantly decreases galactosylation and sialylation levels. Moreover, these effects appeared to be stable and specific for secreted IgG1 as no parallel changes of the corresponding glycans in the cellular glycan pool were observed. Interestingly, several other cytokines and molecules known to affect B-cell biology and antibody production did not have an impact on IgG1 Fc-coupled glycan profiles. Together, these data indicate that different stimuli received by B cells during their activation and differentiation can modulate the Fc-linked glycosylation of secreted IgG1 without affecting the general cellular glycosylation machinery. Our study, therefore, furthers our understanding of the regulation of IgG1 glycosylation at the cellular level.
Antibodies play an important role in the host defense against pathogenic microorganisms, whereas those directed against self-antigens (i.e. auto antibodies) can mediate unwanted immune responses leading to the destruction of tissues and organs. Although the specificity of antibodies is determined by the variable region, antibody-mediated effector functions are crucially dependent on the interaction of its constant region (Fc part) with the complement system as well as with Fc receptors (FcR). The latter receptors are expressed on a variety of innate immune cells including mast cells, NK cells, and neutrophils (1). It has been well established that the Fc-mediated effects of antibodies such as complement activation and antibody-dependent cellular cytotoxicity (ADCC)1 are influenced not only by the antibody isotype (IgM and IgG etc) and subclass (IgG1–4 etc), which is determined by the amino acid sequence of the constant region, but also by Fc-linked carbohydrate structures (2).
Human IgG antibodies share a conserved N-glycosylation site within the CH2 domain of their Fc moieties, where the sugar side chain is attached to the asparagine 297 (Asn297) residue (2, 3). The Asn297-linked carbohydrate chain consists of a common biantennary glycan structure of four N-acetylglucosamine (GlcNAc) and three mannose residues, with variable additions of fucose, galactose, sialic acid, and/or bisecting GlcNAc residues (2–4). Depending on the presence or absence of galactose on one or both arms of the glycan moiety, three highly prevalent, core-fucosylated glycoforms, called G0F (no galactose), G1F (one galactose), and G2F (two galactoses), have been defined. Lack of galactose residues results in a concomitant absence of terminal sialic acid from the core polysaccharide chain (2–4).
It has been shown that these Asn-linked carbohydrate structures, interposed between the heavy chains, are crucial for the structure and biological activity of IgG (2, 5, 6). Whereas agalactosylated IgGs show an increased inflammatory activity (7–9), IgGs with glycans containing terminal sialic acid display an anti-inflammatory activity in mouse models for autoimmune diseases (10, 11). For example, several studies demonstrated that the potent anti-inflammatory activity of intravenous gamma globulin (IVIG) is a direct result of Fc sialylation (10, 11). In addition, IgG1 lacking the branching fucose or with an additional bisecting GlcNAc shows an enhanced ADCC through increased interaction with Fcγ receptors (12–16). Moreover, removal of the sugar moiety by glycosidase-mediated hydrolysis or mutational deletion of the attaching site Asn297 changes the structure of the Fc part, resulting in a nonimmunogenic antibody that is unable to interact with Fcγ receptors and to trigger significant cytokine release (5, 17, 18).
Given the functional relevance of these glycan moieties described above, it is not surprising that changes in IgG glycosylation have been shown to be associated with various physiological and pathological conditions. For example, decreased galactosylation of serum IgG was observed in patients with autoimmune diseases and tumors, and the level of IgG-G0 correlated with disease activity in rheumatoid arthritis (RA) patients (2, 4, 7, 8, 19–24). Moreover, we recently showed that antibodies thought to be involved in RA-pathogenesis exhibit a different glycan moiety than that of irrelevant IgG present in the same sera of diseased individuals (25–27). In addition, autoreactive IgG in synovial fluid of RA patients displays further decreased galactosylation and sialylation levels as compared with the serum counterpart. This difference is specific for these autoantibodies as no difference was observed in total IgG (26). Collectively, these findings not only underline the significance of Fc-coupled glycan structures for antibody-mediated immune responses, but also indicate that glycosylation profiles of IgG are tightly regulated by physiological and pathological determinants in an antigen-specific manner. In line with the latter notion, active immunization reduces sialylation of IgG in mice, thereby switching the steady-state serum IgG from an anti-inflammatory to a pro-inflammatory phenotype with the capacity to elicit an effective inflammatory response. This effect is most prominent for IgG targeting the antigen used for immunization (11).
However, because studies on the modification of IgG Fc-glycan profiles have mainly focused on the enzyme-mediated treatment of purified IgG (2, 10, 11, 17, 28) and/or the genetic engineering of enzyme expression levels in antibody producing cell lines (12, 16, 29), so far little is known on the factors that could drive the composition of Fc-coupled carbohydrate structures of (secreted) IgG during the activation and differentiation of B lymphocytes. For the activation of naive B cells, signals delivered through B-cell receptors (BCR) are required, and in case of T cell-dependent antigens, additional signals provided by antigen-specific CD4+ T helper cells (Th) through CD40-CD40L interaction are indispensable as well. Additionally, cytokines produced by Th cells and other soluble factors in the microenvironment of B cells largely determine the subsequent differentiation of B cells into antibody-secreting cells (ASCs) as well as the type and amount of antibodies they produce (30–33). In the present study, we investigated whether such microenvironmental factors present during the activation and differentiation of B cells toward ASCs could influence the carbohydrate structures in the Fc domain of secreted IgG1. Likewise, we determined the nature of the glycan changes induced and analyzed whether such changes were present in all protein pools expressed by B cells or could mainly be found on IgG1.
MATERIALS AND METHODS
Culture Medium
Cells were cultured in serum-free medium consisting of X-VIVO 15 (Lonza) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin (Cambrex), and 2 mm GlutaMAX (Invitrogen).
Isolation of Human B Cells
Human peripheral blood was obtained from healthy blood bank donors after informed consent in accordance with procedures approved by the local human ethics committee. Peripheral blood mononuclear cells (PBMCs) were prepared by centrifugation over Ficoll-Hypaque gradients. CD19+ B cells were purified by positive selection with the Dynabeads CD19 Pan B and then detached by DETACHaBEAD CD19 (Invitrogen). Preparations typically resulted in > 98% CD19+CD20+ B cells as determined by flow cytometry analysis (see Fig. 1B, top left panel).
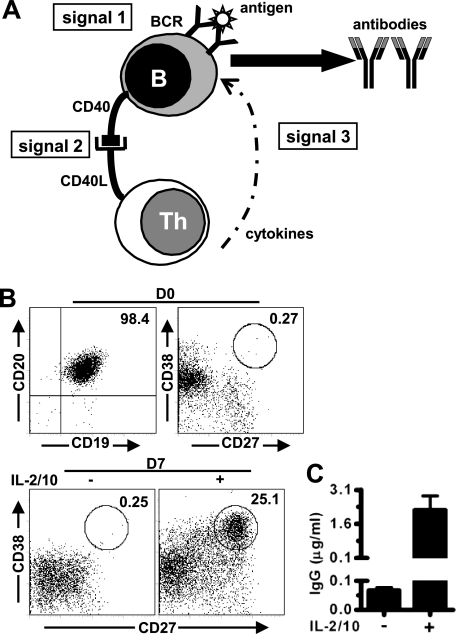
Fig. 1.
Purification and differentiation of human B cells in vitro. Human CD19+ B cells were purified with anti-CD19 beads and subsequently cultured with anti-IgM and CD40L-transfected cells in the absence or presence of additional IL-2 and IL-10 (IL-2/10). Following 7 days, cells were collected and processed for the analysis of CD27 and CD38 expression. A, a schematic overview of the signals required for the T-cell dependent antibody production from human naive B cells. B, representative dot plots showing the CD19 versus CD20 (top left panel) and CD27 versus CD38 (top right and bottom panels) expressions on purified B cells before (D0, top panel) and following 7 days culture (D7, bottom panel). Numbers indicate the percentage of CD19+CD20+ cells (top left panel) or CD27highCD38high antibody-secreting cells (ASCs) within circles. C, levels of IgG in supernatants collected on day 7 were determined by ELISA. Results are expressed as mean ± S.E. of data obtained from three different donors. Th, CD4+ helper T cells.
Flow Cytometric Analysis
Single-cell suspensions were prepared and surface molecules were stained for 30 min at 4 °C with optimal dilutions of antibodies against CD19, CD20, CD27, and CD38 (all from BD Biosciences). Expression of these cell surface markers were assessed on a FACSCalibur flow cytometer and data were analyzed using CellQuest Pro software (BD Biosciences).
Culture of Human B Cells In Vitro
Purified CD19+ B cells were suspended in medium at 8 × 105 cells/ml and 50-μl cell suspensions per well were plated at flat-bottom 96-well plates. Cells were incubated in a total volume of 200 μl/well with a combination of stimuli, comprising BCR-triggering, CD40 stimulation, human interleukin (IL)-2 (50 U/ml), IL-10 (25 ng/ml), and other reagents. BCR was crosslinked by 5 μg/ml F(ab′)2 goat anti-human IgM (Jackson ImmunoResearch Laboratories), and CD40-mediated stimulation was provided by a pre-adhered monolayer of mouse fibroblasts stably transfected with human CD40 ligand (CD40L) (34, 35). Following lethal irradiation with 7500 rad, CD40L-transfected cells were plated at 3000 cells/well in 50 μl. CpG oligodeoxynucleotide (CpG, 2.5 μg/ml), all-trans retinoic aicd (ATRA, Sigma) and cytokines, including IL-4/6/17/21, tumor necrosis factor (TNF)-α, transforming growth factor-β (TGF-β), interferon-γ (IFN-γ), and lymphotoxin-α (LT-α), were also added to some cultures when indicated. All stimuli and cytokines were added at the initiation of the culture. Cytokines were obtained from Peprotech unless indicated otherwise. ATRA was first dissolved in dimethylsulfoxide (DMSO) at 10 mm and further diluted in medium as previously reported (36). No effect of DMSO on the glycoforms of IgG1 was observed (data not shown). Following 7–9 days of incubation, cell culture supernatants were collected and used for the IgG1 glycosylation analysis. In addition, in three donors the cells were harvested, washed, and stored at −20 °C until they were used for cellular N-glycosylation analysis.
Measurement of IgG by ELISA
Levels of total IgG in supernatants collected following 7–9 days culture were measured by ELISA.
Purification of IgG by Protein A beads
Protein A-Sepharose beads (GE Healthcare, Eindhoven, The Netherlands) were washed three times with 10 volumes of PBS. Fifteen microliters of beads were incubated with 150 μl of culture supernatants (collected following 7–9 days of culture) in a 96-well filter plate (Multiscreen Solvinert, 0.45 μm pore-size low-binding hydrophilic PTFE; Millipore, Billerica, MA) on a shaker for 1 h. Beads were thoroughly washed five times with 200 μl of PBS and then twice with 200 μl of water under vacuum (pressure reduction to ∼900 mbar). IgG-molecules (IgG1, IgG2, and IgG4) were eluted into a 96-well V-bottom plate using 100 μl formic acid (100 mm). Samples were dried by vacuum centrifugation.
IgG Digestion with Trypsin
A 20 μg aliquot of trypsin (sequencing grade; Promega, Leiden, The Netherlands) was dissolved in 4 ml of 25 mm ammonium bicarbonate. Within 1 min after preparation, 40 μl of this mixture was added per well to the dried purified antibodies. Samples were shaken (1 min), incubated overnight at 37 °C, and stored at −20 °C until usage.
Nano-Liquid Chromatography Electrospray ionization (LC-ESI)-Ion Trap-MS
Prior to analysis, microtitration plates containing tryptic digests of IgG were subjected to centrifugation (10 min at 3000 × g). Two-tenths microliter aliquots of trypsinized Protein A eluates (corresponding to IgG1, IgG2, and IgG4 from 10 nL of serum) and 2-μl aliquots of trypsinized samples obtained from culture supernatants were applied to a reverse-phase column (C18 PepMap 100Å, 3 μm, 75 μm × 150 mm; Dionex/LC Packings, Amsterdam, the Netherlands) using an Ultimate 3000 nano-LC system (Dionex). The column was equilibrated at room temperature with eluent A (0.1% formic acid in water and 0.4% acetonitrile (ACN)) at a flow rate of 150 nL/min. Following injection of the samples, a gradient was applied to 25% eluent B (95% ACN, 5% water containing 0.1% formic acid) in 15 min and 70% eluent B at 25 min followed by an isocratic elution with 70% eluent B for 5 min. The eluate was monitored by UV absorption at 215 nm.
The LC system was coupled via an online nanospray source to an Esquire HCT ultra ESI-IT-MS (Bruker Daltonics, Bremen, Germany) equipped with an electron transfer dissociation module (PTM Discovery SystemTM) and was operated in the positive ion mode. For electrospray (1100–1250 V), capillaries (360 μm OD, 20 μm ID with 10 μm opening) from New Objective (Cambridge, MA, USA) were used. The solvent was evaporated at 165 °C employing a nitrogen stream of 7 L/min. Ions from m/z 600 to m/z 1800 were monitored. For glycosylation profiling, the mass spectrometer was used in the MS mode. The HPLC method resulted in a separation of the glycopeptides based on the peptide moiety with IgG1 glycopeptides eluting first, followed by IgG4 and IgG2 glycopeptides. Moreover, glycopeptides with neutral glycan moieties tended to elute earlier than glycopeptides with antennae sialylation, as described before (37). For both the neutral and the acidic glycopeptides of each IgG subclass, average mass spectra were generated over a 1 min elution range as described previously (27, 37). Peak heights for 10 unambiguously assigned IgG1 Fc N-glycopeptides (triple protonated species; see Table I) were annotated in DataAnalysis 4.0 (Bruker Daltonics, Bremen, Germany) followed by background subtraction and normalization to the overall intensity of these glycoforms.
Table I. Mass spectrometric detection of the tryptic IgG1 Fc N-glycopeptides and corresponding 2AA-labeled N-glycans. H, hexose; N, N-acetylhexosamine; F, fucose; S, sialic acid.
| Glycan | Glycan composition | Glycopeptidea ([M+3H]3+) |
2AA-labeled glycan ([M-H]−) |
||
|---|---|---|---|---|---|
| Detected | Calculated | Detected | Calculated | ||
| G0F | H3N4F1 | 878.8 | 878.69 | 1582.86 | 1582.60 |
| G1F | H4N4F1 | 932.8 | 932.71 | 1744.92 | 1744.65 |
| G0FN | H3N5F1 | 946.5 | 946.39 | 1785.95 | 1785.68 |
| G2F | H5N4F1 | 986.8 | 986.73 | 1906.99 | 1906.70 |
| G1FN | H4N5F1 | 1000.5 | 1000.40 | 1948.02 | 1947.73 |
| G1FS | H4N4F1S1 | 1029.8 | 1029.74 | 2036.08 | 2035.74 |
| G2FN | H5N5F1 | 1054.5 | 1054.42 | 2110.09 | 2109.78 |
| G2FS | H5N4F1S1 | 1083.8 | 1083.76 | 2198.06 | 2197.80 |
| G1FNS | H4N5F1S1 | 1097.5 | 1097.43 | 2239.19 | 2238.82 |
| G2FNS | H5N5F1S1 | 1151.5 | 1151.45 | 2401.26 | 2400.88 |
a Monoisotopic masses are given throughout.
N-Glycan Release
Cell pellets were suspended in 20 μl (1 volume) of phosphate-buffered saline (PBS) (pH 7.2). Two volumes of 2% SDS (40 μl) were added followed by incubation on a shaker for 10 min. Subsequently, the samples were incubated for 7 min at 90 °C and then cooled down to room temperature. For N-glycan release 2 volumes of Nonidet P-40/PBS/PNGase F (1 volume 4% Nonidet P-40 mixed with 1 volume 5× PBS containing 0.05 U PNGase F per μl; Roche Diagnostics, Mannheim, Germany) followed by overnight incubation at 37 °C.
Glycan Labeling
The N-glycans released by PNGase F treatment were labeled with 2-aminobenzoic acid (2-AA) by reductive amination (12). In short, the labeling mixture was prepared by dissolving 2-AA in a mixture of 15% glacial acetic acid/DMSO to a final concentration of 48 mg/ml. The reducing agent, 2-picolin borane, was dissolved in DMSO to a final concentration of 107 mg/ml. One volume of sample following N-glycan release (100 μl) was mixed with 0.5 volume of 2-AA mixture (50 μl) and 0.5 volume reducing agent (50 μl) for 10 min on a shaker followed by a 2 h incubation at 65 °C.
The labeled glycans were purified by graphitized carbon solid-phase extraction (SPE; Carbograph-cartridge; Alltech, Breda, The Netherlands). Cartridges were prewashed with 5 ml of ACN 0.1% trifluoroacetic acid (TFA), 5 ml of H2O/ACN (50/50) 0.1% TFA, and finally equilibrated with 10 ml of H2O. The N-glycan samples following 2-AA labeling were diluted in 3 ml H2O and applied to the SPE cartridges, followed by a wash with 10 ml of H2O. Subsequently, 2-AA-labeled N-glycans were eluted with 3 ml of H2O/ACN (50/50) 0.1% TFA. ACN was removed by vacuum centrifugation, and the samples were freeze-dried and redissolved in 30 μl of H2O for further analysis by matrix-assisted laser desorption ionization (MALDI)-time-of-flight (TOF)-MS and high-performance liquid chromatography (HPLC) with fluorescence detection.
HPLC Profiling
2-AA-labeled glycans were profiled by hydrophilic interaction liquid chromatography (HILIC)-HPLC (TSK amide 80, 3 μm, 150 mm × 4.6 mm inner diameter column; Tosoh Bioscience, Stuttgart, Germany) at a 1 ml/min flow rate with fluorescence detection (360 nm/420 nm). A binary gradient was applied using 80% ACN, 20% 50 mm ammonium formate pH 4.4 (solvent A), and 50 mm ammonium formate pH 4.4 (solvent B) from 3% solvent B (0 min) to 43% solvent B (50 min). For injection N-glycan samples (1 μl) were brought to 77% ACN by addition of 3.6 μl H2O and 15.4 μl of ACN.
MALDI-TOF-MS
Prior to mass spectrometric analysis 2-AA-labeled N-glycan samples following graphitized carbon SPE were purified by HILIC micro-SPE. HILIC microtips were prepared by putting ∼0.5 mg cotton wool obtained from cotton wool pads into a pipet tip. HILIC microtips were washed with H2O and equilibrated with 90% ACN 0.1%TFA. 3 μl of sample was mixed with 10 μl of ACN to a final concentration of 77% ACN. Samples were applied by pipeting the solution 10 times up and down, followed by washing with 40 μl of 90% ACN, 0.1% TFA and eluted with 2 μl of H2O onto a stainless steel MALDI target plate (Bruker Daltonics, Bremen, Germany). Subsequently 1.3 μl of 10 mg/ml 2,5-dihydroxybenzoic acid in H2O/ACN (50/50) 0.1% TFA was applied on top of each sample and allowed to dry at room temperature. MALDI-TOF-MS was performed on an UltrafleXtremeTM mass spectrometer (Bruker Daltonics). The instrument was externally calibrated using the Bruker peptides calibration kit. Spectra were acquired in the negative-ion reflector mode over the m/z range from 700 to 5000 for a total of 5000 shots.
Statistical Analysis
The level of galactosylation was calculated on the basis of the normalized intensities for the various IgG1 Fc N-glycoforms (see Table I) according to the formula (G1F + G1FN + G1FS + G1FNS) × 0.5 + G2F + G2FN + G2FS + G2FNS. In this formula all the monogalactosylated glycans are weighed with 0.5 (reflecting 50% occupation of their potential galactosylation sites), whereas digalactosylated glycans are fully counted (100% occupation of their galactosylation sites. The prevalence of IgG1 sialylation was determined by summation of all sialylated IgG1 Fc N-glycopeptide species (G1FS, G2FS, G1FNS, and G2FNS). The level of bisecting N-GlcNAc was evaluated by summation of all bisected IgG1 Fc N-glycopeptide species (G0FN, G1FN, G2FN, G1FNS, and G2FNS). The nonparametric Mann-Whitney paired test was used to compare the difference among different groups by using GraphPad Prism 5.00 software (GraphPad, San Diego, CA), and values at p < 0.05 were considered significant.
RESULTS
Differentiation of B Cells into ASCs In Vitro
At least three signals are important for T-cell dependent antibody production by naive B cells (Fig. 1A). By and large, the activation of B cells is initiated by cross-linking of the BCR through antigen (signal 1), which is subsequently further stimulated and modulated through CD40-triggering (signal 2) and cytokine-signaling (signal 3) mediated by Th cells. Together, these signals play a pivotal role in the activation, proliferation, and differentiation of B cells into ASCs upon exposure to T-cell dependent antigens (30–33). Thus, in order to study the factors influencing the glycan pattern of antibodies produced by B cells, we first set up an in vitro culture system containing these three signals to activate and differentiate human B cells toward ASCs.
To this end, purified human CD19+CD20+ B cells (Fig. 1B) were cultured with anti-IgM to crosslink BCR (signal 1) and a pre-adhered monolayer of cells stably expressing human CD40L (signal 2). Because IL-2 and IL-10 have been reported to potently enhance the growth and differentiation of human B cells, they were also included in some cultures to provide the third signal (31–33). Differentiation of B cells was determined by flow cytometry for the percentage of CD27highCD38high ASCs and by ELISA for the titers of secreted IgG in the culture supernatant (30).
As shown in Figs. 1B and 1C, addition of IL-2 and IL-10 greatly increased anti-IgM and CD40L-induced antibody production from purified human B cells, as evidenced by both the percentages of ASCs (25.1% versus 0.25%) and IgG levels in culture supernatants (2.2 ± 0.62 versus 0.067 ± 0.011 μg/ml). Therefore, these three signals (anti-IgM + CD40L + IL-2/10) were used as the basic culture condition (referred as M condition) in all the following experiments.
Glycosylation Profiles of Secreted IgG1 Among Different B-Cell Cultures
Several cytokines, vitamins, and microbial products have previously been reported to modulate the biological functions of B cells. Moreover, it has been recently recognized that Toll-like receptor (TLR)-triggering is crucial for the proper activation of some B cells (38). In a preliminary analysis, we therefore intended to assess the effect of a selection of different factors, belonging to different “families” of molecules known to influence B-cell activation and antibody production, on the Fc-glycosylation profile of IgG1 produced by in vitro stimulated B cells (30, 35, 39–47). These factors include “environment” derivatives such as ATRA (a natural metabolite of vitamin A), innate immune triggers (TLR-9 ligands, CpG), as well as T-cell derived and/or inflammatory cytokines (IL-4/6/17/21, IFN-γ, TNF-α, LT-α, and TGF-β). Therefore, the above-mentioned substances were added during the activation and differentiation of B cells as described in Fig. 1. Following 7–9 days of culture, IgG1 in culture supernatants was purified by Protein A beads and digested with trypsin. Subsequently, Fc N-glycosylation profiles were determined by LC-MS analysis of the tryptic glycopeptides using an established approach, which is suitable for detecting differences in IgG glycosylation (37). As described previously, IgG1 Fc N-glycopeptides carrying neutral N-glycans eluted early in the chromatogram, whereas sialylated IgG1 Fc glycopeptides showed slightly more retention (37). Sum mass spectra of both nonsialylated (neutral) and sialylated glycopeptides of IgG1 were obtained for all samples, and relative intensities of the various glycoforms were determined.
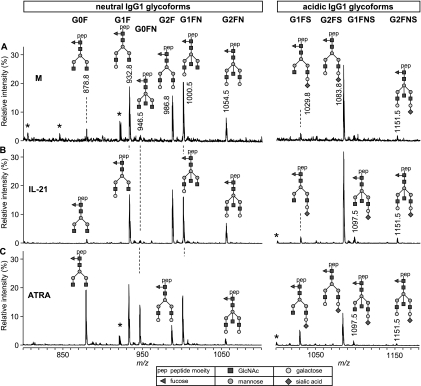
An example of the mass spectrometric data obtained for B cells from one donor under three different culture conditions is given in Fig. 2. Under control conditions (Fig. 2A), the IgG1 showed a high degree of galactosylation (e.g. high G2F at m/z 986.8) and sialylation (high G2FS at m/z 1083.8; see Table I for assignment). All the observed glycoforms appeared to be decorated with a core fucose. Moreover, the incidence of bisected species was rather high (e.g. signals at m/z 1000.5 and 1054.5). Addition of IL-21 resulted in a similar glycosylation profile (Fig. 2B). Notably, in the sample with IL-21 the G2F signal (m/z 986.8) was found to be more intense than the G1F signal (m/z 932.8; Fig. 2B), whereas in the control condition (M) this was the other way around (Fig. 2A), indicating some minor changes in IgG1 Fc galactosylation. Addition of ATRA, in contrast, resulted in the reduction of galactosylation, as evidenced by an increase in the agalactosylated glycoform G0F (m/z 878.8; Fig. 2C). These results indicate that external factors, such as ATRA, could have a strong impact on the glycosylation profile of antibodies produced by B cells.
Fig. 2.
A representative mass spectrometric picture for the Fc glycosylation of IgG1 purified from in vitro B-cell cultures. Purified human CD19+ B cells were cultured as described in the legend to Fig. 1 in the absence (M) (A) or presence of additional IL-21 (B) or ATRA (C) for 7–9 days. IgG1 were subsequently purified from the culture supernatants and their Fc glycosylation profiles were analyzed as described in the Materials and Methods. Mass spectrometric signals are shown for the triple protonated glycopeptides with neutral N-glycan chains (left panels) and acidic N-glycan chains (right panels). *, nonglycopeptide signal.
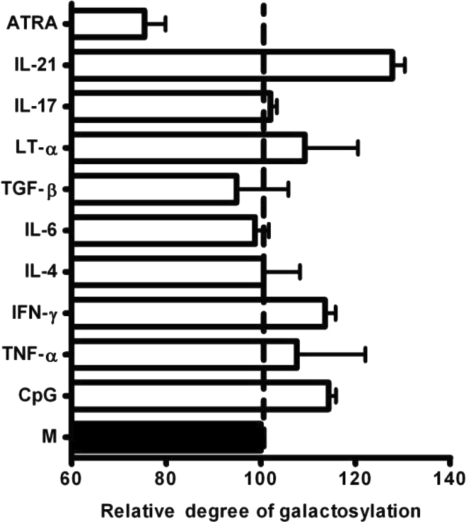
Next, the incidence of IgG1 Fc-linked galactose residues in cultures with or without the above-mentioned set of 10 different mediators (CpG, ATRA TNF-α, LT-α, IFN-γ, TGF-β, IL-4, IL-6, IL-17, and IL-21) was quantified. As shown in Fig. 3, higher frequencies of IgG1 Fc-coupled galactose residues were observed consistently in B cell cultures with CpG, IFN-γ, and IL-21, whereas addition of ATRA significantly reduced IgG1 Fc-linked galactosylation levels. No significant effect was observed in cultures containing IL-4, IL-6, TGF-β, TNF-α, LT-α, and IL-17 (Fig. 3). Together, these data indicate that microenvironmental factors present during the activation and differentiation of B cells could modulate the galactosylation of antibodies they produce.
Fig. 3.
Fc-linked glycosylation of IgG1 produced by B cells among different culture conditions. Purified human CD19+ B cells were cultured as described in the legend to Fig. 1 in the absence (M) or presence of additional CpG (2.5 μg/ml), ATRA (10 nm) and cytokines (50 ng/ml) as indicated. Following 7–9 days, galactosylation patterns of IgG1 in the culture supernatant were analyzed. Results were expressed as mean ± S.E. of the relative degree of galactosylation from 2–4 different experiments. The abundance of Fc-attached galactose residues in IgG1 purified from cultures with M (black bar) in each donor was set as 100%.
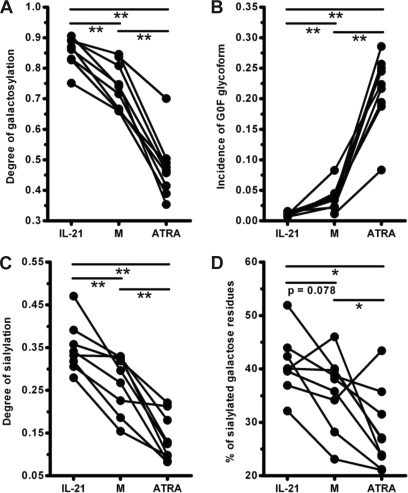
Because CpG, IL-21, and ATRA represent triggers from microbial products, T-cell derived cytokines and food metabolites, respectively, we decided to perform a second set of experiments to confirm their effect on IgG1 galactosylation in an additional set of subjects. Moreover, we investigated their effect on other Fc-linked glycan moieties, such as sialylation and the incidence of bisecting GlcNAc. For reasons of clarity, we only describe the results obtained for IL-21 and ATRA in more detail, whereas the data obtained for CpG are summarized in supplemental Fig. 1. In keeping with the results presented in Figs. 2 and 3, consistently increased or decreased galactosylation levels of IgG1 were observed in B-cell cultures with IL-21/ATRA in all donors tested (Fig. 4A). Accordingly, the abundance of the core-fucosylated IgG1 Fc glycopeptide lacking galactose (G0F) was reduced significantly by IL-21, whereas an opposite pattern was obtained by ATRA (Fig. 4B). For IgG1 purified from cultures with IL-21, M and ATRA, the degrees of galactosylation were 0.85 ± 0.018, 0.74 ± 0.024 and 0.47 ± 0.033, and the incidence of G0F was 0.011 ± 0.0012, 0.037 ± 0.0067 and 0.22 ± 0.020, respectively (mean ± S.E., n = 9).
Fig. 4.
Fc-coupled galactosylation and sialylation levels of IgG1 purified from B-cell cultures with IL-21 or ATRA. Purified human CD19+ B cells were cultured as described in the legend to Fig. 1 in the absence (M)/presence of additional IL-21 or ATRA as indicated. Following 7–9 days, glycosylation patterns of IgG1 in the culture supernatant were analyzed. A and C, comparison of the galactosylation (A) or sialylation (C) levels of IgG1 purified from indicated culture conditions. B and D, comparison of the incidence of IgG1 lacking galactose residues but with core fucose (G0F, B) or the percent of sialylated galactose residues on IgG1 purified from indicated cultures (D). Each dot represents an independent experiment with B cells isolated from different healthy individuals. The missing values indicate that corresponding conditions were not tested in these donors. *, p < 0.05; **, p < 0.01.
Given the reported role of terminal sialic acid residues on the effector function of antibodies (10, 11, 48), we subsequently determined the frequency of sialic acid residues in the Fc portion of IgG1. A representative mass spectra picture for the glycoforms containing sialic acid residues was shown in the right panels of Fig. 2. As shown in Fig. 4C, sialylation levels of IgG1 in cultures with IL-21 were significantly increased. Similar to the effect on galactosylation, addition of ATRA in the culture significantly reduced the percentage of IgG1 Fc-glycans decorated with a sialic acid residue (Fig. 4C). The average sialylation levels of IgG1 were 0.35 ± 0.021, 0.27 ± 0.022 and 0.14 ± 0.018 for cultures with IL-21, M and ATRA, respectively (mean ± S.E., n = 9). Because terminal sialic acid is attached to galactose and, therefore, the absence of galactoses will result in the concurrent absence of former residue, it is possible that the observed effect of IL-21 and ATRA on sialylation was simply because of the increased/decreased galactosylation levels. In order to ascertain the specific effect of IL-21 and ATRA on sialylation, we compared the percentage of galactose residues that contain terminal sialic acid. As shown in Fig. 4D, addition of ATRA significantly decreased the percentage of sialylated galactose residues in eight out of nine donors tested (36.0 ± 2.28% versus 28.27 ± 2.47% in cultures with M and ATRA, respectively). In contrast, IL-21 slightly increased the frequency of galactoses bearing terminal sialic acid in eight out of nine donors (40.86 ± 2.02% versus 36.0 ± 2.28% in cultures with IL-21 and M, respectively, p = 0.078). Collectively, these data indicate that the changes on sialylation levels of IgG1, induced by IL-21 and ATRA as depicted in Fig. 4C, cannot be solely attributed to the increased/decreased number of galactose residues (Fig. 4C and D).
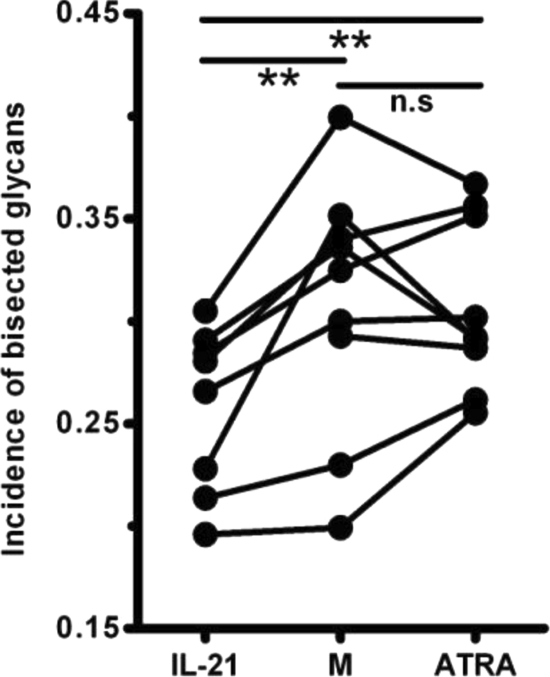
We next wished to study the potential effects of these reagents on the presence of the bisecting GlcNAc on complex Fc N-glycans as it has been shown that expression of this sugar moiety is associated with enhanced ADCC activity (12, 16). As shown in Fig. 5, the percentage of IgG1 purified from cultures with IL-21 with an additional bisecting GlcNAc decreased significantly, whereas no effect was observed with ATRA (0.26 ± 0.014, 0.31 ± 0.021, and 0.31 ± 0.014 for cultures with IL-21, M, and ATRA, respectively). The decrease of bisecting GlcNAc is reflected in the exemplifying mass spectra shown in Fig. 2 (signal at m/z 1000.5 is higher for M than for IL-21).
Fig. 5.
IL-21 reduces the percentage of IgG1 with an additional bisecting GlcNAc. Purified human CD19+ B cells were cultured as described in the legend to Fig. 1 in the absence (M)/presence of IL-21 or ATRA as indicated. Following 7–9 days, the incidence of IgG1 in culture supernatants with bisecting GlcNAc was quantified. Each dot represents an independent experiment with B cells isolated from different healthy individuals. The missing values indicate that corresponding conditions were not tested in these donors. **, p < 0.01; n.s, not significant.
In summary, our data show that several, but not all, factors present in the microenvironment during the activation and differentiation of B cells can influence the glycan patterns of secreted IgG1 (Figs. 1–5). IL-21 increases the levels of both galactosylation and sialylation. In contrast, IgG1 produced by B cells cultured with ATRA displays a significantly reduced incidence of Fc-linked galactose residues as well as a decreased frequency of sialylation. Moreover, IL-21 reduces the incidence of IgG1 bearing bisecting GlcNAc, whereas no effect of ATRA was observed. The effect of CpG on the Fc-linked glycan pattern of IgG1 is similar to that of IL-21 (supplemental Fig. 1).
Glycosylation Profiles of Proteins Isolated from Cell Pellets
To investigate whether the observed changes in glycan moieties among different culture conditions were specific for secreted IgG1 or were similarly affecting the complex mixture of cellular glycoproteins, we extracted proteins from the cell pellets and released N-glycans by PNGase F treatment. Glycans were labeled with 2-AA and analyzed by MALDI-TOF-MS and HPLC with fluorescence detection. Because irradiated mouse fibroblasts stably expressing human CD40L were used to activate and differentiate B cells, they were also included in the analyses to control for a potential contribution of these cells to the total protein-pool isolated. HPLC profiles revealed that the CD40L control sample contained only very limited amounts of N-glycans compared with other samples with B cells (supplemental Fig. 2). However, when we prolonged the collection time we managed to obtain sufficient materials from the CD40L control samples for the subsequent glycan analysis. Mass spectrometric analysis of the N-glycan pools demonstrated that although oligomannosidic N-glycans were present in both B-cell samples and CD40L control cells (supplemental Fig. 3A), a group of complex-type glycans of composition H5–9N5–8F1S1 in the mass range m/z 2400 to 3800 was only found in samples with B cells, but not in the control sample with CD40L cells only (supplemental Fig. 3B). The major species of these complex-type glycans showed compositions of H5N4F1S1, H5N5F1S1, H6N5F1S1, H6N6F1S1, H7N6F1S1, and H7N7F1S1, which are interpreted as sialylated, fucosylated di-, tri-, and tetraantennary structures that may carry a bisecting GlcNAc (supplemental Figs. 3A and 3B). Notably the B-cell specific complex-type N-glycans were characterized by a very high degree of core-fucosylation, which is in line with the complete core-fucosylation of the IgG1 Fc-glycopeptides (Table I). Although MALDI-TOF-MS is a very suitable method for N-glycan profiling, it is well-known that even structurally related N-glycans may show markedly different response factors (49). Because response factors were not taken into account in this study, the stated relative intensities therefore merely reflect the ratios of mass spectrometric signals, but do not correspond to molar ratios of glycans.
Notably, the type of glycans that were identical in composition to those found on IgG1 Fc-portions (Table I) was only present in the B-cell samples, but not in the CD40L control cells (supplemental Fig. 3C). In addition, in the range m/z 1500 to 2000 glycans of composition H3N4F1, H3N3F1S1, and H4N3F1S1 and presumably sulfated versions thereof were selectively detected in the B-cell samples but not in the CD40L cells.
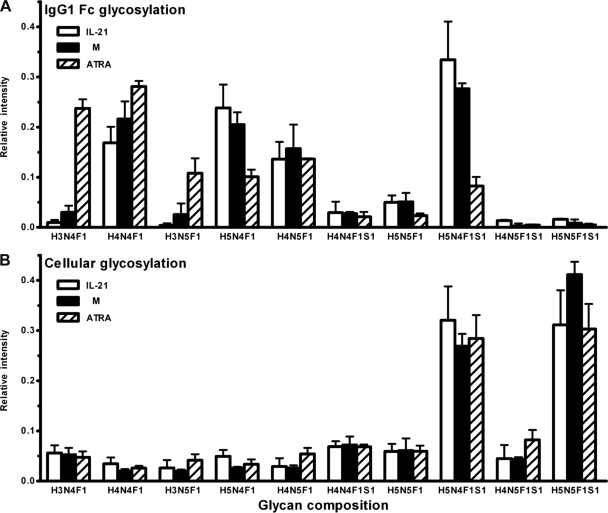
Based on these data, we conclude that the glycoforms depicted in Table I are derived from B cell proteins, and the presence of CD40L cells, therefore, does not interfere with the subsequent analysis. Next, the glycan profiles of secreted IgG1 and cellular proteins, purified from different culture conditions, were compared side-by-side. Only those glycan signals that were found to be shared between IgG1 and the cellular proteins were considered in this comparison, whereas all glycoforms absent on the IgG1 Fc portions and only present on cellular proteins were excluded. In accordance with the above-mentioned observations, IgG1 Fc glycosylation profiles, which comprised 10 core-fucosylated, partially truncated, biantennary glycans that may carry sialic acid and bisecting GlcNAc, were modulated by the addition of ATRA and IL-21 (Fig. 6A). In contrast, the same set of glycans released from cell homogenates showed a very different profile and did not show the above-mentioned responsiveness to ATRA and IL-21 (Fig. 6B). In particular, the glycan of composition H3N4F1 (G0F) on IgG1 was vastly increased upon ATRA treatment, whereas no similar increase was observed for this glycan in the cellular glycosylation profile. Moreover, G2F (H5N4F1) and G2FS (H5N4F1S1) glycans, which were decreased on IgG1 upon ATRA treatment, were not affected by ATRA in the overall cellular glycosylation profiles (Figs. 6A and 6B). Together, these data indicate that although soluble factors such as CpG, IL-21, and ATRA present during the activation and differentiation of B cells can significantly alter the Fc-linked glycosylation profiles of secreted IgG1, they do not significantly affect the overall cellular N-glycosylation patterns of ASCs.
Fig. 6.
Comparison of the glycoforms between secreted IgG1 and cellular proteins. Purified human CD19+ B cells were cultured as described in the legend to Fig. 1 in the absence (M)/presence of additional IL-21 or ATRA. Following 7–9 days, glycan profiles of IgG1 purified from culture supernatants (A) or proteins extracted from cell pellets (B) were analyzed. Only those glycan signals that were found to be shared between secreted IgG1 and the cellular proteins were considered in this comparison. Results are expressed as mean ± S.D. of the relative intensity of each glycoform from three different individuals.
Long-lasting Effect of IL-21 and ATRA on IgG1 Glycan Profiles
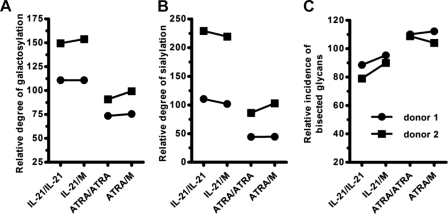
Although most antibody-producing cells are short-lived plasma cells or plasma blasts in vivo, long-lived plasma cells are present in survival niches in the bone marrow and spleen and are capable of continuously secreting antibodies for a long period independent of antigens (50). To analyze whether the effect of IL-21 and ATRA on the glycan profile of IgG1 is transient or more long-lasting, we collected cells on day 5 following the initial stimulation with anti-IgM, IL-2/10 and CD40L-transfected cells in the absence or presence of IL-21 or ATRA. The cells were washed extensively, and subsequently cultured in medium containing IL-2/10 with or without IL-21/ATRA for additional 5 days (no signals through BCR and CD40 were provided during this period). Because plasma cells, following their differentiation from naive/memory B-cells, stop dividing and die within days in in vitro cultures (50), cells were not cultured for prolonged time-periods. Glycosylation profiles of IgG1 produced in the continued presence or absence of stimuli were analyzed and compared. As shown in Fig. 7, ASCs differentiated in the presence of IL-21 or ATRA during the first 5 days continuously produce IgG1 with the same altered Fc-glycosylation pattern in the next 5 days, even in the absence of IL-21/ATRA. Likewise, removing IL-21 or ATRA on day 5 does not significantly change the glycosylation profile of IgG1 as compared with IgG1 under continued IL-21 or ATRA stimulation, respectively. Together, these data indicate that the effect of IL-21 and ATRA on the glycoforms of IgG1 is not transient but rather stable, at least during the time frame analyzed.
Fig. 7.
Long-lasting effect of IL-21 and ATRA on IgG1 glycan profiles. Purified human CD19+ B cells were cultured as described in the legend to Fig. 1 in the absence or presence of additional IL-21 or ATRA (indicated by IL-21 and ATRA before “/”). Following 5 days, cells were collected, washed extensively and then cultured in medium with/without IL-21 or ATRA for an additional 5 days (indicated by M, IL-21, and ATRA after “/”). Supernatants were collected and glycan profiles of IgG1 were analyzed. Galactosylation (A), sialylation (B) and bisecting GlcNAc (C) levels are given following normalization (samples cultured continuously in medium for 10 days were set as 100%).
DISCUSSION
It has been well documented that changes in IgG glycosylation profiles are associated with various physiological (i.e. age and/or pregnancy) and pathological conditions (i.e. patients with autoimmune diseases and/or tumors) (2, 4, 7, 8, 19–24, 51–53). In addition, we and others recently found that auto-antigen specific IgG exhibits a further different glycosylation pattern compared with that of total or irrelevant IgG in patients with autoimmune diseases, indicating that structural features of glycan moieties in the Fc portion of IgG are regulated in an antigen specific fashion (25–27, 54). However, the nature of the stimuli received by B cells to modulate IgG glycosylation remains unknown. In this study, by using an in vitro B-cell culture system resembling the in vivo T-cell dependent antibody production from B cells, we show that treatment of B cells with IL-21 and CpG significantly increases IgG1 Fc-linked galactosylation and sialylation levels, whereas an opposite effect was observed following the addition of ATRA. Moreover, IL-21 and CpG reduce the incidence of IgG1 containing bisecting GlcNAc. Furthermore, it appears that these effects mediated by IL-21 and ATRA are stable for at least another 5 days and therefore the initial stimuli are not required to maintain the glycan profiles of secreted IgG1 once ASCs are generated (which have, in general, a limited life-span). Intriguingly, the changes in IgG1 Fc-glycosylation were not paralleled by similar changes of the expression levels of the corresponding glycans in the cellular glycan pool. Together, these data indicate that soluble microenvironmental factors present during the activation and differentiation of B cells into ASCs modulate the carbohydrate structures of secreted IgG1 without affecting the general cellular glycosylation machinery. Our study could, therefore, further our understandings on the regulation of IgG1 glycosylations at the cellular level under different conditions and could open up possibilities for the identification of pharmaceutical mediators that can modulate Fc-glycosylation and thereby “introduce” a desired function to harmful (auto-) antibodies.
For the production of antibodies against T-cell dependent antigens, activation and proliferation of B cells as well as their subsequent differentiation into ASCs are indispensable. Crosslinking of BCR by antigens and help from Th cells through CD40-CD40L interaction trigger the initial activation and proliferation of B cells, whereas soluble factors, secreted by Th cells or other cells and organisms further determine the outcome of antibody production, such as the amount and/or type (i.e. IgM, IgG1–4, IgA, or IgE) of antibodies produced. Therefore, to study the factors capable of modulating IgG1 glycosylation during the above-mentioned process, we used an in vitro culture system to differentiate B cells into ASCs by anti-IgM (to crosslink cell surface IgM, which serves as BCR), CD40L-transfected cells and IL-2/10 (31–35). In addition to this basic mix of stimuli (Fig. 1), various reagents that have been previously reported to affect the biological function of B cells were included to investigate their effect on IgG1 Fc glycosylations as well (30, 35, 38–47). No significant changes in galactosylation were observed in the Fc portion of IgG1 purified from cultures exposed to TNF-α, LT-α, IL-4/6/17, and TGF-β. In contrast, exposure of B cells to IFN-γ, IL-21, CpG, and ATRA significantly altered the glycan moieties of secreted IgG1 (Figs. 2–6). Notably, TNF-α, IL-4, IFN-γ, and CpG could all function as a growth factor for human B cells (38, 39, 42, 44, 45), whereas only the latter two affect IgG1 glycan profiles (Figs. 3–5 and supplemental Fig. 1). These data demonstrate that not all factors known to modulate the outcome of B-cell responses have an impact on the Fc-coupled glycosylation of IgGs produced by B cells. Moreover, although IL-21 and ATRA both augment the differentiation of B cells toward ASCs and thereby increase the production of antibodies (30, 35, 47), their effect on IgG1 galactosylation and sialylation is opposite (Figs. 2–4 and supplemental Fig. 4). Together, these data indicate that the posttranslational modification of IgG1 sugar chains is a process independent of cell growth and antibody titers. Indeed, in further support of this notion, no correlation of the glycan profiles of IgG1 with cell proliferation or levels of IgG was observed (supplemental Fig. 4).
The observation that IL-21 and CpG have an opposite effect, compared with ATRA, on Fc-glycosylation of IgG1 is intriguing, as they all have been shown previously to enhance B-cell mediated immune responses. IL-21 plays a dominant role in inducing ASCs differentiation during T cell-dependent B cell responses (30, 35). CpG, an innate immune trigger detected by TLR-9, enhances the activation, proliferation of naive B cells as well as their differentiation into ASCs (38, 39). Likewise, ATRA not only enhances the antibody responses against T-cell dependent and independent antigens, but also locally stimulates IgA production in mucosal tissues (47, 55). Given that reduced galactosylation or sialylation levels or the presence of an additional bisecting GlcNAc could lead to increased pro-inflammatory activity of IgG (7–12, 16), our data indicate that IgGs produced by ASCs in the presence of IL-21/CpG bear different effector functions as compared with those produced in the presence of ATRA (Figs. 2–5 and supplemental Fig. 1). Therefore, next to the regulation of the amount and type of antibodies, modulation of the carbohydrate structures of IgG may represent an additional and independent checkpoint to control the strength of humoral immune responses by some factors known to influence the outcome of B-cell responses.
Moreover, given the different sources of IL-21, CpG, and ATRA (Th cells, bacterial DNA and food metabolites, respectively) and locations of their receptors (cell-surface expressed receptor for IL-21, TLR-9 in plasma membrane of cytosolic endosome and nuclear-located retinoic acid receptors, respectively) (38, 39, 55, 56), our data also indicate that IgG1 glycan profiles can be regulated at the cellular level by factors from various origins that signal through different cellular organelles and by different pathways (Figs. 2–5 and supplemental Fig. 1).
Because glycosylation is an enzyme-mediated post-translational process, changes in glycan moieties are supposed to reflect altered enzyme expression levels and/or activities. Accordingly, it has been suggested that different subsets of plasma cells differently process IgG glycans, and therefore, altered glycan IgG profiles in patients could be attributable to a selective expansion of particular subsets of plasma cells with aberrant levels or activities of corresponding enzymes (57). Our data are compatible with this notion as they suggest that microenvironmental factors present during the activation and differentiation of B cells are responsible for the generation of such different subsets of plasma cells (Figs. 1–5 and supplemental Fig. 1). Moreover, in contrast to the significantly different glycosylation patterns of secreted IgG1, similar prevalences of the corresponding glycans in the cellular protein pool were obtained among different culture conditions (Fig. 6 and supplemental Figs. 2 and 3). These data are intriguing as they suggest that environmental factors could modulate IgG1 glycan residues without significantly changing the global cellular glycosylation machinery. The mechanisms underlying the targeted structural changes of IgG1 Fc glycans could include (1) a temporal separation in the production of cellular proteins and secreted IgG1 (with the synthesis of most cellular glycoproteins being accomplished during B-cell differentiation, whereas IgG1 being predominantly produced by the terminally differentiated plasma cells); and (2) a selective modulation of (co)factors that bring glycosyltransferases and IgG1 together in the Golgi apparatus by ATRA and IL-21. Established examples for factors determining the localization and activity of glycosyltransferases are the β4GalNAcTB pilot (GABPI), which recruits the Drosophila melanogaster β4GalNAcTB involved in glycosphingolipid synthesis to the Golgi apparatus (58), and the molecular chaperone Cosmc regulating protein O-glycosylation (59). Nonetheless, significant changes in individual cellular glycoproteins among different culture conditions could still occur because of the heterogeneity of the cell population (memory cells versus ASCs) and proteins (constitutively versus newly expressed) as well as to the possibly continuous turnover of the glycans. Accordingly, a detailed, differential glycoproteomics analysis of B-cells cultured under the various stimulation conditions will be required to identify these potentially regulated cellular glycoproteins.
Footnotes
* Financial support: This work was supported by the Prof. A. A. H. Kassenaar Foundation, the European Community's FP6 funding Project 018661 Autocure and FP7 Project HEALTH-F2-2007-2.4.5-12 Masterswitch, the Dutch Arthritis Foundation (Grant 0801021), and the Netherlands Organization for Scientific Research VIDI and VICI grant (to R. E. M. T.).
 This article contains supplemental Figs. S1 to S4.
This article contains supplemental Figs. S1 to S4.
1 The abbreviations used are:
- ADCC
- antibody-dependent cellular cytotoxicity
- 2-AA
- 2-aminobenzoic acid
- ACN
- acetonitrile
- ASCs
- antibody-secreting cells
- ATRA
- all-trans retinoic acid
- BCR
- B-cell receptors
- CpG
- CpG oligodeoxynucleotide
- GlcNAc
- N-acetylglucosamine
- HILIC
- hydrophilic interaction liquid chromatography
- IFN-γ
- interferon-γ
- IL
- interleukin
- LT-α
- lymphotoxin-α
- RA
- rheumatoid arthritis
- SPE
- solid-phase extraction
- TGF-β
- transforming growth factor-β
- Th
- CD4+ T helper cells
- TNF
- tumor necrosis factor
- TLR
- toll-like receptors
- PTFE
- polytetrafluroethylene.
REFERENCES
- 1. Nimmerjahn F., Ravetch J. V. (2006) Fcgamma receptors: old friends and new family members. Immunity. 24, 19–28 [DOI] [PubMed] [Google Scholar]
- 2. Arnold J. N., Wormald M. R., Sim R. B., Rudd P. M., Dwek R. A. (2007) The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 25, 21–50 [DOI] [PubMed] [Google Scholar]
- 3. Huhn C., Selman M. H., Ruhaak L. R., Deelder A. M., Wuhrer M. (2009) IgG glycosylation analysis. Proteomics. 9, 882–913 [DOI] [PubMed] [Google Scholar]
- 4. Parekh R. B., Dwek R. A., Sutton B. J., Fernandes D. L., Leung A., Stanworth D., Rademacher T. W., Mizuochi T., Taniguchi T., Matsuta K. (1985) Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 316, 452–457 [DOI] [PubMed] [Google Scholar]
- 5. Krapp S., Mimura Y., Jefferis R., Huber R., Sondermann P. (2003) Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J. Mol. Biol. 325, 979–989 [DOI] [PubMed] [Google Scholar]
- 6. Yamaguchi Y., Nishimura M., Nagano M., Yagi H., Sasakawa H., Uchida K., Shitara K., Kato K. (2006) Glycoform-dependent conformational alteration of the Fc region of human immunoglobulin G1 as revealed by NMR spectroscopy. Biochim. Biophys. Acta. 1760, 693–700 [DOI] [PubMed] [Google Scholar]
- 7. Malhotra R., Wormald M. R., Rudd P. M., Fischer P. B., Dwek R. A., Sim R. B. (1995) Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat. Med. 1, 237–243 [DOI] [PubMed] [Google Scholar]
- 8. Matsumoto A., Shikata K., Takeuchi F., Kojima N., Mizuochi T. (2000) Autoantibody activity of IgG rheumatoid factor increases with decreasing levels of galactosylation and sialylation. J. Biochem. 128, 621–628 [DOI] [PubMed] [Google Scholar]
- 9. Rademacher T. W., Williams P., Dwek R. A. (1994) Agalactosyl glycoforms of IgG autoantibodies are pathogenic. Proc. Natl. Acad. Sci. U.S.A. 91, 6123–6127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anthony R. M., Nimmerjahn F., Ashline D. J., Reinhold V. N., Paulson J. C., Ravetch J. V. (2008) Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 320, 373–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaneko Y., Nimmerjahn F., Ravetch J. V. (2006) Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 313, 670–673 [DOI] [PubMed] [Google Scholar]
- 12. Davies J., Jiang L., Pan L. Z., LaBarre M. J., Anderson D., Reff M. (2001) Expression of GnTIII in a recombinant anti-CD20 CHO production cell line: Expression of antibodies with altered glycoforms leads to an increase in ADCC through higher affinity for FC gamma RIII. Biotechnol. Bioeng. 74, 288–294 [PubMed] [Google Scholar]
- 13. Nimmerjahn F., Ravetch J. V. (2005) Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 310, 1510–1512 [DOI] [PubMed] [Google Scholar]
- 14. Shields R. L., Lai J., Keck R., O'Connell L. Y., Hong K., Meng Y. G., Weikert S. H., Presta L. G. (2002) Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277, 26733–26740 [DOI] [PubMed] [Google Scholar]
- 15. Shinkawa T., Nakamura K., Yamane N., Shoji-Hosaka E., Kanda Y., Sakurada M., Uchida K., Anazawa H., Satoh M., Yamasaki M., Hanai N., Shitara K. (2003) The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 278, 3466–3473 [DOI] [PubMed] [Google Scholar]
- 16. Umaña P., Jean-Mairet J., Moudry R., Amstutz H., Bailey J. E. (1999) Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat. Biotechnol. 17, 176–180 [DOI] [PubMed] [Google Scholar]
- 17. Bolt S., Routledge E., Lloyd I., Chatenoud L., Pope H., Gorman S. D., Clark M., Waldmann H. (1993) The generation of a humanized, non-mitogenic CD3 monoclonal antibody which retains in vitro immunosuppressive properties. Eur. J. Immunol. 23, 403–411 [DOI] [PubMed] [Google Scholar]
- 18. Woodle E. S., Xu D., Zivin R. A., Auger J., Charette J., O'Laughlin R., Peace D., Jollife L. K., Haverty T., Bluestone J. A., Thistlethwaite J. R., Jr. (1999) Phase I trial of a humanized, Fc receptor nonbinding OKT3 antibody, huOKT3gamma1(Ala-Ala) in the treatment of acute renal allograft rejection. Transplantation. 68, 608–616 [DOI] [PubMed] [Google Scholar]
- 19. Parekh R. B., Roitt I. M., Isenberg D. A., Dwek R. A., Ansell B. M., Rademacher T. W. (1988) Galactosylation of IgG associated oligosaccharides: reduction in patients with adult and juvenile onset rheumatoid arthritis and relation to disease activity. Lancet. 1, 966–969 [DOI] [PubMed] [Google Scholar]
- 20. Alavi A., Arden N., Spector T. D., Axford J. S. (2000) Immunoglobulin G glycosylation and clinical outcome in rheumatoid arthritis during pregnancy. J. Rheumatol. 27, 1379–1385 [PubMed] [Google Scholar]
- 21. Pekelharing J. M., Hepp E., Kamerling J. P., Gerwig G. J., Leijnse B. (1988) Alterations in carbohydrate composition of serum IgG from patients with rheumatoid arthritis and from pregnant women. Ann. Rheum. Dis. 47, 91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saldova R., Royle L., Radcliffe C. M., Abd Hamid U. M., Evans R., Arnold J. N., Banks R. E., Hutson R., Harvey D. J., Antrobus R., Petrescu S. M., Dwek R. A., Rudd P. M. (2007) Ovarian cancer is associated with changes in glycosylation in both acute-phase proteins and IgG. Glycobiology. 17, 1344–1356 [DOI] [PubMed] [Google Scholar]
- 23. Rook G. A., Steele J., Brealey R., Whyte A., Isenberg D., Sumar N., Nelson J. L., Bodman K. B., Young A., Roitt I. M. (1991) Changes in IgG glycoform levels are associated with remission of arthritis during pregnancy. J. Autoimmun. 4, 779–794 [DOI] [PubMed] [Google Scholar]
- 24. Selman M. H., Niks E. H., Titulaer M. J., Verschuuren J. J., Wuhrer M., Deelder A. M. (2011) IgG Fc N-Glycosylation Changes in Lambert-Eaton Myasthenic Syndrome and Myasthenia Gravis. J. Proteome. Res. 10, 143–152 [DOI] [PubMed] [Google Scholar]
- 25. Scherer H. U., Wang J., Toes R. E. M., van der, Woude D., Koeleman C. A., de Boer A. R., Huízinga T. W., Deelder A. M., Wuhrer M. (2009) Immunoglobulin 1 (IgG1) Fc-glycosylation profiling of anti-citrullinated peptide antibodies from human serum. Proteomics Clin. Appl. 3, 106–115 [DOI] [PubMed] [Google Scholar]
- 26. Scherer H. U., van der, Woude D., Ioan-Facsinay A., el Bannoudi H., Trouw L. A., Wang J., Häupl T., Burmester G. R., Deelder A. M., Huizinga T. W., Wuhrer M., Toes R. E. (2010) Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum. 62, 1620–1629 [DOI] [PubMed] [Google Scholar]
- 27. Wuhrer M., Porcelijn L., Kapur R., Koeleman C. A., Deelder A., de Haas M., Vidarsson G. (2009) Regulated glycosylation patterns of IgG during alloimmune responses against human platelet antigens. J. Proteome. Res. 8, 450–456 [DOI] [PubMed] [Google Scholar]
- 28. Albert H., Collin M., Dudziak D., Ravetch J. V., Nimmerjahn F. (2008) In vivo enzymatic modulation of IgG glycosylation inhibits autoimmune disease in an IgG subclass-dependent manner. Proc. Natl. Acad. Sci. U.S.A. 105, 15005–15009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iida S., Misaka H., Inoue M., Shibata M., Nakano R., Yamane-Ohnuki N., Wakitani M., Yano K., Shitara K., Satoh M. (2006) Nonfucosylated therapeutic IgG1 antibody can evade the inhibitory effect of serum immunoglobulin G on antibody-dependent cellular cytotoxicity through its high binding to FcgammaRIIIa. Clin. Cancer Res. 12, 2879–2887 [DOI] [PubMed] [Google Scholar]
- 30. Bryant V. L., Ma C. S., Avery D. T., Li Y., Good K. L., Corcoran L. M., de Waal M. R., Tangye S. G. (2007) Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J. Immunol. 179, 8180–8190 [DOI] [PubMed] [Google Scholar]
- 31. Kindler V., Zubler R. H. (1997) Memory, but not naive, peripheral blood B lymphocytes differentiate into Ig-secreting cells after CD40 ligation and costimulation with IL-4 and the differentiation factors IL-2, IL-10, and IL-3. J. Immunol. 159, 2085–2090 [PubMed] [Google Scholar]
- 32. Rousset F., Garcia E., Defrance T., Péronne C., Vezzio N., Hsu D. H., Kastelein R., Moore K. W., Banchereau J. (1992) Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 89, 1890–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rousset F., Peyrol S., Garcia E., Vezzio N., Andujar M., Grimaud J. A., Banchereau J. (1995) Long-term cultured CD40-activated B lymphocytes differentiate into plasma cells in response to IL-10 but not IL-4. Int. Immunol. 7, 1243–1253 [DOI] [PubMed] [Google Scholar]
- 34. Duddy M. E., Alter A., Bar-Or A. (2004) Distinct profiles of human B cell effector cytokines: a role in immune regulation? J. Immunol. 172, 3422–3427 [DOI] [PubMed] [Google Scholar]
- 35. Ettinger R., Sims G. P., Fairhurst A. M., Robbins R., da Silva Y. S., Spolski R., Leonard W. J., Lipsky P. E. (2005) IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J. Immunol. 175, 7867–7879 [DOI] [PubMed] [Google Scholar]
- 36. Wang J., Huizinga T. W., Toes R. E. (2009) De novo generation and enhanced suppression of human CD4+CD25+ regulatory T cells by retinoic acid. J. Immunol. 183, 4119–4126 [DOI] [PubMed] [Google Scholar]
- 37. Wuhrer M., Stam J. C., van de Geijn F. E., Koeleman C. A., Verrips C. T., Dolhain R. J., Hokke C. H., Deelder A. M. (2007) Glycosylation profiling of immunoglobulin G (IgG) subclasses from human serum. Proteomics 7, 4070–4081 [DOI] [PubMed] [Google Scholar]
- 38. Ruprecht C. R., Lanzavecchia A. (2006) Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur. J. Immunol. 36, 810–816 [DOI] [PubMed] [Google Scholar]
- 39. Huggins J., Pellegrin T., Felgar R. E., Wei C., Brown M., Zheng B., Milner E. C., Bernstein S. H., Sanz I., Zand M. S. (2007) CpG DNA activation and plasma-cell differentiation of. Blood 109, 1611–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Anolik J. H., Ravikumar R., Barnard J., Owen T., Almudevar A., Milner E. C., Miller C. H., Dutcher P. O., Hadley J. A., Sanz I. (2008) Cutting edge: anti-tumor necrosis factor therapy in rheumatoid arthritis inhibits memory B lymphocytes via effects on lymphoid germinal centers and follicular dendritic cell networks. J. Immunol. 180, 688–692 [DOI] [PubMed] [Google Scholar]
- 41. Borsutzky S., Cazac B. B., Roes J., Guzmán C. A. (2004) TGF-beta receptor signaling is critical for mucosal IgA responses. J. Immunol. 173, 3305–3309 [DOI] [PubMed] [Google Scholar]
- 42. Boussiotis V. A., Nadler L. M., Strominger J. L., Goldfeld A. E. (1994) Tumor necrosis factor alpha is an autocrine growth factor for normal human B cells. Proc. Natl. Acad. Sci. U.S.A. 91, 7007–7011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Burdin N., Van Kooten C., Galibert L., Abrams J. S., Wijdenes J., Banchereau J., Rousset F. (1995) Endogenous IL-6 and IL-10 contribute to the differentiation of CD40-activated human B lymphocytes. J. Immunol. 154, 2533–2544 [PubMed] [Google Scholar]
- 44. Defrance T., Aubry J. P., Vanbervliet B., Banchereau J. (1986) Human interferon-gamma acts as a B cell growth factor in the anti-IgM antibody co-stimulatory assay but has no direct B cell differentiation activity. J. Immunol. 137, 3861–3867 [PubMed] [Google Scholar]
- 45. Defrance T., Vanbervliet B., Aubry J. P., Takebe Y., Arai N., Miyajima A., Yokota T., Lee F., Arai K., de Vries J. E. (1987) B cell growth-promoting activity of recombinant human interleukin 4. J. Immunol. 139, 1135–1141 [PubMed] [Google Scholar]
- 46. Doreau A., Belot A., Bastid J., Riche B., Trescol-Biemont M. C., Ranchin B., Fabien N., Cochat P., Pouteil-Noble C., Trolliet P., Durieu I., Tebib J., Kassai B., Ansieau S., Puisieux A., Eliaou J. F., Bonnefoy-Bérard N. (2009) Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat. Immunol. 10, 778–785 [DOI] [PubMed] [Google Scholar]
- 47. Morikawa K., Nonaka M. (2005) All-trans-retinoic acid accelerates the differentiation of human B lymphocytes maturing into plasma cells. Int. Immunopharmacol. 5, 1830–1838 [DOI] [PubMed] [Google Scholar]
- 48. Anthony R. M., Wermeling F., Karlsson M. C., Ravetch J. V. (2008) Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc. Natl. Acad. Sci. U.S.A. 105, 19571–19578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harvey D. J. (2011) Analysis of carbohydrates and glycoconjugates by matrix-assisted laser desorption/ionization mass spectrometry: an update for the period 2005–2006. Mass Spectrom. Rev. 30, 1–100 [DOI] [PubMed] [Google Scholar]
- 50. Shapiro-Shelef M., Calame K. (2005) Regulation of plasma-cell development. Nat. Rev. Immunol. 5, 230–242 [DOI] [PubMed] [Google Scholar]
- 51. Parekh R., Roitt I., Isenberg D., Dwek R., Rademacher T. (1988) Age-related galactosylation of the N-linked oligosaccharides of human serum IgG. J. Exp. Med. 167, 1731–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shikata K., Yasuda T., Takeuchi F., Konishi T., Nakata M., Mizuochi T. (1998) Structural changes in the oligosaccharide moiety of human IgG with aging. Glycoconj. J. 15, 683–689 [DOI] [PubMed] [Google Scholar]
- 53. Ruhaak L. R., Uh H. W., Beekman M., Koeleman C. A., Hokke C. H., Westendorp R. G., Wuhrer M., Houwing-Duistermaat J. J., Slagboom P. E., Deelder A. M. (2010) Decreased levels of bisecting GlcNAc glycoforms of IgG are associated with human longevity. Plos. One. 5, e12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ercan A., Cui J., Chatterton D. E., Deane K. D., Hazen M. M., Brintnell W., O'Donnell C. I., Derber L. A., Weinblatt M. E., Shadick N. A., Bell D. A., Cairns E., Solomon D. H., Holers V. M., Rudd P. M., Lee D. M. (2010) Aberrant IgG galactosylation precedes disease onset, correlates with disease activity, and is prevalent in autoantibodies in rheumatoid arthritis. Arthritis Rheum. 62, 2239–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ertesvåg A., Naderi S., Blomhoff H. K. (2009) Regulation of B cell proliferation and differentiation by retinoic acid. Semin. Immunol. 21, 36–41 [DOI] [PubMed] [Google Scholar]
- 56. Leonard W. J., Spolski R. (2005) Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat. Rev. Immunol. 5, 688–698 [DOI] [PubMed] [Google Scholar]
- 57. Omtvedt L. A., Royle L., Husby G., Sletten K., Radcliffe C. M., Harvey D. J., Dwek R. A., Rudd P. M. (2006) Glycan analysis of monoclonal antibodies secreted in deposition disorders indicates that subsets of plasma cells differentially process IgG glycans. Arthritis Rheum. 54, 3433–3440 [DOI] [PubMed] [Google Scholar]
- 58. Johswich A., Kraft B., Wuhrer M., Berger M., Deelder A. M., Hokke C. H., Gerardy-Schahn R., Bakker H. (2009) Golgi targeting of Drosophila melanogaster beta4GalNAcTB requires a DHHC protein family-related protein as a pilot. J. Cell Biol. 184, 173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ju T., Aryal R. P., Stowell C. J., Cummings R. D. (2008) Regulation of protein O-glycosylation by the endoplasmic reticulum-localized molecular chaperone Cosmc. J. Cell Biol. 182, 531–542 [DOI] [PMC free article] [PubMed] [Google Scholar]