Abstract
Optimization of the specific affinity of cardiac delivery vector could significantly improve the efficiency of gene/protein delivery, yet no cardiac vectors to date have sufficient target specificity for myocardial infarction (MI). In this study, we explored bacterial tropism for infarcted myocardium based on our previous observations that certain bacteria are capable of targeting the hypoxic regions in solid tumors. Out of several Escherichia coli or Salmonella typhimurium strains, the S. typhimurium defective in the synthesis of ppGpp (ΔppGpp S. typhimurium) revealed accumulation and selective proliferation in the infarcted myocardium without spillover to noncardiac tissue. The Salmonellae that were engineered to express a variant of Renilla luciferase gene (RLuc8), under the control of the E. coli arabinose operon promoter (PBAD), selectively targeted and delivered RLuc8 in the infarcted myocardium only upon injection of -arabinose. An examination of the infarct size before and after infection, and estimations of C-reactive protein (CRP) and procalcitonin indicated that intravenous injection of ΔppGpp S. typhimurium did not induce serious local or systemic immune reactions. This current proof-of-principle study demonstrates for the first time the capacity of Salmonellae to target infarcted myocardium and to serve as a vehicle for the selective delivery of therapeutic agents in MI.
Introduction
Coronary artery disease is a leading cause of morbidity and mortality in the world.1 The detailed investigation of the molecular pathways involved in the development and progression of the most refractory cardiac disease has led to the identification of numerous causative genes and proteins.2 Thus, molecular therapy to modify the levels of these proteins through control of their production offers a potential to improve myocardial function in the patients with refractory angina who have failed conventional medical or surgical treatment.3
Gene-based treatments involve direct cardiac delivery of potent angiogenic factors using catheter or surgical technique, either in naked form or enclosed in nanoparticles, to stimulate new vessel growth in hypoxic regions.4 Although the delivery technique via direct cardiac delivery may improve cardiac targeting, highly cardiotropic vector would be most desirable. Thus, optimizing the tropism of cardiac delivery vectors would help to improve gene/protein delivery and to reduce unwanted extracardiac transfection. However, no vector to date has sufficient specificity for the infarcted myocardium.5
In this study, we examined the feasibility of using avirulent bacteria as drug delivery vehicles with target specificity for the hypoxic/infarcted myocardium based on our previous observations that certain bacteria are capable of targeting the hypoxic regions in solid tumors.6,7,8,9 Most notably, Salmonella typhimurium defective in the synthesis of Magic Spot, ppGpp (ΔppGpp S. typhimurium)10 has been shown to exhibit strong tropism for the infarcted myocardium. This feature was exploited for targeted delivery of protein drugs to the infarcted myocardium. Precise triggering of expression could induce greater therapeutic effects of protein drugs while minimizing systemic toxicity.11 Thus, the Salmonella was implemented to equip with an inducible gene expression system that was controlled in remote. Here, we demonstrated a controlled expression of a bioluminescent protein as a model by employing molecular imaging technique.
Results
Selective localization of attenuated S. typhimurium in the infarcted myocardium
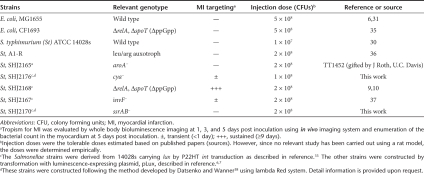
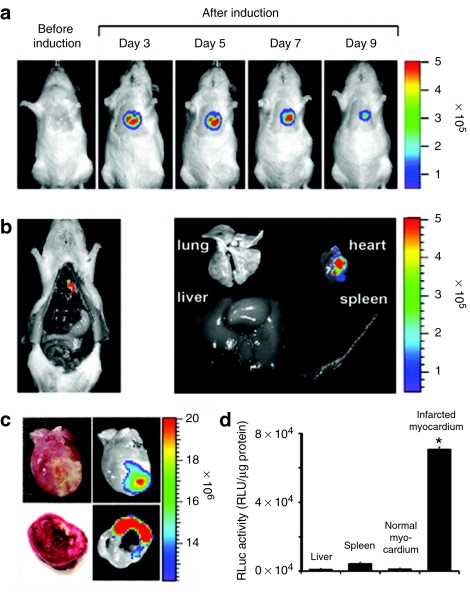
The Sprague–Dawley (SD) rats with myocardial infarction (MI) were injected with the bacteria listed in Table 1 through tail-vein and localized in the animal on 1, 3, 5 days post inoculation (dpi) by whole body bioluminescent imaging. Wild type strain of Escherichia coli and S. typhimurium failed to accumulate in the infarcted myocardium (Supplementary Figure S1). Subsequently, several mutant E. coli and S. typhimurium known to be attenuated were constructed and tested (Table 1). Surprisingly, the S. typhimurium strain defective in ppGpp synthesis (ΔppGpp S. typhimurium) showed great accumulation in the infarcted myocardium. The ΔppGpp S. typhimurium strain lacks both the relA and spoT genes that encode ribosome-bound and cytosolic ppGpp synthetase, respectively.12 The lethal dose, 50% (LD50) of ΔppGpp S. typhimurium is 106-fold higher than that of the wild type parental strain.13 For noninvasive visualization, we constructed a variant that expressed bacterial luciferase from the lux operon.6,7,14,15 The lux operon encodes all of the proteins (i.e., luciferase, substrate, and substrate regenerating enzymes) necessary to generate bioluminescence.7 The engineered ΔppGpp S. typhimurium was injected intravenously (i.v.) into MI rats (n = 5) at a dose of 2 × 108 colony forming units (CFUs). Afterwards whole body bioluminescence imaging was performed at indicated date using in vivo imaging system. Bioluminescence was detected in the heart and spleen of MI rats at 1 dpi (Figure 1a, top panel). Three days after injection, bioluminescence persisted in the heart but was no longer detected in the spleen and liver. Serial monitoring of the rats revealed an initial increase in cardiac bioluminescence up to 5 dpi, followed by a progressive decline in signal intensity. This decline likely reflected a decrease in the number of bacteria in the heart, presumably due to host immune response. In sham operated control rats, bioluminescence was detected only in the spleen and liver at 1 and 3 dpi (Figure 1a, bottom panel), reflecting the reticuloendothelial nature of these organs.
Table 1. Bacterial strains and tropism for myocardial infarction.
Figure 1.
Molecular imaging of bacterial tropism for infarcted myocardium (MI). (a) ΔppGpp S. typhimurium expressing lux (2 × 108 CFUs) was injected through the tail-vein into Sprague–Dawley rats with or without MI (n = 5, each group) 6 hours after surgery. (b) Direct comparison between photon flux and bacterial colony counts in MI (n = 21). A robust correlation exists between bacterial colony counts and bacterial bioluminescence signals (R2 = 0.89). (c) Cross-sections of heart from MI rats at 5 days post inoculation of Salmonellae injection. Thin slice (1.5 mm) cross-sections were prepared and then subjected to triphenyltetrazolium chloride staining (top). Bioluminescence imaging was performed using a cooled charge-coupled device camera for 30 seconds (bottom). Note the dull white area of reduced dehydrogenase activity. CFU, colony forming units; MI, myocardial infarction; ROI, region of interest.
To correlate the imaging data with bacterial load in the heart, spleen, and liver, we counted number of bacteria (CFUs) in the organs of MI rats (n = 21) (Table 2). Early after injection (12 hours), bacterial load was found primarily in the spleen and liver, most likely captured by phagocytic macrophages,16 with a relatively small bacterial burden in the heart. After 24 hours, however, the number of CFUs in myocardial tissue increased dramatically, reaching a maximum at 3 and 5 dpi of ~106 CFU/g, whereas the bacterial burden in the liver and spleen declined over the same period of time to undetectable levels. No bacteria was detected in the liver and spleen after 5 dpi, confirming the ability of ΔppGpp S. typhimurium to specifically target and proliferate in the infarcted myocardium. As shown in Figure 1b, the intensity of bacterial bioluminescence from the heart (Figure 1a) correlated robustly with the bacterial count (R2 = 0.89) in the MI region (Table 2). Analysis of the myocardial tissue sections from the MI rats revealed that bioluminescence was specifically located in the left ventricular wall (Figure 1c, bottom panel), and corresponds to the infarcted region revealed by triphenyltetrazolium chloride (TTC) staining17 (Figure 1c, top panel).
Table 2. Quantification of ΔppGpp S. typhimurium in different organs following intravenous injection in rat myocardial infarction models (n = 21). Numbers represents the number of colony forming units in a gram of tissue (colony forming units/g tissue).
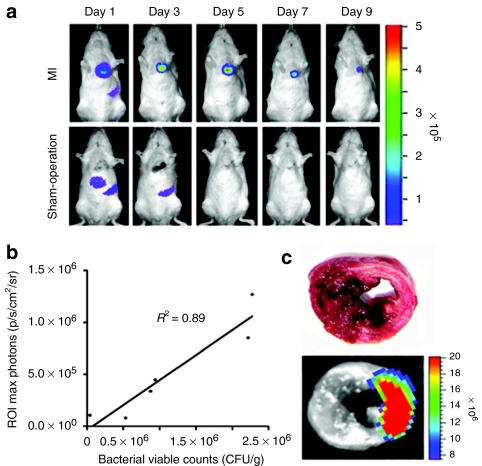
The location of the bacteria within infarcted myocardium was determined by histological analysis (Figure 2a–c). The infarcted areas were delineated by desmin immunohistochemical staining18, which revealed decreased immunoreactivity for desmin in the anterolateral and inferior wall of the left ventricular myocardium (Figure 2a). The infarcted area defined by desmin immunohistochemical staining closely correlated with that defined by TTC staining. The location of Salmonellae was evaluated at 5 dpi by staining the whole myocardial cross-sections with specific antibody and then analyzing the images (Figure 2b). The bacteria were found in the area of infarcted myocardium with decreased desmin immunoreactivity (area 2 and 3), but not in the contralateral normal myocardium (area 1) (Figure 2c). Thus, the results from bioluminescence imaging, bacterial enumeration, and histological analyses all strongly suggest that the i.v. administration of ΔppGpp S. typhimurium into MI rats leads to selective bacterial colonization at the infarcted myocardium.
Figure 2.
Microscopic features of bacterial tropism for infarcted myocardium. ΔppGpp S. typhimurium (2 × 108 CFUs) was injected through the tail-vein into Sprague–Dawley rats with MI (n = 5, each group). (a) Distinct delineation between healthy myocardium and infarcted myocardium from heart after immunohistochemical detection of desmin at 5 days post inoculation. Positive detection of desmin corresponds to the healthy region (brown), while negative detection corresponds to an infarcted lesion. (b) Hematoxylin-eosin (H&E) staining of a cardiac cross-section that corresponds to desmin immunoreactivity. (c) Immunofluorescence staining of indicated H&E stained areas. Sections were stained with antidesmin antibody (red) and anti-Salmonella antibody (green). Bottom right panel is a magnification of the boxed area in the bottom left panel (area 3). Bars = 100 µm. CFU, colony forming units; MI, myocardial infarction.
Remote control of protein expression and secretion in vitro
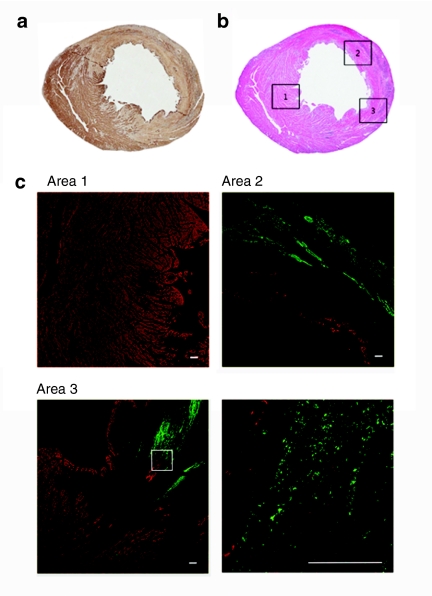
We explored the possibility of using ΔppGpp S. typhimurium as a vehicle for the targeted delivery of therapeutic proteins to the infarcted myocardium. A bacterial expression plasmid encoding a variant of Renilla luciferase (RLuc8)19,20 was constructed, in which the pelB leader sequence21 and a histidine tag (6× His)22 were fused to the amino- and carboxy-terminus, respectively, of RLuc8. The pelB leader sequence consists of the first 22 codons of the gene for pectate lyase B from Erwinia carotovora and directs protein secretion into the bacterial periplasm before being cleaved to mature protein. To create an inducible system, RLuc8 was placed under the control of the PBAD promoter from the E. coli arabinose operon (Figure 3a), which is inactive except in the presence of the inducer -arabinose.9 ΔppGpp S. typhimurium was transformed with pBAD-pelB-RLuc8, and the induction of RLuc8 expression in cultured cells in the presence of -arabinose was examined using a cooled charge-coupled device camera. Light intensity was found to be proportional to the concentration of -arabinose (R2 = 0.90), and no light was detected in the absence of inducer (Figure 3b). Western blot analysis revealed that a 36.9 kDa protein corresponding to RLuc819 was expressed only in the presence of -arabinose (0.2%). No protein was identified in the absence of -arabinose, which indicates that the regulation of PBAD is tightly controlled by the inducer. The presence of RLuc8 in both the bacterial pellet and culture medium suggests that RLuc8 was selectively expressed and secreted upon addition of -arabinose (Figure 3c).
Figure 3.
-arabinose-induced expression and secretion of RLuc8 by attenuated S. typhimurium. (a) Map of the bacterial expression plasmid containing the Renilla luciferase variant (pBAD-pelB-RLuc8). (b) ΔppGpp S. typhimurium was transformed with pBAD-pelB-RLuc8 and then different concentrations (0–0.2%) of -arabinose were added when the bacterial culture reached an OD600 of 0.5–0.7. After 4 hours incubation, coelenterazine (2 µg) was added to the cultures and bioluminescence was measured immediately using a cooled charge-coupled device camera (top panel). Bottom panel shows the quantification of total photon flux. (c) The expression and secretion of RLuc8 (36.9 kDa) was analyzed by western blot using an anti-RLuc antibody. Bacterial pellets (bottom) and culture medium (top) were collected at the indicated times from 2 to 8 hours after treatment with or without 0.2% -arabinose. S.t.+pBAD-pelB-RLuc8 represents S. typhimurium (ΔppGpp) transformed with pBAD-pelB-RLuc8. S.t. represents ΔppGpp S. typhimurium carrying no plasmid (4 hours after fresh culture) as a negative control.
Specific delivery of RLuc8 by ΔppGpp S. typhimurium in infarcted myocardium
Subsequently, we i.v. administered ΔppGpp S. typhimurium carrying pBAD-pelB-RLuc8 (2 × 108 CFUs) to MI rats (n = 6) at 6 hours after MI modeling. -arabinose was administered on 3 dpi to induce RLuc8 expression. This induction protocol was based on previous results showing that the number of Salmonellae in both liver and spleen declined significantly while reaching a maximum in the infarcted myocardium from 3 dpi (Figure 1 and Table 2). The expression of RLuc8 was monitored using a cooled charge-coupled device camera after daily administration of -arabinose and the luciferase substrate coelenterazine. Bioluminescence was detected throughout the length of the experimental period (Figure 4a) peaking at 5 dpi and declining thereafter, most likely due to the decreased bacterial cell number (Table 2). Notably, light signal from the cardiac region was observed only after the administration of -arabinose, and no bioluminescence was detected in the spleen or any other organ. These results were verified by imaging of gross necropsy and of isolated organs at 5 dpi, confirming that Salmonellae had been cleared from organs other than the heart (Figure 4b). Examination of the excised hearts at 5 dpi revealed strong bioluminescence in the anterolateral wall of the myocardium, within the infarcted region as delineated by TTC staining (Figure 4c). Quantitation of RLuc8 activity in various tissues showed that luciferase activity was significantly higher in the infarcted myocardium than in the contralateral normal myocardium (>50-fold higher, P < 0.001) or in any other organ (P < 0.001, Figure 4d).
Figure 4.
Specific expression of RLuc8 by -arabinose in myocardial infarction. (a) A representative in vivo bioluminescence image after tail-vein injection of ΔppGpp S. typhimurium carrying pBAD-pelB-RLuc8 (2 × 108 CFUs) into MI rats (n = 6). Images were acquired at the indicated day after bacterial injection, before and after (4 hours) intraperitoneal injection of -arabinose (1.2 g). The image before induction was obtained at 3 days post inoculation (dpi) immediately before administration of -arabinose. All images were acquired immediately after intravenous injection of coelenterazine (0.7 mg/kg body weight). (b) Bioluminescence images of gross necropsy (left) and of the indicated isolated organs (right) from a representative animal (a) at 5 dpi. (c) Hearts from MI rats (n = 6) were excised at 5 dpi and then subjected to triphenyltetrazolium chloride staining (left) and bioluminescence imaging (right). Representative images of the whole extracted heart (top) and cross-sections (bottom) are shown. (d) Bioluminescence in infarcted myocardium, noninfarcted myocardium, liver, and spleen was measured using a luminometer at 5 dpi (n = 6). The Y-axis indicates relative light units (RLU). Luciferase activity was normalized to total protein concentration in tissue homogenates. *P < 0.001. CFU, colony forming units; MI, myocardial infarction.
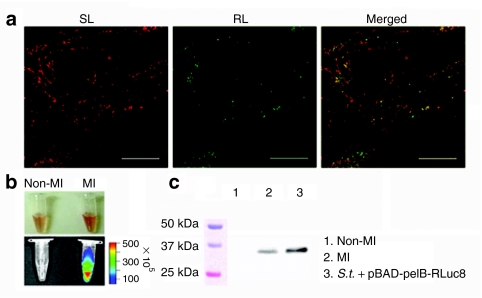
We also examined the selective expression of RLuc8 in the infarcted myocardium by immunofluorescence staining. RLuc8 was identified in the infarcted myocardium where bacteria had colonized (Figure 5a). Taking these histological findings together with the results presented in Figure 2, it was concluded that systemically injected engineered ΔppGpp S. typhimurium could specifically target and successfully deliver cargo molecules to the infarcted myocardium. The presence of RLuc8 in the infarcted tissue was further verified by bioluminescence imaging of tissue homogenates, which revealed that light was produced only from infarcted myocardium but not the contralateral normal myocardium (Figure 5b). Similarly, western blot analysis revealed the presence of a 36.9 kDa RLuc8 protein specifically in the infarcted myocardium (Figure 5c). Taken together, these results suggested a feasibility of engineered bacteria to selectively express a gene of interest in the infarcted tissue.
Figure 5.
Specific expression and secretion of RLuc8 from attenuated S. typhimurium in MI. Infarcted hearts were excised at 5 days post inoculation of ΔppGpp S. typhimurium carrying pBAD-pelB-RLuc8 (2 × 108 CFUs) and 4 hours after -arabinose induction. (a) Immunofluorescence staining of S. typhimurium (red, SL) and RLuc (green, RL) demonstrated bacterial expression and secretion of RLuc8 respectively. The merged image of SL and RL immunofluorescence is shown (Merged). Bar = 50 µm. (b) Bioluminescence in homogenates of infarcted myocardium (MI) and the contralateral normal myocardium (Non-MI) was determined using a cooled charge-coupled device camera after the addition of 2 µg of coelenterazine (top, bright field image; bottom, bioluminescence image). (c) The expression of RLuc8 (36.9 kDa) by the Salmonellae in the infarcted myocardium (lane 2, MI) was analyzed by western blot using an anti-RLuc antibody. The contralateral normal myocardium (Non-MI) was included as a negative control (lane 1). ΔppGpp S. typhimurium expressing RLuc8 (S.t.+pBAD-pelB-RLuc8) in the presence of 0.2% -arabinose in culture medium was included as a positive control (lane 3). CFU, colony forming units.
Bacterial tropism for inflammation and myocardial infarction
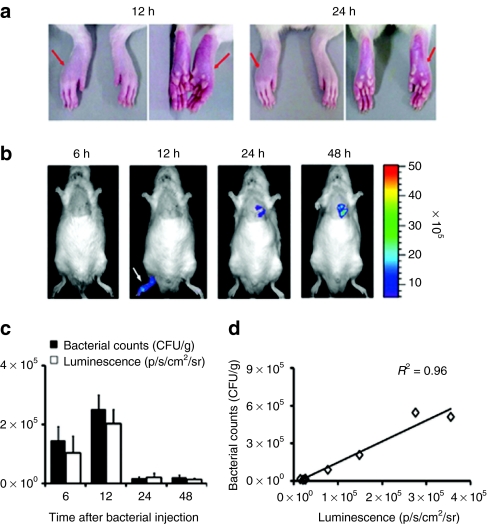
To test bacterial tropism toward inflammatory tissue, bioluminescent ΔppGpp S. typhimurium (2 × 108 CFUs) was i.v. injected into MI rats in which an inflammatory reaction was induced in the right hind paw by localized injection of complete Freund's adjuvant (CFA) (Figure 6a). By using whole body imaging, we observed a similar targeting of the Salmonellae to the site of inflammation; however, it was not sustained for >24 hours (Figure 6b). The bioluminescent signal from MI was clearly visible at 48 hours. To obtain more detailed information on bacterial accumulation in the inflammatory lesion, we counted the number of bacteria (CFUs) in the right hind paw at different time points and compared it with the intensity of the bioluminescence signal. The two measurements correlated strongly with each other (Figure 6c,d). The inflammatory environment may contribute to the targeting by ΔppGpp S. typhimurium, although the colonization at the local inflammatory site is only transient.
Figure 6.
Bacterial tropism for inflammation and infarcted myocardia. An inflammatory reaction was induced by subcutaneous injection of 100 µl of complete Freund's adjuvant (10 mg/ml) in the right hind paw (arrows) of Sprague–Dawley rats (n = 11). After 4 days, the animals were subjected to surgical occlusion of the left anterior descending artery. After 6 hours, ΔppGpp S. typhimurium expressing lux (2 × 108 CFUs) was intravenous injected. Maximum photon intensity was recorded (p/s/cm2/sr). (a) Bright field images of inflammation in the right hind paw (arrows). Images correspond to the bioluminescence images of 12 and 24 hours, respectively. (b) Bioluminescence images of a representative animal. Images were acquired 6, 12, 24, and 48 hours post inoculation (n = 3). (c) Direct comparison of the bacterial number and intensity of bioluminescence signal of the inflamed right hind paw (n = 8). (d) Correlation graph between the bacterial number and intensity of bioluminescence signal of the inflamed right hind paw (n = 8). CFU, colony forming units.
Toxicity of bacterial infection
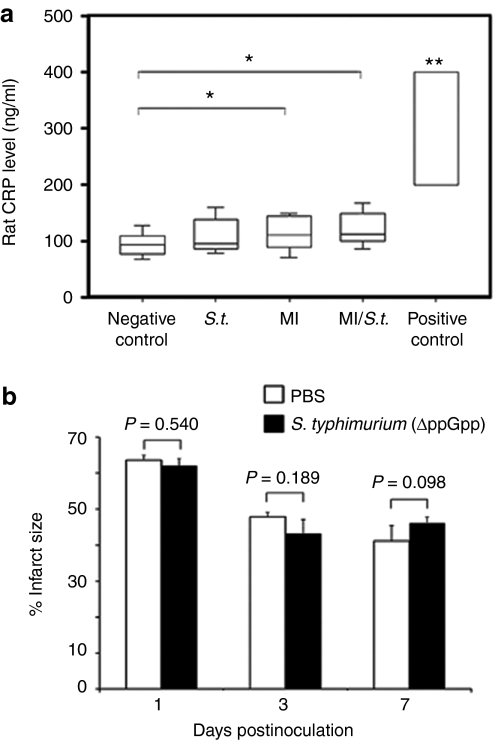
The therapeutic administration of live bacteria often raises concerns of potential toxicity. We sought to characterize the acute and short-term toxicity of attenuated ΔppGpp S. typhimurium following i.v. injection into rats (n = 5 for each group) by monitoring the levels of plasma C-reactive protein (CRP) and procalcitonin (PCT) (Figure 7a and Supplementary Figure S2). CRP is an acute phase protein that is elevated in plasma and serum as a result of injury, infection, or disease.23 PCT is a sensitive and specific marker for bacterial infection, particularly sepsis.24 The plasma levels of CRP in sham operated and MI rats were 95.2 ± 20.7 ng/ml and 115.7 ± 28.5 ng/ml, respectively (P = 0.035), which indicated that local inflammatory reactions were induced following surgical induction of MI in rats. However, there were no significant differences in plasma CRP levels after i.v. injection of ΔppGpp S. typhimurium (2 × 108 CFUs) into MI or sham rats (P = 0.358 for MI rats, P = 0.12 for sham rats, Figure 7a). Plasma PCT was also undetectable after administration of ΔppGpp S. typhimurium (Supplementary Figure S2; a positive test band indicates a plasma PCT level above 0.5 ng/ml). In addition, we examined whether or not bacterial localization in myocardium would give rise to further myocardial damage. The size of infarcted area as assessed by TTC staining was compared in MI rats with (ΔppGpp S. typhimurium) or without (phosphate-buffered saline (PBS)) bacterial infection (Figure 7b). There were no significant differences in the infarct size between two groups as determined at 1 (P = 0.540), 3 (P = 0.189), and 7 dpi (P = 0.098). These results strongly suggest that i.v. administration of attenuated ΔppGpp S. typhimurium does not lead to serious local or systemic inflammatory reactions.
Figure 7.
Systemic and local toxicity associated with ΔppGpp S. typhimurium injection in rats. (a) The levels of C-reactive protein (CRP) after intravenous injection of ΔppGpp S. typhimurium. Plasma CRP levels in sham operated and MI rats (n = 3 each) before and after the injection of Salmonellae (2 × 108 CFUs) were measured at 1 and 5 days post inoculation (dpi). The results are expressed as averages of CRP levels at 1 and 5 dpi. Rats (n = 3) were intravenous injected with lipopolysaccharide (LPS, 5 mg/kg for 3 minutes) as a positive control, and blood was drawn 4 hours after injection. Negative control indicates sham operated rats; S.t., sham operated rats with bacterial injection; MI, MI rats without bacterial injection; MI/S.t., MI rats with bacterial injection; positive control, LPS injected rats. Boxes represent the quartiles and whiskers mark the 10th and 90th percentiles. *P = 0.035; **P < 0.01. (b) Change of infarct size associated with ΔppGpp S. typhimurium injection in MI rats. Sprague–Dawley rats (n = 30) with MI were intravenously injected with PBS or ΔppGpp S. typhimurium (2 × 108 CFUs). At 1, 3, or 7 dpi (n = 5 for each group), rats were sacrificed and hearts were excised for triphenyltetrazolium chloride staining. The infarct size was measured using image processing software. MI, myocardial infarction; PBS, phosphate-buffered saline.
Discussion
In this study, we demonstrated for the first time that attenuated ΔppGpp S. typhimurium exhibits a specific tropism for infarcted myocardial tissue and can be engineered to secrete a target protein into the infarcted myocardium. This novel finding suggests that the Salmonellae can be exploited as a vehicle for the delivery of therapeutic proteins in MI patients. The engineered S. typhimurium used in this study exhibited several useful features: (i) an intrinsic tropism for infarcted myocardium, as demonstrated by bioluminescence imaging of reporter gene expression (Figures 1 and 2); (ii) inducible gene expression and secretion of proteins into the infarcted myocardium (Figures 3–5); (iii) confined gene expression in infarcted myocardium without spillover to noncardiac tissue (Figures 1a and 4a and Table 2).
We showed here that i.v. injected bacteria gained entry and replicated only in the infarcted myocardium, that was followed in real time in live animals through bioluminescence imaging. Due to rapid bacterial replication, light emission originating from the bacteria within infarcted myocardium became easily detectable in vivo. No vectors have exhibited specificity for infarcted myocardium through i.v. injection, and therefore inadvertent transfection of nontarget organs is inevitable.5 Although systemic injection via peripheral or central venous system remains, in general, a cornerstone of medical therapy, a trouble remains such as extensive first-pass clearance by the liver, lungs, spleen, and kidney, which would limit systemic injection of drug delivery vector for tissue-specific targeted delivery. Studies have shown poor cardiac uptake of vector i.v. injected, which is not surprisingly, given that only 4–5% of cardiac output passes through the coronary arteries.25 Nonspecific targeting of drug delivery vector is another potential issue, and therefore, increased exposure to nontargeted organ is problematic. In the context of targeted gene or protein therapy, this can be obviated in some degree with regulating gene expression using cardiac specific promoters26 and by employing viral vectors that express relative cardiac tropism.27,28 Nevertheless, most obvious trouble associated with i.v. delivery of such agents is compromised by marked (conspicuous) dilution in the total blood volume.29 A variety of catheter- or surgical-based techniques also have been developed to directly target the myocardium, but systemic spread of the vector can occur through the bloodstream or the lymphatic system (washout), most often to the liver or spleen.5 All these problems would be solved by employing ΔppGpp S. typhimurium, that specifically localized to regions of MI, and did not leak out from the original site for at least 9 days, and actively proliferated in MI (Figure 1a and Table 2).
No sign of serious local or systemic inflammatory reactions was noted following i.v. administration of ΔppGpp S. typhimurium: the levels of CRP and PCT were not significantly changed (Figure 7a and Supplementary Figure S2). In most, though not all, diseases, the value of circulating CRP reflects ongoing inflammation and/or tissue damage much more accurately than other laboratory parameters of the acute-phase response.23 We found that the plasma levels of CRP increased after surgical induction of MI, but did not show a further increase after the bacterial injection. It was speculated that bacterial colonization in the infarcted myocardium induced a minute, localized inflammation that led to the reduction of bacterial number in the infarcted myocardium.
Furthermore, antibiotic treatment (ciprofloxacin) resulted in a complete eradication of the Salmonellae residing at the MI site within 2 days (Supplementary Figure S3), which means that we could easily terminate bacterial colonization in the infarcted tissue deliberately. These characteristics represent significant advantages of using ΔppGpp S. typhimurium as a vector for gene therapy, in that gene expression is confined to the targeted infarct tissue without significant local or systemic inflammatory effects. Furthermore, the ability to manipulate gene expression through the use of an inducible promoter (PBAD) enables a powerful system for deliberate gene expression in vivo (Figures 3–5).
The mechanism of bacterial targeting of infarct tissue is an intriguing question. It may be the case that excess infiltration of neutrophils associated with MI might be associated with the tropism of the ΔppGpp S. typhimurium. We observed a similar targeting of the Salmonellae to sites of inflammation created by localized injection of CFA, although it was not sustained for >24 hours (Figure 6). Thus, it could be plausible that inflammatory environment might attract the Salmonellae. Another possibility is that the infarct provides a favorable metabolic environment for bacteria to persist and proliferate, although the tropic element(s) has yet to be identified. Interestingly, to date, a strong bacterial targeting to the MI has been observed only with ΔppGpp S. typhimurium, and no other S. typhimurium strain including A1-R strain that is markedly attenuated in virulence and selected for increased tumor targeting capability30 or E. coli MG1655-strain31 (Table 1 and Supplementary Figure S1). Thus, mere attenuation of virulence or inflammatory environment of MI would not be responsible for the specific MI targeting by ΔppGpp S. typhimurium. The identification of inflammatory cells accumulated in hypoxic sites and also of secreted cytokines in infarcted myocardium may provide clues to the mechanism underlying MI targeting by ΔppGpp Salmonella. Whatever the mechanism, the MI targeting is different from the tumor targeting, the latter is due largely to immune privileged microenvironment of tumor, which would provide protection of residing microbes against the host immune system.14 Thus, at the moment, we can only speculate that altered gene expression in ΔppGpp S. typhimurium32 might be responsible for the MI targeting. Understanding the mechanism by which ΔppGpp S. typhimurium targets the infarcted myocardium will require extensive analysis that is beyond the scope of the present work, although nature of which can be exploited to other gene therapy or targeting strategies, if revealed. Overall, the current proof-of-principle study highlights the potential for ΔppGpp S. typhimurium to serve as a targeted gene delivery vehicle for the treatment of MI. Further studies are necessary for the translation of this application to other species including humans.
The current study focused on bacterial delivery of a reporter gene RLuc8. However, the strategy illustrated here could be applied to any number of therapeutic genes including angiogenic growth factors. Given that the bioluminescence reaction requires oxygen, the detection of RLuc8 activity in MI rats suggests that the engineered bacteria were located in the peri-infarct zone, where oxygen is present. Thus, MI targeting bacteria can potentially deliver therapeutic proteins to salvageable myocardium.
In conclusion, we reported the bacterial strain (ΔppGpp S. typhimurium) with specific tropism for MI that may be able to open many new avenues for molecular imaging and therapy, including tissue-specific targeting with signal amplification based on bacterial proliferation, in vivo tissue-specific drug delivery, and the design of imageable therapeutic probes. This is a first such demonstration of taking advantage of specific host–microbe interaction for targeted detection and potential treatment of MI.
Materials and Methods
Plasmids. The expression plasmid pBAD-pelB-RLuc8 has been previously described.19
Bacterial strains. ΔppGpp S. typhimurium, SHJ2037 (relA::cat, spoT::kan), has been previously described.10 Salmonellae were grown in Luria–Bertani broth medium (Becton Dickinson, Franklin Lakes, NJ) with vigorous aeration at 37 °C. For the imaging of bioluminescence, the bacterial luciferase gene (lux) from S. typhimurium-Xen26 (Caliper Life Sciences, Hopkinson, MA) was transduced into strain SHJ2037 by P22HT int transduction (SHJ2168).33 All bacterial strains explored for this study was listed in Table 1.
Animal surgery to induce myocardial infarction. Eight-week old, male SD rats (250–260 g; OrientBio, Kyunggi-do, Korea) underwent sham operation (open thoracotomy only) or left anterior descending artery ligation, as previously described.34 Animal care, all experiments and euthanasia were performed in accordance with protocols approved by the Chonnam National University Animal Research Committee and the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication 85–23, revised 1985). Six hours after surgery, MI rats were injected through the lateral tail-vein with a fresh culture of Salmonellae (2 × 108 CFUs) resuspended in 100 µl of 1× PBS, (Gibco/Invitrogen, Carlsbad, CA). Sham-operated rats were injected with bacteria at the same dose.
Optical bioluminescence imaging of RLuc8 expression. Bioluminescence imaging was performed using the in vivo imaging system 100 (Caliper Life Sciences). Before imaging, coelenterazine (Biotium, Hayward, CA) dissolved in methanol (stock solution of 2 mg/ml) was injected i.v. at a dose of 0.7 mg/kg body weight in a final volume of 200 µl. Imaging signals were quantified in units of maximum photons per second per centimeter square per steradian (p/s/cm2/sr) within the region of interest as described.6,7
Validation of in vivo bioluminescence imaging with ex vivo enzyme assays. After whole body imaging, rat organs (spleen, liver, and heart) were extracted and homogenized in lysis buffer (4 ml/g; Promega, Madison, WI), and subjected to five cycles of freezing and thawing. After centrifugation at 18,500g for 30 minutes at 4 °C, the supernatants were assayed for RLuc activity. Briefly, 20 µl of sample were mixed with 100 µl of 1× RLuc assay reagent (Promega) in a 96-well plate, and luciferase activity was quantitated immediately using a luminometer (Microlumat Plus LB96V; Berthold Technologies, Badwildbad, Germany). Light intensity was normalized to protein concentration (as measured by the Bradford assay), and expressed as relative light units.
Enumeration of S. typhimurium in different organs. At specific times after bacterial injection, SD rats were euthanized and placed in 70% ethanol for 3 minutes. The heart, liver, and spleen were removed, and placed individually into sterile tubes containing PBS at 4 °C, and weighed. Samples were transferred to sterile homogenization tubes, homogenized, and returned to the original tubes for the preparation of serial dilutions with PBS. Agar plates containing kanamycin (50 µg/ml) were inoculated with the homogenate, and the plates were incubated overnight at 37 °C. Colonies were counted and bacterial load was expressed as CFU/g tissue.
Western blot analysis. Bacterial cell lysate and the culture medium were quantified using the Bradford protein assay. Total protein (40 µg) was analyzed by electrophoresis and blotted, as previously described.10
For the analysis of RLuc8 expression in myocardial tissue, homogenized tissue samples were standardized according to protein content, and 200 µg protein samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 12% linear gradient gels. Proteins were transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA), and the membrane was probed with a mouse anti-RLuc monoclonal antibody (1:5,000 dilution) (Chemicon, Temecula, CA), followed by horseradish peroxidase-conjugated goat anti-mouse IgG (immunoglobulin G) (1:80,000 dilution) (Santa Cruz Biotechnology, Santa Cruz, CA). Immunoreactive proteins were detected using luminol reagent (Santa Cruz Biotechnology).
Histological delineation of ischemic lesion. TTC (Sigma, St Louis, MO) staining was performed as previously described.17 Using image processing software (ImageJ, http://rsb.info.nih.gov/ij/), the size of infarcted area (nonstained, pale white region) was measured on both sides and averaged for each slice, and summed from all slices. The size of the infarct was expressed as the fraction of infarcted region relative to the total size of the myocardium.
Immunostaining for Salmonella and RLuc8 location. The infarcted heart was blocked in cross-section and processed for paraffin embedding. Representative sections were sliced into 4 µm-thickness sections and stained with hematoxylin-eosin. Adjacent sections were processed simultaneously for immunohistochemistry. Pretreatment of the tissues for heat-induced epitope retrieval was performed for 5 minutes in a 125 °C pressure cooker with 10 mmol/l citrate buffer, pH 6.0 for mouse antidesmin (1:100; Dako, Copenhagen, Denmark), rabbit anti-Salmonella (1:200; AbD Serotec, Oxford, UK), and mouse anti-RLuc antibodies (1:50; Millipore, Billerica, MA). And then, the slides were incubated with each primary antibody overnight at 4 °C. To detect the damaged myocardium using desmin antibody, streptavidin–horseradish peroxidase detection system was applied and visualized by chromogen reactions of the tissue sections that were initially treated with 0.02% diaminobenzidine.
To detect S. typhimurium overlying damaged myocardium, anti-Salmonella antibody and antidesmin antibody stained with AlexaFluor 488-conjugated chicken anti-rabbit antibody (Molecular Probes, Eugene, OR) and Cy3-conjugated goat anti-mouse antibody (Jackson Immunoresearch Laboratories, Westgrove, PA) to emit green and red fluorescence, respectively. To detect the secretory RLuc protein, anti-Salmonella antibody and anti-RLuc antibody stained with AlexaFluor 568-conjugated goat anti-rabbit antibody (Molecular Probes) and fluorescein isothiocyanate-conjugated goat anti-mouse antibody (Jackson Immunoresearch Laboratories) to emit red and green fluorescence, respectively. After a fluorescently labeled secondary antibody, sliced were mounted with 4′,6-diamidino-2-phenylindole/Antifade solution (Millipore). Antibody diluent (Dako) was applied as a negative control stain. Sections were analyzed using a Zeiss LSM 510 META confocal laser-scanning microscope (Carl Zeiss International, Göttingen, Germany).
Toxicity of attenuated S. typhimurium. Systemic or local inflammation and infection after administration of ΔppGpp S. typhimurium were determined by measuring the levels of CRP, using a rat CRP enzyme-linked immunosorbent assay kit (Life Diagnostics, West Chester, PA), and PCT (Brahms PCT-Q; B.R.A.H.M.S Diagnostica, Hennigsdorg, Germany) in rat plasma, according to manufacturers' protocols.
Inflammatory modeling. To characterize the accumulation of bacteria in inflammation, an inflammatory reaction was induced by subcutaneous injection of 100 µl CFA (inactivated Mycobacterium tuberculosis, 10 mg/ml, Sigma, St Louis, MO) in the right hind paw of SD rats (n = 6). The left hind paw was injected with physiologic saline as a negative control. After 4 days, the animals were subjected to surgical occlusion of the left anterior descending artery. After 6 hours, ΔppGpp S. typhimurium carrying pBAD-pelB-RLuc8 (2 × 108 CFUs) was i.v. injected into inflammatory animals. Images were acquired 4 hours after intraperitoneal injection of -arabinose (1.2 g).
To quantify viable bacteria which maybe located at inflammatory lesions, other rats (n = 8) with inflamed paws but not infarcted hearts received ΔppGpp S. typhimurium carrying pBAD-pelB-RLuc8 (2 × 108 CFUs), simultaneously with 1.2 g/rat of -arabinose. Bioluminescence imaging was acquired at 6, 12, 24, and 48 hours post bacterial administration, then at each time point, paw muscles (n = 2) were isolated, homogenized, and diluted with 1× PBS. Homogenates were spread onto Luria–Bertani agar plates supplemented with ampicillin (50 µg/ml). Overnight grown colonies were determined and normalized to gram of tissue.
Statistical analyses. Differences in one factor between two groups were determined using the two-sided Student's t-test or by Mann–Whitney U-test (for nonparametric data), and among more than two groups using analysis of variance (ANOVA) with a post-hoc test or by Kruskal–Wallis analysis of variance (for nonparametric data). A P value of <0.05 was considered statistically significant for all analyses. Data are expressed as means ± standard deviation (SD).
Additional method. Details of antibiotic treatment is available in the Supplementary Materials and Methods.
SUPPLEMENTARY MATERIAL Figure S1. Tropism of different bacterial strains for infarcted myocardium. SD rats (n = 5 for each group) with MI were injected with 2 x 108 CFUs of wild type E. coli (MG1655) (a), A1-R strain of S. typhimurium (b) or wild type S. typhimurium (14028s) expressing lux (c). Whole body bioluminescence images were obtained at 1, 3, 5 dpi (left panel). Hearts from the MI rats were excised at 5 dpi and subjected to TTC staining and bioluminescence imaging (BLI). BLI was performed using a cooled CCD camera for 30 seconds (right). Maximum photon intensity was recorded (p/s/cm2/sr). Figure S2. Systemic toxicity associated with ΔppGpp S. typhimurium injection in rats. Immunochromatographic analysis of PCT levels in rat plasma 4 hours, 5 days and 10 days after bacteria injection (2 x 108 CFUs). Rats (n = 3) were i.v. injected with LPS (5 mg/kg for 3 minutes) as a positive control. Blood was drawn 4 hours after injection for analysis. Figure S3. Clearance of bacterial infection by antibiotics. (a) Three days after injecting ΔppGpp S. typhimurium expressing lux (2 x 108 CFUs), ciprofloxacin was i.p. injected at a dose of 30 mg/kg/day (bottom, n = 3) or PBS (top, n = 3) twice daily. Efficacy was monitored by bioluminescence imaging acquired at indicated day. (b) Quantification of maximum photon intensity in (a). *P < 0.01. The bioluminescence from the heart significantly decreased from 5 dpi, eventually to undetectable levels in ciprofloxacin-treated rats, a time-point at which bioluminescence peaked in non-treated control rats, which indicated that there was a complete eradication of bacteria by the antibiotic treatment. Materials and Methods.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) (No. 2010-0028750) and by Bio R&D program (2010-0020658) funded by the Ministry of Education, Science and Technology, and in part by the Bioimaging Research Center at GIST. Y.H. was supported by the Pioneer Research Center Program “Bacteriobot” through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (No. 2010-0002241). J.H.R. was supported by grant No. RTI05-01-01 from the Regional Technology Innovation Program of the Ministry of Knowledge Economy (MKE). H.E.C. was supported by MOST through KOSEF (No. 2010-0020539). Y.A. was supported by the Stem Cell Research Program of MOST, Republic of Korea (M10641450001-06N4145-0011). H.-S. B. was supported by KOSEF grant funded by MOST, Republic of Korea, through its National Nuclear Technology Program (M20702010003-07N0201-00300).
Supplementary Material
Tropism of different bacterial strains for infarcted myocardium. SD rats (n = 5 for each group) with MI were injected with 2 x 108 CFUs of wild type E. coli (MG1655) (a), A1-R strain of S. typhimurium (b) or wild type S. typhimurium (14028s) expressing lux (c). Whole body bioluminescence images were obtained at 1, 3, 5 dpi (left panel). Hearts from the MI rats were excised at 5 dpi and subjected to TTC staining and bioluminescence imaging (BLI). BLI was performed using a cooled CCD camera for 30 seconds (right). Maximum photon intensity was recorded (p/s/cm2/sr).
Systemic toxicity associated with ΔppGpp S. typhimurium injection in rats. Immunochromatographic analysis of PCT levels in rat plasma 4 hours, 5 days and 10 days after bacteria injection (2 x 108 CFUs). Rats (n = 3) were i.v. injected with LPS (5 mg/kg for 3 minutes) as a positive control. Blood was drawn 4 hours after injection for analysis.
Clearance of bacterial infection by antibiotics. (a) Three days after injecting ΔppGpp S. typhimurium expressing lux (2 x 108 CFUs), ciprofloxacin was i.p. injected at a dose of 30 mg/kg/day (bottom, n = 3) or PBS (top, n = 3) twice daily. Efficacy was monitored by bioluminescence imaging acquired at indicated day. (b) Quantification of maximum photon intensity in (a). *P < 0.01. The bioluminescence from the heart significantly decreased from 5 dpi, eventually to undetectable levels in ciprofloxacin-treated rats, a time-point at which bioluminescence peaked in non-treated control rats, which indicated that there was a complete eradication of bacteria by the antibiotic treatment.
REFERENCES
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., and, Alitalo K. Gene transfer as a tool to induce therapeutic vascular growth. Nat Med. 2003;9:694–701. doi: 10.1038/nm0603-694. [DOI] [PubMed] [Google Scholar]
- Kim MC, Kini A., and, Sharma SK. Refractory angina pectoris: mechanism and therapeutic options. J Am Coll Cardiol. 2002;39:923–934. doi: 10.1016/s0735-1097(02)01716-3. [DOI] [PubMed] [Google Scholar]
- Müller OJ, Katus HA., and, Bekeredjian R. Targeting the heart with gene therapy-optimized gene delivery methods. Cardiovasc Res. 2007;73:453–462. doi: 10.1016/j.cardiores.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Lyon AR, Sato M, Hajjar RJ, Samulski RJ., and, Harding SE. Gene therapy: targeting the myocardium. Heart. 2008;94:89–99. doi: 10.1136/hrt.2007.116483. [DOI] [PubMed] [Google Scholar]
- Min JJ, Kim HJ, Park JH, Moon S, Jeong JH, Hong YJ.et al. (2008Noninvasive real-time imaging of tumors and metastases using tumor-targeting light-emitting Escherichia coli Mol Imaging Biol 1054–61. [DOI] [PubMed] [Google Scholar]
- Min JJ, Nguyen VH, Kim HJ, Hong Y., and, Choy HE. Quantitative bioluminescence imaging of tumor-targeting bacteria in living animals. Nat Protoc. 2008;3:629–636. doi: 10.1038/nprot.2008.32. [DOI] [PubMed] [Google Scholar]
- Jiang SN, Phan TX, Nam TK, Nguyen VH, Kim HS, Bom HS.et al. (2010Inhibition of tumor growth and metastasis by a combination of Escherichia coli-mediated cytolytic therapy and radiotherapy Mol Ther 18635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VH, Kim HS, Ha JM, Hong Y, Choy HE., and, Min JJ. Genetically engineered Salmonella typhimurium as an imageable therapeutic probe for cancer. Cancer Res. 2010;70:18–23. doi: 10.1158/0008-5472.CAN-09-3453. [DOI] [PubMed] [Google Scholar]
- Song M, Kim HJ, Kim EY, Shin M, Lee HC, Hong Y.et al. (2004ppGpp-dependent stationary phase induction of genes on Salmonella pathogenicity island 1 J Biol Chem 27934183–34190. [DOI] [PubMed] [Google Scholar]
- Forbes NS. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer. 2010;10:785–794. doi: 10.1038/nrc2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M, Gentry DR, Hernandez VJ., and, Vinella D.1996The stringent responseIn: Neidhardt, FC et al. (eds; Escherichia Coli and Salmonella: Cellular and Molecular Biology vol. 1American Society for Microbiology Press: Washington DC; 1458–1496. [Google Scholar]
- Na HS, Kim HJ, Lee HC, Hong Y, Rhee JH., and, Choy HE. Immune response induced by Salmonella typhimurium defective in ppGpp synthesis. Vaccine. 2006;24:2027–2034. doi: 10.1016/j.vaccine.2005.11.031. [DOI] [PubMed] [Google Scholar]
- Yu YA, Shabahang S, Timiryasova TM, Zhang Q, Beltz R, Gentschev I.et al. (2004Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins Nat Biotechnol 22313–320. [DOI] [PubMed] [Google Scholar]
- Yu YA, Timiryasova T, Zhang Q, Beltz R., and, Szalay AA. Optical imaging: bacteria, viruses, and mammalian cells encoding light-emitting proteins reveal the locations of primary tumors and metastases in animals. Anal Bioanal Chem. 2003;377:964–972. doi: 10.1007/s00216-003-2065-0. [DOI] [PubMed] [Google Scholar]
- Aderem A., and, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- Acton PD, Thomas D., and, Zhou R. Quantitative imaging of myocardial infarct in rats with high resolution pinhole SPECT. Int J Cardiovasc Imaging. 2006;22:429–434. doi: 10.1007/s10554-005-9046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogné JM, Rolin S, Pétein M, Tchana-Sato V, Ghuysen A, Lambermont B.et al. (2005Characterization of an original model of myocardial infarction provoked by coronary artery thrombosis induced by ferric chloride in pig Thromb Res 116431–442. [DOI] [PubMed] [Google Scholar]
- Loening AM, Fenn TD, Wu AM., and, Gambhir SS. Consensus guided mutagenesis of Renilla luciferase yields enhanced stability and light output. Protein Eng Des Sel. 2006;19:391–400. doi: 10.1093/protein/gzl023. [DOI] [PubMed] [Google Scholar]
- Loening AM, Wu AM., and, Gambhir SS. Red-shifted Renilla reniformis luciferase variants for imaging in living subjects. Nat Methods. 2007;4:641–643. doi: 10.1038/nmeth1070. [DOI] [PubMed] [Google Scholar]
- Lei SP, Lin HC, Wang SS, Callaway J., and, Wilcox G. Characterization of the Erwinia carotovora pelB gene and its product pectate lyase. J Bacteriol. 1987;169:4379–4383. doi: 10.1128/jb.169.9.4379-4383.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnau J, Lauritzen C, Petersen GE., and, Pedersen J. Current strategies for the use of affinity tags and tag removal for the purification of recombinant proteins. Protein Expr Purif. 2006;48:1–13. doi: 10.1016/j.pep.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Pepys MB., and, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ-Crain M, Jaccard-Stolz D, Bingisser R, Gencay MM, Huber PR, Tamm M.et al. (2004Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial Lancet 363600–607. [DOI] [PubMed] [Google Scholar]
- Lazarous DF, Shou M, Stiber JA, Dadhania DM, Thirumurti V, Hodge E.et al. (1997Pharmacodynamics of basic fibroblast growth factor: route of administration determines myocardial and systemic distribution Cardiovasc Res 3678–85. [DOI] [PubMed] [Google Scholar]
- Chen IY, Gheysens O, Ray S, Wang Q, Padmanabhan P, Paulmurugan R.et al. (2010Indirect imaging of cardiac-specific transgene expression using a bidirectional two-step transcriptional amplification strategy Gene Ther 17827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak CA, Mah CS, Thattaliyath BD, Conlon TJ, Lewis MA, Cloutier DE.et al. (2006Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo Circ Res 99e3–e9. [DOI] [PubMed] [Google Scholar]
- Inagaki K, Fuess S, Storm TA, Gibson GA, Mctiernan CF, Kay MA.et al. (2006Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8 Mol Ther 1445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani JA., and, Kaye DM. Delivery of gene and cellular therapies for heart disease. J Cardiovasc Transl Res. 2010;3:417–426. doi: 10.1007/s12265-010-9190-x. [DOI] [PubMed] [Google Scholar]
- Finlay BB, Fry J, Rock EP., and, Falkow S. Passage of Salmonella through polarized epithelial cells: role of the host and bacterium. J Cell Sci Suppl. 1989;11:99–107. doi: 10.1242/jcs.1989.supplement_11.8. [DOI] [PubMed] [Google Scholar]
- Blattner FR, Plunkett G., 3rd, , Bloch CA, Perna NT, Burland V, Riley M.et al. (1997The complete genome sequence of Escherichia coli K-12 Science 2771453–1462. [DOI] [PubMed] [Google Scholar]
- Song M, Kim HJ, Ryu S, Yoon H, Yun J., and, Choy HE. ppGpp-mediated stationary phase induction of the genes encoded by horizontally acquired pathogenicity islands and cob/pdu locus in Salmonella enterica serovar Typhimurium. J Microbiol. 2010;48:89–95. doi: 10.1007/s12275-009-0179-6. [DOI] [PubMed] [Google Scholar]
- Davis RW, Botstein D., and, Roth JR. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA; 1980. Advanced Bacterial Genetics: A Manual for Genetic Engineering. [Google Scholar]
- Samsamshariat SA, Samsamshariat ZA., and, Movahed MR. A novel method for safe and accurate left anterior descending coronary artery ligation for research in rats. Cardiovasc Revasc Med. 2005;6:121–123. doi: 10.1016/j.carrev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Xiao H, Kalman M, Ikehara K, Zemel S, Glaser G., and, Cashel M. Residual guanosine 3',5'-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J Biol Chem. 1991;266:5980–5990. [PubMed] [Google Scholar]
- Zhao M, Yang M, Ma H, Li X, Tan X, Li S.et al. (2006Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice Cancer Res 667647–7652. [DOI] [PubMed] [Google Scholar]
- Jones BD., and, Falkow S. Identification and characterization of a Salmonella typhimurium oxygen-regulated gene required for bacterial internalization. Infect Immun. 1994;62:3745–3752. doi: 10.1128/iai.62.9.3745-3752.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA., and, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tropism of different bacterial strains for infarcted myocardium. SD rats (n = 5 for each group) with MI were injected with 2 x 108 CFUs of wild type E. coli (MG1655) (a), A1-R strain of S. typhimurium (b) or wild type S. typhimurium (14028s) expressing lux (c). Whole body bioluminescence images were obtained at 1, 3, 5 dpi (left panel). Hearts from the MI rats were excised at 5 dpi and subjected to TTC staining and bioluminescence imaging (BLI). BLI was performed using a cooled CCD camera for 30 seconds (right). Maximum photon intensity was recorded (p/s/cm2/sr).
Systemic toxicity associated with ΔppGpp S. typhimurium injection in rats. Immunochromatographic analysis of PCT levels in rat plasma 4 hours, 5 days and 10 days after bacteria injection (2 x 108 CFUs). Rats (n = 3) were i.v. injected with LPS (5 mg/kg for 3 minutes) as a positive control. Blood was drawn 4 hours after injection for analysis.
Clearance of bacterial infection by antibiotics. (a) Three days after injecting ΔppGpp S. typhimurium expressing lux (2 x 108 CFUs), ciprofloxacin was i.p. injected at a dose of 30 mg/kg/day (bottom, n = 3) or PBS (top, n = 3) twice daily. Efficacy was monitored by bioluminescence imaging acquired at indicated day. (b) Quantification of maximum photon intensity in (a). *P < 0.01. The bioluminescence from the heart significantly decreased from 5 dpi, eventually to undetectable levels in ciprofloxacin-treated rats, a time-point at which bioluminescence peaked in non-treated control rats, which indicated that there was a complete eradication of bacteria by the antibiotic treatment.