Abstract
Recent studies have demonstrated that plasticity of naturally occurring CD4+Foxp3+ regulatory T cells (nTregs) may account for their inability to control chronic inflammation in established autoimmune diseases. All-trans retinoic acid (atRA), the active derivative of vitamin A, has been demonstrated to promote Foxp3+ Treg differentiation and suppress Th17 development. In this study, we report a vital role of atRA in sustaining the stability and functionality of nTregs in the presence of IL-6. We found that nTregs treated with atRA were resistant to Th17 and other Th cell conversion and maintained Foxp3 expression and suppressive activity in the presence of IL-6 in vitro. atRA decreased IL-6R expression and signaling by nTregs. Of interest, adoptive transfer of nTregs even from arthritic mice treated with atRA suppressed progression of established collagen-induced arthritis. We suggest that nTregs treated with atRA may represent a novel treatment strategy to control established chronic immune-mediated inflammatory diseases.
Naturally occurring CD4+Foxp3+ regulatory T cells (nTregs) play crucial roles in controlling autoimmune disease by maintaining immunological homeostasis and self-tolerance (1). Adoptive transfer of nTregs has been proven to prevent many autoimmune diseases; however, transfer of nTregs once the disease is established is less predictable. In lupus, their effect was only modest (2). In collagen-induced arthritis (CIA), nTreg transfer could prevent but was unable to modify established disease (3, 4). The reasons for lack of effect are poorly understood and may include instability of Foxp3 in an inflammatory milieu (5, 6), conversion to proinflammatory effector cells (5, 6) or an acquired resistance of T effector cells to Tregs (7).
Recent studies have documented the instability of nTregs in the presence of certain proinflammatory cytokines. For instance, we and others (5, 6) have recently reported that TCR-stimulated nTregs can be converted to Th17 cells in the presence of IL-6 in vitro. Strong TCR stimulation also drives the conversion of nTregs to Th1 cells (8). Additionally, decreased Foxp3 expression can cause immune disease by subverting the suppressive function of nTregs and converting them into Th2 cells (9). Moreover, the suppressive activities of nTregs can be abrogated by IL-6 (10). Therefore, it is desirable to find an approach that can sustain the stability and functionality of nTregs in the inflammatory condition.
All-trans retinoic acid (atRA), the major vitamin A metabolite, has been proven to not only enhance the de novo generation of naive CD4+ cells to Foxp3+ Tregs but also suppress de novo differentiation of naive CD4+cells to Th17 cells (11–13). We have addressed in this paper the effects of atRA on nTregs. We found that atRA not only maintains the phenotypic stability of nTregs but also sustains their functional activities in the presence of IL-6. Pretreatment of nTregs with atRA can downregulate IL-6R expression and IL-6R signaling, therefore restraining nTregs to Th17 conversion and sustaining Foxp3 expression of nTregs. Of note, we found that adoptive transfer of nTregs treated with atRA to established CIA markedly suppressed the progression and ameliorated the severity of arthritis. More importantly, atRA can alter the stability and function of nTregs from autoimmune arthritic mice, implicating that this strategy may have an important clinical value.
Materials and Methods
Mice
Female DBA/1 mice (6–8 wk) were purchased from The Jackson Laboratory (Bar Harbor, ME). Foxp3gfp knockin mice on the DBA/1 background were developed by backcrossing of Foxp3gfp knockin mice on the C57BL/6 background (provided by Dr. Rudensky, Memorial Sloan-Kettering Cancer Center, New York, NY) to DBA/1 mice for 13 generations. All animals were treated according to National Institutes of Health guidelines for the use of experimental animals with the approval of University of Southern California Committee for the Use and Care of Animals (Los Angeles, CA).
Cell purification, in vitro cell stimulation, and suppressor assay
nTregs were sorted from thymus or spleens in naive DBA/1, CIA model in DBA/1, or Foxp3gfp knockin DBA/1 mice by gating on CD4+CD25+ or CD4+GFP+ cells with 99% purity. To activate nTregs, these cells were stimulated with anti-CD3/CD28–coated beads (1 bead:5 cells) (Invitrogen, Carlsbad, CA), IL-2 (100 U/ml; R&D Systems, Minneapolis, MN) with DMSO, or atRA (0.05 μM; Sigma-Aldrich, St. Louis, MO) for 3 d. nTregs were cultured under a condition polarizing Th17 as previously reported (5). Suppressive activities of these cells were measured by a standard assay as previously described (14).
Intracellular and soluble cytokine production, and IL-6R and signaling molecule expression
To determine the cytokine production, the cells were restimulated with PMA (0.25 μg/ml) and ionomycin (0.25 μg/ml) for 5 h and brefeldin A (5 μg/ml) for 4 h. Cells were stained for surface CD4, IL-6R (anti-CD126; BD Pharmingen, San Diego, CA), anti-CD130 (MBL Medical & Biological Laboratories, Nagoya, Japan), and CRCX-5 (eBioscience, San Diego, CA). These cells were further fixed, permeabilized, and then stained for IL-17, Foxp3, retinoic acid-related orphan receptor (ROR) γt (eBioscience), and anti–phospho-STAT3 (BD Pharmingen). Soluble IL-17 and IFN-γ in the supernatants were determined by ELISA.
Induction of CIA and adoptive transfer
CIA was induced in DBA/1 mice according to standard protocol (15). nTregs treated with or without atRA (1–3 × 106) were adoptively transferred to mice with established CIA at day 28 postimmunization. The severity of disease and clinical score were judged as described above (15). Type II collagen (CII)-specific IgG subsets in the sera were determined using ELISA (15).
Statistical analysis
Statistical comparison between various groups was performed by the Student t test using GraphPad Prism software (GraphPad, San Diego, CA). Differences were considered significant when p values were <0.05.
Results and Discussion
Addition of atRA makes nTregs resistant to Th17 cell conversion and sustains Foxp3 expression when stimulated with IL-6
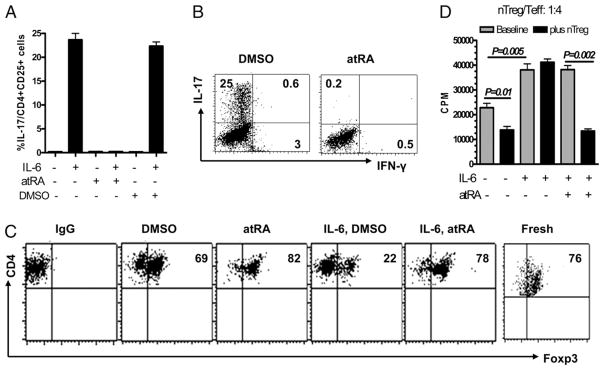
Splenic CD4+CD25+ nTregs sorted from naive DBA/1 mice were stimulated with anti-CD3/CD28 Abs with or without IL-6. As described by previous reports, some nTregs TCR activated with IL-6 can become Th17 cells (5, 6). Fig. 1A, 1B, and Supplemental Fig. 1A show, however, that when atRA but not DMSO control was added to cultures containing IL-6, both intracellular and soluble IL-17 production was completely blocked. We have also observed that addition of atRA did not affect the activation and proliferation status of nTregs, suggesting that atRA may specifically inhibit Th17 conversion from IL-6–treated nTregs.
FIGURE 1.
Addition of atRA confers nTregs resistant to Th17 conversion and sustains Foxp3 expression following stimulation with IL-6. A, Splenic nTregs were stimulated with immobilized anti-CD3 (1 μg/ml), soluble anti-CD28 (1 μg/ml), and IL-6 (10 ng/ml) ± atRA (0.05 μM). Three days later, these cells were collected for intracellular IL-17 and IFN-γ staining. Values indicate mean ± SEM of four separate experiments and representative of these experiments (B). C, nTregs were activated with anti-CD3/CD28–coated beads ± atRA solvent (DMSO) or atRA (0.05 μM) or IL-6 (10 ng/ml) for 3 d, and Foxp3 expression was determined by FACS staining. Data are representative of four independent experiments. D, The suppressive activities of nTregs against dep-CD25 T cells ± IL-6 and/or atRA were determined by the inhibition of tritiated thymidine ([3H]thymidine deoxyribose) uptake. Values indicate the mean ± SEM of four independent experiments (D). The p values were calculated by Student t test and indicate significant differences between cultures ± atRA (p < 0.05; below is same).
Because IL-6 suppresses Foxp3 induction and atRA promotes TGF-β–induced Foxp3 (16), we sought to determine whether addition of atRA can overcome the effect of IL-6 on phenotype of nTregs. Although TCR-stimulated ex vivo nTregs slightly decreased Foxp3 expression, addition of exogenous IL-6 markedly decreased Foxp3 expression (Fig. 1C, Supplemental Fig. 1B). Interestingly, the addition of atRA to nTregs in the presence of IL-6 almost completely prevented the downregulation of the Foxp3 expression seen in DMSO cultures (Fig. 1C, Supplemental Fig. 1B). Previous study has confirmed Th17 conversion came from purified CD25+Foxp3+ but not CD25+-Foxp3− cells (5), and addition of atRA still suppressed Th17 conversion from purified Tregs and sustained Foxp3 expression when stimulated with IL-6 using Foxp3gfp knockin mice (Supplemental Fig. 1B, 1C).
IL-6 also markedly decreases the suppressive activities by nTregs (5, 10). This effect is shown in Fig 1D. The suppressive activity of nTregs against T responder cell proliferation was completely abolished in the presence of IL-6. It is not surprising that addition of IL-6 actually increased responder T cell proliferation in the presence or absence of nTregs because T cells highly express IL-6R (5). Conversely, addition of atRA to the cultures maintained the suppressive activity of nTregs. In addition, addition of atRA alone did not suppress the T cell response in the presence of IL-6 when nTregs were absent (Fig. 1D), suggesting that atRA does not directly interfere with the role of IL-6 in immune response of T responder cells. Taken together, these data suggest that atRA can overcome the proinflammatory effects of IL-6 and sustain the stability and suppressive function of nTregs.
nTregs expanded with atRA are resistant to the inhibitory effects of IL-6 on Foxp3 expression and prevent Th17 conversion
The presence of atRA was not necessary for nTregs to become resistant to the inhibitory effects of IL-6. Unlike nTregs expanded with IL-2 only in which Foxp3 expression gradually decreased (17), Foxp3 expressed by nTregs pretreated with atRA remained stable, and the suppressive activities of these cells were even superior to nTregs expanded without atRA (Fig. 2A, 2B). Although atRA did not increase total Foxp3+ cell numbers (Supplemental Fig. 1D), it prevented Foxp3 from downregulation by expanded nTregs and may inhibit the expansion of CD25+Foxp3− cells, leading to the enrichment of Foxp3+ Tregs. This is consistent with previous reports that suppressive activity of Tregs is closely associated with their Foxp3 levels (14, 18). Given that atRA sustained the phenotype and function of nTregs in the presence of IL-6, we next asked if nTregs expanded with atRA also conferred resistance to the inhibitory effects of IL-6. As shown in Fig. 2C, when expanded nTregs were restimulated with TCR and IL-6, ~20–30% nTregs converted to Th17 or Th1. We did not observe any Th2 and/or follicular Th cell conversion from nTregs (not shown). In contrast, nTregs expanded with atRA were completely resistant to Th17 and Th1 conversion (Fig. 2C). IL-17 and IFN-γ secreted into the supernatants were consistent with intracellular cytokine expression (not shown). In addition, we also observed that the Foxp3 expression by expanded nTregs was markedly decreased following restimulation with IL-6, whereas nTregs previously treated with atRA mostly maintained Foxp3 expression that was similar to Fig. 1C (Fig. 2D). Although expanded nTregs with intact suppressive activity completely lost this activity, the suppressive function of expanded nTregs treated with atRA was completely intact in the presence of IL-6 (Fig. 2E). These nTregs were washed exhaustively postharvesting, and atRA measured by HPLC in the supernatants in suppressive assay cultures was undetectable (not shown). Thus, there was no carryover of atRA in the suppressive activity. These results provide strong evidence that treatment of nTregs with atRA can stabilize their phenotype and suppressive activity.
FIGURE 2.
Expanded nTregs treated with atRA maintain Foxp3 expression and suppressive function while restraining to Th17 and Th1 conversion in the presence of IL-6. A, nTregs were stimulated as in Fig. 1B for 7 d, and Foxp3 expression was determined. B, The suppressive activity of these cells was similarly analyzed by inhibition of [3H]thymidine deoxyribose incorporation as in Fig. 1D. Values indicate the mean ± SEM of triplicate wells and data representative of three independent experiments. nTregs were activated as in A and restimulated with IL-6 as in Fig. 1A. Intracellular IL-17 and IFN-γ expression (C) and Foxp3 expression (D) were determined by FACS staining. Results are either representative or mean ± SEM of three independent experiments. E, The suppressive activities of atRA or DMSO treated nTregs ± IL-6 in vitro was determined by similar methods as Fig. 1D.
nTregs treated with atRA can ameliorate the progression of established CIA in mice
Because IL-6 is often a component of inflammatory infiltrates, the ability of atRA to stabilize nTregs in the presence of IL-6 offers the possibility that transfer of atRA-treated nTregs can be therapeutic in the established chronic immune-mediated diseases, such as CIA. Previous studies have indicated that adoptive transfer of nTregs can prevent the development of CIA, but their therapeutic effect on the established CIA is unsatisfactory (3, 19).
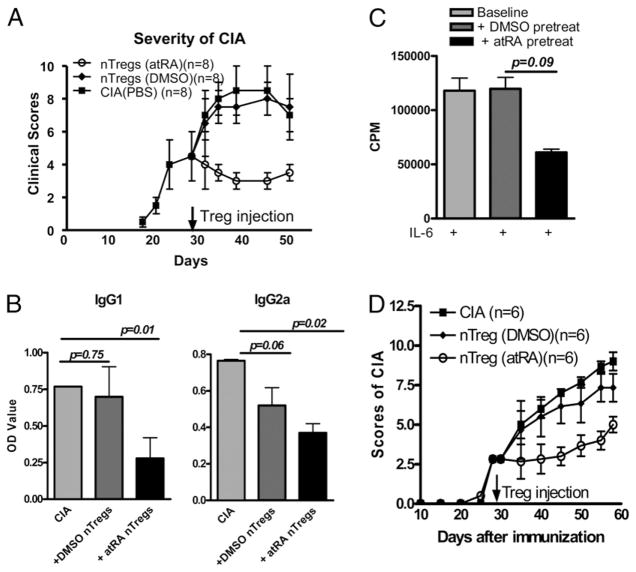
Accordingly, we immunized DBA/1 mice with CII/CFA, and when the animals had developed arthritis around day 28, we transferred 1 × 106 nTregs previously stimulated with or without atRA. We used this dose of nTregs because others have used similar cell numbers to prevent CIA (3). As shown in Fig. 3A, transfer of atRA-treated nTregs completely blocked the progression of arthritis symptoms and could even decrease the clinical score compared with mice at day 28. Conversely, like control mice injected with PBS, mice injected with nTregs activated without atRA developed increasingly more severe arthritis (Fig. 3A). The data in Fig. 3A summarize two independent experiments inducing a total of eight mice in each group. Injection of atRA-treated nTregs but not control cells also significantly suppressed Th17 cell production in draining lymph nodes (Supplemental Fig. 2). In another set of experiments, we injected 3 × 106 nTregs treated with or without atRA, and effects of these cells on CIA were similar to mice treated with 1 × 106 cells (not shown). Thus, the absence of a protective effect of these expanded nTregs treated without atRA could not be explained by insufficient cell numbers. Finally, the transfer of atRA-treated nTregs also suppressed the production of CII-specific Abs in established CIA (Fig. 3B). This indicates that the clinical improvement is associated with a reduced CII-specific immune response.
FIGURE 3.
nTregs treated with atRA suppress the progression of established CIA. A, nTregs isolated from naive DBA/1 mice were expanded as in Fig. 1B for 4 d. Mice with established CIA were injected i.v. with 1 × 106 atRA-treated nTregs, DMSO-treated nTregs, or PBS (control group) (n = 8/group). The mice were examined every 3 d postinjection, and the clinical scores are indicated. B, CII-specific IgG1 and IgG2a levels in sera on day 45 after CII/CFA immunization were measured by ELISA. Values indicate the mean ± SEM of two independent experiments (n = 8/group). C, nTregs isolated from CIA were treated ± atRA as described in Fig. 2A, and their suppressive activity was determined by similar methods as Fig. 2A. Values indicate the mean ± SEM of three independent experiments. D, nTregs from CIA mice were expanded with anti-CD3/CD28 beads (1:5) and IL-2 (100 U/ml) ± atRA (0.05 μM) for 4 d. Mice with established CIA were injected i.v. with 1 × 106 atRA-treated nTregs, DMSO-treated nTregs, or PBS (control group) (n = 6/group). The mice were examined every 5 d postinjection, and the clinical scores are indicated.
Using a similar strategy, we also observed that atRA can stabilize the phenotype and function of nTregs in arthritic mice. Arthritis was evident in DBA/1 mice around day 28 post-immunization. These mice displayed moreCD4+CD25+ cells in spleen (Supplemental Fig. 3A) and lymph nodes (not shown). Conversely, the frequency of Foxp3+ cells between CD4+ and CD4+CD25+ subsets were significantly decreased (Supplemental Fig. 3A–C). These splenic CD4+CD25+ cells still significantly suppressed the proliferation of splenic responder T cells isolated from arthritic mice, although their suppressive activities were less efficient compared with nTregs from naive mice (Supplemental Fig. 3D). Of note, these cells can be converted into Th17 cells when stimulated with IL-6, although their conversion was slightly lower than nTregs isolated from naive mice, and addition of atRA suppressed Th17 conversion (Supplemental Fig. 4A). Similarly, treatment with atRA sustained Foxp3 expression and maintained the suppressive activities by these nTregs (Fig. 3C, Supplemental Fig. 4B). More importantly, nTregs isolated from established CIA resulted in significant suppression on the arthritis progress when they were treated with atRA but not DMSO (Fig. 3D). This finding is very important because atRA-treated nTregs from patients could potentially be used to control disease development.
nTregs treated with atRA maintain their phenotype and function by downregulating IL-6R expression and signaling
We next sought to determine the mechanisms by which atRA sustains the stability of nTregs in the inflammatory milieu. As reported by Xiao et al. (20), atRA not only strongly inhibits the upregulation of IL-6 Rα mRNA induced by TGF-β, but also decreases the levels of phospho-STAT3 expression induced by IL-6 plus TGF-β. We examined whether atRA can affect IL-6R and its signaling expression in nTregs. nTregs were stimulated with TCR with or without atRA for 4 d. We observed that, similar to naive T cells, freshly isolated nTregs expressed substantial amounts of IL-6R α-chain (CD126) that slightly decreased after TCR activation (Fig. 4A). The addition of atRA markedly decreased the CD126 expression in activated nTregs (Fig. 4A), which is consistent with previous finding that atRA decreased CD126 expression on naive CD4+ cells (20). Although IL-6 Rβ (CD130) is not highly expressed by nTregs, addition of atRA also significantly decreased its expression (Fig. 4B). The IL-6R expression reduction is likely associated with downregulation of IL-6R signaling because addition of atRA also significantly decreased STAT3 activation in nTregs (Fig. 4B). When atRA-treated nTregs were restimulated with IL-6, the decrease in IL-6 signaling was accompanied by a decrease of expression of RORγt (Fig. 4B), the crucial transcription factor required for Th17 cell differentiation (21). This finding is in agreement with the previous observation that the combination of IL-2 and TGF-β had dramatic effects on both IL-6R expression and signaling on nTregs (5).
FIGURE 4.

nTregs treated with atRA maintain their phenotype and function via downregulation of IL-6R expression and phospho-STAT3 activation. A, nTregs were treated ± atRA as described in Fig. 2A, and CD126 (IL-6R α-chain) expression was determined by FACS. The figure shows data from one of four separate experiments. B indicates the mean ± SEM of surface CD126, CD130 (IL-6R β-chain), intracellular phosphorylated STAT3, and transcription factor RORγt expression as determined by FACS (n = 4).
It is now evident that instability and plasticity of nTregs in a proinflammatory cytokine milieu like IL-6 is an important factor for their inability to control diseases, such as established CIA. This study extends the observation that retinoic acid can enhance Foxp3 expression and inhibit Th17 differentiation on nTregs. This treatment makes them resistant to Th17 and Th1 cell conversion when stimulated with IL-6 by decreasing IL-6R expression, signaling, and RORγt production. This reduction in IL-6R signaling may be responsible for the maintenance of Foxp3 expression and suppressive activity of nTregs in the presence of IL-6. Although some workers have reported that in some autoimmune diseases T effector cells have become resistant to Tregs, our finding that atRA-treated nTregs, even those cells from arthritic mice, halted the progression of established CIA suggests that, at least in this model, T effector cells can be controlled. Thus, nTregs pretreated with atRA may have special therapeutic potential in immune-mediated chronic inflammatory diseases.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R01 AR059103, American College of Rheumatology Within Our Reach, the Arthritis Foundation, an Outstanding Youth Scientist Award from the National Natural Science Foundation of China (30728007) (all to S.G.Z), and National Natural Science Foundation of China Grant 30772150 (to Z.L.).
Abbreviations used in this paper
- atRA
all-trans retinoic acid
- CIA
collagen-induced arthritis
- CII
type II collagen
- nTreg
naturally occurring CD4+Foxp3+ regulatory T cell
- ROR
retinoic acid-related orphan receptor
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 2.Scalapino KJ, Tang Q, Bluestone JA, Bonyhadi ML, Daikh DI. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J Immunol. 2006;177:1451–1459. doi: 10.4049/jimmunol.177.3.1451. [DOI] [PubMed] [Google Scholar]
- 3.Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM, de Vries RR, Toes RE. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52:2212–2221. doi: 10.1002/art.21195. [DOI] [PubMed] [Google Scholar]
- 4.Bardos T, Czipri M, Vermes C, Finnegan A, Mikecz K, Zhang J. CD4+CD25+ immunoregulatory T cells may not be involved in controlling autoimmune arthritis. Arthritis Res Ther. 2003;5:R106–R113. doi: 10.1186/ar624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+ CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 6.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25−Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 7.Parietti V, Monneaux F, Décossas M, Muller S. Function of CD4+, CD25+ Treg cells in MRL/lpr mice is compromised by intrinsic defects in antigen-presenting cells and effector T cells. Arthritis Rheum. 2008;58:1751–1761. doi: 10.1002/art.23464. [DOI] [PubMed] [Google Scholar]
- 8.Lu L, Wang J, Zhang F, Chai Y, Brand DD, Wang X, Horwitz DA, Shi W, Zheng SG. Role of SMAD and non-SMAD signals in the development of Th17 and regulatory T cells. J Immunol. 2010;184:4295–4306. doi: 10.4049/jimmunol.0903418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 10.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+ CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 11.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 13.Nolting J, Daniel C, Reuter S, Stuelten C, Li P, Sucov H, Kim BG, Letterio JJ, Kretschmer K, Kim HJ, von Boehmer H. Retinoic acid can enhance conversion of naive into regulatory T cells independently of secreted cytokines. J Exp Med. 2009;206:2131–2139. doi: 10.1084/jem.20090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu L, Ma J, Wang X, Wang J, Zhang F, Yu J, He G, Xu B, Brand DD, Horwitz DA, et al. Synergistic effect of TGF-beta superfamily members on the induction of Foxp3+ Treg. Eur J Immunol. 2010;40:142–152. doi: 10.1002/eji.200939618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007;2:1269–1275. doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]
- 16.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G, Olek S, Dietmaier W, Andreesen R, Edinger M. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol. 2009;39:1088–1097. doi: 10.1002/eji.200838904. [DOI] [PubMed] [Google Scholar]
- 18.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 19.Kelchtermans H, Geboes L, Mitera T, Huskens D, Leclercq G, Matthys P. Activated CD4+CD25+ regulatory T cells inhibit osteoclastogenesis and collagen-induced arthritis. Ann Rheum Dis. 2009;68:744–750. doi: 10.1136/ard.2007.086066. [DOI] [PubMed] [Google Scholar]
- 20.Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181:2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.