Abstract
Despite advances in vector technology, inefficient gene transfer still limits clinical efficacy of cancer gene therapy. Cell-penetrating peptides (CPPs), such as the basic domain of the transactivator of transcription (Tat) protein of HIV-1, are internalized by intact cells and have been used to deliver purified recombinant proteins. A combination of gene therapy with protein transduction technology could induce a strong bystander effect and represent a platform to deliver proteins to target cells. However, whether expressed CPP can facilitate intercellular trafficking, i.e., a bystander effect, is controversial. Our data suggest that expressed fusion proteins that contain the basic domain of Tat do not induce a detectable bystander effect. However, Tat-fusion proteins that also contain a secretory signal peptide (SP) can induce a bystander effect in vitro, although the in vivo effect is small. Surprisingly, despite the presence of a SP, the bystander effect does not seem to be related to secretion of the fusion protein. In fact, Tat-fusion proteins are secreted very inefficiently, and protein transduction seems largely mediated by fusion proteins that are released by cell lysis. Modification of Tat can improve secretion efficacy and prevent cleavage by the endoprotease furin, but passage through the secretory pathway is associated with reduced transduction activity of Tat-fusion proteins.
Introduction
As the biology that underlies cancer development is being unmasked, numerous excellent targets for cancer therapy have been discovered. Gene therapy can exploit known genetic aberrations in cancer, but many obstacles related to vector technology remain unsolved. Inefficient gene transfer has been identified as one of the main hurdles toward clinical success. Even with replication competent viral vectors and direct intratumoral injection, only a minority of tumor cells can be transduced, and limitations of viral spread have proved to be very difficult to overcome. Facilitation of intercellular trafficking of the expressed therapeutic protein, i.e., a bystander effect, could be an alternative strategy to improve the efficacy of cancer gene therapy.
About 20 years ago, cellular uptake of the transactivator of transcription (Tat) protein of HIV-1 was first reported.1 Since then, many cell-penetrating peptides (CPP), such as penetratin, VP22 and synthetic oligoarginine, have been described to cross intact cell membranes and deliver proteins or other macromolecules. Tat remains the most widely studied CPP, and it has become clear that only the basic domain (amino acid 47–57) is required for protein transduction.2 Recombinant CPP are well studied and the number of applications is increasing rapidly.3 However, relatively few studies have investigated the characteristics of nonpurified CPP generated with mammalian expression systems. Furthermore, whether expressed CPP can facilitate intercellular trafficking and improve the spread of the protein product of a transgene is controversial. If feasible, expressed CPP could represent a strong platform to deliver proteins to target cells with many potential therapeutic applications.
Cellular release, the first step of intercellular trafficking, has been reported for HIV-1 Tat,4,5,6,7,8,9 and nanomolar Tat concentrations were detected in the serum of HIV-infected patients.10 Transcellular transactivation activity of HIV Tat has also been demonstrated.9,11 However, intercellular trafficking of green fluorescent protein (GFP) fused Tat has only been demonstrated when fusion proteins were released by cell membrane disruption.12 The basic domain of Tat fused to thymidine kinase (TK) resulted in an enhanced bystander effect serving as indirect evidence for intercellular trafficking.13,14 Tat-fusion of β-glucoronidase, expressed with an adenovirus, has been shown to facilitate significant intercellular trafficking and improved biodistribution of the functional protein.15 In this context, it is important to note that β-glucoronidase contains a strong secretory signal peptide (SP), and other more recent publications support the concept that secreted Tat-fusion proteins can mediate intercellular trafficking.16,17,18,19,20
The data we present here indicate that Tat-fusion proteins that contain a secretory SP can support a bystander effect. Surprisingly, the effect does not seem to be related to secretion of the fusion protein. In fact, Tat-fusion proteins are secreted very inefficiently, and protein transduction is largely mediated by fusion proteins that are released by a mechanism of cell lysis. Modification of Tat can greatly improve secretion efficacy, but secreted Tat-fusion proteins have reduced transduction activity.
Results
The basic domain of HIV-Tat, fused to fluorescent proteins, does not support a bystander effect
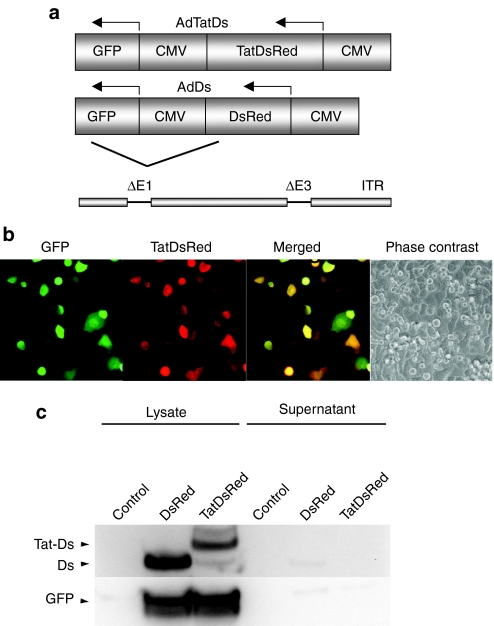
Whether CPP can facilitate cell-to-cell transfer of a fused protein is controversial. To examine this question, an adenoviral vector (AdTatDs) that expresses DsRed-monomer fused to the basic domain of Tat was constructed (Figure 1a). The vector also expresses GFP from a separate promoter. This dual expression system facilitates differentiation between infected cells (green and red fluorescent) and cells that are subject to Tat-mediated intercellular transport of DsRed (red-fluorescent only). A control virus that expresses DsRed without fusion to Tat (AdDs) was also generated. A549 cells were infected with AdTatDs and mixed with uninfected cells. Fluorescent images of living cells were obtained at 24 hour after cell mixing (Figure 1b). Many infected cells coexpressing GFP and TatDsRed were seen. However, no exclusively red-fluorescent cells were detected, indicating the absence of a bystander effect. Similar results were obtained using H1299 and SCC15 cells (data not shown). Continued observation for up to 72 hour also did not show any evidence for intercellular transfer of TatDsRed (data not shown).
Figure 1.
The basic domain of HIV-1 Tat, fused to fluorescent proteins, does not support a bystander effect. (a) Schematic representation of the genome of the constructed adenoviral vectors. AdTatDs contains a DsRed transgene with an n-terminal fusion to amino acid 47–57 of Tat, inserted into the E1 deletion site. A control virus, AdDs, lacks Tat but is otherwise identical. Both viruses also contain a GFP transgene. (b) A549 cells were infected with AdTatDs and mixed with uninfected cells. Fluorescent images of living cells were obtained 24 hour after cell mixing. Infected cells were identified by red and green cofluorescence. No exclusively red-fluorescent cells (which would indicate Tat-mediated transduction) were observed. (c) Immunoblot of lysates and supernatants of infected cells. H1299 cells were infected with AdDs or AdTatDs. After 48 hour, cells and supernatants were harvested. Lysates and tenfold concentrated supernatants were analyzed by immunoblotting. CMV, cytomegalovirus; GFP, green fluorescent protein; Tat, transactivator of transcription (Tat) protein of HIV-1.
The first step for intercellular trafficking of a Tat-fusion protein must be its release from the expressing cells. Cellular release of the full-length HIV-1 Tat protein has been described, but whether Tat-fusion proteins that contain only the basic domain can exit intact cells is controversial. Hence, the lack of cellular release of Tat-fusion proteins could be an explanation for the absence of a bystander effect. To test whether TatDsRed is released by intact cells, cell lysates and tenfold concentrated supernatants of AdTatDs and AdDs-infected cells were analyzed by immunoblotting. TatDsRed, DsRed, and GFP were detected in the cell lysates of infected cells, but no signal could be observed in the supernatants, indicating that TatDsRed is not released from cells at detectable levels (Figure 1c). Similar results were obtained when lysates and supernatants were analyzed with fluorometry (data not shown).
These experiments support the conclusion that fusion proteins that contain the basic domain of Tat are not released from intact cells and do not support a bystander effect.
The addition of a secretory SP results in intercellular transfer of Tat-fusion proteins
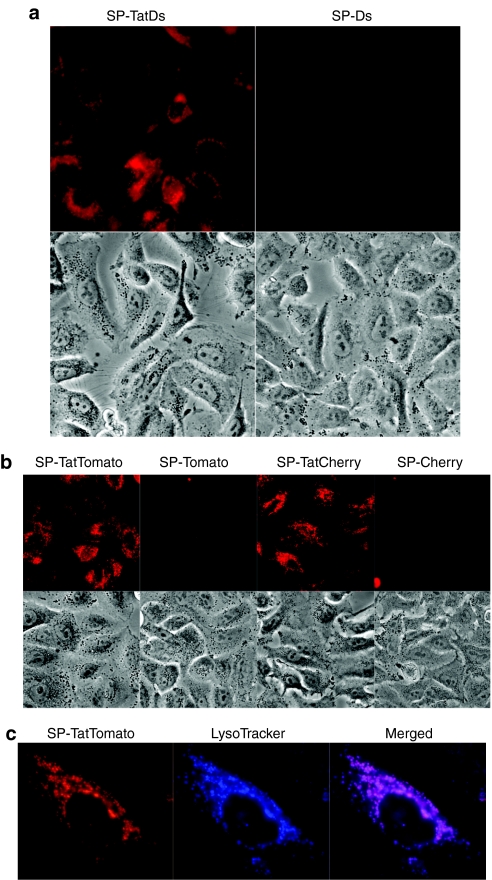
If the lack of cellular release of Tat-fusion proteins prevents intercellular trafficking, the addition of a secretory SP could result in the induction of a bystander effect. To test this hypothesis, adenoviral vectors were created that express TatDsRed or DsRed fused to the SP of the murine immunoglobulin κ-chain (Ad-SP-TatDs, Ad-SP-Ds). Both viruses also coexpress GFP. H1299 cells were infected with the respective viruses. At 24 hour, cells were trypsinized to remove unabsorbed virus and supplied with fresh medium. At 48 hour after infection, the conditioned medium was filtered and applied to fresh A549 cells. Fluorescent images of the treated living cells were obtained 24 hours later. Cells treated with conditioned medium from Ad-SP-TatDs-infected cells, but not those treated with medium from Ad-SP-Ds-infected cells, showed perinuclear red-fluorescent vesicles, suggesting cellular uptake of TatDsRed (Figure 2a). No GFP was observed in any cell, confirming the absence of viral carry over (data not shown).
Figure 2.
Cellular uptake of fluorescent proteins derived from supernatants of SP-Tat-fusion-protein expressing cells. (a) A549 cells were treated with conditioned medium of cells infected with Ad-SP-TatDs or Ad-SP-Ds. Fluorescent and corresponding phase contrast images of the treated living cells were obtained at 24 hour (b) A549 cells were treated with conditioned medium of cells transfected with plasmids expressing SP-TatTomato, SP-Tomato, SP-TatCherry, or SP-Cherry. Fluorescent and corresponding phase contrast images of the treated living cells were obtained at 24 hour (c) A549 cells were exposed to the conditioned medium of SP-TatTomato-transfected cells followed by incubation with LysoTracker Blue, which selectively accumulates in lysosomes. Images of living cells were obtained using fluorescent microscopy. GFP, green fluorescent protein; SP, signal peptide; Tat, transactivator of transcription (Tat) protein of HIV-1.
To confirm that Tat-mediated cell-to-cell transfer is not specific to DsRed or the murine immunoglobulin κ-chain SP, plasmids were constructed expressing TatCherry or TatTomato fused to a strong artificial secretory SP21 (SP-TatCherry, SP-TatTomato). Corresponding control plasmids without Tat were also made. A549 cells treated with conditioned medium from transfected cells were positive for TatCherry and TatTomato, but not the fluorescent proteins without Tat (Figure 2b). The same experimental design also showed uptake of TatTomato (but not Tomato) into HeLa cells, MRC-9 fibroblasts and human umbilical vein endothelial cells (HUVEC) (Supplementary Figure S1).
It is mostly agreed that purified Tat-fusion proteins and other CPP are taken up by a mechanism of endocytosis and accumulate in late endosomal structures. Time-course experiments that we carried out with expressed Tat-fusion proteins were consistent with endocytotic uptake. Cells were treated with conditioned medium from SP-TatTomato-transfected cells. Red-fluorescence was first observed in living cells at 30 minutes, and was markedly intensifying at 2 and 4 hour (Supplementary Figure S2). To further confirm that the Tat-fusion proteins were not merely attached to the cell surface and to determine their intracellular localization, cells were again exposed to the conditioned medium of SP-TatTomato-transfected cells. Cells were then incubated with LysoTracker Blue, which selectively accumulates in lysosomes. Visualization of TatTomato showed fluorescent vesicular structures as observed in previous experiments. Lysosomes, identified with LysoTracker Blue, showed a similar intracellular distribution. Merged images showed almost complete overlap, suggesting late endosomal/lysosomal localization of transduced TatTomato (Figure 2c).
SP-Tat-fusion proteins support a bystander effect in vitro
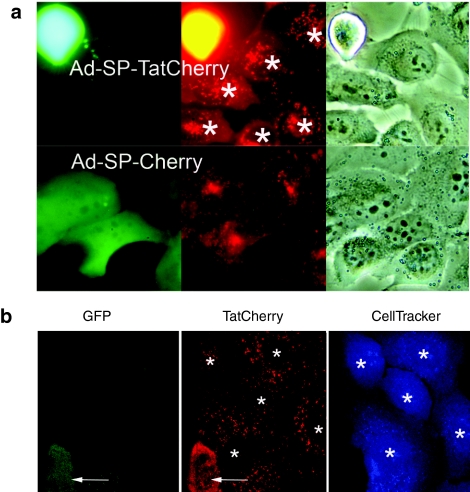
To determine whether expressed SP-Tat-fusion proteins would transduce surrounding cells, i.e., induce a bystander effect; an adenovirus was generated that expresses GFP in addition to SP-TatCherry (Ad-SP-TatCherry). As mentioned, this dual expression system allows for the clear distinction between infected and protein-transduced cells. An identical control virus that lacks Tat (AD-SP-Cherry) was also constructed. H1299 cells were infected and mixed with uninfected A549 cells. Fluorescent images of living cells were taken 48 hour after cell mixing. Infected cells were identified by red and green cofluorescence. Numerous exclusively red-fluorescent cells were observed, indicating Tat-mediated transduction of uninfected cells (Figure 3a). No exclusively red-fluorescent cells were present when uninfected cells were mixed with control-virus-infected (Ad-SP-Cherry) cells.
Figure 3.
SP-Tat-fusion proteins support a bystander effect in vitro. (a) Cells were infected with viruses coexpressing GFP and SP-TatCherry (Ad-SP-TatCherry) or SP-Cherry (Ad-SP-Cherry) and mixed with uninfected cells. Infected cells are indicated by red and green cofluorescence. Transduced cells (*) are exclusively red fluorescent. (b) Cells were infected with Ad-SP-TatCherry and mixed with uninfected cells labeled with CellTracker Blue. Infected cells are indicated by red and green cofluorescence (arrows). Transduced cells (*) are identified by blue and red cofluorescence. GFP, green fluorescent protein; SP, signal peptide; Tat, transactivator of transcription (Tat) protein of HIV-1.
To further confirm this finding and to avoid the potential problem of uneven GFP expression in infected cells, uninfected cells were labeled with CellTracker Blue. For this experiment, H1299 cells were again infected with the virus that expresses SP-TatCherry and GFP. Infected cells were then mixed with uninfected cells labeled with CellTracker Blue. Therefore, protein-transduced cells could be identified by red and blue cofluorescence. When mixed cells were imaged, numerous cells were identified that were blue and red fluorescent, confirming the presence of a bystander effect (Figure 3b).
SP-Tat-fusion proteins support only a small bystander effect in vivo
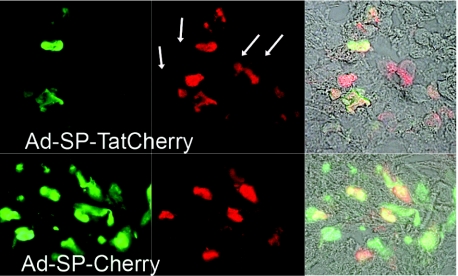
To test whether a bystander effect could also be detected in vivo, xenograft tumors were established in nude mice using A549 cells. Viruses, Ad-SP-TatCherry and Ad-SP-Cherry as control, were injected one time into the center of the tumors. After 7 days tumors were harvested, and frozen sections were examined by fluorescent microscopy. As compared to Cherry, the fluorescent intensity of GFP was considerably lower, possibly due to its poor tissue penetration. After enhancement of the signal with fluorescently labeled antibodies, signal strength for GFP and Cherry was similar. In tissue sections of Ad-SP-Cherry injected tumors, all fluorescent cells were green and red. In sections of tumors infected with Ad-SP-TatCherry, the majority of fluorescent cells showed a similar pattern with green and red cofluorescence, indicating infected cells. Only a few cells were detected that were exclusively red-fluorescent due to Tat-mediated protein transduction. These data suggest that in vivo, a small bystander effect may be induced by SP-Tat fusion of the expressed protein. However, because of the small magnitude of the effect, it is unlikely to translate into a significant therapeutic advantage (Figure 4).
Figure 4.
Secreted Tat-fusion proteins induce a small bystander effect in vivo. Xenograft tumors were injected with viruses coexpressing GFP and SP-TatCherry (Ad-SP-TatCherry) or SP-Cherry (Ad-SP-Cherry). Tumors were harvested at 7 days after injection. Frozen sections were fixed and stained with antibodies against GFP and Cherry. Labeled secondary antibodies were used to maintain a green-fluorescent signal (FITC) for GFP and a red-fluorescent signal (TR) for Cherry. Infected cells are red and green cofluorescent. Transduced cells are red-fluorescent only (arrows). FITC, fluorescein isothiocyanate; GFP, green fluorescent protein; SP, signal peptide; Tat, transactivator of transcription (Tat) protein of HIV-1; TR, texas red.
Tat-fusion proteins are poorly secreted and Tat is cleaved, likely by the endoprotease furin. Modification of Tat improves secretion efficacy and prevents its cleavage
In the in vitro experiments described above, we consistently observed poor secretion efficiency of Tat-fusion proteins. In addition, the basic domain of Tat is substrate to the endoprotease furin.22 Hence, we hypothesized that modification of Tat to improve secretion efficacy and to prevent Tat cleavage would increase the magnitude of a Tat-mediated bystander effect. The basic domain of Tat is highly positively charged, which has been shown to negatively correlate with secretion efficacy.18 A modified Tat (YARAAARQARA) (named Tatm from hereon) with reduced cationic charge and a stabilized α-helical structure has been described. Using a synthesized version of this peptide, a greater than 30-fold increase in transduction activity compared to unmodified Tat has been reported.23 In addition, Tatm does not contain a furin-recognition site.
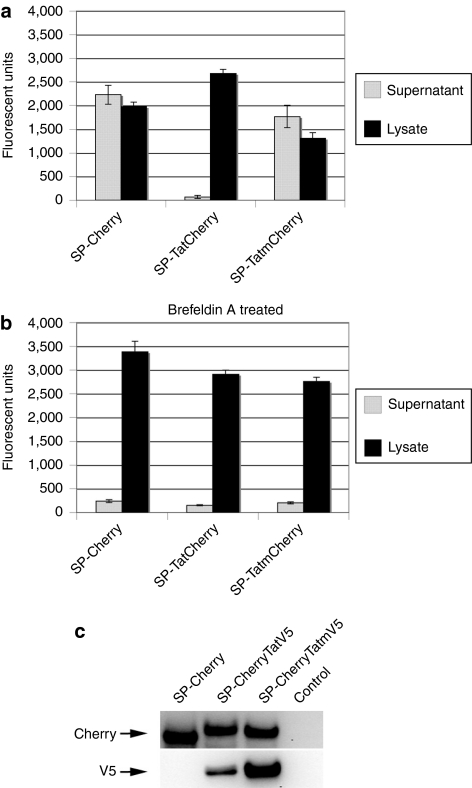
To test secretion efficacy of Tatm-fusion proteins, plasmids expressing SP-TatCherry, SP-TatmCherry and SP-Cherry were transfected into H1299 cells. At 24 hour, cell were rinsed and supplied with fresh medium. After 5 hours incubation time, supernatants and cell fractions were analyzed by fluorometry. Compared to Cherry, TatCherry was released into the supernatant very inefficiently. In contrast, fusion of Cherry to Tatm resulted in uninhibited secretion of the protein (Figure 5a), confirming that Tat modification can improve secretion efficacy.
Figure 5.
Secretion efficacy and resistance to cleavage of wild type and modified Tat fusion proteins. (a) Secretion efficacy of Tat-fusion proteins. SP-TatCherry, SP-TatmCherry and SP-Cherry were transfected into H1299 cells. At 24 hour, cells were supplied with fresh medium, and after 5 hour incubation, supernatants and cell lysates were analyzed for fluorescent intensity. Three independent experiments were performed and SEs are indicated. (b) Identical experiments were carried out in the presence of 1 µg/ml brefeldin A. (c) Tatm is resistant to cleavage. Lysates of cells transfected with plasmids expressing SP-Cherry, SP-CherryTatV5, and SP-CherryTatmV5 were analyzed by immunoblotting with an antibody against Cherry (upper panel) and V5 (lower panel). SP, signal peptide; Tat, transactivator of transcription (Tat) protein of HIV-1.
To investigate the mechanism of secretion, the same experiment was carried out in the presence of brefeldin A, which blocks protein transport from the endoplasmic reticulum (ER) to the Golgi.24 Brefeldin A almost completely inhibited release of Cherry or TatmCherry (Figure 5b), suggesting secretion of these two fusion proteins via the classical pathway.
To test whether modification of Tat would prevent its cleavage in expressing cells, plasmids that express c-terminal Tat or Tatm fused to a V5 Tag (SP-CherryTatV5, SP-CherryTatmV5) were engineered. With these constructs, cleavage within Tat should lead to reduced recognition of the full size fusion-protein with a V5 antibody, whereas recognition with an antibody against Cherry should not be affected. Lysates of transfected cells were normalized for fluorescent activity and analyzed by immunoblotting. An appropriate size band of similar intensity was detected in all samples with an antibody against Cherry. In contrast, when an antibody against V5 was used on the same samples, the signal for CherryTatV5 was considerably weaker compared to CherryTatmV5 (Figure 5c). This suggests that Tatm, in contrast to Tat, is not substrate to cleavage (likely by furin) in this experimental setting.
Secretion of Tatm-fusion proteins is associated with reduced transduction activity
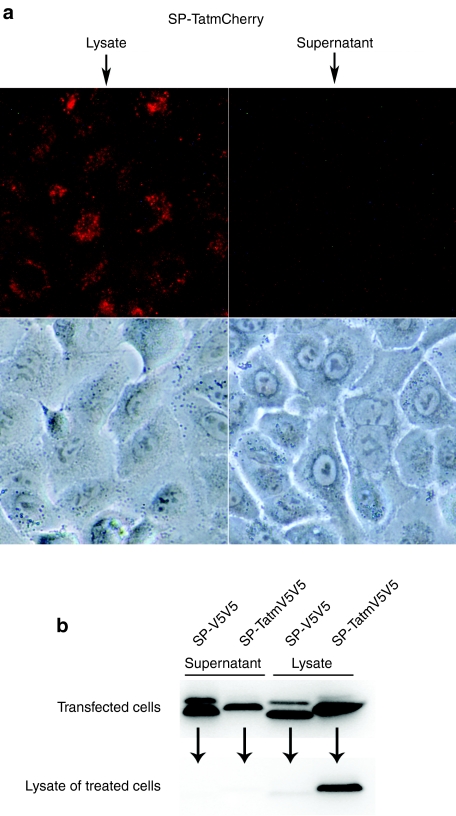
The aim of the following experiment was to test the transduction activity of secreted Tatm-fusion proteins. To analyze transduction activity strictly in the secreted fraction, the experiments were designed to minimize contamination of the secreted fusion proteins with those originating from lysed cells. Forty-eight hours after transfection with a plasmid expressing SP-TatmCherry, cells were rinsed and supplied with fresh medium. The supernatant was carefully aspirated after 3.5 hour and filtered to remove any cellular components. Cell lysates were also prepared. Samples were normalized for fluorescent intensity and applied to fresh cells. SP-TatmCherry, released by a mechanism of cell lysis, was taken up by the treated cells (Figure 6a). However, cells that were treated with the corresponding supernatant showed minimal fluorescent activity, suggesting that secretion of TatmCherry is associated with reduced transduction activity. The experiment was repeated more than three times with similar results. Even when cells were treated with maximum amounts of secreted TatmCherry, little transduction activity was observed, as long as contamination with lysed cells was strictly avoided. This experiment was also carried out using 293 cells as producer cells, again with similar results (data not shown).
Figure 6.
Transduction activity of secreted Tatm-fusion proteins compared to those derived from cell lysis. (a) Cells were treated with the normalized (for fluorescence) lysates or supernatants of cells transfected with SP-TatmCherry. Conditions were optimized to minimize contamination of the supernatants with lysed cells. Fluorescent and corresponding phase contrast images of the treated living cells are shown. (b) Cells were transfected with SP-TatmV5V5 or SP-V5V5. Cell lysates and concentrated supernatants were applied to fresh cells. The upper panel shows an immunoblot with an antibody against V5, analyzing samples of supernatants and lysates of transfected cells, used to treat the fresh cells. The lower panel shows an immunoblot analyzing samples of the treated cells. SP, signal peptide; Tat, transactivator of transcription (Tat) protein of HIV-1.
To examine whether reduction of transduction activity of the secreted fusion-protein was specific to Cherry or a more general phenomenon, Cherry was replaced with a modified double V5 tag. SP-TatmV5V5 or SP-V5V5 expressing plasmids were transfected into H1299 cells, and A549 cells were treated with the lysate or concentrated supernatant of the transfected cells. Normalization, as was done in the experiments with fluorescent fusion proteins, was not possible with this experimental design, but immunoblot analysis of the concentrated supernatant and lysate of transfected cells showed strong expression and secretion of the fusion proteins (Figure 6b upper panel). Analyzing the lysates of treated cells, a strong signal was only detected in samples from cells treated with SP-TatmV5V5 released by cell lysis (Figure 6b lower panel). Only a very faint band was visible in samples from cells treated with the corresponding supernatant, indicating that transduction activity of TatmV5V5 was reduced in the process of being secreted.
These experiments suggest that alteration of Tatm in the secretory pathway leads to reduced transduction activity of the fusion protein. These results also suggest that transduction activity in the previous experiments (Figures 2 and 3) was mediated to a large extent by fusion-protein released through cell lysis, and could be one explanation for the small magnitude of the in vivo bystander effect.
Transduction activity of SP-Tatm, released from expressing cells, is reduced compared to unmodified SP-Tat-fusion proteins. Intracellular localization of the fusion-protein in the expressing cells may influence transduction activity
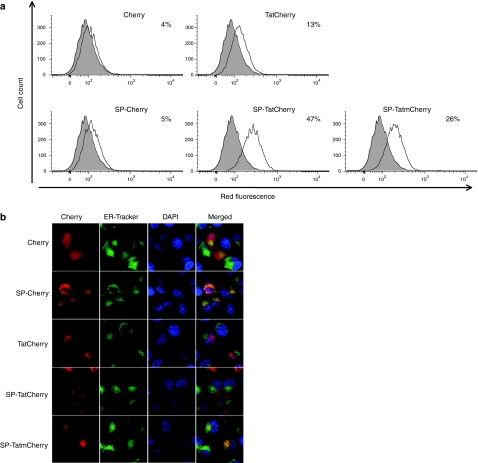
Using synthesized peptides, Tatm has been reported to have substantially increased transduction activity, compared to unmodified Tat.23 To test transduction activity of expressed Tat and Tatm-fusion proteins quantitatively, flow cytometry was used to analyze cells treated with lysates of transfected cells (Figure 7a). SP-TatCherry showed higher transduction activity (47% positive cells) compared to SP-TatmCherry (26%). As mentioned, this observation contrasts published results (obtained using synthesized peptides) and may point toward altered characteristics of expressed CPP compared to synthesized versions.
Figure 7.
Transduction activity and intracellular localization of expressed Tat fusion proteins. (a) Transduction activity of Tat-fusion proteins derived from cell lysis. Cells were treated with the lysate of cells transfected with plasmids expressing the indicated fusion proteins. Treated cells were analyzed by fluorescence-activated cell sorting. The shaded area represents untreated control cells. The experiment was repeated with similar results. (b) Intracellular localization of fusion proteins in transfected cells. Cells were transfected with plasmids expressing the indicated fusion proteins and stained with endoplasmic reticulum (ER)-Tracker Green and 4′,6-diamidino-2-phenylindole (DAPI). Confocal fluorescent images of living cells were obtained with filter sets for Cherry, ER-Tracker and DAPI as indicated. SP, signal peptide; Tat, transactivator of transcription (Tat) protein of HIV-1.
In the absence of a SP (TatCherry), Tat had very low transduction activity (13%). Considering that all fusion proteins in this experiment were released by cell lysis, the cause of increased transduction activity in the presence of a SP is not immediately apparent. However, cell-compartment-dependent alterations of the expressed fusion protein could account for these findings.
To investigate this hypothesis and to correlate transduction activity with intracellular localization of the expressed fusion-protein, cells were transfected with the respective plasmids and costained with ER-Tracker Green and DAPI. Images were obtained using confocal microscopy (Figure 7b). As expected, Cherry was present throughout cytoplasm and nucleus. The addition of a secretory SP to Cherry caused its translocation into the ER. The basic domain of Tat contains a nuclear localization signal,25 and expressed Tat-GFP has been reported to concentrate in nuclear and nucleolar localization.26 In our experiments with TatCherry, nucleolar accumulation was most pronounced. Interestingly, addition of a SP to TatCherry resulted in predominant cytoplasmic localization. Significant colocalization with the ER was not observed. This could be due to competing localization signals from Tat and the SP, and may explain the poor secretion efficacy of Tat-fusion proteins. In contrast, SP-TatmCherry, which does not contain a nuclear localization signal, displayed strong colocalization with the ER, consistent with our experiments shown above indicating secretion via the classical pathway.
These studies suggest that intracellular localization and modification of Tat (or Tatm) in the expressing cells influence transduction activity of the fusion protein. Colocalization with the ER and release through the secretory pathway is associated with reduction of transduction activity.
Discussion
Clinical success of cancer gene therapy has been hampered by inefficient gene transfer and the absence of a strong bystander effect with most vector systems. Recombinant or synthesized CPP have increasingly been used to deliver therapeutic proteins into cells. However, whether CPP can support intercellular transport of fused proteins is controversial. Our data indicate that Tat-fusion proteins without a SP are not released by the expressing cell, and do not induce a significant bystander effect.
These results are in agreement with previous reports that failed to demonstrate evidence for intercellular transport of Tat-GFP and Tat-lymphocytic choriomeningitis virus nucleoprotein.12,27,28 Constructs in these reports did not contain a SP and the expressed fusion proteins were not inducing cell lysis. Protein transduction activity of Tat-GFP was only detected when homogenates prepared from cells infected with the Tat-GFP-expressing adenovirus were applied to fresh cells.12 The effect was small, and is consistent with our results using TatCherry released by cell lysis (Figure 7a). Other studies give indirect evidence that Tat-fusion proteins can support a bystander effect. Tat fused to TK has been shown to increase a bystander effect and therapeutic efficacy of the TK/ganciclovir system.13,14 Although cellular export of Tat-TK was suggested in one study,13 significant amounts of Tat-TK were detected in the supernatant of Tat-TK-expressing cells only in the presence of ganciclovir,14 which induces cell lysis. Full-length Tat is released from infected cells via a nonclassical export mechanism.8 In primary CD4+ T-cells, Tat binds to phosphatidylinositol-4,5-bisphosphate in the cell membrane, which facilitates Tat secretion. Residue 11 seems to be critical for this process.9 Thus, it seems unlikely that fusion proteins containing only the basic domain (amino acids 47–57) are actively exported by this mechanism. In summary, it seems most likely that expressed Tat-fusion proteins that consist of only the basic domain are not actively exported. Hence, the induction of a relatively small bystander effect depends on the release of the fusion-protein by cell lysis, a concept which has also been proposed by others.29
Our data suggest that the addition of a SP to Tat-fusion proteins supports a bystander effect in vitro. However, Tat-fusion proteins are secreted at extremely low levels, and the effect seems largely mediated by fusion proteins that are released by cell lysis. In vivo, the magnitude of the bystander effect is probably not sufficient to be of therapeutic benefit. Poor secretion efficiency of Tat-fusion proteins and cleavage by the endoprotease furin can be overcome with modification of the original Tat sequence. However, localization within the secretory pathway is associated with reduced transduction activity of modified Tat-fusion proteins. Hence, alteration of Tat-fusion proteins in the producer cells may be an additional obstacle to the induction of an efficient bystander effect.
Remarkably, the presence of a SP enhances the transduction activity of fusion proteins that are released by cell lysis. Explanations for these unexpected results remain speculative. The increased activity in the context of a SP could be due to altered intracellular localization. Alternatively, the effect could be due to the SP itself, which is probably not cleaved, as SP-Tat-fusion proteins do not colocalize with the ER. Signal peptides contain a large hydrophobic domain, and hydrophobicity of CPP-fusion peptides has been shown to correlate with transduction activity.30
Other studies also support the concept that signal-peptide-containing Tat-fusion proteins generated with mammalian expression systems can induce a bystander effect. Cells treated with supernatants from SP-TatGFP-expressing cells were shown to be positive for GFP.17,19,20 Other cargo proteins were also used successfully.16,18
Low secretion efficiency of SP-Tat-fusion proteins has been observed by other investigators and may negatively correlate with the cationic charge of the protein transduction domain.17,18 Modified Tat-derived transduction domains, similar to the one used in our studies, have been reported to be secreted efficiently and to support intercellular transport of fused GFP. Using apoptin as cargo protein, function in the target cell was reported.16 Increased apoptotic activity was also seen due to Tat-mediated transduction of secreted Caspase-3 subunits.20 In contrast, Cre recombinase activity (as a marker for nuclear delivery) was only seen in the target cells in the presence of chloroquine, which facilitates endosomal escape.18
Using purified recombinant proteins, trapping of Tat-fusion proteins in late endosomes has been recognized as one of the major factors that limit function of the transduced protein in the target cell.31 Our data indicate that expressed Tat-fusion proteins, similar to purified ones, accumulate in late endosomes/lysosomes in transduced cells. Hence, a strategy to enhance lysosomal escape needs to be employed for optimal protein function, such as the use of the HA2 peptide of the influenza hemagglutinin protein.31 Other investigators reported that internalized (expressed) Tat-fusion proteins concentrate in the Golgi.17 This discrepancy could be related to different cargo proteins or differences in fusion-protein concentration, which has been shown to affect the mechanism of cellular uptake of CPP.32 Cell fixation, which may alter intracellular localization of Tat-fusion proteins,33 was not used in our in vitro experiments evaluating transduced cells.
The modified Tat that was used in this study was originally designed to feature a stabilized α-helical structure. Using synthesized peptides, Tatm was shown to have increased transduction potential,23 although other investigators were unable to reproduce these data using E. coli–expressed fusion proteins.26 Derived from supernatants of transfected cells, a very similar CPP (YARKAARQARA) was reported to be more efficient to transduce target cells compared to the original Tat sequence.16 In our experience, quantitative direct comparison between secreted Tat and Tatm is difficult because of the low secretion efficiency of Tat-fusion proteins. However, using fusion proteins released by cell lysis, transduction potential of Tatm was reduced compared to Tat. In view of the published results mentioned above and the fact that Tatm is not substrate to furin cleavage (in contrast to the original Tat), this observation was not anticipated. These results point toward significant differences between synthetic and expressed CPP, which may be altered in the environment of the producer cells. However, based on the presented experiments, we also cannot rule out intrinsic inferior transduction activity of Tatm.
In summary, SP-Tat-fusion proteins can induce a bystander effect. Surprisingly, the effect seems to be mediated to a large extent by protein that is released by cell lysis, as Tat-fusion proteins are very poorly secreted. Tat modification can improve secretion efficacy, but passage through the secretory pathway is associated with impaired transduction activity.
To improve the efficacy of this technology, the mechanisms that lead to reduced transduction activity of Tat-fusion proteins in the producer cells need to be better understood. Simultaneously, Tat-fusion proteins that incorporate a cancer-cell-lytic protein could be of therapeutic benefit and warrant further evaluation. To be of clinical benefit, the system also needs to incorporate a membrane disrupting peptide, or alternative measure to release the transduced fusion-protein from late endosomes.
Materials and Methods
Cell lines and culture. H1299 (ATCC CRL-5803; American Type Culture Collection, Manassas, VA) nonsmall cell lung cancer cells were cultured in RPMI 1640 medium with 4.5 g/l glucose, 1.0 mmol/l sodium pyruvate plus 10% fetal bovine serum and antibiotics. A549 (ATCC CCL-185) lung adenocarcinoma cells were cultured in F12K medium with 10% fetal bovine serum and antibiotics. Human embryonic kidney cells 293 (Microbix, Toronto, Ontario, Canada) transformed by human adenovirus type 5, MRC-9 (ATCC CCL-212) normal lung fibroblast cells and HeLa (ATCC CCL-2) cervix adenocarcinoma cells were cultured in Eagle's minimum essential medium with Earle's salt and -glutamine plus 10% fetal bovine serum and antibiotics. HUVEC cells were purchased from Cambrex (Baltimore, MD) and cultured in endothelial cell medium as per the manufacturer's instructions.
Adenoviruses. AdTatDs is a replication deficient adenovirus that coexpresses TatDsRed and GFP. The virus contains the complementary DNA (cDNA) for DsRed, cloned from pDsRed-monomer-N1 (Clontech, Mountain View, CA), which was fused to the basic domain of HIV-1 Tat (YGRKKRRQRRR). The cDNA coding for TatDsRed was cloned into pAdTrack (generously provided by Dr Vogelstein, Johns Hopkins, Baltimore, MD). pAdTrack is an E1-deleted adenoviral shuttle plasmid that expresses GFP and the transgene from two separate cytomegalovirus promoters. Virus was generated by homologous recombination in BJ5183-AD1 cells (Adeasy System; Stratagene, La Jolla, CA), following the manufacturer's instructions.
AdDs coexpresses DsRed and GFP and is identical to AdTatDs, except for the omission of Tat.
Ad-SP-TatDs and Ad-SP-Ds are identical to AdTatDs and AdDs, except for the n-terminal fusion of TatDs or Ds to the SP of the murine immunoglobulin κ-chain, which was cloned from pSecTag2 (Invitrogen, Carlsbad, CA).
Ad-SP-TatCherry expresses an artificial secretory SP (MWWRLWW LLLLLLLLWPMVWA)21 fused to the basic domain of Tat and Cherry, cloned from pmCherry-N1 (Clontech). The SP was generated by annealing and cloning of the coding sense and antisense oligonucleotides. The virus also coexpresses GFP.
Ad-SP-Cherry is identical to Ad-SP-TatCherry, except for the omission of Tat.
All viruses were amplified in 293 cells, followed by standard procedure of purification and titration.34
Plasmids. SP-TatCherry expresses an artificial secretory SP (MWWRLWW LLLLLLLLWPMVWA), fused to the basic domain of HIV-1 Tat (YGRKK RRQRRR) and Cherry. The plasmid is based on pmCherry-N1 (Clontech). The SP and Tat were generated by annealing and cloning of the coding sense and antisense oligonucleotides. SP-Cherry is identical to SP-TatCherry, except for the omission of Tat. SP-TatTomato and SP-Tomato are based on ptdTomato-N1 (Clontech) and are otherwise identical to the corresponding Cherry-expressing plasmids. SP-TatmCherry expresses the artificial SP fused to a modified Tat sequence23 (YARAAARQARA) and Cherry. Tatm was generated by cloning of the annealed sense and antisense oligonucleotides.
SP-CherryTatV5 and SP-CherryTatmV5 contain the cDNA for the artificial SP, Cherry, Tat (or Tatm) and V5 (GKPIPNPLLGLDST) all fused in frame in the order as written.
SP-TatmV5V5 contains the cDNA for the artificial SP fused to Tatm and a double modified V5 Tag (GAPIPNPLLGLDAAGAPIPNPLLGLDAA). SP-V5V5 is an identical plasmid without Tatm.
Assessment of the presence of a bystander effect. H1299 cells or A549 cells were infected with the indicated adenoviruses at 50 plaque-forming units per cell. At 24 hour, cells were washed three times with phosphate-buffered saline (PBS) and trypsinized to remove any unabsorbed virus. The infected cells were resuspended and mixed with uninfected H1299 or A549 cells at a ratio of 1:3. A total of 1 × 105 mixed cells per well were seeded in four well chamber slides. Twenty-four hour or 48 hour after cell mixing, images of living cells were obtained with a Nikon (Nikon, Melville, NY) Eclipse TS100 fluorescent microscope and a Nikon DXM1200F digital camera.
To confirm the presence of a bystander effect, uninfected cells were labeled with CellTracker Blue 7-amino-4-chloromethylcoumarin (CMAC) (Invitrogen) before mixing with infected cells. At 37 °C, the uninfected A549 cells were incubated with 10 µmol/l CellTracker Blue CMAC in serum-free medium for 20 minutes, followed by 30 minute incubation with fresh complete medium and two times washing with PBS. Images of mixed living cells were obtained with a Zeiss LSM710 confocal microscope imaging system (Zeiss, Thornwood, NY).
Assessment of protein transduction. 1 × 106 H1299 cells were seeded in 6 cm plates and infected with adenoviruses at 50 plaque-forming units per cell or transfected with 5 µg DNA of constructs as indicated. At 24 hour, plates were rinsed three times with PBS. Infected cells were trypsinized to remove unabsorbed viruses and replated. All plates were supplied with 1.5 ml fresh medium. At 48 hour, the conditioned medium was collected, filtered through 0.22 µm filters and measured for fluorescence with a fluorometer (Bio-TEK FL600; Bio-TEK, Winooski, VT). After normalizing fluorescence, the conditioned medium was applied to fresh A549 cells seeded in four well chamber slides at a density of 1 × 105 per well. Twenty-four hours later, images of treated living A549 cells were obtained.
To measure transduction activity of secreted fusion proteins versus those released by cell lysis, the contact time of the conditioned medium was optimized to minimize contamination with lysed cells. At 48 hour after transfection, plates were rinsed three times with PBS and replenished with 1.5 ml fresh medium. After only 3.5 hour, supernatant was carefully collected, filtered and concentrated threefold using Amicon Ultra (Millipore, Billerica, MA). Cell lysates were harvested by repeatedly freezing and thawing in fresh medium. Supernatants and lysates were normalized for fluorescence and applied to fresh cells.
To evaluate the intracellular localization of transduced Tat-fusion proteins, treated A549 cells were rinsed three times with PBS, incubated with 50 nmol/l LysoTracker Blue DND-22 (Invitrogen) in medium for 30 minutes at 37 °C and rinsed again with PBS. Fluorescent images were obtained with Zeiss Axioplan 2 microscope (×100 oil immersion objective) and Zeiss AxioCam camera (Zeiss).
To localize the ER, 1 × 105 H1299 cells per well were seeded in four well chamber slides and transfected with 0.5 µg DNA of constructs as indicated. At 24 hour, cells were rinsed four times with Hank's balanced salt solution, incubated with 1 µmol/l ER-Tracker Green (Invitrogen) in Hank's balanced salt solution for 30 minutes at 37 °C, rinsed again, and fixed in 4% formaldehyde for 2 minutes at 37 °C followed by two 5-minute washes with PBS. The final slides were loaded with ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (Invitrogen) and sealed. Fluorescent images were obtained with a Zeiss LSM710 confocal microscope imaging system.
Assessment of secretion efficiency. At 24 hour after transfection, H1299 cells were rinsed and supplied with fresh medium. Brefeldin A (Sigma-Aldrich, St Louis, MO) was added at a concentration of 1 µg/ml. After 5 hour incubation, supernatants and cell fractions were harvested. Supernatants were filtered to avoid cellular contamination. Cell lysates were generated using radio immunoprecipitation assay (RIPA) buffer plus protease inhibitors cocktail (Thermo Scientific, Waltham, MA) (RIPA+), and diluted to the same volume as the supernatants. Fluorescence in the cell lysates and supernatants were then measured with a fluorometer (Bio-Rad, Hercules, CA). The experiment was carried out three times and SEs were calculated.
Immunoblot analysis. At 48 hour after infection with AdTatDs or AdDs, 24 hour supernatants were filtered and concentrated tenfold. The cells were lysed in 200 µl RIPA+ buffer on ice for 30 minutes. Proteins from supernatants and lysates were separated by electrophoresis on 4–12% NuPAGE Novex Bis-Tris 1.0 mm sodium dodecyl sulfate gels in duplicates and transferred onto PVDF membranes using the iBlot system (Invitrogen). Membranes were blocked and incubated with either goat anti-DsRed (C-20) polyclonal antibodies (1:1,000) (Santa Cruz Biotechnology, Santa Cruz, CA) or rabbit anti-GFP antibodies (1:1,000) (Santa Cruz Biotechnology) over night at 4 °C, followed by incubation with secondary antibodies donkey anti-goat-HRP (1:1,000) (Santa Cruz Biotechnology) or goat anti-rabbit-HRP (1:1,000) (Santa Cruz Biotechnology) respectively for 1 hour at room temperature. Antibody binding was visualized with SuperSignal West Pico Chemiluminescent substrate (Thermo Scientific).
To test for furin cleavage of Tat or Tatm, lysates of transfected cells were normalized for fluorescence. Samples were subjected to electrophoresis and membrane transfer in duplicates. Membranes were incubated with either goat anti-DsRed (C-20) polyclonal antibodies (1:1,000) followed by appropriate secondary antibodies or anti-V5-HRP (1:5,000) (Invitrogen).
To determine transduction activity of SP-TatmV5V5, 24 hour supernatants of transfected cells were filtered and concentrated greater than tenfold. Lysates were collected by repeated freezing and thawing of cells in 200 µl medium. Supernatants and lysates were applied to A549 cells. After 24 hour, treated cells were washed four times with PBS, trypsinized, pelleted, washed again in PBS, and finally lysed in RIPA+ on ice for 1 hour lysates of treated A549 cells were normalized for total protein and resolved by electrophoresis. Aliquots of the original lysates and concentrated supernatants of transfected cells (used to treat the A549 cells) were also loaded. After transfer, the membrane was probed with anti-V5-HRP (1:5,000) over night at 4 °C.
Fluorescence-activated cell sorting analysis. Lysates of transfected H1299 cells were harvested as described. After normalization for fluorescence, lysates were applied to fresh A549 cells. 24 hours later, cells were washed six times with PBS, trypsinized to eliminate any surface-bound proteins and pelleted by centrifugation (Sorvall RT7 Plus; Thermo Scientific). Cell pellets were resuspended in fresh medium in 5 ml polystyrene tubes (BD falcon; BD Biosciences, Franklin Lakes, NJ) and analyzed with filters for Cherry using a flow cytometer LSRII and FACSDiva software (BD Biosciences). For each sample, 10,000 events of healthy cells were counted. Data were processed and graphed with FlowJo V6.3.3 software (Tree Star, Ashland, OR).
Xenografts in mice and immunohistochemistry. The animal experiments were approved by the New York University School of Medicine Institutional Animal Care and Use Committee. Nude mice (Taconic, NCr-Foxn1nu) aged 6–7 weeks were injected with 3.6 × 106 A549 cells at the right hind flanks. When the tumors reached 5–7 mm in diameter, adenoviruses (7 × 109 plaque-forming units) in 50 µl were injected into the center of the tumors. 7 days after injection, tumors were harvested, frozen in OCT medium, sectioned and subjected to immunostaining. Tissues were fixed in cold methanol for 5 minutes, air dried, washed in PBS for 10 minutes, and blocked with 10% normal donkey serum in PBS in a moisture chamber for 30 minutes. After washing with PBS, samples were incubated with rabbit anti-GFP (1:100) + goat anti-DsRed (1:100) in 5% normal donkey serum in PBS over night in the moisture chamber at 4 °C. The following day, samples were washed with PBS and incubated with secondary antibodies donkey-anti-goat-texas red (1:100) (Santa Cruz Biotechnology) + donkey-anti-rabbit-fluorescein isothiocyanate (1:100) (Santa Cruz Biotechnology) in 5% normal donkey serum in PBS for 1 hour Finally, samples were washed, treated with ProLong Gold antifade reagent (Invitrogen) and sealed with glass coverslips and nail polish. Fluorescent images were obtained with Zeiss Axioplan 2 microscope and Zeiss AxioCam camera (Zeiss).
SUPPLEMENTARY MATERIAL Figure S1. HeLa, MRC-9, or human umbilical vein endothelial cells (HUVEC) cells were treated with conditioned medium from cells infected with Ad-SP-TatTomato. Figure S2. Time course of cellular uptake of TatTomato. Cells were treated with conditioned medium from cells transfected with SP-TatTomato.
Acknowledgments
The study was supported by the Alliance for Cancer Gene Therapy and the Veterans Administration. The authors declare no conflict of interest.
Supplementary Material
HeLa, MRC-9, or human umbilical vein endothelial cells (HUVEC) cells were treated with conditioned medium from cells infected with Ad-SP-TatTomato.
Time course of cellular uptake of TatTomato. Cells were treated with conditioned medium from cells transfected with SP-TatTomato.
REFERENCES
- Frankel AD., and, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Vivès E, Brodin P., and, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- Heitz F, Morris MC., and, Divita G. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br J Pharmacol. 2009;157:195–206. doi: 10.1111/j.1476-5381.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli B, Barillari G, Salahuddin SZ, Gallo RC., and, Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature. 1990;345:84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- Chang HC, Samaniego F, Nair BC, Buonaguro L., and, Ensoli B. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS. 1997;11:1421–1431. doi: 10.1097/00002030-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan RA, et al. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiò S, Alfano M, Primo L, Gramaglia D, Butini L, Gennero L, et al. Cell surface-associated Tat modulates HIV-1 infection and spreading through a specific interaction with gp120 viral envelope protein. Blood. 2005;105:2802–2811. doi: 10.1182/blood-2004-06-2212. [DOI] [PubMed] [Google Scholar]
- Rayne F, Debaisieux S, Bonhoure A., and, Beaumelle B. HIV-1 Tat is unconventionally secreted through the plasma membrane. Cell Biol Int. 2010;34:409–413. doi: 10.1042/CBI20090376. [DOI] [PubMed] [Google Scholar]
- Rayne F, Debaisieux S, Yezid H, Lin YL, Mettling C, Konate K, et al. Phosphatidylinositol-(4,5)-bisphosphate enables efficient secretion of HIV-1 Tat by infected T-cells. EMBO J. 2010;29:1348–1362. doi: 10.1038/emboj.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Neuveut C, Tiffany HL, Benkirane M, Rich EA, Murphy PM, et al. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad Sci USA. 2000;97:11466–11471. doi: 10.1073/pnas.97.21.11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi M, Rusnati M, Presta M., and, Giacca M. Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. J Biol Chem. 2001;276:3254–3261. doi: 10.1074/jbc.M006701200. [DOI] [PubMed] [Google Scholar]
- Cashman SM, Morris DJ., and, Kumar-Singh R. Evidence of protein transduction but not intercellular transport by proteins fused to HIV tat in retinal cell culture and in vivo. Mol Ther. 2003;8:130–142. doi: 10.1016/s1525-0016(03)00131-x. [DOI] [PubMed] [Google Scholar]
- Tasciotti E., and, Giacca M. Fusion of the human immunodeficiency virus type 1 tat protein transduction domain to thymidine kinase increases bystander effect and induces enhanced tumor killing in vivo. Hum Gene Ther. 2005;16:1389–1403. doi: 10.1089/hum.2005.16.1389. [DOI] [PubMed] [Google Scholar]
- Cascante A, Huch M, Rodríguez LG, González JR, Costantini L., and, Fillat C. Tat8-TK/GCV suicide gene therapy induces pancreatic tumor regression in vivo. Hum Gene Ther. 2005;16:1377–1388. doi: 10.1089/hum.2005.16.1377. [DOI] [PubMed] [Google Scholar]
- Xia H, Mao Q., and, Davidson BL. The HIV Tat protein transduction domain improves the biodistribution of β-glucuronidase expressed from recombinant viral vectors. Nat Biotechnol. 2001;19:640–644. doi: 10.1038/90242. [DOI] [PubMed] [Google Scholar]
- Flinterman M, Farzaneh F, Habib N, Malik F, Gäken J., and, Tavassoli M. Delivery of therapeutic proteins as secretable TAT fusion products. Mol Ther. 2009;17:334–342. doi: 10.1038/mt.2008.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsokeras A., and, Kabouridis PS. Secretion and uptake of TAT-fusion proteins produced by engineered mammalian cells. Biochim Biophys Acta. 2009;1790:147–153. doi: 10.1016/j.bbagen.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PA, Catchpole IR, Goddard CA., and, Colledge WH. Comparison of protein transduction domains in mediating cell delivery of a secreted CRE protein. Biochemistry. 2008;47:1157–1166. doi: 10.1021/bi701542p. [DOI] [PubMed] [Google Scholar]
- Barka T, Gresik ES., and, Henderson SC. Production of cell lines secreting TAT fusion proteins. J Histochem Cytochem. 2004;52:469–477. doi: 10.1177/002215540405200405. [DOI] [PubMed] [Google Scholar]
- White MK, Amini S, Khalili K, Kogan M, Donaldson K., and, Darbinian N. Development of a bidirectional caspase-3 expression system for the induction of apoptosis. Cancer Biol Ther. 2008;7:945–954. doi: 10.4161/cbt.7.6.5969. [DOI] [PubMed] [Google Scholar]
- Barash S, Wang W., and, Shi Y. Human secretory signal peptide description by hidden Markov model and generation of a strong artificial signal peptide for secreted protein expression. Biochem Biophys Res Commun. 2002;294:835–842. doi: 10.1016/S0006-291X(02)00566-1. [DOI] [PubMed] [Google Scholar]
- Tikhonov I, Ruckwardt TJ, Berg S, Hatfield GS., and, David Pauza C. Furin cleavage of the HIV-1 Tat protein. FEBS Lett. 2004;565:89–92. doi: 10.1016/j.febslet.2004.03.079. [DOI] [PubMed] [Google Scholar]
- Ho A, Schwarze SR, Mermelstein SJ, Waksman G., and, Dowdy SF. Synthetic protein transduction domains: enhanced transduction potential in vitro and in vivo. Cancer Res. 2001;61:474–477. [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG., and, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben S, Perkins A, Purcell R, Joung K, Sia R, Burghoff R, et al. Structural and functional characterization of human immunodeficiency virus tat protein. J Virol. 1989;63:1–8. doi: 10.1128/jvi.63.1.1-8.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ma J, Song Z., and, Wu M. HIV-1 TAT-mediated protein transduction and subcellular localization using novel expression vectors. FEBS Lett. 2002;532:36–44. doi: 10.1016/s0014-5793(02)03624-4. [DOI] [PubMed] [Google Scholar]
- Yoon JS, Jung YT, Hong SK, Kim SH, Shin MC, Lee DG, et al. Characteristics of HIV-Tat protein transduction domain. J Microbiol. 2004;42:328–335. [PubMed] [Google Scholar]
- Leifert JA, Harkins S., and, Whitton JL. Full-length proteins attached to the HIV tat protein transduction domain are neither transduced between cells, nor exhibit enhanced immunogenicity. Gene Ther. 2002;9:1422–1428. doi: 10.1038/sj.gt.3301819. [DOI] [PubMed] [Google Scholar]
- Leifert JA., and, Whitton JL. “Translocatory proteins” and “protein transduction domains”: a critical analysis of their biological effects and the underlying mechanisms. Mol Ther. 2003;8:13–20. doi: 10.1016/s1525-0016(03)00151-5. [DOI] [PubMed] [Google Scholar]
- Li Y, Rosal RV, Brandt-Rauf PW., and, Fine RL. Correlation between hydrophobic properties and efficiency of carrier-mediated membrane transduction and apoptosis of a p53 C-terminal peptide. Biochem Biophys Res Commun. 2002;298:439–449. doi: 10.1016/s0006-291x(02)02470-1. [DOI] [PubMed] [Google Scholar]
- Wadia JS, Stan RV., and, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- Duchardt F, Fotin-Mleczek M, Schwarz H, Fischer R., and, Brock R. A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic. 2007;8:848–866. doi: 10.1111/j.1600-0854.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, et al. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem. 2003;278:585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- Graham FL., and, Prevec L. Methods for construction of adenovirus vectors. Mol Biotechnol. 1995;3:207–220. doi: 10.1007/BF02789331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HeLa, MRC-9, or human umbilical vein endothelial cells (HUVEC) cells were treated with conditioned medium from cells infected with Ad-SP-TatTomato.
Time course of cellular uptake of TatTomato. Cells were treated with conditioned medium from cells transfected with SP-TatTomato.