Abstract
There are now four genetic mouse models that induce hyperhomocyst(e)inemia by decreasing the activity of an enzyme involved in homocysteine metabolism: cystathionine β-synthase, methylenetetrahydrofolate reductase, methionine synthase and methionine synthase reductase. While each enzyme deficiency leads to murine hyperhomocyst(e)inemia, the accompanying metabolic profiles are significantly and often unexpectedly, different. Deficiencies in cystathionine β-synthase lead to elevated plasma methionine, while deficiencies of the remaining three enzymes lead to hypomethioninemia. The liver [S-adenosylmethionine]:[S-adenosylhomocysteine] ratio is decreased in mice lacking methylenetetrahydrofolate reductase or cystathionine β-synthase, but unexpectedly increased in mice with deficiencies in methionine synthase or methionine synthase reductase. Folate pool imbalances are seen in complete methylenetetrahydrofolate reductase deficiency, where methyltetrahydrofolate is a minor component, and in methionine synthase reductase deficiency, where methyltetrahydrofolate is increased relative to wild-type mice. These differences illustrate the potential diversity among human patients with hyperhomocyst(e)inemia, and strengthen the argument that the pathologies associated with the dissimilar forms of the condition will require different treatments.
Keywords: adenosylhomocysteine, adenosylmethionine, cystathionine β-synthase, folate, genetic mouse models, homocysteine, methionine synthase, methionine synthase reductase, methylenetetrahydrofolate reductase
Introduction
The literature is replete with extensive studies of hyperhomocyst(e)inemia in humans. Hyperhomocyst(e)inemia may arise from a combination of dietary and/or genetic factors, including polymorphisms or severe mutations in enzymes involved in homocysteine metabolism and dietary deficiencies of vitamin B6, riboflavin, cobalamin and/or folate. The enzymes whose deficiency gives rise to hyperhomocyst(e)inemia include cystathionine β-synthase, methylenetetrahydrofolate reductase, methionine synthase and methionine synthase reductase. Despite the common factor of an elevation in plasma homocysteine, patients with these enzyme deficiencies may display rather varied adverse clinical effects. Deficiencies of methylenetetrahydrofolate reductase and methionine synthase give rise to low or normal levels of plasma methionine (1), while deficiencies of cystathionine β-synthase lead to hypermethioninemia (2). The pathologies associated with mutations leading to severe enzyme deficiencies have been attributed to defects in cellular methylation reactions due to altered ratios of [S-adenosylmethionine (AdoMet)]:[S-adenosylhomocysteine (AdoHcy)], to oxidative stress and covalent modifications of proteins induced directly by elevated plasma homocysteine and indirectly by homocysteine-thiolactone, or in the case of methionine synthase deficiency to methyl trapping leading to an imbalance in folate pools. If increased cardiovascular risk were due to a direct effect of elevated plasma homocyst(e)ine, one might expect that protocols that lower plasma homocyst(e)ine such as folate supplementation would ameliorate the risk. In the face of disappointing results of folate fortification during secondary prevention trials (HOPE-2 (3), VISP (4), and NORVIT (5)) in reducing cardiovascular events in patients with a prior history of cardiovascular disease, we are forced to ask whether the increased cardiovascular disease risk associated with hyperhomocyst(e)inemia might not have more complex causes, depending on the associated metabolic abnormalities. For example, methyl trapping, or the accumulation of methyltetrahydrofolate at the expense of 10-formyltetrahydrofolate and methylenetetrahydrofolate needed for the biosynthesis of the nucleotide precursors of RNA and DNA, is expected to be a problem in hyperhomocyst(e)inemia caused by deficiencies of methionine synthase or methionine synthase reductase, but not in deficiencies of methylenetetrahydrofolate reductase, where the level of methyltetrahydrofolate is abnormally low. However, measurements of folate distributions in the tissues of humans that would confirm this hypothesis are lacking. We will begin with a description of how mouse models of hyperhomocyst(e)inemia as a group serve to illustrate the possible complexities associated with the human forms of the condition. We will then discuss relevant human clinical findings in light of these findings in genetic mouse models.
Metabolite measurements in genetic mouse models for hyperhomocyst(e)inemia
Genetic mouse models of hyperhomocyst(e)inemia are extremely relevant to our understanding of hyperhomocyst(e)inemia in humans because they permit tissue metabolite measurements that are difficult if not impossible to obtain in affected humans. To date there are four strains of mice in which hyperhomocyst(e)inemia has been induced by genetic manipulations leading to a deficiency of one of the enzymes involved in homocysteine metabolism.
Cystathionine β-synthase.
The first genetic model for hyperhomocyst(e)inemia lacked the Cbs gene specifying cystathionine β-synthase. Cystathionine β-synthase catalyzes the first step in the conversion of homocysteine to cysteine: the condensation of homocysteine and serine to form cystathionine. As expected, mice with this enzyme deficiency have elevated plasma homocyst(e)ine (6). Cbs heterozygotes show mildly elevated plasma methionine and normal levels of AdoMet, but show elevations in liver AdoHcy resulting in a reduced [AdoMet]:[AdoHcy] ratio in this tissue (7). In the severely hyperhomocyst(e)inemic Cbs mutant homozygote, AdoMet levels in liver are elevated approximately 2-fold, while AdoHcy levels are elevated about 9-fold, again resulting in a reduced AdoMet:AdoHcy ratio and impaired genomic DNA methylation status (8). These authors reported that the effect of the Cbs knockout on AdoMet levels was tissue specific, with almost no effect in brain or kidney.
Methylenetetrahydrofolate reductase.
A knockout of the Mthfr gene specifying methylenetetrahydrofolate reductase was constructed in the Rozen laboratory (9). Mthfr−/− mice have elevated plasma homocyst(e)ine and reduced plasma methionine. Their AdoMet levels were lower in all tissues examined, and AdoHcy levels were higher, resulting in AdoMet:AdoHcy ratios that were dramatically decreased (9). Biological methylation was impaired in all tissues examined. These mice also exhibited an abnormal distribution of folates: methyltetrahydrofolate levels are greatly reduced and the total folate content was unchanged in liver and brain (9) but reduced in plasma (10). While methyltetrahydrofolate is the major folate in the plasma of wild-type animals, it comprises less than 40% of the folate in Mthfr−/− mice.
Methionine synthase.
A knockout of the Mtr gene specifying cobalamin-dependent methionine synthase results in embryonic lethality (11). Heterozygotes show mildly elevated plasma homocyst(e)ine and normal (12) or elevated (11) plasma methionine. In liver, AdoMet levels were slightly increased in heterozygotes as compared to wild-type mice, while AdoHcy levels were slightly decreased, although the changes did not reach statistical significance.
Methionine synthase reductase.
A knockout of the Mtrr gene specifying methionine synthase reductase, an auxiliary protein required for methionine synthase activity, also causes embryonic lethality in homozygotes (unpublished data of Roy Gravel, discussed in (13)). A gene-trap insertion (MtrrGt(pGT1Lxf)XG334Byg; hereafter abbreviated MtrrGt) into this gene results in an MtrrGt/Gt mouse methionine synthase reductase activity that is reduced but not absent (13). The MtrrGt/Gt mouse is hyperhomocyst(e)inemic and hypomethioninemic. However, the liver levels of AdoMet are elevated and the levels of AdoHcy are decreased. This surprising observation suggests unidentified compensatory mechanisms exist in the liver that can spare AdoMet in a hypomethioninemic mouse. [AdoMet]:[AdoHcy] ratios in brain and heart are unchanged by Mtrr disruption. The MtrrGt/Gt mouse shows a tendency towards methyl trapping, with elevated fractions of methyltetrahydrofolate in liver, heart and kidney, but not in brain. The metabolite measurements in these various mouse models are qualitatively summarized in Table 1.
Table 1.
Metabolite levels in genetic mouse models for hyperhomocyst(e)inemiaa
| plasma | plasma | liver | liver | liver | liver | liver | ||
|---|---|---|---|---|---|---|---|---|
| genotype | Hcy | Met | AdoMet | AdoHcy | ratio AdoMet:AdoHcy |
total folate | % CH3- H4folate |
References |
| Cbs−/− |

|
not determined |

|
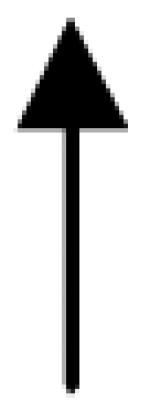
|

|
not determined |
not determined |
Choumenovitch et al., 2002 |
| Cbs−/+ |
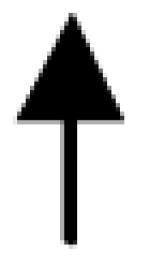
|
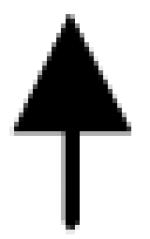
|
unchanged |
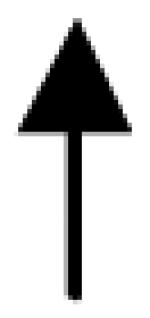
|

|
not determined |
not determined |
Dayal et al, 2001 |
| Mthfr−/− |

|
not determined |

|

|

|
unchanged |

|
Chen et al., 2001 |
| Mthfr−/+ |

|
not determined |

|
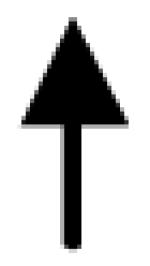
|

|
unchanged |

|
Chen et al, 2001 |
| Mtr−/+ |

|

|

|

|

|
unchanged | not determined |
Swanson et al, 2001; Dayal et al., 2005 |
| Mtrrgt/gt |
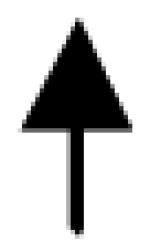
|
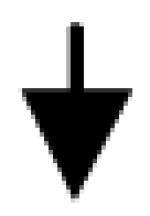
|

|

|

|
unchanged |
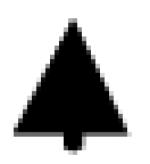
|
Elmore et al, 2007 |
| Mtrr+/gt |

|

|
not determined |
not determined |
not determined | not determined |
not determined |
Elmore et al., 2007 |
Indicated changes in metabolite levels are relative to the wild-type littermates. Unexpected changes are highlighted in red.
What do these models tell us that is of relevance to hyperhomocyst(e)inemia in humans?
Folate metabolism.
None of the mouse models that have been examined show altered levels of total folates in tissues. It will be of great interest to see if their hyperhomocyst(e)inemia responds to folate supplementation of standard lab chow. A preliminary indication of the response of Mthfr-deficient mice to folate deficiency comes from studies in the Rozen laboratory (14). A folate-deficient diet exacerbates hyperhomocyst(e)inemia in both wild-type and Mthfr+/− mice, suggesting that addition of folate to a normal diet might reduce plasma homocyst(e)ine levels in Mthfr-deficient mice, as it is known to do in humans with mild MTHR deficiency due to homozygosity for the 677C>T mutation (15). However, it remains to be determined whether folate supplementation would reduce plasma homocyst(e)ine in mice with deficiencies in methionine synthase or methionine synthase reductase induced by genetic manipulation or by cobalamin deficiency, or in Cbs-deficient mice. The most common genetic cause of human hyperhomocyst(e)inemia is the homozygosity for the 677C>T mutation in MTHFR (15), and we know that the plasma homocyst(e)ine of TT patients is responsive to dietary folate supplementation. However, other causes of hyperhomocyst(e)inemia might not respond to folate supplementation, or might respond without altering the root cause of cardiovascular risk.
Embryonic lethality.
Of the four genetic mouse models for hyperhomocyst(e)inemia discussed here, the only enzyme deficiencies that lead to embryonic lethality are deficiencies in methionine synthase and methionine synthase reductase. Thus clearly, hyperhomocyst(e)inemia in and of itself is not sufficient to cause embryonic lethality. These are the two enzyme deficiencies most likely to lead to methyl trapping, and it is tempting to speculate that intracellular methyl trapping is linked to the embryonic lethality. If so, conditions which lead to milder methyl trapping in humans and in mice (e.g. cobalamin deficiency, or polymorphism in methionine synthase or methionine synthase reductase) may also be associated with increased incidence of fetal loss. Unpublished studies on pregnancy outcome in the Mtrr+/Gt dam in the Rozen laboratory suggest that maternal methionine synthase reductase deficiency is indeed linked to embryonic resorption and fetal abnormalities, including ventricular septal defects. The implications of these results are profound, in light of the recent meta-analysis that indicated maternal low folate status is a risk factor for spontaneous abortion (16), since the critical form(s) of folate are not known.
Neural tube defects.
Finally there is the issue of the linkage between hyperhomocyst(e)inemia and neural tube defects. No studies that we are aware of have reported the incidence of neural tube defects in hyperhomocyst(e)inemic mice. Cardiac abnormalities, particularly ventricular septal defects, are often used as surrogate markers for the much rarer neural tube defects, and ventricular septal defect incidence has been shown to be elevated in wild-type mice fed a folate-deficient diet, and in both Mthfr+/− and MtrrGt/Gt mice (13, 14). But more comprehensive studies might help to elucidate the origin of the dramatic effect of maternal periconceptual folate supplementation in the periconceptional period in lowering the incidence of neural tube defects in the resultant offspring.
Conclusions
Given the very different metabolite profiles observed in genetic mouse models for hyperhomocyst(e)inemia, we need to emphasize the idea that some or all of the pathological risks associated with human hyperhomocyst(e)inemia may arise not from an elevation in homocyst(e)ine per se, but from metabolic abnormalities that are specific to the cause of that hyperhomocyst(e)inemia.
Abbreviations:
- Cbs
Cystathionine β-synthase gene
- Mtr
methionine synthase gene
- Mtrr
methionine synthase reductase gene
- Mthfr
Methylenetetrahydrofolate reductase gene
- AdoHcy
S-adenosylhomocysteine
- AdoMet
S-adenosylmethionine
References
- 1.Rosenblatt DS. Inherited disorders of folate transport and metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. Vol. 1. McGraw Hill; New York: 1995. pp. 3111–28. [Google Scholar]
- 2.Mudd SH, Levy HL, Skovby F. Disorders of transsulfuration. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. seventh ed. Vol. 1. McGraw-Hill; New York: 1995. pp. 1279–327. [Google Scholar]
- 3.The Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–88. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 4.Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: The Vitamin Intervention for Stroke Prevention (VISP) randomized control trial. JAMA. 2004;291:565–75. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 5.Bønaa KH, Njølstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–88. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow MR, Maeda N. Mice deficient in cystathionine-beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci, USA. 1995;92:1585–9. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dayal S, Bottiglieri T, Arning E, Maeda N, Malinow MR, Sigmund CD, et al. Endothelial dysfunction and elevation of S-adenosylhomocysteine in cystathionine beta-synthase-deficient mice. Circ Res. 2001;88:1203–9. doi: 10.1161/hh1101.092180. [DOI] [PubMed] [Google Scholar]
- 8.Choumenovitch S, Selhub J, Bagley PJ, Maeda N, Nadeau Mr, Smith DE, Choi SW. In the cystathionine beta-synthase knockout mouse, elevations in total plasma homocysteine increase tissue S-adenosylhomocysteine, but responses of S-adenosylmethionine and DNA methylation are tissue specific. J Nutr. 2002;132:2157–60. doi: 10.1093/jn/132.8.2157. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Karaplis AC, Ackerman SL, Pogribny IP, Meinyk S, Lussier-Cacan S, et al. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet. 2001;10:433–43. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- 10.Ghandour H, Chen Z, Selhub J, Rozen R. Mice deficient in methylenetetrahydrofolate reductase exhibit tissue-specific distribution of folates. J Nutr. 2004;134:2975–8. doi: 10.1093/jn/134.11.2975. [DOI] [PubMed] [Google Scholar]
- 11.Swanson DA, Liu M-L, Baker PJ, Garrett L, Stitzel M, Wu J, et al. Targeted disruption of the methionine synthase gene in mice. Mol Cell Biol. 2001;21:1058–65. doi: 10.1128/MCB.21.4.1058-1065.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dayal S, Devlin AM, McCaw RB, Liu ML, Arning E, Bottiglieri T, et al. Cerebral vascular dysfunction in methionine synthase-deficient mice. Circulation. 2005;112:737–44. doi: 10.1161/CIRCULATIONAHA.104.529248. [DOI] [PubMed] [Google Scholar]
- 13.Elmore CL, Wu X, Leclerc D, Watson ED, Bottiglieri T, Krupenko NI, et al. Metabolic derangement of methionine and folate metabolism in mice deficient in methionine synthase reductase. Mol Genet Metab. 2007;91:85–97. doi: 10.1016/j.ymgme.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li D, Pickell L, Liu Y, Wu Q, Cohn JS, Rozen R. Maternal methylenetetrahydrofolate reductase deficiency and low dietary folate lead to adverse reproductive outcomes and congenital heart defects in mice. Am J Clin Nutr. 2005;82:188–95. doi: 10.1093/ajcn.82.1.188. [DOI] [PubMed] [Google Scholar]
- 15.Jacques PF, Bostom AG, Williams RR, Ellison RC, Eckfeldt JH, Rosenberg IH, et al. Relationship between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation. 1996;93:7–9. doi: 10.1161/01.cir.93.1.7. [DOI] [PubMed] [Google Scholar]
- 16.George L, Mills JL, Johansson ALV, Nordmark A, Olander B, Granath F, Cnattingius S. Plasma folate levels and risk of spontaneous abortion. JAMA. 2002;288:1867–73. doi: 10.1001/jama.288.15.1867. [DOI] [PubMed] [Google Scholar]


