Abstract
The aim of this phase I/II nonrandomized trial was to assess feasibility, safety as well as immunological and clinical responses of a mRNA-based vaccination in patients with stage IV renal cell cancer using granulocyte-macrophage colony stimulating factor (GM-CSF) as adjuvant. Intradermal injections of in vitro transcribed naked mRNA, which was generated using plasmids coding for the tumor-associated antigens mucin 1(MUC1), carcinoembryonic (CEA), human epidermal growth factor receptor 2 (Her-2/neu), telomerase, survivin, and melanoma-associated antigen 1 (MAGE-A1) were performed in 30 enrolled patients. In the first 14 patients (cohort A) vaccinations were administered on days 0, 14, 28, and 42 (20 µg/antigen) while in the consecutive 16 patients (cohort B) an intensified protocol consisting of injections at days 0–3, 7–10, 28, and 42 (50 µg/antigen) was used. In both cohorts, after this induction period, vaccinations were repeated monthly until tumor progression analyzed by Response Evaluation Criteria In Solid Tumors criteria (RECIST). Vaccinations were well tolerated with no severe side effects and induced clinical responses [six stable diseases (SD) and one partial response in cohort A and nine SD in cohort B]. In cohort A, 35.7% survived 4 years (median survival 24 months) compared to 31.25% in cohort B (median survival 29 months). Induction of CD4+ and CD8+ T cell responses was shown for several tumor-associated antigens (TAA) using interferon-γ (IFN-γ) enzyme-linked immunosorbent spot (ELISpot) and Cr-release assays.
Introduction
Renal cell carcinoma (RCC) is a relatively rare tumor accounting for 2–3% of malignancies in adults. When diagnosed at early stage of disease, surgical resection can be curative. However, ~30% of patients develop metastases resulting in a 5-year survival rate of <10%.1 Despite the use of the recently introduced targeted therapies, management of advanced RCC remains challenging. The fact that RCC can evoke an immune response that occasionally results in spontaneous remissions, the detection of dendritic- and T-cells in RCC tissues,2,3 and advances in tumor immunology have stimulated the development of vaccination strategies for RCC patients. Within the last years, several tumor-associated antigens (TAA) expressed in RCC and recognized by cytotoxic T lymphocytes (CTL) have been defined by using expression cloning, reverse immunology approaches, or the application of DNA micro-array technologies.4,5 Recent reports from several phase I/II trials have shown encouraging results and furthermore demonstrated the safety of specific immunotherapy.6,7,8,9,10,11,12,13 Numerous studies have shown that dendritic cells (DC) transfected with mRNA coding for a TAA or whole tumor RNA are able to induce potent antigen- and tumor-specific T cell responses.14,15,16,17,18,19,20,21,22,23,24,25 This technology was pioneered by E. Gilboa and later confirmed by several other groups. As a promising alternative to vaccination with transfected DC, direct intradermal application of naked mRNA was demonstrated to be effective in the expression of the encoded protein26,27 and the subsequent generation of antigen-specific T cell responses in several mouse models.28,29,30,31 We conducted a phase I/II trial to investigate feasibility, safety, and immunological responses of a naked mRNA-based vaccination adjuvanted with granulocyte-macrophage colony stimulating factor (GM-CSF) in advanced RCC patients.
Results
Patient characteristics and study design
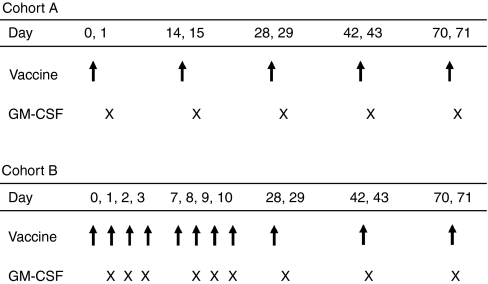
Between August 2003 and November 2005, 30 patients aged 36–79 years were enrolled in the study. Intradermal injections of in vitro transcribed naked mRNA, which was generated using plasmids coding for the tumor-associated antigens mucin 1 (MUC1), carcinoembryonic (CEA), human epidermal growth factor receptor 2 (Her-2/neu), telomerase, survivin and melanoma-associated antigen 1 (MAGE-1) were performed. In the first 14 patients (cohort A), vaccinations were administered on days 0, 14, 28, and 42 (20 µg/antigen) whereas in the consecutive 16 patients (cohort B) an intensified protocol consisting of injections at days 0–3, 7–10, 28, and 42 (50 µg/antigen) was used. In both cohorts, after this induction period, vaccinations were repeated monthly until tumor progression analyzed by Response Evaluation Criteria In Solid Tumors (RECIST) criteria. An overview of the injection schedules is given in Figure 1.
Figure 1.
Study design (injection schedule). Vaccinations were performed on days 0, 14, 28, and 42 in cohort A and on days 0–3, 7–10, 28, and 42 in cohort B (marked by the arrow). Vaccinations were repeated monthly until tumor progression. On the day following mRNA-injection, granulocyte-macrophage colony stimulating factor (GM-CSF) was applied subcutaneously (marked by “x”).
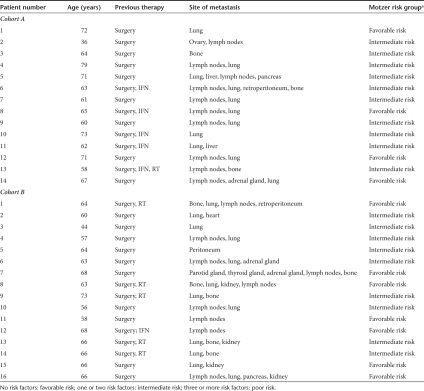
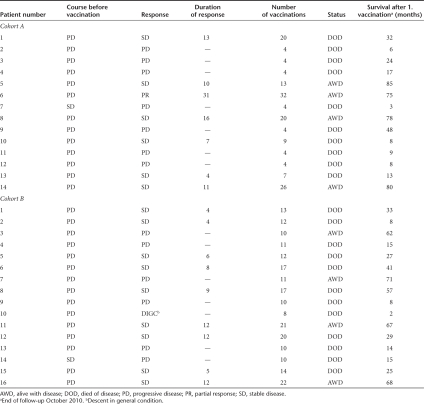
Three patients in cohort A and five in cohort B were female. The median age in cohort A was 64.5 compared to 64 years in cohort B (Table 1). All 30 patients had received prior therapy, including surgery (all patients), interferon-α (five patients in cohort A, one patient in cohort B), and radiotherapy (one patient in cohort A and five patients in cohort B). 19 patients (9 in cohort A, 10 in cohort B) had surgery only. Histology of renal cell carcinoma was mostly that of clear cell carcinoma or papillary carcinoma and metastatic disease was present at numerous anatomic sites including lung, lymph nodes, kidney, heart, bone, peritoneum, parotid- and, adrenal gland, pancreas, and retroperitoneum (Table 1). According to Motzer risk criteria (MSKCC risk model),32 four patients in cohort A were at favorable risk (no risk factors) and 10 had one or two risk factors (intermediate risk). In cohort B, seven patients were at favorable risk (no risk factors) the remaining nine patients had an intermediate risk.
Table 1. Patient characteristics.
The following risk factors were used, as assessed by Motzer: low Karnofsky index (<80%), high lactate dehydrogenase-levels (>1.5 × upper normal level), low hemoglobin level, “corrected” calcium >10 mg/dl, absence of prior nephrectomy. No risk factors: favorable risk; one or two risk factors: intermediate risk; three or more risk factors: poor risk.
Safety and toxicity
The toxicity associated with our vaccination therapy was minimal, always reversible and mainly restricted to swelling, redness, and itching at the injection site of GM-CSF, fever ≤38 °C and headache on the day of GM-CSF administration (no grad III or IV toxicity). One patient developed an allergic reaction to GM-CSF. No autoimmune phenomena were observed.
Immunological responses
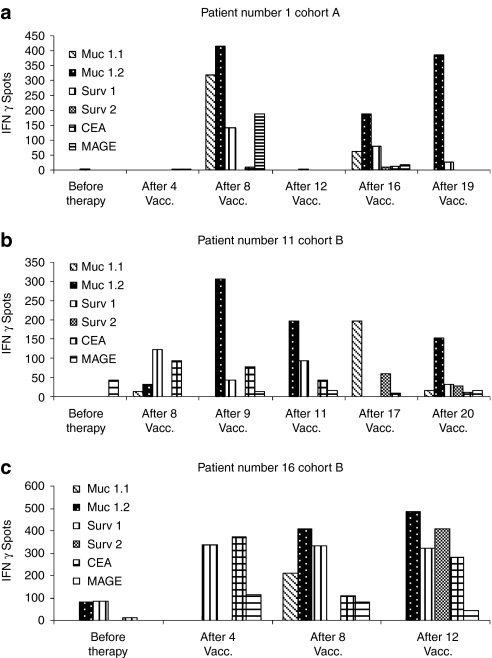
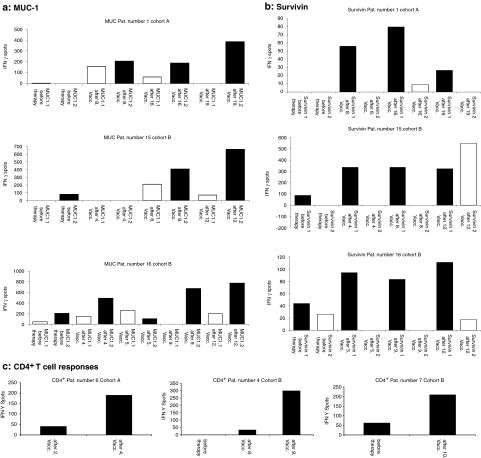
The induction of TAA-specific T cell responses in vivo after mRNA injections was assessed by performing interferon-γ (IFN-γ) enzyme-linked immunosorbent spot (ELISpot) and cytotoxic T cell assays. The vaccination-induced TAA-specific CTL responses in human leukocyte antigen (HLA)-A2 positive patients were determined by analysis of IFN-γ production of T lymphocytes after stimulation with the cognate peptide epitope derived from used TAA in vitro. An immune response to peptides was considered significant when a stimulation index of ≥2 as compared to the number of spots before vaccination was detected at least at two different time points after vaccinations for two or more TAA-derived peptides. In ELISpot assays, antigen-specific T cell induction was observed for several TAA in 8 out of 10 tested patients, in some patients already after the first four vaccinations (1/1 tested patient in arm A and 7/9 tested patients in arm B). In patient number, three of arm B ELISpot assay was performed only at two time points (before vaccination and after 4th vaccination). A stimulation index ≥2 could be shown already after four vaccinations, but by reason of a missing 2nd timepoint we defined this immunological response as negative. Exemplary ELISpot assays of CD8+ T cells reactive to the TAA MUC1, survivin, CEA, and MAGE are shown in Figure 2. As shown in Figure 3 immune responses against the epitopes MUC 1.2 and survivin 1 were pronounced in ELISpot assays as compared to the other (MUC 1.1 and survivin 2) antigenic peptides deduced from these tumor-associated antigens indicating immunodominance of the epitopes MUC 1.2 and survivin 1. Figure 3 demonstrates in detail the exemplary course of responses against HLA-A2 binding MUC 1 and survivin peptides in three patients. Due to lack of blood samples, we could perform ELISpot assays using HLA-A2 binding peptides only for one of the four HLA-A2 positive patients in arm A, but for all nine HLA-A2 positive patients in arm B (Supplementary Figures S1 and S2).
Figure 2.
Generation of immunological responses upon vaccination with in vitro transcribed mRNA; exemplary interferon-γ (IFN-γ) enzyme-linked immunosorbent spot (ELISpot) assays: course of several tumor-associated antigen (TAA). Autologous peripheral blood mononuclear cells from human leukocyte antigen (HLA)-A2+ patients were pulsed with the peptides MUC 1.1, MUC 1.2, MAGE, CEA, survivin 1 and 2 deduced from TAA used in the vaccine and used as stimulators. (a–c) Representative ELISpot assays of several different T cell response patterns of three patients. Samples from prevaccination and representative samples after one or more vaccinations were evaluated simultaneously. CEA, carcinoembryonic; MAGE, melanoma-associated antigen 1; MUC 1, mucin 1.
Figure 3.
Generation of immunological responses upon vaccination with in vitro transcribed mRNA. Exemplary enzyme-linked immunosorbent spot (ELISpot) assays. (a,b) Immunodominant antigens. Of all used antigens, immune responses for the epitopes MUC 1.2 and survivin 1 were pronounced in ELISpot assays indicating that these epitopes are immunodominant. Detailed course of immune responses are displayed for the epitopes (a) MUC 1.1 and 1.2 (b) and survivin 1 and 2 in three patients. (c) Results obtained for CD4+ cells. Autologous CD4+ T cells were isolated by magnetic activated cell sorting technology and stimulated with dendritic cells electroporated with a mixture of RNA coding for tumor-associated antigen used in the vaccine. Enhanced green fluorescent protein (EGFP)-RNA served as negative control, counted spots in EGFP vials were subtracted. MUC 1, mucin 1.
For the detection of CD4+ T cell responses CD4+ T lymphocytes were isolated by magnetic activated cell sorting technology and stimulated with autologous DC electroporated with the TAA-mRNA-mix as described above. The immune response to TAA used for vaccinations was analyzed in IFN-γ ELISpot assays and was considered significant when a stimulation index of ≥2 compared to the spot numbers before vaccination was detected at least at one time point after vaccination. Vaccine-induced CD4+ T cell responses are shown in Figure 3c for three patients. Responses were detected in four of eight tested patients using IFN-γ ELISpot assays.
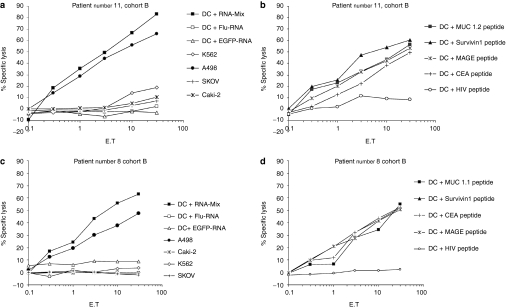
In order to analyze the cytotoxic activity of vaccine-induced T cells, peripheral blood leukocytes from vaccinated HLA-A2 positive patients were restimulated with autologous DC electroporated with the mix of mRNA coding for the used TAA. The lytic activity was determined in chromium-release assays against autologous DC pulsed with HLA-A2-binding peptides derived from the TAA or HLA-A2-positive tumor cell lines expressing the antigens. In addition, DC electroporated with the mRNA-mix coding for the TAA were included as targets in these assays. As a negative control, DC transfected with enhanced green fluorescent protein (EGFP)-coding mRNA were used. Data and results from two patients are presented in Figure 4. CTL obtained after one in vitro restimulation efficiently lysed target cells pulsed with the antigenic peptides (MUC1, survivin, MAGE, CEA), as well as DC electroporated with a mixture of RNA coding for TAA. Lysis was shown to be antigen-specific since no lysis was detected when target cells were loaded with an irrelevant peptide derived from HIV or when electroporated with EGFP-RNA. We could demonstrate antigen-specific lysis of target cells in chromium-release assays in 8 out of 10 tested patients.
Figure 4.
Lytic activity of vaccine-induced T lymphocytes. Cytotoxic T lymphocytes obtained after in vitro restimulation efficiently lysed target cells [dendritic cells (DC)] pulsed with the antigenic peptides (MUC1, survivin, MAGE, CEA), as well as DC electroporated with a mixture of RNA coding for tumor-associated antigen (TAA). Lysis was shown to be antigen-specific since no lysis was detected when target cells were loaded with the peptide derived from HIV or when electroporated with enhanced green fluorescent protein (EGFP) RNA. In addition, the human leukocyte antigen (HLA)-A2 positive tumor cell line A498 [renal cell carcinoma (RCC)], as well as HLA-A2 negative control cell lines like SK-OV-3 cells (ovarian cancer) and CaKi-2 (RCC) were included demonstrating HLA restricted lysis of CTL. K562 [chronic myelogenous leukemia (CML) in blast crisis] was used to exclude natural killer-cell mediated lysis. Data from two patients are presented (a,b: patient number 11 and c,d: patient number 8, cohort B). CEA, carcinoembryonic; MAGE, melanoma-associated antigen 1; MUC 1, mucin 1.
In order to analyze the possible generation of antibody responses we included the mRNA coding for hepatitis-B-surface (HBS) antigen in the experimental vaccine. However, we were not able to determine any seroconversion in any of the vaccinated patients as all remained anti-HBS negative (central laboratory facilities at the University of Tuebingen, data not shown).
Clinical responses to mRNA vaccinations
With the exception of one patient, all of the 30 patients initially enrolled in the study completed the first part of the vaccination schedule before the first staging time point (7 weeks after study entry; 4 injections in cohort A and 10 injections in cohort B). Seven patients in cohort A were still on study treatment after the first four vaccinations due to objective response (n = 1) or stabilization of disease (n = 6) with the number of vaccinations ranging from 7 to 32 (Table 2). Nine of sixteen patients in cohort B achieved stable disease after 7 weeks of treatment and received >10 vaccinations, ranging from 12 to 22 vaccinations (three patients received 20 or more vaccinations). Treatment of one patient in cohort B could not be continued due to a descent in general condition. This patient survived only 2 months after the first vaccination.
Table 2. Clinical responses.
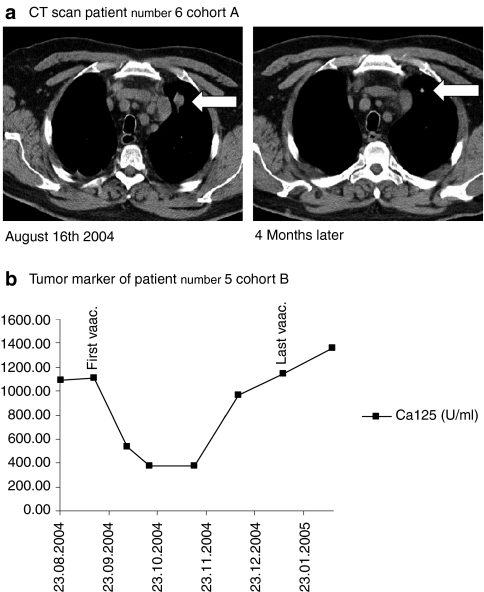
During administration of the mRNA formulation, there was tumor regression or stabilization lasting for >3 months (defined as clinical benefit) in 7 of 14 patients in cohort A and in 9 of 16 patients in cohort B. Partial response was confirmed in patient number six in cohort A and was observed as shrinking of cervical and mediastinal lymph nodes as well as pulmonary metastases (Figure 5a). In one patient in cohort B (patient number five), the need for abdominal paracentesis, that had to be performed every other day due to refractory ascites and extended abdominal tumor sites, diminished during the first vaccinations. This patient remained free of paracentesis for >3 months and the ascites completely resolved in line with a decline of the tumor marker CA-125 and regression of abdominal tumor sites (Figure 5b). Data of clinical outcome are presented in Table 2. The longest duration of response for one patient with partial response was 31 months in cohort A. In cohort B, the duration of response was 12+ months at most at the last follow-up due to later initiation of this cohort. Median duration of response for all responders was 9.5 months. Median progression free survival was 2 months in cohort A, 4 months in cohort B and 4 months for all patients together. 35.7% (5/14) of patients in cohort A showed survival of 4 years or more after the first vaccination (range of 48–85 months, median survival 24 months). Four of fourteen (28.6%) were still alive at the last follow-up (October 2010) with a 5-year survival rate of 21.4% in this cohort. In cohort B, 31.25% (5/16) of patients were alive 4 years after study entry with a survival ranging from 48 to 71 months. Median survival was 29 months. Four of sixteen patients were alive at follow-up in October 2010 and 25% had survived >5 years (Table 2).
Figure 5.
Clinical responses in vaccinated patients. (a) Computed tomography (CT) scan of patient number 6 (cohort A) with pulmonary metastases. Size of metatases diminished after 4 months of vaccination. (b) Course of tumor markers under vaccination (patient number 5, cohort B) The arrow marks the first and the last injection. The need of paracentesis diminished during vaccination.
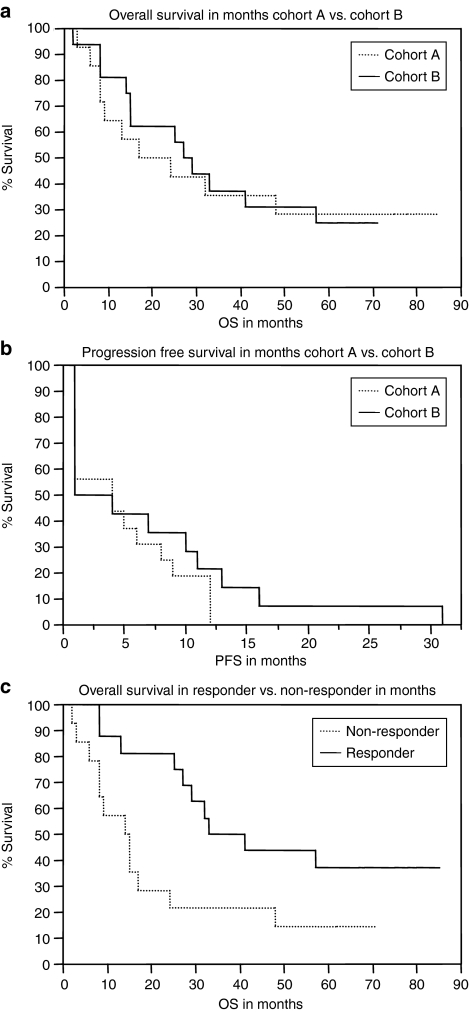
Kaplan–Meier plots for overall survival (a) and progression free survival (b) of the two cohorts are presented in Figure 6. While there was no significant difference in the overall survival between cohort A and cohort B, a significant improvement of the overall survival was found when comparing patients clinically responding to the treatment at the first staging time point with nonresponders (P < 0.02) (Figure 6c).
Figure 6.
Kaplan–Meier graphs: survival data. (a) Overall survival in months of patients in cohort A versus cohort B. Log-rank test showing no significant differences in overall survival (P = 0.85). Median survival cohort A: 24 months, cohort B: 29 months. (b) Progression free survival in months of patients in cohort A versus cohort B. Log rank test showing no significant differences in progression free survival (P = 0.33). (c) Overall survival in clinical responders versus nonresponders. Patients who had shown objective response or at least stable disease at the first staging time point were defined as clinical responders (P = 0.02). Median survival nonresponder: 14 months, responder: 41 months. PFS, progression free survival; OS, overall survival.
Immunological and clinical responses correlated in most tested patients, though we could not perform statistical analysis, because not all immunological assays could be performed in all patients. This was due to HLA-type and lack of blood samples. Taken together, 12 patients showed immunological responses in one or more of performed assays. Five patients showed no immunological response. Furthermore in two patients immunological responses could be detected in one assay (ELISpot) but not in performed chromium-release assays. As mentioned above in one further patient ELISpot assay was performed only at two time points (before vaccination and after four vaccinations) due to lack of blood samples. A stimulation index >2 could be detected already after four vaccinations, but due to missing 2nd time point, we defined this immunological response as negative. Taken together median survival was 40.5 months in the 12 patients showing immunological response versus 14 months in the 5 patients showing no response in immunological assays (Supplementary Table S1).
Discussion
The discovery of new TAA and the development of various vaccination methods led to the evaluation of multiple experimental immunotherapies in RCC patients. Several studies have proven feasibility and safety6,7,9 of these vaccines and have proven to result in the generation of antigen-specific immune responses. Direct injection of naked mRNA was demonstrated to elicit immune response against the encoded antigens.29,30,31 A clinical application of naked mRNA in melanoma patients was recently published by Weide et al. Total autologous tumor RNA was extracted, reversely transcribed, and administered intradermally. The treatment was shown to be safe and feasible but lacked clear clinical effectiveness.33
We used naked mRNA molecules in our vaccination study since naturally transient and cytosolically active mRNA molecules are considered to be a possibly safer and more potent alternative to DNA for gene vaccination. Patients suffering from metastatic RCC were repeatedly injected intradermally with naked mRNA coding for the TAA MUC1, CEA, Her-2/neu, telomerase, survivin- and melanoma-associated antigen 1 (MAGE-A1). RNA applications were feasible, safe and well tolerated with no relevant side effects. Even though we were able to detect T cell responses capable of recognizing and lyzing target cells expressing the used tumor-associated antigens that represent self-antigens we did not observe any side effects with regard to autoimmune reactions or organ toxicity. This is in line with the results from our previous vaccination studies8,34,35 and studies performed by others.6,7,33 The only so far reported self-reactions were documented in patients with malignant melanoma where vaccine-induced vitiligo was described.
Clinical responses [one partial response and six stable diseases (SD)] were achieved in 7 of 14 patients in cohort A and 9 of 16 patients had SD in the intensified cohort B, with a median survival of 24 months in cohort A and 29 months in cohort B, respectively. Survival correlated with the MSKCC risk model and showed promising results under vaccination in this small group of patients. Patients without risk factors survived 8–80 months (1-year survival rate 90.9%, median survival: 57 months). The expected 1 year survival rate for these patients is 83% with a suspected median survival of 20 months according to the MSKCC risk model. For patients with intermediate risk our treatment resulted in a 1-year survival rate of 63% (expected according to MSKCC risk model: 58%) and a median survival of 15 months compared to an expected median survival of 10 months.32 Currently, 8 of our 30 patients are still alive (October 2010). As frequently observed in former studies, immunological responses were detected in almost all patients with clinical response or stabilization of disease. It is of special interest and importance that T cell responses elicited in patients upon vaccinations lasted for several months during treatment and were directed against several epitopes derived from a defined antigen demonstrating that this approach generates polyclonal immune responses with a broad specificity against multiple antigens and T cell epitopes. In addition, our study convincingly shows that a mixture of several mRNA can be safely and efficiently applied in order to generate specific antitumor immunity that consists of both CD4+ and CD8+ effector cells. We did not specifically address the induction of regulatory T cells in our study. However, the CD4+ cells detected and expanded in our patients secreted IFN-γ, thus excluding their regulatory function and indicating a helper T cells 1 (Th-1) phenotype. In order to analyze the possible generation of antibody responses, we included the RNA coding for HBS antigen. However, we were not able to determine any seroconversion in vaccinated patients as all remained anti-HBS antigen negative. A correlation between immunological responses and survival was found in most of the tested patients, though this observation has limited expressiveness, because we could not perform all assays in every patient. In this study, we demonstrate that specific immunotherapy using intradermal injections of naked in vitro transcribed mRNA coding for several TAA can elicit immunological responses and result in a clinical benefit in patients with metastatic RCC. Messenger RNA application allows targeting multiple TAA and epitopes independent of patients HLA-type. Thus, it represents a safe and versatile vaccination strategy in the context of anticancer immunotherapies.
Materials and Methods
Patient characteristics and clinical protocol. This is a nonrandomized phase I/II trial using naked mRNA transcribed from DNA plasmids and coding for MUC1, CEA, Her-2/neu, telomerase, survivin, and MAGE-A1 tumor antigens, as well as influenza-matrix-M1 protein (IMP) and hepatitis-B-surface antigen (HBS antigen) serving as potential controls. The primary end point was to assess feasibility and safety. The secondary end points were the analysis of immunological responses and potential antitumor activity. Patients with histologically confirmed advanced RCC after accomplishing surgery, radiation or IFN-α therapy or without prior therapy were included in the study (Table 1). Except for two patients all had progressive disease (Table 2). Inclusion criteria were bidimensionally measurable lesions, willingness, and ability to give informed consent, aged 18–79 years, 6 weeks interval to last chemotherapy/immunotherapy and/or radiation, bilirubin <2 mg/dl, creatinine <2 mg/dl, and a Karnofsky score >70%. Exclusion criteria were chemotherapy or intake of immune-modulating drugs like corticosteroids within 6 weeks before onset of vaccination, severe heart disease (NYHA ≥3), neurological or psychiatric disorders, brain metastases, pregnancy, underage patients or inability to give informed consent, and history of secondary malignancies. The protocol was approved by the local institutional ethics committee at Eberhard-Karls-University Tuebingen, Tuebingen, Germany. Written informed consent was given by each patient prior to study inclusion. The Declaration of Helsinki protocols were followed. Preceding the first vaccination, all patients underwent an extended clinical evaluation including physical examination, hematological and biochemical parameters, and computed tomography scans. Messenger RNA were transcribed from plasmids and coded for MUC1, CEA, Her-2/neu, telomerase, survivin, and MAGE-A1 tumor antigens as well as IMP and HBS as potential controls (in collaboration with CureVac, Tuebingen, Germany). 20 µg (cohort A) or 50 µg (cohort B) of coding naked RNA per antigen dissolved in 150 µl phosphate-buffered saline as injection solution were injected intradermally at two different sites. This was not a randomized trial. In the first group of patients vaccinations were performed on days 0, 14, 28, and 42 (cohort A). In the following patients, vaccination was applied in an intensified manner on days 0–3, 7–10, 28, and 42 (cohort B). Based on previous results from animal studies30 on the day following RNA vaccination, GM-CSF 100 µg/m2 (cohort A) and 250 µg (cohort B) was administered subcutaneously at one of the injection sites of RNA. Injection sites were the lower abdomen or upper thigh and sites and localizations could be changed during treatment. Restaging was performed around week 7. Further restaging was performed every 6–8 weeks according to RECIST criteria. In case of tumor regression or stable disease, vaccination therapy was repeated monthly until tumor progression (Figure 1).
According to the approach used (intradermal application of RNA and subcutaneous application of GM-CSF) we assumed typical rate of risk for local and systemic infection as well as a possible allergic reaction. Furthermore, we expected an inflammatory skin reaction at the injection site consisting of erythema, pruritus, and swelling. Systemic flu-like symptoms such as myalgia, headache, or fever were assumed mainly due to application of the adjuvants.
Every adverse event was to be documented at every visit according to its character, time point, intensity, and duration of appearance as well as probable causality (Common Toxicity Criteria version 2.0). Serious adverse events were to be immediately reported to the principle investigator and local institutional ethics committee. In case of death due to side effects patients should be autopsied.
Cell isolation and cultures. Patient-derived peripheral blood mononuclear cells (PBMC) were isolated by FICOLL-Paque (Life Technologies, Grand Island, NY) density gradient centrifugation of 50-ml heparinized blood. Generation and characterization of patients monocyte-derived dendritic cells was performed as described previously.34,36 The following cell lines were used: A498 (RCC, HLA-A2+, American Type Culture Collection, Manassas, VA), SK-OV-3 cells (ovarian cancer, HLA-A2-, kindly provided by O.J. Finn, University of Pittsburgh, Pittsburgh, PA), CaKi-2 (RCC, HLA-A2-, kindly provided by colleagues of Eberhard-Karls-University Tuebingen), and K562 [chronic myelogenous leukemia (CML) in blast crisis; American Type Culture Collection]. Morphology of all used cell lines was checked by microscope once or twice a week during culturing periods at both high and low density. HLA-typing was performed for indicated cell lines. Furthermore used cell lines had been tested for several transcription factors as well as tumor-associated antigens in our laboratory.8,37,38,39
Peptides. The HLA-A2 binding peptides derived from MUC1.1 (sequence STAPPVHNV), MUC1.2 (sequence LLLLTVLTV), survivin 1 (sequence ELTLGEFLKL), survivin 2 (sequence TLPPAWQPFL), CEA 8 (sequence YLSGANLNL), MAGE-A1 (sequence KVLEYVIKV), VMT-1_IAPUE (influenza matrix protein, sequence: GILGFVFTL) were synthesized using standard F-moc chemistry on a peptide synthesizer (432A; Applied Biosystems, Weiterstadt, Germany) and analyzed by reverse-phase high-performance liquid chromatography and mass spectrometry (http://www.syfpeithi.de). Peptides were kindly provided by Prof Stefan Stevanovic, Department for Immunology, Eberhard-Karls-University Tuebingen.
ELISpot assays. To analyze vaccine-induced expansion of T cells specific for the used TAA-RNA, we performed IFN-γ ELISpot assays in HLA-A2 positive patients as described previously.8,38 In brief, peripheral blood was obtained before the first vaccination and at various time points during the vaccination period. PBMC were isolated by FICOLL gradient centrifugation and were cryopreserved. After completion of treatment, samples from prevaccination and after one and more applications of the vaccine were evaluated simultaneously. PBMC obtained before the first vaccination and PBMC from individual time points during treatment were incubated in 24-well plates with HLA-A2-binding peptides derived from the TAA used in the vaccine or dimethyl sulfoxide (used for peptide dilution, negative control) for 7 days at 37 °C. One peptide was used per well in a concentration of 50 µg/ml. For the readout, autologous PBMC were pulsed with the corresponding peptide and used as stimulators in ELISpot assays. Each sample was tested in duplicate. Spots obtained in dimethyl sulfoxide wells (without peptide) were considered as background activity and subtracted from values obtained with the tested epitopes. Phorbol myristate acetate (PMA) and ionomycin were used as positive controls. For CD4+ ELISpot assays, CD4+ cells were sorted from isolated PBMC by magnetic activated cell sorting technology according to the manufacturers' instructions. Autologous DC were generated from by monocyte plastic-adherence from PBMC as previously described.40 DC were electroporated with the mRNA-mix (as described below) consisting of all TAA were used as stimulators for the analysis of vaccine-induced CD4+ T lymphocyte responses ex vivo. Electroporation of DC was performed as described previously.15 The physical parameters used for electroporation were as follows: voltage of 300 V, capacitance of 150 µF, resistance of 1,540 ω, and pulse time of 231 ms. After electroporation, cells were transferred immediately into RP10 medium. DC and CD4+ T cells from different time points were incubated in ELISpot assays for 40 hours at 37 °C. Each sample was tested in duplicate. DC electroporated with EGFP-RNA served as a negative control and the spots of these T cell responses were considered as background activity and subtracted from values obtained with the RNA-mix.
Standard 51Cr-release assay. To analyze the lytic activity of the induced CTL, autologous PBMC were restimulated twice with autologous DC, electroporated with the mRNA mixture consisting of all five mRNA coding for TAA used in the vaccine, and the lytic activity was determined in a standard 51Cr-release assay. This assay was performed with some modifications as described previously.34 Following targets were used: A498 (RCC, HLA-A2+), SK-OV-3 cells (ovarian cancer, HLA-A2−), and CaKi-2 (RCC, HLA-A2−), peptide-pulsed autologous DC or autologous DC electroporated with RNA coding for the specific antigens and autologous DC electroporated with a mixture of TAA RNA. Enhanced green fluorescent protein (EGFP)-coding mRNA served as a negative control. The physical parameters used for electroporation were as described above. After electroporation, cells were transferred immediately into RP10 medium supplemented with the cytokines GM-CSF (100 ng/ml) and IL-4 (20 ng/ml) and returned to the incubator.
Generation of mRNA by in vitro transcription. EGFP-coding in vitro transcript was synthesized from the plasmid pSP64–Poly(A)–EGFP-2 (generously provided by V.F.I. Van Tendeloo, Antwerp, Belgium) as described previously.14,15 The generation of mRNA used in the assays was performed by CureVac as recently described.30,41
Statistics. For statistical analyses, we used Jmp (ver 7). Survival data were based on information given by patients, family members, or the family doctor. For survival analyses, we used the Kaplan–Meier method including evaluation of median and mean survival. To compare survival functions, we used a log-rank test.
SUPPLEMENTARY MATERIAL Figure S1. Scatter plots of ELISpots. a-f: Complete ELISpot data presented as scatter plots for each HLA-A2 binding peptide (a: MUC 1.1, b: MUC 1.2, c: Survivin 1, d: Survivin 2, e: CEA, f: MAGE) derived from TAA used in the vaccine. Each dot represents the number of ELISpot INF-γ spots from samples obtained either pre-vaccination or after one and more applications for a single patient. Means and standard error bars are presented in the figure. g: Analysis of vaccine-induced CD4+ T lymphocyte responses. Complete ELISpot data presented as scatter plots. Stimulation was performed using autologous DC electroporated with a mRNA-Mix containing all TAA used in the vaccine. Each dot represents the number of ELISpot INF-γ spots from samples obtained either pre-vaccination or after one and more applications for a single patient. Means and standard error bars are presented in the figure. Figure S2. ELISpot data. In order to give the reader an idea of the frequency of immune responses we provided original ELISpot data for all measured patients. a: Analysis of vaccine-induced expansion of T cells specific for the used TAA-RNA. b: Analysis of vaccine-induced CD4+ T lymphocyte responses. Table S1. Immunological assays.
Acknowledgments
Potential conflicts of interest: I.H.: Employment and Leadership Position at CureVac GmbH, Role Held: Managing Director, Stock Ownership: CureVac GmbH. We thank Sylvia Stephan (1), Bruni Schuster (1), Solveig Daecke (1), Renate Dreher (1) and Corinna Jaeth (1) for excellent technical assistance. S.M.R. was supported by the European Social Fund in Baden-Württemberg. The work was performed in Tuebingen, Germany.
Supplementary Materials
Scatter plots of ELISpots. a-f: Complete ELISpot data presented as scatter plots for each HLA-A2 binding peptide (a: MUC 1.1, b: MUC 1.2, c: Survivin 1, d: Survivin 2, e: CEA, f: MAGE) derived from TAA used in the vaccine. Each dot represents the number of ELISpot INF-γ spots from samples obtained either pre-vaccination or after one and more applications for a single patient. Means and standard error bars are presented in the figure. g: Analysis of vaccine-induced CD4+ T lymphocyte responses. Complete ELISpot data presented as scatter plots. Stimulation was performed using autologous DC electroporated with a mRNA-Mix containing all TAA used in the vaccine. Each dot represents the number of ELISpot INF-γ spots from samples obtained either pre-vaccination or after one and more applications for a single patient. Means and standard error bars are presented in the figure.
ELISpot data. In order to give the reader an idea of the frequency of immune responses we provided original ELISpot data for all measured patients. a: Analysis of vaccine-induced expansion of T cells specific for the used TAA-RNA. b: Analysis of vaccine-induced CD4+ T lymphocyte responses.
Immunological assays.
REFERENCES
- Motzer RJ, Bander NH., and, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- Belldegrun A, Muul LM., and, Rosenberg SA. Interleukin 2 expanded tumor-infiltrating lymphocytes in human renal cell cancer: isolation, characterization, and antitumor activity. Cancer Res. 1988;48:206–214. [PubMed] [Google Scholar]
- Thurnher M, Radmayr C, Ramoner R, Ebner S, Böck G, Klocker H, et al. Human renal-cell carcinoma tissue contains dendritic cells. Int J Cancer. 1996;68:1–7. doi: 10.1002/(SICI)1097-0215(19960927)68:1<1::AID-IJC1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Michael A., and, Pandha HS. Renal-cell carcinoma: tumour markers, T-cell epitopes, and potential for new therapies. Lancet Oncol. 2003;4:215–223. doi: 10.1016/s1470-2045(03)01044-1. [DOI] [PubMed] [Google Scholar]
- Renkvist N, Castelli C, Robbins PF., and, Parmiani G. A listing of human tumor antigens recognized by T cells. Cancer Immunol Immunother. 2001;50:3–15. doi: 10.1007/s002620000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltl L, Zelle-Rieser C, Gander H, Papesh C, Ramoner R, Bartsch G, et al. Immunotherapy of metastatic renal cell carcinoma with tumor lysate-pulsed autologous dendritic cells. Clin Cancer Res. 2002;8:3369–3376. [PubMed] [Google Scholar]
- Su Z, Dannull J, Heiser A, Yancey D, Pruitt S, Madden J, et al. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res. 2003;63:2127–2133. [PubMed] [Google Scholar]
- Wierecky J, Müller MR, Wirths S, Halder-Oehler E, Dörfel D, Schmidt SM, et al. Immunologic and clinical responses after vaccinations with peptide-pulsed dendritic cells in metastatic renal cancer patients. Cancer Res. 2006;66:5910–5918. doi: 10.1158/0008-5472.CAN-05-3905. [DOI] [PubMed] [Google Scholar]
- Wierecky J, Mueller M., and, Brossart P. Dendritic cell-based cancer immunotherapy targeting MUC-1. Cancer Immunol Immunother. 2006;55:63–67. doi: 10.1007/s00262-005-0673-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhartsberger N, Ramoner R, Putz T, Gander H, Rahm A, Falkensammer C, et al. Antigen-independent immune responses after dendritic cell vaccination. Cancer Immunol Immunother. 2007;56:897–903. doi: 10.1007/s00262-006-0245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltl L, Ramoner R, Zelle-Rieser C, Gander H, Putz T, Papesh C, et al. Allogeneic dendritic cell vaccination against metastatic renal cell carcinoma with or without cyclophosphamide. Cancer Immunol Immunother. 2005;54:663–670. doi: 10.1007/s00262-004-0629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Märten A, Renoth S, Heinicke T, Albers P, Pauli A, Mey U, et al. Allogeneic dendritic cells fused with tumor cells: preclinical results and outcome of a clinical phase I/II trial in patients with metastatic renal cell carcinoma. Hum Gene Ther. 2003;14:483–494. doi: 10.1089/104303403321467243. [DOI] [PubMed] [Google Scholar]
- Avigan D, Vasir B, Gong J, Borges V, Wu Z, Uhl L, et al. Fusion cell vaccination of patients with metastatic breast and renal cancer induces immunological and clinical responses. Clin Cancer Res. 2004;10:4699–4708. doi: 10.1158/1078-0432.CCR-04-0347. [DOI] [PubMed] [Google Scholar]
- Dörfel D, Appel S, Grünebach F, Weck MM, Müller MR, Heine A, et al. Processing and presentation of HLA class I and II epitopes by dendritic cells after transfection with in vitro-transcribed MUC1 RNA. Blood. 2005;105:3199–3205. doi: 10.1182/blood-2004-09-3556. [DOI] [PubMed] [Google Scholar]
- Grünebach F, Müller MR, Nencioni A., and, Brossart P. Delivery of tumor-derived RNA for the induction of cytotoxic T-lymphocytes. Gene Ther. 2003;10:367–374. doi: 10.1038/sj.gt.3301901. [DOI] [PubMed] [Google Scholar]
- Milazzo C, Reichardt VL, Müller MR, Grünebach F., and, Brossart P. Induction of myeloma-specific cytotoxic T cells using dendritic cells transfected with tumor-derived RNA. Blood. 2003;101:977–982. doi: 10.1182/blood-2002-04-1273. [DOI] [PubMed] [Google Scholar]
- Schaft N, Dorrie J, Thumann P, Beck VE, Muller I, Schultz ES, Kampgen E, et al. Generation of an optimized polyvalent monocyte-derived dendritic cell vaccine by transfecting defined RNAs after rather than before maturation. J Immunol. 2005;174:3087. doi: 10.4049/jimmunol.174.5.3087. [DOI] [PubMed] [Google Scholar]
- Melhem NM, Liu XD, Boczkowski D, Gilboa E., and, Barratt-Boyes SM. Robust CD4+ and CD8+ T cell responses to SIV using mRNA-transfected DC expressing autologous viral Ag. Eur J Immunol. 2007;37:2164–2173. doi: 10.1002/eji.200636782. [DOI] [PubMed] [Google Scholar]
- Nair S, Boczkowski D, Fassnacht M, Pisetsky D., and, Gilboa E. Vaccination against the forkhead family transcription factor Foxp3 enhances tumor immunity. Cancer Res. 2007;67:371–380. doi: 10.1158/0008-5472.CAN-06-2903. [DOI] [PubMed] [Google Scholar]
- Schuler G. Dendritic cell-based vaccination against cancer. J Gene Med. 2003. pp. S28–S29..
- Thumann P, Moc I, Humrich J, Berger TG, Schultz ES, Schuler G, et al. Antigen loading of dendritic cells with whole tumor cell preparations. J Immunol Methods. 2003;277:1–16. doi: 10.1016/s0022-1759(03)00102-9. [DOI] [PubMed] [Google Scholar]
- Grünebach F, Kayser K, Weck MM, Müller MR, Appel S., and, Brossart P. Cotransfection of dendritic cells with RNA coding for HER-2/neu and 4-1BBL increases the induction of tumor antigen specific cytotoxic T lymphocytes. Cancer Gene Ther. 2005;12:749–756. doi: 10.1038/sj.cgt.7700842. [DOI] [PubMed] [Google Scholar]
- Heine A, Grünebach F, Holderried T, Appel S, Weck MM, Dörfel D, et al. Transfection of dendritic cells with in vitro-transcribed CMV RNA induces polyclonal CD8+- and CD4+-mediated CMV-specific T cell responses. Mol Ther. 2006;13:280–288. doi: 10.1016/j.ymthe.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Müller MR, Tsakou G, Grünebach F, Schmidt SM., and, Brossart P. Induction of chronic lymphocytic leukemia (CLL)-specific CD4- and CD8-mediated T-cell responses using RNA-transfected dendritic cells. Blood. 2004;103:1763–1769. doi: 10.1182/blood-2003-06-2097. [DOI] [PubMed] [Google Scholar]
- Nencioni A, Müller MR, Grünebach F, Garuti A, Mingari MC, Patrone F, et al. Dendritic cells transfected with tumor RNA for the induction of antitumor CTL in colorectal cancer. Cancer Gene Ther. 2003;10:209–214. doi: 10.1038/sj.cgt.7700557. [DOI] [PubMed] [Google Scholar]
- Probst J, Weide B, Scheel B, Pichler BJ, Hoerr I, Rammensee HG, et al. Spontaneous cellular uptake of exogenous messenger RNA in vivo is nucleic acid-specific, saturable and ion dependent. Gene Ther. 2007;14:1175–1180. doi: 10.1038/sj.gt.3302964. [DOI] [PubMed] [Google Scholar]
- Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247 4949 Pt 1:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Pascolo S. Messenger RNA-based vaccines. Expert Opin Biol Ther. 2004;4:1285–1294. doi: 10.1517/14712598.4.8.1285. [DOI] [PubMed] [Google Scholar]
- Hoerr I, Obst R, Rammensee HG., and, Jung G. In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. Eur J Immunol. 2000;30:1–7. doi: 10.1002/1521-4141(200001)30:1<1::AID-IMMU1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Carralot JP, Probst J, Hoerr I, Scheel B, Teufel R, Jung G, et al. Polarization of immunity induced by direct injection of naked sequence-stabilized mRNA vaccines. Cell Mol Life Sci. 2004;61:2418–2424. doi: 10.1007/s00018-004-4255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conry RM, LoBuglio AF, Wright M, Sumerel L, Pike MJ, Johanning F, et al. Characterization of a messenger RNA polynucleotide vaccine vector. Cancer Res. 1995;55:1397–1400. [PubMed] [Google Scholar]
- Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A., and, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- Weide B, Carralot JP, Reese A, Scheel B, Eigentler TK, Hoerr I, et al. Results of the first phase I/II clinical vaccination trial with direct injection of mRNA. J Immunother. 2008;31:180–188. doi: 10.1097/CJI.0b013e31815ce501. [DOI] [PubMed] [Google Scholar]
- Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L., and, Brugger W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000;96:3102–3108. [PubMed] [Google Scholar]
- Wierecky J, Muller MR, Hantschel M, Horger MS, Pascolo S, Brugger W, et al. 2005Intradermal RNA-vaccination of patients with metastatic renal cell carcinoma Blood51B.
- Brossart P, Heinrich KS, Stuhler G, Behnke L, Reichardt VL, Stevanovic S, et al. Identification of HLA-A2-restricted T-cell epitopes derived from the MUC1 tumor antigen for broadly applicable vaccine therapies. Blood. 1999;93:4309–4317. [PubMed] [Google Scholar]
- Brauer KM, Werth D, von Schwarzenberg K, Bringmann A, Kanz L, Grünebach F, et al. BCR-ABL activity is critical for the immunogenicity of chronic myelogenous leukemia cells. Cancer Res. 2007;67:5489–5497. doi: 10.1158/0008-5472.CAN-07-0302. [DOI] [PubMed] [Google Scholar]
- Schmidt SM, Schag K, Müller MR, Weinschenk T, Appel S, Schoor O, et al. Induction of adipophilin-specific cytotoxic T lymphocytes using a novel HLA-A2-binding peptide that mediates tumor cell lysis. Cancer Res. 2004;64:1164–1170. doi: 10.1158/0008-5472.can-03-2538. [DOI] [PubMed] [Google Scholar]
- von Schwarzenberg K, Held SA, Schaub A, Brauer KM, Bringmann A., and, Brossart P. Proteasome inhibition overcomes the resistance of renal cell carcinoma cells against the PPARgamma ligand troglitazone. Cell Mol Life Sci. 2009;66:1295–1308. doi: 10.1007/s00018-009-8542-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SM, Schag K, Müller MR, Weck MM, Appel S, Kanz L, et al. Survivin is a shared tumor-associated antigen expressed in a broad variety of malignancies and recognized by specific cytotoxic T cells. Blood. 2003;102:571–576. doi: 10.1182/blood-2002-08-2554. [DOI] [PubMed] [Google Scholar]
- Carralot JP, Weide B, Schoor O, Probst J, Scheel B, Teufel R, et al. Production and characterization of amplified tumor-derived cRNA libraries to be used as vaccines against metastatic melanomas. Genet Vaccines Ther. 2005;3:6. doi: 10.1186/1479-0556-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatter plots of ELISpots. a-f: Complete ELISpot data presented as scatter plots for each HLA-A2 binding peptide (a: MUC 1.1, b: MUC 1.2, c: Survivin 1, d: Survivin 2, e: CEA, f: MAGE) derived from TAA used in the vaccine. Each dot represents the number of ELISpot INF-γ spots from samples obtained either pre-vaccination or after one and more applications for a single patient. Means and standard error bars are presented in the figure. g: Analysis of vaccine-induced CD4+ T lymphocyte responses. Complete ELISpot data presented as scatter plots. Stimulation was performed using autologous DC electroporated with a mRNA-Mix containing all TAA used in the vaccine. Each dot represents the number of ELISpot INF-γ spots from samples obtained either pre-vaccination or after one and more applications for a single patient. Means and standard error bars are presented in the figure.
ELISpot data. In order to give the reader an idea of the frequency of immune responses we provided original ELISpot data for all measured patients. a: Analysis of vaccine-induced expansion of T cells specific for the used TAA-RNA. b: Analysis of vaccine-induced CD4+ T lymphocyte responses.
Immunological assays.