Abstract
Oxidative stress has been implicated in aging and many human diseases, notably neurodegenerative disorders and various cancers. The reactive oxygen species that are generated by aerobic metabolism and environmental stressors can chemically modify proteins and alter their biological functions. Cells possess protein repair pathways to rescue oxidized proteins and restore their functions. If these repair processes fail, oxidized proteins may become cytotoxic. Cell homeostasis and viability are therefore dependent on the removal of oxidatively damaged proteins. Numerous studies have demonstrated that the proteasome plays a pivotal role in the selective recognition and degradation of oxidized proteins. Despite extensive research, oxidative stress-triggered regulation of proteasome complexes remains poorly defined. Better understanding of molecular mechanisms underlying proteasome function in response to oxidative stress will provide a basis for developing new strategies aimed at improving cell viability and recovery as well as attenuating oxidation-induced cytotoxicity associated with aging and disease. Here we highlight recent advances in the understanding of proteasome structure and function during oxidative stress and describe how cells cope with oxidative stress through proteasome-dependent degradation pathways.
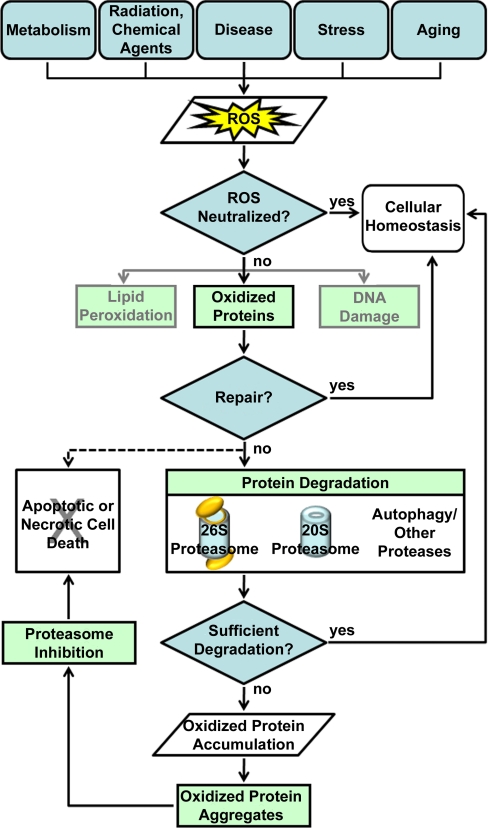
Reactive oxygen species (ROS)1 are routinely produced as a byproduct of aerobic metabolism and oxidative phosphorylation (1–4). Exposure to various environmental stressors (e.g. ionizing and nonionizing radiation, or certain chemical agents) can also result in the production of ROS (5–8). In addition, ROS production and accumulation can be generated during disease pathogenesis (e.g. Abeta-mediated production of ROS in Alzheimer's disease (9)), or even the natural aging process (10, 11) (Fig. 1). Unneutralized ROS cause oxidative damage to lipids, proteins, and DNA, thus leading to aberrant molecular activities (12–14). Protein oxidation is particularly detrimental as the resulting conformational changes to protein structures can render damaged proteins inactive or lead to functional abnormalities.
Fig. 1.
Cellular Response to Oxidative Stress. Shown here is a flow chart detailing the production of reactive oxygen species (ROS) and the subsequent cellular response resulting in either the return to normal cellular homeostasis or apoptotic/necrotic cell death.
To maintain cell viability and normal homeostasis, aerobic organisms have evolved several defense mechanisms for reducing the deleterious effects of oxidative stress, including the production of antioxidants (e.g. glutathione, vitamins A, C, and E, and flavenoids) and enzymatic scavengers of ROS (e.g. superoxide dismutases (SOD), catalase, and glutathione peroxide). Cells also possess oxidation-reduction (redox)-dependent protein repair pathways, which are triggered by oxidation of redox proteins (15, 16). Redox signaling pathways activate kinase cascades and gene transcription aimed at rescuing oxidized proteins and restoring their functions (15–18). If cellular defense and repair processes fail, oxidatively damaged proteins can undergo direct chemical fragmentation, or form large aggregates (19, 20). Although the pathogenicity of protein aggregates remains uncertain (21), it is known that unrestricted accumulation of damaged proteins can disrupt important cellular processes, including proteasome-mediated protein degradation (22). Therefore, timely removal of oxidatively damaged proteins is of critical importance to maintain normal cellular homeostasis and viability. Although there is evidence suggesting that chaperone mediated autophagy is activated during oxidative stress response (23), the proteasome represents the major proteolytic machinery for the removal of oxidized and misfolded proteins (19, 24–27). If homeostasis is not restored, cells ultimately undergo apoptotic or necrotic cell death (28, 29).
Oxidative stress has been implicated in aging and many human diseases including Alzheimer's disease, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis (ALS), cataract formation, and human cancers (30–36). In particular, pathological developments in neurodegenerative diseases have been strongly linked to oxidation triggered protein aggregation partly because of elevated ROS levels in the brain (37–39). To prevent cytotoxicity induced by oxidized proteins, normal proteasome-dependent degradation is essential for cells to cope with oxidative stress (25, 40, 41). Proteasomal dysfunction can lead to decreased degradation of misfolded proteins, thus resulting in accumulation of oxidized proteins and subsequent protein aggregation. Protein aggregates can then feedback to further inhibit proteasome activities, generate additional cellular stress, and lead to cytotoxicity and human pathologies. Such phenomena have been implicated in many oxidative stress-associated disorders (42, 43).
Despite the proteasome's critical role in oxidative stress response, our current understanding of how proteolysis of oxidized proteins is regulated and how oxidative stress modulates proteasome structure and function remains limited. Further understanding of how proteasome-dependent degradation pathways are regulated in response to oxidative stress may provide a molecular basis for developing new strategies for curbing oxidative stress and preventing the formation of intracellular protein aggregates during aging and disease. Although other types of cellular stress, such as ubiquitin stress and metal stress, share overlapping components and response pathways as those involved with oxidative stress, the differing overall responses and distinct requirements for signaling and survival indicate these types of stress are not functionally synonymous with oxidative stress (44–47), and are beyond the scope of this review. This review focuses on the recent developments in our understanding of proteasomal regulation during oxidative stress.
Proteasomes and Oxidative Stress
The 26S proteasome is a multicatalytic protease responsible for ubiquitin/ATP dependent protein degradation (48–50). This macromolecular protein complex is composed of the 20S core particle (CP), capped by a 19S regulatory particle (RP, also known as CAP or PA700) on one or both sides (51, 52). The eukaryotic 20S CP is composed of two copies each of 14 subunits, 7α and 7β, which form a conserved barrel-shaped structure with four stacked seven-member rings in the order of αββα (48, 53). Three of the β subunits (i.e. β1(Y), β2(Z), and β5(X)) are catalytically active and are responsible for the various proteolytic activities of the proteasome (e.g. chymotrypsin-like, trypsin-like, and caspase-like activities) (54). Upon Interferon-γ induction, mammalian 20S proteasomes can incorporate three alternative catalytic β subunits, β1i (LMP2), β2i (MECL), and β5i (LMP7), to constitute immunoproteasomes that are best known for generating immunopeptides for MHC class I antigen presentation (55, 56). Although α subunits are catalytically inactive, they are critical for gating the opening of the 20S core particle and for forming associations with regulatory complexes (49, 53).
The 19S regulatory complex is composed of at least 19 different subunits, which are arranged into two subcomplexes, the base and the lid (57, 58). The base complex contains six ATPases (Rpt1–6) plus four non-ATPase subunits (Rpn1, Rpn2, Rpn10, and Rpn13) and directly associates with the 20S core. The lid is found distal to the base and contains nine non-ATPase subunits (Rpn3, Rpn5–9, Rpn11–12, and Rpn15). The 19S particle carries several biochemical functions including recognition of polyubiquitinated substrates, cleavage of polyubiquitin chains to recycle ubiquitin, unfolding of substrates, assisting in opening the 20S core, and subsequent translocation of unfolded substrates into the catalytic chamber (49, 59–61). The activities of the 19S regulatory complex and its assembly with the 20S proteasome have been shown to be strictly ATP-dependent.
In addition to association with 19S regulatory particles, 20S proteasome can bind to alternative activator proteins. Three mammalian 20S activators have been identified to date: PA28αβ, PA28γ, and PA200 (Blm10 in yeast) (58, 62–65). These proteasome activators modulate 20S proteasome structure and generate “active” 20S proteasomes by opening the α ring channel, thereby facilitating the entry of protein substrates for degradation. Because these alternative regulatory proteins lack deubiquitinases and ATPase activity, they promote protein degradation in an ubiquitin/ATP-independent manner.
Although the degradation of oxidatively damaged proteins can occur by both ubiquitin/ATP-dependent (i.e. 26S-dependent) and ubiquitin/ATP-independent (i.e. 20S-dependent) mechanisms (25, 66), various studies have implied that 20S proteasomes may be more critical for the removal of damaged proteins (19, 24, 26, 67). This may be in part because of the fact that the 20S proteasome is more resistant to oxidative stress than the 26S proteasome as the 20S complex can maintain activity even upon treatment with moderate to high concentrations of H2O2, whereas the 26S proteasome is much more vulnerable (68, 69). Recently, it has been shown that 20S proteasomes can degrade oxidized proteins (e.g. histones, hemoglobin, superoxide dismutase) in vitro, independent of ubiquitin/ATP (19, 24, 26, 70, 71). This phenomenon has been attributed to 20S proteasome recognition of, and interaction with, abnormally exposed hydrophobic patches in oxidatively damaged and unfolded proteins that induce conformational changes in the 20S structure and promote channel opening followed by protein degradation (19, 24, 26). It remains unclear, however, if degradation of oxidatively damaged proteins by the 20S proteasome can occur in vivo in a similar manner as was shown in vitro.
The combination of associating regulatory complexes, post-translational modifications, proteasome interacting proteins (PIPs), and subunit composition define the structure and activity of a given proteasome entity (50, 58, 72–79). The diverse range of regulatory and activating complexes that modulate 20S core activity supports the idea that the proteasome is a highly dynamic protein complex, capable of adjusting its proteolytic activity depending on the needs of the cell. Accordingly, protein-protein interactions, post-translational modifications, and proteasome gene regulation represent additional levels of regulation for fine tuning the collective proteasome activity upon oxidative stress.
Regulation of the Proteasome by Interacting Proteins
Protein-protein interaction is one of the major mechanisms regulating protein functions. Therefore, characterizing PIPs is important for understanding the regulation of proteasome function. Various approaches have been developed to capture and identify PIPs using genetic and biochemical techniques. Among them, mass spectrometry coupled with affinity purification has evolved as an attractive and powerful tool (80, 81), which has led to the discovery of hundreds of PIPs (46, 75, 76, 82–91). In addition to the proteins that form the proteasome holocomplex, a broad class of PIPs have been identified, including ubiquitin receptors, ubiquitin ligases, deubiquitinases, proteasome activators and inhibitors, chaperones, and other types of modulators (46, 50, 58, 74–76, 82–94). These proteins associate with proteasomes dynamically in response to environmental changes and affect the function and structure of proteasome complexes.
Ecm29-dependent Disassembly of the 26S Proteasome
We recently employed biochemical and quantitative mass spectrometry-based proteomic approaches to monitor the structural dynamics of the 26S proteasome in yeast and mammalian cells in an effort to understand the molecular mechanisms underlying the regulation of 26S proteasomes upon H2O2-induced oxidative stress (77). In this study, we determined that acute H2O2 stress disrupts the integrity of the 26S proteasome complex and causes the dissociation of the 20S core from the 19S particle in a dose-dependent manner. We also detected H2O2-induced loss of 26S proteasome proteolytic activities, likely because of the observed separation of the 19S particle from the 20S core. Additionally, we characterized the dynamic changes of PIPs using stable isotope labeling with amino acid in cell culture (SILAC)-based quantitative mass spectrometry, and identified that one of the yeast PIPs, Ecm29, is substantially recruited to the 19S particle in response to H2O2 stress. Biochemical and genetic experiments revealed that the H2O2 stress-induced attenuation of yeast 26S proteasome activity is because of Ecm29-dependent disassembly of the 26S proteasome complex, indicating that Ecm29 is a key regulator of 26S proteasome structure in response to H2O2 stress. Ecm29-dependent proteasome dissociation has proven important for cell survival, particularly for recovery following oxidative stress. This phenomenon is independent of yeast activator protein 1 (Yap1), a transcription factor critical for oxidative stress response in yeast, and therefore functions as a parallel defense pathway against H2O2-induced stress. In addition to the previously established Ecm29 functions (83, 95, 96), our results describe a role for Ecm29 in the response to oxidative stress in yeast, suggesting that Ecm29 may have multiple functionalities in controlling 26S proteasome structure.
H2O2 stress-induced disassembly of the 26S proteasome was observed in both yeast and mammalian cells (77), suggesting that this is a conserved mechanism for regulating proteasome activities in an effort to cope with oxidative insults. Several studies have suggested that degradation of oxidized proteins is likely more dependent on 20S than 26S proteasomes (19, 24, 26, 67). Therefore, we suspect that disassembly of 26S proteasomes during oxidative stress serves to increase 20S proteasome abundance, allowing cells to more effectively clear irreparably damaged proteins and mitigate the cytotoxic effects of their accumulation (19, 71, 97). This notion is further supported by studies using mutants defective in 26S proteasome assembly (98), or activities (99), which demonstrated that mutant cells are more resistant to H2O2 exposure, and are able to degrade oxidized proteins more effectively than their wild-type controls. Despite its identification as a PIP in mammalian cells, mammalian Ecm29 appears to be functionally distinct from its yeast ortholog (100, 101). Extensive analyses by Gorbea et al. revealed that mammalian Ecm29 associates with various molecular motors and endosomal components, and serves as an adaptor protein, recruiting 26S proteasomes to specific cellular compartments such as flotillin-positive endosomes, endoplasmic reticulum (ER), and the centrosome (100, 101). In addition, studies in HeLa cells demonstrated that human 26S proteasomes remain assembled even following detergent-induced dissociation of Ecm29 (100). Furthermore, the levels and distribution of Ecm29 vary markedly among mouse organs, and can be absent in some tissues (100). These results indicate that Ecm29 is not necessary for the association of the 20S core and the 19S particle. From these studies, it is evident that Ecm29 has some distinct functions in higher eukaryotes that are not present in lower eukaryotic systems. This brings into question whether the reverse is also true. Consequently, the question of whether mammalian Ecm29 is involved in modulating the stability of 26S proteasome assembly in response to oxidative stress, like its yeast ortholog, remains unanswered, and the details regarding the regulator(s) responsible for the observed H2O2-triggered dissociation of the 20S core from the 19S particle in mammalian cells (77) are in need of further elucidation.
Usp14-dependent Modulation of Proteasomal Degradation
Human Usp14 is a proteasome-associated deubiquitinating enzyme that disassembles polyubiquitin chains from the end distal to the substrate, thus shortening chains rather than removing them together (84, 102, 103). Usp14 and its yeast ortholog, Ubp6, have been identified as potent inhibitors of proteasomal degradation of selected ubiquitinated substrates in vitro and in cells by two different modes of action (47, 84). The decreased degradation of some proteasome substrates is dependent on Usp14 deubiquitinase activity; whereas other substrates are stabilized by a mechanism that is independent of Usp14 deubiquitinase activity (47). Lee et al. has recently identified a selective small molecule (IU1) that inhibits the deubiquitinating activity of Usp14 (47). It has been shown that IU1 strongly reduces the accumulation of oxidized proteins by accelerating their degradation in cells exposed to oxidants (e.g. menadione, H2O2), thus promoting cell survival and enhancing cell resistance to proteotoxic stress. However, the IU1 inhibitor had little to no effect on ubiquitin-independent proteasomal degradation indicating that modulation of proteasomal degradation by Usp14 is mediated by changing the accessibility of ubiquitinated substrates for proteasomal degradation, rather than directly altering proteasome catalytic activity. This represents a very different mechanism from Ecm29-dependent regulation of the 26S proteasome in response to oxidative stress as discussed above (77). Together, these results demonstrate that regulation of proteasomal degradation is a very complex process and multiple mechanisms exist in cells that target various aspects of the degradation process in response to cytotoxic stress. Whether and how these regulatory steps work independently or together require further clarification.
Chaperone-mediated Proteasome Regulation
Given the association of chaperone proteins with unfolded and misfolded proteins, and the contribution of oxidative stress to protein misfolding, it is not surprising that chaperone PIPs contribute to proteasomal regulation in an effort to protect cells from oxidative damage (104–109). For example, it has been shown that neural cells overexpressing the human chaperone protein HDJ-1/Heat shock protein 40 (Hsp40) are more resistant to cytotoxicity associated with both oxidative stressors and general proteasome inhibitors. This suggests that heat shock proteins may confer resistance to oxidative stress by preserving proteasome function and attenuating the toxicity of proteasome inhibition (105). Similarly, Hsp90 and α-crystalline both associate with the proteasome and are important regulators of specific 20S proteasome activities when cells are submitted to oxidative challenge (106–108). Interestingly, under non-stressed conditions Hsp90 and α-crystalline inhibit 20S proteasome activity (108, 110, 111), but upon oxidative stress, these chaperones protect activated 20S proteasomes from oxidative inactivation (106–108). Hsp90 also appears to selectively promote the degradation of oxidized substrates by the 20S proteasome in vitro (112). Taken together, these results suggest that molecular chaperones may play a role in regulating proteasome activity in response to oxidative stress by both stabilizing specific proteolytic activities and by aiding the recognition and degradation of oxidized substrates. However, the molecular mechanisms by which chaperone proteins regulate proteasome activity in response to oxidative stress have yet to be determined.
Regulation of the Proteasome by Post-translational Modifications
Protein post-translational modifications can regulate protein functions by changing their structures and physiochemical properties (113, 114), including their biochemical activity, intracellular localization, turnover rate, and protein-protein interactions. Identification and characterization of protein post-translational modifications is therefore important for defining how proteins are regulated in various cellular environments. With the vast and rapid improvements in mass spectrometry-based proteomic approaches (81, 114, 115), various post-translational modifications of proteasome subunits have been reported, including phosphorylation, acetylation, oxidation, and myristoylation (86, 116–123). Most of these modifications were identified from large scale analyses at the proteome level or studies of purified proteasome complexes. Following the identification of proteasomal post-translational modifications, further analyses using genetic and/or biochemical approaches are required to determine the functional and biological significance of each modification. This review will focus on those post-translational modifications that have been linked to proteasome function associated with oxidative stress.
Oxidative Modifications
Oxidative modification refers to a process by which ROS attack proteins, leading to fragmentation of the polypeptide backbone, modification of amino acid side chains, and/or the generation of protein-protein cross-linkages. Side chain modifications include β-scission of alanine, valine, leucine, and aspartic acid, oxidation of methionine, and carbonylation (124). Intra- and interprotein cross linking can occur through a variety of mechanisms, including the formation of Schiff base cross-linkage (e.g. resulting from 4-hydroxy-2-nonenal (HNE) modification), and the formation disulfide bridges between oxidized and reduced thiol groups (124). Recent studies have shown that 19S and 20S proteasome subunits are susceptible to oxidative modifications, including carbonylation, HNE modification, and S-glutathionylation (27, 125–128). It has been shown that carbonylation of Rpt3 resulted in impaired Rpt3 ATPase activity and a subsequent decrease in ubiquitin/ATP-dependent proteolysis of the 26S proteasome (126). In addition, carbonylation or HNE modification of the 20S proteasome has been shown to suppress its proteolytic activities (125). These results suggest that oxidative modifications of proteasomes can contribute to the regulation of proteasome functions in response to oxidative stress.
S-glutathiolation is the covalent attachment of glutathione (GSH) to protein thiol groups. There are two mechanisms by which proteins can be S-glutathiolated: GSH can react with oxidized thiol groups (e.g. Cys-SOH or Cys-S-S-Cys), or oxidized glutathione (GSSG) can react with reduced thiol residues (e.g. Cys-SH) (129). GSH is considered to have antioxidant function, by stabilizing oxidized protein thiol groups, preventing further, possibly irreversible thiol oxidation through S-glutathiolation, but S-glutathiolaton is also known to regulate protein activity (130). Upon H2O2-induced oxidative stress in yeast, S-glutathiolation of 20S subunits was demonstrated both in vitro and in vivo (127). Further functional studies determined that treatment of purified 20S proteasomes with GSH lead to the inhibition of chymotrypsin-like and trypsin-like activities (127). In comparison, mammalian proteasomes appear to have a biphasic response to S-glutathiolation, as low concentrations of GSH or GSSG increased the chymotrypsin-like activity of purified mammalian proteasomes whereas high levels of GSH or GSSG led to decreased activity (128). Although S-glutathiolation of the 20S proteasome generally inhibits proteasome activity, the biphasic response observed for S-glutathiolation of mammalian proteasome may be evidence of proteasome S-glutathiolation acting as a redox signaling trigger through which proteasome activity is regulated depending on the redox status of mammalian cells.
ADP-Ribosylation
In addition to oxidative modifications, other types of modifications may be involved in altering proteasome activities during oxidative stress. Poly [ADP-ribose] polymerase 1 (PARP1), a nuclear enzyme that transfers ADP-ribose moieties from NAD+ to glutamic acid, aspartic acid, or lysine residues, is activated in response to oxidative stress (70, 131–133). Interestingly, evidence exists suggesting that nuclear 20S proteasomes can be ADP-ribosylated by PARP1 in human hematopoietic K562 cells, resulting in increased chymotrypsin-like activity of the nuclear 20S proteasome (70). Given the nuclear localization of PARP1 and its role in DNA repair (134), ADP-ribosylation is likely unique to nuclear proteasomes and may function to enhance proteasomal degradation of oxidized nuclear proteins (70, 135).
Phosphorylation
The proteasome is extensively and dynamically phosphorylated, though only a few phosphorylation events have been linked to the regulation of proteasome activity (136–139). One recent study revealed that Rpt5 (19S subunit) can be phosphorylated by human apoptosis signal-regulating kinase 1 (Ask1) (136). Although the specific Rpt5 functional sites have yet to be identified, phosphorylation did result in the inhibition of Rpt5 ATPase activity and in the reduction of 26S proteasome proteolytic activities (136). The impairment of the 26S proteasome activity is not because of changes in the 26S proteasome assembly. It is interesting to note that Ask1 is required for the H2O2 stress-induced inhibition of 26S proteasome activity in mouse fibroblasts and that Ask1 is activated by Thioredoxin in response to various stresses including oxidative stress (140–143). Therefore, Ask1-dependent proteasome phosphorylation may act as a regulatory mechanism of proteasome activities during various stress responses.
Apart from Ask1, additional kinases have been found to phosphorylate proteasome subunits including CK2 (formerly casein kinase II), cyclic AMP-dependent kinase (PKA), Ca2+/calmodulin-dependent kinase (CaM-K) II, AMP-activated protein kinase (AMPK), and c-Abl and abl-related gene (Arg) tyrosine kinases (137, 139, 144–148). Phosphorylation of proteasome subunits by these kinases appears to be involved in several proteasomal related functions and regulations including proteasome assembly (137, 144–146, 149), and proteolytic activities (139, 147). For example, CK2 phosphorylation of α7 is important for stabilizing the association of the 20S CP to the 19S RP (144, 145, 149). Although α7 phosphorylation, is not required for assembly of the 26S proteasome, it was reported that dephosphorylation of α7 following INFγ treatment correlated with decreased 26S proteasome stability. Several putative PKA target substrates have also been identified from murine cardiac and hepatic tissue (147). In this study it was shown that proteasomal peptidase activities were elevated following in vitro phosphorylation of the 20S CP, at multiple sites, by PKA (147). Another recent report demonstrated that CaMKII can directly phosphorylate Rpt6, and that constitutive activation of CaMKII results increased proteasome activity, whereas pharmalogical inhibition of CaMKII decreases the degradation of a GFP reporter protein in vivo, suggesting that Rpt6 phosphorylation may regulate proteasome activity (137). Proteasome activity can also be negatively regulated by phosphorylation, as Liu et al. conclusively demonstrated that c-Abl and Arg phosphorylation of α4 results in suppressed 20S and 26S proteasome proteolytic activities (139). Although proteasome phosphorylation by these kinases has not been directly linked to oxidative stress, activities of CK2, PKA, CaM-KII, c-Abl, and Arg have been shown to be modulated during oxidative stress (150–157). Given the biological significance of proteasome phosphorylation by these kinases, we speculate that these phosphorylation events may provide additional means of regulating proteasome activities upon oxidative insult.
Oxidative Stress-Mediated Proteasome Gene Regulation
Oxidative stress-mediated gene regulation is a known component of the defense mechanism for cellular responses to proteotoxic stress (158, 159). In yeast, much of the oxidative stress-driven transcriptional activation is controlled by the redox reactive transcription factor Yap1 (160). Rpn4, the transcriptional activator for proteasome genes, is a Yap1 targeted gene (161–164). Upon oxidative stress, transient Yap1-mediated Rpn4 mRNA up-regulation (163) and Yap1-dependent expression of several yeast proteasome components (165) have been observed, however the biological consequences of these changes were not evaluated. Nevertheless, overexpression of proteasome catalytic subunits β1 or β5 in mammalian cells increased proteasome catalytic activities that correlated with enhanced cell viability and reduced accumulation of oxidized proteins following oxidative stress (166). It has also been shown that overexpression of proteasome assembly protein UMP1 improves cell viability following exposure to various oxidants (167, 168). The increased resistance to oxidative stress by UMP1 overexpression may be because of increased levels of proteasome activity (167, 168) resulting from up-regulation of proteasome β-subunits (168). Together, these studies suggest that increased 20S expression and assembly would enhance a cell's capacity to cope with oxidative stress. Alternatively, disruption of Rpn4-mediated proteasome induction leads to reduced viability in response to oxidative stress (169), demonstrating the critical role of proteasome gene regulation for combating oxidative insult.
In higher eukaryotes, nuclear factor κB (NFκB) and activator protein-1 (AP-1; Yap1 homolog) are the most widely accepted transcriptional regulators of mammalian oxidative stress response, but they are not responsible for activation of proteasome gene transcription (170, 171). Instead, transcription factor 11 (TCF11; long isoform of Nrf1) and NF-E2-related factor 2 (Nrf2) have been shown to promote the expression of several proteasome genes (171, 172), and may act as functional orthologs of yeast Rpn4 (78, 173, 174). Information detailing how these transcription factors are regulated under stress is still unknown and needs to be further investigated. Although the transcriptional control of proteasome expression in the mammalian system appears to be more complex than the yeast system, up-regulation of proteasome expression has also been observed in mammalian cells as an adaptive cellular response to prolonged exposure of oxidative stress (67, 175).
In addition to standard proteasome subunits, mammalian systems, unlike their yeast counterparts, also contain IFN-γ inducible catalytic β subunits that are integral parts of immunoproteasomes. Recently it has been recognized that immunoproteasomes are up-regulated under ROS attack and also contribute to the removal of oxidized proteins in mammalian cells (67, 175–177). Interestingly, it has been suggested that immunoproteasomes are more resistant to oxidative stress than standard proteasomes (67). Cells and mice deficient for immunoproteasome subunits are more susceptible to oxidation-induced cell death because of reduced proteasome activity and accumulation of oxidized proteins (176, 177). It appears that increased immunoproteasome expression not only helps preserve proteasome function, but also makes cells more resistant to oxidative insult (67, 175–177). Whether the same class of transcription factors regulates expression of standard and inducible proteasomal subunits remains to be determined.
Proposed Model of Oxidative Stress-dependent Regulation of the 26S Proteasome
In order to effectively defend the cell against oxidative insults, cells must coordinate repair systems with proteasome-dependent degradation. Based on recent findings (19, 41, 67, 68, 71, 77, 97, 165, 175, 176, 178, 179), we propose a working model to illustrate how compositional and structural changes of proteasomes modulate their proteolytic activities in response to ROS attack (Fig. 2). In the absence of stress, the 26S proteasome represents the major cellular degradation machinery and carries out ATP-dependent degradation of ubiquitinated substrates. At the onset of oxidative stress, it has been suggested that activities of the 26S proteasome can be initially stimulated by unknown mechanisms for degrading mildly oxidized proteins, thus protecting cells from oxidative damage (175, 178). However, when the oxidative challenge persists, or acute oxidative stress is applied, partial inhibition of 26S activity occurs, leading to an accumulation of ubiquitinated substrates (41, 68, 77). Although inhibition of 26S proteasomes could be caused by oxidation products such as protein aggregates or oxidized lipids (178, 179), it is most likely because of oxidative stress-triggered 26S disassembly as shown recently (41, 77). The dissociation of the 20S core from the 19S particle allows the liberation of 20S complexes and therefore increases cellular capacity for ATP/ubiquitin-independent removal of oxidized proteins. Whether other types of regulatory proteins are required for such 20S-dependent degradation in vivo requires further investigation. In yeast cells, 26S proteasome disassembly is regulated by proteasome interacting protein Ecm29, and we hypothesize that a similar type of regulator exists in mammalian cells. Because mammalian cells have more regulatory proteins and proteasomal components, we suspect that the molecular details underlying the regulation of the mammalian 26S proteasome are likely much more complicated than the yeast system. At this stage, 26S proteasome disassembly is reversible (77); once the oxidative stress is removed, the reassembly of the 26S proteasome occurs and the degradation of ubiquitinated substrates can resume, leading to cellular recovery.
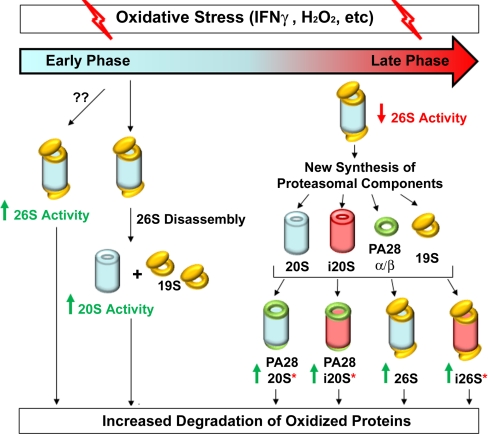
Fig. 2.
Model of oxidative stress-dependent regulation of proteasomes. In the early phase of cellular response to oxidative insult, various changes occur to modulate 26S and 20S proteasome activity in order to promote the degradation of oxidized proteins, and limit the damage of oxidative stress. Initially, under milder stress conditions, 26S proteasomes are activated by mechanisms still unknown. With persistent oxidative insult, or application of acute oxidative stress, proteasomes disassemble into 20S CPs and 19RPs. In yeast, the PIP Ecm29 is required for this disassembly (77). Following dissociation, free 20S proteasomes are activated and oxidized proteins are degraded independently of ATP and ubiquitin. If cells undergo prolonged exposure to oxidative stress (at least 12 h), cells enter the late phase of cellular response to oxidative stress. Though the exact mechanism is unknown, 26S proteasome inhibition ultimately signals the synthesis of new proteasome components and the formation of functional proteasome degradation units. Of note * 20S, i20S, and i26S proteasomes are more effective than standard 26S proteasomes for degrading oxidized proteins.
During prolonged exposure of oxidative stress (i.e. later phase—at least 12 h following stress induction), proteasomal activities are inhibited and de novo proteasome synthesis is activated (67, 165, 175, 176). Up-regulation of both standard and inducible proteasomal components leads to the formation of more functional 20S and i20S proteasomes, respectively. The newly produced 20S and i20S complexes can associate with PA28 and/or 19S regulatory complexes respectively to form diverse functional proteasome complexes for ubiquitin/ATP- independent and/or dependent degradation of oxidized proteins (41, 67). It has been suggested that activated 20S, i20S, and i26S proteasomes are all better able to degrade oxidized proteins than the standard 26S proteasome (41, 67), and the production of immunoproteasomes may be of particular importance for mounting a cellular response against oxidative stress (176). Ultimately, the heterogeneous populations of proteasomes act in concert to degrade toxic oxidized proteins and protect cells from oxidative damage.
CONCLUSION
The proteasome is regulated by complex and poorly understood mechanisms. Attempts to clarify proteasome functional dynamics in response to oxidative stress are complicated by the presence of heterogeneous proteasome populations and multiple regulatory pathways. Additionally, cells exhibit diverse, often contrasting, responses to oxidative stress that are dependent on the type, dose, and duration of oxidative insults. Despite well-established knowledge that proteasomes are important for the removal of oxidatively damaged proteins and the more recently proposed model whereby proteasome activities are modulated by elevated ROS levels, many key questions remain unanswered. These include the following: (1) how do the subtypes of proteasome complexes work together to effectively degrade damaged proteins; (2) what are the mechanisms controlling proteasomal activities and how do these adapt to oxidative stress; (3) how is proteolysis of oxidatively damaged proteins regulated; (4) how are 20S proteasomes activated in vivo for the degradation of oxidized proteins; (5) what molecular mechanisms link proteasome inhibition and/or activation to oxidative stress-associated human pathologies. Although several recent studies have provided new insights that shed light on some of these questions, we have only just begun to unravel the molecular details underlying oxidative stress-triggered regulation of proteasome complexes. To fully address these questions, systematic analyses using biochemical, genetic and proteomic approaches are required. This will not only allow the understanding of ROS-induced regulation of proteasomes, but also provide potential molecular targets for screening proteasome inhibitors and activators. Given that oxidative stress-induced human diseases are associated with the accumulation of misfolded proteins and the loss of proteasome activities, strategies that enhance endogenous proteasome activity would be beneficial. Recent success of using a Usp14 inhibitor to accelerate proteasomal degradation of oxidized proteins (47) demonstrates the possibility of developing proteasome activating reagents for preventing protein aggregation in aging and/or neurodegenerative disorders.
Footnotes
* This work was supported by National Institutes of Health grants (Epilepsy Research Training Program at UCI_T32 NS045540-06A1 to C. A. and GM-74830 to L. H.).
1 The abbreviations used are:
- ROS
- reactive oxygen species
- CP
- core particle
- PIP
- proteasome interacting protein
- GSH
- glutathione.
REFERENCES
- 1. Boveris A. (1984) Determination of the production of superoxide radicals and hydrogen peroxide in mitochondria. Methods Enzymol. 105, 429–435 [DOI] [PubMed] [Google Scholar]
- 2. Chance B., Sies H., Boveris A. (1979) hydroperoxide metabolism in mammalian organs. Physiol. Rev. 59, 527–605 [DOI] [PubMed] [Google Scholar]
- 3. Hansford R. G., Hogue B. A., Mildaziene V. (1997) Dependence of H2o2 formation by rat heart mitochondria on substrate availability and donor age. J. Bioenerg. Biomembr. 29, 89–95 [DOI] [PubMed] [Google Scholar]
- 4. Turrens J. F., Boveris A. (1980) Generation of superoxide anion by the nadh dehydrogenase of bovine heart mitochondria. Biochem. J. 191, 421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vile G. F., Tanew-Ilitschew A., Tyrrell R. M. (1995) Activation of Nf-kappa B in human skin fibroblasts by the oxidative stress generated by UVa Radiation. Photochem. Photobiol. 62, 463–468 [DOI] [PubMed] [Google Scholar]
- 6. Leach J. K., Van Tuyle G., Lin P. S., Schmidt-Ullrich R., Mikkelsen R. B. (2001) Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 61, 3894–3901 [PubMed] [Google Scholar]
- 7. Chou A. P., Li S., Fitzmaurice A. G., Bronstein J. M. (2010) Mechanisms of rotenone-induced proteasome inhibition. Neurotoxicology 31, 367–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watanabe Y., Suzuki O., Haruyama T., Akaike T. (2003) Interferon-gamma induces reactive oxygen species and endoplasmic reticulum stress at the hepatic apoptosis. J. Cell Biochem. 89, 244–253 [DOI] [PubMed] [Google Scholar]
- 9. Hureau C., Faller P. (2009) Abeta-mediated ros production by cu ions: structural insights, mechanisms and relevance to Alzheimer's disease. Biochimie 91, 1212–1217 [DOI] [PubMed] [Google Scholar]
- 10. Leutner S., Eckert A., Müller W. E. (2001) Ros generation, lipid peroxidation and antioxidant enzyme activities in the aging brain. J. Neural Transm. 108, 955–967 [DOI] [PubMed] [Google Scholar]
- 11. Finkel T., Holbrook N. J. (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247 [DOI] [PubMed] [Google Scholar]
- 12. Sedelnikova O. A., Redon C. E., Dickey J. S., Nakamura A. J., Georgakilas A. G., Bonner W. M. (2010) Role of oxidatively induced DNA lesions in human pathogenesis. Mutat. Res. 704, 152–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adibhatla R. M., Hatcher J. F. (2010) Lipid oxidation and peroxidation in cns health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid. Redox. Signal 12, 125–169 [DOI] [PubMed] [Google Scholar]
- 14. Bochkov V. N., Oskolkova O. V., Birukov K. G., Levonen A. L., Binder C. J., Stöckl J. (2010) Generation and biological activities of oxidized phospholipids. Antioxid. Redox. Signal 12, 1009–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finkel T. (2000) Redox-dependent signal transduction. FEBS Lett. 476, 52–54 [DOI] [PubMed] [Google Scholar]
- 16. Barford D. (2004) The role of cysteine residues as redox-sensitive regulatory switches. Curr. Opin. Struct. Biol. 14, 679–686 [DOI] [PubMed] [Google Scholar]
- 17. Martindale J. L., Holbrook N. J. (2002) cellular response to oxidative stress: signaling for suicide and survival. J. Cell. Physiol. 192, 1–15 [DOI] [PubMed] [Google Scholar]
- 18. Chen D., Wilkinson C. R., Watt S., Penkett C. J., Toone W. M., Jones N., Bähler J. (2008) Multiple pathways differentially regulate global oxidative stress responses in fission yeast. Mol. Biol. Cell 19, 308–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davies K. J. (2001) Degradation of oxidized proteins by the 20s proteasome. Biochimie 83, 301–310 [DOI] [PubMed] [Google Scholar]
- 20. Davies K. J. (1987) Protein damage and degradation by oxygen radicals. I. General aspects. J. Biol. Chem. 262, 9895–9901 [PubMed] [Google Scholar]
- 21. Tyedmers J., Mogk A., Bukau B. (2010) Cellular strategies for controlling protein aggregation. Nat. Rev. Mol. Cell Biol. 11, 777–788 [DOI] [PubMed] [Google Scholar]
- 22. Bence N. F., Sampat R. M., Kopito R. R. (2001) Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292, 1552–1555 [DOI] [PubMed] [Google Scholar]
- 23. Kiffin R., Christian C., Knecht E., Cuervo A. M. (2004) Activation of chaperone-mediated autophagy during oxidative stress. Mol. Biol. Cell 15, 4829–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Breusing N., Grune T. (2008) Regulation of proteasome-mediated protein degradation during oxidative stress and aging. Biol. Chem. 389, 203–209 [DOI] [PubMed] [Google Scholar]
- 25. Goldberg A. L. (2003) Protein degradation and protection against misfolded or damaged proteins. Nature 426, 895–899 [DOI] [PubMed] [Google Scholar]
- 26. Jung T., Grune T. (2008) The Proteasome and its role in the degradation of oxidized proteins. IUBMB Life 60, 743–752 [DOI] [PubMed] [Google Scholar]
- 27. Farout L., Mary J., Vinh J., Szweda L. I., Friguet B. (2006) Inactivation of the proteasome by 4-hydroxy-2-nonenal is site specific and dependant on 20s proteasome subtypes. Arch. Biochem. Biophys. 453, 135–142 [DOI] [PubMed] [Google Scholar]
- 28. Buttke T. M., Sandstrom P. A. (1994) Oxidative stress as a mediator of apoptosis. Immunol. Today 15, 7–10 [DOI] [PubMed] [Google Scholar]
- 29. Boldyrev A. A. (2000) Discrimination between apoptosis and necrosis of neurons under oxidative stress. Biochemistry 65, 834–842 [PubMed] [Google Scholar]
- 30. Multhaup G., Ruppert T., Schlicksupp A., Hesse L., Beher D., Masters C. L., Beyreuther K. (1997) Reactive oxygen species and Alzheimer's disease. Biochem. Pharmacol. 54, 533–539 [DOI] [PubMed] [Google Scholar]
- 31. Jenner P. (2003) Oxidative stress in Parkinson's disease. Ann. Neurol. 53 Suppl 3, S26–36; discussion S36–38 [DOI] [PubMed] [Google Scholar]
- 32. Browne S. E., Ferrante R. J., Beal M. F. (1999) Oxidative stress in Huntington's disease. Brain Pathol. 9, 147–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jackson C. E., Bryan W. W. (1998) Amyotrophic lateral sclerosis. Semin. Neurol. 18, 27–39 [DOI] [PubMed] [Google Scholar]
- 34. Spector A. (1995) Oxidative stress-induced cataract: mechanism of action. FASEB J. 9, 1173–1182 [PubMed] [Google Scholar]
- 35. Kumar B., Koul S., Khandrika L., Meacham R. B., Koul H. K. (2008) Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 68, 1777–1785 [DOI] [PubMed] [Google Scholar]
- 36. Brown N. S., Bicknell R. (2001) Hypoxia and oxidative stress in breast cancer. oxidative stress: its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res. 3, 323–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Butterfield D. A., Kanski J. (2001) Brain protein oxidation in age-related neurodegenerative disorders that are associated with aggregated proteins. Mech. Ageing Dev. 122, 945–962 [DOI] [PubMed] [Google Scholar]
- 38. Keller J. N., Mattson M. P. (1998) Roles of lipid peroxidation in modulation of cellular signaling pathways, cell dysfunction, and death in the nervous system. Rev. Neurosci. 9, 105–116 [DOI] [PubMed] [Google Scholar]
- 39. Sayre L. M., Smith M. A., Perry G. (2001) Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Curr. Med. Chem. 8, 721–738 [DOI] [PubMed] [Google Scholar]
- 40. Ding Q., Dimayuga E., Martin S., Bruce-Keller A. J., Nukala V., Cuervo A. M., Keller J. N. (2003) Characterization of chronic low-level proteasome inhibition on neural homeostasis. J. Neurochem. 86, 489–497 [DOI] [PubMed] [Google Scholar]
- 41. Seifert U., Bialy L. P., Ebstein F., Bech-Otschir D., Voigt A., Schröter F., Prozorovski T., Lange N., Steffen J., Rieger M., Kuckelkorn U., Aktas O., Kloetzel P. M., Krüger E. (2010) Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell 142, 613–624 [DOI] [PubMed] [Google Scholar]
- 42. Ciechanover A., Brundin P. (2003) the ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron 40, 427–446 [DOI] [PubMed] [Google Scholar]
- 43. Dahlmann B. (2007) Role of proteasomes in disease. BMC Biochem. 8 Suppl 1, S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Valko M., Morris H., Cronin M. T. (2005) Metals, Toxicity and oxidative stress. Curr. Med. Chem. 12, 1161–1208 [DOI] [PubMed] [Google Scholar]
- 45. Rodríguez-Gabriel M. A., Russell P. (2005) Distinct signaling pathways respond to arsenite and reactive oxygen species in schizosaccharomyces pombe. Eukaryot. Cell 4, 1396–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hanna J., Meides A., Zhang D. P., Finley D. (2007) A ubiquitin stress response induces altered proteasome composition. Cell 129, 747–759 [DOI] [PubMed] [Google Scholar]
- 47. Lee B. H., Lee M. J., Park S., Oh D. C., Elsasser S., Chen P. C., Gartner C., Dimova N., Hanna J., Gygi S. P., Wilson S. M., King R. W., Finley D. (2010) Enhancement of proteasome activity by a small-molecule inhibitor of Usp14. Nature 467, 179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Voges D., Zwickl P., Baumeister W. (1999) The 26s proteasome: a molecular machine designed for controlled proteolysis. Annu. Rev. Biochem. 68, 1015–1068 [DOI] [PubMed] [Google Scholar]
- 49. Pickart C. M., Cohen R. E. (2004) Proteasomes and their kin: proteases in the machine age. Nat. Rev. Mol. Cell Biol. 5, 177–187 [DOI] [PubMed] [Google Scholar]
- 50. Finley D. (2009) Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu. Rev. Biochem. 78, 477–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Murata S., Yashiroda H., Tanaka K. (2009) Molecular mechanisms of proteasome assembly. Nat. Rev. Mol. Cell Biol. 10, 104–115 [DOI] [PubMed] [Google Scholar]
- 52. Kim H. M., Yu Y., Cheng Y. (2011) Structure characterization of the 26s proteasome. Biochim Biophys Acta 1809, 67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Groll M., Ditzel L., Löwe J., Stock D., Bochtler M., Bartunik H. D., Huber R. (1997) Structure of 20s proteasome from yeast at 2.4 a resolution. Nature 386, 463–471 [DOI] [PubMed] [Google Scholar]
- 54. Ciechanover A. (1998) The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J. 17, 7151–7160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Goldberg A. L., Cascio P., Saric T., Rock K. L. (2002) The importance of the proteasome and subsequent proteolytic steps in the generation of antigenic peptides. Mol. Immunol. 39, 147–164 [DOI] [PubMed] [Google Scholar]
- 56. Klare N., Seeger M., Janek K., Jungblut P. R., Dahlmann B. (2007) Intermediate-type 20 S proteasomes in Hela cells: “Asymmetric” subunit composition, diversity and adaptation. J. Mol. Biol. 373, 1–10 [DOI] [PubMed] [Google Scholar]
- 57. Glickman M. H., Rubin D. M., Fried V. A., Finley D. (1998) The regulatory particle of the saccharomyces cerevisiae proteasome. Mol. Cell. Biol. 18, 3149–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schmidt M., Hanna J., Elsasser S., Finley D. (2005) Proteasome-associated proteins: regulation of a proteolytic machine. Biol. Chem. 386, 725–737 [DOI] [PubMed] [Google Scholar]
- 59. Verma R., Oania R., Graumann J., Deshaies R. J. (2004) Multiubiquitin chain receptors define a layer of substrate selectivity in the ubiquitin-proteasome system. Cell 118, 99–110 [DOI] [PubMed] [Google Scholar]
- 60. Elsasser S., Finley D. (2005) Delivery of ubiquitinated substrates to protein-unfolding machines. Nat. Cell Biol. 7, 742–749 [DOI] [PubMed] [Google Scholar]
- 61. Verma R., Aravind L., Oania R., McDonald W. H., Yates J. R., 3rd, Koonin E. V., Deshaies R. J. (2002) Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26s proteasome. Science 298, 611–615 [DOI] [PubMed] [Google Scholar]
- 62. Dubiel W., Pratt G., Ferrell K., Rechsteiner M. (1992) Purification of an 11 S regulator of the multicatalytic protease. J. Biol. Chem. 267, 22369–22377 [PubMed] [Google Scholar]
- 63. Ma C. P., Slaughter C. A., DeMartino G. N. (1992) Identification, purification, and characterization of a protein activator (Pa28) of the 20 S proteasome (Macropain). J. Biol. Chem. 267, 10515–10523 [PubMed] [Google Scholar]
- 64. Gao X., Li J., Pratt G., Wilk S., Rechsteiner M. (2004) purification procedures determine the proteasome activation properties of reg gamma (Pa28 gamma). Arch. Biochem. Biophys. 425, 158–164 [DOI] [PubMed] [Google Scholar]
- 65. Ustrell V., Hoffman L., Pratt G., Rechsteiner M. (2002) Pa200, a nuclear proteasome activator involved in DNA repair. EMBO J. 21, 3516–3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shang F., Gong X., Taylor A. (1997) Activity of ubiquitin-dependent pathway in response to oxidative stress. ubiquitin-activating enzyme is transiently up-regulated. J. Biol. Chem. 272, 23086–23093 [DOI] [PubMed] [Google Scholar]
- 67. Pickering A. M., Koop A. L., Teoh C. Y., Ermak G., Grune T., Davies K. J. (2010) The immunoproteasome, the 20s proteasome, and the PA28αβ proteasome regulator are oxidative stress-adaptive proteolytic complexes. Biochem. J. 432, 585–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Reinheckel T., Sitte N., Ullrich O., Kuckelkorn U., Davies K. J., Grune T. (1998) Comparative resistance of the 20s and 26s proteasome to oxidative stress. Biochem. J. 335 (Pt 3), 637–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Reinheckel T., Ullrich O., Sitte N., Grune T. (2000) Differential impairment of 20s and 26s proteasome activities in human hematopoietic K562 Cells during oxidative stress. Arch. Biochem. Biophys. 377, 65–68 [DOI] [PubMed] [Google Scholar]
- 70. Ullrich O., Reinheckel T., Sitte N., Hass R., Grune T., Davies K. J. (1999) Poly-Adp ribose polymerase activates nuclear proteasome to degrade oxidatively damaged histones. Proc. Natl. Acad. Sci. U.S.A. 96, 6223–6228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shringarpure R., Grune T., Mehlhase J., Davies K. J. (2003) Ubiquitin conjugation is not required for the degradation of oxidized proteins by proteasome. J. Biol. Chem. 278, 311–318 [DOI] [PubMed] [Google Scholar]
- 72. Zhang F., Hu Y., Huang P., Toleman C. A., Paterson A. J., Kudlow J. E. (2007) Proteasome function is regulated by cyclic Amp-dependent protein kinase through phosphorylation of Rpt6. J. Biol. Chem. 282, 22460–22471 [DOI] [PubMed] [Google Scholar]
- 73. Wang X., Guerrero C., Kaiser P., Huang L. (2007) Proteomics of proteasome complexes and ubiquitinated proteins. Expert Rev. Proteomics 4, 649–665 [DOI] [PubMed] [Google Scholar]
- 74. Gomes A. V., Zong C., Edmondson R. D., Li X., Stefani E., Zhang J., Jones R. C., Thyparambil S., Wang G. W., Qiao X., Bardag-Gorce F., Ping P. (2006) Mapping the murine cardiac 26s proteasome complexes. Circ. Res. 99, 362–371 [DOI] [PubMed] [Google Scholar]
- 75. Wang X., Huang L. (2008) Identifying dynamic interactors of protein complexes by quantitative mass spectrometry. Mol. Cell Proteomics 7, 46–57 [DOI] [PubMed] [Google Scholar]
- 76. Kaake R. M., Milenkovic T., Przulj N., Kaiser P., Huang L. (2010) Characterization of cell cycle specific protein interaction networks of the yeast 26s proteasome complex by the Qtax strategy. J. Proteome Res. 9, 2016–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang X., Yen J., Kaiser P., Huang L. (2010) Regulation of the 26s Proteasome Complex During Oxidative Stress. Sci Signal, In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Xie Y. (2010) Structure, assembly and homeostatic regulation of the 26s proteasome. J Mol. Cell. Biol. 2, 308–317 [DOI] [PubMed] [Google Scholar]
- 79. Glickman M. H., Raveh D. (2005) Proteasome plasticity. FEBS Lett. 579, 3214–3223 [DOI] [PubMed] [Google Scholar]
- 80. Kaake R. M., Wang X., Huang L. (2010) Profiling of protein interaction networks of protein complexes using affinity purification and quantitative mass spectrometry. Mol Cell Proteomics 9, 1650–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Washburn M. P. (2010) Driving biochemical discovery with quantitative proteomics. Trends Biochem. Sci. 3, 170–177 [DOI] [PubMed] [Google Scholar]
- 82. Verma R., Chen S., Feldman R., Schieltz D., Yates J., Dohmen J., Deshaies R. J. (2000) Proteasomal proteomics: identification of nucleotide-sensitive proteasome-interacting proteins by mass spectrometric analysis of affinity-purified proteasomes. Mol. Biol. Cell 11, 3425–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Leggett D. S., Hanna J., Borodovsky A., Crosas B., Schmidt M., Baker R. T ., Walz T., Ploegh H., Finley D. (2002) Multiple associated proteins regulate proteasome structure and function. Mol. Cell. 10, 495–507 [DOI] [PubMed] [Google Scholar]
- 84. Hanna J., Hathaway N. A., Tone Y., Crosas B., Elsasser S., Kirkpatrick D. S., Leggett D. S., Gygi S. P., King R. W., Finley D. (2006) Deubiquitinating enzyme Ubp6 functions noncatalytically to delay proteasomal degradation. Cell 127, 99–111 [DOI] [PubMed] [Google Scholar]
- 85. Crosas B., Hanna J., Kirkpatrick D. S., Zhang D. P., Tone Y., Hathaway N. A., Buecker C., Leggett D. S., Schmidt M., King R. W., Gygi S. P., Finley D. (2006) Ubiquitin chains are remodeled at the proteasome by opposing ubiquitin ligase and deubiquitinating activities. Cell 127, 1401–1413 [DOI] [PubMed] [Google Scholar]
- 86. Wang X., Chen C. F., Baker P. R., Chen P. L., Kaiser P., Huang L. (2007) Mass spectrometric characterization of the affinity-purified human 26s proteasome complex. Biochemistry 46, 3553–3565 [DOI] [PubMed] [Google Scholar]
- 87. Guerrero C., Tagwerker C., Kaiser P., Huang L. (2006) An integrated mass spectrometry-based proteomic approach: quantitative analysis of tandem affinity-purified in vivo cross-linked protein complexes (Qtax) to decipher the 26 S proteasome-interacting network. Mol. Cell Proteomics 5, 366–378 [DOI] [PubMed] [Google Scholar]
- 88. Guerrero C., Milenkovic T., Przulj N., Kaiser P., Huang L. (2008) Characterization of the proteasome interaction network using a Qtax-based tag-team strategy and protein interaction network analysis. Proc. Natl. Acad. Sci. U.S.A. 105, 13333–13338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Besche H. C., Haas W., Gygi S. P., Goldberg A. L. (2009) Isolation of mammalian 26s proteasomes and P97/Vcp complexes using the ubiquitin-like domain from Hhr23b reveals novel proteasome-associated proteins. Biochemistry 48, 2538–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Scanlon T. C., Gottlieb B., Durcan T. M., Fon E. A., Beitel L. K., Trifiro M. A. (2009) Isolation of human proteasomes and putative proteasome-interacting proteins using a novel affinity chromatography method. Exp. Cell Res. 315, 176–189 [DOI] [PubMed] [Google Scholar]
- 91. Tai H. C., Besche H., Goldberg A. L., Schuman E. M. (2010) Characterization of the brain 26s proteasome and its interacting Proteins. Front Mol Neurosci 3, pii: 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Meng F., Forbes A. J., Miller L. M., Kelleher N. L. (2005) Detection and localization of protein modifications by high resolution tandem mass spectrometry. Mass Spectrom. Rev. 24, 126–134 [DOI] [PubMed] [Google Scholar]
- 93. Hartmann-Petersen R., Gordon C. (2004) Proteins interacting with the 26s proteasome. Cell Mol. Life Sci. 61, 1589–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zong C., Gomes A. V., Drews O., Li X., Young G. W., Berhane B., Qiao X., French S. W., Bardag-Gorce F., Ping P. (2006) Regulation of murine cardiac 20s proteasomes: role of associating partners. Circ. Res. 99, 372–380 [DOI] [PubMed] [Google Scholar]
- 95. Kleijnen M. F., Roelofs J., Park S., Hathaway N. A., Glickman M., King R. W., Finley D. (2007) Stability of the proteasome can be regulated allosterically through engagement of its proteolytic active sites. Nat. Struct. Mol. Biol. 14, 1180–1188 [DOI] [PubMed] [Google Scholar]
- 96. Lehmann A., Niewienda A., Jechow K., Janek K., Enenkel C. (2010) Ecm29 fulfils quality control functions in proteasome assembly. Mol. Cell 38, 879–888 [DOI] [PubMed] [Google Scholar]
- 97. Grune T., Reinheckel T., Davies K. J. (1997) Degradation of oxidized proteins in mammalian cells. FASEB J. 11, 526–534 [PubMed] [Google Scholar]
- 98. Inai Y., Nishikimi M. (2002) Increased degradation of oxidized proteins in yeast defective in 26 s proteasome assembly. Arch. Biochem. Biophys. 404, 279–284 [DOI] [PubMed] [Google Scholar]
- 99. Kurepa J., Smalle J. A. (2008) Structure, function and regulation of plant proteasomes. Biochimie 90, 324–335 [DOI] [PubMed] [Google Scholar]
- 100. Gorbea C., Goellner G. M., Teter K., Holmes R. K., Rechsteiner M. (2004) Characterization of mammalian Ecm29, a 26 S proteasome-associated protein that localizes to the nucleus and membrane vesicles. J. Biol. Chem. 279, 54849–54861 [DOI] [PubMed] [Google Scholar]
- 101. Gorbea C., Pratt G., Ustrell V., Bell R., Sahasrabudhe S., Hughes R. E., Rechsteiner M. (2010) A protein interaction network for ecm29 Links the 26 S proteasome to molecular motors and endosomal components. J. Biol. Chem. 285, 31616–31633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lam Y. A., Xu W., DeMartino G. N., Cohen R. E. (1997) Editing of ubiquitin conjugates by an isopeptidase in the 26s proteasome. Nature 385, 737–740 [DOI] [PubMed] [Google Scholar]
- 103. Hu M., Li P., Song L., Jeffrey P. D., Chenova T. A., Wilkinson K. D., Cohen R. E., Shi Y. (2005) Structure and mechanisms of the proteasome-associated deubiquitinating enzyme Usp14. EMBO J. 24, 3747–3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chen L., Thiruchelvam M. J., Madura K., Richfield E. K. (2006) Proteasome dysfunction in aged human alpha-synuclein transgenic Mice. Neurobiol Dis 23, 120–126 [DOI] [PubMed] [Google Scholar]
- 105. Ding Q., Keller J. N. (2001) Proteasome inhibition in oxidative stress neurotoxicity: implications for heat shock proteins. J. Neurochem. 77, 1010–1017 [DOI] [PubMed] [Google Scholar]
- 106. Conconi M., Szweda L. I., Levine R. L., Stadtman E. R., Friguet B. (1996) Age-related decline of rat liver multicatalytic proteinase activity and protection from oxidative inactivation by heat-shock protein 90. Arch. Biochem. Biophys. 331, 232–240 [DOI] [PubMed] [Google Scholar]
- 107. Conconi M., Friguet B. (1997) Proteasome inactivation upon aging and on oxidation-effect of Hsp 90. Mol. Biol. Rep. 24, 45–50 [DOI] [PubMed] [Google Scholar]
- 108. Conconi M., Petropoulos I., Emod I., Turlin E., Biville F., Friguet B. (1998) Protection from oxidative inactivation of the 20s proteasome by heat-shock protein 90. Biochem. J. 333 (Pt 2), 407–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pratt W. B., Morishima Y., Peng H. M., Osawa Y. (2010) Proposal for a role of the Hsp90/Hsp70-based chaperone machinery in making triage decisions when proteins undergo oxidative and toxic damage. Exp. Biol. Med. (Maywood) 235, 278–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tsubuki S., Saito Y., Kawashima S. (1994) Purification and characterization of an endogenous inhibitor specific to the Z-Leu-Leu-Leu-Mca degrading activity in proteasome and its identification as heat-shock protein 90. FEBS Lett. 344, 229–233 [DOI] [PubMed] [Google Scholar]
- 111. Wagner B. J., Margolis J. W. (1995) Age-dependent association of isolated bovine lens multicatalytic proteinase complex (proteasome) with heat-shock protein 90, an endogenous inhibitor. Arch Biochem. Biophys 323, 455–462 [DOI] [PubMed] [Google Scholar]
- 112. Whittier J. E., Xiong Y., Rechsteiner M. C., Squier T. C. (2004) Hsp90 enhances degradation of oxidized calmodulin by the 20 S proteasome. J. Biol. Chem. 279, 46135–46142 [DOI] [PubMed] [Google Scholar]
- 113. Mann M., Jensen O. N. (2003) Proteomic analysis of post-translational modifications. Nat. Biotechnol. 21, 255–261 [DOI] [PubMed] [Google Scholar]
- 114. Witze E. S., Old W. M., Resing K. A., Ahn N. G. (2007) Mapping protein post-translational modifications with mass spectrometry. Nat. Methods 4, 798–806 [DOI] [PubMed] [Google Scholar]
- 115. Dowling P., Meleady P., Henry M., Clynes M. (2010) Recent advances in clinical proteomics using mass spectrometry. Bioanalysis 2, 1609–1615 [DOI] [PubMed] [Google Scholar]
- 116. Ballif B. A., Carey G. R., Sunyaev S. R., Gygi S. P. (2008) Large-scale identification and evolution indexing of tyrosine phosphorylation sites from murine brain. J. Proteome Res. 7, 311–318 [DOI] [PubMed] [Google Scholar]
- 117. Rikova K., Guo A., Zeng Q., Possemato A., Yu J., Haack H., Nardone J., Lee K., Reeves C., Li Y., Hu Y., Tan Z., Stokes M., Sullivan L., Mitchell J., Wetzel R., Macneill J., Ren J. M., Yuan J., Bakalarski C. E., Villen J., Kornhauser J. M., Smith B., Li D., Zhou X., Gygi S. P., Gu T. L., Polakiewicz R. D., Rush J., Comb M. J. (2007) global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 131, 1190–1203 [DOI] [PubMed] [Google Scholar]
- 118. Mayya V., Lundgren D. H., Hwang S. I., Rezaul K., Wu L., Eng J. K., Rodionov V., Han D. K. (2009) quantitative phosphoproteomic analysis of T cell receptor signaling reveals system-wide modulation of protein-protein interactions. Sci. Signal. 2, ra46. [DOI] [PubMed] [Google Scholar]
- 119. Rush J., Moritz A., Lee K. A., Guo A., Goss V. L., Spek E. J., Zhang H., Zha X. M., Polakiewicz R. D., Comb M. J. (2005) Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat. Biotechnol. 23, 94–101 [DOI] [PubMed] [Google Scholar]
- 120. Beausoleil S. A., Jedrychowski M., Schwartz D., Elias J. E., Villén J., Li J., Cohn M. A., Cantley L. C., Gygi S. P. (2004) Large-scale characterization of Hela cell nuclear phosphoproteins. Proc. Natl. Acad. Sci. U.S.A. 101, 12130–12135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Dephoure N., Zhou C., Villén J., Beausoleil S. A., Bakalarski C. E., Elledge S. J., Gygi S. P. (2008) A quantitative atlas of mitotic phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 105, 10762–10767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Daub H., Olsen J. V., Bairlein M., Gnad F., Oppermann F. S., Körner R., Greff Z., Kéri G., Stemmann O., Mann M. (2008) Kinase-selective enrichment enables quantitative phosphoproteomics of the kinome across the cell cycle. Mol. Cell 31, 438–448 [DOI] [PubMed] [Google Scholar]
- 123. Brill L. M., Salomon A. R., Ficarro S. B., Mukherji M., Stettler-Gill M., Peters E. C. (2004) Robust phosphoproteomic profiling of tyrosine phosphorylation sites from human T cells using immobilized metal affinity chromatography and tandem mass spectrometry. Anal. Chem. 76, 2763–2772 [DOI] [PubMed] [Google Scholar]
- 124. Stadtman E. R. (2006) Protein oxidation and aging. Free Radic. Res. 40, 1250–1258 [DOI] [PubMed] [Google Scholar]
- 125. Bulteau A. L., Lundberg K. C., Humphries K. M., Sadek H. A., Szweda P. A., Friguet B., Szweda L. I. (2001) Oxidative modification and inactivation of the proteasome during coronary occlusion/reperfusion. J. Biol. Chem. 276, 30057–30063 [DOI] [PubMed] [Google Scholar]
- 126. Ishii T., Sakurai T., Usami H., Uchida K. (2005) Oxidative modification of proteasome: identification of an oxidation-sensitive subunit in 26 S proteasome. Biochemistry 44, 13893–13901 [DOI] [PubMed] [Google Scholar]
- 127. Demasi M., Silva G. M., Netto L. E. (2003) 20 S proteasome from Saccharomyces cerevisiae is responsive to redox modifications and is S-glutathionylated. J. Biol. Chem. 278, 679–685 [DOI] [PubMed] [Google Scholar]
- 128. Demasi M., Shringarpure R., Davies K. J. (2001) Glutathiolation of the proteasome is enhanced by proteolytic inhibitors. Arch Biochem. Biophys. 389, 254–263 [DOI] [PubMed] [Google Scholar]
- 129. Pompella A., Visvikis A., Paolicchi A., De, Tata V., Casini A. F. (2003) The changing faces of glutathione, a cellular protagonist. Biochem. Pharmacol 66, 1499–1503 [DOI] [PubMed] [Google Scholar]
- 130. Shackelford R. E., Heinloth A. N., Heard S. C., Paules R. S. (2005) Cellular and molecular targets of protein s-glutathiolation. Antioxid. Redox. Signal. 7, 940–950 [DOI] [PubMed] [Google Scholar]
- 131. Duan Y., Gross R. A., Sheu S. S. (2007) Ca2+-dependent generation of mitochondrial reactive oxygen species serves as a signal for poly(Adp-ribose) polymerase-1 activation during glutamate excitotoxicity. J. Physiol. 585, 741–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Banasik M., Komura H., Shimoyama M., Ueda K. (1992) Specific inhibitors of poly(Adp-Ribose) synthetase and mono(Adp-Ribosyl)transferase. J. Biol. Chem. 267, 1569–1575 [PubMed] [Google Scholar]
- 133. Bürkle A. (2005) Poly(Adp-Ribose). The most elaborate metabolite of Nad+. FEBS J. 272, 4576–4589 [DOI] [PubMed] [Google Scholar]
- 134. Satoh M. S., Lindahl T. (1992) Role of poly(Adp-Ribose) formation in DNA repair. Nature 356, 356–358 [DOI] [PubMed] [Google Scholar]
- 135. Catalgol B., Wendt B., Grimm S., Breusing N., Ozer N. K., Grune T. (2010) Chromatin repair after oxidative stress: role of parp-mediated proteasome activation. Free Radic. Biol. Med. 48, 673–680 [DOI] [PubMed] [Google Scholar]
- 136. Um J. W., Im E., Park J., Oh Y., Min B., Lee H. J., Yoon J. B., Chung K. C. (2010) Ask1 negatively regulates the 26s proteasome. J. Biol. Chem. 285, 36434–36446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Djakovic S. N., Schwarz L. A., Barylko B., DeMartino G. N., Patrick G. N. (2009) Regulation of the proteasome by neuronal activity and calcium/calmodulin-dependent protein kinase Ii. J. Biol. Chem. 284, 26655–26665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Moreno D., Viana R., Sanz P. (2009) Two-hybrid analysis identifies Psmd11, a non-ATPase subunit of the proteasome, as a novel interaction partner of AMP-activated protein kinase. Int J Biochem. Cell Biol. 41, 2431–2439 [DOI] [PubMed] [Google Scholar]
- 139. Liu X., Huang W., Li C., Li P., Yuan J., Li X., Qiu X. B., Ma Q., Cao C. (2006) Interaction between C-Abl and Arg tyrosine kinases and proteasome subunit Psma7 regulates proteasome degradation. Mol. Cell 22, 317–327 [DOI] [PubMed] [Google Scholar]
- 140. Matsukawa J., Matsuzawa A., Takeda K., Ichijo H. (2004) The Ask1-Map kinase cascades in mammalian stress response. J. Biochem. 136, 261–265 [DOI] [PubMed] [Google Scholar]
- 141. Saitoh M., Nishitoh H., Fujii M., Takeda K., Tobiume K., Sawada Y., Kawabata M., Miyazono K., Ichijo H. (1998) Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (Ask) 1. EMBO J. 17, 2596–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Noguchi T., Takeda K., Matsuzawa A., Saegusa K., Nakano H., Gohda J., Inoue J., Ichijo H. (2005) Recruitment of tumor necrosis factor receptor-associated factor family proteins to apoptosis signal-regulating kinase 1 signalosome is essential for oxidative stress-induced cell death. J. Biol. Chem. 280, 37033–37040 [DOI] [PubMed] [Google Scholar]
- 143. Fujino G., Noguchi T., Matsuzawa A., Yamauchi S., Saitoh M., Takeda K., Ichijo H. (2007) Thioredoxin and Traf family proteins regulate reactive oxygen species-dependent activation of Ask1 through reciprocal modulation of the N-terminal homophilic interaction of Ask1. Mol. Cell. Biol. 27, 8152–8163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Castaño J. G., Mahillo E., Arizti P., Arribas J. (1996) Phosphorylation of C8 and C9 subunits of the multicatalytic proteinase by casein kinase Ii and identification of the C8 phosphorylation sites by direct mutagenesis. Biochemistry 35, 3782–3789 [DOI] [PubMed] [Google Scholar]
- 145. Bose S., Stratford F. L., Broadfoot K. I., Mason G. G., Rivett A. J. (2004) Phosphorylation of 20s proteasome alpha subunit C8 (Alpha7) stabilizes the 26s proteasome and plays a role in the regulation of proteasome complexes by gamma-interferon. Biochem. J. 378, 177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Satoh K., Sasajima H., Nyoumura K. I., Yokosawa H., Sawada H. (2001) Assembly of the 26s proteasome is regulated by phosphorylation of the P45/Rpt6 Atpase subunit. Biochemistry 40, 314–319 [DOI] [PubMed] [Google Scholar]
- 147. Lu H., Zong C., Wang Y., Young G. W., Deng N., Souda P., Li X., Whitelegge J., Drews O., Yang P. Y., Ping P. (2008) Revealing the dynamics of the 20 S proteasome phosphoproteome: a combined CID and electron transfer dissociation approach. Mol. Cell. Proteomics 7, 2073–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Mirkin N., Jaconcic J., Stojanoff V., Moreno A. (2007) High Resolution X-Ray crystallographic structure of bovine heart cytochrome C and its application to the design of an electron transfer biosensor. Proteins 70, 83–92 [DOI] [PubMed] [Google Scholar]
- 149. Bose S., Mason G. G., Rivett A. J. (1999) Phosphorylation of proteasomes in mammalian cells. Mol. Biol. Rep. 26, 11–14 [DOI] [PubMed] [Google Scholar]
- 150. Persad S., Elimban V., Kaila J., Dhalla N. S. (1997) Biphasic alterations in cardiac beta-adrenoceptor signal transduction mechanism due to oxyradicals. J. Pharmacol. Exp. Ther. 282, 1623–1631 [PubMed] [Google Scholar]
- 151. Humphries K. M., Pennypacker J. K., Taylor S. S. (2007) Redox regulation of Camp-dependent protein kinase signaling: kinase versus phosphatase inactivation. J. Biol. Chem. 282, 22072–22079 [DOI] [PubMed] [Google Scholar]
- 152. Takahashi M., Ko L. W., Kulathingal J., Jiang P., Sevlever D., Yen S. H. (2007) Oxidative stress-induced phosphorylation, degradation and aggregation of alpha-synuclein are linked to upregulated Ck2 and cathepsin D. Eur. J. Neurosci. 26, 863–874 [DOI] [PubMed] [Google Scholar]
- 153. Murtaza I., Wang H. X., Feng X., Alenina N., Bader M., Prabhakar B. S., Li P. F. (2008) Down-regulation of catalase and oxidative modification of protein kinase Ck2 lead to the failure of apoptosis repressor with caspase recruitment domain to inhibit cardiomyocyte hypertrophy. J. Biol. Chem. 283, 5996–6004 [DOI] [PubMed] [Google Scholar]
- 154. Sayed M., Kim S. O., Salh B. S., Issinger O. G., Pelech S. L. (2000) Stress-induced activation of protein kinase Ck2 by direct interaction with P38 mitogen-activated protein kinase. J. Biol. Chem. 275, 16569–16573 [DOI] [PubMed] [Google Scholar]
- 155. Howe C. J., Lahair M. M., McCubrey J. A., Franklin R. A. (2004) Redox regulation of the calcium/calmodulin-dependent protein kinases. J. Biol. Chem. 279, 44573–44581 [DOI] [PubMed] [Google Scholar]
- 156. Baskaran R., Wood L. D., Whitaker L. L., Canman C. E., Morgan S. E., Xu Y., Barlow C., Baltimore D., Wynshaw-Boris A., Kastan M. B., Wang J. Y. (1997) Ataxia telangiectasia mutant protein activates C-Abl tyrosine kinase in response to ionizing radiation. Nature 387, 516–519 [DOI] [PubMed] [Google Scholar]
- 157. Sun X., Wu F., Datta R., Kharbanda S., Kufe D. (2000) Interaction between protein kinase C delta and the C-Abl tyrosine kinase in the cellular response to oxidative stress. J. Biol. Chem. 275, 7470–7473 [DOI] [PubMed] [Google Scholar]
- 158. Allen R. G., Tresini M. (2000) Oxidative Stress and Gene Regulation. Free Radic Biol Med 28, 463–499 [DOI] [PubMed] [Google Scholar]
- 159. Sone H., Akanuma H., Fukuda T. (2010) Oxygenomics in environmental stress. Redox Rep 15, 98–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Delaunay A., Isnard A. D., Toledano M. B. (2000) H2o2 Sensing through oxidation of the Yap1 transcription factor. EMBO J. 19, 5157–5166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Mannhaupt G., Schnall R., Karpov V., Vetter I., Feldmann H. (1999) Rpn4p acts as a transcription factor by binding to Pace, a nonamer box found upstream of 26s proteasomal and other genes in yeast. FEBS Lett. 450, 27–34 [DOI] [PubMed] [Google Scholar]
- 162. Dohmen R. J., Willers I., Marques A. J. (2007) Biting the hand that feeds: Rpn4-dependent feedback regulation of proteasome function. Biochim. Biophys. Acta 1773, 1599–1604 [DOI] [PubMed] [Google Scholar]
- 163. Hahn J. S., Neef D. W., Thiele D. J. (2006) A stress regulatory network for co-ordinated activation of proteasome expression mediated by yeast heat shock transcription factor. Mol. Microbiol. 60, 240–251 [DOI] [PubMed] [Google Scholar]
- 164. Owsianik G., Balzi l L., Ghislain M. (2002) Control of 26s proteasome expression by transcription factors regulating multidrug resistance in Saccharomyces cerevisiae. Mol. Microbiol. 43, 1295–1308 [DOI] [PubMed] [Google Scholar]
- 165. Lee J., Godon C., Lagniel G., Spector D., Garin J., Labarre J., Toledano M. B. (1999) Yap1 and Skn7 control two specialized oxidative stress response regulons in yeast. J. Biol. Chem. 274, 16040–16046 [DOI] [PubMed] [Google Scholar]
- 166. Chondrogianni N., Stratford F. L., Trougakos I. P., Friguet B., Rivett A. J., Gonos E. S. (2003) Central role of the proteasome in senescence and survival of human fibroblasts: induction of a senescence-like phenotype upon its inhibition and resistance to stress upon its activation. J. Biol. Chem. 278, 28026–28037 [DOI] [PubMed] [Google Scholar]
- 167. Chen Q., Thorpe J., Dohmen J. R., Li F., Keller J. N. (2006) Ump1 extends yeast lifespan and enhances viability during oxidative stress: central role for the proteasome? Free Radic. Biol. Med. 40, 120–126 [DOI] [PubMed] [Google Scholar]
- 168. Chondrogianni N., Gonos E. S. (2007) Overexpression of Hump1/Pomp proteasome accessory protein enhances proteasome-mediated antioxidant defence. Exp. Gerontol. 42, 899–903 [DOI] [PubMed] [Google Scholar]
- 169. Wang X., Xu H., Ju D., Xie Y. (2008) Disruption of Rpn4-induced proteasome expression in saccharomyces cerevisiae reduces cell viability under stressed conditions. Genetics 180, 1945–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Du J., Mitch W. E., Wang X., Price S. R. (2000) Glucocorticoids induce proteasome C3 subunit expression in L6 muscle cells by opposing the suppression of its transcription by Nf-Kappa B. J. Biol. Chem. 275, 19661–19666 [DOI] [PubMed] [Google Scholar]
- 171. Takabe W., Matsukawa N., Kodama T., Tanaka K., Noguchi N. (2006) Chemical structure-dependent gene expression of proteasome subunits via regulation of the antioxidant response element. Free Radic. Res. 40, 21–30 [DOI] [PubMed] [Google Scholar]
- 172. Kwak M. K., Wakabayashi N., Greenlaw J. L., Yamamoto M., Kensler T. W. (2003) Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol. Cell. Biol. 23, 8786–8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Kraft D. C., Deocaris C. C., Wadhwa R., Rattan S. I. (2006) Preincubation with the proteasome inhibitor Mg-132 enhances proteasome activity via the Nrf2 transcription factor in aging human skin fibroblasts. Ann. N.Y. Acad. Sci. 1067, 420–424 [DOI] [PubMed] [Google Scholar]
- 174. Steffen J., Seeger M., Koch A., Krüger E. (2010) Proteasomal degradation is transcriptionally controlled by Tcf11 via an Erad-dependent feedback loop. Mol Cell 40, 147–158 [DOI] [PubMed] [Google Scholar]
- 175. Ding Q., Reinacker K., Dimayuga E., Nukala V., Drake J., Butterfield D. A., Dunn J. C., Martin S., Bruce-Keller A. J., Keller J. N. (2003) Role of the proteasome in protein oxidation and neural viability following low-level oxidative stress. FEBS Lett. 546, 228–232 [DOI] [PubMed] [Google Scholar]
- 176. Hussong S. A., Kapphahn R. J., Phillips S. L., Maldonado M., Ferrington D. A. (2010) Immunoproteasome deficiency alters retinal proteasome's response to stress. J. Neurochem. 113, 1481–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Ding Q., Martin S., Dimayuga E., Bruce-Keller A. J., Keller J. N. (2006) Lmp2 knock-out mice have reduced proteasome activities and increased levels of oxidatively damaged proteins. Antioxid. Redox. Signal. 8, 130–135 [DOI] [PubMed] [Google Scholar]
- 178. Grune T., Jung T., Merker K., Davies K. J. (2004) Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and ‘aggresomes’ during oxidative stress, aging, and disease. Int. J. Biochem. Cell Biol. 36, 2519–2530 [DOI] [PubMed] [Google Scholar]
- 179. Sitte N., Merker K., von Zglinicki T., Grune T. (2000) Protein oxidation and degradation during proliferative senescence of human Mrc-5 fibroblasts. Free Radic. Biol. Med. 28, 701–708 [DOI] [PubMed] [Google Scholar]