Abstract
The UN1 monoclonal antibody recognized the UN1 antigen as a heavily sialylated and O-glycosylated protein with the apparent molecular weight of 100–120 kDa; this antigen was peculiarly expressed in fetal tissues and several cancer tissues, including leukemic T cells, breast, and colon carcinomas. However, the lack of primary structure information has limited further investigation on the role of the UN1 antigen in neoplastic transformation.
In this study, we have identified the UN1 antigen as CD43, a transmembrane sialoglycoprotein involved in cell adhesion, differentiation, and apoptosis. Indeed, mass spectrometry detected two tryptic peptides of the membrane-purified UN1 antigen that matched the amino acidic sequence of the CD43 intracellular domain. Immunological cross-reactivity, migration pattern in mono- and bi-dimensional electrophoresis, and CD43 gene-dependent expression proved the CD43 identity of the UN1 antigen. Moreover, the monosaccharide GalNAc-O-linked to the CD43 peptide core was identified as an essential component of the UN1 epitope by glycosidase digestion of specific glycan branches. UN1-type CD43 glycoforms were detected in colon, sigmoid colon, and breast carcinomas, whereas undetected in normal tissues from the same patients, confirming the cancer-association of the UN1 epitope. Our results highlight UN1 monoclonal antibody as a suitable tool for cancer immunophenotyping and analysis of CD43 glycosylation in tumorigenesis.
Glycoproteins play a major role in cell signaling, immune recognition, and cell-cell interaction because of their glycan branches conferring structure variability and binding specificity to lectin ligands (1). Mucin-type glycoproteins are characterized by a high content of O-linked carbohydrate chains (O-glycans), and are secreted or expressed on the membrane of hematopoietic and epithelial cells. O-glycan biosynthesis initiates in the Golgi apparatus with the attachment of N-acetyl-galactosamine (GalNAc) to serine or threonine residues by an UDP-N-acetyl-d galactosamine: polipeptide N-acetylgalactosaminyltransferase (GalNAc-transferase) generating the Tn antigen structure of O-glycans. Subsequent elongation of the O-linked glycan branch is catalyzed by tissue-specific glycosyltransferases through the addition of other carbohydrates, such as galactose, fucose, and sialic acid, which results in the synthesis of a complex array of O-glycan structures that differ for the nature and length of O-linked carbohydrate chains (2). In addition, the oligosaccharides can be modified by sialylation, fucosylation, sulfatation, methylation, or acetylation. Truncation of O-glycan structures as well as abnormal expression of specific O-glycans occur in cancer cells, suggesting that aberrant glycosylation may contribute to cancer progression by modifying cell signaling, adhesion, and antigenicity (3, 4).
The UN1 monoclonal antibody (mAb)1, which was initially selected for high reactivity with human immature thymocytes (CD3dim) (5), recognized the UN1 antigen as a highly sialylated and O-glycosylated protein with the apparent molecular weight of 100–120 kDa and binding specificity to the lectins Maackia Amurensis (MAL II) and Peanut Agglutinin (PNA), which are binders of the sialic acid in (α2–3) linkage and the disaccharide Galβ1,3GalNAc, respectively (6). The UN1 antigen was immunodetected on the membrane of human thymocytes, a subpopulation of peripheral blood CD4+ T-lymphocytes and leukemic T-cell lines, such as HPB-ALL, H9, and MOLT-4 (5, 6). In addition, UN1 was expressed at early stages of development in fetal tissues, including thymus, spleen, adrenal cortex, bronchial epithelium, and skin, and was down-regulated in ontogeny (7). UN1 was also expressed in a variety of solid tumors, including breast, colon, gastric, and squamous cell lung carcinomas, whereas undetected in normal tissues and benign lesions (7). In particular, a direct correlation was observed between the expression level of UN1 and the stage of malignancy in breast tissue (8). In fact, UN1 was not expressed in normal cells and nonproliferative lesions, whereas it was poorly expressed in fibroadenoma, moderately expressed in atypical hyperplasia, and highly expressed in proliferative lesions of in situ breast carcinoma (stage 0 of disease) and infiltrating breast carcinoma (stages I–III) with the highest expression level in metastatic lesions (stage IV) (8). The expression pattern in primary cells suggested that UN1 behaved as an oncofetal antigen with a potential value for cancer immunophenotyping and clinical applications; however, the role of UN1 in tumorigenesis was not further addressed because of the lack of knowledge of its primary structure.
In this study, we demonstrate that UN1 is the transmembrane CD43 glycoprotein. The primary structure of UN1 was determined by mass spectrometry through the identification of two tryptic peptides that matched the intracytoplasmic domain of CD43. The CD43 identity of UN1 antigen was confirmed by immunological cross-reactivity of the two proteins and strict dependence of UN1 detection on CD43 gene expression. We also show that the single monosaccharide GalNAc-O-linked to the CD43 peptide core is a component of the UN1 epitope, and that CD43 molecules harboring the UN1 epitope are peculiarly expressed in breast, colon, and sigmoid colon carcinomas. The evidence that UN1-type glycosylated CD43 molecules are expressed in cancer tissues supports the possibility of using the UN1 mAb for cancer immunophenotyping and analysis of CD43 glycosylation in tumorigenesis.
EXPERIMENTAL PROCEDURES
Plasmids
The plasmid pCMV-CD43 expressing the human CD43 was purchased from Origene Company (Rockville, MD). The CD43 nucleotide sequence was amplified by PCR from pCMV-CD43 using the forward primer CCCAAGCTTATGGCCACGCTTCTCCTTCTCCTT and reverse primer GGGGTACCGAAGGGGCAGCCCCGTCTCC; the PCR product was digested with HindIII and KpnI and ligated to HindIII-KpnI-digested p3xFLAG-CMV-14 (Sigma-Aldrich) to generate p3xFLAG-CMV-CD43 expressing 3xFLAG fused to C terminus of CD43.
Antibodies
The UN1 mAb (IgG1, k) was produced by the hybridoma technology and selected for high reactivity with human immature thymocytes (5); immunoglobulins were purified from hybridoma supernatant by affinity chromatography on protein G-Sepharose (GE Healthcare, Uppsala, Sweden). CD43 (C-20) against a C terminus peptide of human CD43, CD43 (MEM-59) against a neuraminidase-sensitive epitope of CD43, donkey anti-goat IgG-HRP and normal mouse IgG were from Santa Cruz Biotechnology (Santa Cruz, CA); anti-FLAG M2 and anti-γ-Tubulin were from Sigma-Aldrich; sheep anti-mouse IgG horseradish peroxidase conjugated was from GE Healthcare.
Cell Lines, Transfection and Flow Cytometry
HPB-ALL and CEM, human leukemic T-cell lines expressing the UN1 antigen (5), were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum/phosphate-buffered saline (PBS), 2 mm l-glutamine, 100U/ml penicillin, 100 μg/ml streptomycin, at 5% CO2 and 37 °C. Media were purchased from GIBCO, Invitrogen, Carlsbad, CA. HPB-ALL cells (5 × 106) were transfected with p3xFLAG-CMV-CD43 (40 μg) by electroporation (0.2 kV, 950 μF) using a Bio-Rad Gene Pulser apparatus (Bio-Rad, Hercules, CA), as previously reported (9). For flow cytometry, HPB-ALL cells (5 × 106) were electroporated with CD43 siRNA or control siRNA (500 pmol) (Dharmacon, Lafayette, CO), and 48 h later the transfected cells (1 × 106) were 1 h-incubated with the UN1 mAb (1 μg) or CD43 (MEM-59) antibody (1 μg) in 100 μl of PBS at 4 °C, washed in PBS, and incubated with Alexa Fluor 488 rabbit anti-mouse IgG (Molecular Probes, Eugene, Oregon) for 30 min at 4 °C. Following extensive washing in PBS, cells were analyzed by flow cytometry using FacScan (Becton Dickinson, Franklin Lakes, NJ).
Western Blotting and Immune Blotting
Proteins were separated by 8% SDS-PAGE and transferred to nitrocellulose membranes (GE Healthcare) by electroblotting in 150 mm glycine-20 mm Tris at 20 mA for 16 h. Following pre-incubation in PBS plus 5% blocking reagent (Bio-Rad), membranes were incubated with primary antibody in PBS plus 5% blocking reagent for 2 h at room temperature, followed by incubation with secondary antibody in PBS plus 5% blocking reagent for 1 h at room temperature. Strips were washed, treated with ECL Western blotting kit (GE Healthcare), and exposed to x-ray film.
Purification of the UN1 Antigen
The UN1 antigen was purified from HPB-ALL membrane extracts by sequential steps, which included anion exchange chromatography, immunoprecipitation, mimotope-based displacement from immunocomplex, and lectin affinity binding, as previously described (10) (supplemental Fig. S1). Briefly, HPB-ALL cells (2.4 × 1010) were suspended in 230 ml of Buffer R containing 20 mm Tris/HCl, pH 7.8, supplemented with Protease Inhibitor Mixture (Roche, Mannheim, Germany), and sonicated using Bandelin Sonoplus GM70 (Bandelin Electronic, Berlin, Germany). Cell lysate was 10 min-centrifuged at 800 × g to remove nuclei and intact cells. Supernatant was further centrifuged for 2 h at 100,000 × g at 4 °C. The pellet (membrane fraction) was lysed in buffer R containing 1% Triton X-100 for 16 h on ice, and centrifuged for 60 min at 15,000 × g to recover the supernatant. Membrane proteins were separated by anion-exchange chromatography on a diethylaminoethyl (DEAE)-Sepharose Fast Flow (Sigma-Aldrich) column connected to the AKTA FPLC System (GE Healthcare). The column (2.6 cm × 28 cm) was equilibrated with 20 mm Tris/HCl, pH 7.8, containing 0.1% Triton X-100 (buffer A) at a flow rate of 2 ml/min. Membrane proteins (1 g) were applied to the column, washed with buffer A, and bound proteins were eluted with 500 mm NaCl in buffer A; the elution profile was monitored by absorbance at 280 nm. Collected fractions (24 ml) were analyzed for the presence of the UN1 antigen by Western blotting using the UN1 mAb. For UN1 quantization, films were analyzed by scanning densitometry using NIH Image Software (http://rsbweb.nih.gov/nih-image/); specific signal was evaluated as number of pixels/μg of protein. UN1-positive fractions were pooled and dialyzed against PBS buffer containing 0.1% Triton X-100. Dialyzed sample was adjusted to 0.5% Triton X-100 final concentration and preincubated with normal mouse IgG (474 μg) coupled to 4.5 ml of a 50% (v/v) slurry of Protein G-Sepharose (GE Healthcare) on a rotating agitator for 16 h at 4 °C. Following centrifugation at 800 × g, the pellet was recovered as a negative control for mass spectrometry, whereas supernatant (about 240 mg of proteins) was incubated with UN1 mAb (474 μg) bound to 4.5 ml of a 50% (v/v) slurry of Protein G-Sepharose for 16 h on a rotating agitator at 4 °C. The immunocomplex was collected by centrifugation at 800 × g for 5 min at 4 °C, and the pellet was washed and resuspended in 15 ml of PBS buffer containing 0.5% Triton X-100. By screening a random peptide library displayed on filamentous fd phages with UN1 mAb, we previously identified the G-23 peptide (SFAATPHTCKLLDECVPLWPAEG) as a mimotope of the UN1 antigen (10). The UN1 antigen was displaced from the binding to the UN1 mAb by incubation with G23 peptide at a peptide/UN1 mAb molar ratio of 1 × 103 for 16 h at 4 °C; the displaced UN1 antigen was recovered in supernatant following centrifugation at 800 × g for 5 min at 4 °C, as previously described (10). The UN1 antigen was separated from contaminant G-23 peptide by 16 h-incubation with biotinylated MAL II (5 μg/ml; Vector Laboratories, Burlingame, CA), for which sialic acid (α2–3) is a ligand, followed by 2 h-incubation with Streptavidin MagneSphere Paramagnetic Particles (Promega, Madison, WI) on a rotating agitator at 4 °C. The UN1 antigen/MAL II complex was collected with a magnetic separator and, following extensive washing in PBS buffer containing 0.5% Triton X-100, the lectin binding to the UN1 antigen was competed with 250 mm sialic acid in 3.6 ml of PBS containing 0.1% Triton X-100, which released the purified UN1 sample for mass spectrometry.
Nano Liquid Chromatography Tandem MS (LC-MS/MS) Analysis
The membrane purified UN1 antigen was trichloroacetic acid-precipitated and resuspended in 50 μl of 200 mm Tris-HCl buffer, pH 8.0, containing 0.1% Triton X-100. UN1-positive DEAE fractions immunoprecipitated with IgG were used as control sample of mass spectrometry. Protein samples were 1 h-reduced with 10 mm dithiothreitol (DTT) at 37 °C followed by 1 h-incubation with 30 mm iodoacetamide at 37 °C for cysteine alkylation. Iodoacetamide was neutralized by 20 min incubation with DTT (15 mm final concentration) and calcium chloride was added to 1 mm final concentration. Protein samples were digested with sequencing-grade modified trypsin (3.2 ng/μl) (Sigma-Aldrich) overnight at 37 °C, as previously reported (11). To avoid nonionic detergent Triton X-100 contamination, a two-step purification method was applied based on reversed-phase solid phase extraction (SPE) followed by strong-cation exchange (SCX) chromatography (11). Briefly, tryptic peptides were purified by reversed-phase SPE with Oasis HLB cartridges (10 mg packing bed, Waters, Milford, MA). SPE column was conditioned with 500 μl of H2O/methanol 1/1 (v/v); the column was equilibrated with 500 μl of H2O/methanol/trifluoroacetic acid 97.9/2/0.1 (v/v/v) (Wash A). The peptide solution (62 μl) was diluted to a final volume of 500 μl in Wash A, and loaded onto the SPE cartridge. Following two consecutive 400 μl washings with Wash A and H2O/methanol/formic acid mixture 97.9/2/0.1 (v/v/v), respectively, peptides were eluted off the SPE cartridge with 250 μl of H2O/methanol/formic acid 19.9/80/0.1 (v/v/v). The eluted peptides were evaporated to dryness in a vacuum centrifuge and stored at 4 °C until use. Peptides were dissolved in 30 μl of H2O/methanol/formic acid mixture 84/15/1 (v/v/v) (Wash SCX) and then applied to SCX Zip TipsTM (Millipore, Billerica, MA), previously equilibrated with Wash SCX. Following extensive washing with Wash SCX, the detergent-free peptide mixture was eluted in 2 μl of H2O/methanol/ammonium hydroxide 80/15/5 (v/v/v) and diluted with 28 μl of H2O/acetonitrile/trifluoroacetic acid 97.95/2/0.05 (v/v/v) (loading pump solvent).
Nanoscale liquid chromatography (NanoLC) was performed on Ultimate nanoLC system (Dionex, Sunnyvale, CA), using a valveless setup, as previously described (11). Briefly, a peptide sample (10 μl) was loaded at the rate of 12 μl/min onto Integra FritTM trapping column (100 μm bore ID; packing bed length 0.5 cm) (New Objective, Cambridge, MA) in-house packed with 5 μm C18 particles (Dr. Maisch GmbH, Entringen, Germany). Following 4-min washing with loading pump solvent, the trapping column was switched on-line to a Pico FritTM column (50 μm bore ID; packing bed length 10 cm) (New Objective) in-house packed with 3 μm C18 particles. Peptides were separated at 100 nL/min with a binary gradient consisting of H2O/acetonitrile/formic acid/trifluoroacetic acid 97.9/2/0.09/0.01 (v/v/v/v) (mobile phase A), and H2O/acetonitrile/formic acid/trifluoroacetic acid 29.9/70/0.09/0.01 (v/v/v/v) (mobile phase B). Gradient was from 0 to 40% mobile phase B in 25 min. Following 5 min at 95% mobile phase B, the column was re-equilibrated at 0% mobile phase B for 15 min before the following injection.
MS detection was performed on a QSTAR XL hybrid quadrupole time-of-flight mass spectrometer (Applied Biosystems, Foster City, CA) operating in positive ion mode, with nanoelectrospray potential at 1300 V, curtain gas at 15 units, collision-activated dissociation gas at 3 units. Information-dependent acquisition was performed by selecting the two most abundant peaks for MS/MS analysis following a full TOF-MS scan from 400 to 1200 m/z lasting 2 s. MS/MS analysis was performed in enhanced mode (2 s/scan). Threshold value for peak selection for MS/MS was 10 counts.
Database Search of MS/MS Spectra
MS/MS spectra were converted in Mascot Generic Format by the Analyst software (Applied Biosystems, version 1.1). A script running on Analyst was used to determine peptide charge state and to perform centroiding and de-isotoping on MS/MS data. Data were analyzed on the local Mascot search engine (www.matrixscience.com), version 1.9, against the International Protein Index Human Database (version 3.38; 70856 entries; accessed on January 2008) using the following parameters: MS tolerance 20 ppm; MS/MS tolerance 0.2 Da; fixed modifications carbamidomethyl cysteine; variable modifications methionine oxidized; enzyme trypsin; maximum missed cleavages 1.
Two-dimensional Gel Electrophoresis
HPB-ALL membrane extracts (300 μg) were precipitated by adding nine sample volumes of ice-cold acetone for 2 h at - 20 °C. Following centrifugation at 15,000 × g for 30 min, pellets were resuspended in 125 μL of rehydration buffer containing 8 m urea, 2% Triton X-100, 70 mm DTT, 0.8% (v/v) IPG buffer, pH 3–10 nonlinear (GE Healthcare) by mixing (800 rpm, 30 min, 30 °C) in thermomixer (Eppendorf, Hamburg, Germany), and loaded onto 7-cm Immobiline DryStrip gels (pH 3–10 nonlinear) (GE Healthcare). Isoelectric focusing was conducted on an IPGphor (GE Healthcare) with a current limit of 50 μA per strip at 20 °C. Following 16 h-rehydration, the isoelectric focusing was performed by applying 300 V for 30 min, a gradient to 1000 V for 30 min, a gradient to 5000 V for 90 min, and finally 5000 V for 90 min. Following isoelectric focusing, strips were equilibrated in SDS equilibration buffer [50 mm Tris-HCl, pH 8.8, 6 m urea, 30% glycerol, 2% SDS, and 0.002% (w/v) bromphenol blue] containing 2% DTT for 15 min at room temperature followed by further incubation in fresh SDS equilibration buffer containing 2.5% (w/v) iodoacetamide for 15 min at room temperature. The equilibrated strips were then applied to 8% SDS-PAGE for 2-DE gel separation, transferred to nitrocellulose membranes, and immunoblotted with UN1 or anti-CD43 antibodies.
Glycosidase Digestions
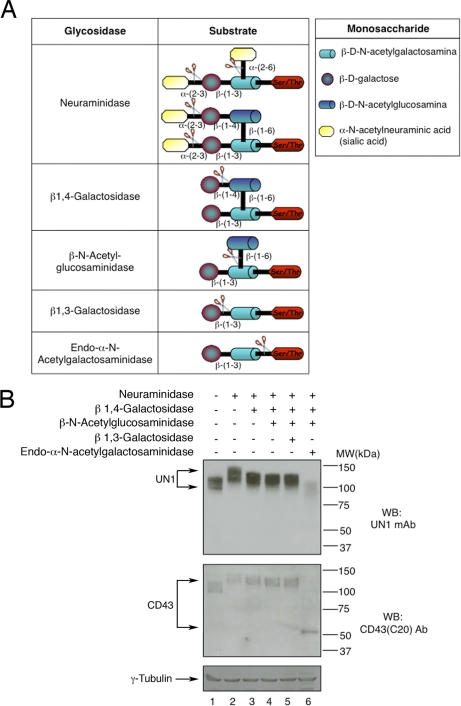
HPB-ALL membrane extracts were sequentially digested with glycosidases (CalBiochem brand Deglycosylation Kit, EMD Biosciences, San Diego, CA), according to the manufacturer's instructions. Briefly, protein extract (100 μg) was incubated in 100 μl with α2–3,6,8,9-neuraminidase (2.5 mU) for 16 h at 37 °C followed by sequential digestion with β1,4-galactosidase (1.5 mU), β-N-acetylglucosaminidase (23 mU), β1,3-galactosidase (2000 mU) or endo-α-N-acetylgalactosaminidase (0.6 mU) for 24 h at 37 °C. Aliquots of digested samples were analyzed by Western blotting using CD43 (C-20) and UN1 antibodies.
Glycan Array Screening
The Consortium for Functional Glycomics (www.functionalglycomics.org) performed the glycan array screening, as previously described (12). The printed array version 3.2 consisted of 406 glycans in six replicates (http://www.functionalglycomics.org/static/consortium/resources/resourcecoreh12.shtml). Further details are given in supplemental Fig. S2.
Confocal Microscopy
Tissues were obtained from patients of the Oncology Surgery, Cancer Center of Excellence, Fondazione Tommaso Campanella, Catanzaro, upon the written consent of the patients and the approval of the Ethics Committee Board. Surgical specimens were derived from cancer tissues of sigmoid colon, colon, and breast; noncancerous tissues were resected 5–10 cm away from the cancer tissues of the same patient. Samples were suspended in OCT compound (Sakura Finetek, Torrance, CA) and frozen at −80 °C. Serial 4-μm sections were obtained from the block using a Leica CM 1950 cryostat (Leica Mycrosystems, Wetzlar, Germany). For optical microscopy, cryostat sections were stained with hematoxylin and eosin (H&E) and analyzed for cell morphology. For confocal microscopy, sections were 1-h incubated with the UN1 mAb (1 μg/ml) or normal mouse IgG (1:400) (Santa Cruz) diluted in PBS on ice in a humidified chamber, washed in PBS, and incubated with rabbit anti-mouse-Alexa Fluor488 (1:400) for 30 min in the dark. Following washing in PBS, sections were fixed and permeabilized using Cytofix/CytoPerm kit (BD Biosciences Pharmingen, San Diego, CA) for 20 min on ice, and then washed in washing buffer (PBS containing 1% BSA, 0.2% Triton X-100). Then, sections were 1-h incubated with CD43 (C-20) antibody (1:200) or normal goat IgG (1:400) (Santa Cruz) in a humidified chamber on ice; following washing, sections were incubated with donkey anti-goat-Alexa Fluor568 (1:400) (Molecular Probes) for 30 min in the dark. Nuclei were stained with DAPI (Sigma-Aldrich) diluted in PBS (2 μg/ml). Following washing with PBS, coverslips were mounted on glass slides by using ProLong Antifade Kit (Molecular Probes). Pictures were captured with a Leica TCS SP2 confocal microscope (Leica Mycrosystems) with a HC PL FLUOTAR 40× Apo PLA oil immersion objective (NA 1.4) in glycerol and acquired with Leica Confocal Software Version 2.61. Z stacks of images were collected using a step increment of 0.2 μm between planes. UN1 was visualized by excitation with an argon laser at 488 nm and photomultiplier tube voltage of 420 mV. CD43 was detected using a krypton laser at 543 nm and photomultiplier tube voltage of 650 mV. Nuclei were detected using an argon laser at 405 nm and photomultiplier tube voltage of 450 mV. Single optical sections using 4× averaging were acquired by sequential scanning to collect the images in three channels. Scan format was 1024 × 1024 pixels. Images were processed with Adobe Photoshop CS.
RESULTS
Mass Spectrometry Identifies the UN1 Antigen as CD43
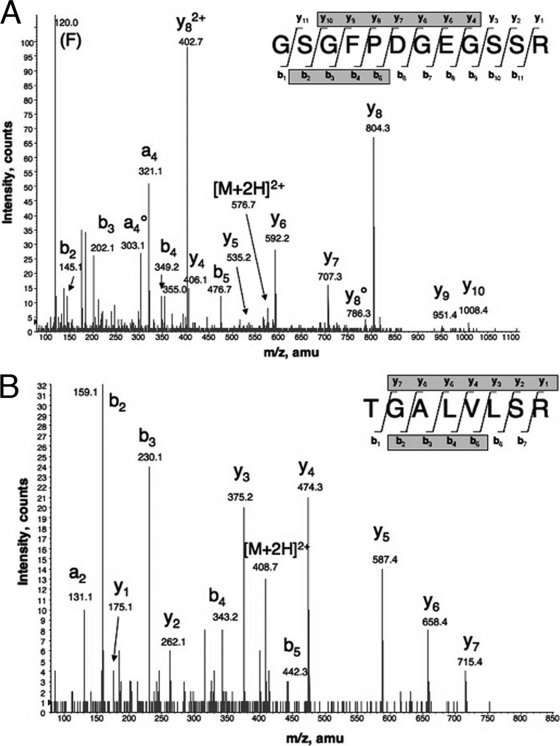
Purification of the UN1 antigen from membranes of HPB-ALL cells was performed in sequential biochemical steps, as previously described (10) (supplemental Fig. S1). In the first step, the membrane extract was fractionated by anion-exchange chromatography followed by immunoprecipitation of pooled UN1-positive fractions with the UN1 mAb. Then, the UN1 antigen was displaced from the immunocomplex using the G-23 peptide, a UN1 mimotope (10), and further purified by MAL II lectin-affinity chromatography. Following tryptic digestion, the UN1 peptides were separated by nanoscale liquid chromatography and analyzed by tandem mass spectrometry (nanoLC-MS/MS). MS spectra identified the peptides GSGFPDGEGSSR and TGALVLSR (Fig. 1A, B). Mascot search analysis of the International Protein Index Human Database indicated that CD43 was the putative candidate of the UN1 antigen based on statistically significant identification scores of the two identified peptides that matched the sequence from amino acids 285 to 292 and amino acids 327 to 338 of human CD43, also named leukosialin or sialophorin (Table I and Fig. 2).
Fig. 1.
NanoLC-MS/MS spectra of the UN1 antigen. The UN1 antigen was digested with trypsin and analyzed by nanoLC-MS/MS. MS/MS spectra of doubly charged ions detected at 576.75 m/z (A) and 408.75 m/z (B) are shown. Database search of MS/MS spectra lead to a statistically significant identification of the following peptide sequences: GSGFPDGEGSSR (Mascot ion score 70) (A) and TGALVLSR (Mascot ion score 57) (B).
Table I. Mascot search-based identification of CD43 as putative candidate of the UN1 antigen. Mascot search analysis of the International Protein Index Human Database indicated that CD43 was a putative candidate of the UN1 antigen based on the peptide amino acidic sequences identified by nanoLC-MS/MS. *Mascot ion score threshold for 95% confidence was 30.
| Name | Accession number | MW (Da) | Sequence coverage | Number of identified peptides | Peptide amino acidic sequence | MASCOT Score* |
|---|---|---|---|---|---|---|
| Sialophorin/Leukosialin/CD43 [Homo sapiens] | IPI00027430 | 40297 | 5.3% | 2 | TGALVLSR | 70 |
| GSGFPDGEGSSR | 57 |
Fig. 2.
Primary structure of CD43. The amino acidic sequence of CD43 with the extracellular (blue), transmembrane (red) and intracellular (black) domains is shown. The UN1 peptides identified by mass spectrometry are shown in bold square.
Immunological Cross-reactivity and Expression Pattern Confirm the CD43 Identity of the UN1 Antigen
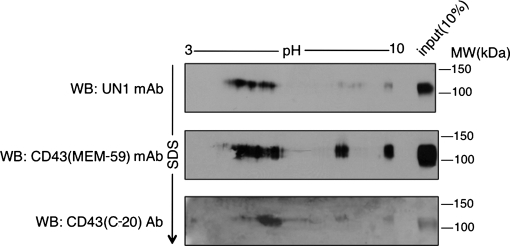
The human CD43 precursor includes 400 amino acids with the predicted molecular weight of 40 kDa and apparent molecular weight of 54 kDa upon electrophoresis (13). CD43 is converted by O-glycosylation to two major glycoforms with the apparent molecular weight of 115 and 135 kDa, which are differentially expressed depending on cellular activation, and are recognized by specific antibodies (14). To verify the identity of UN1 antigen as CD43, we tested the immunodetection of purified UN1 glycoprotein by CD43 (C-20) antibody, which detects a glycosylation-independent epitope of the CD43 C terminus. On mono-dimensional electrophoresis followed by Western blotting analysis, both the CD43 (C-20) and UN1 antibodies detected a protein with the apparent molecular weight of 110–120 kDa, indicating that the UN1 antigen was immunologically related to CD43 (Fig. 3). Further analysis of protein migration on two-dimensional electrophoresis (2-DE) followed by Western blotting was performed with the UN1 and CD43 (C-20) antibodies, including as additional antibody CD43 (MEM-59) that recognizes a sialic acid-dependent CD43 epitope. Several and pH-coincident protein spots were detected by the UN1 and anti-CD43 antibodies over the 4–10 pH interval, although a preferential detection for acidic forms of the antigen was observed in the case of UN1 mAb (Fig. 4). Thus, based on the two-dimensional electrophoresis (2-DE) migration pattern, UN1 behaved as CD43 glycoforms with higher content of sialic acid.
Fig. 3.
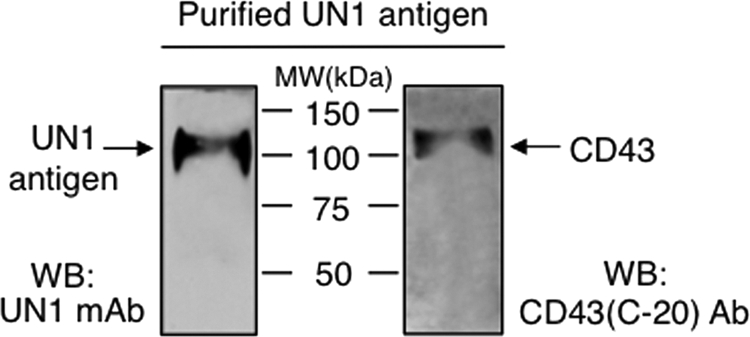
Purified UN1 antigen is recognized by anti-CD43 antibody. UN1 antigen (0.08% of the total purified sample) was separated on 8% SDS-PAGE and analyzed by Western blotting with UN1 or CD43 (C-20) antibodies. The CD43 (C-20) antibody recognizes an epitope mapping at the C terminus of human CD43. One representative experiment out of three is shown.
Fig. 4.
Migration patterns of the UN1 antigen and CD43 on 2-DE. Total extract of HPB-ALL cells was fractionated on 2-DE; slab gels were analyzed by Western blotting with UN1, CD43 (MEM-59) and CD43 (C-20) antibodies. The CD43 (MEM-59) antibody recognizes a neuraminidase-sensitive epitope on CD43 molecule. An aliquot (10%) of the protein sample was applied in the lateral well of each gel and subject to second dimension SDS-PAGE. One representative experiment out of three is shown.
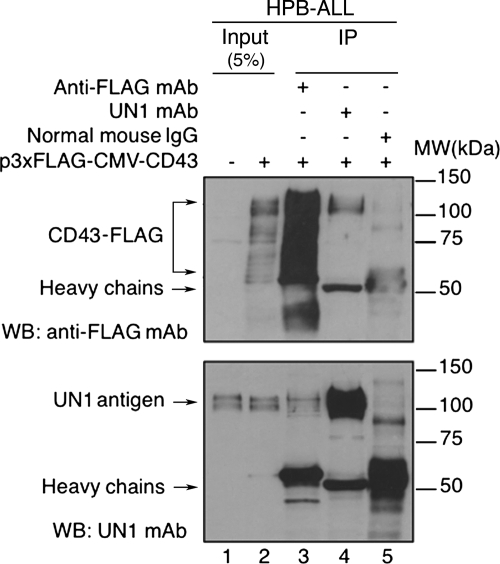
Next, we tested whether the UN1 mAb recognized CD43 upon cell transfection. To this end, HPB-ALL cells were stably transfected with p3XFLAG-CMV-CD43 expressing CD43 fused to the 3XFLAG epitope; total cell extracts were immunoprecipitated with the antibodies anti-FLAG, UN1, or normal mouse IgG, and each immunocomplex was analyzed by Western blotting with anti-FLAG and UN1 antibodies. A wide range of proteins with SDS-PAGE mobility between 55 and 120 kDa were detected by the anti-FLAG antibody in CD43-FLAG-transfected cells, while undetected in untransfected cells, which corresponded to the expected sizes of precursor and mature glycoforms of CD43 (13) (Fig. 5, top panel, lanes 1 and 2). Two major protein bands of 100–120 kDa were detected by the UN1 mAb in both untransfected and CD43-FLAG-transfected cells, which likely corresponded to glycoforms of endogenous CD43 and transfected CD43-FLAG with high molecular weights (Fig. 5, bottom panel, lanes 1 and 2). CD43-FLAG immunoprecipitated with anti-FLAG was recognized by the UN1 mAb as protein bands with the apparent molecular weight of 100–120 kDa (Fig. 5, bottom panel, lane 3). Similarly, UN1 antigen immunoprecipitated with the UN1 mAb was recognized by anti-FLAG antibody as protein bands of the apparent molecular weight of 100–120 kDa (Fig. 5, top panel, lane 4). Both anti-FLAG and UN1 antibodies failed to detect protein bands in the range of 100–120 kDa in immunoprecipitated proteins with mouse IgG control (Fig. 5, top and bottom panels, lane 5), indicating the specific immunological cross-reactivity of UN1 antigen and CD43. These results indicated that highly glycosylated isoforms of CD43-FLAG were immunoprecipitated and detected by the UN1 mAb, proving the in vivo recognition of distinct CD43 glycoforms as UN1 antigen.
Fig. 5.
CD43 is recognized by the UN1 mAb upon transfection. HPB-ALL cells were stably transfected with p3xFLAG-CMV-CD43; cell extracts were immunoprecipitated (IP) with anti-FLAG antibody (lanes 3), UN1 mAb (lanes 4) or normal mouse IgG (lanes 5). Immunocomplexes were separated by 8% SDS-PAGE and analyzed by Western blotting with anti-FLAG and UN1 antibodies. One representative experiment out of three is shown.
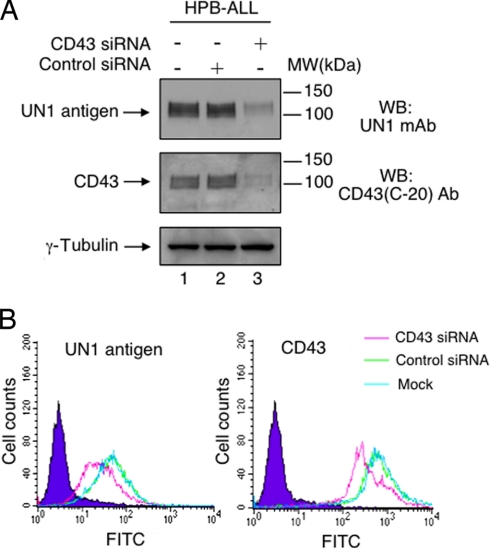
Next, we asked whether the UN1 antigen detection was dependent on CD43 gene expression. To this end, we analyzed the expression pattern of the UN1 antigen in wild type and CD43−/−CEM cells, a human leukemic T-cell line. The UN1 antigen and CD43 were detected by the antibodies UN1 and CD43 (C-20), respectively, as proteins with the apparent molecular weight of 140–150 kDa in wild-type CEM cells, whereas they were both undetected in CD43−/−CEM cells (Fig. 6). In HPB-ALL cells, RNA interference of CD43 abolished the detection of both CD43 and UN1 antigen, as observed by Western blotting analysis of cell extracts (Fig. 7A), and flow cytometry (Fig. 7B). Based on the immunological cross-reactivity, similarity of migration pattern, and CD43-dependent gene expression, we concluded that the UN1 antigen corresponded to CD43 glycoforms with high molecular weights.
Fig. 6.
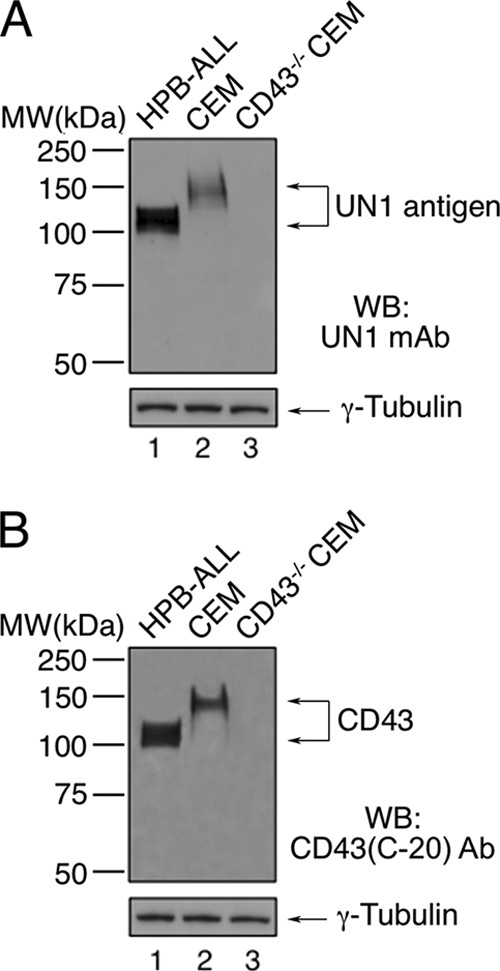
The UN1 antigen expression is dependent on CD43 gene. Total protein extracts (50 μg) of wild type and CD43−/−CEM cells were separated by 8% SDS-PAGE and analyzed by Western blotting with the antibodies UN1 (A), CD43 (C-20) (B), or anti-γ-Tubulin (A and B). As independent control, HPB-ALL cell extract (50 μg) was run in both gels. The apparent molecular weight of UN1 and CD43 was in the range of 100–120 kDa in HPB-ALL cells, and 140–150 kDa in CEM cells, because of cell-type differential CD43 glycosylation. One representative experiment out of three is shown.
Fig. 7.
CD43 silencing by RNA interference causes the loss of UN1 detection. A, HPB-ALL cells were transfected with CD43 siRNA (500 pmol), scrambled siRNA (500 pmol), or left untransfected. Forty-eight hours later, protein extracts (50 μg) were separated by 8% SDS-PAGE and analyzed by Western blotting with antibodies CD43 (C-20), UN1, or anti-γ-Tubulin. B, HPB-ALL cells were transfected with CD43 siRNA (500 pmol), scrambled siRNA (500 pmol), or left untransfected. Forty-eight hours later, cells were stained with the UN1 or CD43 (MEM-59) antibodies, and analyzed by flow cytometry. Histograms show the expression profile of UN1-positive (left panel) and CD43-positive (right panel) cells. One representative experiment out of three is shown.
The Monosaccharide N-Acetylgalactosamine O-linked to CD43 is an Essential Component of the UN1 Epitope
The UN1 antigen was initially characterized as a heavily sialylated and O-glycosylated protein with binding affinity for the PNA lectin, a ligand of the core 1 disaccharide Galβ1,3GalNAc (6). To determine whether glycan branches are critical for recognition of the UN1 epitope of CD43, HPB-ALL cell extract was sequentially digested with glycosidases that removed distinct glycan branches (Fig. 8A), and analyzed by Western blotting for the expression level of the UN1 antigen. The CD43 (C-20) antibody recognizing an epitope at the C terminus of CD43 was included to monitor the CD43 expression levels independently of glycosylation. Neuraminidase digestion reduced the SDS-PAGE mobility of both CD43 and UN1 antigen in a similar manner, as expected for the loss of negatively charged sialic acid residues (6, 15) (Fig. 8B, compare lanes 1 and 2). Accordingly with previous observation (6), the neuraminidase treatment did not affect the UN1 detection, confirming that the UN1 epitope is sialic acid-independent. Sequential digestion with β1,4-galactosidase and β-N-acetylglucosaminidase slightly increased the SDS-PAGE mobility of UN1 and CD43 in a similar manner without affecting the recognition of the UN1 epitope (Fig. 8B, lanes 3 and 4), indicating that the Galβ1,4GlcNAcβ1,6 disaccharide of the core 2 O-glycans was present in UN1-type CD43 molecules but was not a component of the UN1 epitope. Further digestion with β1,3-galactosidase, which removes the galactose of the (Galβ1,3GalNAc) core 1 structure, did not significantly affect the SDS-PAGE mobility of UN1 and CD43 molecules, and the UN1 recognition (Fig. 8B, lane 5). Differently, the digestion with endo-α-N-acetylgalactosaminidase, which removes the Galβ1,3GalNAc disaccharide, reduced the size of CD43 to the molecular weight of about 50 kDa, which is the expected size of unglycosylated CD43, and determined the loss of UN1 detection (Fig. 8B, lane 6). Altogether these results indicated that the monosaccharide N-acetylgalactosamine (GalNAc) O-linked to CD43 was required for detection of the UN1 epitope. However, the screening of 406-glycan array, including GalNAc, failed to identify ligands of the UN1 mAb (supplemental Fig. S2), suggesting that the monosaccharide GalNAc must be O-linked to the CD43 peptide core in order to generate the UN1 epitope.
Fig. 8.
Removal of single monosaccharide GalNAc-O-Ser/Thr causes the loss of UN1 detection. A, Scheme of the glycosidases used to remove specific glycan branches of the UN1 antigen. B, Total extract (100 μg) of HPB-ALL cells was left untreated (lane 1), digested with neuraminidase alone (lane 2), or neuraminidase followed by sequential digestion with β 1,4-galactosidase (lane 3), β-N-acetylglucosaminidase (lane 4), and β1,3-galactosidase (lane 5) or endo-α-N-acetylgalactosaminidase (lane 6). Digest aliquots (50 μg) were separated on 8% SDS-PAGE and analyzed by Western blotting with UN1, CD43 (C-20), or anti-γ-Tubulin antibodies. One representative experiment out of four is shown.
UN1-type CD43 Glycoforms are Expressed in Cancer Tissues
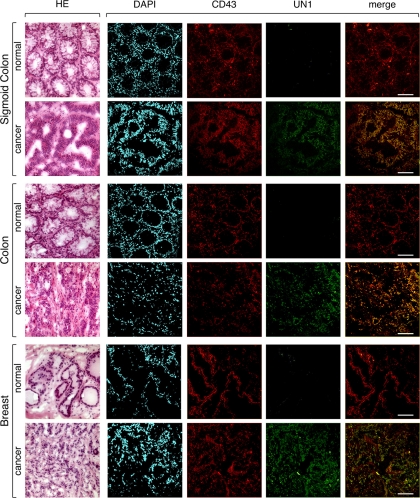
The UN1 antigen was mostly expressed in leukemic T-cell lines and solid tumor tissues (7, 8). Thus, we wonder whether the expression pattern of CD43 and UN1 antigen was correlated in human tissues. To this end, serial sections of normal and cancer tissues were collected from patients, including colon, sigmoid colon, and breast carcinomas. By optical microscopy, normal mucosal samples of colon and sigmoid colon showed normal maturation and architecture of glands with small mature lymphocytes in the stroma; in contrast, tumor samples showed poorly differentiated carcinoma in colon and well-differentiated carcinoma in sigmoid colon, respectively (Fig. 9). Normal breast samples showed lobules and acinar structures immersed in fibrous stroma, whereas the neoplastic counterparts showed poorly differentiated ductal carcinoma with lymphomonocitary infiltrate in the stroma (Fig. 9). By confocal microscopy, CD43 was detected at similar levels in normal and tumor tissues of colon, sigmoid colon and breast using the CD43 (C-20) antibody recognizing a glycosylation-independent epitope of CD43; differently, UN1 was exclusively detected in cancer tissues, where it colocalized with CD43 (Fig. 9). Occasionally, a slight UN1-positive staining was observed at the level of infiltrating lymphocytes in normal tissues, which agreed with the previous observation of UN1 expression in mature peripheral blood T lymphocytes (5, 6). These results demonstrated that the UN1-type CD43 glycoforms were associated with the tumor phenotype.
Fig. 9.
UN1-type CD43 glycoforms are expressed in cancer tissues. Serial cryosections of surgical specimens were derived from normal and cancer tissues of sigmoid colon, colon, and breast. For optical microscopy, sections were stained with hematoxylin and eosin (HE). For confocal microscopy, sections were stained with DAPI (blue), or the immunofluorescent antibodies CD43 (C-20) (red) and UN1 (green). Image superposition of UN1 and CD43 (merge) indicates colocalization by yellow color. Bars, 47.61 μm.
DISCUSSION
The UN1 mAb detected the UN1 antigen as a membrane-associated and highly sialylated glycoprotein expressed in fetal tissues and several cancer tissues (5, 7, 8). In this study, we have demonstrated that the UN1 antigen is CD43, a trans-membrane mucin-like glycoprotein (13). The primary structure of the UN1 antigen was identified by mass spectrometry of purified UN1 from HPB-ALL cell membranes. In fact, mass spectrometry detected two tryptic peptides of the UN1 antigen that matched the amino acidic sequence of CD43 within its intracellular domain. Significant signals for other peptides were not retrieved likely because of the heavy glycosylation of CD43.
The CD43 identity of UN1 antigen was demonstrated by the following evidence: (1) recognition of the membrane purified UN1 with anti-CD43 antibodies; (2) recognition of transfected CD43-FLAG by the UN1 mAb; (3) lack of UN1 detection in T cells deleted of the CD43 gene, or silenced for CD43 by RNA interference. Altogether these results indicated that CD43 and UN1 were the same protein as they were immunologically related and transcribed from the same gene.
CD43 is a highly conserved mucin-type glycoprotein, which is expressed on the surface of thymocytes and hematopoietic cells with the exception of erythrocytes and resting B cells (16); similar cellular distribution was reported for the UN1 antigen (5, 6). CD43 contains 400 amino acids and showed heterogeneity in SDS-PAGE migration with the apparent molecular weight of 115–130 kDa because of extensive O-glycosylation with one site of N-linked glycosylation (14, 17). Indeed, CD43 can harbor about 80 O-linked glycan chains with a high content in sialic acid conferring negative charge on cell surface (14, 18); desialylation reduces the CD43 migration on SDS-PAGE because of loss of acidic charges (15). Consistently with CD43 identity, the UN1 glycoprotein shows heterogeneous migration on SDS-PAGE in the same range of CD43, and slower migration following desialylation (6, 8) (Fig. 8).
Glycosylation of CD43 generates different glycoforms depending on cell-type and cellular activation. In resting human T cells, the major CD43 glycoform migrates on SDS-PAGE with the apparent molecular weight of 115 kDa and mainly harbors the O-linked tetrasaccharide side chain NeuNAcα 2,3 Galβ1,3 (NeuNAcα2,6) GalNAcα1-Ser/Thr (core 1 glycan structure). In activated human T cells, the major CD43 glycoform has an SDS-PAGE mobility of the apparent molecular weight of 130 kDa and harbors the hexasaccharide side chain NeuNAcα 2,3 Galβ1,3 (NeuNAcα2,3 Galβ1,4 GlcNAcβ1,6) GalNAcα1-Ser/Thr (core 2 glycan structure) (16). Conversion from unbranched core 1 to branched core 2 structure is catalyzed by core 2 β1,6N-acetylglucosaminyltransferase, and is promoted in T-cell activation (19–21).
CD43 glycoforms generate different epitopes that are recognized by distinct monoclonal antibodies (17). By glycosidase digestion, we have shown that the UN1 mAb recognizes a CD43 epitope that includes the monosaccharide GalNAc-O-linked to the peptide core. These findings agree with our previous observation of specific binding of UN1 to the PNA lectin, which is a selective binder of core 1 structure Galβ1,3GalNAc disaccharide of O-glycans (6). In addition, we have now demonstrated that the removal of the β-d-galactose from the core 1 Galβ1,3GalNAc disaccharide does not affect the recognition of UN1 epitope, indicating that the monosaccharide GalNAc-O-linked to the peptide core, corresponding to the Tn antigen of O-glycans, is the minimal carbohydrate component of the UN1 epitope. The observation that the single monosaccharide GalNAc is undetected by the UN1 mAb in a glycan array supports the possibility that GalNAc-O-linked to CD43 peptide core generates the UN1 conformation. Whether GalNAc-O-Ser/Thr is the contact binding for the UN1 mAb or rather it modifies linear epitopes of CD43 has yet to be characterized.
The extracellular domain of CD43 binds molecules involved in cell adhesion, such as intercellular adhesion molecule (ICAM)-1 (22) and E-selectin (23), whereas the intracytoplasmic tail of CD43 interacts with cytoskeleton linker proteins, Src kinases and zeta-chain of CD3 upon CD43 activation (24). Biochemical features and physical interactions are strictly related to the function of CD43 in cell signaling. Remodelling of sugar chains by cell-type specific glycosyltransferases, or shedding by proteases releasing partial protein domains, modulate the CD43 activity in cell differentiation, adhesion, and survival (14, 19, 20, 25–27).
Aberrant expression of certain glycan structures as well as occurrence of truncated structures, precursors, or novel structures of glycans may affect ligand-receptor interactions and thus interfere with regulation of cell adhesion, migration and proliferation (1). Indeed, altered expression or distinct glycoforms of CD43 have been associated with neoplastic transformation (28–30). Consistently with these observations, we show that UN1-type glycosylated CD43 molecules are specifically expressed in colon, sigmoid colon and breast carcinomas, while undetected in normal tissues from the same individuals (Fig. 9). Addressing the role of CD43 glycosylation in cell signaling and cancer development has proved to be a difficult task as a consequence of the in vivo heterogenicity of CD43 glycoforms. The evidence that UN1-type glycoforms of CD43 are specifically expressed in cancer tissues suggests that these molecules may promote cell proliferation and invasion by aberrant signaling. Consistently with this hypothesis, CD43 signaling leads to the activation of NF-κB and AP1 (31), which are pro-survival transcription factors that promote a cancer phenotype when deregulated. Aberrant O-glycosylation of CD43 in cancer cells could modulate the binding to alternative ligands, and may result in altered CD43 signaling that promotes cancer progression. In support of this possibility, the increased expression of the UN1 antigen, here identified as distinct CD43 glycoforms, correlated with breast cancer progression (8). The evidence that GalNAc-O-Ser/Thr, the initial monosaccharide O-linked to the CD43 peptide core, is a key component of the tumor-associated UN1 epitope of CD43 suggests that either overexpression of the truncated Tn antigen O-glycan structure, or site-specific O-glycosylation of CD43 generating the glycan-peptide UN1 epitope, may deregulate CD43 signaling and contribute to cancer progression. Accordingly with this hypothesis, the Tn antigen was detected in 90% of human carcinomas with high metastatic potential and poor diagnosis (32), and behaved as carbohydrate biomarker of cancer (33–38). In this context, the UN1 mAb detects cancer-associated CD43 molecules bearing the Tn determinant, and may function as an additional tool for cancer immunophenotyping and analysis of CD43 glycosylation in cancer.
Acknowledgments
This study is dedicated to the memory of Prof. Dr. Salvatore Venuta, Chancellor of University “Magna Graecia” of Catanzaro.
Footnotes
* This work was supported by grants from FIRB-MIUR, COFIN-MIUR, AIRC, and Istituto Superiore di Sanità, Rome. The glycan-array analysis was performed by the Protein-Glycan Interaction Core (core H) of The Consortium for Functional Glycomics supported by NIH GM62116.
 This article contains supplemental Figs. S1 and S2.
This article contains supplemental Figs. S1 and S2.
1 The abbreviations used are:
- mAb
- monoclonal antibody
- GalNAc
- N-acetyl-galactosamine
- GalNAc-transferase
- UDP-N-acetyl-D galactosamine: polipeptide N-acetylgalactosaminyltransferase
- MAL II
- Maackia Amurensis lectin
- PNA
- Peanut Agglutinin
- DEAE
- diethylaminoethyl
- SPE
- reversed-phase solid phase extraction
- SCX
- strong-cation exchange
- nanoLC
- nanoscale liquid chromatography
- MS/MS
- tandem mass spectrometry.
REFERENCES
- 1. Ohtsubo K., Marth J. D. (2006) Glycosylation in cellular mechanisms of health and disease. Cell 126, 855–867 [DOI] [PubMed] [Google Scholar]
- 2. Wopereis S., Lefeber D. J., Morava E., Wevers R. A. (2006) Mechanisms in protein O-glycan biosynthesis and clinical and molecular aspects of protein O-glycan biosynthesis defects: a review. Clin. Chem. 52, 574–600 [DOI] [PubMed] [Google Scholar]
- 3. Hakomori S. (2002) Glycosylation defining cancer malignancy: new wine in an old bottle. Proc. Natl. Acad. Sci. U.S.A. 99, 10231–10233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brockhausen I. (2006) Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep. 7, 599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tassone P., Bond H., Bonelli P., Tuccillo F., Valerio G., Petrella A., Lamberti A., Cecco L., Turco M. C., Cerra M., et al. (1994) UN1, a murine monoclonal antibody recognizing a novel human thymic antigen. Tissue Antigens 44, 73–82 [DOI] [PubMed] [Google Scholar]
- 6. Cecco L., Bond H. M., Bonelli P., Tuccillo F., Cerra M., Tassone P., Sorice R., Lamberti A., Morrone G., Venuta S. (1998) Purification and characterization of a human sialoglycoprotein antigen expressed in immature thymocytes and fetal tissues. Tissue Antigens 51, 528–535 [DOI] [PubMed] [Google Scholar]
- 7. Tassone P., Tuccillo F., Bonelli P., D'Armiento F. P., Bond H. M., Palmieri C., Tagliaferri P., Venuta S. (2002) Fetal ontogeny and tumor expression of the early thymic antigen UN1. Int. J. Oncol. 20, 707–711 [PubMed] [Google Scholar]
- 8. Tassone P., Bonelli P., Tuccillo F., Bond H. M., D'Armiento F. P., Galea E., Palmieri C., Tagliaferri P., Natali P. G., Venuta S. (2002) Differential expression of UN1, early thymocyte-associated sialoglycoprotein, in breast normal tissue, benign disease and carcinomas. Anticancer Res. 22, 2333–2340 [PubMed] [Google Scholar]
- 9. Mallardo M., Dragonetti E., Baldassarre F., Ambrosino C., Scala G., Quinto I. (1996) An NF-kappaB site in the 5′-untranslated leader region of the human immunodeficiency virus type 1 enhances the viral expression in response to NF-kappaB-activating stimuli. J. Biol. Chem. 271, 20820–20827 [DOI] [PubMed] [Google Scholar]
- 10. de Laurentiis A., Caterino M., Orrù S., Ruoppolo M., Tuccillo F., Masullo M., Quinto I., Scala G., Pucci P., Palmieri C., Tassone P., Salvatore F., Venuta S. (2006) Partial purification and MALDI-TOF MS analysis of UN1, a tumor antigen membrane glycoprotein. Int. J. Biol. Macromol. 39, 122–126 [DOI] [PubMed] [Google Scholar]
- 11. Gaspari M., Abbonante V., Cuda G. (2007) Gel-free sample preparation for the nanoscale LC-MS/MS analysis and identification of low-nanogram protein samples. J. Sep. Sci. 30, 2210–2216 [DOI] [PubMed] [Google Scholar]
- 12. Blixt O., Head S., Mondala T., Scanlan C., Huflejt M. E., Alvarez R., Bryan M. C., Fazio F., Calarese D., Stevens J., Razi N., Stevens D. J., Skehel J. J., van Die I., Burton D. R., Wilson I. A., Cummings R., Bovin N., Wong C. H., Paulson J. C. (2004) Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc. Natl. Acad. Sci. U.S.A. 101, 17033–17038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carlsson S. R., Fukuda M. (1986) Isolation and characterization of leukosialin, a major sialoglycoprotein on human leukocytes. J. Biol. Chem. 261, 12779–12786 [PubMed] [Google Scholar]
- 14. Fukuda M., Tsuboi S. (1999) Mucin-type O-glycans and leukosialin. Biochim. Biophys. Acta 1455, 205–217 [DOI] [PubMed] [Google Scholar]
- 15. Remold-O'Donnell E., Kenney D. M., Parkman R., Cairns L., Savage B., Rosen F. S. (1984) Characterization of a human lymphocyte surface sialoglycoprotein that is defective in Wiskott-Aldrich syndrome. J. Exp. Med. 159, 1705–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fukuda M. (1991) Leukosialin, a major O-glycan-containing sialoglycoprotein defining leukocyte differentiation and malignancy. Glycobiology 1, 347–356 [DOI] [PubMed] [Google Scholar]
- 17. Cyster J. G., Shotton D. M., Williams A. F. (1991) The dimensions of the T lymphocyte glycoprotein leukosialin and identification of linear protein epitopes that can be modified by glycosylation. EMBO J. 10, 893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Dyken S. J., Green R. S., Marth J. D. (2007) Structural and mechanistic features of protein O-glycosylation linked to CD8+ T-cell apoptosis. Mol. Cell. Biol. 27, 1096–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Piller F., Le Deist F., Weinberg K. I., Parkman R., Fukuda M. (1991) Altered O-glycan synthesis in lymphocytes from patients with Wiskott-Aldrich syndrome. J. Exp. Med. 173, 1501–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higgins E. A., Siminovitch K. A., Zhuang D. L., Brockhausen I., Dennis J. W. (1991) Aberrant O-linked oligosaccharide biosynthesis in lymphocytes and platelets from patients with the Wiskott-Aldrich syndrome. J. Biol. Chem. 266, 6280–6290 [PubMed] [Google Scholar]
- 21. Bierhuizen M. F., Maemura K., Fukuda M. (1994) Expression of a differentiation antigen and poly-N-acetyllactosaminyl O-glycans directed by a cloned core 2 beta-1,6-N-acetylglucosaminyltransferase. J. Biol. Chem. 269, 4473–4479 [PubMed] [Google Scholar]
- 22. Rosenstein Y., Park J. K., Hahn W. C., Rosen F. S., Bierer B. E., Burakoff S. J. (1991) CD43, a molecule defective in Wiskott-Aldrich syndrome, binds ICAM-1. Nature 354, 233–235 [DOI] [PubMed] [Google Scholar]
- 23. Matsumoto M., Atarashi K., Umemoto E., Furukawa Y., Shigeta A., Miyasaka M., Hirata T. (2005) CD43 functions as a ligand for E-Selectin on activated T cells. J. Immunol. 175, 8042–8050 [DOI] [PubMed] [Google Scholar]
- 24. Pedraza-Alva G., Mérida L. B., Burakoff S. J., Rosenstein Y. (1998) T cell activation through the CD43 molecule leads to Vav tyrosine phosphorylation and mitogen-activated protein kinase pathway activation. J. Biol. Chem. 273, 14218–14224 [DOI] [PubMed] [Google Scholar]
- 25. Mambole A., Baruch D., Nusbaum P., Bigot S., Suzuki M., Lesavre P., Fukuda M., Halbwachs-Mecarelli L. (2008) The cleavage of neutrophil leukosialin (CD43) by cathepsin G releases its extracellular domain and triggers its intramembrane proteolysis by presenilin/gamma-secretase. J. Biol. Chem. 283, 23627–23635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seo W., Ziltener H. J. (2009) CD43 processing and nuclear translocation of CD43 cytoplasmic tail are required for cell homeostasis. Blood 114, 3567–3577 [DOI] [PubMed] [Google Scholar]
- 27. Hernandez J. D., Nguyen J. T., He J., Wang W., Ardman B., Green J. M., Fukuda M., Baum L. G. (2006) Galectin-1 binds different CD43 glycoforms to cluster CD43 and regulate T cell death. J. Immunol. 177, 5328–5336 [DOI] [PubMed] [Google Scholar]
- 28. Santamaría M., López-Beltrán A., Toro M., Peña J., Molina I. J. (1996) Specific monoclonal antibodies against leukocyte-restricted cell surface molecule CD43 react with nonhematopoietic tumor cells. Cancer Res. 56, 3526–3529 [PubMed] [Google Scholar]
- 29. Sikut R., Nilsson O., Baeckström D., Hansson G. C. (1997) Colon adenoma and cancer cells aberrantly express the leukocyte-associated sialoglycoprotein CD43. Biochem. Biophys. Res. Commun. 238, 612–616 [DOI] [PubMed] [Google Scholar]
- 30. Sikut R., Andersson C. X., Sikut A., Fernandez-Rodriguez J., Karlsson N. G., Hansson G. C. (1999) Detection of CD43 (leukosialin) in colon adenoma and adenocarcinoma by novel monoclonal antibodies against its intracellular domain. Int. J. Cancer 82, 52–58 [DOI] [PubMed] [Google Scholar]
- 31. Santana M. A., Pedraza-Alva G., Olivares-Zavaleta N., Madrid-Marina V., Horejsi V., Burakoff S. J., Rosenstein Y. (2000) CD43-mediated signals induce DNA binding activity of AP-1, NF-AT, and NFkappa B transcription factors in human T lymphocytes. J. Biol. Chem. 275, 31460–31468 [DOI] [PubMed] [Google Scholar]
- 32. Springer G. F. (1997) Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J. Mol. Med. 75, 594–602 [DOI] [PubMed] [Google Scholar]
- 33. Terasawa K., Furumoto H., Kamada M., Aono T. (1996) Expression of Tn and sialyl-Tn antigens in the neoplastic transformation of uterine cervical epithelial cells. Cancer Res. 56, 2229–2232 [PubMed] [Google Scholar]
- 34. Konno A., Hoshino Y., Terashima S., Motoki R., Kawaguchi T. (2002) Carbohydrate expression profile of colorectal cancer cells is relevant to metastatic pattern and prognosis. Clin. Exp. Metastasis 19, 61–70 [DOI] [PubMed] [Google Scholar]
- 35. Kumar S. R., Sauter E. R., Quinn T. P., Deutscher S. L. (2005) Thomsen-Friedenreich and Tn antigens in nipple fluid: carbohydrate biomarkers for breast cancer detection. Clin. Cancer Res. 11, 6868–6871 [DOI] [PubMed] [Google Scholar]
- 36. Kawaguchi T., Takazawa H., Imai S., Morimoto J., Watanabe T., Kanno M., Igarashi S. (2006) Expression of Vicia villosa agglutinin (VVA)-binding glycoprotein in primary breast cancer cells in relation to lymphatic metastasis: is atypical MUC1 bearing Tn antigen a receptor of VVA? Breast Cancer Res. Treat. 98, 31–43 [DOI] [PubMed] [Google Scholar]
- 37. Pinho S., Marcos N. T., Ferreira B., Carvalho A. S., Oliveira M. J., Santos-Silva F., Harduin-Lepers A., Reis C. A. (2007) Biological significance of cancer-associated sialyl-Tn antigen: modulation of malignant phenotype in gastric carcinoma cells. Cancer Lett. 249, 157–170 [DOI] [PubMed] [Google Scholar]
- 38. Li Q., Anver M. R., Butcher D. O., Gildersleeve J. C. (2009) Resolving conflicting data on expression of the Tn antigen and implications for clinical trials with cancer vaccines. Mol. Cancer Ther. 8, 971–979 [DOI] [PMC free article] [PubMed] [Google Scholar]