Abstract
Gene therapy approaches to enhance endothelial progenitor cell (EPC) homing may augment cell engraftment to ischemic tissue and lead to a greater therapeutic response. Therefore, we assessed the effects of ultrasound-mediated (UM) transfection of the chemokine stromal cell–derived factor-1 (SDF-1) on homing and engraftment of intravenously administered EPCs and the subsequent angiogenic response in chronically ischemic skeletal muscle. Bone marrow–derived EPCs were isolated from donor Fisher 344 rats, cultured and labeled in preparation for injection into recipient animals via a jugular vein. Using a model of chronic hindlimb ischemia in rats, we demonstrated that UM destruction of intravenous carrier microbubbles loaded with SDF-1 plasmid DNA resulted in targeted transfection of the vascular endothelium within ischemic muscle and greater local engraftment of EPCs. The combination of SDF-1gene therapy and EPCs lead to the greatest increase in tissue perfusion and microvascular density within ischemic muscle, compared to no treatment or either monotherapy alone. Our results demonstrate that UM transfection of SDF-1 improves EPC targeting to chronically ischemic tissue, enhancing vascular engraftment and leading to a more robust neovascularization response.
Introduction
Circulating endothelial progenitor cells (EPCs) originating from the bone marrow have been shown to home to sites of tissue injury, facilitating endogenous tissue repair, and vascular regeneration.1 Once differentiated, ex vivo expanded EPCs have been reported to integrate into blood vessels and induce neovascularization of ischemic hindlimbs and hearts in animal models of ischemia and infarction.2 Although several clinical studies have now demonstrated the therapeutic potential of exogenously administered EPCs to improve left ventricular ejection fraction after acute myocardial infarction (MI),3,4 other studies have yielded negative results.5,6 Given that patients at the highest cardiovascular risk have the lowest number and poorest migratory and homing capacity of endogenous EPCs, the use of autologous EPCs for neovascularization in the clinical setting may prove less effective.7 In this setting, the ability to increase the homing of exogenously administered EPCs to specific target sites may potentially improve the angiogenic response to cell therapy.
Several factors have been shown to influence EPC mobilization and homing to ischemic tissue, including chemokines,8 angiogenic cytokines,9 and pharmacologic agents.10 Stromal cell–derived factor-1 (SDF-1) is one such chemokine considered to play an important role in progenitor cell homing and recruitment for ischemic neovascularization.8,11 We have previously demonstrated that ultrasound-mediated (UM) destruction of carrier microbubbles bearing vascular endothelial growth factor (VEGF) plasmid DNA results in improved perfusion within chronically ischemic skeletal muscle.12 Furthermore, this delivery strategy may be more effective than direct intramuscular injections, resulting in directed vascular transfection over a wider distribution, leading to a more efficient angiogenic response.13 In this study, we hypothesized that UM delivery of microbubbles bearing genes encoding for human SDF-1 would result in localized transfection of the vascular endothelium and surrounding myocytes, and facilitate maximal homing and engraftment of intravenously delivered EPCs, thus acting synergistically to promote neovascularization of chronically ischemic muscle.
Results
EPC characterization and functional analysis
We first characterized the bone marrow–derived EPCs used in our experiments, and confirmed their functionality after labeling. After 10 days in culture, EPCs developed an endothelial-like phenotype, expressing endothelial cell surface markers UEA-1, VEGFR-II and the receptor for SDF-1, CXCR4 (Supplementary Figure S1a), with no change in cell phenotype after labeling with the fluorophore chloromethyl trimethyl rhodamine (CMTMR) (Supplementary Figure S1b,c). After CMTMR labeling, cell loss was minimal, with ~90% cell viability. CMTMR labeling did not affect EPC function in vitro, with similar migratory capacity in response to both SDF-1 and VEGF and angiogenic potential by matrigel tubule formation assay (Supplementary Figure S1d,e).
Time course of EPC circulation
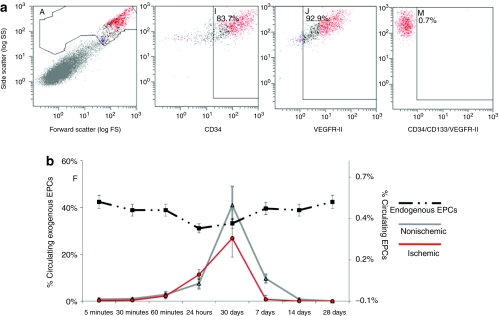
Fluorescence-activated cell sorting (FACS) analysis was used to track CMTMR-labeled EPCs after intravenous injection, to determine the time of maximum concentration within the circulation. Figure 1 shows the percentage of injected cells that are circulating at each time point in control nonligated animals, and in animals after induction of chronic ischemia (see Animal preparation). Immediately after intravenous injection (within 30 minutes), very few circulating EPCs were detected. Histological analysis immediately postinjection demonstrates that the majority of the cells lodge in the lungs. After 24 hours, the numbers of cells found in the systemic circulation begin to rise, with a clear peak in exogenous circulating EPCs 3 days postinjection. The presence of chronic ischemia lead to minimal changes in the time course of EPC circulation, with lower circulating numbers at day 3, perhaps related to greater trafficking of EPCs from the circulation into the ischemic muscle. In comparison, there were little changes in % circulating endogenous EPCs over time (Figure 1b).
Figure 1.
In vivo exogenous and endogenous endothelial progenitor cell (EPC) tracking by fluorescence-activated cell sorting (FACS) analysis in control and ischemic animals. (a) FACS plots showing the cell population and marker positivity. (b) Data (mean ± SD) on in vivo exogenous and endogenous EPC tracking by FACS. Peak exogenous EPC circulation (solid lines—left axis) occurred between 1 and 7 days with maximal circulation at day 3 in both control and ischemic groups. The dashed line—right axis represents endogenous EPCs. Endogenous EPC levels remained stable throughout the time course of the study.
SDF-1 gene transfection efficacy and localization
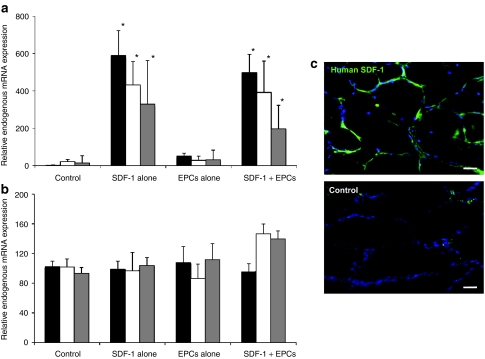
Quantitative real-time reverse transcriptase (RT)-PCR data for exogenous SDF-1 mRNA from ischemic muscle at various time points postdelivery in SDF-1 gene treatment groups is shown in Figure 2a. Using specific primers, exogenous transgene expression (normalized to the contralateral nonischemic muscle) was detected in both SDF-1–treated and SDF-1 + EPC treated ischemic muscles, being similar for both groups. For both groups, transfection was greatest at day 3 postdelivery, decreasing over time. The SDF-1 transgene was not detectable in control or EPC alone treated muscle. Endogenous SDF-1 mRNA expression is shown in Figure 2b, showing no significant changes in the groups over time.
Figure 2.
Data (mean ± SD) on stromal cell–derived factor-1 (SDF-1) transfection efficacy and localization in hindlimb skeletal muscle after ultrasound-mediated transfection, and effects on endogenous SDF-1 expression. Black bars—day 3, white bars—day 7, gray bars—day 14. (a) Exogenous (human) SDF-1 expression by reverse transcriptase (RT)-PCR in the various groups at all time points (*P < 0.05 versus both control and endothelial progenitor cells (EPCs) alone). Maximal transgene expression can be seen at day 3 in the groups receiving SDF-1 gene therapy. (b) Endogenous (rat) SDF-1 expression by RT-PCR in the various groups at all time points. There is no difference in endogenous SDF-1 expression along all groups and time points. (c) Immunostaining for exogenous human SDF-1. In SDF-1–treated muscle, positive SDF-1 staining (green) is present in the vascular endothelium of capillaries and arterioles, with some staining also evident in the myocytes. No discernable staining is present in control untreated muscle. Bar = 50 µm. Blue—TO-PRO-3 (nuclear counterstain), green—human SDF-1.
Immunohistochemical analysis for exogenous human SDF-1 expression was performed 3 days postdelivery (Figure 2c). There was a strong positive signal (green staining) in the SDF-1 delivered ischemic muscle, with little to no staining in controls. The majority of SDF-1 immunostaining was localized to the endothelium of arterioles and capillaries with some staining associated with myocytes.
Time course of EPC engraftment and cell retention
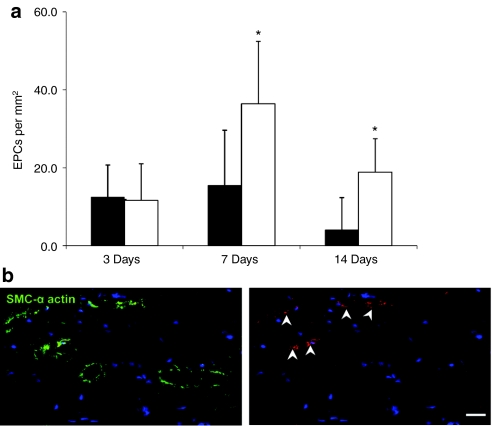
At each of the study time points EPC engraftment was assessed by immunostaining. Sections of the proximal hindlimb adductor muscle were visualized under confocal microscopy and cell engraftment/retention was quantified. EPC retention within the ischemic hindlimb adductor muscle at various time points after delivery was quantified by cell counting. The peak of exogenous retained EPCs occurred at day 7 postinjection in all EPC-treated animals (Figure 3a). Maximal exogenous EPC number was found in the SDF-1 + EPC group with nearly a twofold increase over the EPC alone group (P < 0.01) at both day 7 and day 14. There was no difference in EPC number in the SDF-1 + EPC and EPC alone groups at day 3. Figure 3b illustrates a section of hindlimb adductor muscle stained for smooth muscle α-actin (green), showing several CMTMR-labeled EPCs (red) engrafted within medium-sized arterioles at day 7 after delivery within an SDF-1 + EPC treated animal.
Figure 3.
Data on exogenous endothelial progenitor cell (EPC) engraftment and retention. (a) Quantitative data (mean ± SD) on exogenous chloromethyl trimethyl rhodamine (CMTMR)-labeled EPC engraftment and retention in ischemic muscle from all treatment groups, at multiple time points postdelivery. Data is expressed as number of cells per mm2. Maximal EPC engraftment occurred at 7-day postinjection in both groups, with the highest number of cells seen in the stromal cell–derived factor-1 (SDF-1) + EPC group at both the 7-day and 14-day time points (*P < 0.01 versus EPCs alone). Black bar—EPCs alone, white bar—EPCs + SDF-1. (b) Immunostaining of medium-sized arterioles at day 7 postdelivery from the SDF-1 + EPCs group showing exogenously delivered CMTMR-labeled EPCs (red staining) associated with smooth muscle α-actin stained arterioles (green staining). Bar = 50 µm. Blue = TO-PRO-3 (nuclear stain), green—smooth muscle α-actin, red—CMTMR-labeled exogenous EPCs. Arrow—exogenous EPCs.
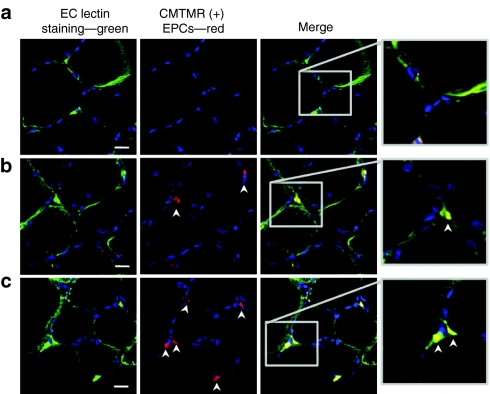
As expected there was no detectable exogenous EPC retention in control ischemic hindlimbs (Figure 4a). In the EPC alone treated group (Figure 4b), there were numerous cells seen associated with capillaries. In the SDF-1 + EPC treated group there were increased numbers of engrafted cells (Figure 4c), predominantly engrafted within small arterioles and capillaries within the ischemic hindlimb muscle.
Figure 4.
Representative images of exogenous endothelial progenitor cell (EPC) engraftment within ischemic muscle by fluorescent microscopy, at 14-day postinjection. (a) No exogenous EPC engraftment in control untreated muscle. (b) EPC engraftment in the EPC alone treated ischemic muscle. (c) A greater number of engrafted EPCs in the stromal cell–derived factor-1 + EPC ischemic muscle with engrafted EPCs associated with the vessel walls of capillaries. Bar = 50 µm. Blue—TO-PRO-3 (nuclear stain), green—endothelial cell (EC)-lectin, red—chloromethyl trimethyl rhodamine (CMTMR)–labeled exogenous EPCs. Single arrow—exogenous EPCs.
Muscle perfusion and vascular density
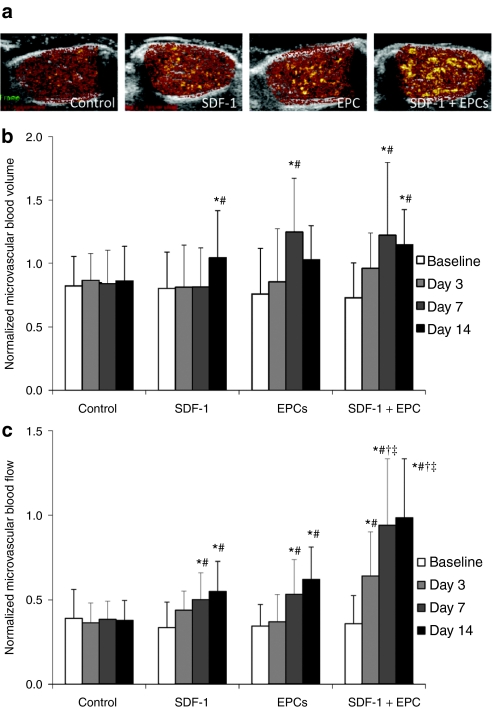
Perfusion within ischemic adductor muscle in all groups was measured using contrast-enhanced ultrasound (CEU). Representative CEU-perfusion images at plateau signal intensity for all treatment groups prior to and after gene delivery are shown in Figure 5. At 2 weeks post-iliac artery ligation, immediately prior to gene delivery, microvascular blood volume (MBV) was reduced to ~75–80% and microvascular blood flow (MBF) to ~30–35% in the ischemic leg normalized to the contralateral normal leg in all four groups. In the control group there was no change in normalized MBV and MBF over time (Figure 5). After treatment with SDF-1 gene therapy or intravenous EPCs alone, there were significant increases in normalized MBV and MBF that began at day 7 postdelivery and extending to day 14. In comparison, the improvement in MBF was significantly greater with combined SDF-1 gene therapy coupled with intravenous EPC administration, as compared to either monotherapy alone (P < 0.005 versus SDF-1 alone, and P < 0.01 versus EPCs alone, at days 7 and 14 after delivery) (Figure 5). Increases in MBV and MBF also occurred at an earlier time point, day 3, compared to other treatment groups.
Figure 5.
Proximal hindlimb contrast-enhanced ultrasound (CEU) perfusion data in all treatment groups. (a) Representative color-coded CEU-perfusion images of ischemic hindlimb muscle blood flow from all groups at 14-day postdelivery. CEU signal from microbubbles was greatest for combined stromal cell–derived factor-1 (SDF-1) + endothelial progenitor cell (EPC)-treated ischemic muscle. (b) Microvascular blood volume (mean ± SD) in the ischemic muscle, normalized to contralateral nonischemic muscle for the four treatment groups; at baseline (pretreatment) and at 3, 7, and 14 days postdelivery. *P < 0.01 compared to corresponding data in control untreated animals, #P < 0.005 compared to corresponding baseline (predelivery) data. (c) Microvascular blood flow (mean ± SD) in the ischemic muscle, normalized to contralateral nonischemic muscle for the four treatment groups; at baseline (pretreatment) and at 3, 7, and 14 days postdelivery. *P <0.005 compared to corresponding data in control untreated animals, #P < 0.005 compared to corresponding baseline (predelivery) data, †P < 0.005 compared to corresponding data in SDF-1–treated animals, ‡P < 0.01 compared to corresponding data in EPC-treated animals.
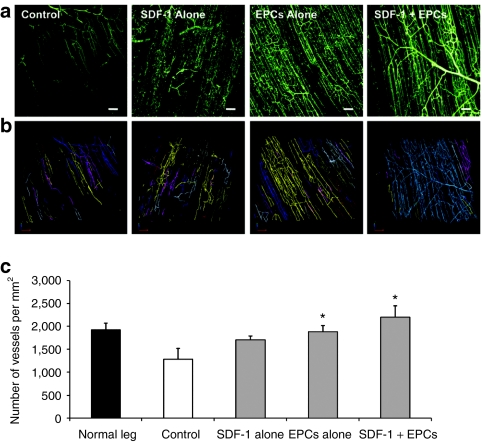
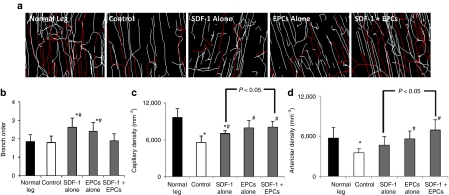
Fluorescent microangiography (FMA) analysis revealed reduced vessel density in the control group's ischemic proximal hindlimb adductor muscle 4 weeks post-iliac artery ligation. Consistent with CEU-perfusion data, at 14 days after gene delivery there were increases in vessel density in all treatment groups with the greatest increases seen with combination SDF-1 gene therapy plus intravenous EPCs (Figure 6). Further FMA analysis was performed using branch ordering to distinguish changes in capillary density versus arteriolar density after therapies (Figure 7). Both SDF-1 alone and EPC alone treated muscle showed increases in branch order ratio, indicating a higher relative density of capillaries versus arterioles. In comparison, the combined SDF-1 + EPC group showed a restoration to normal branch order ratio (Figure 7b). While both SDF-1 + EPC and EPC alone treated ischemic muscle showed significant increases in capillary density versus control nontreated muscle (Figure 7c), the combined SDF-1 + EPC treated animals were the only group that demonstrated a significantly increased arteriole density at day 14 postdelivery.
Figure 6.
Data on proximal hindlimb vascular density in all treatment groups. (a) Representative stacked fluorescent microangiographic (FMA) images of microvessels in ischemic hindlimb muscle after no treatment (control), ultrasound-mediated delivery of stromal cell–derived factor-1 (SDF-1) alone, exogenous endothelial progenitor cells (EPCs) administration and a combination of SDF-1 gene delivery and exogenous EPC delivery. Bar = 50 µm. (b) Computerized rendering of the above image stacks, from which vascular density is quantified. Note the greater vascular density in the SDF-1 + EPC group versus all other groups. (c) Quantitative microvessel density by FMA (mean ± SD) of ischemic hindlimb muscle at day 14 postdelivery, in all treatment groups. At 14-day postdelivery, FMA revealed a significant increase in the density of microvessels in the ischemic leg of both EPC delivery groups, with maximal vessel density occurring in the SDF-1 + EPC group. *P < 0.05 compared to corresponding data from control ischemic muscle.
Figure 7.
Data (mean ± SD) on branch order, capillary and arteriolar density of ischemic hindlimb muscle at day 14 postdelivery from all groups, by fluorescent microangiography and 3D branch order analysis using the Strahler Method. (a) Representative color-coded images of arteriolar and capillary density from hindlimb skeletal muscle in normal rats, and ischemic rats from all four groups, at day 14 postdelivery. Red—branch order 1–3 (arterioles), white—branch order 0 (capillaries). (b) Both stromal cell–derived factor-1 (SDF-1) and endothelial progenitor cells (EPCs) alone groups had increases in branch order ratio, indicating a relatively higher density of capillaries:arterioles. The combined SDF-1 + EPC treated ischemic muscles showed restoration to normal branch order. (c) The combined SDF-1 + EPC and EPC alone treated ischemic muscles showed significant increases in capillary density versus control, (d) while the combined EPC +SDF-1 group was the only treatment group that demonstrated a significantly increased arteriolar density compared to control untreated animals. *P < 0.05 versus normal leg, #P < 0.05 versus control.
Discussion
While the success of progenitor cell therapy relies on the ability of cells to repair damaged tissue, it is also critically dependent on their homing, migration and retention to sites of injury, regardless of mode of delivery. The novel finding of this study is that a noninvasive gene- and cell-based therapeutic approach, using vascular gene transfer of SDF-1 by UM destruction of plasmid bearing microbubbles to augment homing and engraftment of exogenously administered EPCs, leads to a greater angiogenic response as compared to SDF-1 gene therapy or intravenous EPCs alone.
The physiologic and pathologic factors that influence EPC homing to ischemic tissue remain complex and poorly understood, with a number of potential factors postulated.14 SDF-1 is a chemokine considered to play an important role in hematopoietic stem cell trafficking,11 and has been shown to play a critical role in stem cell recruitment for ischemic neovascularization. Several studies have investigated the therapeutic role of SDF-1 for endogenous EPC recruitment and repair. In a mouse model of coronary ligation, gene delivery of SDF-1 using an adenoviral vector after acute MI more than doubled endogenous bone marrow–derived EPC recruitment into ischemic myocardium.8 Similarly, gene transfer of SDF-1 promotes EPC mobilization and homing for ischemic neovascularization via VEGF/endothelial nitric oxide synthase–related pathways.15 Finally, SDF-1 protein administration into wounds reversed the nitric oxide–mediated EPC homing impairment in diabetes, enhancing wound healing.16 This study suggests that SDF-1 could potentially be used to overcome the functional impairment of EPCs in disease states that may limit their therapeutic applications.7 In the only study that examined the effects of SDF-1 on the therapeutic potential of exogenously administered EPCs, Yamaguchi et al.17 performed intramuscular injections of SDF-1 protein combined with intravenous human EPC transplantation into the acutely ischemic hindlimb muscle of athymic nude mice. They found an increased local accumulation of fluorescence-labeled EPCs within ischemic muscle in the SDF-1 treatment group, which was associated with an increase in tissue perfusion and capillary density. We have now extended this concept, demonstrating that a combined gene- and cell-based therapy approach, using SDF-1 gene delivery in conjunction with intravenous EPC administration, results in an enhanced therapeutic neovascularization in the setting of chronic ischemia, compared to either therapy alone.
The basal incorporation rate of exogenous EPCs into normal tissue, in the absence of injury, is extremely low.18 EPC engraftment is enhanced in the setting of tissue injury or ischemia, with engraftment being greater in the setting of acute as compared to chronic ischemia.19 Thus, strategies to enhance homing and engraftment would be valuable for improving the therapeutic effect of EPC-based therapies, particularly in the setting of chronic ischemia, as in our study. In the majority of studies to date, exogenous EPC delivery has been via local administration, mainly intramuscular and intracoronary injections. These relatively invasive techniques make them less suitable for repeated administrations. The ability to use an intravenous administration of EPCs would be an attractive, noninvasive technique to deliver EPCs. However, studies have shown a lack of targeting with this method, with the absolute level of radiolabeled EPCs homing to the normal heart being ~1% after intravenous injection.20 This increases approximately twofold to 2% after acute MI, a strong stimulus for homing and engraftment. When EPCs are injected intra-arterially, however, the engraftment rate is significantly higher, ~4.7-fold, after acute MI.20 Data from human studies have confirmed these findings. After intracoronary administration of radiolabeled EPCs, the average activity within the first 24 hours was highest among patients with acute MI, and progressively decreasing with time from infarction, with lowest activity after intracoronary injection seen in patients with chronic (>1 year post) MI. This marked difference in EPC engraftment and retention in the setting of acute versus chronic ischemia is in keeping with studies demonstrating that the chemotactic factors VEGF and SDF-1 are downregulated in chronic ischemia as opposed to upregulated in more acute ischemia.21 Thus, a method to increase targeting and engraftment of intravenously delivered EPCs, specifically in the setting of chronic ischemia or infarction where endogenous homing signals are low, would be an important first step in developing a noninvasive method of EPC delivery for therapeutic angiogenesis.
An important aspect of our study was the determination of the time course of systemic EPC circulation after an intravenous injection in normal and chronically ischemic animals, which was not examined in the prior study of combined SDF-1 protein and EPC delivery.17 Using FACS, we were able to track our CMTMR-labeled EPCs, showing that immediately after injection into the jugular vein there were very few detectable exogenous EPCs in the peripheral circulation, due predominantly to lodging and retention within the lungs.22 After initial pulmonary retention, exogenous EPCs slowly reappeared in the peripheral circulation, beginning at 24 hours, and peaking at day 3 postinjection, eventually tapering off by day 14. In the presence of chronic ischemia, the time course of EPC circulation remained unchanged. We subsequently designed our delivery protocols to allow for peak SDF-1 gene transfection to coincide with maximal systemic circulation of our exogenously administered EPCs. We have previously shown that maximal gene transfection after UM microbubble destruction occurs at day 3 postdelivery.12,13 This allowed us to administer intravenous EPCs simultaneously with UM SDF-1 plasmid DNA delivery. Gene transfer strategies yield a longer duration of therapeutic action than localized protein delivery, as noted in our study where SDF-1 transgene expression was detectable out to 14 days postdelivery, thus providing a continuous homing signal from our targeted ischemic tissue for the full duration of EPC circulation after intravenous administration. Another advantage of our technique is the specific site of targeted gene delivery. As compared to direct intramuscular injections, UM gene delivery results in directed vascular transfection over a wider distribution, leading to a more effective gene delivery strategy.13 Similar to our previous studies,12,13 SDF-1 gene transfection by UM destruction of DNA-bearing microbubbles was seen to localize to the vascular endothelium and surrounding myocytes, where augmentation of signaling for circulating EPC homing and transmigration may be fully maximized. In our study, the vascular gene transfer of SDF-1 more than doubled exogenous EPC engraftment into the vasculature of ischemic hindlimb muscle out to 14 days after delivery, resulting in an enhanced angiogenic response, a greater density of neovessels and increases in both arteriolar and capillary density while preserving normal branch order ratios. Increases in tissue perfusion were evident as early as 3 days postdelivery in our combined SDF-1 plus EPC treated animals as compared to EPC treatment alone, despite similar numbers of engrafted EPCs at that early time point. This finding is in keeping with data suggesting that activation of the SDF-1/CXCR4 axis enhances collateral flow, at least in part, via direct effects on vascular endothelial cells.23
Our study has several important limitations. While we were able to follow the engraftment of our exogenous CMTMR-labeled EPCs, we were unable to track endogenous EPCs that may also home to sites of SDF-1 gene transfer. Thus we cannot determine the relative effects of exogenous versus endogenous EPC engraftment on the neovascularization response to SDF-1 gene therapy. Regardless, the addition of exogenous EPCs to SDF-1 gene therapy resulted in a further increment over SDF-1 therapy alone, suggesting an important contribution by cell transplantation. We do not have any data on long term EPC engraftment, beyond 14 days after delivery. We did not study the effect of microbubble destruction alone on progenitor cell recruitment, as has been previously studied.24 In our prior study of UM gene delivery, destruction of microbubbles bearing control plasmid DNA did not result in upregulation of endogenous angiogenic genes or restoration in hindlimb perfusion as compared to no treatment, suggesting a lack of a significant biologic effect.12
In summary, UM transfection of SDF-1 improves intravenous EPC targeting to ischemic tissue, enhancing vascular EPC engraftment into chronically ischemic tissue and leading to greater neovascularization response. Strategies for combined gene- and EPC-based therapies may be more effective than either therapy alone, for promoting angiogenesis and improving perfusion to chronically ischemic tissue.
Materials and Methods
Animal preparation. The study protocol was approved by the Animal Care and Use Committee at St Michael's Hospital Research Centre, University of Toronto (Toronto, Ontario, Canada). Proximal hindlimb adductor muscle ischemia was produced in 126 anesthetized Fisher F344 rats. Under sterile conditions, the left common iliac artery and small proximal branches were exposed and ligated with 4–0 silk sutures. The incision was closed in layers and animals were recovered. In this model, while flow is immediately reduced to ~25% normal, endogenous angiogenesis occurs over the subsequent 2 weeks, after which perfusion remains chronically reduced in the proximal adductor muscles at ~40–50% of normal,12,13,25 without limb necrosis or auto-amputation.
Cell preparation and labeling. EPCs used for all our experiments were isolated from the tibias and femurs of 3–5-week-old syngeneic Fisher 344 rats (Charles River Laboratories, Sherbrooke, Quebec, Canada). The aspirated marrow was centrifuged and bone marrow cells plated on fibronectin coated flasks at a density of >1 × 106 cells/ml and grown in supplemented endothelial cell basal medium 2 (Cedarlane Laboratories, Burlington, Ontario, Canada) for 10 days, to promote differentiation into an endothelial phenotype.26 EPCs were labeled using the viable fluorophore CMTMR (Invitrogen, Burlington, Ontario, Canada), which provides an accurate method of detecting ex vivo–labeled cells out to several months.22 The cells were allowed to recover for 24 hours after labeling, before being injected (see Supplementary Materials and Methods for further details).
EPC characterization and functional analysis. Unlabeled and CMTMR-labeled EPCs were stained for the presence of mature endothelial markers, endothelial specific lectin, UEA-1 (1:200) (Sigma, Oakville, Ontario, Canada), VEGFR-II (1:50) (R&D Systems, Minneapolis, MN) and CXCR4 (1:150) (abcam, Cambridge, MA). Percent marker positivity was determined using FACS analysis. In vitro EPC function was assessed by standard Boyden Migration and Matrigel Tubule formation assays27 (see Supplementary Materials and Methods for further details).
Time course of exogenous EPC circulation. The time course of circulating exogenous EPCs after an intravenous injection was studied in 12 rats, six control nonligated rats, and six rats at 2 weeks after induction of hindlimb ischemia by left iliac artery ligation. CMTMR-labeled EPCs (1 × 106 in 1 ml sterile saline) were injected via the right jugular vein over 1 minute. Blood samples (100 µl) were serially taken at 5, 30, and 60 minutes for short-term tracking. For longer term tracking 100 µl samples were taken at 1, 3, 7, 14, and 28 days postinjection. Whole blood samples were lysed with BD FACS lysing solution (BD Biosciences, Mississauga, Ontario, Canada), and analyzed by FACS to quantify circulating CMTMR-labeled EPCs, as well as endogenous non-CMTMR-labeled EPCs using specific EPC markers—VEGFR-II, CD34 and CD133 (see Supplementary Materials and Methods for further details).
Microbubble and DNA preparation and assembly. Microbubbles with a cationic lipid shell were created as previously described.12,13 For perfusion imaging, lipid-shelled decafluorobutane microbubbles were used. Microbubble concentrations were determined using a Coulter Multisizer IIe (Beckman-Coulter, Mississauga, Ontario, Canada), prior to intravenous administration (see Supplementary Materials and Methods for further details).
Perfusion imaging. CEU imaging of the proximal hindlimb adductor muscles was performed as previously described,12,13,25,27,28 to obtain perfusion data on MBV, velocity, and MBF12,28 (see Supplementary Materials and Methods for further details).
Gene delivery. For UM gene delivery, ultrasound transmission was performed with a phased array transducer (Sonos 5500, Philips Ultrasound) at 1.3 MHz and a transmit power of 0.9W (120 V, 9 mA), using a pulsing interval of 5 seconds. Cationic microbubbles (1.5 ml; 1 × 109) charge-coupled with 500 µg of human SDF-1 cDNA (InvivoGen, San Diego, CA) was infused over 10 minutes intravenously. To allow a wider field of delivery, ultrasound was transmitted during a slow sweep along the length of the proximal ischemic hindlimb, for a total of 20 minutes12,13 (see Supplementary Materials and Methods for further details).
FMA. FMA, a method of postmortem vascular casting, was performed to quantify vessel density, as previously described.12,13 Capillary and arteriolar density was calculated by branch order analysis based on the Strahler Method,29 using Neurolucida Software package (MBF Bioscience, Williston, VT) (see Supplementary Materials and Methods and Supplementary Figure S2 for further details).
Immunohistochemistry. In vivo EPC engraftment and spatial localization were determined using immunohistochemistry. Cryo-blocks of explanted tissue were sectioned (15 µm thick) every 25 µm and rehydrated, fixed in 2% paraformaldehyde (Sigma) in phosphate-buffered saline, and washed. Cell surface antigens were identified using: mouse anti-human CD31 (Cedarlane Laboratories), rabbit anti-human SDF-1 (Upstate Biotechnology, Waltham, MA), mouse anti-rat α-actin (abcam) and endothelial cell specific lectin (UEA-1) (Sigma). The presence of antibody was confirmed by exposure to a phycoerythrin or fluorescein isothiocyanate conjugated secondary antibody. TO-PRO-3 (Invitrogen) was used as a nuclear marker (see Supplementary Materials and Methods for further details).
RT-PCR. Quantitative real-time RT-PCR for exogenous SDF-1 transcript and endogenous SDF-1 were performed, using previously described methods12,13 (see Supplementary Materials and Methods for further details).
Experimental protocol. CEU-perfusion imaging of the ischemic and contralateral control hindlimb adductor muscles was performed 14 days after ligation, at a time when endogenous angiogenesis was complete. Delivery was then performed, according to assigned treatment groups: group 1: control group, no treatment; group 2: UM SDF-1 plasmid microbubble delivery; group 3: intravenous EPC delivery; group 4: SDF-1 plasmid microbubble delivery plus intravenous EPC delivery (n = 30 per group). CEU imaging was performed at days 3, 7, and 14 postdelivery (n = 10 per group). In five rats per group (day 14), FMA was performed before being killed. Tissue for immunohistochemistry and RT-PCR was obtained from the remaining rats' ischemic and nonischemic hindlimbs, lungs, heart, and liver.
Statistical methods. Data are expressed as mean ± SD. Comparisons of data recorded at a single time point was performed by two-tailed unpaired Student t-tests that assumed unequal variance. Analyses of data acquired at several time points for multiple groups were performed by two-way analysis of variance; if significant, Bonferroni correction for multiple comparisons was applied when post hoc analysis between different time points or between different groups at the same time point was performed.
SUPPLEMENTARY MATERIAL Figure S1. Phenotypic characterization and functional analysis of endothelial progenitor cells (EPCs), before and after CMTMR labeling. (a) Immunostaining of EPCs for endothelial cell markers, showing expression of VEGFR-II, UEA-1 and CXCR4 on EPCs (Red – Propidium Iodide (nuclear stain), Green – VEGFR-II, UEA-1 and CXCR4, scale bar = 50μm). (b) Immunostaining of CMTMR labeled EPCs, showing co-expression of UEA-1 and CMTMR (Red – CMTMR, Green - UEA-1, Yellow – co-localization of UEA-1 and CMTMR, scale bar = 50μm) (c) Quantitative data (mean ± SD) on cell viability, surface marker (UEA-1, VEGFR-II and CXCR4) expression and marker positivity after CMTMR labeling. There was ~90% viability of cells after CMTMR labeling, with no change in cell surface markers (d) Quantitative EPC migration data (mean ± SD) for un-labeled and CMTMR-labeled EPCs, showing no differences in cell migratory function pre- and post-CMTMR labeling. (e) Quantitative matrigel tubule formation data (mean ± SD) for un-labeled and CMTMR-labeled EPCs, showing no differences in angiogenic capacity pre- and post-CMTMR labeling. Figure S2. Color-coded diagram illustrating branch order analysis based on the Strahler Method. Vascular structures are classified starting at terminal end points (Branch order 0 - blue). The labeling of segments progresses toward segment origins or nodes. At each node, the parent segment is labeled with an order number one larger than the daughter segment. This results in the following breakdown according to branch order; Distal Segments = 0 (blue), i.e. capillaries, while arterioles carry progressively higher branch orders of 1 (red), 2 (yellow) and 3 (green). Materials and Methods.
Acknowledgments
This work was supported by an Operating Grant (FRN 62763) from the Canadian Institutes of Health Research, Ottawa, Ontario, Canada, and an Equipment Grant from the Canadian Foundation for Innovation, Ottawa, Ontario, Canada. J.R.L. is supported by grants R01-HL-074443, R01-HL-078610, and R01-DK-063508 from the National Institutes of Health, Bethesda, MD. H.L.-P. is supported by a Phase II Clinician Scientist Award, Heart and Stroke Foundation of Ontario, Ottawa, Ontario, Canada and an Early Researcher Award from the Ministry of Research and Innovation, Ontario, Canada. The authors declared no conflict of interest.
Supplementary Material
Phenotypic characterization and functional analysis of endothelial progenitor cells (EPCs), before and after CMTMR labeling. (a) Immunostaining of EPCs for endothelial cell markers, showing expression of VEGFR-II, UEA-1 and CXCR4 on EPCs (Red – Propidium Iodide (nuclear stain), Green – VEGFR-II, UEA-1 and CXCR4, scale bar = 50μm). (b) Immunostaining of CMTMR labeled EPCs, showing co-expression of UEA-1 and CMTMR (Red – CMTMR, Green - UEA-1, Yellow – co-localization of UEA-1 and CMTMR, scale bar = 50μm) (c) Quantitative data (mean ± SD) on cell viability, surface marker (UEA-1, VEGFR-II and CXCR4) expression and marker positivity after CMTMR labeling. There was ~90% viability of cells after CMTMR labeling, with no change in cell surface markers (d) Quantitative EPC migration data (mean ± SD) for un-labeled and CMTMR-labeled EPCs, showing no differences in cell migratory function pre- and post-CMTMR labeling. (e) Quantitative matrigel tubule formation data (mean ± SD) for un-labeled and CMTMR-labeled EPCs, showing no differences in angiogenic capacity pre- and post-CMTMR labeling.
Color-coded diagram illustrating branch order analysis based on the Strahler Method. Vascular structures are classified starting at terminal end points (Branch order 0 - blue). The labeling of segments progresses toward segment origins or nodes. At each node, the parent segment is labeled with an order number one larger than the daughter segment. This results in the following breakdown according to branch order; Distal Segments = 0 (blue), i.e. capillaries, while arterioles carry progressively higher branch orders of 1 (red), 2 (yellow) and 3 (green).
REFERENCES
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M.et al. (1999Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization Circ Res 85221–228. [DOI] [PubMed] [Google Scholar]
- Hirata K, Li TS, Nishida, M, Ito, H, Matsuzaki, M, Kasaoka, S.et al. (2003Autologous bone marrow cell implantation as therapeutic angiogenesis for ischemic hindlimb in diabetic rat model Am J Physiol Heart Circ Physiol 284H66–H70. [DOI] [PubMed] [Google Scholar]
- Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H.et al. (2006Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction N Engl J Med 3551210–1221. [DOI] [PubMed] [Google Scholar]
- Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA.et al. (2007Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis Arch Intern Med 167989–997. [DOI] [PubMed] [Google Scholar]
- Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T.et al. (2006Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction N Engl J Med 3551199–1209. [DOI] [PubMed] [Google Scholar]
- Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W.et al. (2006Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial Lancet 367113–121. [DOI] [PubMed] [Google Scholar]
- Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H.et al. (2001Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease Circ Res 89E1–E7. [DOI] [PubMed] [Google Scholar]
- Abbott JD, Huang Y, Liu D, Hickey R, Krause DS., and, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H.et al. (1999VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells EMBO J 183964–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser U, Engberding N, Bahlmann FH, Schaefer A, Wiencke A, Heineke A.et al. (2004Statin-induced improvement of endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival after experimental myocardial infarction requires endothelial nitric oxide synthase Circulation 1101933–1939. [DOI] [PubMed] [Google Scholar]
- De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A.et al. (2004SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells Blood 1043472–3482. [DOI] [PubMed] [Google Scholar]
- Leong-Poi H, Kuliszewski MA, Lekas M, Sibbald M, Teichert-Kuliszewska K, Klibanov AL.et al. (2007Therapeutic arteriogenesis by ultrasound-mediated VEGF165 plasmid gene delivery to chronically ischemic skeletal muscle Circ Res 101295–303. [DOI] [PubMed] [Google Scholar]
- Kobulnik J, Kuliszewski MA, Stewart DJ, Lindner JR., and, Leong-Poi H. Comparison of gene delivery techniques for therapeutic angiogenesis ultrasound-mediated destruction of carrier microbubbles versus direct intramuscular injection. J Am Coll Cardiol. 2009;54:1735–1742. doi: 10.1016/j.jacc.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Hristov M, Erl W., and, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23:1185–1189. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- Hiasa K, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S.et al. (2004Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization Circulation 1092454–2461. [DOI] [PubMed] [Google Scholar]
- Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG.et al. (2007Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha J Clin Invest 1171249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S.et al. (2003Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization Circulation 1071322–1328. [DOI] [PubMed] [Google Scholar]
- Crosby JR, Kaminski WE, Schatteman G, Martin PJ, Raines EW, Seifert RA.et al. (2000Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation Circ Res 87728–730. [DOI] [PubMed] [Google Scholar]
- Urbich C., and, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- Aicher A, Brenner W, Zuhayra M, Badorff C, Massoudi S, Assmus B.et al. (2003Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling Circulation 1072134–2139. [DOI] [PubMed] [Google Scholar]
- van Weel V, Seghers L, de Vries MR, Kuiper EJ, Schlingemann RO, Bajema IM.et al. (2007Expression of vascular endothelial growth factor, stromal cell-derived factor-1, and CXCR4 in human limb muscle with acute and chronic ischemia Arterioscler Thromb Vasc Biol 271426–1432. [DOI] [PubMed] [Google Scholar]
- Campbell AI, Kuliszewski MA., and, Stewart DJ. Cell-based gene transfer to the pulmonary vasculature: Endothelial nitric oxide synthase overexpression inhibits monocrotaline-induced pulmonary hypertension. Am J Respir Cell Mol Biol. 1999;21:567–575. doi: 10.1165/ajrcmb.21.5.3640. [DOI] [PubMed] [Google Scholar]
- Carr AN, Howard BW, Yang HT, Eby-Wilkens E, Loos P, Varbanov A.et al. (2006Efficacy of systemic administration of SDF-1 in a model of vascular insufficiency: support for an endothelium-dependent mechanism Cardiovasc Res 69925–935. [DOI] [PubMed] [Google Scholar]
- Zen K, Okigaki M, Hosokawa Y, Adachi Y, Nozawa Y, Takamiya M.et al. (2006Myocardium-targeted delivery of endothelial progenitor cells by ultrasound-mediated microbubble destruction improves cardiac function via an angiogenic response J Mol Cell Cardiol 40799–809. [DOI] [PubMed] [Google Scholar]
- Leong-Poi H, Christiansen J, Heppner P, Lewis CW, Klibanov AL, Kaul S.et al. (2005Assessment of endogenous and therapeutic arteriogenesis by contrast ultrasound molecular imaging of integrin expression Circulation 1113248–3254. [DOI] [PubMed] [Google Scholar]
- Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q., and, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res. 2005;96:442–450. doi: 10.1161/01.RES.0000157672.70560.7b. [DOI] [PubMed] [Google Scholar]
- Kuliszewski MA, Fujii H, Liao C, Smith AH, Xie A, Lindner JR.et al. (2009Molecular imaging of endothelial progenitor cell engraftment using contrast-enhanced ultrasound and targeted microbubbles Cardiovasc Res 83653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM., and, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97:473–483. doi: 10.1161/01.cir.97.5.473. [DOI] [PubMed] [Google Scholar]
- Peirce SM, Price RJ., and, Skalak TC. Spatial and temporal control of angiogenesis and arterialization using focal applications of VEGF164 and Ang-1. Am J Physiol Heart Circ Physiol. 2004;286:H918–H925. doi: 10.1152/ajpheart.00833.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phenotypic characterization and functional analysis of endothelial progenitor cells (EPCs), before and after CMTMR labeling. (a) Immunostaining of EPCs for endothelial cell markers, showing expression of VEGFR-II, UEA-1 and CXCR4 on EPCs (Red – Propidium Iodide (nuclear stain), Green – VEGFR-II, UEA-1 and CXCR4, scale bar = 50μm). (b) Immunostaining of CMTMR labeled EPCs, showing co-expression of UEA-1 and CMTMR (Red – CMTMR, Green - UEA-1, Yellow – co-localization of UEA-1 and CMTMR, scale bar = 50μm) (c) Quantitative data (mean ± SD) on cell viability, surface marker (UEA-1, VEGFR-II and CXCR4) expression and marker positivity after CMTMR labeling. There was ~90% viability of cells after CMTMR labeling, with no change in cell surface markers (d) Quantitative EPC migration data (mean ± SD) for un-labeled and CMTMR-labeled EPCs, showing no differences in cell migratory function pre- and post-CMTMR labeling. (e) Quantitative matrigel tubule formation data (mean ± SD) for un-labeled and CMTMR-labeled EPCs, showing no differences in angiogenic capacity pre- and post-CMTMR labeling.
Color-coded diagram illustrating branch order analysis based on the Strahler Method. Vascular structures are classified starting at terminal end points (Branch order 0 - blue). The labeling of segments progresses toward segment origins or nodes. At each node, the parent segment is labeled with an order number one larger than the daughter segment. This results in the following breakdown according to branch order; Distal Segments = 0 (blue), i.e. capillaries, while arterioles carry progressively higher branch orders of 1 (red), 2 (yellow) and 3 (green).