Abstract
Adeno-associated virus vectors (AAV) show promise for liver-targeted gene therapy. In this study, we examined the long-term consequences of a single intravenous administration of a self-complementary AAV vector (scAAV2/ 8-LP1-hFIXco) encoding a codon optimized human factor IX (hFIX) gene in 24 nonhuman primates (NHPs). A dose–response relationship between vector titer and transgene expression was observed. Peak hFIX expression following the highest dose of vector (2 × 1012 pcr-vector genomes (vg)/kg) was 21 ± 3 µg/ml (~420% of normal). Fluorescent in-situ hybridization demonstrated scAAV provirus in almost 100% of hepatocytes at that dose. No perturbations of clinical or laboratory parameters were noted and vector genomes were cleared from bodily fluids by 10 days. Macaques transduced with 2 × 1011 pcr-vg/kg were followed for the longest period (~5 years), during which time expression of hFIX remained >10% of normal level, despite a gradual decline in transgene copy number and the proportion of transduced hepatocytes. All macaques developed serotype-specific antibodies but no capsid-specific cytotoxic T lymphocytes were detected. The liver was preferentially transduced with 300-fold more proviral copies than extrahepatic tissues. Long-term biochemical, ultrasound imaging, and histologic follow-up of this large cohort of NHP revealed no toxicity. These data support further evaluation of this vector in hemophilia B patients.
Introduction
Hemophilia B, an X-linked bleeding disorder, is ideally suited for gene replacement approaches. This is partly because its clinical manifestations are attributable to the lack of a single gene product, clotting factor IX (FIX) and because the therapeutic goal is modest, as 1% of physiological levels would ameliorate the severe bleeding phenotype. Furthermore, the availability of animal models, including the ability to assess transduction in nonhuman primate (NHP) model, allows extensive preclinical evaluation of gene transfer strategies.1,2,3,4 Several approaches for FIX replacement have been evaluated (reviewed in ref. 5); however, recombinant adeno-associated viral vectors (rAAV) currently appear most promising. These vectors have an excellent safety profile and can direct long-term transgene expression from postmitotic tissues such as the liver and muscle.6,7 To further improve the potency and safety of rAAV-mediated gene transfer for hemophilia B, we have incorporated three distinct aspects to our study design. The first involves the use of a novel self-complementary AAV vector (scAAV) encoding human FIX (hFIX) designed to achieve therapeutic hFIX expression with lower doses of vector. This is an important safety feature given that the occurrence of capsid-specific CD8+ T cell activation and transaminitis appears to be vector dose-dependent.7,8 Secondly, we have pseudotyped these vectors with AAV8 capsid protein, which offers several potential advantages over AAV2. These include: (i) an ability to mediate effective transduction in animals with immunity to AAV2, (ii) reduced virus uptake by antigen-presenting cells, and (iii) a lower seroprevalence in humans.9,10 Finally, because of the unique tropism of AAV8, efficient and selective transduction of the liver is possible following systemic administration of scAAV vector via the peripheral venous route, a simple noninvasive approach that is safer and highly desirable for patients with a bleeding diathesis.11
Thus far, characterization of the consequences of systemic delivery of scAAV vectors has been limited, with follow-up of efficacy and safety usually for <2 years, a time period inadequate for a chronic disorder such as hemophilia B.11 The minimum vector dose required for therapeutic expression as well as safety and stability of peripheral vein delivery of scAAV2/8-LP1-hFIXco in primates remains undefined. In this study, we describe the consequences of peripheral vein administration of our novel scAAV2/8-LP1-hFIXco vector in a relatively large cohort of NHP (n = 24) over an extended period of follow-up of over 5 years. Our results indicate that peripheral vein delivery of scAAV2/8-LP1-hFIXco at a variety of different doses is safe and not associated with acute or delayed toxicity. In addition, stable transgene expression was observed, even in animals with pre-existing immunity to other AAV serotypes. Ultrasound imaging as well as histological evaluation did not reveal an increased incidence of malignancy.
Results
Therapeutic dose range for self-complementary vectors encoding hFIX in rhesus macaques
The preclinical stock of scAAV2/8 was extensively characterized to ensure that the key release criteria for clinical grade vector were met. The yield of scAAV was ~2 × 1012 pcr-vector genomes (vg)/10-stack cell factory (~5,000 particles/293T cell). Between 2 × 109 and 2 × 1012 pcr-vg/kg of this clinical grade scAAV2/8-LP1-hFIXco vector was administered as a bolus infusion in the saphenous vein of five cohorts of juvenile male macaques (Table 1). These animals had been carefully screened and selected to ensure that they had undetectable baseline anti-AAV8 titers by both enzyme-linked immunosorbent assay and neutralizing antibody assays, thereby reducing the risk of immune-mediated blockade of gene transfer. Administration of scAAV2/8-LP1-hFIXco at all five dose levels was well tolerated without perturbation of vital signs including pulse, blood pressure, respiratory rate, and temperature or change in behavior during the observation period after infusion.
Table 1. Relationship between dose of scAAV2/8-LP1-hFIXco and gene transfer efficiency.
Peripheral vein administration of the highest dose of vector (2 × 1012 pcr-vg/kg) resulted in a significant viremia in all macaques in this dose cohort. An average of 3.6 ± 0.9 × 109 vg/ml of scAAV2/8-LP1-hFIXco DNA was detectable in the plasma on day 1 following vector administration, which gradually declined to undetectable levels by day 10, as measured by quantitative PCR (qPCR). Vector genomes were also detectable in the saliva, urine, and stool of all animals in this dose cohort but at levels that were at least a log lower than those in plasma (Figure 1). Complete blood count, coagulation studies, and serum biochemistry studies (including liver function tests) remained within the normal range for the duration of the study in the transduced animals, as well as two control animals (Supplementary Figure S1).
Figure 1.
Clearance of self-complementary AAV vector (scAAV)2/8-LP1-hFIXco vector after peripheral vein administration of 2 × 1012 pcr-vector genomes (vg)/kg. Clearance of the vector from rhesus plasma, urine, saliva, and stool was determined using a quantitative PCR (qPCR) assay on samples collected following peripheral vein administration of 2 × 1012 pcr-vg/kg scAAV2/8-LP1-hFIXco. Standards consisting of serial dilutions of scAAV2/8-LP1-hFIXco in rhesus plasma were used to define the sensitivity of the assay. Results are expressed as mean transgene copy number (vg)/ml ± SE of sample obtained from three animals in this dose cohort and indicate that the vector genomes have cleared from the body fluids by day 10.
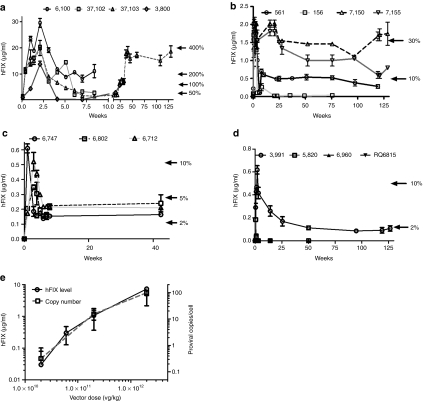
Within 24 hours of peripheral vein administration of 2 × 1012 pcr-vg/kg of scAAV2/8-LP1-hFIXco, hFIX expression was detectable at ~20% (1.0 ± 0.2 µg/ml) of normal levels, reaching supraphysiologic levels of 21.0 ± 3 µg/ml (420% of normal) 15 days after gene transfer (Figure 2a). Two animals, 6100 and 37102 were sacrificed at 56 days for histopathologic assessment. hFIX expression in their plasma at this point was 11.0 ± 3 and 3.0 ± 3 µg/ml, respectively. Similarly, expression of hFIX in the two surviving animals had a trough level of 17% (0.8 ± 0.1 µg/ml) at 8 weeks. Expression in both animals increased over time to steady-state levels that have been maintained for between 22 and 130 weeks (study still ongoing) after vector administration without any overt toxicity.
Figure 2.
Expression of human factor IX (hFIX) in rhesus macaques transduced with different doses of adeno-associated viral (AAV) vectors. Plasma hFIX levels (mean ± SEM) measured by enzyme-linked immunosorbent assay (ELISA) at the stated time points following peripheral vein administration of scAAV-LP1-hFIX of (a) 2 × 1012 pcr-vg/kg, (b) 2 × 1011 pcr-vg/kg, (c) 6 × 1010 pcr-vg/kg, and (d) 2 × 1010 pcr-vg/kg. (e) The relationship between vector dose and plasma hFIX levels and scAAV transgene copy number in the liver at 4 weeks after gene transfer.
The profile of hFIX expression was broadly similar when a tenfold lower dose (2 × 1011 pcr-vg/kg) of scAAV2/8-LP1-hFIXco was administered into the peripheral vein of four macaques. Once again, hFIX expression at therapeutic levels (2 ± 0.8% of normal, range = 1–4%) was detectable within 24 hours of peripheral vein administration of scAAV2/8-LP1-hFIXco (Figure 2b). Transgene expression continued to rise, reaching peak values (1.8 ± 0.1 µg/ml = ~36%) by 7 days after gene transfer, before declining to mean steady-state levels of around 0.85 ± 0.4 µg/ml (17 ± 8%), which were sustained for the duration of the study (>2.5 years) in three out of four animals, consistent with our previous report.11 In the fourth animal (macaque 156), hFIX was detectable for 143 days before being abrogated by anti-hFIX antibodies with a peak titer of 12 Bethesda inhibitor assay units /ml. This animal subsequently developed nephrotic syndrome, associated with progressive edema, low serum albumin (3.1 g/dl), raised serum creatinine (2.2 mg/dl), and heavy proteinuria (3+ by dipstick method). It was euthanized 4 weeks later and necropsy findings showed marked diffuse glomerulosclerosis in both kidneys (Supplementary Figure S2). Interestingly, nephrotic syndrome in association with a persistent humoral response to FIX has also been reported in humans with hemophilia B.12
An approximately half log decrease in the scAAV2/8-LP1-hFIXco vector dose to 6 × 1010 pcr-vg/kg resulted in a significant drop in peak-plasma hFIX levels to 7 ± 2% (0.35 ± 0.1 µg/ml) of normal in the three macaques in this dose cohort. As with the other two dose levels, expression subsequently declined to a steady-state level, in this case of ~3.7 ± 0.4% (0.19 ± 0.02 µg/ml, Figure 2c).
In contrast to the previous dose levels, peripheral vein delivery of 2 × 1010 pcr-vg/kg scAAV2/8-LP1-hFIXco vector to four macaques resulted in variable levels of transgene expression. One of the four animals expressed hFIX at nearly 12% (0.6 ± 0.04 µg/ml) of normal at around 3 weeks. Expression then dropped to steady-state levels of ~2% (0.11 ± 0.02 µg/ml). A second animal had peak expression of hFIX at 1% of physiological levels at around 3 weeks, but this then declined to undetectable levels within 10 weeks of vector administration (Figure 2d). This variable level of gene transfer (0–12% of normal) suggests that this dose may be at the threshold required for stable transduction, a concept described for other serotypes of AAV in mice. Peripheral vein administration of 2 × 109 pcr-vg/kg of scAAV2/8-LP1-hFIXco in one macaque did not result in any detectable hFIX over a 3-month period (Table 1).13 No humoral response to the hFIX xenoprotein, as assessed by a modified Bethesda assay, was detected in any of the animals in these last two dose cohorts. Therefore, a dose-dependent, increase in plasma hFIX levels followed peripheral vein administration of scAAV2/8-LP1-hFIXco in macaques with no evidence of saturation kinetics even at the highest dose of 2 × 1012 pcr-vg/kg (Figure 2e).
Molecular and pathological consequences of scAAV2/8-LP1-hFIXco gene transfer
Biopsies of the liver, spleen, kidney, and testes were obtained from each of the animals in the 2 × 1012 pcr-vg/kg cohort 1 week following vector administration. In addition, a more extensive tissue collection (>40 different tissues) was performed at necropsy of two of the macaques (6100 and 37102) 56 days after administration of 2 × 1012 pcr-vg/kg of scAAV2/8-LP1-hFIXco, and of a control animal that received only excipient. All tissue samples had a normal appearance macroscopically. Histologic evaluation did not reveal any pathological lesions in the scAAV-transduced or control macaques; specifically, no ischemic, apoptotic, inflammatory, or mitotic changes were noted.
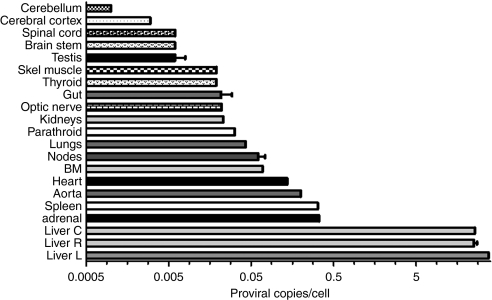
Vector biodistribution studies using a qPCR assay (Figure 3) showed that the scAAV2/8-LP1-hFIXco proviral DNA was detectable in all tissue samples obtained from the two necropsied animals in the highest dose cohort (2 × 1012 pcr-vg/kg). The liver, however, was preferentially transduced with an average of 98 ± 12 proviral copies per diploid genome (c/dg) 8 weeks after gene transfer. In contrast, the genome copy number per cell in the adrenal gland, spleen, aorta, and the heart was 300-fold lower, at an average of 0.25 ± 0.01 c/dg, and was even lower in the other organs at the same time point.
Figure 3.
Biodistribution of vector following peripheral vein administration of 2 × 1012 pcr-vg/kg scAAV2/8-LP1-hFIXco. Results of quantitative PCR (qPCR) analysis of genomic DNA, isolated from the indicated organs at 8 weeks after administration of 2 × 1012 pcr-vg/kg of scAAV2/8 particles via the peripheral venous route using primers unique to hFIXco. Shown is transgene copy number per diploid genome ± SE corrected for variation in loading and amplification efficiency using GAPDH primers. BM, bone marrow.
An almost linear, dose-dependent, increase in scAAV2/8-LP1-hFIXco proviral copy number in the liver was observed with a regression coefficient (r) = 0.915 (Figure 2e). The lowest proviral copy number at week 1 was detected in the liver of animals in the 2 × 1010 pcr-vg/kg dose cohort with proviral copy numbers of 0.54 and 0.1 c/dg in animals 3991 and 5820, respectively. In contrast, the transgene copy number (18 ± 2 c/dg) was 50-fold higher in animals transduced with 2 × 1011 pcr-vg/kg of scAAV2/8-LP1-hFIXco. The highest level of gene transfer was observed in the 2 × 1012 pcr-vg/kg with a proviral copy number of 109 ± 16 c/dg. Interestingly, the amount of hFIX per copy of scAAV2/8-LP1-hFIXco genome was consistent over the dose cohorts and ranged from 0.07 to 0.09 µg/proviral copy.
The scAAV2/8-LP1-hFIXco genome was detected by fluorescent in-situ hybridization in almost 100% of hepatocytes derived from macaques that received 2 × 1012 pcr-vg/kg. Approximately 90% and 30% of hepatocytes derived from macaques transduced at the 2 × 1011 and 2 × 1010 pcr-vg/kg dose levels showed the presence of the proviral DNA, respectively. The scAAV genome was not detected in hepatocytes in the animal that received 2 × 109 pcr-vg/kg or those that received excipient. These data suggest that a minimum dose of 2 × 1011 pcr-vg/kg is required to ensure gene transfer to at least 50% of hepatocytes.
Relationship between vector dose and immunological response
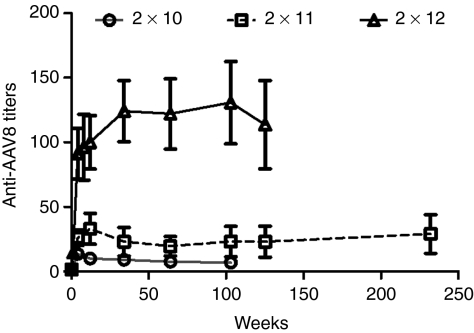
Following peripheral vein administration of 2 × 1012 pcr-vg/kg, the relative anti-AAV8 antibody titers went up to an average of 15 ± 2 relative units (RU) at week 1 and were an average of 91 ± 19 RU at week 4 (Figure 4). The peak anti-AAV8 antibody titers were 33 ± 12 and 14 ± 0.5 RU in the 2 × 1011 and 2 × 1010 pcr-vg/kg dose cohorts, respectively. The antibody titers have remained above pre-AAV infusion values in all dose cohorts over a period that extends up to 5 years.
Figure 4.
Humoral immune response after peripheral vein administration of self-complementary AAV vector (scAAV) vector. Plasma obtained from macaques after peripheral administration of scAAV2/8-LP1-hFIXco at the stated doses and at different time points after vector administration was analyzed for the presence of adeno-associated virus (AAV)8-specific immunoglobulin G (IgG) by enzyme-linked immunosorbent assay (ELISA) ± SE. Assays were performed in triplicate.
To determine whether peripheral vein administration of scAAV2/8-LP1-hFIXco caused an expansion of AAV capsid-specific T cells in rhesus macaques, peripheral blood mononuclear cells were harvested at various time points from the macaques in the 2 × 1012 pcr-vg/kg dose cohort as well as monkeys treated with excipient alone for interferon-γ enzyme-linked immunosorbent spot assay. Incubation of rhesus peripheral blood mononuclear cells with phorbol myristate acetate as positive control resulted in robust IFN-γ expression with detection of >1,000 spot-forming units/106 peripheral blood mononuclear cell. None of the animals transduced with 2 × 1012 pcr-vg/kg of scAAV2/8-LP1-hFIXco had a detectable T cell response to AAV8 capsid (peptide pools or empty capsid) at any of the time points studied (data not shown).
Relatively stable long-term expression of hFIX in NHP
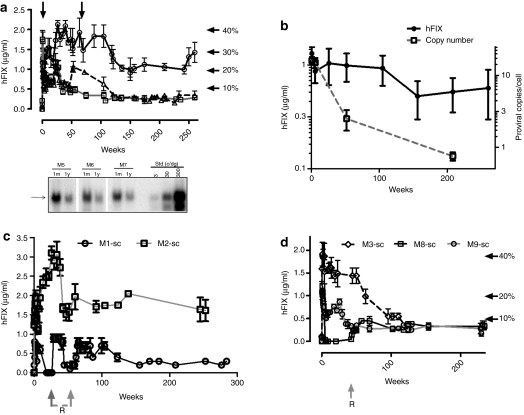
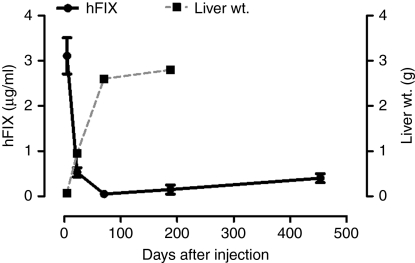
Previously, we have reported efficient transduction of the liver following mesenteric and peripheral vein administration of research grade scAAV-LP1-hFIXco pseudotyped with serotype 5 or 8 capsid in eight juvenile macaques.8,11 The estimated vector titer by the slot blot technique used in these studies differed from the current qPCR method which is now routinely used for quantification of research and clinical grade vector, by approximately fivefold. Therefore, based on our current qPCR titering methodology, the macaques described (M1-2sc mesenteric vein and M5-7sc peripheral vein, serotype 8 and M3, M8-9 sc peripheral vein, serotype 5) in our previous studies most likely received the same number of vector particles/kg as did those in our present study who received ~2 × 1011 pcr-vg/kg. This is supported by comparable peak (1.3 ± 0.2 µg/ml compared with 1.8 ± 0.1 µg/ml) and steady-state (1.1 ± 0.5 µg/ml compared with 0.85 ± 0.4 µg/ml) plasma hFIX levels in the cohort that received research grade vector (M5-7sc, Figure 5a,b) when compared with macaques that received 2 × 1011 pcr-vg/kg of clinical grade scAAV2/8-LP1-hFIXco vector in the present study. Furthermore, both groups of animals had a comparable proviral copy number in the liver. These older cohorts of macaques provide an opportunity to extend the safety and efficacy observation to over 5 years following either mesenteric or peripheral vein administration of scAAV2/8-LP1-hFIXco. Importantly, there have been no adverse events during this period. The developmental profiles, heights, and weights of these animals are comparable to those of untransduced age-matched male controls. Their hematological parameters and serum biochemistries, including renal and liver function tests, have remained within the normal range. M1-sc, who received mesenteric vein administration of scAAV2/8-LP1-hFIXco, developed a neutralizing anti-hFIX antibody within 3 months of gene transfer. This antibody was successfully eradicated using a combination of rituximab and cyclosporine initially, followed by rituximab and cyclophosphamide a month later (Figure 5c). This animal has since remained free of inhibitors and continues to express hFIX at 0.3 ± 0.1 µg/ml (~6% of normal), with a follow-up that extends to 5.5 years. The second animal in this mesenteric vein cohort continues to express 1.6 ± 0.3 µg/ml (~32% of normal) over a similar duration. Another animal (M8-sc) developed neutralizing anti-hFIX antibody within 2 months of peripheral vein administration of scAAV2/5-LP1hFIXco vector. This macaque was treated with a combination of rituximab and cyclophosphamide, which again resulted in eradication of the inhibitor. This animal also continues to express hFIX at 6% of physiologic levels without any further immunosuppression (Figure 5d).
Figure 5.
Long-term expression of human factor IX (hFIX) following a single bolus infusion of research grade self-complementary adeno-associated viral vector (scAAV)-LP1-hFIXco. (a) Plasma human FIX (hFIX) levels (mean ± SEM) measured by enzyme-linked immunosorbent assay (ELISA) at the stated time points following a single bolus infusion of scAAV-LP1-hFIXco into the peripheral vein using serotype 8 pseudotyped vector (arrows show points when liver biopsies were obtained). Macaques in these studies have been described previously.8,11 The bottom panel shows Southern blot analysis of genomic DNA extracted from the liver obtained at the stated time points after peripheral vein administration of scAAV2/8-LP1-hFIXco and digested with BsrDI followed by hybridization with an hFIXco-specific probe at the stated time. Arrow shows the expected ~1.5 kb hybridization band. (b) The relationship between plasma hFIX levels and transgene copy number in the liver. Plasma hFIX levels (mean ± SEM) following a single bolus infusion of scAAV-LP1-hFIXco into the (c) mesenteric vein using serotype 8 pseudotyped vector, and (d) peripheral vein using serotype 5 pseudotyped vector. Gray arrows with “R” in Figure 5c,d denote administration of the combination of rituximab (325 mg/m2) and cyclophosphamide (250 mg/m2) as a bolus weekly infusion for a period of 4 weeks.
The mean hFIX levels in the three animals (M5-7sc) that received peripheral vein delivery of research grade scAAV2/8-LP1-hFIXco has declined from the previous steady-state expression of 1.1 ± 0.5 µg/ml at around 9 months to an average of 0.6 ± 0.3 µg/ml at 5 years, although this difference is not statistically significant (P = 0.12) (Figure 5a,b). During this time, the average weight of these animals has increased by 2.6-fold (average at injection = 5.0 kg, current = 13.0 kg). In contrast, the transgene copy number, as assessed by qPCR, analysis declined markedly from 15 ± 2.4 c/dg at 6 weeks to 2.5 ± 1.2 c/dg at 12 months and 0.75 ± 0.1 c/dg toward the end of year 4 (Figure 5b). Southern blot analysis of genomic DNA extracted from liver biopsies obtained at 6 weeks and 1 year after peripheral vein delivery confirmed the loss of scAAV2/8-LP1-FIXco proviral genomes over time (Figure 5a). The percentage of hepatocytes transduced also decreased over time, as assessed by fluorescent in-situ hybridization, from a mean of 69 ± 1% at 6 weeks to 14 ± 4% at 1 year. The expression of hFIX/copy of scAAV2/8-LP1-FIXco in the liver increased by 12-fold from 0.06 µg of FIX/copy at 6 weeks to 0.73 µg of FIX/copy at 4 years.
A modified ligation-mediated PCR technique was used to isolate integrated forms of AAV following delivery of scAAV vector into macaque liver in the absence of selection. Over 100 clones have been isolated and sequenced. Most of these clones contained an average 250 bp of scAAV LP1-hFIXco DNA sequence consisting of either partial scAAV sequence or scAAV-scAAV junctions separated by varying lengths of intervening AAV inverted terminal repeat sequence, as reported previously in mice.14 However, we did not find any evidence of rAAV integration into genomic sequence. Whereas this may be a reflection of the sensitivity of the assay used, it is consistent with observation in humans following wild-type infection with AAV2.15
No evidence of malignant transformation following scAAV2/8-LP1-hFIX-mediated gene transfer
Macaques underwent abdominal ultrasound scanning to look for the possible development of liver tumors following peripheral vein delivery of scAAV2/8-LP1-hFIXco. High-quality ultrasound studies performed by an experienced diagnostic radiologist showed that the echotexture of each of the livers was normal. Two macaques were found to have gallstones, but no tumors were detected in the livers or any other abdominal organs of any of the macaques with a follow-up period ranging from 22 to 66 months. Similarly, laparotomy and histological assessment of random liver biopsies did not show any evidence of tumor or mitotic transformation of hepatocytes following peripheral or mesenteric vein delivery of scAAV2/8-LP1-hFIXco over a period of 5 years.
To evaluate the possibility that the developing liver was more susceptible to events that enhance the generation of tumors,16 a high dose (1 × 1013 pcr-vg/kg) of scAAV2/8-LP1-hFIXco was injected into the peritoneal cavity of day 17 fetal mice (MF-1 strain, n = 62). This route of administration is technically easier and safer to perform than intravenous injection in fetuses although we acknowledge that the biodistribution of the vector may be distinct for the two routes of administration. hFIX was detectable in all animals after birth at an average of 3.1 ± 0.4 µg/ml (~60% of normal) within 4 days of birth (i.e., 7 days after vector administration, Figure 6). Transgene expression then declined over time to reach trough levels of 0.05 ± 0.02 µg/ml (~1% of normal) on day 70 after vector administration. Murine liver titering weight during this period increased by almost 40-fold. hFIX expression was then maintained at a steady-state level of 0.4 ± 0.1 µg/ml (~8% of normal) at ~14 months. All 62 animals were healthy up to 18 months after in-utero vector administration, at which point they were all sacrificed to survey for subclinical tumors, as reported before.16 A nodule of ~0.5 cm was found in the livers of two animals, which were confirmed by histopathological examination to be hepatocellular carcinomas (HCC). A similar incidence (~3%) of HCC was observed in animals that did not receive an AAV vector (1 out of 34 control animals) but were followed for the same duration. Twenty-nine scAAV-specific ligation-mediated PCR products were isolated from genomic DNA extracted from the two HCC samples and 27 from genomic DNA extracted from the liver biopsies obtained from tissues surrounding the HCC. However, we did not find any vector-host integration junctions amongst these clones, consistent with previous reports and the fact that AAV is maintained mainly in an episomal form in postmitotic tissues such as the liver.17
Figure 6.
Expression of human factor IX (hFIX) following in-utero delivery of self-complementary adeno-associated viral vector (scAAV)2/8-LP1-hFIXco in mice. Correlation between mean body mass (weight in grams) and plasma human FIX (hFIX) levels (mean ± SEM) measured by enzyme-linked immunosorbent assay (ELISA) at the stated time points following a single in-utero administration of scAAV2/8-LP1-hFIXco.
We also performed an extensive survey at necropsy in adult male C57Bl/6 mice 1½–2½ years following tail vein administration of 8 × 1010 vg/kg of scAAV-LP1-hFIXco. The vector was injected when these mice were 4–6 weeks old. From an initial cohort of 82 mice, 20 mice were sacrificed at various time points within 12 months of gene transfer as described previously for assessment of gene transfer by immunohistochemistry or Southern blot analysis.8,11 Abnormal pathology was not detected in the organs of any of these animals. Of the remaining 62 animals, 26 were followed up for 1.75 years, 17 for 2 years, and 19 for 2.25 years after gene transfer prior, to being sacrificed for analysis. In total, six tumors were found with all being detected in the oldest cohort. Two of these tumors were pinhead size, three were between 3 and 5 mm and one extrahepatic lesion was 6 mm in maximum diameter. The histopathology of these lesions was quite mixed but consistent with that described before for an aging colony of mice.18,19,20 Two of the discrete lesions were composed of what appeared to be a proliferation of Kupffer cells and dilated sinusoids, along with areas of necrosis; the third was a hemangiosarcoma. One of the tiny tumors was a histiocytic sarcoma, the other was a benign lesion. The extrahepatic tumor in the mesentery was a lymphoma. Overall, the incidence of tumor in these mice is in keeping with previous reports for this strain.18,19,20 In addition, qPCR analysis of DNA extracted from three of the larger tumors and surrounding normal liver revealed a low vector copy number that ranged from 0.03 to 0.6 c/dg in the normal liver tissue and only 10–25% that amount in the tumors, suggesting that clonal expansion had not occurred. Liver in mice that had not developed a tumor had between 0.5 and 5-vector c/dg. AAV integration analysis was not performed on these tissue samples.
Discussion
In this study, we used clinical grade scAAV vector to extensively evaluate the safety and efficacy of peripheral vein administration of five different doses of scAAV2/8-LP1-hFIXco in macaques. A dose-dependent increase in hFIX levels in rhesus plasma was observed following systemic administration of scAAV2/8-LP1-hFIXco vector between a dose range of 2 × 1010 to 2 × 1012 pcr-vg/kg, with no evidence of saturation kinetics. A linear relationship was noted between vector dose and transgene copy number in the liver, which was preferentially transduced following peripheral vein delivery of scAAV2/8-LP1-hFIXco.
hFIX expression at or above 1% of normal was observed in two of four macaques transduced with 2 × 1010 pcr-vg/kg and was maintained stably in one of these macaques for the duration of the study (almost 2.5 years). More consistent hFIX expression above 1% of normal was found following a single peripheral vein administration of 6 × 1010 pcr-vg/kg. At this dose level, the mean hFIX expression in three macaques was 3.7 ± 0.4% of normal levels, which would be sufficient to change a hemophilia B patient's phenotype from severe to moderate. Comparison and standardization of titering methods are currently ongoing in order to determine how this dose compares to that used in the clinical trial of liver-directed administration of single-stranded AAV2 into patients with severe hemophilia B, where a dose of 2 × 1012 vg/kg was required for expression of hFIX at about 10% of normal.7 In addition, we are currently developing and validating new titering methods that are independent of vector configuration and expression cassette.
In our study, peripheral vein administration of 2 × 1011 pcr-vg/kg into macaques resulted in stable hFIX expression at ~17 ± 8% of physiological levels over a period of 4 years, whereas a tenfold higher dose (2 × 1012 pcr-vg/kg) mediated supraphysiological levels of hFIX at 300 ± 0.4% of normal. If these NHP data could be extrapolated directly to humans, scAAV2/8-LP1-hFIXco-mediated gene transfer with a dose of 2 × 1011 pcr-vg/kg could potentially eliminate the risk of spontaneous bleeding in a patient with severe hemophilia B as a result of continuous endogenous expression of hFIX at levels that are observed in patients with mild hemophilia B. This would have a great impact on the quality and duration of life and result in significant economic benefits through savings on the use of factor IX concentrates and time spent in hospital.
Expression of hFIX/copy of provirus in the liver was similar over the dose cohorts when assessed early after gene transfer (within 2 months). However, longer follow-up demonstrated a decline in transgene copy number as well as in the number of hepatocytes harboring the scAAV genome, with an associated increase in the expression of hFIX per copy of provirus. The reason for the increased efficiency of transgene expression is not clear. One explanation could be the saturation of a pathway involved in hFIX expression at the early time points. An alternate explanation could be that the presence of multiple homologous copies of scAAV2/8-LP1-hFIXco within a concatemeric array may have resulted in repression of transgene expression, which may potentially be reversible as copy number decreases over time. Such “repeat-induced gene silencing” has been documented in mice,21 fungi,22 plants,23 Drosophila,24 and nematodes25 and likely represents a host defense response to viral infection that often results in multiple copies of the viral genome in the same cell.26 Another possible explanation that is at variance with reports from our group and that of others, is that stable FIX expression is mediated by integrated copies of the AAV provirus that are present at frequencies below the threshold of detection by the techniques currently available.13 Further studies are, therefore, required to resolve this important issue.
As expected, all macaques developed a prompt and lasting capsid-specific humoral immune response following peripheral vein delivery of scAAV2/8-LP1-hFIXco. Although this does not have direct clinical consequences, it does preclude these animals from subsequently being successfully transduced with vector of the same serotype in the event that transgene expression should fall below therapeutic levels.8,27,28,29 We and others, have, however, established that it is possible to achieve successful transduction in animals with pre-existing anti-AAV antibodies following administration of AAV vector pseudotyped with an alternate serotype.11,30,31 Based on our current NHP data this is likely to be required infrequently and at periods that extend beyond 5 years.8,27,28
We have previously reported that neutralizing antibodies to hFIX can develop in some macaques following AAV-mediated gene transfer.3,8,11,28 Epitope mapping studies suggest that these anti-hFIX antibodies are provoked by expression of a xenoprotein in NHP.8 Furthermore, we have established that the hFIX-specific immune response can be abrogated with the use of a rituximab-containing regimen resulting in sustained long-term expression of hFIX without the need for repeated or persistent treatment with immunosuppressive therapy.13,32 In the context of gene therapy for hemophilia B, eligible patients will have received numerous exposures to factor IX protein concentrates, thus making them less likely to develop inhibitors. In fact, none of the patients with hemophilia that have been subjected to various gene transfer approaches have developed inhibitors.33
We did not observe any acute or delayed vector-induced hepatotoxicity, immune-mediated, or otherwise, in any of the macaques despite systematic evaluation of serum biochemistries, liver histopathology, and testing for the development of AAV capsid-specific cytotoxic T cells. This likely reflects the fact that the macaques selected for this study did not have any evidence of previous infection with wild-type AAV8.34 Moreover, the biological properties of AAV8 capsid are substantially different from AAV2 vectors used in previous trials.6,7 AAV8 vectors uncoat more rapidly35,36 and, therefore, its capsid proteins may be cleared more efficiently than those of AAV2, thereby reducing the potential for persistent presentation of the capsid protein following gene transfer.
A recent report noted that AAV vectors may be associated with an increased incidence of HCC in mice, possibly due to integration of the provirus in an imprinted region of murine chromosome 12 that is rich in miRNAs and snoRNAs.16 The tumors noted in this study were very small, did not produce clinical disease and were found toward the end of the natural life span of mice (2 years) that had received the AAV during the neonatal period. We have not seen an increased incidence of liver tumors following systemic delivery of scAAV2/8-LP1-hFIXco at high doses in mice (including vector administration in utero) or rhesus macaques. Two additional large studies have been published in which an increased incidence of tumor formation was also not observed following AAV administration.32,37 Thus, although insertional mutagenesis and/or tumorigenesis may be a potential risk to study participants following scAAV-mediated gene transfer, our data suggest that the risk is probably quite low.
Thus, we have demonstrated, in a systematic manner, the long term safety of AAV-mediated liver-targeted hFIX gene transfer in a relevant preclinical animal model. Rhesus macaques maintained a stable level of transgene expression for the duration of study (often >5 years) whereas showing no evidence of toxicity. Included in the assessment for toxicity were liver injury, particularly immune-mediated hepatoxicity, and the development of liver tumors. Therefore, cautiously extending this approach to human subjects in the context of a clinical trial appears justified. Based on these data, we have recently opened a phase I/II clinical trial of AAV-mediated gene transfer for hemophilia B that has begun to accrue participants.
Materials and Methods
Vector production and characterization. Clinical grade scAAV vector stocks were made by an adenovirus-free transient transfection method described before.38,39 In brief, scAAV particles were produced by transfecting HEK293T cells (~8 × 108 in a 10-stack cell factory) with the plasmids pscAAV-LP1-hFIXco+help3 and pCR21+LTAAVhelp2-8 (both prepared to large scale by Aldevron, Fargo, ND). The first plasmid contains the entire scAAV vector genome,8 along with the necessary adenovirus helper sequences. The second plasmid contains AAV serotype 2 rep and serotype 8 cap genes. Detailed sequences are available upon request. Forty-eight hours after transfection, cells were harvested, lysed with a microfluidizer, and treated with benzonase (EMD Chemicals, Gibbstown, NJ). The vector was purified by a three-step chromatography method involving group separation, anion exchange, and gel filtration before concentration by tangential flow filtration. Purified particles were formulated in phosphate-buffered saline containing 0.25% human serum albumin, vialed, and stored at −80 °C at a concentration of ~5 × 1011 pcr-vg/ml. The stocks were characterized as described before.38,39,40,41 Vector titers were determined by a qPCR assay using primers spanning the junction of the SV40 polyA signal and the AAV sequences at the beginning of the 3′ inverted terminal repeat, and are reported as “pcr-vg.” The standard was prepared by linearizing the vector plasmid pscAAV-LP1-hFIXco with EcoRI. The purified digest was then quantified by UV spectrophotometry.
Animal procedures. All animal procedures were performed in accordance with institutional guidelines under protocols approved by the Institutional Biosafety Committee and the Institutional Animal Care and Use Committee at St Jude Children's Research Hospital, and the University of Tennessee Health Science Center, Memphis, TN. All animal work carried out in the United Kingdom was performed under the authority of the UK Home Office Project and Personal Licenses regulations and was compliant with the guidelines of the University College London ethical review committee. In utero, intraperitoneal, delivery of vector into MF-1 mice, 16 days after conception was performed as described previously.42,43 Male rhesus macaques were purchased from either Charles River Laboratories (Sparks, NV) or Covance Research Products (Alice, TX) and transduced by administering the vector into the saphenous vein as per our previously described procedures.8,11
Efficacy and toxicity assessments. Plasma levels of hFIX in mice and NHPs were detected by enzyme-linked immunosorbent assay assays as described before.3,44 Gene transfer efficiency at a molecular level and the presence of anti-AAV and anti-hFIX antibodies were assessed as per our previously described methods.8,11 Anti-AAV8 antibody response was expressed as the end-point titer (RU/ml) defined as the reciprocal of the interpolated dilution with an absorbance value equal to five times the mean absorbance background value. Testing of the T cell response for AAV capsid was performed by interferon-γ enzyme-linked immunosorbent spot in which pools of peptides derived from the AAV capsid protein sequence were analyzed in triplicate with 1–3 × 105 peripheral blood mononuclear cells isolated from macaques at baseline and at various time points after vector delivery.7,45
Fluorescent in-situ hybridization was performed as described before.46 In brief, frozen liver biopsy specimens were thawed, minced, fixed, and hybridized with digoxigenin deoxyuridine triphosphate-labeled LP1-hFIXco or a randomly selected macaque BAC clone (CH250–279C2) probe. Specific hybridization signals were detected using fluorescein isothiocyanate-conjugated anti-digoxigenin antibodies. The slides were then counterstained with DAPI and analyzed.
Integration of the scAAV provirus into the host genome was assessed using ligation-mediated PCR. In brief, genomic DNA extracted from the liver was digested with BglII, HindIII, NcoI, KpnI, StuI, or SwaI, which do not cut within the scAAV-LP1-hFIXco genome, to reduce background. This was followed by ligation to enzyme-specific linkers and amplification of putative integration junctions by nested PCR using linkers (5′ACTGACAGCGGAGATAATCG3′ and 5′GTGC GAGTAGCATACTAGAG 3′) and scAAV (5′ GGAGTCATTCCCCTCT CACACTAC 3′ and 5′ TAAGTCCACTGGCTGGGATCTGAG 3′) specific primers. The amplification product was cloned into TA cloning vector and sequenced.
Our previously described method was used for detection of the rAAV genome in bodily fluids (plasma, urine, oral swabs, and stool) collected from each macaque at several time points after peripheral vein administration of scAAV-LP1-hFIXco.11 This method could reliably detect 500 vector particles in 1 ml of rhesus plasma. Complete blood count, coagulation studies, and serum biochemistry (including renal and liver function tests) were assessed by a Good Laboratory Practices-certified laboratory (Antech Diagnostics, Morrisville, NC). Ultrasound images of rhesus macaque livers were obtained with a Biosound MyLab 30 (Universal Medical Systems, Bedford Hills, NY) ultrasound machine. The images were produced with a 5.0–9.0 MHz Microconvex Probe (CA123).
SUPPLEMENTARY MATERIAL Figure S1. Selected serial laboratory values obtained in the three macaques following peripheral vein administration of 2 × 1012 vg/kg of scAAV2/8-LP1-hFIXco over the 8 weeks compared to control animals that received excipient alone (PBS + 0.25% human serum albumin). (A) Hemoglobin (HgB). (B) White blood cell count (WBC). (C) Aspartate aminotransferase (AST). (D) Alanine aminotransferase (ALT). Figure S2. A representative photomicrograph (×10) showing changes consistent with glomerular sclerosis in the kidney of macaque 156A. Many of the glomeruli are hyalinized. There is marked interstitial and periglomerular fibrosis and many of the tubules have formed microcysts filled with proteinaceous fluid. Small multifocal aggregates of chronic inflammation are also present. Figure S3. Images of macaque hepatocytes observed with a fluorescence microscope using a digoxigenin (dUTP-labeled LP1-hFIXco) probe. Specific hybridization signals were detected using FITC-conjugated antidigoxigenin antibodies. The slides were then counterstained with DAPI. AAV genomes appear as green spots in transduced cells but are not visible in hepatocytes that are not transduced.
Acknowledgments
This work was supported by The Katharine Dormandy Trust, U.K., Medical Research Council, UK, Department of Health's NIHR Biomedical Research Centers funding award to UCLH/UCL, UK, The ASSISI Foundation of Memphis, the American Lebanese Syrian Associated Charities (ALSAC), the National Heart, Lung, and Blood Institute grant HL073838 and the National Cancer Institute Cancer Center Support Grant CA027165. This report/article presents independent research commissioned by the National Institute for Health Research (NIHR), UK under its Programme Grants for Applied Research scheme (RP-PG-0310-1001). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. The authors declared no conflict of interest.
Supplementary Material
Selected serial laboratory values obtained in the three macaques following peripheral vein administration of 2 × 1012 vg/kg of scAAV2/8-LP1-hFIXco over the 8 weeks compared to control animals that received excipient alone (PBS + 0.25% human serum albumin). (A) Hemoglobin (HgB). (B) White blood cell count (WBC). (C) Aspartate aminotransferase (AST). (D) Alanine aminotransferase (ALT).
A representative photomicrograph (×10) showing changes consistent with glomerular sclerosis in the kidney of macaque 156A. Many of the glomeruli are hyalinized. There is marked interstitial and periglomerular fibrosis and many of the tubules have formed microcysts filled with proteinaceous fluid. Small multifocal aggregates of chronic inflammation are also present.
Images of macaque hepatocytes observed with a fluorescence microscope using a digoxigenin (dUTP-labeled LP1-hFIXco) probe. Specific hybridization signals were detected using FITC-conjugated antidigoxigenin antibodies. The slides were then counterstained with DAPI. AAV genomes appear as green spots in transduced cells but are not visible in hepatocytes that are not transduced.
REFERENCES
- Evans JP, Brinkhous KM, Brayer GD, Reisner HM., and, High KA. Canine hemophilia B resulting from a point mutation with unusual consequences. Proc Natl Acad Sci USA. 1989;86:10095–10099. doi: 10.1073/pnas.86.24.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu RK, Sangiorgi F, Wu LY, Kurachi K, Anderson WF, Maxson R, et al. Targeted inactivation of the coagulation factor IX gene causes hemophilia B in mice. Blood. 1998;92:168–174. [PubMed] [Google Scholar]
- Nathwani AC, Davidoff AM, Hanawa H, Hu Y, Hoffer FA, Nikanorov A, et al. Sustained high-level expression of human factor IX (hFIX) after liver-targeted delivery of recombinant adeno-associated virus encoding the hFIX gene in rhesus macaques. Blood. 2002;100:1662–1669. doi: 10.1182/blood-2002-02-0589. [DOI] [PubMed] [Google Scholar]
- Wang L, Zoppè M, Hackeng TM, Griffin JH, Lee KF., and, Verma IM. A factor IX-deficient mouse model for hemophilia B gene therapy. Proc Natl Acad Sci USA. 1997;94:11563–11566. doi: 10.1073/pnas.94.21.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Davidoff AM., and, Tuddenham EG. Prospects for gene therapy of haemophilia. Haemophilia. 2004;10:309–318. doi: 10.1111/j.1365-2516.2004.00926.x. [DOI] [PubMed] [Google Scholar]
- Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, Ng CY, Zhou J, Spence Y, Waddington SN, et al. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107:2653–2661. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J., and, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe LH, Wang L, Somanathan S, Zhi Y, Figueredo J, Calcedo R, et al. Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat Med. 2006;12:967–971. doi: 10.1038/nm1445. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, McIntosh J, Ng CY, Zhou J, Spence Y, et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMichele D. Inhibitor development in haemophilia B: an orphan disease in need of attention. Br J Haematol. 2007;138:305–315. doi: 10.1111/j.1365-2141.2007.06657.x. [DOI] [PubMed] [Google Scholar]
- Nakai H, Yant SR, Storm TA, Fuess S, Meuse L., and, Kay MA. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J Virol. 2001;75:6969–6976. doi: 10.1128/JVI.75.15.6969-6976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai H, Wu X, Fuess S, Storm TA, Munroe D, Montini E, et al. Large-scale molecular characterization of adeno-associated virus vector integration in mouse liver. J Virol. 2005;79:3606–3614. doi: 10.1128/JVI.79.6.3606-3614.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnepp BC, Jensen RL, Chen CL, Johnson PR., and, Clark KR. Characterization of adeno-associated virus genomes isolated from human tissues. J Virol. 2005;79:14793–14803. doi: 10.1128/JVI.79.23.14793-14803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW, et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- Nakai H, Fuess S, Meuse L, Storm TA., and, Kay MA. Persistent hF.IX expression in mouse hepatocytes from episomal rAAV circular intermediates does not rely on the presence of AAV-ITR but the structure of expression cassette itself. Blood. 2000;96:431a. [Google Scholar]
- Rowlatt C, Chesterman FC., and, Sheriff MU. Lifespan, age changes and tumour incidence in an ageing C57BL mouse colony. Lab Anim. 1976;10:419–442. doi: 10.1258/002367776780956917. [DOI] [PubMed] [Google Scholar]
- Tittiger M, Ma X, Xu L., and, Ponder KP. Neonatal intravenous injection of a gammaretroviral vector has a low incidence of tumor induction in mice. Hum Gene Ther. 2008;19:1317–1323. doi: 10.1089/hum.2008.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CH, Ward JM., and, Chandra M. The morphology, immunohistochemistry, and incidence of hematopoietic neoplasms in mice and rats. Toxicol Pathol. 1993;21:206–218. doi: 10.1177/019262339302100213. [DOI] [PubMed] [Google Scholar]
- Garrick D, Fiering S, Martin DI., and, Whitelaw E. Repeat-induced gene silencing in mammals. Nat Genet. 1998;18:56–59. doi: 10.1038/ng0198-56. [DOI] [PubMed] [Google Scholar]
- Faugeron G. Diversity of homology-dependent gene silencing strategies in fungi. Curr Opin Microbiol. 2000;3:144–148. doi: 10.1016/s1369-5274(00)00066-7. [DOI] [PubMed] [Google Scholar]
- Flavell RB. Inactivation of gene expression in plants as a consequence of specific sequence duplication. Proc Natl Acad Sci USA. 1994;91:3490–3496. doi: 10.1073/pnas.91.9.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorer DR., and, Henikoff S. Expansions of transgene repeats cause heterochromatin formation and gene silencing in Drosophila. Cell. 1994;77:993–1002. doi: 10.1016/0092-8674(94)90439-1. [DOI] [PubMed] [Google Scholar]
- Kelly WG., and, Fire A. Chromatin silencing and the maintenance of a functional germline in Caenorhabditis elegans. Development. 1998;125:2451–2456. doi: 10.1242/dev.125.13.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney MW, Mai T, Yang X., and, Jardine K. Evidence for repeat-induced gene silencing in cultured Mammalian cells: inactivation of tandem repeats of transfected genes. Exp Cell Res. 2002;274:1–8. doi: 10.1006/excr.2001.5443. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Cochrane M, McIntosh J, Ng CY, Zhou J, Gray JT, et al. Enhancing transduction of the liver by adeno-associated viral vectors. Gene Ther. 2009;16:60–69. doi: 10.1038/gt.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff AM, Gray JT, Ng CY, Zhang Y, Zhou J, Spence Y, et al. Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5, and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models. Mol Ther. 2005;11:875–888. doi: 10.1016/j.ymthe.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Wang L, Calcedo R, Wang H, Bell P, Grant R, Vandenberghe LH, et al. The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol Ther. 2010;18:126–134. doi: 10.1038/mt.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff AM, Gray JT, Ng CY, Zhang Y, Zhou J, Spence Y, et al. Adeno-associated viral vectors pseudotyped with serotype 8 capsid trnsduce murine and nonhuman primate liver more efficiently and through a different mechanism than vectors based on serotypes 2 and 5. Mol Ther. 2004;11:875–888. doi: 10.1016/j.ymthe.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Arruda VR, Stedman HH, Haurigot V, Buchlis G, Baila S, Favaro P, et al. Peripheral transvenular delivery of adeno-associated viral vectors to skeletal muscle as a novel therapy for hemophilia B. Blood. 2010;115:4678–4688. doi: 10.1182/blood-2009-12-261156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell P, Moscioni AD, McCarter RJ, Wu D, Gao G, Hoang A, et al. Analysis of tumors arising in male B6C3F1 mice with and without AAV vector delivery to liver. Mol Ther. 2006;14:34–44. doi: 10.1016/j.ymthe.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Benjamin R, Nienhuis AW., and, Davidoff AM. Current status and prospects for gene therapy. Vox Sang. 2004;87:73–81. doi: 10.1111/j.1423-0410.2004.00543.x. [DOI] [PubMed] [Google Scholar]
- Hernandez YJ, Wang J, Kearns WG, Loiler S, Poirier A., and, Flotte TR. Latent adeno-associated virus infection elicits humoral but not cell-mediated immune responses in a nonhuman primate model. J Virol. 1999;73:8549–8558. doi: 10.1128/jvi.73.10.8549-8558.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai H, Fuess S, Storm TA, Muramatsu S, Nara Y., and, Kay MA. Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. J Virol. 2005;79:214–224. doi: 10.1128/JVI.79.1.214-224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CE, Storm TA, Huang Z., and, Kay MA. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J Virol. 2004;78:3110–3122. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell P, Wang L, Lebherz C, Flieder DB, Bove MS, Wu D, et al. No evidence for tumorigenesis of AAV vectors in a large-scale study in mice. Mol Ther. 2005;12:299–306. doi: 10.1016/j.ymthe.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Hanawa H, Vandergriff J, Kelly P, Vanin EF., and, Nienhuis AW. Efficient gene transfer into human cord blood CD34+ cells and the CD34+CD38- subset using highly purified recombinant adeno-associated viral vector preparations that are free of helper virus and wild-type AAV. Gene Ther. 2000;7:183–195. doi: 10.1038/sj.gt.3301068. [DOI] [PubMed] [Google Scholar]
- Allay JA, Coleman JS, Nienhuis AW, Davidoff A, Nathwani AC, Sleep S, et al. Production of self-complementary serotype 8 AAV vector for treatment of Hemophilia B. Mol Ther. 2010;18 [Google Scholar]
- Wright JF. Manufacturing and characterizing AAV-based vectors for use in clinical studies. Gene Ther. 2008;15:840–848. doi: 10.1038/gt.2008.65. [DOI] [PubMed] [Google Scholar]
- Zen Z, Espinoza Y, Bleu T, Sommer JM., and, Wright JF. Infectious titer assay for adeno-associated virus vectors with sensitivity sufficient to detect single infectious events. Hum Gene Ther. 2004;15:709–715. doi: 10.1089/1043034041361262. [DOI] [PubMed] [Google Scholar]
- Waddington SN, Buckley SM, Nivsarkar M, Jezzard S, Schneider H, Dahse T, et al. In utero gene transfer of human factor IX to fetal mice can induce postnatal tolerance of the exogenous clotting factor. Blood. 2003;101:1359–1366. doi: 10.1182/blood-2002-03-0779. [DOI] [PubMed] [Google Scholar]
- Waddington SN, Nivsarkar MS, Mistry AR, Buckley SM, Kemball-Cook G, Mosley KL, et al. Permanent phenotypic correction of hemophilia B in immunocompetent mice by prenatal gene therapy. Blood. 2004;104:2714–2721. doi: 10.1182/blood-2004-02-0627. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Davidoff A, Hanawa H, Zhou JF, Vanin EF., and, Nienhuis AW. Factors influencing in vivo transduction by recombinant adeno-associated viral vectors expressing the human factor IX cDNA. Blood. 2001;97:1258–1265. doi: 10.1182/blood.v97.5.1258. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, Edmonson SA, Hui DJ, Sabatino DE, et al. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110:2334–2341. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KJ, Danks MK, Ragsdale ST, Valentine MB., and, Valentine VA. Translocations of 17q21 approximately qter in neuroblastoma cell lines infrequently include the topoisomerase IIα gene. Cancer Genet Cytogenet. 2006;167:92–94. doi: 10.1016/j.cancergencyto.2005.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Selected serial laboratory values obtained in the three macaques following peripheral vein administration of 2 × 1012 vg/kg of scAAV2/8-LP1-hFIXco over the 8 weeks compared to control animals that received excipient alone (PBS + 0.25% human serum albumin). (A) Hemoglobin (HgB). (B) White blood cell count (WBC). (C) Aspartate aminotransferase (AST). (D) Alanine aminotransferase (ALT).
A representative photomicrograph (×10) showing changes consistent with glomerular sclerosis in the kidney of macaque 156A. Many of the glomeruli are hyalinized. There is marked interstitial and periglomerular fibrosis and many of the tubules have formed microcysts filled with proteinaceous fluid. Small multifocal aggregates of chronic inflammation are also present.
Images of macaque hepatocytes observed with a fluorescence microscope using a digoxigenin (dUTP-labeled LP1-hFIXco) probe. Specific hybridization signals were detected using FITC-conjugated antidigoxigenin antibodies. The slides were then counterstained with DAPI. AAV genomes appear as green spots in transduced cells but are not visible in hepatocytes that are not transduced.