Abstract
Most animals can distinguish two distinct types of touch stimuli: gentle (innocuous) and harsh (noxious/painful) touch, but the underlying mechanisms are not well understood. C. elegans is a highly successful model for the study of gentle touch sensation. However, little is known about harsh touch sensation in this organism. Here we characterize harsh touch sensation in C. elegans. We show that C. elegans exhibits differential behavioral responses to harsh touch and gentle touch. Laser ablations identify distinct sets of sensory neurons and interneurons required for harsh touch sensation at different body segments. Optogenetic stimulation of the circuitry can drive behavior. Patch-clamp recordings reveal that TRP family and amiloride-sensitive Na+ channels mediate touch-evoked currents in different sensory neurons. Our work identifies the neural circuits and characterizes the sensory channels mediating harsh touch sensation in C. elegans, establishing it as a genetic model for studying this sensory modality.
Introduction
The sense of touch is a universal phenomenon found in nearly every organism1. In animals, specialized sensory systems have been evolved to detect and react to touch, which are essential for their survival and reproduction2. Most animals can distinguish at least two distinct types of touch stimuli: gentle touch and harsh touch, with the former being harmless and sometimes pleasant and the latter unpleasant and often painful2. In mammals, the myelinated Aβ sensory nerves detect gentle touch, while the lightly myelinated Aδ and non-myelinated C fibers sense harsh touch2. However, unlike photosensation and chemosensation that have been extensively characterized in mammals, little is known about the molecular mechanisms of touch sensation1. For example, the central players in touch sensation, the mechanosensitive channels that are believed to convert forces into electrical responses, have not been identified in mammals.
The nematode C. elegans is a widely used model organism for sensory transduction such as touch sensation, chemosensation and photosensation1, 3–6. In particular, C. elegans is renowned for its success in elucidating the mechanisms of gentle touch sensation1. A group of sensory neurons (ALM, AVM and PLM) sense gentle touch in the worm body, while ASH, OLQ and FLP detect gentle touch at the nose tip1, 7. A saturated forward genetic screen has identified a mechanotransduction channel complex that senses gentle body touch, with the ENaC/degenerin family Na+ channel MEC-4/MEC-10 forming the channel pore, and MEC-6 and MEC-2 linking the channel to the extracellular matrix and intracellular cytoskeleton, respectively1, 8. Remarkably, many of the genes in this complex are evolutionarily conserved, which has prompted characterization of the function of their homologues in gentle touch sensation in mammals. Indeed, the mammalian homologue of MEC-2 and MEC-4 has recently been reported to play an important role in gentle touch sensation and pressure sensation in mice, respectively9, 10.
However, unlike gentle touch sensation, little is known about harsh touch sensation in C. elegans. The behavioral responses to harsh touch have not been clearly defined. Though one neuron (PVD) has been found to act in harsh touch sensation11, 12, the majority of the circuitry is unknown. Here, we characterize harsh touch sensation in C. elegans by defining the behavioral responses to harsh touch and by dissecting the underlying neural circuits and genes. We show that as is the case with mammals, C. elegans exhibits differential behavioral responses to harsh and gentle touch and employs distinct sensory neurons to detect harsh and gentle touch. Optogenetic stimulation of the circuitry can mimic behavioral responses. Through electrophysiological recording, we show that both TRP (transient receptor potential) family and amiloride-sensitive Na+ channels mediate mechanosensitive currents, but in different sensory neurons. Calcium imaging of the circuitry reveals a neuronal correlate for harsh and gentle touch sensation. Our results establish a framework for understanding the mechanisms of harsh touch sensation in a genetic model organism.
Results
Differential behavioral responses to harsh and gentle touch
Eyelash is commonly used to deliver gentle touch to the anterior and posterior animal body with a force in the range of 1~10μN, which triggers backward and forward movement, respectively13. To deliver harsh touch, we used a platinum wire pick with a force of 100–200 μN (Figure 1a). Stronger forces (>500 μN) often caused physical damages to the animal, manifested by a sluggish or loss of response in following trials. Animals reacted to anterior harsh touch by initiating backward movement and to posterior harsh touch by initiating forward movement (Figure 1b, Supplementary Figure S1a and Movie 1–2); however, the responses were much more robust than gentle touch responses. As a direct comparison, we focused on the anterior touch response since it is relatively straightforward to quantify this response by measuring the distance (# of head swings) of backward movement. Under our conditions, gentle touch-triggered backward movement lasted an average of 1.7±0.2 head swings, which was usually followed by forward movement without a direction change (Figure 1c–d). By contrast, harsh touch-evoked backward movement lasted an average of 5.1±0.4 head swings, which was often followed by a direction change (Figure 1c–d). Such direction change ensures that the animal would not return to its previous location. This avoidance response may help animals to effectively avoid hazardous environments. Thus, C. elegans shows differential behavioral responses to gentle and harsh touch.
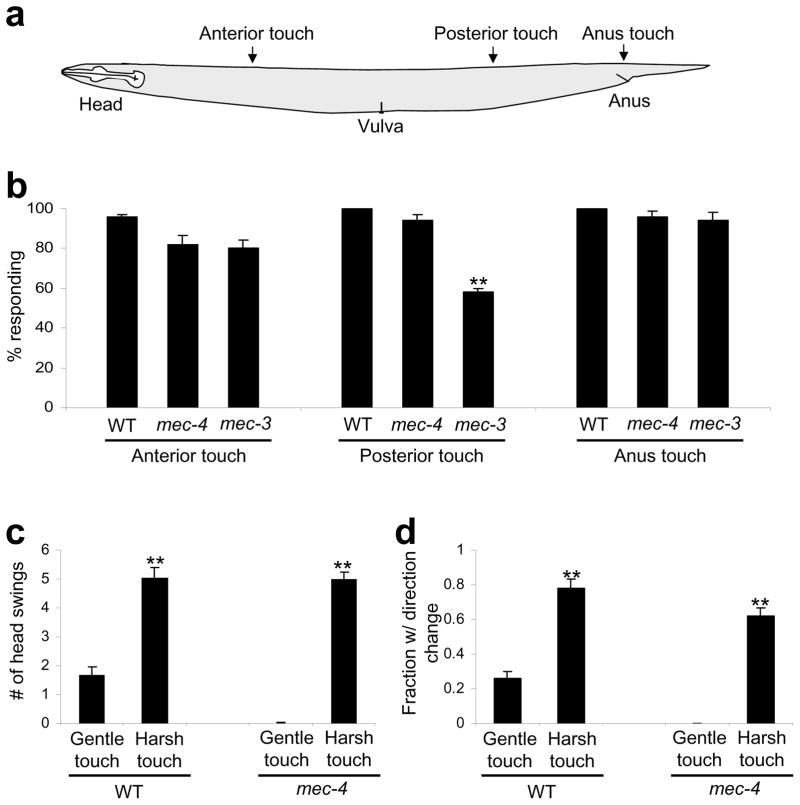
Figure 1. Animals exhibit differential behavioral responses to harsh touch and gentle touch.
(a) A schematic illustrating the location of harsh touch stimuli delivered to different body segments.
(b) Response of WT, mec-4(e1611) and mec-3(e1338) worms to anterior, posterior and anus harsh touch. mec-4(e1611) and mec-3(e1338) worms were outcrossed for six and four times, respectively. The non-outcrossed CGC strain CB1338 of mec-3(e1338) was a bit unhealthy and showed reduced responses (Supplementary Figure S1). n=10. **p<0.0001 (ANOVA with Bonferroni test). Error bars: SEM.
(c) Animals respond to harsh touch more robustly than gentle touch. The number of head swings of backward movement triggered by anterior gentle and harsh touch was scored. **p<0.0001 (t test; harsh touch vs. gentle touch). However, harsh touch data from mec-4(e1611) mutant and wild-type under were not significantly different. n=10. Error bars: SEM.
(d) The frequency of direction change following backward movement triggered by anterior harsh touch and gentle touch. A direction change was defined as a ≥90 degree turn from the previous locomotion direction. **p<0.0001 (t test; harsh touch vs. gentle touch). However, harsh touch data from mec-4(e1611) mutant strain and wild-type were not significantly different. n=10. Error bars: SEM.
We also tested the anus area, as C. elegans recruits a different set of neurons for harsh touch sensation in this area (see below). Animals reacted to anus touch by initiating forward movement (Figure 1b).
Finally, we tested the middle segment (vulva touch). Unlike other body segments, animals reacted to vulva touch by initiating either forward or backward movement with a bias towards forward movement (Supplementary Figure S1b). Because of this, we focused our efforts on characterizing anterior, posterior and anus touch responses.
Gentle touch mutants retain harsh touch responses
We tested the gentle touch-insensitive mutant mec-4(e1611) in which all gentle touch sensory neurons degenerate14. As reported11, though gentle touch insensitive, this mutant was sensitive to harsh touch (Figure 1b; Supplementary Movie 3–4). Specifically, the number of head swings of backward movement triggered by anterior harsh touch and the frequency of direction change following backward movement were similar to those observed in wild-type, suggesting that animals lacking gentle touch sensitivity retain normal harsh touch responses (Figure 1b–d). Thus, C. elegans may depend on different neurons for harsh touch sensation.
Interneurons required for harsh touch responses
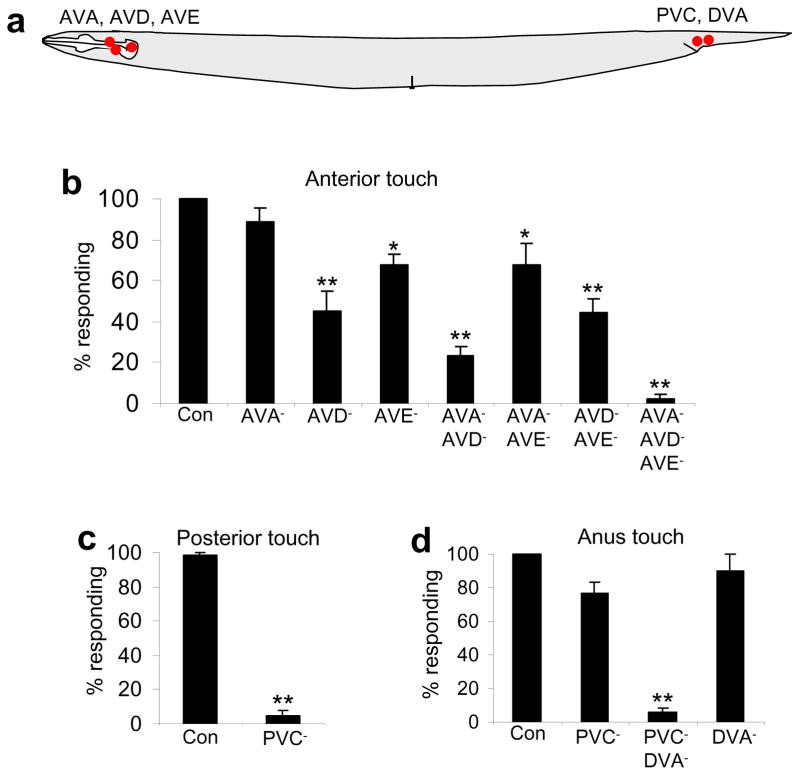
To identify the neural circuits underlying harsh touch sensation, we applied a laser ablation approach. We first examined the interneurons. The interneuron circuits underlying gentle touch behavior have been well characterized15. The backward command interneurons AVA and AVD together are required for gentle touch response, while the forward command interneuron PVC mediates posterior gentle touch response15. The other backward command interneuron AVE, however, is dispensable for gentle touch behavior15 (Figure 2a). The backward and forward command interneurons drive backward and forward movement by controlling downstream ventral cord motor neurons, respectively15. We killed these interneurons individually and in combination with a laser microbeam. While ablation of AVA, AVD and AVE individually led to a modest defect in anterior harsh touch response, animals lacking AVA, AVD and AVE in combination failed to move backwards upon anterior harsh touch (Figure 2b); yet these animals maintained normal posterior and anus harsh touch responses (Supplementary Figure S2a–b). Thus, AVA, AVD and AVE are specifically required for anterior harsh touch response.
Figure 2. Identification of the interneurons required for harsh touch behavior.
(a) A schematic illustrating the position of interneuron cell bodies.
(b) AVA, AVD and AVE are required for mediating anterior but not posterior or anus harsh touch response. *p<0.02. **p<0.0001 (ANOVA with Bonferroni test compared to control). n≥5. Error bars: SEM.
(c) PVC is required for mediating posterior but not anterior or anus harsh touch response. **p<0.0001 (t test). n≥12. Error bars: SEM.
(d) PVC and DVA together are required for mediating anus harsh touch response. **p<0.0001 (ANOVA with Bonferroni test compared to control). n≥4. Error bars: SEM.
Similarly, animals lacking the forward command interneuron PVC failed to move forward upon posterior harsh touch (Figure 2c), but responded normally to anterior harsh touch (Supplementary Figure S2c–d). Thus, PVC is required for posterior harsh touch response.
However, PVC-ablated animals can still move forward upon anus touch, albeit at a reduced frequency (Figure 2d). Apparently, additional interneurons must contribute to anus response. We considered those in the anus area that form synaptic connections with ventral cord motor neurons. DVA came to our attention, as it synapses onto the B type ventral cord motor neurons that drive forward locomotion16. While ablation of DVA alone led to a slight reduction in anus response, animals with both PVC and DVA ablated were unable to move forward upon anus touch, indicating that DVA and PVC together are required for anus response (Figure 2d and S2e–f).
Sensory neurons required for anterior harsh touch sensation
We then sought to identify the sensory neurons required for harsh touch sensation. To eliminate gentle touch responses, we killed the anterior gentle touch neurons ALM/AVM and the posterior gentle touch neuron PLM on top of all of the ablations aimed at identifying anterior and posterior/anus harsh touch sensory neurons, respectively.
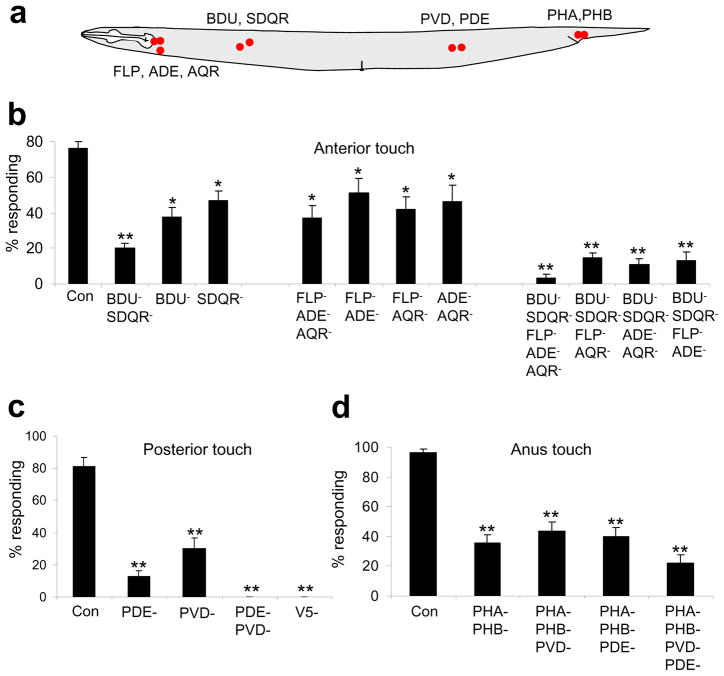
To identify the sensory neurons required for detecting anterior harsh touch, we considered candidates that are located in the anterior body and also form synaptic connections with the anterior interneurons AVA/AVD/AVE (Figure 3a). BDU and SDQR fall into this category16. Laser ablation of BDU or SDQR led to a significant reduction in anterior harsh touch response (Figure 3b). Killing BDU together with SDQR, but not with SDQL, yielded a more severe defect (Figure 3b and Supplementary Figure S3). Nevertheless, these animals retained a ~20% response rate (Figure 3b). These results indicate that while BDU and SDQR play an important role in anterior harsh touch sensation, other sensory neurons are also involved.
Figure 3. Identification of the sensory neurons required for harsh touch behavior.
(a) A schematic illustrating the location of sensory neuron cell bodies.
(b) Two groups of sensory neurons are required for mediating anterior harsh touch response. The anterior gentle touch sensory neurons ALM and AVM were killed in all ablations. *p<0.03. **p<0.0001 (ANOVA with Bonferroni test compared to control). n≥9. Error bars: SEM.
(c) PVD and PDE together are required for mediating posterior harsh touch response. The posterior gentle touch sensory neuron PLM was killed in all ablations. V5 is the precursor cell of PVD and PDE. **p<0.0001 (ANOVA with Bonferroni test compared to control). n≥7. Error bars: SEM.
(d) PHA/PHB are important for mediating anus harsh touch response. PLM was killed in all ablations. **p<0.0001 (ANOVA with Bonferroni test compared to control). n≥9. Error bars: SEM.
To identify the sensory neurons that function redundantly with BDU and SDQR in the anterior circuit, we considered FLP, AQR and ADE which form synaptic connections with AVA/AVD/AVE and are located close to the terminal end of the pharynx16. Unlike BDU and SDQR, these three sensory neurons are ciliated neurons16. While the sensory cilium of FLP is located at the nose tip, this neuron has multiple dendritic branches extending into the anterior body16, 17. This appears to be a distinct feature pertaining to these three sensory neurons, as other head sensory neurons send their sensory endings to the nose tip16. Laser ablation of FLP, AQR and ADE together resulted in a significant reduction in anterior response, but did not abolish it (Figure 3b). Thus, these three ciliated sensory neurons are important, though not required, for anterior harsh touch sensation.
We therefore ablated these two distinct groups of neurons together. Laser ablation of BDU, SDQR, FLP, ADE and AQR together virtually eliminated anterior response (Figure 3b). As a control, the ablated animals (also lacking gentle touch neurons) responded normally to posterior and anus harsh touch (Supplementary Figure S4 and S5). Thus, these sensory neurons together are specifically required for anterior harsh touch sensation.
Sensory neurons required for posterior harsh touch sensation
To identify the sensory neurons required for posterior harsh touch sensation, we examined those that are located in the posterior body and form synaptic connections with the posterior interneuron PVC. PVD and PDE fall into this category16 (Figure 3a). PVD is a non-ciliated multidendritic neuron regulating harsh touch sensation12, 17, 18, while PDE bears a sensory cilium16. PVD ablation led to a reduction in posterior response, but again did not abolish it, indicating that additional sensory neurons are involved (Figure 3c). Notably, ablation of PVD (together with gentle touch neurons) did not yield a defect in anterior response (Supplementary Figure S3a, S4a, and S6a); nor did it affect anus response (Supplementary Figure S4c and S6b), indicating that PVD plays a specific role in posterior harsh touch sensation. We also checked mec-3 mutant animals in which PVD is not fully differentiated11. Similarly, while mec-3 mutant animals displayed a severe defect in sensing vulva touch as previously reported11 (Supplementary Figure S1b), and exhibited a reduced response to posterior touch, this mutant retained normal sensitivity to anterior and anus harsh touch (Figure 1b; Supplementary Figure S1a). Thus, PVD is not required for anterior, posterior or anus harsh touch sensation.
Laser ablation of PDE also led to a strong defect in posterior response (Figure 3c), indicating that PDE plays an important role in this area. The observation that the loss of PVD or PDE reduced rather than abolished posterior response prompted us to reason that these two neurons may play a redundant role in posterior harsh touch sensation. Indeed, animals lacking both PVD and PDE failed to move forward upon posterior harsh touch (Figure 3c). As a control, we killed PVD together with the other two posterior neurons PVM and SDQL, and found that these animals retained posterior response at a level similar to that observed in animals lacking PVD only (Supplementary Figure S3b). Finally, animals lacking PVD, PDE and all gentle touch neurons, though defective in sensing posterior touch, responded normally to anterior and anus harsh touch (Supplementary Figure S4). Thus, PVD and PDE together are specifically required for posterior harsh touch response.
Notably, instead of moving forward, animals lacking both PVD and PDE briefly “jerked” backwards upon posterior harsh touch (Supplementary Movie 5). A similar phenomenon was observed in animals lacking the forward interneuron PVC. This suggests the presence of a competing circuit. As the behavioral output of this putative competing circuit was quite modest and only manifested in the absence of PVD and PDE, we did not explore it further in this study.
Sensory neurons required for anus harsh touch sensation
To identify the sensory neurons required for harsh touch sensation in the anus area, we first ablated the T cell, a precursor to a number of neurons in the anus area19, but did not detect a severe defect (Supplementary Figure S3c). We then examined the ciliated sensory neurons in this area: PQR and PHA/PHB. While loss of PQR did not lead to a notable defect (Supplementary Figure S3c), ablation of PHA/PHB resulted in a significant reduction in anus response (Figure 3d). As a control, no defect was detected in anterior or posterior responses in ablated animals (Supplementary Figure S4 and S7), indicating that PHA/PHB have a specific role in anus touch sensation.
The observation that PHA/PHB-ablated animals retained residual (~35%) anus response suggests the presence of additional sensory neurons in anus touch sensation. We considered several candidates. First, laser ablation of PHA/PHB and PQR together did not yield a more severe defect (Supplementary Figure S3c), suggesting that PQR may not play an important role. This is consistent with the wiring pattern that PHA/PHB rather than PQR form synaptic connections with PVC and DVA16. Second, we tested DVA. DVA is unique in that it is both an interneuron and a sensory neuron (a proprioceptive neuron for body posture control)20, 21. However, laser ablation of PHA/PHB and DVA together did not reveal a more severe defect in anus response (Supplementary Figure S3c). Given that DVA and PVC are required for anus response (Figure 2d), this result indicates that DVA may mainly act as an interneuron in the anus circuit. Finally, we considered the possibility that the posterior neurons PVD and PDE may have a role in anus touch sensation since the receptive field of these two neurons may extend to the anus area. PVD has sensory branches close to the anus17, 22. While PDE is physically distant from the anus, anus touch could slightly displace PDE cilium in distance. Indeed, laser ablation of PHA/PHB together with PVD and PDE further reduced the anus response rate to ~20% (Figure 3d). Although additional neurons are involved, these data identify PHA/PHB as the primary sensory neurons in mediating anus harsh touch sensation.
Optogenetic stimulation of the circuitry can drive behavior
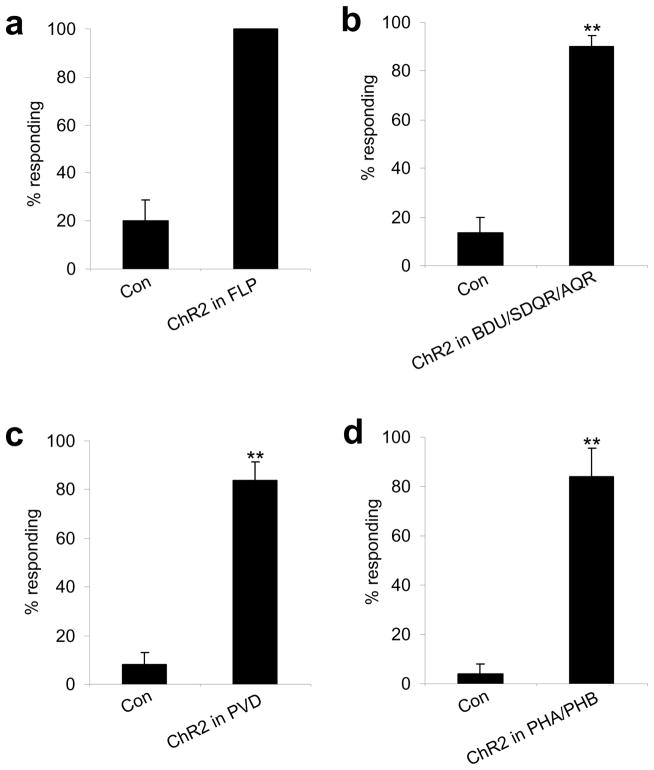
We tested whether stimulation of the identified sensory neurons can mimic harsh touch behavioral responses. We took an optogenetic approach by expressing ChR2, a light-gated non-selective cation channel23, 24, in sensory neurons. ChR2 stimulation of the anterior neuron FLP initiated backward movement (Figure 4a). Stimulation of the anterior neurons AQR, BDU and SDQR by ChR2 also triggered backward movement (Figure 4b).
Figure 4. Optogenetic stimulation of sensory neurons by ChR2 can drive behavioral responses.
(a) ChR2 stimulation of FLP can drive backward movement. ChR2 was expressed in FLP as a transgene under the mec-3 promoter in the lite-1(xu7)mec-4(e1611) background. The mec-3 promoter labels FLP, PVD and gentle touch neurons. lite-1(xu7) abolishes intrinsic phototaxis response5. mec-4(e1611) eliminates all gentle touch neurons14. We thus analyzed FLP-positive animals in which PVD was killed by laser. Control animals were transgene-free siblings. **p<0.0001 (t test). n≥5. Error bars: SEM.
(b) ChR2 stimulation of BDU, SDQR and AQR can drive backward movement. ChR2 was expressed as a transgene under the gcy-35 promoter in the lite-1(xu7) background. This promoter also labels URX and AVM in the head and anterior body 45, so we analyzed animals with URX and AVM ablated. Control animals were transgene-free siblings. **p<0.0001 (t test). n≥6. Error bars: SEM.
(c) ChR2 stimulation of PVD can drive forward movement. ChR2 was expressed in PVD as a transgene under the mec-3 promoter in the lite-1(xu7)mec-4(e1611) background. We analyzed PVD-positive animals with FLP killed by laser. Control animals were transgene-free siblings. **p<0.0001 (t test). n≥5. Error bars: SEM.
(d) ChR2 stimulation of PHA/PHB can drive forward movement. ChR2 was expressed as a transgene under the ocr-2 promoter in the lite-1(xu7) background 31. To avoid turning on the head neurons labeled by the ocr-2 promoter, we directed light pulses to the animal tail. Control animals were transgene-free siblings. **p<0.0001 (t test). n≥5. Error bars: SEM.
Likewise, ChR2 stimulation of the posterior sensory neurons PVD can trigger forward movement (Figure 4c). A similar observation was made in animals expressing ChR2 in the anus sensory neuron PHA/PHB (Figure 4d). However, we did not observe such a phenomenon in animals expressing ChR2 in PDE. It is possible that ChR2-mediated cation influx cannot fully mimic the kinetics and/or ion selectivity of the conductance carried by the endogenous mechanosensitive channels in these neurons. Nonetheless, these results provide additional evidence supporting a role for the identified sensory neurons in harsh touch sensation.
A mechano-TRP channel regulates harsh touch sensation
We next asked what genes are important for harsh touch sensation. The fact that animals lacking the gentle touch channel MEC-4/MEC-10 are sensitive to harsh touch suggests that other types of channels could be involved.
TRP family channels are conserved between C. elegans and humans25. Many TRP channels have been implicated in mechanosensation, though the exact role of these channels in mechanosensation remains largely unclear26, 27. The C. elegans genome encodes 17 TRP channel homologues covering all of the seven TRP subfamilies28, 29. As the expression patterns of most TRP channels have been reported28, 29, we examined those known to be expressed in harsh touch sensory neurons, including TRPV (OSM-9 and OCR-2), TRPA (TRPA-1), and TRPN (TRP-4) channels. TRPV channels have been implicated in mechanosensation in both invertebrates and vertebrates27, 30. The C. elegans TRPV channel OCR-2 is expressed in the anus sensory neurons PHA/PHB and is required for mechanosensation mediated by the head neuron ASH31. We did not detect a defect in anus response in ocr-2 mutant animals (Supplementary Figure S8a); nor did we observe a deficit in posterior response (Supplementary Figure S8b). A similar result was obtained with animals lacking OSM-9, another TRPV channel expressed in PHA/PHB32, as well as animals lacking both OSM-9 and OCR-2 (Supplementary Figure S8). Thus, OCR-2/OSM-9 channels are not essential for anus harsh touch sensation.
TRPA channels have been implicated in mechanosensation in both invertebrates and vertebrates27, 33–35. The TRPA channel TRPA-1 is expressed in the anus sensory neurons PHA/PHB and posterior sensory neurons PVD and PDE35. However, we did not observe a noticeable defect in anus response; nor did we detect a phenotype in posterior response, suggesting that TRPA-1 may not be required for harsh touch sensation in these areas (Figure 5 and S8).
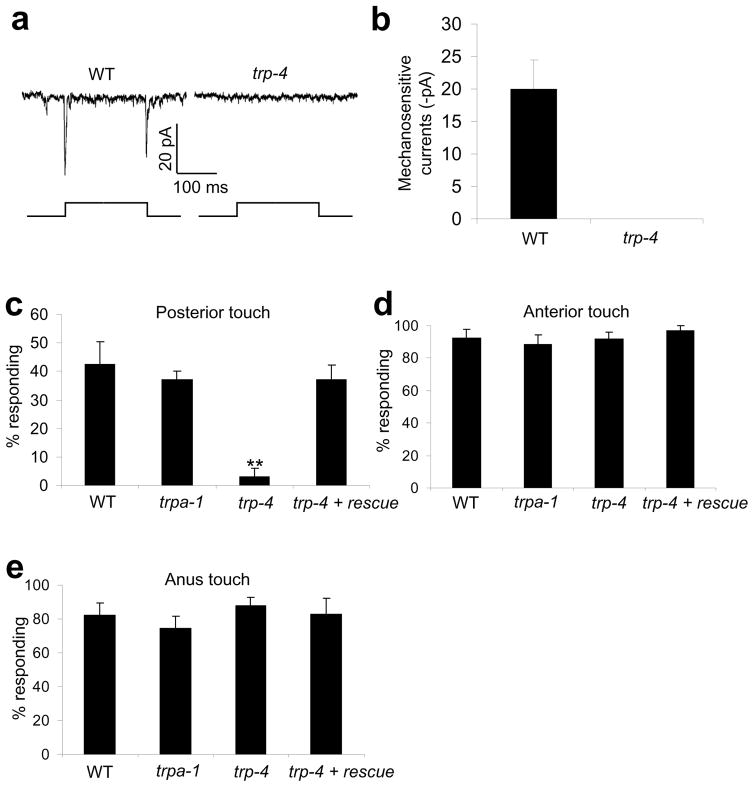
Figure 5. The TRPN channel TRP-4 regulates posterior harsh touch response.
(a) Touch evokes mechanosensitive currents in PDE in a TRP-4-dependent manner. Clamping voltage: −75 mV. Touch was directed to the cilium of PDE. Displacement: 10 μm. 1 μm displacement was sufficient to evoke mechanosensitive currents in PDE.
(b) Bar graph summarizing the data in (a). n≥5. Error bars: SEM.
(c) TRP-4 is required for posterior harsh touch behavioral response mediated by PDE. To rescue the defect, wild-type trp-4 cDNA was expressed as a transgene in PDE under the dat-1 promoter in trp-4(sy695) mutant animals20, 46. PVD and PLM were both killed in each genotype. **p<0.0001 (ANOVA with Bonferroni test compared to control). n≥7. Error bars: SEM.
(d) Responses to anterior harsh touch in worms described in (c).
(e) Responses to anus harsh touch in worms described in (c).
TRPN channels have also been implicated in mechanosensation in a number of invertebrate and vertebrate organisms, including C. elegans, Drosophila and zebra fish20, 27, 36–38. The C. elegans TRPN channel TRP-4 represents the first-identified TRP family member that functions as a mechanically-gated channel39. TRP-4 is required for proprioception mediated by the DVA neuron20. However, trp-4 mutant animals did not exhibit a defect in anus response (Figure 5e). TRP-4 is also expressed in dopamine neurons, including PDE20, 36. We therefore recorded PDE in response to touch by patch-clamp. A glass probe driven by a piezo actuator was used to deliver touch to the cilium of PDE39. Similar to the other dopamine neuron CEP39, mechanical stimulation of the cilium of PDE evoked mechanosensitive currents with both an “on” and “off” response in a TRP-4-dependent manner (Figure 5a–b).
We further asked whether TRP-4 has a role in posterior harsh touch behavior. To eliminate functional redundancy from PVD which does not seem to express TRP-4 (Supplementary Figure S8d), we tested PVD-ablated animals and found that trp-4 mutant animals lost posterior response (Figure 5c). As a control, no such defect was detected in trpa-1 mutant animals (Figure 5c). Importantly, expression of wild-type trp-4 cDNA in PDE fully rescued the phenotype (Figure 5c). We did not test the role of TRP-4 in ADE, as it is merely one of the five anterior sensory neurons; but presumably TRP-4 may play a similar role in this neuron. These observations indicate that TRP-4 regulates harsh touch sensation at least posteriorly.
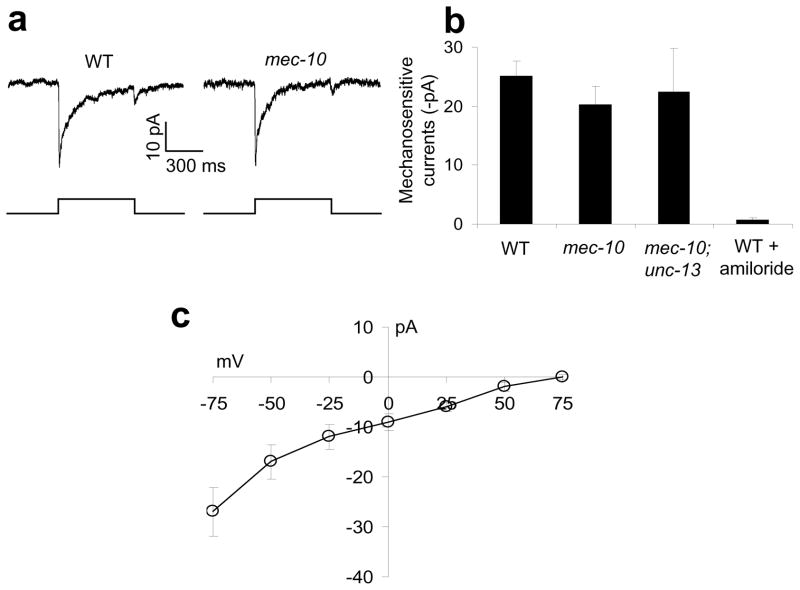
Amiloride-sensitive channels mediate mechano-currents in PVD
We also recorded by patch-clamp the mechanosensitivity of PVD, the other posterior sensory neuron. Mechanical stimulation of the dendrite of PVD evoked mechanosensitive currents with “on” and “off” components (Figure 6a). Unlike dopamine neurons39, mechanosensitive currents in PVD are amiloride-sensitive (Figure 6b). The amiloride sensitivity suggests that the channel is encoded by a member(s) in the ENaC family as reported for gentle touch neurons8. Indeed, PVD mechanosensitive currents were inwardly rectifying with a reversal potential around +70 mV, close to the equilibrium potential of Na+, consistent with the view that they are carried by an ENaC channel(s) (Figure 6c).
Figure 6. An amiloride-sensitive channel(s) mediates mechanosensitive currents in PVD.
(a) Mechanical stimulation evokes mechanosensitive currents in PVD. Such currents persisted in mec-10(tm1552). Clamping voltage: −75 mV. Touch was directed to the primary dendrite of PVD. Displacement: 20 μm. A 10 μm displacement was needed to evoke mechanosensitive currents in PVD, a much higher threshold than that in PDE. PVD in mec-10(tm1552) animals displayed a similar sensitivity (threshold).
(b) Bar graphs. Touch-evoked mechanosensitive currents in PVD were sensitive to amiloride (200 μM) but persisted in mec-10(tm1552), as well as in mec-10(tm1552); unc-13(e51) double mutant animals. n≥5. Error bars: SEM.
(c) I–V relations of mechanosensitive currents in PVD. n≥5. Error bars: SEM.
The above result prompted us to examine MEC-10, an ENaC channel recently found to be essential for PVD mechanosensitivity in a calcium imaging assay12. Notably, mechanosensitive currents remained in PVD of mec-10(tm1552) mutant animals (Figure 6a–b). These mutant animals also retained behavioral responses to harsh touch (Supplementary Figure S9). The mechanosensitive currents in PVD persisted in mec-10;unc-13 double mutant animals in which synaptic transmission was abrogated, indicating that the recorded currents originated in PVD (Figure 6b). We did not examine the other candidate mechanosensitive ENaC channel DEGT-1 expressed in PVD12, as no mutant is available for this channel. It is possible that another ENaC channel or DEGT-1 carries the mechanosensitive currents in PVD; alternatively, multiple ENaC channels may function redundantly in PVD. The discrepancy in MEC-10 between the two studies may result from the use of different functional and behavioral assays.
Animals lacking harsh touch neurons respond to gentle touch
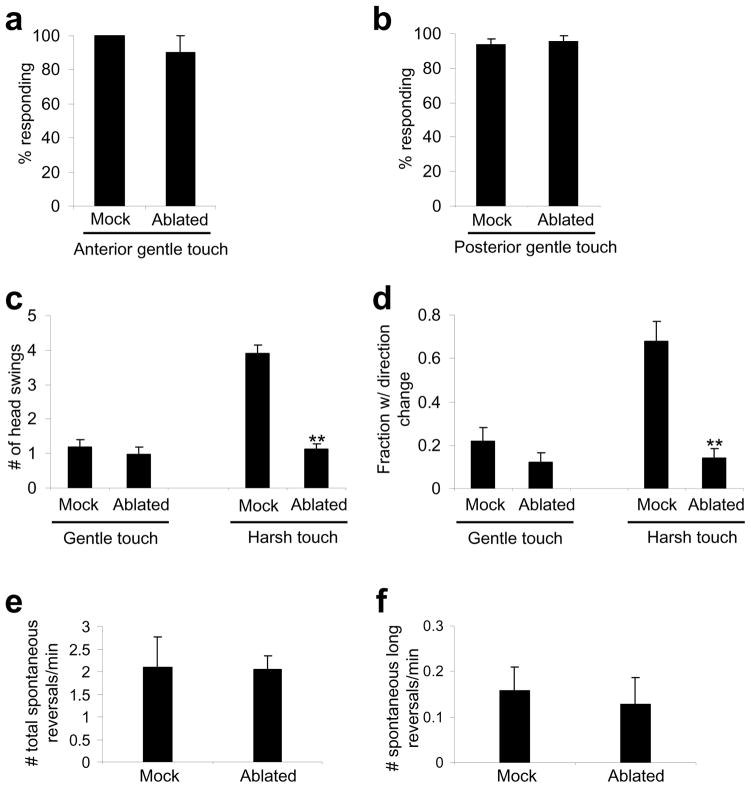
Considering that gentle touch-insensitive mutant animals are sensitive to harsh touch, one might ask whether animals lacking harsh touch sensory neurons respond to gentle touch. To test this, we ablated all of the anterior harsh touch sensory neurons, but left intact the anterior gentle touch sensory neurons ALM and AVM. These animals responded normally to anterior gentle touch (Figure 7a). We also killed the posterior harsh touch sensory neurons PVD and PDE, but spared the posterior gentle touch neuron PLM. These animals responded normally to posterior gentle touch (Figure 7b). Thus, animals lacking harsh touch sensory neurons retained gentle touch responses, providing additional evidence that C. elegans employs distinct sensory neurons to detect harsh touch and gentle touch.
Figure 7. Animals lacking harsh touch sensory neurons can no longer distinguish harsh touch from gentle touch.
(a) Worms lacking anterior harsh touch sensory neurons retain sensitivity to anterior gentle touch. All five anterior sensory neurons (BDU, SDQR, FLP, ADE and AQR) were ablated. Anterior gentle touch neurons (ALM and AVM) were not killed. n=10. Error bars: SEM.
(b) Worms lacking posterior harsh touch sensory neurons retain sensitivity to posterior gentle touch. PDE and PVD were ablated. The posterior gentle touch neuron PLM was not killed. n=10. Error bars: SEM.
(c) Worms lacking harsh touch sensory neurons responded similarly to harsh touch and gentle touch. All anterior harsh touch sensory neurons were ablated, and the ablated animals were assayed for anterior gentle and harsh touch responses. The number of head swings of backward movement triggered by anterior harsh and gentle touch was scored. Gentle touch sensory neurons were not killed. n=10. **p<0.0001 (ANOVA with Bonferroni test compared to mock). Error bars: SEM.
(d) The same worms in (c) were scored for the frequency of direction change following backward movement. n=10. **p<0.0001 (ANOVA with Bonferroni test compared to mock). Error bars: SEM.
(e) Animals lacking all anterior harsh touch sensory neurons show a normal frequency of spontaneous reversals. n=10. Error bars: SEM.
(f) Animals lacking all anterior harsh touch sensory neurons show a normal frequency of long reversals during spontaneous locomotion. Long reversals are defined as backward movement events with >3 head swings. n=10. Error bars: SEM.
But these animals interpret harsh touch as gentle touch
Having demonstrated that animals lacking harsh touch sensory neurons retained gentle touch responses, we wondered how these animals respond to harsh touch (Figure 7c–f). Again, we focused on the anterior response by quantifying the number of head swings of backward movement triggered by anterior harsh and gentle touch. In ablated animals (lacking anterior harsh touch neurons but retaining anterior gentle touch neurons), the number of head swings evoked by harsh touch was similar to that triggered by gentle touch (Figure 7c). This defect was not caused by an inability of ablated animals in executing long reversals, as they exhibited long reversals in spontaneous locomotion at a frequency similar to that observed in mock-ablated animals (Figure 7f). In addition, following reversals, ablated animals typically moved forward without a direction change, a phenomenon resembling gentle touch response (Figure 7d). These results suggest that animals lacking harsh touch sensory neurons can no longer distinguish harsh touch from gentle touch, providing further evidence that C. elegans employs distinct sensory neurons to sense harsh touch and gentle touch.
A neuronal correlate for harsh and gentle touch sensation
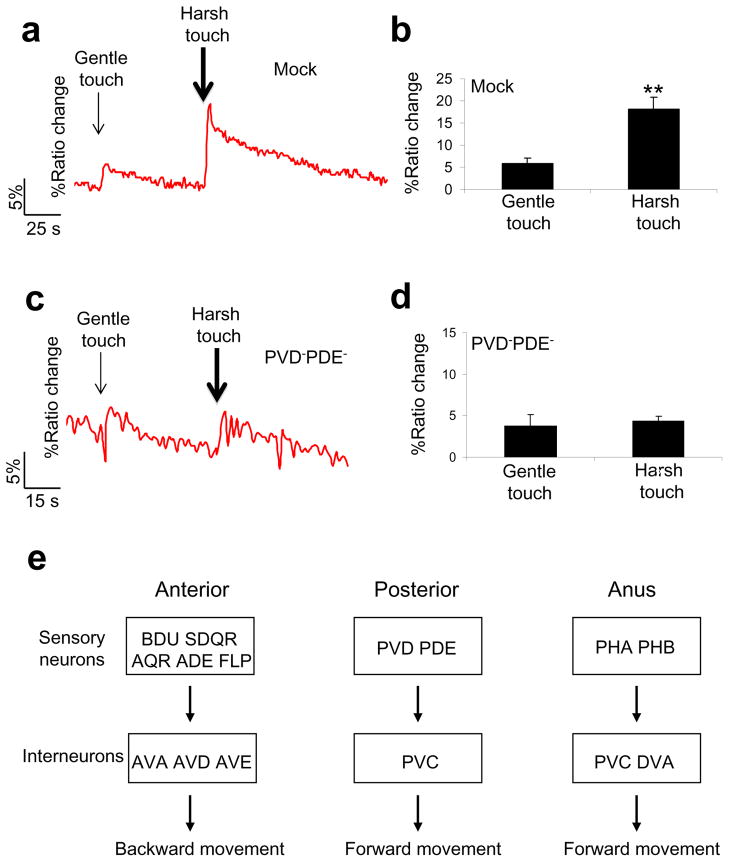
Lastly, we explored the neural basis by which C. elegans distinguishes harsh touch from gentle touch. Clearly, the interneurons mediating gentle and harsh behavior largely overlap. This suggests that gentle and harsh touch sensory neurons may send differential outputs to the interneurons. We tested this model on the interneuron PVC, as we found it relatively easy to record the response of this neuron to touch by calcium imaging. Both gentle and harsh touch can stimulate PVC (Figure 8a–b). Importantly, the amplitude of PVC calcium response evoked by harsh touch was significantly greater than that induced by gentle touch (Figure 8a–b). This is consistent with our behavioral data showing that harsh touch responses were more robust than gentle touch responses.
Figure 8. Differential stimulation of the interneuron PVC by gentle and harsh touch.
(a) Harsh touch evokes a greater calcium response in PVC. Gentle touch (5μm at 20 Hz; 3 s) and harsh touch (20 μm at 20 Hz; 3 s) were sequentially delivered to the posterior body of immobilized animals.
(b) Bar graph summarizing the data in (a). **p<0.0001 (t test). n=21. Error bars: SEM.
(c) Animals lacking harsh touch sensory neurons responded similarly to harsh and gentle touch in PVC.
(d) Bar graph summarizing the data in (c). n=5. Error bars: SEM.
(e) A diagram summarizing the neural circuits underlying harsh touch sensation. The sensory neurons and interneurons required for harsh touch sensation in each body segment are listed.
As our behavioral experiments revealed that animals lacking harsh touch sensory neurons interpret harsh touch as gentle touch, we sought to provide a neuronal correlate for this observation. We repeated the calcium imaging experiment on animals lacking the posterior harsh touch sensory neurons (Figure 8c–d). PVC in these animals no longer displayed differential calcium responses to gentle and harsh touch but instead responded similarly to these two types of stimuli (Figure 8c–d), providing a neuronal correlate for our behavioral observation.
Discussion
In this study, we show that C. elegans exhibits differential behavioral responses to harsh touch and gentle touch, an observation also seen in higher organisms2. That C. elegans exhibits robust harsh touch responses may allow it to effectively avoid this hazardous cue, providing a mechanism for its survival. C. elegans recruits a distinct set of sensory neurons to detect harsh touch, a phenomenon that is also observed in higher organisms (Figure 8e). Thus, harsh touch sensation in nematodes and mammals appears to bear some interesting analogies at the behavioral and neural circuit level.
Unlike gentle touch sensory neurons that all belong to the same class of non-ciliated neurons containing 15-protofilament microtubules1, the sensory neurons required for harsh touch sensation include both ciliated and non-ciliated cells. Despite this morphological difference, many of these neurons share the same precursors in lineage, and some are in fact sister neurons. For example, the posterior sensory neuron PVD and PDE both are descendents of V5; BDU and ALM are sister neurons; SDQR and AVM share the same precursor QL19. It is not clear whether this contributes to their functional identity in touch sensation.
PVD and FLP are both multidendritic neurons17, with one involved in detecting posterior touch and the other in anterior touch. As the dendrites of these neurons cover a large surface area of C. elegans body, they may possess a large receptive field. Then how would ciliated neurons compensate for the small size of their sensory cilia? Notably, the sensory cilia of ciliated neurons such as PDE exhibit a higher mechanosensitivity than the dendrites of PVD. Unlike in patch-clamp recordings, in behavioral assays the majority of touch stimuli are probably delivered at a position quite distant to rather than right on top of the cilia; in this case, their high mechanosensitivity would allow them to sense those nearby or distantly positioned touch stimuli. In this regard, these ciliated neurons may also be considered as light-touch neurons that sense harsh touch in distance. Finally, it should be noted that although we have identified the sensory neurons required for harsh touch sensation, whether they all are truly mechanosensory neurons like PVD and PDE require functional confirmation, and we also do not exclude the possibility that other sensory neurons may play a role in this behavior.
Unlike sensory neurons, the interneurons required for harsh touch and gentle touch behaviors largely overlap, suggesting that these interneurons may receive differential inputs from gentle and harsh touch sensory neurons. This model is supported by calcium imaging analysis of the interneuron PVC in response to gentle and harsh touch. This suggests that the sensory information may be encoded at least partly in the sensory neurons in the posterior circuit.
The observation that gentle touch mutants are sensitive to harsh touch suggests that C. elegans may not rely on the same genes to regulate harsh touch responses. Indeed, we show that both TRP and ENaC family channels are involved in regulating harsh touch sensation. Although the activity of mechanically-gated channels has been detected in many cell types in metazoans, very little is known about their molecular identities27, 30. The ENaC family channel MEC-4/MEC-10, K2P channel TREK-1, newly identified Piezos, and TRPN family channel TRP-4 arguably represent the only exceptions30, 39, 40. We have tested a number of TRP channel mutants in harsh touch behavior. Although most of the tested TRP channels are not essential for behavioral responses to harsh touch, it does not preclude the possibility that they do play a role in harsh touch sensation. The identification of TRP and ENaC family channels provides a proof-of-principle that C. elegans can be used as a model to identify genes regulating harsh touch sensation. Our studies lay the foundation for future work that promises to elucidate the molecular mechanisms underlying harsh touch sensation in C. elegans, which may facilitate our understanding of this sensory modality in higher organisms.
Methods
Strains
WT: N2. CB1338: mec-3(e1338) - CGC strain, not outcrossed. TQ526: mec-3(e1338) 4x outcrossed. TQ1243: mec-4(e1611) 6x outcrossed. TQ428: akIs3[Pnmr-1::gfp] 8x outcrossed. TQ509: daf-19(m86); mec-4(e1611). TQ519: xuEx320[Pmec-3::gfp + Pdat-1::yfp2 + Pdat-1::dsred]. TQ945: xuEx16[Pgcy-35::yfp + Pmec-3::gfp]. TQ1380: xuEx847[Ptwk-16::dsred]. TQ1415: xuEx471[Pdat-1::dsred + Punc-122::gfp]. TQ1991: xuEx536[Pmec-3::dsred + Pocr-2::dsred]; xuEx320. TQ1436: xuEx368[Pdat-1::ChR2 + Pdat-1::dsred + lin-15(+)]; lite-1(xu7). TQ1438: xuEx474[Pocr-2::ChR2 + Pocr-2::dsred + lin-15(+)]; lite-1(xu7). TQ1443: xuEx479[Pgcy-35::ChR2 + Pgcy-35::dsred + lin-15(+)]; lite-1(xu7). TQ1576: lite-1(xu7); mec-4(e1611); xuEx531[Pmec-3::ChR2 + Pmec-3::dsred + lin-15(+)]. TQ1737: trp-4(sy695); xuEx584[Pdat-1::trp-4::SL2::yfp + Punc-122::gfp]; xuEx12. TQ1732: trp-4(sy695); xuEx320. TQ1569: mec-10(tm1552); xuEx320. TQ1988: trpa-1(ok999); xuEx320. TQ1989: ocr-2(ak47); xuEx320. TQ1990: osm-9(ky10); xuEx320. TQ2177: ocr-2(ak47)osm-9(ky10); xuEx320. TQ2179: mec-10(tm1552); unc-13(e51); xuEx12. TQ2290: lite-1(xu7); xuEx648[Pnmr-1::G-CaMP3.0 + Pnmr-1::dsred]; xuEx471.
Behavioral analysis and molecular biology
Harsh touch was delivered with a platinum wire pick (2 cm in length, 99.95% purity, 0.25 mm in diameter; cat# PTP101 from WPI) mounted to a glass Pasteur pipette. The tip of the pick was flattened to 20 μm in thickness and cut to make an edge with a width of 30 μm. We touched the worm body with the edge in a top-down manner. One should avoid scratching the surface of the assay plate. One hour prior to testing, day 1 adult animals were transferred to an NGM plate spread with a thin layer of freshly grown OP50 bacteria. Each worm was tested five times with a 2–10 min interval between each trial, and a response rate for each worm was tabulated. We tested anterior response on animals slowly moving forward, and examined posterior, anus and middle-segment (vulva touch) responses on animals that were non-moving or nearly non-moving. To estimate forces, we touched animals on an NGM testing plate, which was placed on top of an analytical balance. The applied forces can be estimated by recording the readings on the analytical balance. In this case, the worm was semi-immobilized by gluing its vulva area with cyanoacrylate glue to facilitate force recording. This protocol estimated that the average force applied to the anterior, posterior and anus area was: 140±40μN, 150±30μN, and 80±40μN, respectively (n=10 each, ± indicates SD). Before scoring freely-moving animals, we found it helpful to practice touching animals on an analytical balance. We recommend not starting to score animals unless the practice shows that applied forces are within the range as listed above.
Gentle touch was delivered with an eyelash. mec-4 mutant animals may respond to some “stiff” eyelash if large forces are applied. Thus, prior to each new experiment, we always practiced on both wild-type and mec-4 mutant animals to ensure that wild-type animals responded but mec-4 animals did not.
To quantify spontaneous reversal rate, we used an automated worm tracking system41, 42. Day 1 adult animals were tracked for 10 min on NGM plates spread with a thin layer of freshly-grown OP50 bacteria.
Laser ablation was performed on L1–L3 animals with a MicroPoint Laser system (Photonic Instruments) on 2% agarose pad, and ablated animals were allowed to grow to adulthood before behavioral analysis43 (Supplementary Figure S10). A GFP transgene driven by the nmr-1 promoter was used to aid ablation of interneurons44. To ablate BDU, SDQ, AQR and PQR, we used a YFP transgene driven by the gcy-35 promoter45. A GFP transgene under the mec-3 promoter was used to ablate gentle touch neurons and PVD and FLP11, and a similar transgene under the dat-1 promoter was used to help ablate PDE and ADE46. To ablate PHA/PHB, we used a GFP transgene expressed under the ocr-2 promoter31. To rescue the trp-4 phenotype in PDE, wild-type trp-4 cDNA was expressed as a transgene under the dat-1 promoter46, and the plasmid was directly injected into the trp-4(sy695) mutant background.
Optogenetics
Animals were grown on NGM plates containing 5 μM of all-trans retinal. Day 1 adult animals were tested on retinal-free plates spread with a thin layer of freshly grown OP50 bacteria. All experiments were performed on lite-1(xu7) animals that lack intrinsic phototaxis responses to short-wavelength light 4, 5. Each worm was tested five times with a 5–8 min interval between each trial, and a response rate was tabulated for each worm. Blue light pulses (2 sec from an EXFO Xcite lamp) at an intensity of 5–10 mW/mm2 were delivered to the anterior, posterior or tail of the worm via a 10x objective in combination with a zoom lens on a Zeiss microscope (Zeiss Discovery). We closed the diaphragm of the microscope to direct light to individual body parts. To test posterior and anus responses, we challenged non-moving animals. A positive response was scored if the animal began to move forward (for posterior and anus responses) or stopped forward movement (for anterior response) within 3 sec after the cessation of light illumination and also initiated backward movement that lasted at least half a head swing. Background light used to visualize animals was filtered into red. Light intensity was determined with a radiometric sensor head (268LP) coupled to an optometer (S471; UDT Instruments).
Electrophysiology and calcium imaging
Whole-cell recordings were performed on an Olympus microscope (BX51WI) with an EPC-10 amplifier controlled by the Patchmaster software (HEKA). A glass stimulus probe was driven by a Piezo actuator (PI) mounted on a micromanipulator (Sutter). Animals were glued to a sylgard-coated coverglass covered with bath solution, and a small piece of cuticle in the posterior body was cut open and pinned down to the coverglass to expose the cell body of PDE and PVD, which can be identified by a green or red fluorescent protein marker expressed as a transgene driven by the dat-1 and mec-3 promoter, respectively11, 47. Touch was directed to the cilium of PDE or primary dendrite of PVD by pressing the cuticle overlaying the cilium or primary dendrite, respectively. Recording pipettes were pulled from borosilicate glass. The bath solution contains 145 mM NaCl, 10 mM HEPES, 20 mM glucose, 1 mM MgCl2, 1 mM CaCl2, and 2.5 mM KCl (335 mOsm; pH adjusted to 7.3 with NaOH). The pipette solution contains 145 mM CsCl, 10 mM HEPES, 20 mM glucose, 0.25 mM CaCl2, and 5 mM EGTA, 5 mM Na2ATP, 0.5 mM GTP (325 mOsm; pH adjusted to 7.2 with CsOH). Voltages were clamped at −75 mV. Current data were sampled at 20–40 kHz. Series resistance and membrane capacitance were both compensated during recording. Liquid junction potentials were also corrected.
Calcium imaging was performed on the same hardware system used for patch-clamp recording. MetaFlour (Molecular Devices) was used for imaging acquisition and analysis. G-CaMP3.0 and DsRed2 were co-expressed in PVC as a transgene driven by the nmr-1 promoter to allow for ratiometric imaging. Animals were immobilized on a 5% agarose pad with cyanoacrylate glue in bath solution containing 2.5 mM serotonin. G-CaMP and DsRed2 fluorescence was excited at 484 nm and 565 nm, respectively. The peak percentage ratio change (484/565 nm) was used to quantify calcium levels in PVC.
Supplementary Material
Acknowledgments
We thank A. Kumar for comments, L. Looger for G-CaMP3.0, W. Schafer for strains, and A. Gottschalk for ChR2 plasmid. Some strains were obtained from the Caenorhabditis Genetics Center and Knockout Consortiums in the U.S.A. and Japan. This work was supported by an AHA postdoctoral fellowship (W.L.) and grants from the NIGMS (X.Z.S.X.) and Pew Scholars Program (X.Z.S.X).
Footnotes
Author contributions
W.L. performed the experiments and analyzed the data. L.K. carried out the electrophysiological recordings and analyzed the data. B.J.P. and Z.F. contributed reagents and tools, respectively. X.Z.S.X. supervised the project and wrote the paper with help from all other authors.
Competing financial interests: The authors declare no competing financial interests.
References
- 1.Bounoutas A, Chalfie M. Touch sensitivity in Caenorhabditis elegans. Pflugers Arch. 2007;454:691–702. doi: 10.1007/s00424-006-0187-x. [DOI] [PubMed] [Google Scholar]
- 2.Purves D, et al. Sensation and sensory processsing. In: Fitzpatrick D, editor. Neuroscience. Sinauer Associates, Inc; Sunderland: 2008. pp. 207–393. [Google Scholar]
- 3.Bargmann CI. Chemosensation in C. elegans. WormBook; 2006. pp. 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward A, Liu J, Feng Z, Xu XZ. Light-sensitive neurons and channels mediate phototaxis in C. elegans. Nature Neurosci. 2008;11:916–922. doi: 10.1038/nn.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, et al. C. elegans phototransduction requires a G protein–dependent cGMP pathway and a taste receptor homolog. Nature neuroscience. 2010;13:715–722. doi: 10.1038/nn.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards SL, et al. A novel molecular solution for ultraviolet light detection in Caenorhabditis elegans. PLoS Biol. 2008;6:e198. doi: 10.1371/journal.pbio.0060198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan JM, Horvitz HR. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:2227–2231. doi: 10.1073/pnas.90.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nature neuroscience. 2005;8:43–50. doi: 10.1038/nn1362. [DOI] [PubMed] [Google Scholar]
- 9.Wetzel C, et al. A stomatin-domain protein essential for touch sensation in the mouse. Nature. 2007;445:206–209. doi: 10.1038/nature05394. [DOI] [PubMed] [Google Scholar]
- 10.Lu Y, et al. The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron. 2009;64:885–897. doi: 10.1016/j.neuron.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Way JC, Chalfie M. The mec-3 gene of Caenorhabditis elegans requires its own product for maintained expression and is expressed in three neuronal cell types. Genes Dev. 1989;3:1823–1833. doi: 10.1101/gad.3.12a.1823. [DOI] [PubMed] [Google Scholar]
- 12.Chatzigeorgiou M, et al. Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nature neuroscience. 2010;13:861–868. doi: 10.1038/nn.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodman MB. Mechanosensation. WormBook; 2006. pp. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Driscoll M, Chalfie M. The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature. 1991;349:588–593. doi: 10.1038/349588a0. [DOI] [PubMed] [Google Scholar]
- 15.Chalfie M, et al. The neural circuit for touch sensitivity in Caenorhabditis elegans. J Neurosci. 1985;5:956–964. doi: 10.1523/JNEUROSCI.05-04-00956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 17.Albeg A, et al. C. elegans multi-dendritic sensory neurons: morphology and function. Molecular and cellular neurosciences. 2011;46:308–317. doi: 10.1016/j.mcn.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oren-Suissa M, Hall DH, Treinin M, Shemer G, Podbilewicz B. The fusogen EFF-1 controls sculpting of mechanosensory dendrites. Science (New York, NY) 2010;328:1285–1288. doi: 10.1126/science.1189095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Feng Z, Sternberg PW, Xu XZS. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature. 2006;440:684–687. doi: 10.1038/nature04538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wicks SR, Rankin CH. Integration of mechanosensory stimuli in Caenorhabditis elegans. J Neurosci. 1995;15:2434–2444. doi: 10.1523/JNEUROSCI.15-03-02434.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jose AM, Bany IA, Chase DL, Koelle MR. A specific subset of transient receptor potential vanilloid-type channel subunits in Caenorhabditis elegans endocrine cells function as mixed heteromers to promote neurotransmitter release. Genetics. 2007;175:93–105. doi: 10.1534/genetics.106.065516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagel G, et al. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol. 2005;15:2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 24.Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nature reviews. 2007;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 25.Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 26.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nature reviews. 2007;8:510–521. doi: 10.1038/nrn2149. [DOI] [PubMed] [Google Scholar]
- 28.Xiao R, Xu XZS. Function and regulation of TRP family channels in C. elegans. Pflugers Arch. 2009;458:851–860. doi: 10.1007/s00424-009-0678-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao R, Xu XZC. elegans TRP Channels. Advances in experimental medicine and biology. 2011;704:323–339. doi: 10.1007/978-94-007-0265-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharif-Naeini R, et al. TRP channels and mechanosensory transduction: insights into the arterial myogenic response. Pflugers Arch. 2008;456:529–540. doi: 10.1007/s00424-007-0432-y. [DOI] [PubMed] [Google Scholar]
- 31.Tobin D, et al. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35:307–318. doi: 10.1016/s0896-6273(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 32.Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwan KY, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 34.Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 35.Kindt KS, et al. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nature neuroscience. 2007;10:568–577. doi: 10.1038/nn1886. [DOI] [PubMed] [Google Scholar]
- 36.Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science (New York, NY) 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- 37.Sidi S, Friedrich RW, Nicolson T. NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science (New York, NY) 2003;301:96–99. doi: 10.1126/science.1084370. [DOI] [PubMed] [Google Scholar]
- 38.Kindt KS, et al. Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans. Neuron. 2007;55:662–676. doi: 10.1016/j.neuron.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 39.Kang L, Gao J, Schafer WR, Xie Z, Xu XZSC. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 2010;67:381–391. doi: 10.1016/j.neuron.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coste B, et al. Piezo1 and Piezo2 Are Essential Components of Distinct Mechanically Activated Cation Channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng Z, et al. A C. elegans model of nicotine-dependent behavior: regulation by TRP family channels. Cell. 2006;127:621–633. doi: 10.1016/j.cell.2006.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu AL, Feng Z, Hsieh MY, Xu XZ. Identification by machine vision of the rate of motor activity decline as a lifespan predictor in C. elegans. Neurobiol Aging. 2009;30:1498–1503. doi: 10.1016/j.neurobiolaging.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bargmann CI, Avery L. Laser killing of cells in Caenorhabditis elegans. Methods Cell Biol. 1995;48:225–250. doi: 10.1016/s0091-679x(08)61390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brockie PJ, Madsen DM, Zheng Y, Mellem J, Maricq AV. Differential expression of glutamate receptor subunits in the nervous system of Caenorhabditis elegans and their regulation by the homeodomain protein UNC-42. J Neurosci. 2001;21:1510–1522. doi: 10.1523/JNEUROSCI.21-05-01510.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gray JM, et al. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- 46.Nass R, Hall DH, Miller DM, 3rd, Blakely RD. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3264–3269. doi: 10.1073/pnas.042497999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lints R, Emmons SW. Patterning of dopaminergic neurotransmitter identity among Caenorhabditis elegans ray sensory neurons by a TGFbeta family signaling pathway and a Hox gene. Development. 1999;126:5819–5831. doi: 10.1242/dev.126.24.5819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.