Abstract
Ex-vivo regional gene therapy with bone marrow cells (BMCs) overexpressing bone morphogenetic protein-2 (BMP-2) has demonstrated efficacy in healing critical sized bone defects in preclinical studies. The purpose of this preclinical study was to compare the osteoinductive potential of a novel “same day” ex-vivo regional gene therapy versus a traditional two-step approach, which involves culture expansion of the donor cells before implantation. In the “same day” strategy buffy coat cells were harvested from the rat bone marrow, transduced with a lentiviral vector-expressing BMP-2 for 1 hour and implanted into a rat femoral defect in the same sitting. There was no significant difference (P = 0.22) with respect to the radiographic healing rates between the femoral defects treated with the “same day” strategy (13/13; 100%) versus the traditional two-step approach (11/14; 78%). However, the femoral defects treated with the “same day” strategy induced earlier radiographic bone healing (P = 0.004) and higher bone volume (BV) [micro-computed tomography (micro-CT); P < 0.001]. The “same day” strategy represents a significant advance in the field of ex-vivo regional gene therapy because it offers a solution to limitations associated with the culture expansion process required in the traditional ex vivo approach. This strategy should be cost-effective when adapted for human use.
Introduction
Autologous bone graft is considered as the gold standard for graft material in treating difficult bone repair scenarios because it possesses osteogenic and osteoconductive properties.1 However, the limited availability of autogenous bone graft and the morbidity associated with the graft harvest procedure has required the orthopedic surgeon to identify alternative bone graft strategies.2,3,4
Recombinant bone morphogenetic proteins are the most potent osteoinductive agents available today and have demonstrated efficacy in promoting bone healing in tibial fractures and spinal fusions in prospective randomized controlled trials.5,6,7 Recombinant bone morphogenetic protein-2 is approved by the US Food and Drug Administration for treatment of acute open tibial shaft fractures and for fusion of lumbar spine in patients with degenerative disk disease at one level.8 However, the high cost of recombinant bone morphogenetic proteins, the variability of its efficacy in humans, a search for a more optimal carrier for local delivery and recent reports of serious side effects associated with its use are important factors limiting its clinical utility.9,10,11,12,13
There is still a critical need for a regimen that can be used to heal large bone defects or enhance bone repair in a compromised biological environment. Ex-vivo regional gene therapy involves the use of genetically modified cells to deliver the protein of interest to a specific bone repair site.14,15,16,17 Successful bone healing requires four critical components including cells that can respond to osteogenic signals, osteoinductive growth factors that can drive the mesenchymal stem cells toward an osteoblastic pathway (osteoinduction), matrix that provides attachment for the cellular constituents so that they can bridge bone defects and deposit bone (osteoconduction) and an intact vascular supply.18,19 Regional gene therapy has been proposed as a more efficient growth factor delivery system because it provides an osteoinductive signal of variable duration, responding cells and the cells that overexpress the protein of interest, and these cells can be placed on an osteoconductive matrix that facilitates bone repair.20,21,22 Both viral and nonviral vectors have been used to deliver the complementary DNA (cDNA). Nonviral vectors are nonpathogenic but have low efficacy. Adenovirus, oncoretrovirus, lentivirus, and adeno-associated virus are commonly described viral vectors that have been used in preclinical gene therapy studies and differ from each other with respect to immunogenic response, packaging capacity, ability to induce dividing and nondividing cells, ability to insert into host chromosome and ease of manufacturing.23,24
In a prior study from our laboratory using a traditional two-step approach, BMP producing bone marrow cells (BMCs) were associated with a more robust bone repair compared to a large dose of recombinant protein in a rat critical sized femoral defect.14 We hypothesized that the enhanced bone repair was secondary to both the overexpression of osteoinductive proteins by the transduced mesenchymal stem cells (paracrine effect) and the ability of these same cells to respond to the secreted protein (autocrine effect).25,26,27 However, the conventional ex vivo gene therapy involves harvest and culture expansion of mesenchymal stem cells, which is time consuming and requires special tissue culture facilities.18,28,29,30 Although this therapy is not yet clinically available, the cost and inconvenience associated with culture expansion is a major impediment to the clinical adaptability of ex vivo gene therapy. Therefore, we decided to develop a novel ex vivo gene therapy strategy that does not require culture expansion of stem cells and has the potential to be a more cost-effective and convenient way of delivering both osteogenic cells and osteoinductive proteins to successfully treat the most difficult bone healing scenarios.
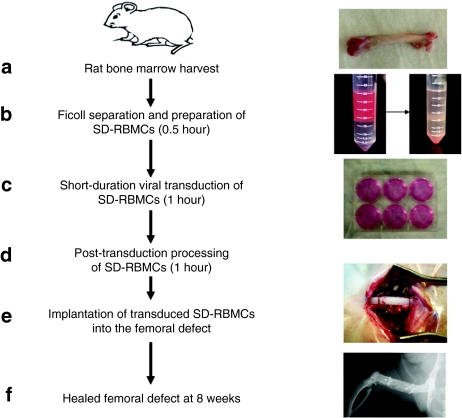
The purpose of this study was twofold: (i) to develop a novel “same day” strategy for ex-vivo regional gene therapy in which the harvest and transduction of BMCs and their implantation would be performed at same sitting to avoid culture expansion of donor marrow cells (Figure 1) and (ii) to compare the quality of bone repair with the “same day” versus the conventional two-step regimen using rat BMCs (RBMCs) transduced with a lentiviral vector-expressing BMP-2 in a rat femoral defect model. Transgene expression in our standard lentiviral vector was enhanced by employing a two-step transcriptional gene amplification system.31 The results of this study demonstrate a solution to problems that are associated with the traditional two-step ex vivo approach.
Figure 1.
Steps involved in the “same day” ex vivo gene therapy. (a) Harvest of bone marrow from the rat femur; (b) Ficoll separation and preparation of rat “same day” bone marrow cells (SD-RBMCs) for viral transduction (time required 0.5 hour); (c) Short-duration viral transduction of SD-RBMCs (time required 1 hour); (d) Post-transduction preparation of SD-RBMCs (time required 1 hour); (e) Placement of transduced SD-RBMCs on a collagen-ceramic matrix and implantation into the femoral defect; (f) Healed femoral defect at 8 weeks after cell implantation. SD-RBMCs, “same day” rat bone marrow cells.
Results
Transduced cultured RBMCs and transduced “same day” cells produce a consistent and similar amount of BMP-2 in vitro
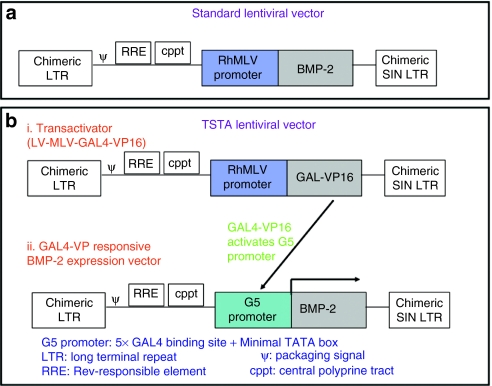
In order to get enhanced BMP-2 production, the standard lentiviral vector was modified using a two-step transcriptional amplification (TSTA) system (Figure 2). The efficacy of the newly constructed LV-TSTA-BMP-2 vector was tested in the cultured RBMCs by measuring the BMP-2 production in tissue culture following transduction. The in vitro BMP-2 production in RBMCs transduced with the LV-TSTA-BMP-2 vector (725.4 ng/day/mg cell protein) was more than tenfold higher when compared to that in the RBMCs transduced with standard lentiviral vector (LV-RhMLV-BMP-2; 64 ng/day/mg cell protein) for an multiplicity of infection (MOI) of 25.
Figure 2.
Structure of lentiviral two-step transcription amplification (TSTA) vector system. (a) The Top panel depicts the original lentiviral vector used in our laboratory which encodes bone morphogenetic protein-2 (BMP-2) complementary DNA (cDNA) downstream of RhMLV promoter. (b) The bottom panel shows the construct of the two-step transcriptional activation system (TSTA) that consists of two different lentiviral vectors namely the transactivator vector and the transgene expression vector. The cells are transduced with both vectors simultaneously. The GAL4-VP16 activates the G5 promoter in the transgene expression vector to amplify the expression of BMP-2. Both constructs contain the Rev-responsive element (RRE) and the central polyprine tract (cPPT), which enhance the efficiency of gene expression. LTR, long-terminal repeat; SIN, self-inactivating.
Aliquots were taken from the “same day” and the cultured BMCs that were implanted into the rat femoral defect. These cells were maintained in culture for a period of 4 weeks and the conditioned media was used to measure the in vitro BMP-2 production at weekly interval for 4 weeks. There was a sustained BMP-2 production by the transduced “same day” cells (SD-RBMCs + LV-TSTA-BMP-2) and the transduced cultured BMCs (C-RBMCs + LV-TSTA-BMP-2) over the 4-week time period of in vitro measurement (Supplementary Table S2). Furthermore, there were no significant differences (P > 0.05) with respect to the in vitro BMP-2 production at any time point between the transduced “same day” cells and the transduced cultured BMCs. The nontransduced rat cultured BMCs (C-RBMCs) and nontransduced “same day” RBMCs (SD-RBMCs) did not produce any significant amount of in vitro BMP-2.
Comparison of the quality of bone repair with a “same day” strategy versus a traditional two-step ex vivo strategy
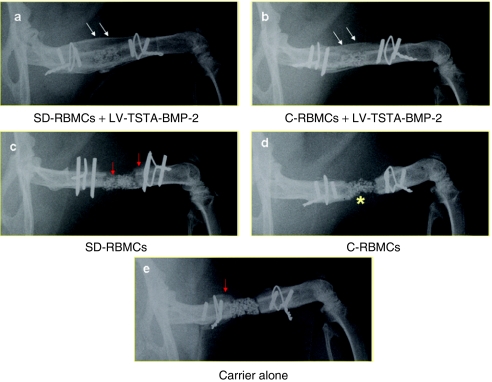
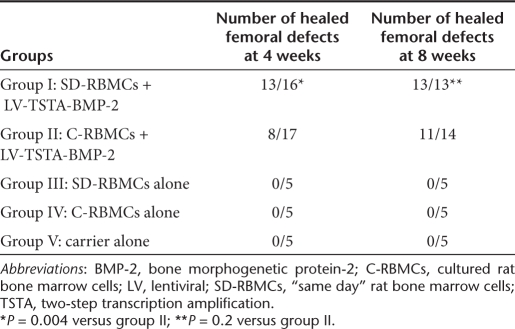
Femoral defects treated with transduced “same day” cells demonstrate earlier radiographic healing when compared to the femoral defects treated with transduced cultured BMCs. Radiographic healing was defined as osseous continuity across the femoral defect.14 There was a very good agreement (kappa statistic, k = 0.88) among the three independent observers with respect to the evaluation of radiographic healing. At 8 weeks, all of the 13 femoral defects in group I (SD-RBMCs + LV-TSTA-BMP-2) animals demonstrated new bone formation that bridged across the femoral defects (complete radiographic healing) (Figure 3). Two animals in group I demonstrated new bone formation that extended into the surrounding muscles of the thigh (heterotopic new bone) at 4 weeks (Supplementary Figure S1). The radiographs at 8 weeks in these two animals did not demonstrate any further increase in the amount of this heterotopic bone. In group II (C-RBMCs + LV-TSTA-BMP-2) animals, 11 out of 14 defects demonstrated complete radiographic healing at 8 weeks. The radiographic healing rates in group I defects (13/16; 81%; P = 0.004) were significantly higher at 4 weeks when compared to the group II defects (8/17; 47%; Table 1). However, at 8 weeks the radiographic healing rates in group I (13/13; 100 percent) were not statistically different (P = 0.22) when compared to the group II defects (11/14; 79%). In the control groups [group III (C-RBMCs), group IV (SD-RBMC), and group V (carrier alone]) there was some new bone formation present but none of the defects (0 out of 15) had healed at 8 weeks.
Figure 3.
Plain radiographs. (a) Representative radiographic images of healed femoral defect in animals treated with transduced “same day” cells (SD-RBMCs + LV-TSTA-BMP-2). Double white arrows depict bridging bone across femoral defect and restoration of cortex. (b) Femoral defects treated with transduced cultured bone marrow cells (C-RBMCs + LV-TSTA-BMP-2) demonstrate bridging new bone formation (double white arrows) across the femoral defect 8 weeks after the cell implantation. The defects treated with (c) nontransduced “same day” cells (SD-RBMCs), (d) nontransduced cultured bone marrow cells (C-RBMCs) or (e) carrier alone demonstrated some periosteal new bone formation (red arrows) but none of these defects demonstrated complete radiographic healing. The composite carrier (compression resistant matrix) was used to deliver the cells in the femoral defect (yellow asterisk in d). BMP-2, bone morphogenetic protein-2; LV, lentiviral; SD-RBMCs, “same day” rat bone marrow cells; TSTA, two-step transcription amplification.
Table 1. Radiographic results.
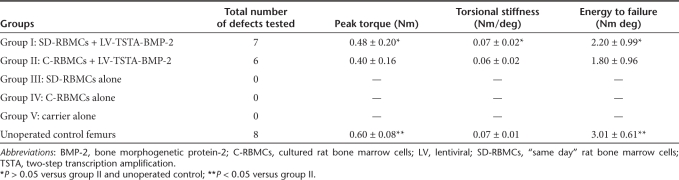
Femoral defects treated with the transduced “same day” cells demonstrate a biomechanical equivalence to the unoperated control femurs. In order to assess the mean energy to failure (ETF), maximum torque, and torsional stiffness of the bone formed in the defects, torsional biomechanical testing was performed on healed femurs at 8 weeks following surgery. There were no significant differences (P > 0.05) with respect to the ETF, maximum torque, and torsional stiffness in the group I defects (SD-RBMCs + LV-TSTA-BMP-2) when compared to the group II (C-RBMCs + LV-TSTA-BMP-2) defects (Table 2).
Table 2. Torsional biomechanical testing.
The mean ETF, maximum torque, and torsional stiffness in unoperated age-matched controls were 3.01 ± 0.61 Nm deg, 0.6 ± 0.08 Nm, 0.07 ± 0.01 Nm/deg, respectively. The ETF in group I defects at 8 weeks was 73% of the intact control femora (P > 0.05) whereas the ETF in group II defects was 60% of the intact control femora (P < 0.05). In group I defects, the peak torque at 8 weeks was 80% of the untreated controls (P > 0.05) where as the peak torque in group II defects reached 67% of the intact controls (P < 0.05) at the end of 8 weeks. There were no significant differences with respect to torsional stiffness in the unoperated controls when compared to either the group I or the group II defects. None of the femoral defects in group III (SD-RBMCs), group IV (C-RBMCs), or group V (carrier alone) healed, and consequently biomechanical testing was not performed in these specimens.
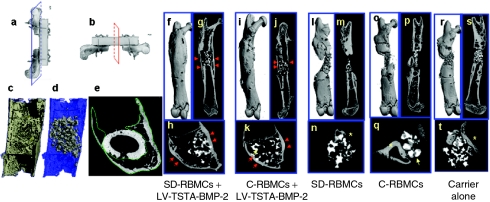
Femoral defects treated with the transduced “same day” cells demonstrate significantly increased bone formation on micro-CT when compared to the femoral defects treated with transduced cultured BMCs. The volumetric assessment of the total new bone formed in the region of the femoral defect was performed by the micro-computed tomography (micro-CT) scans of the harvested femoral specimens. The healed femoral defects in group I (SD-RBMCs + LV-TSTA-BMP-2) and group II (C-RBMCs + LV-TSTA-BMP-2) demonstrated osseous continuity and reconstitution of the cortices in the region of femoral defect (Figure 4). There were no significant differences (P > 0.05) with respect to the bone volume fraction (bone volume/total volume; BV/TV) in the healed group I (38 ± 11.1%) and group II defects (39 ± 8.4%) (Supplementary Table S3). However, the BV in group I defects (93 ± 6.5 mm3) was significantly higher (P < 0.001) when compared to the BV in group II defects (64.1 ± 15.5 mm3). None of the animals demonstrated a continuous cortex across the femoral defect in groups III, IV, and V and there was less new bone formation in the region of femoral defect compared when compared to groups I and II.
Figure 4.
Micro-computed tomography (micro-CT) images. Representative micro-CT images obtained in the (a) longitudinal plane (blue color) and (b) axial planes (red color) of femur specimens from each study group at 8 weeks. (c–e) The delineation of quantified callus bone volume (enclosed within green color contour in e) from the native host bone and the scaffold material (gray scale material in d). The healed femora in the group I (f–h; RBMCs + LV-TSTA-BMP-2) and the group II (i–k; C-RBMCs + LV-TSTA-BMP-2) demonstrate abundant new bone bridging the femoral defect. There is formation of new cortices (red arrows in g, h, j, and k) and reconstitution of the medullary canal across the femoral defect. The carrier scaffold (yellow arrows in k and q) used to deliver the cells has higher radiodensity and is easily distinguished from the original cortex and the new bone formed in the defect. There is some periosteal new bone formed at the host defect interface and under the polyethylene plate (yellow asterisk) in the defects treated with the nontransduced “same day” cells (SD-RBMCs; l–n), nontransduced cultured bone marrow cells (C-RBMCs; o–q), or carrier alone (r–t) but none of these defects demonstrated any bridging new bone formation (longitudinal images l–p, r, and s). BMP-2, bone morphogenetic protein-2; LV, lentiviral; SD-RBMCs, “same day” rat bone marrow cells; TSTA, two-step transcription amplification.
Histologic and histomorphometric analysis
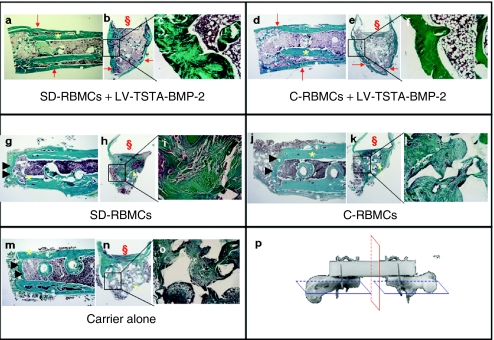
Histologic analyses of the femoral defects in group I animals (SD-RBMCs + LV-TSTA-BMP-2) demonstrated a new bony cortex that bridged across the proximal and distal ends of the femoral defect in two out of three animals sacrificed at 4 weeks. None of the three femoral defects examined histologically at 4 weeks in group II (C-RBMCs + LV-TSTA-BMP-2) animals were healed and they had variable amount of new bone formation at the proximal or distal end of the femoral defect. At 8 weeks, a continuous bony cortex was present between the proximal and distal end of the femoral defects in group I and group II animals (Figure 5). At either end of the femoral defect, trabecular new bone was present between the original host cortex and the new bony cortex. Furthermore, there was reconstitution of the femoral canal in the region of femoral defect. The femurs that did not show complete radiographic healing in group II animals (3 out of 14) demonstrated a variable amount of new bone formation in the femoral defect. In contrast, the femoral defects in group III (SD-RBMCs), group IV (C-RBMCs), and group V (carrier alone) animals demonstrated new bone formation at the proximal and distal ends of the femoral defect but there was no continuous bony cortex across the femoral defect (Figure 5). Furthermore, the medullary canal in the proximal and distal end of the femoral defect was obliterated by the fibrous tissue and the trabecular bone.
Figure 5.
Histologic analysis of study groups at 8 weeks. Representative longitudinal and transverse histologic sections stained with Mason trichrome were obtained in the longitudinal (blue color, p) and axial plane (red color, p) of the femur. The original host cortex is labeled with yellow asterisk in the longitudinal sections. The position of the polyethylene plate (construct shown in p) is labeled (§) in the transverse sections for the orientation purpose. Within each panel there are three images: a longitudinal section (×1), followed by a transverse section through the middle of the defect (×1) and lastly a higher magnification image (×10) of the inset. The healed femora in group I (a–c; SD-RBMCs + LV-TSTA-BMP-2) and group II (d–f; C-RBMCs + LV-TSTA-BMP-2) animals demonstrate abundant new bone formation at the host defect interface (red arrow in a and d) and across the defect (red arrows in b and e). There is reconstitution of the new cortices and the medullary canal in the region of the defect in groups I and II signifying complete healing. In contrast to the healed defects in groups I and II, the defects in group III (SD-RBMCs; g–i), group IV (C-RBMCs; j–l), and group V (carrier alone; m–o) demonstrated obliteration of the medullary canal at the host defect interface (black arrow heads in g, j, and m). There was fibrous tissue mixed with the composite carrier (yellow arrows in h, k, and n) and some new bone in the center of the defects in groups III, IV, and V (control groups). BMP-2, bone morphogenetic protein-2; LV, lentiviral; SD-RBMCs, “same day” rat bone marrow cells; TSTA, two-step transcription amplification.
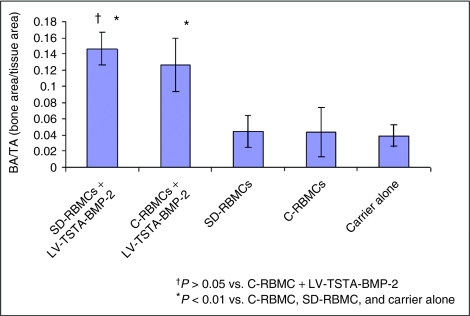
There were no significant differences (P > 0.05) with respect to the bone area to tissue area fraction (BA/TA) in the group I defects (0.15 ± 0.02) when compared to the group II defects (0.13 ± 0.03; Figure 6). Furthermore, there were no significant differences (P > 0.05) with respect to the total bone area and the tissue area in group I defects when compared to the group II defects. The BA/TA in group III (0.04 ± 0.02), group IV (0.04 ± 0.03) and group V (0.04 ± 0.01) femoral defects were significantly smaller than group I (P < 0.01 versus groups III or IV or V) or group II femoral defects (P < 0.01 versus groups III or IV or V).
Figure 6.
Histomorphometric analysis (bone area/tissue area). The bone area/tissue area (BA/TA) in defects treated with transduced “same day” cells that overexpress BMP-2 (SD-RBMCs + LV-TSTA-BMP-2) was significantly higher (P < 0.01) than the defects treated with the nontransduced “same day” cells (SD-RBMCs) or defects treated with carrier alone. The defects treated with the transduced cultured bone marrow cells that overexpress BMP-2 (C-RBMCs + LV-TSTA-BMP-2) were significantly higher (P < 0.01) than the defects treated with nontransduced bone marrow cells (C-RBMCs) or treated with carrier alone. Furthermore, there was no significant difference (P > 0.05) between the defects treated with transduced “same day” cells and defects treated with transduced cultured bone marrow cells with respect to BA/TA. BMP-2, bone morphogenetic protein-2; LV, lentiviral; SD-RBMCs, “same day” rat bone marrow cells; TSTA, two-step transcription amplification.
Discussion
The results of this study demonstrate the clinical potential of a novel “same day” ex-vivo regional gene therapy approach to promote bone repair, which may make gene therapy cost-effective when adapted for human use. The “same day” strategy eliminates the cost and inconvenience associated with the traditional two-step ex vivo approach, which limited the clinical potential of gene therapy as a therapeutic regimen to treat nonlethal bone repair problems. In this study, the processed RBMCs (“same day” cells) were harvested, transduced, and implanted in <3 hours into a critical sized femoral defect that healed completely at 8 weeks. The defects treated with the transduced “same day” cells demonstrated earlier radiographic healing (P = 0.004) and significantly increased BV (micro-CT; P < 0.001) compared to the defects treated with cultured BMCs. Furthermore, at 8 weeks the peak torque and the ETF in the femoral defects treated with the transduced “same day” cells were 80 and 73%, respectively of the untreated controls (P > 0.05) whereas the peak torques and the ETF in defects treated with transduced cultured BMCs were 67 and 60%, respectively of the untreated controls (P < 0.05). The superior quality of bone repair seen with the “same day” strategy could be related to higher BMP-2 production by the “same day” cells in vivo. It is also possible that the hematopoietic fraction in the “same day” cells (buffy coat cells) provides a favorable milieu similar to a fracture hematoma and leads to better bone repair. The hematopoietic fraction in the “same day” cells contain platelets, monocytes and macrophages which are also present in the fracture hematoma and have been shown to be a source of osteoinductive and angiogenic factors during fracture repair.32
In vivo gene therapy involves direct injection of viral vector into the desired anatomic site and is one of the options that can provide prolonged delivery of BMPs. In vivo gene therapy is easy to use but presence of lower transduction efficiency, lack of control over the target cell population and potential risks associated with direct viral inoculation make this strategy less attractive.18 In addition, this therapy may not be effective in clinical situations where there is a compromised biological environment such as a large bone defect and devascularized soft tissue. There may be an insufficient number of responding cells at the bone defect site. Ex-vivo regional gene therapy provides delivery of osteoprogenitor cells and sustained delivery of transgene product and has a potential to be used clinically in the future for healing large bone defects in a compromised host environment. One of the potential limitations of the adoption of ex-vivo regional gene therapy for clinical use is the cost, time, and inconvenience associated with the culture expansion of stem cells. This is the major reason that we developed “same day” gene therapy even though we had success with the traditional “two stage” approach in preclinical models.14,21,33,34 The other concerns associated with in vitro expansion of the bone marrow stem cells include alteration in the morphology as well as biological characteristics of the cultured cells and a potential risk of infection.35,36,37
It has been proposed that strategies that can eliminate or minimize the culture expansion will make ex vivo gene therapy more clinically adaptable. In a preclinical study using a rat critical sized femoral defect model, Betz et al. and Evans et al. recently demonstrated that treatment of femoral defects with syngeneic muscle or adipose tissue grafts that were transduced with a BMP-2-expressing cDNA or adenoviral vector for 24 hours consistently healed femoral bone defects in rats. The femoral defects that were treated with nontransduced muscle or adipose tissue grafts did not heal.28,30,38 The strategy proposed in these studies minimizes the harvest of cells from the tissue and obviates the need to expand the cells in culture for weeks. However, when translated to clinical use this strategy will require two separate surgical procedures performed at least 24 hours apart. One procedure will involve harvest of the graft and the second one will allow for the implantation of the genetically modified graft to the desired anatomic site. This technique shows clinical promise but it is more technically difficult to harvest adipose or muscle tissue grafts from a patient when compared to a bone marrow harvest, which is very simple to perform. The critical element of the “same day” strategy is that cell harvest and genetic manipulation of the donor cells occurs in one sitting so that the transduced cells will be implanted into the bone defect as a single composite procedure on the same day within 3 hours of the cell harvest. In addition, optimization of the viral transduction process will lead to a further reduction in the overall transduction time and could make this strategy applicable for not only complex but simple bone graft procedures.
Vector selection is a critical component in the development of regional ex vivo gene therapy. We have used both lentiviral and adenoviral vectors in a preclinical model to evaluate bone repair. There has been a significant interest in using nonviral vectors to avoid concerns regarding insertional mutagenesis and the immunogenic response to viral particles.24,39 However, in preclinical studies in our laboratory (J.R. Lieberman unpublished results) using liposomes we were unable to produce sufficient BMP to heal a critical bone defect.
Although, we and others have demonstrated that gene therapy with an adenoviral vector can heal segmental defects in animal models, there is concern using this strategy in humans because of the immunogenic response to adenoviral proteins and a limited healing potential because of the short duration of protein expression.14,15,16,21,33 This hypothesis was supported by a prior study using the same femoral defect model. We noted better quality of bone repair induced by BMP-2-producing RBMCs using a lentiviral vector compared with the BMCs transduced with an adenoviral vector containing the BMP-2 cDNA.21
Our hypothesis was that a successful “same day” gene therapy strategy requires a vector with high transduction efficiency and stronger transgene expression so that sufficient protein production can be induced in the donor BMCs following a short duration of transduction. We used a lentiviral vector in this study because these vectors have the ability to induce several months of protein production, which will be necessary to successfully heal the large bone defects in which regional gene therapy will be most appropriate.20,21,34 Moreover lentiviral vectors insert into the host chromosome, have high packaging capacity and transduction is not limited by cell division.23 In order to attain enhanced transgene expression, we designed a lentiviral vector in our laboratory using the two-step amplification system (TSTA). TSTA-based vector systems have been successfully used in transgenic mice to enhance the expression of reporter genes, which are driven by weak promoters.40 Iyer et al. demonstrated a six- to sevenfold increase in the protein activity in prostate cancer cells transduced with a TSTA-based lentiviral vector in the presence of androgens.41 The lentiviral TSTA system used in our study induced more than a tenfold increase in BMP-2 production compared to the standard lentiviral vector which is in accordance with the study by Iyer et al.41
There are safety concerns related to the use of lentiviral vectors in humans. These include the replication competence of viral vectors, insertional mutagenesis, and immune response to viral proteins.42 Introduction of self-inactivating HIV-1-based vectors, deletion of the promoter/enhancer domain within the long-terminal repeat, and elimination of accessory genes from the packaging construct are important modifications that have been incorporated into the vector systems to improve their safety profile but further studies are necessary.43 In addition, the safety profile of our regional gene therapy related to the biodistribution of the virus to other anatomic sites and potential for prolonged gene expression with excessive new bone formation needs to be established before this strategy can be adapted to treat bone healing problems in humans.
The ease of harvest and the fact that bone marrow contains mesenchymal stem cells make autologous bone marrow an attractive cell-based therapy to enhance bone healing without any fear of immune rejection. Connolly et al. have demonstrated that percutaneous administration of autologous bone marrow aspirate can stimulate healing of tibial nonunions but multiple injections over time were necessary.44 Hernigou et al. used percutaneous autologous bone marrow injection to heal 53 of 60 tibial nonunions. All the seven nonunions that failed to heal had significantly lower number and concentration of progenitors compared to the nonunions that healed.45 In a preclinical study from our laboratory, Cuomo et al. found a wide variability in the concentration of mesenchymal stem cells among the young healthy donors. Furthermore, the number of mesenchymal cells in the aspirated bone marrow can be increased approximately sevenfold via stem cell concentration. However, the healing rate of critical sized femoral defects in nude rats that were treated with human bone marrow was very low (2 out of 34 animals) and it was concluded that a higher number of stem cells or a stronger osteoinductive signal was required to heal the defects.46 The aforementioned studies suggest the importance of delivering an adequate osteogenic signal at the bone repair site to produce effective bone healing. By transducing the cells with a vector-containing cDNA for BMP-2, we are enhancing the biologic potential of the cells and delivering the protein to the specific anatomic site.
Two femoral defects treated with transduced “same day” cells demonstrated heterotopic ossification surrounding the healed femoral defects. The number of cells and the amount of in vitro BMP-2 production by the transduced cells that were implanted in these two animals were not significantly different from the transduced “same day” cells used for other animals in this experimental group. This exuberant callus may be secondary to the leaking of cells from the carrier into the adjacent muscle or due to excessive soft tissue trauma at the time of the procedure. Alternatively, this finding could be a reflection of the enhanced migratory capabilities of the processed marrow used in the “same day” strategy that includes hematopoietic cells in addition to the mesenchymal stem cells. In the culture expanded BMCs, the hematopoietic cell fraction is eliminated over serial passages because of its inability to adhere to the culture plates. Although the lentiviral vector containing the cDNA was used successfully in both the “same day” and traditional two-step approach, there is a concern that constitutive expression of BMP-2 may lead to excessive bone formation with the vector. Therefore, we are interested in limiting BMP production after the bone healing has occurred. Multiple drug-dependent regulatable systems (e.g., tet-on and tet-off inducible gene expression system) have been developed for fine regulation of gene expression but these systems have limitations, which include basal leakiness, pleiotropic effects of the inducer, toxicity of inducing agents, and low levels of expression.47 Suicide gene therapy using HSV-Tk system is another potential option for termination of constitutive transgene expression. We have designed a bicistronic lentiviral vector that has the ability to constitutively express the therapeutic transgene and also has an inducible suicide gene system that can be activated by ganciclovir. Therefore, the cells carrying the viral vector can be killed by administration of ganciclovir once the therapeutic response has been achieved. This strategy needs to be evaluated in appropriate animal models.
A limitation of this study is that we do not know the ultimate fate and contribution of the transduced donor hematopoietic cells to the bone repair in the “same day” strategy. Nor do we know the duration of survival of the transduced donor cells following implantation. A prior study from our laboratory has demonstrated that the transduced cells do get incorporated into the new bone formed at the site of bone repair.48 In addition, we have noted that lentiviral gene expression persists for at least 2 months in vivo.21
In conclusion, we have demonstrated that a novel “same day” ex-vivo regional gene therapy strategy using transduced BMCs can effectively heal a critical sized femoral defect in an animal model without the requirement to expand these cells in tissue culture. The “same day” regional gene therapy has the potential to be a convenient, cost-effective, and clinically adaptable treatment option for promoting bone repair in challenging clinical scenarios characterized by large bone defects and a compromised host environment.
Materials and Methods
Isolation and culture expansion of RBMCs (C-RBMCs) for the traditional two-step ex vivo strategy. Bone marrow was harvested from the femurs and tibias of 8–10-week-old male Lewis rats (Charles River Laboratories, Wilmington, MA) under sterile conditions according to a previously published protocol.14 The RBMCs were cultured in Iscove's modified Dulbecoco's media (GIBCO Invitrogen, Grand Island, NY) supplemented with 15% fetal bovine serum (Omega Scientific, Tarzana, CA), penicillin 100 U/ml and streptomycin 100 µg/ml. The RBMCs were maintained in a humidified atmosphere and 5% CO2 at 37 °C. The culture medium was replaced every 3–4 days, and all nonadherent cells were then removed. When confluent, the adherent BMCs were trypsinized and passaged approximately at a 1:3 split. We used passage 3 cells for viral transduction.
Isolation and processing of rat bone marrow for the “same day” ex vivo strategy (SD-RBMCs). Bone marrow was harvested from the femurs and tibias of 8–10-week-old male Lewis rats as described above. Bone marrow was centrifuged for 5 minutes and resuspended with phosphate-buffered saline (PBS; GIBCO, Invitrogen). The cell suspension was layered over the Histopaque-1083 solution (Sigma-Aldrich, St Louis, MO) and centrifuged at 400g for 30 minutes at 4 °C. Buffy coat cells (SD-RBMCs) at the interface were collected and washed twice in PBS.
Lentiviral vector construction using TSTA system. In order to obtain enhanced BMP-2 production, a standard lentiviral vector (LV-RhMLV-BMP-2) was modified to a TSTA system.31 The TSTA system requires two different lentiviral vectors namely the transactivator vector and the transgene expression vector (Figure 2). Two plasmids namely, the TSTA-NSN plasmid-containing GAL4-VP16 sequence and the TSTA-fl-hrl PBIL plasmid-containing G5 promoter (five Gal4-binding site) were used to create lentiviral vectors for the TSTA system. The transactivator vector plasmid of the TSTA system (Lenti-RhMLV-GAL4-VP16) was created by replacing enhanced green fluorescent protein (EGFP) in our original lentiviral vector (Lenti-RhMLV-EGFP) with GAL4-VP16 cDNA. We then constructed the GAL4 responsive transgene expression vector (Lenti-G5-EGFP) by replacing the RhMLV promoter with G5 promoter in the Lenti-RhMLV-EGFP. The GAL4-VP responsive BMP-2 expression vector was constructed using the Lenti-G5-BMP-2 plasmid, which was created by replacing EGFP with BMP-2 cDNA in the Lenti-G5-EGFP vector. Both constructs contain the Rev-responsive element and the central polyprine tract, which enhance the efficiency of gene expression.
All lentiviral vector stocks were generated by calcium phosphate-mediated transfection of 293T cells (American Type Culture Collection, Manassas, VA) as described previously.49 The titer of TSTA lentiviral vectors was determined by comparison of p24 protein contents between the TSTA lentiviral vectors and LV-RhMLV-GFP vector. The p24 content in each viral lot was quantified by enzyme-linked immunosorbent assay (ELISA). The titer of control LV-RhMLV-GFP vector was calculated from ratio of GFP+ cells determined by flow cytometric analysis. The GFP+ cells were counted at 2 days after transduction of 293T cells. The titers of all TSTA vector stocks were ~1–2 × 108 transducing units/ml. Optimal MOI for cultured BMCs was determined by green fluorescent protein (GFP) expression and cytotoxicity induced by serial MOI of transduction. When RBMCs were transduced with LV-RhMLV-GFP vector at MOI of 1, 5, 25, or 125, transduction at MOI of 1 demonstrated GFP expression in ~60% of the cells by flow cytometry. Although over 90% of the cells were positive at MOI of 5 and 25, MOI of 25 induced greater fluorescence intensity of GFP than MOI of 5. At higher MOI (125), more than half of the cells died secondary to the toxicity of the virus. Therefore, we used an MOI of 25 in this study because we could obtain higher transduction efficiency without causing viral toxicity-related cell death.
Viral transduction of cultured RBMCs. Culture expanded RBMCs (C-RBMCs) were plated on 10-cm culture dishes at a density of 1×106 cells in 5-ml Iscove's modified Dulbecoco's media supplemented with 15% fetal bovine serum/dish. Viral transductions were carried out in the presence of 8-µg/ml polybrene at MOI of 25/25 for Lenti-RhMLV-GAL4-VP16/Lenti-G5-BMP-2. The transduced cells were centrifuged (1,000 r.p.m. × 5 minutes) and washed with PBS three times before implantation to remove the extra virus suspended in cell culture medium and minimize contamination of final cell mixture with extracellular virus. The C-RBMCs were then resuspended in 60 µl of PBS and placed on ice until implantation into a femoral defect.
Viral transduction of “same day” cells. The “same day” RBMCs (SD-RBMCs) were plated into a 6-well plate with 5 × 106 cells per well in Iscove's modified Dulbecoco's media supplemented with 15% fetal bovine serum. Viral transductions were carried out in the presence of 8-µg/ml polybrene at MOI of 25/25 for Lenti-RhMLV-GAL4-VP16/Lenti-G5-BMP-2. The cells were incubated in a humidified atmosphere and 5% CO2 at 37 °C for 1 hour. The transduced cells were centrifuged (1,000 r.p.m. × 5 minutes) and washed with PBS three times before implantation to remove the extra virus suspended in cell culture medium and minimize contamination of final cell mixture with extracellular virus. The transduced SD-RBMCs were then resuspended in 60 µl of PBS and placed on ice until implantation into the femoral defect. The total time required for the cell preparation using the “same day” strategy was <3 hours and this included a viral transduction time of 1 hour (Figure 1).
ELISA for BMP-2 production. In vitro BMP-2 production in C-RBMCs and SD-RBMCs was quantified using an ELISA kit (Quantikine; R&D Systems, Minneapolis, MN) at weekly intervals for 4 weeks according to the manufacturer's instructions. ELISA was performed on the conditioned media that was collected from the cells cultured for 24 hours and the BMP-2 production was reported as ng/ml.
In vivo bone formation at an orthotopic site. After appropriate approval from the institutional animal review committee, femoral defect procedures were performed on 14-week old, male Lewis rats according to a previously published protocol.14 Briefly, all the animals were anesthetized using inhalational anesthesia (2–3% isoflurane and oxygen) and hind limbs were prepared for the operation under standard sterile conditions. The femur was then approached posterolaterally with the rat in the prone position. The periosteum was incised and elevated circumferentially. A polyethylene plate, measuring 23 × 4 × 4 mm was secured to the femur with four 0.99-mm transverse-threaded Kirschner wires and two 1.0-mm surgical steel cerclage wires. An 8-mm critical size complete defect was then created in the middle of the femur with the use of a 1-mm side-cutting carbide burr under irrigation with sterile saline solution. After the segmental defects were created, an 8 × 4 × 4 mm compression resistant matrix (calcium-phosphate ceramic plus type 1 bovine collagen; Medtronic Sofamor Danek, Memphis, TN) was placed in all the defects. Overlying muscle was closed with Vicryl suture securing the carrier in place followed by skin closure. Following surgery, the animals were placed in a recovery chamber and allowed to bear weight immediately.
Study groups. A total of 48 femoral defects were divided into 1 of the 5 treatment groups (Supplementary Table S1). The femoral defects in group I (n = 16) animals received 15 × 106 SD-RBMCs transduced with lentiviral vector-expressing BMP-2 (LV-TSTA-BMP-2). The femoral defects in group II animals (n = 17) received 5 × 106 C-RBMCs transduced with LV-TSTA-BMP-2 vector. The femoral defects in group III (n = 5) and group IV (n = 5) animals received nontransduced SD-RBMCs (15 × 106) and nontransduced C-RBMCs (5 × 106), respectively. The animals in group III and group IV served as controls for group I and group II animals, respectively. The femoral defects in group V (n = 5) animals received the carrier (compression resistant matrix) alone without any cells. In addition, age-matched femora from eight unoperated male Lewis rats were used as controls for the torsional biomechanical testing. All but six animals (three each in groups I and II) were sacrificed at 8 weeks. Three animals in group I and group II were sacrificed at 4 weeks.
Radiographic analysis. Radiographs were performed using Faxitron equipment (Field Emission, McMinnville, OR) at 4- and 8-weeks time points (Supplementary Table S1). The radiographs were analyzed by three-blinded independent observers to assess the healing response within the bone defect using a previously established protocol.21 Kappa statistic was calculated to asses the interobserver variability among the three observers.
Micro-CT scan. The femoral specimens were analyzed with the micro-focus X-ray computed tomography CT scans (µCT40; Scanco Medical AG, Bruttisellen, Switzerland) to assess the volume of new bone formed at the defect site at 8 weeks. Serial tomographic images were acquired at 55 kV and 145 µA, collecting 1,000 projections per rotation at 300 msec integration time. Three-dimensional 16-bit grayscale images were reconstructed using standard convolution back projection algorithms with Shepp and Logan filtering, and rendered at a discrete density of 244,141 voxels/mm3 (isometric16-µm voxels). Native cortical bone, callus formation, and scaffold material were identified on the basis of X-ray attenuation signatures, and segmented from marrow and soft tissue into discrete volumetric components. We measured both the cortical new bone as well as the trabecular new bone that had formed in the femoral defect region. The innermost pins of the surgical fixation construct (plate, pins, and cerclage wire) on either side of the femoral defect were used as a landmark for reproducible measurement of the region of interest.
Mechanical testing. Torsional testing of operated femora was performed to assess the quality of new bone that had formed to heal the femoral defect according to a previously published protocol.21 Unoperated femurs of age and gender matched Lewis rats (n = 8) were used as controls. The animals were euthanized 8 weeks after the surgery and the surrounding soft tissue, hardware, and polyethylene plate were removed. The proximal and distal ends of the femur specimen were embedded in polymethylmethacrylate blocks centering the longitudinal bone axis with the axis of torsion. The embedded specimen was then mounted in a torsional-testing fixture that was attached to a mechanical test machine (MTS, Minneapolis, MN). The distal end of the specimen was rotated laterally at a rate of 15 degrees per minute until bone failure was observed. Peak torque, ETF, and torsional stiffness parameters were calculated from the torque-rotation curves.
Histology and histomorphometric analysis. A total of 35 femora were analyzed histologically (Supplementary Table S1). After the animals were sacrificed at the 4- or 8-week time points, the quadriceps muscle and overlying soft tissue was resected, and internal hardware removed. Histologic specimens were fixed in 10% buffered formalin at 4 °C for 5 days followed by decalcification in 10% EDTA for 8 weeks at room temperature with gentle mechanical stirring. The specimens were then dehydrated and embedded in paraffin.
The histologic sections were performed in two planes according to a previously established protocol (Figure 5).14,33 The specimen was cut through the center of the femoral defect to generate two separate specimens each having one half of the femoral defect at its end. Axial/transverse histologic sections were then obtained from the side of the specimen that had the femoral defect. After the axial sections were obtained, the specimens were re-embedded and histologic sections were performed in a plane that was parallel to the long axis of the femur (longitudinal section). Using the Bioquant analysis software the new bone formed (bone area) and the total tissue area was selected using the threshold tools. The mean bone area/tissue area was reported. All the sections were stained with hematoxylin and eosin and Mason's trichrome stain. Cross-sections from each study group were analyzed histomorphometrically using the Olympus imaging system with the Bioquant Osteo system (Bioquant Image Analysis, Nashville, TN) according to a previously published protocol.14
Statistical analysis. The number of animals in the experimental and control groups were determined with power analysis based on previous preclinical gene therapy studies performed in our laboratory. We anticipated both the “same day” ex vivo gene therapy and the traditional ex vivo gene therapies to have a high percent of healing. With α set at 0.05, 14 animals in each experimental group would give us a power of >80% to allow comparison of statistical equivalence between the “same day” and traditional ex vivo treatment groups. It was determined that five animals would be adequate for each of the control groups as they are expected to have no healing.
The radiographic results were compared using a Fischer exact test. A kappa statistic was calculated as a measure of interobserver reliability for the three-blinded independent observers. The data collected during the ELISA, biomechanical testing, micro-CT imaging, and histomorphometric analysis was expressed as mean ± SD. One-way analysis of variance analysis with a post hoc (Newman–Keuls multiple comparison) test or a Student's t-test was used to compared means among different groups. Statistical significance was reported to be present when P < 0.05.
SUPPLEMENTARY MATERIAL Figure S1. Heterotopic ossification in the “same day” gene therapy. Representative plain radiograph of animals (n=2) in the SD-RBMC+LV-TSTA-BMP-2 treatment group that developed heterotopic new bone formation (white arrow heads). There was no new bone formation visible elsewhere in the body on plain radiographs in these two animals at 4 or 8 weeks. Table S1. Study groups. Table S2. In vitro BMP-2 production. Table S3. Micro-CT analyses.
Acknowledgments
This work was supported by a research grant from the National Institute of Health (RO1 AR057076-01A1 to J.R.L). The authors declared no conflict of interest.
Supplementary Material
Heterotopic ossification in the “same day” gene therapy. Representative plain radiograph of animals (n=2) in the SD-RBMC+LV-TSTA-BMP-2 treatment group that developed heterotopic new bone formation (white arrow heads). There was no new bone formation visible elsewhere in the body on plain radiographs in these two animals at 4 or 8 weeks.
Study groups.
In vitro BMP-2 production.
Micro-CT analyses.
REFERENCES
- Gamradt SC., and, Lieberman JR. Bone graft for revision hip arthroplasty: biology and future applications. Clin Orthop Relat Res: 2003. pp. 183–194. [DOI] [PubMed]
- St John TA, Vaccaro AR, Sah AP, Schaefer M, Berta SC, Albert T.et al. (2003Physical and monetary costs associated with autogenous bone graft harvesting Am J Orthop (Belle Mead NJ) 3218–23. [PubMed] [Google Scholar]
- Heneghan HM., and, McCabe JP. Use of autologous bone graft in anterior cervical decompression: morbidity & quality of life analysis. BMC Musculoskelet Disord. 2009;10:158. doi: 10.1186/1471-2474-10-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Rhim R, Li L, Martha J, Swaim BH, Banco RJ.et al. (2009Prospective study of iliac crest bone graft harvest site pain and morbidity Spine J 9886–892. [DOI] [PubMed] [Google Scholar]
- Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R.et al. (2002Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients J Bone Joint Surg Am 84-A2123–2134. [DOI] [PubMed] [Google Scholar]
- Swiontkowski MF, Aro HT, Donell S, Esterhai JL, Goulet J, Jones A.et al. (2006Recombinant human bone morphogenetic protein-2 in open tibial fractures. A subgroup analysis of data combined from two prospective randomized studies J Bone Joint Surg Am 881258–1265. [DOI] [PubMed] [Google Scholar]
- Glassman SD, Carreon LY, Djurasovic M, Campbell MJ, Puno RM, Johnson JR.et al. (2008RhBMP-2 versus iliac crest bone graft for lumbar spine fusion: a randomized, controlled trial in patients over sixty years of age Spine 332843–2849. [DOI] [PubMed] [Google Scholar]
- Betz OB, Betz VM, Abdulazim A, Penzkofer R, Schmitt B, Schröder C.et al. (2010The repair of critical-sized bone defects using expedited, autologous BMP-2 gene-activated fat implants Tissue Eng Part A 161093–1101. [DOI] [PubMed] [Google Scholar]
- Vaidya R, Carp J, Sethi A, Bartol S, Craig J., and, Les CM. Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur Spine J. 2007;16:1257–1265. doi: 10.1007/s00586-007-0351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford CH., 3rd, , Carreon LY, McGinnis MD, Campbell MJ., and, Glassman SD. Perioperative complications of recombinant human bone morphogenetic protein-2 on an absorbable collagen sponge versus iliac crest bone graft for posterior cervical arthrodesis. Spine. 2009;34:1390–1394. doi: 10.1097/BRS.0b013e3181a2da08. [DOI] [PubMed] [Google Scholar]
- Haidar ZS, Hamdy RC., and, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A: Current challenges in BMP delivery. Biotechnol Lett. 2009;31:1817–1824. doi: 10.1007/s10529-009-0099-x. [DOI] [PubMed] [Google Scholar]
- Shields LB, Raque GH, Glassman SD, Campbell M, Vitaz T, Harpring J.et al. (2006Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion Spine 31542–547. [DOI] [PubMed] [Google Scholar]
- Garrison KR, Donell S, Ryder J, Shemilt I, Mugford M, Harvey I.et al. (2007Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review Health Technol Assess (Winchester, England) 111–150.iii–iv. [DOI] [PubMed] [Google Scholar]
- Lieberman JR, Daluiski A, Stevenson S, Wu L, McAllister P, Lee YP.et al. (1999The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats J Bone Joint Surg Am 81905–917. [DOI] [PubMed] [Google Scholar]
- Dragoo JL, Lieberman JR, Lee RS, Deugarte DA, Lee Y, Zuk PA.et al. (2005Tissue-engineered bone from BMP-2-transduced stem cells derived from human fat Plast Reconstr Surg 1151665–1673. [DOI] [PubMed] [Google Scholar]
- Lee JY, Peng H, Usas A, Musgrave D, Cummins J, Pelinkovic D.et al. (2002Enhancement of bone healing based on ex vivo gene therapy using human muscle-derived cells expressing bone morphogenetic protein 2 Hum Gene Ther 131201–1211. [DOI] [PubMed] [Google Scholar]
- Rutherford RB, Moalli M, Franceschi RT, Wang D, Gu K., and, Krebsbach PH. Bone morphogenetic protein-transduced human fibroblasts convert to osteoblasts and form bone in vivo. Tissue Eng. 2002;8:441–452. doi: 10.1089/107632702760184709. [DOI] [PubMed] [Google Scholar]
- Carofino BC., and, Lieberman JR. Gene therapy applications for fracture-healing. J Bone Joint Surg Am. 2008;90 Suppl 1:99–110. doi: 10.2106/JBJS.G.01546. [DOI] [PubMed] [Google Scholar]
- Gamradt SC., and, Lieberman JR. Genetic modification of stem cells to enhance bone repair. Ann Biomed Eng. 2004;32:136–147. doi: 10.1023/b:abme.0000007798.78548.b8. [DOI] [PubMed] [Google Scholar]
- Feeley BT, Conduah AH, Sugiyama O, Krenek L, Chen IS., and, Lieberman JR. In vivo molecular imaging of adenoviral versus lentiviral gene therapy in two bone formation models. J Orthop Res. 2006;24:1709–1721. doi: 10.1002/jor.20229. [DOI] [PubMed] [Google Scholar]
- Virk MS, Conduah A, Park SH, Liu N, Sugiyama O, Cuomo A.et al. (2008Influence of short-term adenoviral vector and prolonged lentiviral vector mediated bone morphogenetic protein-2 expression on the quality of bone repair in a rat femoral defect model Bone 42921–931. [DOI] [PubMed] [Google Scholar]
- Li G, Corsi-Payne K, Zheng B, Usas A, Peng H., and, Huard J. The dose of growth factors influences the synergistic effect of vascular endothelial growth factor on bone morphogenetic protein 4-induced ectopic bone formation. Tissue Eng Part A. 2009;15:2123–2133. doi: 10.1089/ten.tea.2008.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PD., and, Ghivizzani SC. Viral vectors for gene therapy. Pharmacol Ther. 1998;80:35–47. [PubMed] [Google Scholar]
- Evans CH, Ghivizzani SC., and, Robbins PD. The 2003 Nicolas Andry Award. Orthopaedic gene therapy. Clin Orthop Relat Res. 2004. pp. 316–329. [DOI] [PubMed]
- Gazit D, Turgeman G, Kelley P, Wang E, Jalenak M, Zilberman Y.et al. (1999Engineered pluripotent mesenchymal cells integrate and differentiate in regenerating bone: a novel cell-mediated gene therapy J Gene Med 1121–133. [DOI] [PubMed] [Google Scholar]
- Lieberman JR, Le LQ, Wu L, Finerman GA, Berk A, Witte ON.et al. (1998Regional gene therapy with a BMP-2-producing murine stromal cell line induces heterotopic and orthotopic bone formation in rodents J Orthop Res 16330–339. [DOI] [PubMed] [Google Scholar]
- Krebsbach PH, Gu K, Franceschi RT., and, Rutherford RB. Gene therapy-directed osteogenesis: BMP-7-transduced human fibroblasts form bone in vivo. Hum Gene Ther. 2000;11:1201–1210. doi: 10.1089/10430340050015248. [DOI] [PubMed] [Google Scholar]
- Betz OB, Betz VM, Abdulazim A, Penzkofer R, Schmitt B, Schröder C.et al. (2009Healing of large segmental bone defects induced by expedited bone morphogenetic protein-2 gene-activated, syngeneic muscle grafts Hum Gene Ther 201589–1596. [DOI] [PubMed] [Google Scholar]
- Baltzer AW., and, Lieberman JR. Regional gene therapy to enhance bone repair. Gene Ther. 2004;11:344–350. doi: 10.1038/sj.gt.3302195. [DOI] [PubMed] [Google Scholar]
- Betz OB, Betz VM, Abdulazim A, Penzkofer R, Schmitt B, Schröder C.et al. (2009The Repair of Critical Size Bone Defects using Expedited, Autologous BMP-2 Gene Activated Fat Implants Tissue Eng Part Aepub ahead of print). [DOI] [PubMed]
- Iyer M, Wu L, Carey M, Wang Y, Smallwood A., and, Gambhir SS. Two-step transcriptional amplification as a method for imaging reporter gene expression using weak promoters. Proc Natl Acad Sci USA. 2001;98:14595–14600. doi: 10.1073/pnas.251551098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JR, Daluiski A., and, Einhorn TA. The role of growth factors in the repair of bone. Biology and clinical applications. J Bone Joint Surg Am. 2002;84-A:1032–1044. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- Peterson B, Zhang J, Iglesias R, Kabo M, Hedrick M, Benhaim P.et al. (2005Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue Tissue Eng 11120–129. [DOI] [PubMed] [Google Scholar]
- Hsu WK, Sugiyama O, Park SH, Conduah A, Feeley BT, Liu NQ.et al. (2007Lentiviral-mediated BMP-2 gene transfer enhances healing of segmental femoral defects in rats Bone 40931–938. [DOI] [PubMed] [Google Scholar]
- Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A., and, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio D, Garcia-Castro J, Martín MC, de la Fuente R, Cigudosa JC, Lloyd AC.et al. (2005Spontaneous human adult stem cell transformation Cancer Res 653035–3039. [DOI] [PubMed] [Google Scholar]
- Izadpanah R, Kaushal D, Kriedt C, Tsien F, Patel B, Dufour J.et al. (2008Long-term in vitro expansion alters the biology of adult mesenchymal stem cells Cancer Res 684229–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CH, Liu FJ, Glatt V, Hoyland JA, Kirker-Head C, Walsh A.et al. (2009Use of genetically modified muscle and fat grafts to repair defects in bone and cartilage Eur Cell Mater 1896–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein SA. In vivo nonviral delivery factors to enhance bone repair. Clin Orthop Relat Res. 2000. pp. S113–S119. [DOI] [PubMed]
- Iyer M, Salazar FB, Lewis X, Zhang L, Wu L, Carey M.et al. (2005Non-invasive imaging of a transgenic mouse model using a prostate-specific two-step transcriptional amplification strategy Transgenic Res 1447–55. [DOI] [PubMed] [Google Scholar]
- Iyer M, Salazar FB, Lewis X, Zhang L, Carey M, Wu L.et al. (2004Noninvasive imaging of enhanced prostate-specific gene expression using a two-step transcriptional amplification-based lentivirus vector Mol Ther 10545–552. [DOI] [PubMed] [Google Scholar]
- Connolly JB. Lentiviruses in gene therapy clinical research. Gene Ther. 2002;9:1730–1734. doi: 10.1038/sj.gt.3301893. [DOI] [PubMed] [Google Scholar]
- Sinn PL, Sauter SL., and, McCray PB., Jr Gene therapy progress and prospects: development of improved lentiviral and retroviral vectors–design, biosafety, and production. Gene Ther. 2005;12:1089–1098. doi: 10.1038/sj.gt.3302570. [DOI] [PubMed] [Google Scholar]
- Connolly JF, Guse R, Tiedeman J., and, Dehne R. Autologous marrow injection for delayed unions of the tibia: a preliminary report. J Orthop Trauma. 1989;3:276–282. doi: 10.1097/00005131-198912000-00002. [DOI] [PubMed] [Google Scholar]
- Hernigou P, Poignard A, Beaujean F., and, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87:1430–1437. doi: 10.2106/JBJS.D.02215. [DOI] [PubMed] [Google Scholar]
- Cuomo AV, Virk M, Petrigliano F, Morgan EF., and, Lieberman JR. Mesenchymal stem cell concentration and bone repair: potential pitfalls from bench to bedside. J Bone Joint Surg Am. 2009;91:1073–1083. doi: 10.2106/JBJS.H.00303. [DOI] [PubMed] [Google Scholar]
- Stieger K, Belbellaa B, Le Guiner C, Moullier P., and, Rolling F. In vivo gene regulation using tetracycline-regulatable systems. Adv Drug Deliv Rev. 2009;61:527–541. doi: 10.1016/j.addr.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamradt SC, Abe N, Bahamonde ME, Lee YP, Nelson SD, Lyons KM.et al. (2006Tracking expression of virally mediated BMP-2 in gene therapy for bone repair Clin Orthop Relat Res 450238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama O, An DS, Kung SP, Feeley BT, Gamradt S, Liu NQ.et al. (2005Lentivirus-mediated gene transfer induces long-term transgene expression of BMP-2 in vitro and new bone formation in vivo Mol Ther 11390–398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Heterotopic ossification in the “same day” gene therapy. Representative plain radiograph of animals (n=2) in the SD-RBMC+LV-TSTA-BMP-2 treatment group that developed heterotopic new bone formation (white arrow heads). There was no new bone formation visible elsewhere in the body on plain radiographs in these two animals at 4 or 8 weeks.
Study groups.
In vitro BMP-2 production.
Micro-CT analyses.