Summary
Introduction
We hypothesized that the combination of the EGFR tyrosine kinase inhibitor (TKI) gefitinib with the powerful chemopreventive manipulation of lung specific transgenic prostacyclin synthase (PGIS) overexpression on tumorigenesis in FVB/N mice would result in augmented chemoprevention.
Materials and Methods
Wildtype and littermate PGIS overexpressors (OE) were given urethane, 1 mg/kg i.p. followed by thrice weekly i.p. injections of gefitinib, 50 or 100 mg/kg, or vehicle. Pulmonary adenomas were enumerated and measured.
Results
Gefitinib at either 50 or 100 mg/kg administered i.p. three times weekly was effective in inhibiting EGF induced EGFR tyrosine phosphorylation and downstream signaling. The PGIS overexpressors showed significant decreases in tumor multiplicity consistent with prior studies. Gefitinib had no effect on tumor multiplicity or volume in wildtype mice. Among the PGIS overexpressors, a significant reduction in tumor multiplicity was shown in the 50 mg/kg, but not the 100 mg/kg, gefitinib treatment group versus vehicle control animals (1.13 +/− 0.29 vs. 2.29 +/− 0.32 tumors/mouse, p=0.015). We examined the phosphorylation status in selected downstream effectors of EGFR (Erk, Akt, Src, PTEN). The major difference in the 50 mg/kg vs. 100 mg/kg group was an increase in p-Src in the PGIS OE mice receiving the higher dose.
Conclusion
We conclude that gefitinib alone has no chemopreventive efficacy in this model; it augmented the effect of PGIS overexpression at 50 mg/kg but not 100 mg/kg. Increased p-Src is correlated with loss of efficacy at the higher dose, suggesting the potential for combined EGFR and Src inhibition strategies in chemoprevention.
Keywords: Chemoprevention, lung cancer, transgenic mice
Introduction
Lung cancer remains the leading cause of cancer death in both men and women in the United States(1). With poor 5-year lung cancer survival rates and a growing at-risk population of former smokers, it is critical to understand the molecular mechanisms that govern neoplastic progression, devise effective early detection strategies, and identify potential pharmacologic interventions to chemoprevent lung cancer. The majority of US lung cancer cases are diagnosed in former smokers, so beyond smoking cessation, more effective prevention strategies are needed(2). Chemically induced murine adenocarcinoma models contain many of the same histological, gene expression and genetic alterations found in human lung tumors, and these models are critical for the evaluation of agents in pre-clinical testing(3, 4). Increased pulmonary PGI2 (prostacyclin) by lung-specific overexpression of prostacyclin synthase (PGIS) chemoprevents lung cancer in chemically induced and cigarette smoke exposure models, suggesting that PGI2 plays an important role in lung tumorigenesis(5, 6). Previous studies have evaluated the balance of PGI2 and PGE2 as an indicator of chemoprevention in PGIS overexpressors. While a decrease in PGE2 levels may be one explanation for the chemopreventive effects (by decreasing immune surveillance) observed in colon cancer studies, significant alterations in PGE2 levels have not proven critical in our experiments with PGIS overexpression in lung cancer chemoprevention(5). Recent data suggest PPARγ activation as one explanation for prostacyclin's chemopreventive effects(7).
The epidermal growth factor receptor (EGFR) is a membrane bound receptor tyrosine kinase that forms homo and heterodimers upon ligand binding, leading to phosphorylation of tyrosine residues on the cytosolic domains of certain proteins. This leads to the activation of cellular signaling pathways (notably the Ras/Raf/mitogen-activated protein kinase (MAPK) and phosphatidyl inositol 3'-kinase (PI3K)-Akt pathways) that regulate cell proliferation and survival. Non-small cell lung cancer (NSCLC) may contain EGFR mutations or increased copy number, and targeted therapies with tyrosine kinase inhibitors (TKIs) are currently approved for treating NSCLC. Increased EGFR expression is frequent in endobronchial metaplasias and dysplasias, as is EGFR gene copy amplification(8–10). EGFR mutations have likewise been demonstrated in premalignant lung epithelium, supporting the potential of EGFR TKIs for chemoprevention(11). Mouse adenomas and adenocarcinomas characteristically contain activating mutations of K-ras, which have been linked to lack of clinical response to EGFR TKIs in some studies. However, this association does not necessarily have relevance to chemoprevention of less advanced lesions. In fact, three groups have reported varying efficacy of gefitinib in chemoprevention of lung tumors in mice using protocols that result in activated K-ras(12–14). More recently, gefitinib has been reported to prevent tumor growth in a model of lung adenocarcinoma in transgenic mice expressing activated EGFR driven by the lung epithelial specific surfactant apoprotein C (SP-C) promoter(15, 16). An additional rationale for combining an EGFR TKI with PGIS overexpressionis based on the findings that EGFR inhibition has proven effective in limiting growth in E-cadherin expressing NSCLC tumors and strategies to increase E-cadherin expression have resulted in sensitivity to EGFR TKIs(17). PGI2 is a PPARγ ligand and both PPARγ overexpression and ligands have been demonstrated to increase E-cadherin expression in NSCLC cell lines(18). We hypothesized that the combination of PGIS overexpression and EGFR TKI would lead to augmented chemoprevention.
Materials and Methods
Development of Transgenic Prostacyclin Synthase Overexpressors
Transgenic mice were developed using a construct consisting of the human SP-C promoter and full-length rat prostacyclin synthase cDNA(19). Transgenic mice and their wild-type littermates were bred in the animal facilities at the Denver VA Medical Center. Mice were genotyped by performing PCR on genomic DNA isolated from tails as described previously.
Confirming Biologic Activity of Gefitinib in vivo
To confirm that gefitinib was effectively inhibiting EGFR, PGIS OE (n=12) and wild-type animals (n=12) received gefitinib at 50 mg/kg or 100 mg/kg dissolved in Tween 80R vehicle alone intraperitoneally (i.p.) thrice weekly for two weeks. Two hours after the last gefitinib dose, mice were injected i.p. with either EGF (Sigma, 10 ng/g) in saline or saline alone, then sacrificed 15 minutes later and lung tissue harvested for analysis of EGFR and ERK phosphorylation.
Carcinogenesis and Gefitinib Treatment
FVB/N mice were fed standard chow (Test Diet/Purina Mills, Richmond, IN) in conventional caging in a controlled environment (12 h light-dark cycle, food and water ad libitum). PGIS OE and their wild-type littermates aged 8–10 weeks were given a single i.p. injection of urethane (1mg/kg mouse weight) in saline. One week after urethane exposure, i.p. injections of gefitinib dissolved in Tween 80R (Sigma, St. Louis, Il), were given in doses of 50 mg/kg, 100 mg/kg, as well as a control of vehicle alone, three times per week. Serial body weights were followed during the experiment and 18 weeks after urethane injection animals were euthanized by pentobarbital overdose. At the time of sacrifice, tumors were dissected from the surrounding lung parenchyma, enumerated and measured using a digital calipers. The presence of pulmonary adenomas and condition of the lung parenchyma were assessed and confirmed by microscopic examination. Lung tissue was snap frozen or immediately used for analysis of PGE2 and 6-keto PGF1α (the stable metabolite of PGI2) eicosanoid levels. All animal procedures were IACUC approved.
Western analysis for E-cadherin, p-Erk, p-Akt, p-Src, PTEN
Lung homogenates were prepared in buffer containing (50 mM Tris HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 2.5 mM EGTA, 10% glycerol, 1% Nonidet P-40, 1 mM dithiothreitol, 10 mM glycerophosphate, 10 mM NaF, 1 mM sodium orthovanadate, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml pepstatin A, and 1 mM phenylmethylsulfonyl fluoride). The homogenates were centrifuged for 10 min at 10,000 rpm and the supernatant collected. Protein concentration was determined using the BCA protein assay. Proteins were separated on SDS-PAGE, transferred to nitrocellulose membranes (GE HealthCare). Membranes were blocked in PBS containing 0.1% Tween 20 and 1% BSA for 1 h. Membranes were incubated with primary antibodies overnight at 4 °C, and with secondary antibodies for 1 h at room temperature. Antibodies to Akt, p-Akt, Erk, p-Erk, Src and p-Src (Tyr416) were obtained from Cell Signalling Technology and that for E-cadherin from BD Biosciences. Enhanced chemiluminescent reagent (ECL) antibodies (Amersham Biosciences) were used for immunodetection.
Immunopreciptitation of EGFR
400 μg of lung lysate was incubated with 10 μg of anti-EGFR antibody (Santa Cruz) at 4°C overnight. The immunoprecipitate was collected using protein A/G beads, washed and denatured for 5 minutes at 95°C and then separated by SDS-polyacrilamide gel electrophoresis (7% w/v acrilamide). Phosphorylated EGFR was visualized by western blotting probing with anti-pTyr (4G10 antibody, BD Biosciences).
PGE2 and PGI2 Estimation by Enzyme Immunoassay (EIA)
Due to PGI2's short half-life, the more stable metabolite 6-keto PG F1α is commonly used as an indicator for PGI2 levels. PGE2 and 6-keto PGF1α EIA assays (Cayman Chemical, Ann Arbor, MI) were performed as previously described to determine the effect of gefitinib on prostaglandin levels(5). At time of sacrifice, two samples of lung tissue from mice from each treatment group were collected and analyzed. Samples were homogenized in 500μL Earle's salt solution (Invitrogen, Carlsbad, CA), incubated 1–2 hrs. on ice, and stabilized in 1500 μL 100% methanol. Samples were centrifuged (16000g ×10 min.) and supernatants used for EIA. Calculations of prostaglandin levels were based on measurements taken in the linear portion of a standard curve. Assays were performed in a blinded fashion using coded sample tubes and normalized for protein concentration.
Statistical Analysis
Data for tumor multiplicity and volume were collected and analyzed using GraphPad Prism 4.02 for Windows (GraphPad Software for Science Inc., San Diego, CA). Results are presented as means ± SE. The significance of differences between two measurements was determined by unpaired, two-tailed t-tests; one-way analysis of variance was used for multiple comparisons.
Results
PGIS OE mice expressed increased levels of E-cadherin
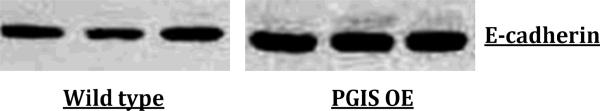
Western blots of PGIS OE and wildtype mouse lung showed increased expression of E-cadherin in the PGIS OE lungs (Figure 1).
Figure 1. E-cadherin protein expression in PGIS OE and wildtype mouse lungs.
E-cadherin protein expression is increased in PGIS OE compared to wildtype mouse whole lung extracts.
Experimental mice tolerated gefitinib and the dosage was biologically active
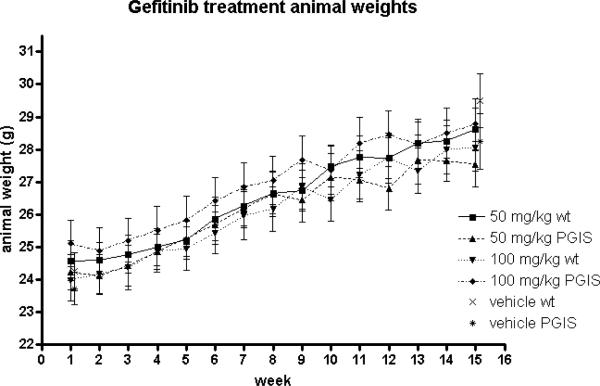
Experimental mice in the treatment groups exhibited low mortality rates with no significant differences seen in comparison with the control group. The survival rates for wildtype mice were 24/25 (96%) for vehicle, 28/31 (90%) for 50 mg/kg gefitinib and 22/28 (79%) for 100 mg/kg gefintinib. For PGIS OE, survival rates were 21/28 (75%) vehicle, 17/21 (81%) 50 mg/kg gefitinib and 20/25 (80%) for 100 mg/kg gefitinib. We did not determine the cause of death of mice. Tolerability of gefitinib was additionally monitored through biweekly measurements of body weight (Figure 2). There were no significant changes seen in these measurements between experimental and control animals.
Figure 2. Weight curves of various treatment groups.
All treatment groups had similar weight gain throughout the experiment.
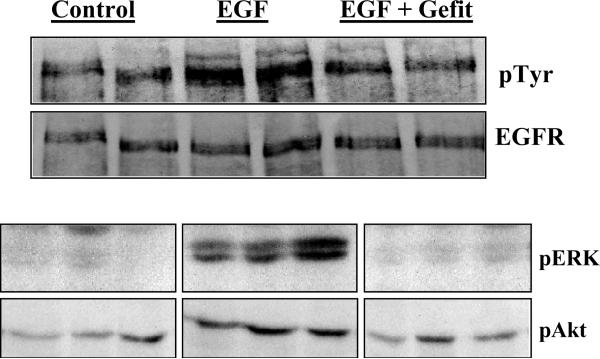
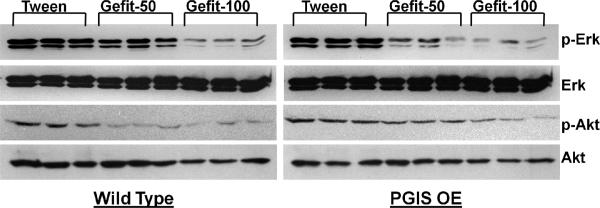
To confirm that gefitinib had achieved EGFR inhibition, PGIS OE and wildtype littermates were sacrificed after 14 days of thrice weekly gefitinib (50 or 100 mg/kg) or Tween-80R treatment. At the time of sacrifice, i.p. epidermal growth factor (EGF) at a dose of 10 ng/g was given. Lungs were harvested 15 minutes after EGF administration and protein collected for analysis. Figure 3 shows a Western blot from control and gefinitib treated animals and confirms that the presence of gefitinib partially blocked EGF induced EGFR autophosphorylaton. To further confirm that EGFR inhibition had limited downstream signaling, these lung homogenates were also analyzed for pERK and pAkt. Gefitinib treatment led to decreases in phosphorylation of both ERK and Akt after the administration of EGF (Figure 3).
Figure 3.
Upper panel: Gefitinib inhibits EGF induced EGFR phosphorylation in mice treated for a week with the drug: Mice were treated with gefitnib (50 or 100 mg/kg thrice weekly) or Tween for two weeks and challenged with 10 ng/gm of EGF ip for 15 min before collection of lungs for analysis. EGFR was immunopreciptated and probed for tyrosine phosphophorylation. Gefitinib blocks EGF induced tyrosine phosphorylation of the EGFR. Only results of the 100 mg/kg gefitinib dose are shown.
Lower panel: Inhibition of EGF signaling in mice treated with gefitinib for a week: Figure shows the effect of gefitinib on the phosphorylation of Akt, p44/42 Erk in lungs of mice treated with gefitnib thrice weekly for two weeks and challenged with EGF 10 ng/gm ip for 15 min. Gefitinib blocks EGF induced Erk phosphorylation and diminishes Akt phosphorylation. Only results of the 100 mg/kg gefitinib dose are shown.
PGIS Overexpressors treated with 50 mg/kg gefitinib exhibited decreased tumor multiplicity versus the vehicle and 100 mg/kg treatment groups
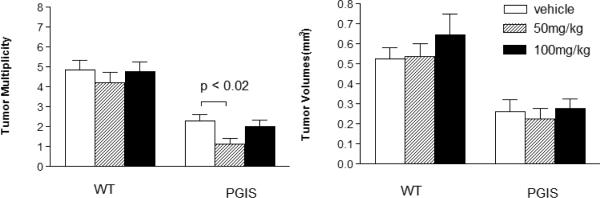
As previously reported, tumor multiplicity and volume was significantly lower in PGIS overexpressor compared to wildtype mice regardless of gefitinib treatment (Figure 4)(5). All mice injected with urethane developed tumors. Among the wild-types treated with gefitinib, multiplicity was not significantly different between vehicle (n = 24 mice) group (4.22 +/− 0.53 tumors/mouse), the 50 mg/kg (n = 28 mice) group (4.86 +/− 0.48 tumors/mouse), or the 100 mg/kg (n = 22 mice) group (4.77 +/− 0.49 tumors/mouse). Within the PGIS overexpressors, treatment with 50 mg/kg gefitinib (n = 17 mice) resulted in a statistically significant (p=0.015) reduction in tumor multiplicity (1.13 +/− 0.29 tumors/mouse) compared to vehicle (n = 21 mice) only (2.29 +/− 0.32 tumors/mouse). Unexpectedly, treatment with 100 mg/kg gefitinib (n = 20 mice) (2.00 +/− 0.32 tumors/mouse) was not statistically different (p=0.534) from vehicle alone (2.29 +/− 0.32 tumors/mouse). Gefitinib treatment did not result in significant changes in tumor volume in either the PGIS overexpressor or wildtype mice (Figure 4).
Figure 4. 50 mg/kg gefitinib, in addition to PGIS overexpression, significantly decreases tumor multiplicity.
PGIS overexpressors treated with gefitinib 50 mg/kg thrice weekly show decreased tumor multiplicity, while a dose of 100 mg/kg did not alter tumor development. All PGIS overexpressors had lower tumor multiplicity and volume compared to wildtype mice, as previously described.
Analysis of downstream tyrosine kinase signaling pathways in lung and tumors from treatment and control animals
Based on the known intracellular signaling pathways activated by the EGFR, we chose to analyze lung homogenates and tumors from all treatment groups for p-Erk, p-Akt, p-Src, and PTEN. Figure 5 shows the basal phosporylation of Erk1/Erk2 (p44/42 MAP kinase) and Akt in the lung of both PGIS OE and wt mice after 17 weeks of gefitinib treatment. Both doses of gefitinib resulted in decreases in p-Erk and p-Akt. Tumors from PGIS OE showed similar decreases in p-Erk and p-Akt. These same decreases were not consistently seen in tumors from wt mice.
Figure 5. Effect of gefitinib on basal phosphorylation of Akt, p44/42 Erk in lungs of wild type and PGIS OE mice treated with urethane.
Figure shows the effect of gefitinib on the phosphorylation of Akt and p44/42 Erk in lungs of mice treated with urethane and tween/ gefitinib. Both p-Erk and p-Akt show a dose dependent decrease in intensity.
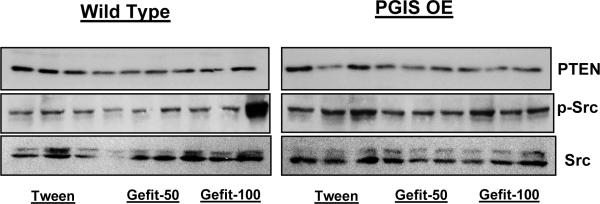
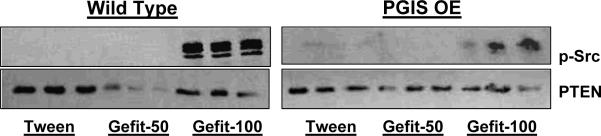
We next examined the effects of chronic gefitinib treatment on the basal phosphorylation of Src and PTEN in both lung tissue and urethane-induced tumors after 17 weeks of gefitinib treatment. Of note, in the PGIS OE animals, gefitinib at a dose of 50 mg/kg was associated with a decrease in p-Src in lung tissue, and this decrease was not seen in the PGIS OE receiving control vehicle or the higher gefitinib dose (Figure 6). Wildtype animals receiving the lower gefitinib dose also exhibited a selective decrease in p-Src (Figure 6). When tumors from the experimental groups were analyzed, the higher dose of gefitinib resulted in Src phosphorylation (Figure 7). Taken together, the PGIS OE mice who exhibited significant additional decreases in tumor multiplicity with gefitinib 50 mg/kg (as opposed to control or gefitinib 100 mg/kg) showed a corrresponding inhibition of Src activation.
Figure 6. Effect of gefitinib on basal phosphorylation of Src and expression of PTEN in lungs of wild type and PGIS OE mice treated with urethane.
Figure shows the effect of gefitinib on the phosphorylation of src kinase and expression of PTEN in lungs of mice treated with urethane and tween/ gefitinib.
Figure 7. Effect of gefitnib on basal phosphorylation of Src and PTEN in tumors from wild type and PGIS OE mice treated with urethane.
Figure shows the effect of gefitinib on the phosphorylation of src kinase and PTEN in tumors obtained from lungs of mice treated with urethane and tween/ gefitinib. Src phosphorylation is markedly increased in mice given 100 mg/kg gefitinib thrice weekly.
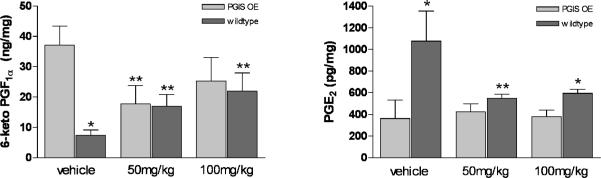
PGI2 and PGE2 levels with gefitinib treatment
To better determine if the additional decrease in tumor multiplicity was due to alterations in prostaglandin levels, PGE2 and 6-keto PGF1α were measured. As previously reported, the presence of the PGIS transgene resulted in increased levels of 6-keto PGF1α and a decrease in PGE2 levels(5). The addition of gefitinib abrogated these changes (Figure 8).
Figure 8. Whole lung levels of 6-keto PGF1α and PGE2 in different experimental groups.
As previously described, PGIS overexpression raises 6-keto PGF1α and depresses PGE2 levels. Gefitinib blocks this effect, but does not block PGIS induced chemoprevention.
*p < 0.05 in comparision of tg+ and wt of same treatment group
**p < 0.05 in comparision with same genotype of vehicle group
Discussion
Prior to moving chemopreventive agents to definitive phase III trials, it is important to perform a comprehensive evaluation using evidence from epidemiology, cell biology, preclinical models and phase II trials with intermediate endpoint outcomes. The EGFR signaling pathway has been strongly implicated in the development of NSCLC. Five reports of positive results for gefitinib in murine models of lung cancer chemoprevention exist(12–16). Three of the models had K-ras mutations as early events(12–14). However, in two of these reports, only modest chemopreventive effects were demonstrated(13, 14). Gefitnib dosages ranged from 5 mg/kg/d to 250 mg/kg/d and there is no clear association between dose and efficacy. In contrast, in a transgenic model driven by alveolar epithelial expression of mutant EGFR, gefitnib was highly effective at a dose of 5 mg/kg/d in two separate reports(15, 16). One group has described an early treatment result for erlotinib in the urethane mouse model, in which tumor volume was decreased in male mice and increased in females(20). For the highest chemopreventive efficacy, combinations of multiple interventions may be necessary, as has been demonstrated in xenograft models of squamous cell carcinoma of the head and neck(21, 22). Prostacyclin analogs are another promising chemopreventive strategy, based on a high degree of efficacy in mouse models, and are currently being evaluated in a phase II clinical trial with endobronchial histology as the primary endpoint. The EGFR TKIs and prostacyclin analogs have different mechanisms of action; the former blocking the EGFR pathway and the latter promoting epithelial differentiation. We therefore hypothesized that the combination of PGIS overexpression and EGFR TKI administration would have increased chemopreventive efficacy.
We assessed the chemopreventive effect of gefitinib in PGIS overexpressor and wildtype littermate FVB/N mice using a model based on the tobacco smoke carcinogen, urethane, which induces K-ras mutations as an early event (23). Both PGIS overexpressor and wildtype mice tolerated gefitinib at doses of 50 mg/kg and 100 mg/kg i.p. thrice weekly without excess weight loss or mortality compared to controls injected with vehicle alone. In order to demonstrate that the dosages were effective in inhibiting the EGFR pathway, we performed analysis of EGFR and ERK1/2 phosphorylation in response to intravenous EGF 2 hours after gefitinib administration and biologic efficacy was shown.
As previously reported, PGIS overexpression caused an approximate 50% decrease in tumor multiplicity in mice treated with vehicle alone, as well as across all gefitinib dose groups(5). Gefitinib had no effect on either tumor multiplicity or volume in wildtype mice at either dose, but did produce a statistically significant reduction of approximately 50% (1.13 vs. 2.29, p = 0.015) in tumor multiplicity in PGIS overexpressor mice at the 50 mg/kg dose, but not at the 100 mg/kg dose. No reduction in tumor volume was seen with gefitinib. The lack of chemopreventive effect of gefitinib alone is at odds with results published by other groups. Differences in carcinogenesis protocols, or background mouse strains make it difficult to evaluate these differing results; however, it at least two of the other reports, the chemopreventive efficacy of gefitinib was weak(13, 14). Gefitinib in our experiment was administered i.p. and demonstrated to have biologic activity in the experimental animals.
While gefitinib at a dose of 50 mg/kg thrice weekly approximately doubled the chemopreventive effect of PGIS overexpression, the higher dose of gefitinib (100 mg/kg thrice weekly) did not augment the chemopreventive efficacy of PGIS overexpression and was accompanied by an increase in src phosphorylation and loss of efficacy. Increased activity of src has been shown to be a mechanism for resistance to EGFR TKIs and we speculate that this may be responsible for the lack of efficacy of the 100 mg/kg dose(24–26). In addition, gefitinib at either dose abrogated the increase in PGF1α and decrease in PGE2 previously described. In spite of these changes in whole lung prostaglandin levels, PGIS overexpression maintained a chemopreventive effect.
We conclude that in the urethane model of mouse lung carcinogenesis, gefitinib has no chemopreventive effect in wildtype FVB/N mice. The combination of EGFR TKI and prostacyclin analogs for chemoprevention of lung cancer was not highly effective in this K-ras dependent model and the loss of activity at higher gefitinib doses is counterintuitive and problematic. We do not fully understand the effect of differing doses of gefitinib in our experiments and those of others, but induction of src phosphorylation may play a role in resistance to chemoprevention and should be measured in future experiments. Combining EGFR and src inhibition may be a useful strategy for lung cancer chemoprevention.
Acknowledgments
Supported by Department of Veterans Affairs Merit Review Grants (YEM and RLK) and NCI P50 CA58187 (SPORE in Lung Cancer, YEM and RLK)
Footnotes
Conflict of interest statement RLK and YEM are coinventors on a patent for the use of prostacyclin analogs for the prevention of cancer. VK, ABM, TMH and ML have no conflicts to declare.
Reference List
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J.Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Tong L, Spitz MR, Fueger JJ, Amos CA. Lung carcinoma in former smokers. Cancer. 1996;78:1004–1010. doi: 10.1002/(SICI)1097-0142(19960901)78:5<1004::AID-CNCR10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Malkinson AM. Primary lung tumors in mice as an aid for understanding, preventing, and treating human adenocarcinoma of the lung. Lung Cancer. 2001;32:265–279. doi: 10.1016/s0169-5002(00)00232-4. [DOI] [PubMed] [Google Scholar]
- 4.Stearman RS, Dwyer-Nield L, Zerbe L, Blaine SA, Chan Z, Bunn PA, Jr., Johnson GL, Hirsch FR, Merrick DT, Franklin WA, Baron AE, Keith RL, Nemenoff RA, Malkinson AM, Geraci MW. Analysis of orthologous gene expression between human pulmonary adenocarcinoma and a carcinogen-induced murine model. Am.J Pathol. 2005;167:1763–1775. doi: 10.1016/S0002-9440(10)61257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keith RL, Miller YE, Hoshikawa Y, Moore MD, Gesell TL, Gao B, Malkinson AM, Golpon HA, Nemenoff RA, Geraci MW. Manipulation of pulmonary prostacyclin synthase expression prevents murine lung cancer. Cancer Res. 2002;62:734–740. [PubMed] [Google Scholar]
- 6.Keith RL, Miller YE, Hudish TM, Girod CE, Sotto-Santiago S, Franklin WA, Nemenoff RA, March TH, Nana-Sinkam SP, Geraci MW. Pulmonary prostacyclin synthase overexpression chemoprevents tobacco smoke lung carcinogenesis in mice. Cancer Res. 2004;64:5897–5904. doi: 10.1158/0008-5472.CAN-04-1070. [DOI] [PubMed] [Google Scholar]
- 7.Nemenoff R, Meyer AM, Hudish TM, Mozer AB, Snee A, Narumiya S, Stearman RS, Winn RA, Weiser-Evans M, Geraci MW, Keith RL. Prostacyclin prevents murine lung cancer independent of the membrane receptor by activation of peroxisomal proliferator--activated receptor gamma. Cancer Prev.Res. 2008;1:349–356. doi: 10.1158/1940-6207.CAPR-08-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merrick DT, Kittelson J, Winterhalder R, Kotantoulas G, Ingeberg S, Keith RL, Kennedy TC, Miller YE, Franklin WA, Hirsch FR. Analysis of c-ErbB1/epidermal growth factor receptor and c-ErbB2/HER-2 expression in bronchial dysplasia: evaluation of potential targets for chemoprevention of lung cancer. Clin.Cancer Res. 2006;12:2281–2288. doi: 10.1158/1078-0432.CCR-05-2291. [DOI] [PubMed] [Google Scholar]
- 9.Jonsson S, Varella-Garcia M, Miller YE, Wolf HJ, Byers T, Braudrick S, Kiatsimkul P, Lewis M, Kennedy TC, Keith RL, Bjornsson J, McWilliams A, Lam S, Hirsch FR, Franklin WA. Chromosomal aneusomy in bronchial high-grade lesions is associated with invasive lung cancer. Am.J.Respir.Crit Care Med. 2008;177:342–347. doi: 10.1164/rccm.200708-1142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurie JM, Shin HJ, Lee JS, Morice RC, Ro JY, Lippman SM, Hittelman WN, Yu R, Lee JJ, Hong WK. Increased epidermal growth factor receptor expression in metaplastic bronchial epithelium. Clin.Cancer Res. 1996;2:1787–1793. [PubMed] [Google Scholar]
- 11.Tang X, Shigematsu H, Bekele BN, Roth JA, Minna JD, Hong WK, Gazdar AF, Wistuba II. EGFR tyrosine kinase domain mutations are detected in histologically normal respiratory epithelium in lung cancer patients. Cancer Res. 2005;65:7568–7572. doi: 10.1158/0008-5472.CAN-05-1705. [DOI] [PubMed] [Google Scholar]
- 12.Yan Y, Lu Y, Wang M, Vikis H, Yao R, Wang Y, Lubet RA, You M. Effect of an epidermal growth factor receptor inhibitor in mouse models of lung cancer. Mol.Cancer Res. 2006;4:971–981. doi: 10.1158/1541-7786.MCR-06-0086. [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto N, Wislez M, Zhang J, Iwanaga K, Dackor J, Hanna AE, Kalyankrishna S, Cody DD, Price RE, Sato M, Shay JW, Minna JD, Peyton M, Tang X, Massarelli E, Herbst R, Threadgill DW, Wistuba II, Kurie JM. High expression of ErbB family members and their ligands in lung adenocarcinomas that are sensitive to inhibition of epidermal growth factor receptor. Cancer Res. 2005;65:11478–11485. doi: 10.1158/0008-5472.CAN-05-1977. [DOI] [PubMed] [Google Scholar]
- 14.Kishino D, Kiura K, Takigawa N, Katayama H, Kuyama S, Sato K, Okada T, Ohashi K, Tanimoto M. Effect of gefitinib on N-nitrosamine-4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induced lung tumorigenesis in A/J mice. Lung Cancer. 2009;65:284–289. doi: 10.1016/j.lungcan.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Ohashi K, Rai K, Fujiwara Y, Osawa M, Hirano S, Takata K, Kondo E, Yoshino T, Takata M, Tanimoto M, Kiura K. Induction of lung adenocarcinoma in transgenic mice expressing activated EGFR driven by the SP-C promoter. Cancer Sci. 2008;99:1747–1753. doi: 10.1111/j.1349-7006.2008.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohashi K, Takigawa N, Osawa M, Ichihara E, Takeda H, Kubo T, Hirano S, Yoshino T, Takata M, Tanimoto M, Kiura K. Chemopreventive effects of gefitinib on nonsmoking-related lung tumorigenesis in activating epidermal growth factor receptor transgenic mice. Cancer Res. 2009;69:7088–7095. doi: 10.1158/0008-5472.CAN-08-4205. [DOI] [PubMed] [Google Scholar]
- 17.Witta SE, Gemmill RM, Hirsch FR, Coldren CD, Hedman K, Ravdel L, Helfrich B, Dziadziuszko R, Chan DC, Sugita M, Chan Z, Baron A, Franklin W, Drabkin HA, Girard L, Gazdar AF, Minna JD, Bunn PA., Jr. Restoring E-cadherin expression increases sensitivity to epidermal growth factor receptor inhibitors in lung cancer cell lines. Cancer Res. 2006;66:944–950. doi: 10.1158/0008-5472.CAN-05-1988. [DOI] [PubMed] [Google Scholar]
- 18.Wick M, Hurteau G, Dessev C, Chan D, Geraci MW, Winn RA, Heasley LE, Nemenoff RA. Peroxisome proliferator-activated receptor-gamma is a target of nonsteroidal anti-inflammatory drugs mediating cyclooxygenase-independent inhibition of lung cancer cell growth. Mol.Pharmacol. 2002;62:1207–1214. doi: 10.1124/mol.62.5.1207. [DOI] [PubMed] [Google Scholar]
- 19.Geraci MW, Gao B, Shepherd DC, Moore MD, Westcott JY, Fagan KA, Alger LA, Tuder RM, Voelkel NF. Pulmonary prostacyclin synthase overexpression in transgenic mice protects against development of hypoxic pulmonary hypertension. J.Clin.Invest. 1999;103:1509–1515. doi: 10.1172/JCI5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zerbe LK, Dwyer-Nield LD, Fritz JM, Redente EF, Shroyer RJ, Conklin E, Kane S, Tucker C, Eckhardt SG, Gustafson DL, Iwata KK, Malkinson AM. Inhibition by erlotinib of primary lung adenocarcinoma at an early stage in male mice. Cancer Chemother.Pharmacol. 2008;62:605–620. doi: 10.1007/s00280-007-0644-z. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Chen ZG, Choe MS, Lin Y, Sun SY, Wieand HS, Shin HJ, Chen A, Khuri FR, Shin DM. Tumor growth inhibition by simultaneously blocking epidermal growth factor receptor and cyclooxygenase-2 in a xenograft model. Clin.Cancer Res. 2005;11:6261–6269. doi: 10.1158/1078-0432.CCR-04-2102. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Zhang H, Tighiouart M, Lee JE, Shin HJ, Khuri FR, Yang CS, Chen ZG, Shin DM. Synergistic inhibition of head and neck tumor growth by green tea (−)-epigallocatechin-3-gallate and EGFR tyrosine kinase inhibitor. Int.J.Cancer. 2008;123:1005–1014. doi: 10.1002/ijc.23585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hecht SS. Tobacco smoke carcinogens and lung cancer. J.Natl.Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 24.Mueller KL, Hunter LA, Ethier SP, Boerner JL. Met and c-Src cooperate to compensate for loss of epidermal growth factor receptor kinase activity in breast cancer cells. Cancer Res. 2008;68:3314–3322. doi: 10.1158/0008-5472.CAN-08-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin B, Ariyama H, Baba E, Tanaka R, Kusaba H, Harada M, Nakano S. Activated Src and Ras induce gefitinib resistance by activation of signaling pathways downstream of epidermal growth factor receptor in human gallbladder adenocarcinoma cells. Cancer Chemother.Pharmacol. 2006;58:577–584. doi: 10.1007/s00280-006-0219-4. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Kalyankrishna S, Wislez M, Thilaganathan N, Saigal B, Wei W, Ma L, Wistuba II, Johnson FM, Kurie JM. SRC-family kinases are activated in non-small cell lung cancer and promote the survival of epidermal growth factor receptor-dependent cell lines. Am.J Pathol. 2007;170:366–376. doi: 10.2353/ajpath.2007.060706. [DOI] [PMC free article] [PubMed] [Google Scholar]