Abstract
Glycans present on glycoproteins from the eggs of the parasite Schistosoma mansoni are mediators of various immune responses of the human host, including T-cell modulation and granuloma formation, and they are the target of glycan-specific antibodies. Here we have analyzed the glycosylation of kappa-5, a major glycoprotein antigen from S. mansoni eggs using a targeted approach of lectin purification followed by mass spectrometry of glycopeptides as well as released glycans. We demonstrate that kappa-5 has four fully occupied N-glycosylation sites carrying unique triantennary glycans composed of a difucosylated and xylosylated core region, and immunogenic GalNAcβ1–4GlcNAc (LDN) termini. Furthermore, we show that the kappa-5 specific IgE antibodies in sera of S. mansoni-infected individuals are directed against the core region of the kappa-5 glycans. Whereas two previously analyzed immunomodulatory egg glycoproteins, IPSE/alpha-1 and omega-1, both express diantennary N-glycans with a difucosylated core and one or two Galβ1–4(Fucα1–3)GlcNAc (Lewis X) antennae, the kappa-5 glycosylation appears unique among the major soluble egg antigens of S. mansoni. The distinct structural and antigenic properties of kappa-5 glycans suggest a specific role for kappa-5 in schistosome egg immunogenicity.
Schistosomiasis, a debilitating disease caused by infection with Schistosoma parasites, forms a major public health burden in many areas in the tropics and subtropics. The main pathological symptoms of Schistosoma mansoni infection are caused by the deposition of parasite eggs into the organs of the host and the subsequent immunological consequences, which include the formation of perioval granulomas associated with a pronounced T helper 2 (Th2) response (1).
Many aspects of these egg-induced immune processes are thought to be at least partly mediated by protein glycosylation (2–5). The role of glycans during granuloma formation has been explored in vivo by hepatic implantation of antigen-coated Sepharose beads as artificial eggs into mice (3). In this model, beads that carry soluble egg antigens (SEA)1 of S. mansoni give rise to granulomas very similar to granulomas around actual schistosome eggs. In contrast, granulomas were not formed around beads coated with SEA of which the glycans had been destroyed by periodate treatment. Intact glycosylation has also been reported to be crucial for Th2-polarizing properties of SEA in a murine model of intranasal sensitization giving rise to antigen-specific IgE production and induction of IL-4 and IL-10 (2).
The structure of SEA glycans has been studied extensively, mainly by mass spectrometric (MS) analysis of released N- and O-glycans (6–8). However, to obtain knowledge about the contribution of glycosylation to the functional properties of individual glycoproteins, protein-specific glycosylation analyses, rather than studies on glycans released from glycoprotein mixtures, are essential. Up to now, only for two schistosome egg glycoproteins, omega-1 (9) and IPSE/alpha-1 (10), a protein-specific in-depth glycosylation analysis has been carried out (11, 12), and both these glycoproteins were found to carry the immunogenic Galβ1–4(Fucα1–3)GlcNAc (Lewis X) motif (13–17). The similar glycosylation patterns of these proteins do not account for the variety of antigenic glycan elements found in schistosome eggs, emphasizing the need for detailed studies on the glycosylation of other subsets of the egg glycoproteome. Recently, kappa-5, an immunogenic egg glycoprotein from S. mansoni putatively involved in host-parasite interactions, has been identified (18). Schramm et al. showed that kappa-5 is the target of a pronounced IgE response in the human host (18). Recombinant kappa-5 expressed in human embryonic kidney (HEK) cells did not reveal any IgE reactivity. Because mammalian-derived HEK cells have a different glycosylation repertoire from schistosomes, this may point to a role of specific glycans as the IgE target. In addition, kappa-5 was shown to be the primary S. mansoni SEA constituent that binds to soybean agglutinin (SBA), a lectin that is specific for terminal α/β-d-N-acetylgalactosamine (GalNAc) (19). Terminal GalNAc is a common monosaccharide in several S. mansoni life stages (6, 20, 21) including eggs (6) as part of GalNAcβ1–4GlcNAc (LDN), a structure to which several immunogenic properties have been attributed (4, 22–24). The selective binding of SBA to kappa-5 suggests that kappa-5 carries such GalNAc-containing glycans.
The significance of schistosome glycans with respect to host-schistosome interactions and the peculiar properties of kappa-5 glycosylation prompted us to perform a detailed analysis of kappa-5 using nanoscale liquid chromatography (LC)-MS(/MS) and matrix-assisted laser disorption ionization-time-of-flight (MALDI-TOF)(/TOF)-MS measurements of released glycans as well as tryptic glycopeptides, in combination with exoglycosidase treatments. We here show that each glycosylation site of kappa-5 to a large degree carries a unique type of core-difucosylated, core-xylosylated triantennary glycan with three terminal LDN motifs, setting it apart from the other members of the egg glycoproteome. Furthermore, we show that IgE reactivity of kappa-5 is attributable to its glycans. These observations underscore the antigenic properties of kappa-5 glycans and emphasize the need for further unraveling the role of glycosylation in the interaction of individual SEA components with the immune system of the host.
EXPERIMENTAL PROCEDURES
Antigens, Glycoconjugates, and Sera
S. mansoni soluble egg antigens (SEA) were prepared as described previously (10). Kappa-5 was isolated by soybean agglutinin (SBA; Sigma, Zwijndrecht, the Netherlands) affinity chromatography as described previously (18) or via a slightly adapted method. For the adapted method, two milligrams of SBA was coupled to 1 ml N-hydroxysuccinimide-activated Sepharose beads (GE Healthcare, Uppsala, Sweden) according to the manufacturer's instructions. SEA in phosphate-buffered saline (PBS), pH 7.4, was applied to a column containing the SBA beads and the bound material was eluted with 0.1 m galactose in PBS, pH 7.4. To maximize extraction efficiency, the effluent was reapplied once to the column. Effluent and eluate fractions were analyzed by SDS-PAGE with silver staining and Western blotting for the presence and purity of kappa-5. Eluate fractions containing purified kappa-5 were pooled, concentrated by ICON concentrators (9K MWCO, Thermo scientific, Rockford, IL, USA), dialyzed against PBS using Slide-a-lyzer dialysis cassettes (3.5 MWCO, Thermo scientific) and stored at −20 °C.
For testing the specificity of exoglycosidases used in this study, the synthetic glycoconjugates Fucα1–3GalNAcβ1–4GlcNAcβ1–3Galα1-(CH2)5-squarate (F-LDN-tag) and GalNAcβ1–4(Fucα1–3)GlcNAcβ1–3Galα1-(CH2)5-squarate (LDN-F-tag), recovered following derivatization with diethylsquarate during a protein coupling procedure (25, 26) were used.
Approval for this study has been granted by the Medical Ethics Committee of the Leiden University Medical Center. The serum samples used to determine the IgE-reactive properties of kappa-5 were provided from an immunoepidemiological study that was previously carried out in the village of Ndombo, Senegal. The study design, epidemiology, sample collection, and ethical procedures have been described in detail (27, 28). In short, consent forms were developed in the local language. The purpose and contents of the study were explained in detail to the community in the local language. Informed consent was obtained from individual adult participants but for children, the parents or guardians consented on their behalf. Each individual signed a consent form before commencement of any activity. All information obtained from participants was kept confidential.
Venous blood samples were collected, allowed to stand at room temperature for 1 h, and centrifuged at 1500 rpm. The serum was carefully removed and stored frozen at −15 °C. The serum samples were transported on dry ice to the Netherlands, aliquoted in 1.5-ml tubes (Eppendorf, Hamburg, Germany) and stored at −80 °C until use.
SDS-PAGE and Western Blotting
Kappa-5 was separated on a 12% polyacrylamide gel by SDS-PAGE under reducing conditions using the Mini-Protean Cell system (Bio-Rad, Veenendaal, the Netherlands). Proteins were detected with silver stain, as previously described (29). For Western blotting, the proteins were transferred onto a nitrocellulose membrane in a Bio-Rad Criterion Blotter system according to manufacturer's instructions. For detection of LDN glycosylation, protein blots were stained as described previously (30). In short, blots were blocked with BSA and incubated with monoclonal antibody (mAb) 273–3F2, which binds to LDN (21). Blots were subsequently incubated with alkaline phosphatase-labeled goat anti-mouse IgG,A,M (Caltag; Invitrogen, Breda, the Netherlands) and stained with nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate.
To test the glycan-dependent binding of IgE antibodies to kappa-5, nitrocellulose membranes carrying kappa-5 were blocked with PBS/0.1% Tween and treated with meta-periodate in acetate buffer or only acetate buffer as a control. Then, membranes were incubated with human infection sera diluted 1:100 and subsequently with alkaline phosphatase-labeled mouse anti-human IgE (Sigma). Antibody binding was visualized by nitro blue tetrazolium /5-bromo-4-chloro-3-indolyl phosphate. To determine the efficacy of meta-periodate treatment, meta-periodate- and mock-treated membranes were stained with SBA-horseradish peroxidase and 3,3′-diaminobenzidine. As a control, membranes were stained with silver stain as described before (31) and the anti-kappa-5 antibody 1G2 ((18) and unpublished results).
IgE Binding ELISA
Maxisorp immuno plates (NUNC, Roskilde, Denmark) were coated with 5 μg/ml of the indicated kappa-5 variants and blocked with PBS/0.05% Tween. Then, plates were incubated with human infection sera diluted 1:100 and subsequently with alkaline phosphatase-labeled mouse anti-human IgE (Sigma). Binding was visualized with para-nitrophenylphosphate.
Sample Preparation for Mass Spectrometry
Tryptic digestions were either performed in solution or in gel following SDS-PAGE. For the in-solution approach, to ≈ 5 μg of kappa-5 in 50 μl 50 mm ammonium bicarbonate, 0.1 μg of trypsin was added (Promega, Leiden, the Netherlands) followed by overnight incubation at 37 °C with or without prior reduction and alkylation. For reduction and alkylation, the sample was mixed with 0.05 volumes of 200 mm dithiothreitol, incubated for 30 min at 56 °C, following which 0.2 volumes of 200 mm iodoacetamide were added and the sample was incubated for 30 min in the dark at room temperature. Following digestion, the sample was stored at −20 °C and used for nano-LC-MS and PNGase A treatment.
In-gel digestion was performed as described previously (32). Individual protein bands of interest were excised from the gel. Following tryptic digestion, (glyco)peptides were collected using two rounds of extraction with 20 μl of 0.1% trifluoroacetic acid (TFA) and stored at −20 °C.
Exo-glycosidase Treatments
Glycoproteins, tryptic glycopeptides, or glycoconjugates were treated with β-N-acetylhexosaminidase from Canavalia ensiformis (62.5 mU; Sigma) in 100 mm sodium phosphate buffer, pH 5.0, for 24 h at 37 °C. α-Fucosidase treatment on these samples was performed with α1-(3,4)-fucosidase from Xanthomonas manihotis (0.5 mU; Sigma) or α-l-fucosidase from bovine kidney (15 mU; Sigma) in 100 mm sodium phosphate buffer, pH 5.0, for 24 h at 37 °C. Untreated and treated samples were subjected to nano-LC-MS(/MS), in case of glycoproteins following trypsin digestion.
PNGase A Release
A tryptic digest of kappa-5 was adjusted to pH 5.0 by addition of TFA and incubated with PNGase A (10 mU; Roche Diagnostics) overnight at 37 °C. The sample was applied to a Zip-TipC18 (Millipore, Billerica, MA, USA), washed five times with 20 μl 0.1% TFA and eluted with 50% acetonitrile (ACN), 0.1% TFA. The flow-through containing released glycans was applied to a self-packed (20 μl) porous graphitized carbon column (Carbograph; Alltech, Deerfield, IL, U.S.A.). The column was first equilibrated with a standard of 10 pmol maltopentaose to prevent irreversible binding of kappa-5-released carbohydrates. The maltopentaose was eluted from the column with 70% aqueous ACN and following washing the carbon column with water, the kappa-5-released carbohydrates were loaded on this column. The glycans were eluted with 50% aqueous ACN.
MALDI-TOF(/TOF)-MS
PNGase A-released and purified glycans of kappa-5 were spotted directly on the MALDI target plate and mixed with 1 μl of matrix solution, 2,5-dihydroxybenzoic acid (Bruker Daltonics, Bremen, Germany), 10 mg/ml 50% ACN/0.1% TFA. Sample spots were dried under a stream of warm air. MALDI-TOF(/TOF)-MS data were obtained using an Ultraflex II time-of-flight mass spectrometer (Bruker Daltonics) equipped with a LIFT-MS/MS facility. Spectra were acquired in the positive-ion reflectron mode. For fragment ion analysis in the tandem time-of-flight (TOF/TOF) mode, precursors were accelerated with 8 kV and selected in a timed ion gate. Fragment ions generated by laser-induced decomposition of the precursor were further accelerated by 19 kV in the LIFT cell, and their masses were analyzed following the ion reflector passage. For confirmation of the identity of the isolated kappa-5, peptides extracted following in-gel digestion were purified by Zip-TipC18, eluted with 1 μl 50% ACN, 0.1% TFA onto the sample target plate, and mixed with 1 μl of matrix solution (10 mg/ml 2,5-dihydroxybenzoic acid in 50% ACN, 0.1% TFA). The peptide mass fingerprint spectrum acquired was processed with FlexAnalysis version 3.3. The baseline subtraction algorithm Median was used, and peaks were detected using the Snap algorithm with following settings: S/N threshold >7, relative intensity threshold 10%, minimum intensity threshold 50, maximal number of peaks 100, quality factor threshold 90, Snap average composition averagine. The PMF data were searched with Mascot version 2.2.06 (Matrix Science, London, UK) against all entries (12,852,469) in the NCBInr database (release 31 Jan 2011), using Biotools version 3.2 as interface. Mascot parameter settings were trypsin digestion, up to two missed cleavages allowed, fixed carbamidomethyl modification of cysteine and variable methionine modification. The maximum mass tolerance was set at 0.1 Da. A protein score greater than 85 was significant (p > 0.05), with the scores for hits to the kappa-5 variants in the NCBInr protein database ranging from 162 to 198.
Nano-High Performance Liquid Chromatography-Electrospray Ionization (HPLC-ESI)-ion trap-MS(/MS)
(Glyco)peptide samples were applied to a reverse-phase column (PepMap, 3 μm, 75 μm × 100 mm; Dionex, Amsterdam, the Netherlands) using a Ultimate 3000 nano-LC system (Dionex). The column was equilibrated with eluent A (0.1% formic acid in water) at a flow rate of 300 nL·min−1. Following injection of the sample, a linear gradient was applied to 25% B (95% ACN, 0.1% formic acid) in 15 min, followed by a gradient to 70% B in 10 min and subsequent isocratic elution of 5 min. The eluate was monitored by absorption at 215 nm.
The LC column was coupled to an Esquire HCT Ultra ESI-IT-MS (Bruker Daltonics) equipped with an online nanospray source operating in the positive-ion mode. Electropolished, stainless steel LC-MS emitters (150 μm OD, 30 μm ID) from Proxeon A/S (Odense, Denmark) were used for electrospray (1100–1250 V). The solvent was evaporated at 170 °C employing a nitrogen stream of 6 L min−1 and ions from m/z 400 to m/z 1800 were registered in the MS mode. When operated in the auto MS/MS mode, registering ions from m/z 140 to 2200, each MS scan was followed by the acquisition of MS/MS spectra of up to five of the most abundant ions in the MS spectrum.
RESULTS
Kappa-5 is the Major LDN-containing Glycoprotein in S. mansoni Soluble Egg Antigens (SEA)
Kappa-5 was purified from SEA by SBA-affinity chromatography and subjected to SDS-PAGE under reducing conditions, giving rise to a prominent kappa-5 band at ∼ 50 kDa (Fig. 1A), as previously shown (18). The peptide mass fingerprint analysis of the tryptic digest matched to the secretory glycoprotein k5 precursor of Schistosoma mansoni (kappa-5), accession AAX83117 (NCBInr) with a Mowse score of 198, a sequence coverage of 58%, and 27 of 59 mass values searched matched. Mass spectrometric analysis demonstrated that the 50 kDa kappa-5 isomer contains four glycosylation sites N116, N143, N174, and N251 (Fig. 2). In a previous study, an additional protein band at a lower apparent molecular weight was found, containing an isomer of kappa-5 with only three glycosylation sites because of a single nucleotide mutation (18). Mass spectrometric analysis of glycopeptides isolated from this protein band (data not shown) indicated that the glycans carried by the kappa-5 isomer with three glycosylation sites were identical to those of the kappa-5 carrying four glycans.
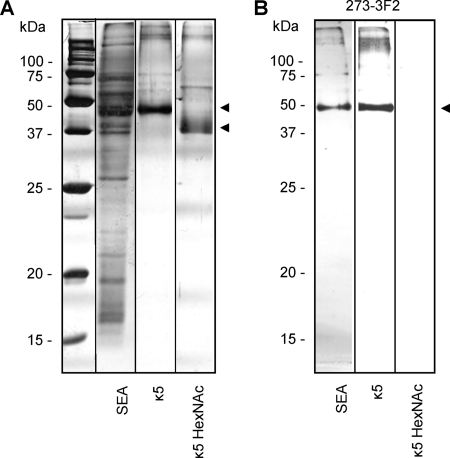
Fig. 1.
Visualization and characterization of kappa-5. SEA, kappa-5 (κ5) and β-N-acetylhexosaminidase-treated kappa-5 (κ5 HexNAc) were separated under reducing conditions by SDS-PAGE and silver stained (A). For testing the presence of terminal LDN, the antigens were subjected to Western blot using a monoclonal antibody against LDN (273–3F2) (B). Kappa-5 is indicated by arrowheads.
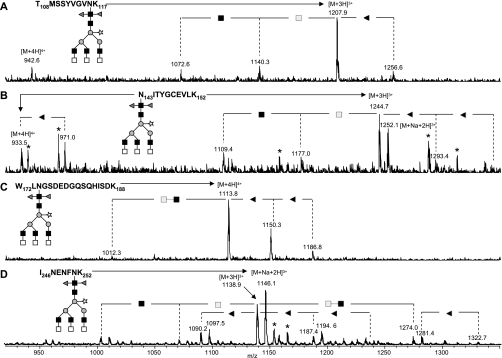
Fig. 2.
Heterogeneity of the glycan moieties on the four glycosylation sites of kappa-5. Kappa-5 was subjected to reduction and alkylation, digested with trypsin and the resulting glycopeptides were analyzed by LC-ion trap-MS. Mass spectra cover the four glycosylation sites N116 (A), N143 (B), N174 (C), and N251 (D). Differences in fucose, N-acetylglucosamine, and N-acetylgalactosamine content of the glycan moiety are indicated. Nonglycopeptide signals are marked with asterisks (*). Gray triangle, fucose; light gray square, N-acetylgalactosamine; dark gray square, N-acetylglucosamine; gray circle, mannose; open star, xylose.
SBA binds terminal GalNAc (19), a monosaccharide that abundantly occurs in S. mansoni glycoconjugates in the context of the disaccharide element GalNAcβ1–4GlcNAc (LDN) (6, 20, 21). To investigate whether kappa-5 carries LDN motifs, SBA-purified kappa-5 was subjected to Western blot analysis. The LDN-reactive mAb 273–3F2 was found to bind to kappa-5 (Fig. 1B), demonstrating the presence of LDN. Treatment of kappa-5 with β-N-acetylhexosaminidase (κ5 HexNAc) to remove terminal β-linked HexNAc residues and thereby LDN completely abrogated binding of the antibody, verifying the presence of a terminal LDN motif on kappa-5. Moreover, on SDS-PAGE the molecular weight of κ5 HexNAc decreased by >5 kDa upon β-N-acetylhexosaminidase treatment (Fig. 1A), which implies the presence of multiple LDN termini on kappa-5. Western blot analysis of SEA with mAb 273–3F2 showed one reactive protein band at the same molecular weight as isolated kappa-5 (Fig. 1B). Together, these data demonstrate that kappa-5 is the major glycoprotein in SEA that carries terminal, unsubstituted LDN.
Kappa-5-reactive IgE Antibodies in Human Infection Sera are Directed Against Glycans
To investigate the hypothesis that glycans are the target of kappa-5-reactive IgE in S. mansoni infection sera (18), SBA-purified kappa-5 was subjected to SDS-PAGE under reducing conditions, blotted onto a nitrocellulose membrane and treated with periodate to destroy its glycan structures. Following treatment, SBA-reactivity of kappa-5 was lost, whereas protein staining using silver stain and reactivity to a mAb against the kappa-5 protein were unaffected (Fig. 3A), indicating that by periodate treatment of kappa-5 on a blotting membrane the integrity of the glycans is destroyed, whereas the protein remains intact. Untreated kappa-5 is bound by IgE antibodies in human S. mansoni infection sera (Fig. 3B), as previously shown by Schramm et al. (18). Upon treatment with periodate, IgE reactivity was lost or strongly diminished (Fig. 3B), confirming that kappa-5 glycans are responsible for the binding of IgE antibodies in human infection sera to kappa-5.
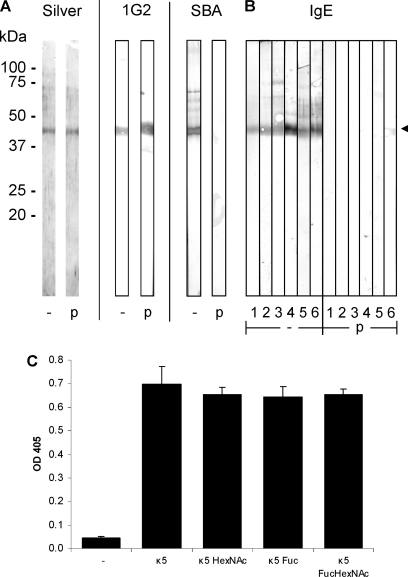
Fig. 3.
Reaction of IgE in human infection sera with kappa-5. (A, B) Blots containing SBA-purified kappa-5 were treated (p) with metaperiodate to destroy glycan structures, or without (-). Kappa-5 is indicated by the arrowhead. A, Treatment efficiency was analyzed with a protein stain (Silver), a monoclonal antibody against kappa-5 (1G2) and SBA reactivity (SBA). B, Sera from six human subjects known to be infected with S. mansoni were used to probe the kappa-5 blots. IgE antibody reactivity was visualized by a secondary antibody specific for IgE. C, Kappa-5 was treated with β-N-acetylhexosaminidase (κ5 HexNAc), α-fucosidase from X. manihotis (κ5 Fuc) or a combination of the enzymes (κ5 FucHexNAc), and the kappa-5 variants were tested in an ELISA for their reactivity with IgE from two human infection sera. One out of two representative experiments is shown.
Next, to investigate whether the reactivity of IgE antibodies from S. mansoni infection sera is targeted against the LDN antennae of kappa-5 or not, we performed an ELISA to measure IgE binding to kappa-5 variants generated by β-N-acetylhexosaminidase and fucosidase treatments. To ensure success of removal of HexNAc and fucose residues, glycopeptides generated following tryptic digestion of an aliquot of the exoglycosidase-treated kappa-5 variants were analyzed by LC-MS (data not shown), similarly as demonstrated for the exoglycosidase-treated glycopeptides (see Fig. 4 and Fig. 5). Fig. 3C shows that β-N-acetylhexosaminidase-treated kappa-5 exhibits similar IgE reactivity as untreated kappa-5, indicating that LDN on kappa-5 is not the primary target for serum IgE. Similarly, kappa-5 of which antenna fucoses were removed by treatment with α1-(3,4)-fucosidase from X. manihotis, and a variant treated sequentially with the fucosidase and β-N-acetylhexosaminidase, respectively, also display IgE reactivity comparable to untreated kappa-5 (κ5 Fuc and κ5 FucHexNAc, respectively, in Fig. 3C). Together, these observations indicate that the core structure rather than the antenna of kappa-5 glycans are associated with the IgE-reactivity of kappa-5.
Fig. 4.
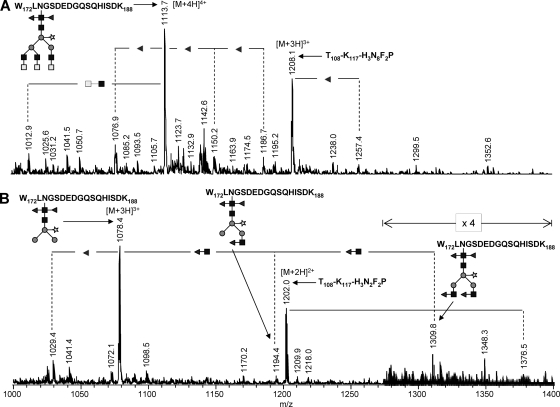
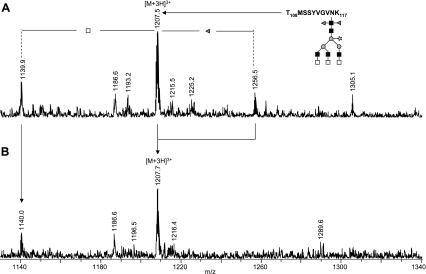
β-N-Acetylhexosaminidase treatment of kappa-5 glycopeptides. Kappa-5 was subjected to reduction and alkylation, digested with trypsin and the resulting glycopeptides W172-K188 and T108-K117 were analyzed by LC-MS before (A) and following (B) treatment with β-N-acetylhexosaminidase. Signals are labeled with monoisotopic masses. Differences in fucose, N-acetylglucosamine and N-acetylgalactosamine content of the glycan moiety are indicated. Gray triangle, fucose; light gray square, N-acetylgalactosamine; dark gray square, N-acetylglucosamine; gray circle, mannose; open star, xylose.
Fig. 5.
α-Fucosidase treatment of kappa-5 glycopeptides. Kappa-5 was subjected to reduction and alkylation, digested with trypsin and the resulting glycopeptide T108-K117 was analyzed by LC-MS before (A) and following (B) treatment with α-fucosidase from X. manihotis. Signals are labeled with monoisotopic masses. Differences in fucose, N-acetylglucosamine, and N-acetylgalactosamine content of the glycan moiety are indicated. Gray triangle, fucose; light gray square, N-acetylgalactosamine; dark gray square, N-acetylglucosamine; gray circle, mannose; open star, xylose.
The structural details of the antigenic kappa-5 glycans were further analyzed using various MS-based techniques and glycosidase treatments.
Analysis of PNGase A-released N-glycans from Kappa-5
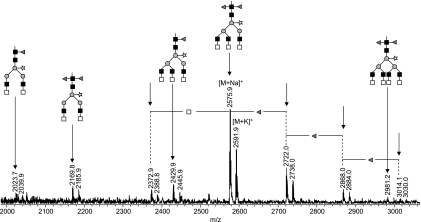
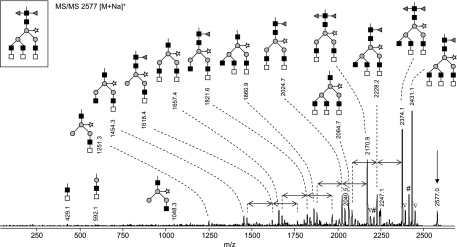
To obtain a general overview of N-glycans on kappa-5, tryptic kappa-5 glycopeptides were treated with PNGase A, which releases all N-glycans including those with the core α3-linked fucose modification frequently occurring in schistosome glycoproteins. The glycan pool was analyzed by MALDI-TOF-MS in the positive reflectron mode (Fig. 6). Single charged sodiated ions were detected (indicated by single-headed arrows in Fig. 6), accompanied by a signal with lower intensity corresponding to a potassium adduct. The major glycan species in the MALDI-TOF-MS spectrum (∼46% of total glycan pool) is observed at m/z 2575.9 [M+Na]+ and m/z 2591.9 [M+K]+ (Fig. 6), which corresponds to a reducing glycan with a mass of 2552.9 Da and a composition of H3N8F2P (H, hexose; N, N-acetylhexosamine; F, deoxyhexose, fucose; P, pentose, xylose). To further assess the glycan structure, the sodiated species at m/z 2575.9 was subjected to MALDI-TOF/TOF-MS fragmentation analysis (Fig. 7). Aided by the fragmentation pattern, we propose that H3N8F2P consists of a trimannosyl N-glycan core containing two fucoses at the reducing N-acetylglucosamine, a xylose residue linked to the β-linked mannose and three terminal LDN branches. The occurrence of LDN termini was indicated by the signal at m/z 429.1 (Fig. 7), which corresponds to sodiated HexNAc2 fragments. Based on the strong reactivity of kappa-5 with an LDN antibody (Fig. 3B), we interpret these fragments as terminal LDN motifs. This interpretation was supported by the presence of signals at m/z 2170.9 and 2374.1 [M+Na]+, corresponding to the loss of respectively one and two terminal HexNAc residues from the parent ion. The positioning of the fucose residues was revealed by a H3N7P fragment at m/z 2064.7 [M+Na-H2O]+ in Fig. 7, indicative of a chitobiose cleavage product that has lost the reducing end GlcNAc with one α3- and one α6-linked fucose. Furthermore, the signal at m/z 1048.3, corresponding to a sodiated H3N2P fragment indicates that the xylose residue is associated with the trimannosyl core, as previously described for S. mansoni egg (6) and cercarial (33) N-glycans.
Fig. 6.
MALDI-TOF-MS of PNGase A-released glycans from kappa-5. SBA- purified kappa-5 was digested with trypsin followed by PNGase A treatment. Released carbohydrates were subsequently purified using carbon adsorption chromatography and measured by MALDI-TOF-MS in the positive-ion reflectron mode. Signals are labeled with monoisotopic masses. m/z Values indicated with an arrow correspond to sodiated species. Gray triangle, fucose; light gray square, N-acetylgalactosamine; dark gray square, N-acetylglucosamine; gray circle, mannose; open star, xylose.
Fig. 7.
MS/MS of the major PNGase A-released glycan from kappa-5. The major PNGase A-released carbohydrate (m/z 2577 [M+Na]+) in Fig. 6 was analyzed by MALDI-TOF/TOF-MS. The parent ion is indicated by an arrow. Differences in fucose content are indicated by double-headed arrows. The given glycan structures are examples. Gray triangle, fucose; light gray square, N-acetylgalactosamine; dark gray square, N-acetylglucosamine; gray circle, mannose; open star, xylose; #, loss of water; ▿, presumably potassium adduct co-isolated during precursor selection.
The remaining PNGase A-released glycans visualized in Fig. 6 were assigned on the basis of mass differences compared with the above described major glycan signal at m/z 2575.9 [M+Na]+. Evidently, heterogeneity of the glycan moieties on kappa-5 is caused by variation in the number of HexNAc and fucose residues, as reflected by corresponding mass differences (203 and 146 Da, respectively) between the registered glycans. The second major glycan (at m/z 2722.0 [M+Na]+ and 2738.0 [M+K]+) (Fig. 6) accounts for ∼17% of glycans on kappa-5 and contains an additional fucose residue compared with the major signal, which may imply the presence of a fucose residue linked to one of the LDN antennae. Other glycan variations include structures containing two or four LDN motifs and a mono-fucosylated core (Fig. 6).
Identification and Characterization of N-glycosylation Sites of Kappa-5
To obtain in-depth, site-specific glycosylation information, kappa-5 was subjected to reduction, alkylation and trypsinization, following which resulting glycopeptides were analyzed by reversed phase LC-ion trap-MS. The most abundant glycopeptide species for all four glycosylation sites was found to be pep-H3N8F2P (m/z 1207.9 [M+3H]3+ for site N116, 1244.7 [M+3H]3+ and 1252.1 [M+Na+2H]3+ for site N143, 1113.8 [M+4H]4+ for site N174 and 1138.9 [M+3H]3+ and 1146.1 [M+Na+2H]3+ for site N251 in Figs. 2A, 2B, 2C, and 2D, respectively), which is in accordance with the major glycan species found among the PNGase A-released glycans (Fig. 6). Moreover, the same heterogeneity of the number of HexNAc and fucose moieties observed in the pool of released glycans (Fig. 6) is observed at the glycopeptide level (Fig. 2).
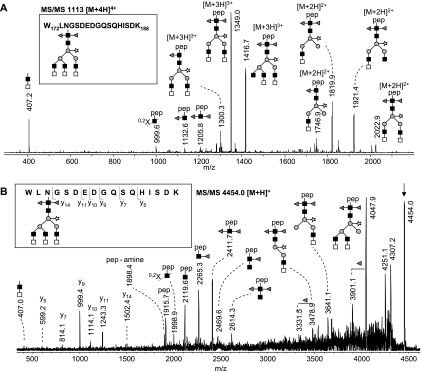
To further substantiate the tentative assignments based on the analysis of the released glycans and to verify the linkage position of HexNAc and fucose residues, kappa-5 glycopeptides were rerun on the reverse phase LC-MS system, including glycopeptides treated with exoglycosidases. In addition, glycopeptides were subjected to fragmentation using LC-MS/MS. Nano-LC-MS/MS of glycan H3N8F2P on peptide W172-K188 (m/z 1113 [M+4H]4+; Fig. 8A) verifies the presence of LDN antennae by intense signals at m/z 407.2 [HexNAc2+H]+, m/z 1349.0 [M-2HexNAc+3H]3+, and 2022.9 [M-2HexNAc+2H]2+ (loss of one LDN from the parent ion), and m/z 1819.9 [M-4HexNAc+2H]2+ (loss of two LDN). Furthermore, the absence of signals at m/z 610.3 [HexNAc3+H]+, 553.2 [HexNAc2Fuc1+H]+, and 813.4 [HexNAc4+H]+ confirms the absence of HexNAc3, fucosylated LDN or dimeric LDN antennae, which is a further indication that H3N8F2P-W172-K188 contains three, nonfucosylated terminal LDN branches. Additionally, data from β-N-acetylhexosaminidase treatment underline this assumption (Fig. 4). The signal of untreated W172-K188-H3N8F2P (m/z 1113.7 [M+4H]4+ in Fig. 4A) completely disappears upon treatment, whereas a similarly intense signal at m/z 1078.4 [M+3H]3+ (Fig. 4B) appears, corresponding to the loss of six HexNAc residues (three LDN antennae). To obtain detailed information on the core structure, a MALDI-TOF/TOF-MS of W172-K188-H3N8F2P was recorded (Fig. 8B). A core cleavage pattern with an intense signal at m/z 2411.7 [peptide+HexNAc1Fuc2+H]+ was observed, which verifies that the two fucoses in H3N8F2P are linked to the core GlcNAc residue. In this respect, it must be noted that the lower intensity signals at m/z 2265.3 [peptide+HexNAc1Fuc1+H]+ and 2119.6 [peptide+HexNAc1+H]+ are interpreted as the result of a chitobiose cleavage in combination with loss of core fucoses. For glycan H3N8F2P on the other three glycosylation sites, similar results were obtained using fragmentation and exoglycosidase derived data (Fig. 4 and supplemental Figs. S1–S4), leading to the conclusion that the N-glycan H3N8F2P is structurally identical for all sites and contains three terminal LDN branches, two fucoses linked to the innermost GlcNAc and a xylose linked to the β-linked mannose.
Fig. 8.
MS/MS of peptide W172-K188 carrying H3N8F2P N-glycans. The MS/MS spectra were acquired by LC-ion trap-MS/MS (A) and MALDI-TOF/TOF-MS/MS (B) from a tryptic digest of kappa-5. Glycopeptides are singly positively charged, unless specified otherwise. The given glycan structures are examples. Gray triangle, fucose; light gray square, N-acetylgalactosamine; dark gray square, N-acetylglucosamine; gray circle, mannose; open star, xylose; pep, peptide. 0,2X, ring fragmentation of the N-linked N-acetylglucosamine.
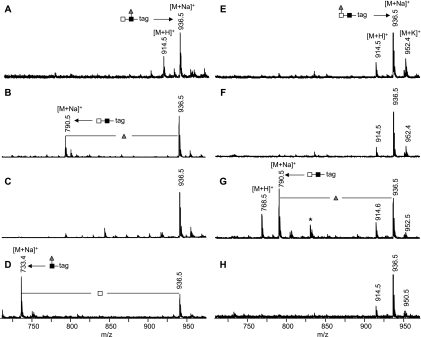
The composition of a substitution present on a minor portion of each glycosylation site, H3N8F3P (Fig. 2), points to an N-glycan with two core fucoses, one core xylose and three LDN antennae of which one is fucosylated, as verified by LC-MS/MS (supplemental Fig. S5). The position of the antenna fucose was determined by enzymatic degradation using a set of three exoglycosidases. First, the specificities of α1-(3,4)-fucosidase from X. manihotis, α-l-fucosidase from bovine kidney, and β-N-acetylhexosaminidase from C. ensiformis were tested using two synthetic glycoconjugates containing the terminal GalNAcβ1–4(Fucα1–3)GlcNAc (LDN-F-tag) and Fucα1–3GalNAcβ1–4GlcNAc (F-LDN-tag) sequences (25, 26). Treatment with α-fucosidase from X. manihotis resulted in the partial loss of one fucose from LDN-F-tag (m/z 936.5 [M+H]+ in Fig. 9A shifts to m/z 790.5 [M+H]+ in Fig. 9B), whereas F-LDN-tag was not affected by the enzyme (m/z 936.5 [M+H]+ in Fig. 9E does not shift in Fig 9F). Interestingly, α-fucosidase from bovine kidney has the opposite specificity. Under the conditions applied, it was able to partially cleave the fucose of F-LDN-tag (Fig. 9G) but not of LDN-F-tag (Fig. 9C). Upon treatment with β-N-acetylhexosaminidase, LDN-F-tag loses one HexNAc (m/z 936.5 [M+Na]+ in Fig. 9A shifts to m/z 733.4 [M+Na]+ in Fig. 9D), whereas the enzyme has no effect on F-LDN-tag (Fig. 9H). The tryptic glycopeptides of kappa-5 were treated with the same glycosidases. We used glycopeptide T108-K117 containing glycosylation site N116 to monitor the effect of exoglycosidase treatment on glycan H3N8F3P by LC-MS. Treatment of kappa-5 glycopeptides with α-fucosidase from X. manihotis results in removal of one fucose from T108-K117-H3N8F3P as the signal at m/z 1256.5 [M+3H]3+ in Fig. 5A completely shifts to the signal at m/z 1207.5 [M+3H]3+ in Fig. 5B, whereas the α-fucosidase derived from bovine kidney has no effect on the glycopeptide (data not shown). In addition, treatment with β-N-acetylhexosaminidase results in the disappearance of the signal at m/z 1257.4 [M+3H]3+ in Fig. 4A and the appearance of a signal at m/z 1376.5 [M+2H]2+ in Fig. 4B, representing the loss of the two (nonfucosylated) LDN structures and one HexNAc from the fucosylated LDN antenna, as confirmed by fragmentation analysis (supplemental Fig. S6). Together, these data demonstrate the presence of the LDN-F but not the F-LDN motif on glycopeptide T108-K117-H3N8F3P.
Fig. 9.
MALDI-TOF-MS of exoglycosidase treated glycoconjugates. To test the specificity of exoglycosidases, GalNAcβ1–4(Fucα1–3)GlcNAcβ1–3Galα1-(CH2)5-squarate (A–D) and Fucα1–3GalNAcβ1–4GlcNAcβ1–3Galα1-(CH2)5-squarate (E–H) were either not treated (A and E) or treated with α1-(3,4)-fucosidase from Xanthomonas manihotis (B and F), α-l-fucosidase from bovine kidney (C and G) or β-N-acetyl-hexosaminidase from Canavalia ensiformis (D and H) and analyzed by MALDI-TOF-MS. Nonglycoconjugate signals are marked with asterisks (*). Gray triangle, fucose; light gray square, N-acetylgalactosamine; dark gray square, N-acetylglucosamine.
Fragmentation spectra and exoglycosidase treatment data were also obtained for the other glycans on kappa-5, as summarized in Table I. Interestingly, the glycosylation patterns were found to be remarkably similar for the four glycosylation sites, although some differences in relative abundances as well as presence or absence of low abundant glycans existed.
Table I. Kappa-5 glycopeptide species detected after trypsin digestion. Kappa-5 was subjected to reduction, alkylation, and subsequent trypsinization. Resulting glycopeptides were analyzed by LC-ESI-MS. Glycan compositions are given in terms of hexose (H), N-acetylhexosamine (N), fucose (F) and pentose/xylose (P). Monoisotopic masses of glycopeptide precursors are given throughout. Proposed glycan structures are deduced from glycopeptide MS after enzyme treatments and fragmentation data. 3,6F, α3/α6-di-fucosylation of the innermost GlcNAc.
| Glycan composition | Glycopeptide signal (m/z) | Structural characteristics |
|---|---|---|
| Peptide T106-K117 with glycosylation site N116 | ||
| H3N6F2P | 1072.6 [M+3H]3+ | 3,6F, Xyl, two LDN |
| H3N7F2P | 1140.3 [M+3H]3+ | 3,6F, Xyl, two LDN, one HexNAc |
| H3N8F2P | 906.1 [M+H]4+ 1207.9 [M+3H]3+ | 3,6F, Xyl, three LDN |
| H3N8F3P | 942.6 [M+4H]4+ 1256.6 [M+3H]3+ | 3,6F, Xyl, two LDN, one LDN-F |
| Peptide N143-K152 with glycosylation site N143 | ||
| H3N6F2P | 1109.4 [M+3H]3+ | 3,6F, Xyl, two LDN |
| H3N7F2P | 1177.0 [M+3H]3+ | 3,6F, Xyl, two LDN, one HexNAc |
| H3N8F2P | 933.5 [M+4H]4+ 1244.7 [M+3H]3+ 1252.1 [M+Na+2H]3+ | 3,6F, Xyl, three LDN |
| H3N8F3P | 971.0 [M+4H]4+ 1293.4 [M+3H]3+ | 3,6F, Xyl, two LDN, one LDN-F |
| H3N8F4P | 1342.4 [M+3H]3+ | 3,6F, Xyl, one LDN, two LDN-F |
| Peptide W172-K188 with glycosylation site N174 | ||
| H3N6F2P | 1012.3 [M+4H]4+ | 3,6F, Xyl, two LDN |
| H3N8F2P | 1113.8 [M+4H]4+ | 3,6F, Xyl, three LDN |
| H3N8F3P | 1150.3 [M+4H]4+ | 3,6F, Xyl, two LDN, one LDN-F |
| H3N8F4P | 1186.8 [M+4H]4+ | 3,6F, Xyl, one LDN, two LDN-F |
| Peptide I246-K252 with glycosylation site N251 | ||
| H3N6F2P | 1003.7 [M+3H]3+ | 3,6F, Xyl, two LDN |
| H3N7F2P | 1071.3 [M+3H]3+ | 3,6F, Xyl, two LDN, one HexNAc |
| H3N8F1P | 1090.2 [M+3H]3+ 1097.5 [M+Na+2H]3+ | Monofucosylated core, Xyl, three LDN |
| H3N8F2P | 1138.9 [M+3H]3+ 1146.1 [M+Na+2H]3+ | 3,6F, Xyl, three LDN |
| H3N8F3P | 1187.4 [M+3H]3+ 1194.6 [M+Na+2H]3+ | 3,6F, Xyl, two LDN, one LDN-F |
| H3N8F4P | 1235.7 [M+3H]3+ 1243.7 [M+Na+2H]3+ | 3,6F, Xyl, one LDN, two LDN-F |
| H3N10F2P | 1274.0 [M+3H]3+ 1281.4 [M+Na+2H]3+ | 3,6F, Xyl, four LDN |
| H3N10F3P | 1322.7 [M+Na+2H]3+ | 3,6F, Xyl, three LDN, one LDN-F |
DISCUSSION
Our data show that the S. mansoni egg antigen kappa-5 carries a unique set of glycans with three LDN antennae on an α3/α6-difucosylated, xylosylated N-glycan core. These glycans appear to be exclusively expressed on kappa-5. No other SEA components were detected that are bound by an LDN-reactive mAb or SBA, the GalNAc-binding lectin used to purify kappa-5 from S. mansoni egg protein extracts. So far, two other abundant S. mansoni egg glycoproteins have been characterized in terms of glycosylation, IPSE/alpha-1 (34) and omega-1 (9). The glycan profiles of IPSE/alpha-1 and omega-1 are highly similar to each other and are characterized by the presence of diantennary glycans with core difucosylation and Lewis X antennae (11, 12), whereas in contrast to kappa-5 core xylosylation is absent and LDN-motifs were observed only in minor amounts in these two proteins.
The striking differences between the glycosylation of kappa-5 on the one hand and IPSE/alpha-1 and omega-1 on the other, emphasize the value of protein-specific MS-based glycosylation analyses. Although glycomics studies of glycans released from protein mixtures derived from cercariae, worms or eggs provide useful information on the glycan repertoire of a cell or organism, such studies usually lack the structural information required to address glycan-related properties of individual glycoproteins.
Schistosome-derived glycoproteins play an important role in immunomodulatory mechanisms associated with schistosomiasis via their action on a range of immune cells including dendritic cells (DC) and T cells (35). Glycosylated SEA components from S. mansoni are internalized by immature monocyte-derived DC primarily via the C-type lectin receptors Mannose receptor (MR), macrophage galactose-type lectin (MGL) and DC-SIGN, leading to suppression of Toll like receptor-induced DC activation (36). It is currently unknown which individual glycoproteins in SEA bind to these different lectin receptors. Binding of pathogen-derived glycans to DC-SIGN and MR can trigger intracellular pathways associated with immunomodulation (37, 38), but MGL-mediated signaling pathways involving pathogen glycans have to our knowledge not yet been described. The structural elucidation of kappa-5 glycans, and the previously reported studies on the glycosylation of IPSE/alpha-1 (12), a basophil modulator (39) and of omega-1 (11), an SEA component recently described to condition DC to drive Th2 responses (40), provide a starting point to further detail the contribution of these abundant SEA glycoproteins to C-type lectin-mediated immunomodulatory processes. Guided by the reported binding characteristics of MR, MGL, and DC-SIGN, the Lewis X elements present on both IPSE/alpha-1 and omega-1 are potential ligands for DC-SIGN (41), and they may also confer MR binding (42, 43). In contrast, kappa-5 is a candidate ligand for MGL that binds to α- and β-linked GalNAc residues (44).
Another special property of kappa-5 is that it is recognized by IgE as well as IgG antibodies in sera from S. mansoni infected individuals but not from uninfected individuals, whereas no other SEA components are as strongly reactive with IgE (18). In serum from S. mansoni-infected mice IgE has been detected that is specific for the N-glycan core modifications α3-fucose and possibly β2-xylose (45), which can both be present on S. mansoni egg glycans (6, 8). Interestingly, recombinant kappa-5 from HEK-cells, a mammalian cell type not capable of producing core α3-fucosylated and/or β2-xylosylated glycans, did not reveal IgE reactivity but had ample IgG reactivity in human sera, suggesting that glycans are the IgE targets (18). Our data confirm that kappa-5 glycans constitute the IgE epitopes (Fig. 3) and moreover demonstrate that the antenna structures on kappa-5 glycans, including the LDN epitopes, are not involved in IgE binding of kappa-5 to human infection sera (Fig. 3C). As the N-glycan core of kappa-5 glycans contains two fucoses linked to the innermost GlcNAc and a xylose (Table I), our data together with the previously published data on the antigenic properties of the α3-core fucose and β2-xylose (45), strongly suggest that the IgE antibodies in S. mansoni infection sera are targeted against the core decorations of kappa-5. Notably, asparagine-linked glycans belong to the most abundant environmental immune determinants and form the basis of the so-called cross-reactive carbohydrate determinants (46). About 20% or more of allergic patients generate specific antiglycan IgE, often accompanied by IgG. Despite some structural variation, the two main motifs in these cross-reactive carbohydrate determinants are the xylose and the core-3-linked fucose, which form the essential part of two independent epitopes. With respect to kappa-5, we believe that in particular the xylose residue forms part of the minimal glycan IgE epitope, because omega-1 and IPSE/alpha-1, which also contain core di-fucosylated glycans but lack the xylose residue, are not bound by IgE in schistosomiasis infection sera (18).
In a global glycomic comparison of glycans from S. mansoni and S. japonicum eggs, N-glycans with a α3-, α6-difucosylated, xylosylated core were found exclusively in the S. japonicum egg-derived glycan fraction (6). We here show that also S. mansoni is capable of expressing this highly unusual combination of core decorations. The specific three LDN antennae-containing glycan of kappa-5 was not observed by Khoo et al. (6). It is possible that within the total PNGase A-released glycan pool of S mansoni eggs, the major kappa-5-derived glycan H3N8F2P forms a minor fraction that could not be detected because it was obscured by other more abundant glycans. However, in a recent MALDI-TOF MS based profiling study of released glycans from several life stages of S. mansoni, the PNGase A-sensitive H3N8F2P glycan species was observed in eggs as well as miracidia (7). Also, kappa-5 was detected in eggs as well as miracidia, both at the protein and mRNA level (18), which is in line with the current data indicating that the H3N8F2P glycan is associated with kappa-5. Interestingly, the core-difucosylated Lewis X-containing glycans observed on IPSE/alpha-1 and omega-1 (11, 12) were detected in the PNGase A-released fraction of eggs, but not of miracidia (7), which is in line with the observation that IPSE/alpha-1 and omega-1 are expressed in the sub shell area of the egg (9, 10). Kappa-5 has been located to the same area, but as yet it is not clear whether kappa-5 is glycosylated differently from IPSE/alpha-1 and omega-1 because it is produced in the context of a different cellular glycosylation machinery, or because protein-specific effects play a role.
The observation that kappa-5 is the major LDN-expressing glycoprotein from schistosome eggs is additionally relevant in view of a previous report that a synthetic LDN-glycoconjugate can induce granulomas in a mouse model based on the implantation of coated Sepharose beads as artificial eggs (4). Liver granulomas with high similarity in terms of cellular constituency and temporal regulation compared with granulomas induced by schistosome eggs in a natural infection were induced by SEA- and LDN-coated beads, but not by a range of fucosylated conjugates including Lewis X (4). The potential role of kappa-5 as an authentic granuloma-inducing component of schistosome eggs should be investigated.
MS techniques are at the basis of structural analysis of glycans, and MS is an indispensible tool to obtain sensitive and detailed structural information on scarce and complex material such as pathogen-derived glycoproteins. It remains particularly difficult, however, to determine the specific positions and linkages of antenna elements in branched glycans. Therefore, validation of the structural assignments by glycosidase treatments and/or antiglycan antibodies is critical for a detailed and solid interpretation of MS data and the determination of glycan structures. To generate a tool to discriminate between the immunogenic LDN-F and F-LDN elements, we investigated the specificity of two α-fucosidases and one β-N-acetylhexosaminidase using synthetic LDN-F- and F-LDN-containing glycoconjugates. Interestingly, under the conditions applied, α1(-3,4)-fucosidase derived from X. manihotis was found to specifically cleave the fucose from LDN-F, whereas α-l-fucosidase from bovine kidney could cleave off the fucose of F-LDN but not LDN-F. In addition, β-N-acetylhexosaminidase from C. ensiformis provided structural information by the cleavage of unsubstituted HexNAc from LDN-F. Using this knowledge, we were able to demonstrate that a minor subset of kappa-5 glycans carries terminal LDN-F motifs, whereas no F-LDN motifs were observed on kappa-5. These specific glycosidases will be useful tools to distinguish F-LDN and LDN-F in a variety of settings, as these isomers form antigenically different motifs. For instance, in schistosome infections, high IgG antibody responses are found against F-LDN, but not against LDN-F (25, 47).
With the completion of the glycosylation analysis of kappa-5, three major S. mansoni egg antigens (48, 49) have now been identified in terms of protein and glycan sequence. This information will contribute to our understanding of the immunological mechanisms induced by these proteins and/or the glycans that they carry. The collective glycans detected on these three major egg antigens seem to make up the majority of N-glycans detected in S. mansoni excretory/secretory antigens (8) and in the specific PNGase A-sensitive fraction of egg N-glycans (7).
Acknowledgments
We thank Rick Versteegh and Magnus Palmblad for technical assistance.
Footnotes
* This work was supported by the Dutch Organization for Scientific Research (NWO), Grant No CW ECHO 700.55.013.
 This article contains supplemental Figs. S1 to S6.
This article contains supplemental Figs. S1 to S6.
1 The abbreviations used are:
- SEA
- soluble egg antigen
- DC
- dendritic cell
- DC-SIGN
- dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin
- HEK
- Human embryonic kidney
- HexNAc
- N-acetylhexosamine
- F-LDN
- Fucα1–3GalNAcβ1–4GlcNAc
- Fuc
- Fucose
- Gal
- Galactose
- GalNAc
- N-acetylgalactosamine
- GlcNAc
- N-acetylglucosamine
- IPSE
- Interleukin-4-inducing principle from schistosome eggs
- LDN
- GalNAcβ1–4GlcNAc
- LDN-F
- GalNAcβ1–4(Fucα1–3)GlcNAc
- Lewis X
- Galβ1–4(Fucα1–3)GlcNAc
- mAb
- monoclonal antibody
- MGL
- Macrophage galactose-type lectin
- MR
- Mannose receptor
- NHS
- N-hydroxysuccinimide
- SBA
- Soy bean agglutinin
- SBA-HRP
- Horseradish peroxidase-linked soy bean agglutinin
- Th2
- T helper 2
- Xyl
- Xylose.
REFERENCES
- 1. Pearce E. J. (2005) Priming of the immune response by schistosome eggs. Parasite Immunol. 27, 265–270 [DOI] [PubMed] [Google Scholar]
- 2. Okano M., Satoskar A. R., Nishizaki K., Abe M., Harn D. A., Jr. (1999) Induction of Th2 responses and IgE is largely due to carbohydrates functioning as adjuvants on Schistosoma mansoni egg antigens. J. Immunol. 163, 6712–6717 [PubMed] [Google Scholar]
- 3. van de, Vijver K. K., Hokke C. H., van Remoortere A., Jacobs W., Deelder A. M., Van Marck E. A. (2004) Glycans of Schistosoma mansoni and keyhole limpet haemocyanin induce hepatic granulomas in vivo. Int. J. Parasitol. 34, 951–961 [DOI] [PubMed] [Google Scholar]
- 4. Van de Vijver K. K., Deelder A. M., Jacobs W., Van Marck E. A., Hokke C. H. (2006) LacdiNAc- and LacNAc-containing glycans induce granulomas in an in vivo model for schistosome egg-induced hepatic granuloma formation. Glycobiology 16, 237–243 [DOI] [PubMed] [Google Scholar]
- 5. Faveeuw C., Mallevaey T., Paschinger K., Wilson I. B., Fontaine J., Mollicone R., Oriol R., Altmann F., Lerouge P., Capron M., Trottein F. (2003) Schistosome N-glycans containing core alpha 3-fucose and core beta 2-xylose epitopes are strong inducers of Th2 responses in mice. Eur. J. Immunol. 33, 1271–1281 [DOI] [PubMed] [Google Scholar]
- 6. Khoo K. H., Chatterjee D., Caulfield J. P., Morris H. R., Dell A. (1997) Structural mapping of the glycans from the egg glycoproteins of Schistosoma mansoni and Schistosoma japonicum: identification of novel core structures and terminal sequences. Glycobiology 7, 663–677 [DOI] [PubMed] [Google Scholar]
- 7. Hokke C. H., Deelder A. M., Hoffmann K. F., Wuhrer M. (2007) Glycomics-driven discoveries in schistosome research. Exp. Parasitol. 117, 275–283 [DOI] [PubMed] [Google Scholar]
- 8. Jang-Lee J., Curwen R. S., Ashton P. D., Tissot B., Mathieson W., Panico M., Dell A., Wilson R. A., Haslam S. M. (2007) Glycomics analysis of Schistosoma mansoni egg and cercarial secretions. Mol. Cell Proteomics 6, 1485–1499 [DOI] [PubMed] [Google Scholar]
- 9. Fitzsimmons C. M., Schramm G., Jones F. M., Chalmers I. W., Hoffmann K. F., Grevelding C. G., Wuhrer M., Hokke C. H., Haas H., Doenhoff M. J., Dunne D. W. (2005) Molecular characterization of omega-1: a hepatotoxic ribonuclease from Schistosoma mansoni eggs. Mol. Biochem. Parasitol. 144, 123–127 [DOI] [PubMed] [Google Scholar]
- 10. Schramm G., Gronow A., Knobloch J., Wippersteg V., Grevelding C. G., Galle J., Fuller H., Stanley R. G., Chiodini P. L., Haas H., Doenhoff M. J. (2006) IPSE/alpha-1: a major immunogenic component secreted from Schistosoma mansoni eggs. Mol. Biochem. Parasitol. 147, 9–19 [DOI] [PubMed] [Google Scholar]
- 11. Meevissen M. H., Wuhrer M., Doenhoff M. J., Schramm G., Haas H., Deelder A. M., Hokke C. H. (2010) Structural characterization of glycans on omega-1, a major schistosoma mansoni egg glycoprotein that drives Th2 responses. J. Proteome. Res. 9, 2630–2642 [DOI] [PubMed] [Google Scholar]
- 12. Wuhrer M., Balog C. I., Catalina M. I., Jones F. M., Schramm G., Haas H., Doenhoff M. J., Dunne D. W., Deelder A. M., Hokke C. H. (2006) IPSE/alpha-1, a major secretory glycoprotein antigen from schistosome eggs, expresses the Lewis X motif on core-difucosylated N-glycans. FEBS J. 273, 2276–2292 [DOI] [PubMed] [Google Scholar]
- 13. Thomas P. G., Carter M. R., Atochina O., Da'Dara A. A., Piskorska D., McGuire E., Harn D. A. (2003) Maturation of dendritic cell 2 phenotype by a helminth glycan uses a Toll-like receptor 4-dependent mechanism. J. Immunol. 171, 5837–5841 [DOI] [PubMed] [Google Scholar]
- 14. Okano M., Satoskar A. R., Nishizaki K., Harn D. A., Jr. (2001) Lacto-N-fucopentaose III found on Schistosoma mansoni egg antigens functions as adjuvant for proteins by inducing Th2-type response. J. Immunol. 167, 442–450 [DOI] [PubMed] [Google Scholar]
- 15. Atochina O., Da'dara A. A., Walker M., Harn D. A. (2008) The immunomodulatory glycan LNFPIII initiates alternative activation of murine macrophages in vivo. Immunology 125, 111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang J., Zhang Y., Wei J., Zhang X., Zhang B., Zhu Z., Zou W., Wang Y., Mou Z., Ni B., Wu Y. (2007) Lewis X oligosaccharides targeting to DC-SIGN enhanced antigen-specific immune response. Immunology 121, 174–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hsu S. C., Tsai T. H., Kawasaki H., Chen C. H., Plunkett B., Lee R. T., Lee Y. C., Huang S. K. (2007) Antigen coupled with Lewis-x trisaccharides elicits potent immune responses in mice. J. Allergy Clin. Immunol. 119, 1522–1528 [DOI] [PubMed] [Google Scholar]
- 18. Schramm G., Hamilton J. V., Balog C. I., Wuhrer M., Gronow A., Beckmann S., Wippersteg V., Grevelding C. G., Goldmann T., Weber E., Brattig N. W., Deelder A. M., Dunne D. W., Hokke C. H., Haas H., Doenhoff M. J. (2009) Molecular characterisation of kappa-5, a major antigenic glycoprotein from Schistosoma mansoni eggs. Mol. Biochem. Parasitol. 166, 4–14 [DOI] [PubMed] [Google Scholar]
- 19. Lis H., Sela B. A., Sachs L., Sharon N. (1970) Specific inhibition by N-acetyl-D-galactosamine of the interaction between soybean agglutinin and animal cell surfaces. Biochim. Biophys. Acta 211, 582–585 [DOI] [PubMed] [Google Scholar]
- 20. Wuhrer M., Koeleman C. A., Fitzpatrick J. M., Hoffmann K. F., Deelder A. M., Hokke C. H. (2006) Gender-specific expression of complex-type N-glycans in schistosomes. Glycobiology 16, 991–1006 [DOI] [PubMed] [Google Scholar]
- 21. van Remoortere A., Hokke C. H., van dam G. J., van Die I., Deelder A. M., van den Eijnden D. H. (2000) Various stages of schistosoma express Lewis(x), LacdiNAc, GalNAcbeta1–4 (Fucalpha1–3)GlcNAc and GalNAcbeta1–4(Fucalpha1–2Fucalpha1–3)GlcNAc carbohydrate epitopes: detection with monoclonal antibodies that are characterized by enzymatically synthesized neoglycoproteins. Glycobiology 10, 601–609 [DOI] [PubMed] [Google Scholar]
- 22. Nyame A. K., Leppanen A. M., DeBose-Boyd R., Cummings R. D. (1999) Mice infected with Schistosoma mansoni generate antibodies to LacdiNAc (GalNAc beta 1–>4GlcNAc) determinants. Glycobiology 9, 1029–1035 [DOI] [PubMed] [Google Scholar]
- 23. Nyame A. K., Lewis F. A., Doughty B. L., Correa-Oliveira R., Cummings R. D. (2003) Immunity to schistosomiasis: glycans are potential antigenic targets for immune intervention. Exp. Parasitol. 104, 1–13 [DOI] [PubMed] [Google Scholar]
- 24. van den, Berg T. K., Honing H., Franke N., van Remoortere A., Schiphorst W. E., Liu F. T., Deelder A. M., Cummings R. D., Hokke C. H., van Die I. (2004) LacdiNAc-glycans constitute a parasite pattern for galectin-3-mediated immune recognition. J. Immunol. 173, 1902–1907 [DOI] [PubMed] [Google Scholar]
- 25. van Remoortere A., Vermeer H. J., van Roon A. M., Langermans J. A., Thomas A. W., Wilson R. A., van Die I., van den, Eijnden D. H., Agoston K., Kerekgyarto J., Vliegenthart J. F., Kamerling J. P., van dam G. J., Hokke C. H., Deelder A. M. (2003) Dominant antibody responses to Fucalpha1–3GalNAc and Fucalpha1–2Fucalpha1–3GlcNAc containing carbohydrate epitopes in Pan troglodytes vaccinated and infected with Schistosoma mansoni. Exp. Parasitol. 105, 219–225 [DOI] [PubMed] [Google Scholar]
- 26. Agoston K., Kerékgyártó J., Hajkó J., Batta G., Lefeber D. J., Kamerling J. P., Vliegenthart J. F. (2002) Synthesis of fragments of the glycocalyx glycan of the parasite Schistosoma mansoni. Chemistry. 8, 151–161 [DOI] [PubMed] [Google Scholar]
- 27. Balog C. I., Mayboroda O. A., Wuhrer M., Hokke C. H., Deelder A. M., Hensbergen P. J. (2010) Mass spectrometric identification of aberrantly glycosylated human apolipoprotein C-III peptides in urine from Schistosoma mansoni-infected individuals. Mol. Cell Proteomics. 9, 667–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stelma F. F., Talla I., Polman K., Niang M., Sturrock R. F., Deelder A. M., Gryseels B. (1993) Epidemiology of Schistosoma mansoni infection in a recently exposed community in northern Senegal. Am. J. Trop. Med. Hyg. 49, 701–706 [DOI] [PubMed] [Google Scholar]
- 29. Heukeshoven J., Dernick R. (1985) Simplified method for silver staining of proteins in polyacrylamide gels and the mechanism of silver staining. ELECTROPHORESIS 6, 103–112 [Google Scholar]
- 30. Robijn M. L., Wuhrer M., Kornelis D., Deelder A. M., Geyer R., Hokke C. H. (2005) Mapping fucosylated epitopes on glycoproteins and glycolipids of Schistosoma mansoni cercariae, adult worms and eggs. Parasitology 130, 67–77 [DOI] [PubMed] [Google Scholar]
- 31. van Oostveen I., Ducret A., Aebersold R. (1997) Colloidal silver staining of electroblotted proteins for high sensitivity peptide mapping by liquid chromatography-electrospray ionization tandem mass spectrometry. Anal. Biochem. 247, 310–318 [DOI] [PubMed] [Google Scholar]
- 32. Steen H., Pandey A., Andersen J. S., Mann M. (2002) Analysis of tyrosine phosphorylation sites in signaling molecules by a phosphotyrosine-specific immonium ion scanning method. Sci. STKE. 2002, L16. [DOI] [PubMed] [Google Scholar]
- 33. Khoo K. H., Huang H. H., Lee K. M. (2001) Characteristic structural features of schistosome cercarial N-glycans: expression of Lewis X and core xylosylation. Glycobiology 11, 149–163 [DOI] [PubMed] [Google Scholar]
- 34. Schramm G., Falcone F. H., Gronow A., Haisch K., Mamat U., Doenhoff M. J., Oliveira G., Galle J., Dahinden C. A., Haas H. (2003) Molecular characterization of an interleukin-4-inducing factor from Schistosoma mansoni eggs. J. Biol. Chem. 278, 18384–18392 [DOI] [PubMed] [Google Scholar]
- 35. Maizels R. M., Yazdanbakhsh M. (2003) Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat. Rev. Immunol. 3, 733–744 [DOI] [PubMed] [Google Scholar]
- 36. van Liempt E., van Vliet S. J., Engering A., García Vallejo J. J., Bank C. M., Sanchez-Hernandez M., van Kooyk Y., van Die I. (2007) Schistosoma mansoni soluble egg antigens are internalized by human dendritic cells through multiple C-type lectins and suppress TLR-induced dendritic cell activation. Mol. Immunol. 44, 2605–2615 [DOI] [PubMed] [Google Scholar]
- 37. Geijtenbeek T. B., Gringhuis S. I. (2009) Signalling through C-type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 9, 465–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Torrelles J. B., Schlesinger L. S. (2010) Diversity in Mycobacterium tuberculosis mannosylated cell wall determinants impacts adaptation to the host. Tuberculosis 90, 84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schramm G., Mohrs K., Wodrich M., Doenhoff M. J., Pearce E. J., Haas H., Mohrs M. (2007) Cutting edge: IPSE/alpha-1, a glycoprotein from Schistosoma mansoni eggs, induces IgE-dependent, antigen-independent IL-4 production by murine basophils in vivo. J. Immunol. 178, 6023–6027 [DOI] [PubMed] [Google Scholar]
- 40. Everts B., Perona-Wright G., Smits H. H., Hokke C. H., van der, Ham A. J., Fitzsimmons C. M., Doenhoff M. J., van der, Bosch J., Mohrs K., Haas H., Mohrs M., Yazdanbakhsh M., Schramm G. (2009) Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J. Exp. Med. 206, 1673–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Liempt E., Bank C. M., Mehta P., Garciá-Vallejo J. J., Kawar Z. S., Geyer R., Alvarez R. A., Cummings R. D., Kooyk Y., van Die I. (2006) Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Lett. 580, 6123–6131 [DOI] [PubMed] [Google Scholar]
- 42. Taylor M. E., Bezouska K., Drickamer K. (1992) Contribution to ligand binding by multiple carbohydrate-recognition domains in the macrophage mannose receptor. J. Biol. Chem. 267, 1719–1726 [PubMed] [Google Scholar]
- 43. Taylor M. E., Drickamer K. (1993) Structural requirements for high affinity binding of complex ligands by the macrophage mannose receptor. J. Biol. Chem. 268, 399–404 [PubMed] [Google Scholar]
- 44. van Vliet S. J., Saeland E., van Kooyk Y. (2008) Sweet preferences of MGL: carbohydrate specificity and function. Trends Immunol. 29, 83–90 [DOI] [PubMed] [Google Scholar]
- 45. van Die I., Gomord V., Kooyman F. N., van den Berg T. K., Cummings R. D., Vervelde L. (1999) Core alpha1–>3-fucose is a common modification of N-glycans in parasitic helminths and constitutes an important epitope for IgE from Haemonchus contortus infected sheep. FEBS Lett. 463, 189–193 [DOI] [PubMed] [Google Scholar]
- 46. Altmann F. (2007) The role of protein glycosylation in allergy. Int. Arch. Allergy Immunol. 142, 99–115 [DOI] [PubMed] [Google Scholar]
- 47. Naus C. W., van Remoortere A., Ouma J. H., Kimani G., Dunne D. W., Kamerling J. P., Deelder A. M., Hokke C. H. (2003) Specific antibody responses to three schistosome-related carbohydrate structures in recently exposed immigrants and established residents in an area of Schistosoma mansoni endemicity. Infect. Immun. 71, 5676–5681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Doenhoff M. J., Dunne D. W., Bain J., Lillywhite J. E., McLaren M. L. (1985) Serodiagnosis of mansonian schistosomiasis with CEF6, a cationic antigen fraction of Schistosoma mansoni eggs. Dev. Biol. Stand. 62, 63–73 [PubMed] [Google Scholar]
- 49. Dunne D. W., Agnew A. M., Modha J., Doenhoff M. J. (1986) Schistosoma mansoni egg antigens: preparation of rabbit antisera with monospecific immunoprecipitating activity, and their use in antigen characterization. Parasite Immunol. 8, 575–586 [DOI] [PubMed] [Google Scholar]