Abstract
Stem cells provide novel sources of cell therapies for motor neuron disease that have recently entered clinical trials. In the present study, we transplanted human neural stem cells (NSCs) into the ventral horn of both the lumbar (L4–L5) and cervical (C4–C5) protuberance of SOD G93A rats, in an effort to test the feasibility and general efficacy of a dual grafting paradigm addressing several muscle groups in the front limbs, hind limbs and the respiratory apparatus. Transplantation was done prior to the onset of motor neuron disease. Compared with animals that had received dead NSC grafts (serving as controls), rats with live NSCs grafted at the two spinal levels lived 17 days longer. Disease onset in dually grafted animals was delayed by 10 days compared to control animals. Disease duration in NSC-grafted animals was longer by 7 days compared to controls. Our results support the potential of NSC grafts at multiple levels of spinal cord as future cellular therapy for motor neuron disease.
Keywords: Amyotrophic lateral sclerosis, grafting, cell therapies, regeneration, animal models
Introduction
Amyotrophic lateral sclerosis (ALS) is featured by progressive degeneration of upper and lower motor neurons [1]. Cell death prevention strategies, including the use of trophic factors and small neuroprotective molecules, have had very limited clinical success, whereas molecular mechanisms proposed on the basis of certain autosomal dominant forms of ALS have been poor predictors of therapeutic targeting [2]. Cell replacement or cell protection strategies using neural stem cell (NSC) grafts have rekindled therapeutic optimism [3] and the first ALS clinical trial using NSCs in one transplantation site (lumbar) has been recently approved by FDA (http://www.alsa.org/news/, http://www.alsa.org/patient/drug.cfm?id=1575).
Although most cases of ALS are sporadic, 10% of cases are familial and a fifth of them are associated with > 90 mutations in the superoxide dismutase 1 (SOD1) gene which encodes a Cu/Zu SOD [4–6]. Several transgenic animal models were established based on these mutations, including SOD1 G93A rats and mice overexpressing an SOD1 mutation at amino acid position 93 (Gly-Ala)[7–11]. Transgenic SOD1 G93A rodents successfully reproduce most clinical features of ALS and have been extensively used to characterize pathology [12–15] and to serve as subjects for experimental therapeutic trials [16–23].
SOD1 G93A rats have become popular because of size and handling advantages when compared to mice. In our previous studies, we found that human NSCs grafted into the lumbar spinal cord of these rats survive in the degenerative environment, undergo extensive neuronal differentiation, and improve several indicators of motor neuron disease including prolongation of life span, improvement of motor function and delay of disease onset time [22]. In addition, with both transsynaptic tracing and immuno-electron microscopy, differentiated human NSCs have been shown to form structurally mature, mostly inhibitory, synapses on host motor neurons, a pattern demonstrating that NSCs integrate into the host motor circuitry [24]. Taken together, the above findings indicate that SOD1 G93A rats are useful for the preclinical evaluation of human NSCs as therapeutic tools for motor neuron disease.
The present investigation is founded on the prior success of lumbar grafts of human NSCs in SOD1 G93A rodents [22]. Although ongoing clinical trials have adopted the one-site lumbar grafting that we espoused in our previous work, it is possible that future cellular therapies will broaden the grafting paradigm to also include cervical segments that innervate front limbs and respiratory muscles such as the diaphragm. Therefore, in the present study, we combined human NSC grafts into the lumbar protuberance as in our previous experiments with grafts into the cervical segment of spinal cord in the same animals. We asked whether combined grafts are feasible, safe and can also afford general clinical benefits for experimental animals. Our results are consistent with the idea that combined lumbar-cervical grafts are safe and effective and can form the basis for multiple-site transplantation therapies for ALS in the future.
Materials and methods
SOD1 G93A rat breeding
SOD1 G93A male rats from Taconic (Germantown, NY) were bred to Tac:N(SD) female rats from the same company as one male: one female. Offspring were weaned and genotyped at 21 days of age and positive transgenic male pups were bred with original females to propagate the colony. The rest of positive pups were used for NSCs grafting. All animal care and surgery procedures were carried out according to protocols approved by the Animal Care and Use Committee of the Johns Hopkins Medical Institutions.
Preparation of human NSCs
Human NSCs used in this study were prepared from the cervical-thoracic cord of a single 8-week human fetus as described [25]. Tissues were donated by the mother in a manner compliant with NIH and FDA guidelines. Dissociation and expansion of cells in monolayer were performed as described [26]. Cryopreserved NSI-566RSC cells [25] from passage 12 were thawed, washed, concentrated, and transplanted directly without re-culturing. Viability of the cells was 80–90% at the time of transplantation. Immediately prior to transplantation, less than 1% of these cells express the neuronal markers TUJ1 and MAP2 or the astroglial marker GFAP. The overwhelming majority of human NSCs express nestin, whereas ~5% are immunoreactive for PSA-NCAM [27]. Dead cells were prepared by three cycles of snap-freezing NSCs in liquid nitrogen and then slow-thawing at room temperature. The completed cell death was confirmed by Trypan Blue staining.
Surgical procedures
Surgical procedures utilized gas anesthesia (isoflurane: oxygen: nitrous oxide = 1:33:66) and aseptic conditions. In order to reduce phenotype variance brought on by litter difference, animals from the same litter were paired in experimental (i.e. live-cell recipient) and control (i.e. dead-cell recipient) groups. Live or dead NSCs were transplanted into both cervical and lumbar segments of the spinal cord of 63-day old animals (220–300 g)(n=11 per group). Briefly, dorsal laminectomies were performed to expose the spinal cord at C4–C5 and L4–L6 levels. Cell suspensions were delivered first via 4 bilateral injections at C4–C5 and then via 8 bilateral injections at L4–L6 (equal number of injections on each side). All injections were aimed at the ventral horn on both sides (2×104 cells/1μL per injection site, total 2.4 ×105 cells per animal from 12 injections) with a 33-gauge needle using 10 μl Hamilton syringes attached to a Kopf stereotaxic device. After surgery, rats were given FK-506 (i.p., q.d., 1mg/kg/day) to prevent immune rejection which is a well-known problem in xenografting. FK-506 has been shown in previous studies to effectively prevent immune rejection of human NSC grafts by rodent hosts [22;25].
Histology, immunocytochemistry, and microscopy
To confirm the survival, migration, differentiation and integration into motor circuit of grafted cells at the two injection sites, immunocytochemical staining of the post-mortem spinal cords was done as previously described [22]. Briefly, spinal cords were dissected and processed after animals were transcardially perfused with 4% neutral-buffered paraformaldehyde. Coronal spinal cord sections (35μm) were incubated in primary antibodies, then in secondary antibodies conjugated with cyanine dyes (Cy3 or Cy2), and then counterstained with DAPI and coverslipped. The primary antibodies used in this study are as follows: mouse anti- human nuclear antigen protein antibody (HNu, 1:800, human specific, Milipore, Billerica, MA) to identify grafted human NSCs; rabbit anti-TUJ1 (1:400; human, rat and mouse specific, Research Diagnostics Inc., Flanders, NJ) to evaluate neuronal differentiation; mouse anti- synaptophysin (SYN, 1:200, human and hamster specific, Milipore, Billerica, MA) to evaluate synaptic terminal formation in human NSC-derived nerve cells; goat anti-ChAT IgG (1:100, human, rat and mouse specific, Milipore, Billerica, MA) to label host motor neurons. Immunostained sections were studied with a Zeiss Axiophot microscope and images were captured with a Spot RT Slider digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI) or a Zeiss LSM 510 inverted confocal microscope (Carl Zeiss, Thornwood, NY). Neuronal differentiation of cervical grafts was evaluated under 100x magnification on randomly selected fields (n=3, 5 fields per subject) by calculating the rate of TUJ1 (+), HNu (+) cells in the entire population of grafted HNu (+) cells.
Animal testing
Rats were weighed twice each week to measure disease onset time as the time point after two consecutive weight decreases. Motor strength tests included the BBB rating [23;28] and the inclined plane [29] scales and were performed as described [22]. For BBB scoring, animals were tested for 4–5 minutes in an open field. For inclined plane scoring, the angle that the animal could stay for at least 5 seconds on the inclined mat was recorded as the subject’s inclined plane score. Rats were sacrificed after BBB score decreased to 3 or lower, i.e. a time point at which only one joint had movement or there was no movement at all. Survival analysis was done with Kaplan Meier curves followed by log-rank test to compare between live- and dead-cell graft groups. End-point analyses of disease-onset time and life span were also used to compare between the two groups with a students’ t test. BBB and incline plane scoring of treatment and control groups were analyzed by two way ANOVA followed by a Fisher LSD post hoc test.
Results
Survival, neuronal differentiation and structural integration of human NSCs in the spinal cord of SOD1 G93A rats
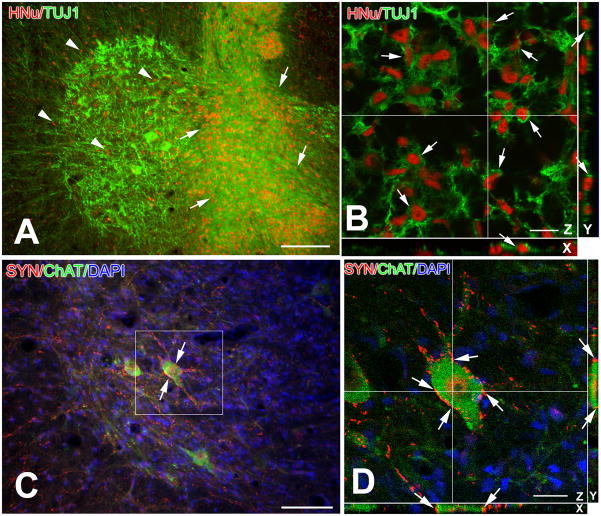
Dual NSC transplantation in the cervical and lumbar cord proved to be safe. With the exception of one fatality in a pilot experiment, there was no surgery-related mortality in either experimental or control groups. Grafted NSCs in cervical and lumbar parenchyma survived, differentiated into neurons and migrated extensively in the spinal cord gray and white matter (Fig. 1A and B). In addition, 68.8±0.07% of NSCs surviving in cervical segments differentiated into neurons, which is very similar to differentiation rates of lumbar cord grafts in our previous studies (68.4%–75.0%) [22;25]. The differentiation and migration in lumbar segments are consistent with previous single-grafting studies [22;25] (data not shown). In C4–C5, differentiated NSCs were seen to form synapses with host motor neurons (Fig. 1C and D), along the same patterns previously observed in the lumbar cord [22;24].
Figure 1.
Survival, migration, neuronal differentiation (A, B) and motor circuit integration (C, D) of human NSC grafts in rat cervical cord parenchyma.
A. Grafted NSCs survive in large numbers and migrated in the gray and white matter of the cervical cord as shown by HNu immunoreactivity (red). Inoculation site is indicated with arrows. Migration of NSCs towards ventral horn is marked with arrowheads. Some grafted cells are located in the white matter (extreme left). Surviving host motor neurons are shown as large TUJ1 (+) nerve cells.
B. This confocal image illustrates the neuronal differentiation of grafted NSCs (arrows) on the basis of dual staining with the graft-selective marker HNu (red) and the generic neuronal marker TUJ1 (green) on X, Y and Z different sectioning planes.
C–D. NSC-derived neurons innervate the cell bodies and proximal dendrites of cervical motor neurons of SOD1 G93A rats. Immunoreactivity for human synaptophysin (SYN, red) serves as a selective marker for graft-derived terminals. Postsynaptic host structures are labeled with the motor neuron phenotypic marker choline acetyltransferase (ChAT, green). In (C), a large number of human synaptophysin (+) boutons (SYN, red; arrows) contacts host ChAT motor neurons (green). B is a confocal image taken from the framed area of (C) to confirm the apposition of human synaptophysin boutons to the cell body and dendrites of the motor neuron at the center of (C). Arrows in (D) depict boutons that are positionally validated with x and y resectioning.
Scale bars: A, 100 μm; B, 20 μm; C, 50 μm, D, 10 μm
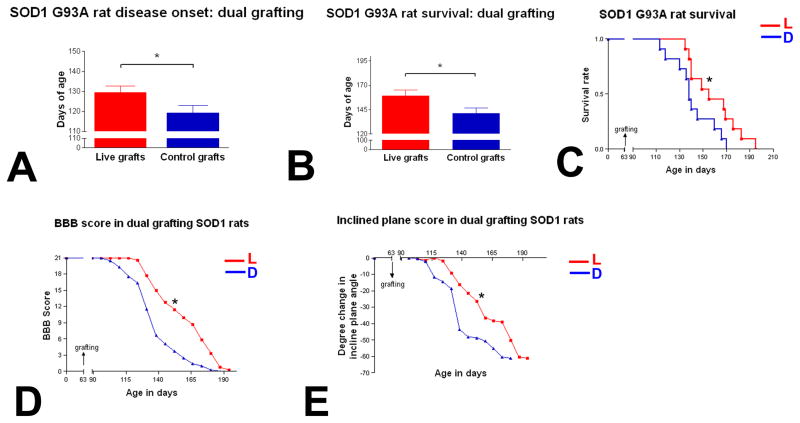
Prolongation of life span, extension of disease duration, delay of disease onset and attenuation of motor weakness after human NSCs grafting
The average disease-onset time for rats with live human NSC grafts was 129.5 ± 3.2 days, i.e. 10 days longer than rats with dead-NSC transplants (119.2 ± 3.7days) (Fig. 1A). Animals with live NSCs showed increased survival by both Kaplan-Meier and end-point analysis. The average life span for animals transplanted with live NSCs was 158.9± 6.3 days, whereas rats grafted with dead NSCs lived for 141.3 ± 5.5 days (Fig. 1B). There is a 17-day difference in survival time between these two groups, which is statistically significant when data are analyzed with student’s t-test. This significance is corroborated with Kaplan-Meier and log rank analysis showing that animals with live NSCs have significant better survival than control animals (Fig. 1C). Disease duration (the time from disease onset to the end point) in animals with live NSC transplants was 29.4 ± 4.6 days, i.e. 7 days longer compared to control animals (22.1 ± 3.5 days). Compared to control animals, muscle weakness in animals with live NSC transplants progressed significantly more slowly as evidenced by time plots of both BBB-open field and inclined-plane strength scores which show a significant separation between animals with live NSCs and controls (Fig. 1D–E).
Taken together, these findings indicate that dual transplantation of human NSCs in the cervical and lumbar spinal cord of SOD1 G93A rats ameliorates motor neuron disease in subjects that reproduce a particularly fulminant type of ALS. Therapeutic effects include delaying disease onset time, slowing disease progression, and prolonging survival and life span, and suggest that dual transplants with human NSCs have a place as experimental cell therapies for patients with ALS.
Discussion
The principal rationale behind the two-site transplantation paradigm implemented here is the need to develop experimental therapeutic models for a disease that involves most somatic muscle groups including hind limbs, front limbs and the diaphragm, and globally affects locomotion and respiratory function [30]. Our findings show that, besides improving motor strength, NSC transplants also extended the animals’ life span by 17 days, i.e. one ninth or so of the life span of these animals. Furthermore, dual transplantation prolonged disease duration in SOD1 G93A rats by 7 days, i.e. 32% disease progression time. These figures compare very favorably to the corresponding ones from our published single-site lumbar grafting experiments [22]. However, the favorable result of dual grafting cannot be directly compared to the outcome from single grafting. Reasons include the fact that dual transplantation is an altogether different surgery protocol involving greater degree of injury to the animal and the spinal cord, and the fact that the two studies use different generations of SOD1 transgenic rats with variable disease severities. The outcome of the present study only demonstrate that dual transplantation involving cervical and lumbar grafting targets is an effective and safe alternative to single transplantation as a potential cellular therapy for ALS.
Consistent with the notion of the importance of cervical targets in stem cell transplantation is recent evidence that focal grafts of glial-restricted precursors in the cervical cord of SOD1 G93A rats ameliorate disease progression and extended survival [31]. Although we did not perform a direct assessment of respiratory function in the present study, such analyses should be the subject of future studies correlating phrenic motor neuron targeting with respiratory improvements.
Transgenic rodents harboring SOD1 mutations that cause certain familial forms of ALS have been extensively used not only to understand disease mechanisms, but also to develop experimental therapeutics [16–23]. In the case of experimental cellular therapies for ALS models, stem cells provide theoretically inexhaustible and very versatile sources of cells for transplantation. Stem cell derivatives, e.g. NSCs, may exert therapeutic effects either by replacing dying motor neurons or by protecting motor neurons in various stages of injury [3]. Based on previous findings with NSC grafts identical to the ones used in the present study [22;24;25;27], motor neuron replacement is not a likely mechanism for the therapeutic effects of human NSCs in SOD1 transgenic rodents. These NSCs differentiate into small neurons that form structurally mature synapses with host motor neurons, but they do not seem to project, as a whole, outside the spinal cord to reach the target musculatures [22;24]. Over 50% these NSC-derived neurons express GABAergic neurotransmitter phenotypes [25] and ~70% of the synapses of these neurons on host motor neurons have a symmetrical morphology typical of inhibitory synapses [24]. Such synapses may contribute to the amelioration of motor neuron disease by “buffering” excessive glutamate release, based on the theory that excitotoxicity contributes to motor neuron degeneration [32;33]. Moreover, human NSCs used in the present study also express and secrete motor neuron trophic factors, i.e. GDNF and BDNF, that can promote the survival of injured motor neurons via classical retrograde or transsynaptic signaling [22;34]. Therapeutic effects of various types of stem cell grafts in transgenic rodent models of ALS have also been observed by other investigators [27;35;36].
Our findings demonstrate that transplantation of human NSCs in multiple segments in the spinal cord is a viable option and an effective alternative to single-segment grafting in experimental therapeutics of ALS. Although mechanisms of therapeutic efficacy of such grafts in ALS and its animal models must be more fully characterized in future research, transplantation strategies presented here may serve as experimental methodologies to inform the ongoing clinical trials of human NSCs in ALS patients.
Figure 2.
Effects of human NSC transplantation on disease onset (A), life span/survival (B–C) and muscle weakness (D–E) in G93A SOD1 rats.
A. Disease onset was 10 days later in the live-cell group (129.5 ± 3.234 days, n=11) compared to the dead-cell group (119.2 ± 3.709 days, N=11) (P=0.0481).
B. End-point analysis shows that the live-cell group has significantly longer life span compared to dead-cell group (158.9 ± 6.256 days and 141.3 ± 5.494 days, respectively, n=11) (P=0.0469).
C. This Kaplan-Meier plot shows a significant separation between live (L) and dead cell group (D) survival throughout the course of the study (p=0.0402), suggesting animals with live NSCs have better survival.
D–E. BBB (D) and inclined plane (E) scores show a significant separation in these two measures of muscle weakness between live-cell (L) and dead-cell (D) groups (p=0.0183 and 0.045, respectively), suggesting muscle weakness progress is significantly slowed down in animals with live NSCs compared with control animals.
Bar= Mean ±SEM, * P<0.05.
Acknowledgments
This work was supported by NIH grant RO1 NS045140-03, the Muscular Dystrophy Association, and the Robert Packard Center for ALS Research at Johns Hopkins.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Price DL, Ackerley S, Martin LJ, Koliatsos VE, Wong PC. Motor neuron diseases. In: Brady ST, Siegel GJ, Albers RW, Price DL, editors. Basic Neurochemistry. Elsevier; Burlington: 2005. pp. 731–743. [Google Scholar]
- 2.Rothstein JD. Of mice and men: reconciling preclinical ALS mouse studies and human clinical trials. Ann Neurol. 2003;53:423–426. doi: 10.1002/ana.10561. [DOI] [PubMed] [Google Scholar]
- 3.Koliatsos VE, Xu L, Yan J. Human stem cell grafts as therapies for motor neuron disease. Expert Opin Biol Ther. 2008;8:137–141. doi: 10.1517/14712598.8.2.137. [DOI] [PubMed] [Google Scholar]
- 4.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 5.Cudkowicz ME, McKenna-Yasek D, Sapp PE, Chin W, Geller B, Hayden DL, Schoenfeld DA, Hosler BA, Horvitz HR, Brown RH. Epidemiology of mutations in superoxide dismutase in amyotrophic lateral sclerosis. Ann Neurol. 1997;41:210–221. doi: 10.1002/ana.410410212. [DOI] [PubMed] [Google Scholar]
- 6.Gaudette M, Hirano M, Siddique T. Current status of SOD1 mutations in familial amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:83–89. doi: 10.1080/14660820050515377. [DOI] [PubMed] [Google Scholar]
- 7.Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 8.Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 9.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 10.Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, DeGennaro LJ, Cleveland DW, Rothstein JD. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proc Natl Acad Sci U S A. 2002;99:1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagai M, Aoki M, Miyoshi I, Kato M, Pasinelli P, Kasai N, Brown RH, Jr, Itoyama Y. Rats expressing human cytosolic copper-zinc superoxide dismutase transgenes with amyotrophic lateral sclerosis: associated mutations develop motor neuron disease. J Neurosci. 2001;21:9246–9254. doi: 10.1523/JNEUROSCI.21-23-09246.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aoki M, Kato S, Nagai M, Itoyama Y. Development of a rat model of amyotrophic lateral sclerosis expressing a human SOD1 transgene. Neuropathology. 2005;25:365–370. doi: 10.1111/j.1440-1789.2005.00611.x. [DOI] [PubMed] [Google Scholar]
- 13.Llado J, Haenggeli C, Pardo A, Wong V, Benson L, Coccia C, Rothstein JD, Shefner JM, Maragakis NJ. Degeneration of respiratory motor neurons in the SOD1 G93A transgenic rat model of ALS. Neurobiol Dis. 2006;21:110–118. doi: 10.1016/j.nbd.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Wengenack TM, Holasek SS, Montano CM, Gregor D, Curran GL, Poduslo JF. Activation of programmed cell death markers in ventral horn motor neurons during early presymptomatic stages of amyotrophic lateral sclerosis in a transgenic mouse model. Brain Res. 2004;1027:73–86. doi: 10.1016/j.brainres.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto A, Okada Y, Nakamichi M, Nakamura M, Toyama Y, Sobue G, Nagai M, Aoki M, Itoyama Y, Okano H. Disease progression of human SOD1 (G93A) transgenic ALS model rats. J Neurosci Res. 2006;83:119–133. doi: 10.1002/jnr.20708. [DOI] [PubMed] [Google Scholar]
- 16.Kriz J, Nguyen MD, Julien JP. Minocycline slows disease progression in a mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2002;10:268–278. doi: 10.1006/nbdi.2002.0487. [DOI] [PubMed] [Google Scholar]
- 17.Kriz J, Gowing G, Julien JP. Efficient three-drug cocktail for disease induced by mutant superoxide dismutase. Ann Neurol. 2003;53:429–436. doi: 10.1002/ana.10500. [DOI] [PubMed] [Google Scholar]
- 18.Acsadi G, Anguelov RA, Yang H, Toth G, Thomas R, Jani A, Wang Y, Ianakova E, Mohammad S, Lewis RA, Shy ME. Increased survival and function of SOD1 mice after glial cell-derived neurotrophic factor gene therapy. Hum Gene Ther. 2002;13:1047–1059. doi: 10.1089/104303402753812458. [DOI] [PubMed] [Google Scholar]
- 19.Wang LJ, Lu YY, Muramatsu S, Ikeguchi K, Fujimoto K, Okada T, Mizukami H, Matsushita T, Hanazono Y, Kume A, Nagatsu T, Ozawa K, Nakano I. Neuroprotective effects of glial cell line-derived neurotrophic factor mediated by an adeno-associated virus vector in a transgenic animal model of amyotrophic lateral sclerosis. J Neurosci. 2002;22:6920–6928. doi: 10.1523/JNEUROSCI.22-16-06920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guillot S, Azzouz M, Deglon N, Zurn A, Aebischer P. Local GDNF expression mediated by lentiviral vector protects facial nerve motoneurons but not spinal motoneurons in SOD1(G93A) transgenic mice. Neurobiol Dis. 2004;16:139–149. doi: 10.1016/j.nbd.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki M, McHugh J, Tork C, Shelley B, Klein SM, Aebischer P, Svendsen CN. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS ONE. 2007;2:e689. doi: 10.1371/journal.pone.0000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu L, Yan J, Chen D, Welsh AM, Hazel T, Johe K, Hatfield G, Koliatsos VE. Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplantation. 2006;82:865–875. doi: 10.1097/01.tp.0000235532.00920.7a. [DOI] [PubMed] [Google Scholar]
- 23.Klein SM, Behrstock S, McHugh J, Hoffmann K, Wallace K, Suzuki M, Aebischer P, Svendsen CN. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther. 2005;16:509–521. doi: 10.1089/hum.2005.16.509. [DOI] [PubMed] [Google Scholar]
- 24.Xu L, Ryugo DK, Pongstaporn T, Johe K, Koliatsos VE. Human neural stem cell grafts in the spinal cord of SOD1 transgenic rats: differentiation and structural integration into the segmental motor circuitry. J Comp Neurol. 2009;514:297–309. doi: 10.1002/cne.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan J, Xu L, Welsh AM, Hatfield G, Hazel T, Johe K, Koliatsos VE. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med. 2007;4:e39. doi: 10.1371/journal.pmed.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johe KK, Hazel TG, Muller T, Dugich-Djordjevic MM, McKay RD. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996;10:3129–3140. doi: 10.1101/gad.10.24.3129. [DOI] [PubMed] [Google Scholar]
- 27.Yan J, Xu L, Welsh AM, Chen D, Hazel T, Johe K, Koliatsos VE. Combined immunosuppressive agents or CD4 antibodies prolong survival of human neural stem cell grafts and improve disease outcomes in amyotrophic lateral sclerosis transgenic mice. Stem Cells. 2006;24:1976–1985. doi: 10.1634/stemcells.2005-0518. [DOI] [PubMed] [Google Scholar]
- 28.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 29.Rivlin AS, Tator CH. Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J Neurosurg. 1977;47:577–581. doi: 10.3171/jns.1977.47.4.0577. [DOI] [PubMed] [Google Scholar]
- 30.Haverkamp LJ, Appel V, Appel SH. Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain. 1995;118(Pt 3):707–719. doi: 10.1093/brain/118.3.707. [DOI] [PubMed] [Google Scholar]
- 31.Lepore AC, Rauck B, Dejea C, Pardo AC, Rao MS, Rothstein JD, Maragakis NJ. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci. 2008;11:1294–1301. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma HS. Interaction between amino acid neurotransmitters and opioid receptors in hyperthermia-induced brain pathology. Prog Brain Res. 2007;162:295–317. doi: 10.1016/S0079-6123(06)62015-3. [DOI] [PubMed] [Google Scholar]
- 33.Woo RS, Li XM, Tao Y, Carpenter-Hyland E, Huang YZ, Weber J, Neiswender H, Dong XP, Wu J, Gassmann M, Lai C, Xiong WC, Gao TM, Mei L. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Rind HB, Butowt R, von Bartheld CS. Synaptic targeting of retrogradely transported trophic factors in motoneurons: comparison of glial cell line-derived neurotrophic factor, brain-derived neurotrophic factor, and cardiotrophin-1 with tetanus toxin. J Neurosci. 2005;25:539–549. doi: 10.1523/JNEUROSCI.4322-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez-Gonzalez R, Knuckles P, Velasco I. Transient Recovery in a Rat Model of Familial Amyotrophic Lateral Sclerosis after Transplantation of Motor Neurons Derived From Mouse Embryonic Stem Cells. Cell Transplant. 2009 doi: 10.3727/096368909X12483162197123. [DOI] [PubMed] [Google Scholar]
- 36.Vercelli A, Mereuta OM, Garbossa D, Muraca G, Mareschi K, Rustichelli D, Ferrero I, Mazzini L, Madon E, Fagioli F. Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2008;31:395–405. doi: 10.1016/j.nbd.2008.05.016. [DOI] [PubMed] [Google Scholar]