Abstract
Urea cycle defects presenting early in life with hyperammonemia remain difficult to treat and commonly necessitate liver transplantation. Gene therapy has the potential to prevent hyperammonemic episodes while awaiting liver transplantation, and possibly also to avert the need for transplantation altogether. Ornithine transcarbamylase (OTC) deficiency, the most prevalent urea cycle disorder, provides an ideal model for the development of liver-targeted gene therapy. While we and others have successfully cured the spfash mouse model of OTC deficiency using adeno-associated virus (AAV) vectors, a major limitation of this model is the presence of residual OTC enzymatic activity which confers a mild phenotype without clinically significant hyperammonemia. To better model severe disease we devised a strategy involving AAV2/8-mediated delivery of a short hairpin RNA (shRNA) to specifically knockdown residual endogenous OTC messenger RNA (mRNA). This strategy proved highly successful with vector-treated mice developing severe hyperammonemia and associated neurological impairment. Using this system, we showed that the dose of an AAV rescue construct encoding the murine OTC (mOTC) cDNA required to prevent hyperammonemia is fivefold lower than that required to control orotic aciduria. This result is favorable for clinical translation as it indicates that the threshold for therapeutic benefit is likely to be lower than indicated by earlier studies.
Introduction
Urea cycle defects presenting early in life with hyperammonemia remain difficult to treat and commonly necessitate liver transplantation.1 Ammonia is highly neurotoxic,2 such that the prognosis for affected infants is closely linked to the severity and duration of hyperammonemic episodes, occurring at the time of presentation and/or while awaiting liver transplantation.3,4 Medical interventions including dietary protein restriction, arginine supplementation, and pharmacological induction of ammonia clearance by alternate pathways, while useful, often fail to control hyperammonemia in the face of catabolic stress occurring in concert with intercurrent illness. Gene therapy has the potential to be curative, in the first instance by preventing hyperammonemic episodes while awaiting liver transplantation, and subsequently by averting the need for transplantation altogether once the challenges of vector safety and persistence of transgene expression have been addressed.3,5
Among urea cycle defects, ornithine transcarbamylase (OTC) deficiency is the most prevalent6 and provides an ideal model for the development of liver-targeted gene therapy.5 We and others have successfully cured the spfash mouse model of OTC deficiency using adeno-associated virus (AAV) vectors.7,8 One of the limitations of the spfash mouse, however, is the presence of residual OTC enzymatic activity, such that affected mice exhibit a mild phenotype with elevated urinary orotic acid levels, but no clinically significant hyperammonemia.9 As a consequence, studies performed to date have used normalization of urinary orotic acid levels and response to ammonium challenge7,8 as measures of metabolic correction rather than control of hyperammonemia, which is the relevant therapeutic end point. This limitation precludes studies designed to determine the minimum level of stable gene transfer required to control hyperammonemia, which we hypothesized to be significantly less than that required to control orotic aciduria.
To address this shortcoming of the OTC-deficient spfash mouse model we devised a strategy involving AAV2/8-mediated delivery of a short hairpin RNA (shRNA) designed to specifically knockdown residual endogenous OTC messenger RNA (mRNA) in the liver with a view to inducing a hyperammonemic phenotype. This strategy proved robust and highly successful with vector-treated mice developing severe hyperammonemia and associated neurological impairment within 4–17 days of treatment. This model system was used to define the minimum levels of OTC gene transfer required to prevent hyperammonemia by dose titration of an AAV rescue construct encoding the murine OTC (mOTC) cDNA. Consistent with our hypothesis, these experiments showed that the vector dose required to prevent hyperammonemia is fivefold lower than that required to control orotic aciduria. This result is favorable for the clinical translation of gene therapy for OTC deficiency from mouse to man as it indicates that the threshold for therapeutic benefit is likely to be lower than indicated by earlier studies. The model also revealed that vector-encoded OTC activity is less efficacious than equivalent levels of endogenous activity, suggesting further gains are likely to be achieved by efforts to mimic endogenous patterns of urea cycle enzyme activity across the hepatic lobule.
In summary, the shRNA-induced hyperammonemic OTC-deficient spfash mouse model reported here more accurately recapitulates the clinical challenge of liver-targeted gene delivery in the treatment of severe OTC deficiency than do currently available models. This model should also prove useful in the study of ammonia neurotoxicity.
Results
Design and screening of shRNAs for knockdown of murine OTC mRNA
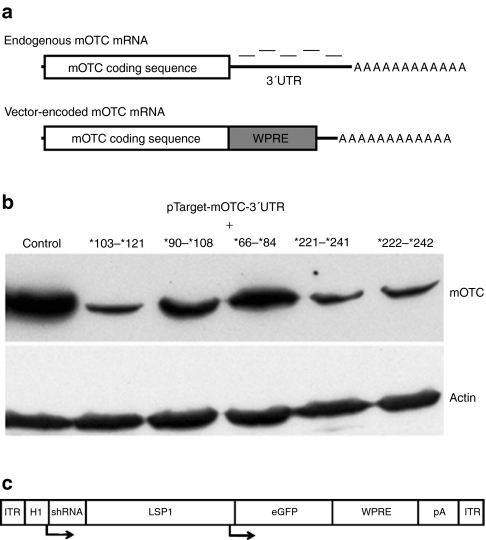
A panel of five shRNA-encoding DNA cassettes were designed to specifically knock down endogenous mOTC mRNA, but not vector-encoded mOTC mRNA. This was achieved by targeting the 3′ untranslated region (UTR) of mOTC mRNA, absent from vector-encoded transcripts (Table 1 and Figure 1a). The shRNA sequences were initially subcloned into PPT.CG.H110 under the transcriptional control of the H1 RNA polymerase III promoter, and screened for relative knockdown efficacy in a transfection assay in human embryonic kidney 293 cells transiently expressing full-length mOTC mRNA transcripts from a plasmid vector (pTarget-mOTC-3′UTR). Western analysis of OTC protein revealed that three of the five shRNAs tested substantially reduced OTC expression (Figure 1b). Of these shRNAs, *103–*121 was chosen for further analysis (designated shRNA-OTC), and along with a nonsense shRNA was subcloned into a previously described AAV2 vector construct11 upstream of the existing liver-specific expression cassette (Figure 1c). The recombinant vector genomes were then encapsidated with the highly murine liver-tropic AAV8 capsid for in vivo studies.
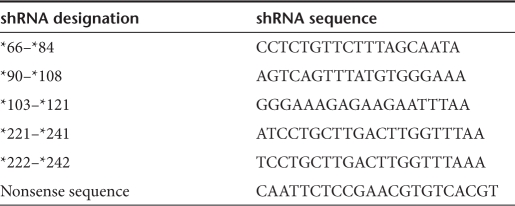
Table 1. shRNA and nonsense sequences.
Figure 1.
Design of short hairpin RNA (shRNA) targeting strategy and in vitro validation. (a) Position of the shRNA target sequences tested within the 3′untranslated region (UTR) of the endogenous murine ornithine transcarbamylase (mOTC) mRNA coding sequence. NB: These sequences are not present in the vector-encoded mOTC transgene. (b) Western blot analysis of OTC protein levels in cell lysates (30 µg protein per lane) following cotransfection of the PPT.CGH1.shRNA constructs with an expression plasmid containing full-length OTC coding sequence (pTarget-mOTC-3′UTR). The shRNA target sequence designations are based on the Human Genome Variation Society (HGVS) nomenclature23 using accession no NM008769. Using this nomenclature, the symbol (*) is used to indicate the 3′UTR, with numbering restarting from *1 after the stop codon. The control lane contains cell lysate from cells which were transfected with PPT.CGH1 and pTarget-mOTC-3′UTR. Actin was included as a loading control. (c) Schematic diagram of the rAAV2/8-shRNA OTC knockdown construct. Arrows indicated transcription start sites. ITR, inverted terminal repeat; LSP1, liver-specific promoter; pA, bovine growth hormone polyadenylation signal; WPRE, woodchuck post-transcriptional regulatory element.
Conversion of OTC-deficient spfash mice to a severe hyperammonemic phenotype
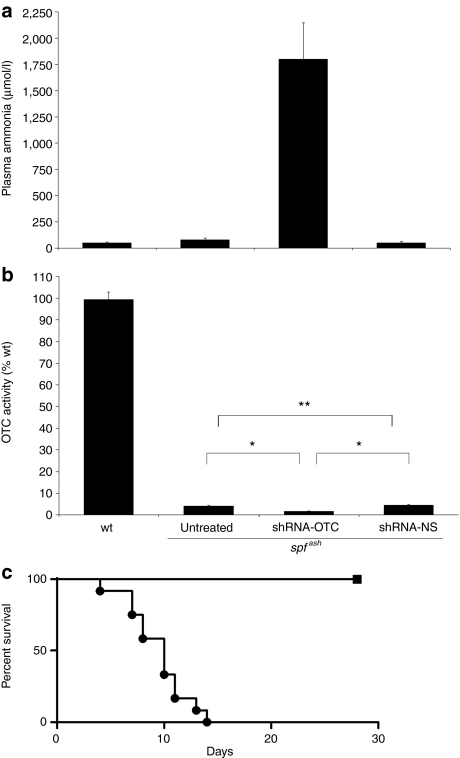
To test in vivo knock down efficacy young adult male spfash mice were injected via the intraperitoneal route with the rAAV2/8 vector encoding shRNA-OTC over a range of doses from 1 × 1011 to 1.5 × 1012 vector genome (vg)/mouse. The negative control vector encoding the nonsense shRNA was delivered at a single high dose of 1 × 1012 vg/mouse. Mice were then observed daily for up to 1 month for evidence of ill health and/or signs of neurological dysfunction. Mice receiving the nonsense shRNA remained clinically well throughout the observation period with normal ammonia levels. In contrast, all mice receiving vector encoding shRNA-OTC either died precipitously or developed severe ataxia, necessitating euthanasia, within 4–14 days with associated severe hyperammonemia (Figure 2 and Table 2). Analysis of plasma alanine transaminase levels collected at time of death excluded nonspecific liver toxicity. Analysis of livers harvested at the time of death or euthanasia revealed that induction of hyperammonemia by treatment with shRNA-OTC was associated with suppression of OTC enzymatic activity to ≤2.5% (Table 2).
Figure 2.
Induction of a severe hyperammonemic phenotype in adult spfash mice. Adult male spfash mice were injected (intraperitoneal) with recombinant adeno-associated virus (rAAV) 2/8-shRNA-OTC over a range of doses from 1.5 × 1012 to 1 × 1011 vector genomes (vg)/mouse. Mice were observed for up to 1 month postinjection and at signs of neurological damage mice were euthanized and blood and liver collected for analysis. (a) Plasma ammonia levels and (b) OTC activity in liver lysate from wild-type (n = 6), untreated spfash (n = 6), and spfash mice injected with shRNA-OTC (n = 12) and shRNA-ns (n = 3). (c) Kaplan–Meier survival analysis of spfash mice injected with shRNA-OTC (n = 12; closed circle) and shRNA-ns (n = 3; closed square). The data presented for the spfash mice injected with shRNA-OTC is the average of all mice with doses from 1.5 × 1012 to 1 × 1011 vg/mouse. The dose of vector containing shRNA-ns was 1.5 × 1012 vg/mouse. Error bars represent mean ± SEM. *P < 0.05; **, not significant (Mann–Whitney U test). OTC, ornithine transcarbamylase; shRNA, short hairpin RNA.
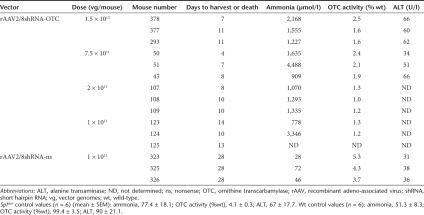
Table 2. Dose titration of rAAV2/8shRNA-OTC in young adult spfash male mice.
Co-delivery of a therapeutic AAV vector prevents hyperammonemia
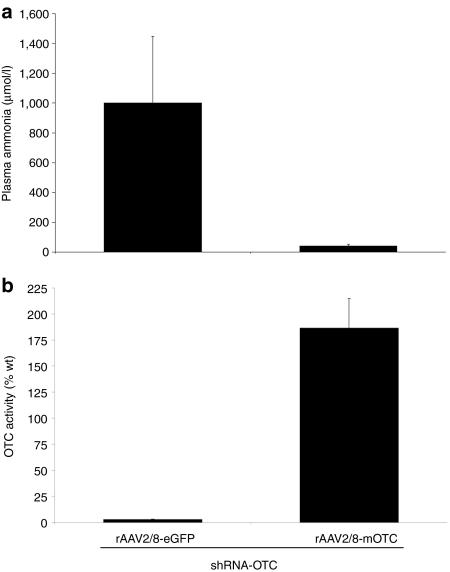
We next sought to establish whether co-delivery of a therapeutic vector encoding the mOTC cDNA open reading frame, rAAV2/8-LSP1mOTC, with delivery of shRNA-OTC could prevent the development of hyperammonemia. Four young adult male spfash mice received simultaneous intraperitoneal injections of vector encoding shRNA-OTC at a dose of 1 × 1011 vg/mouse and rAAV2/8-LSP1mOTC or rAAV2/8-LSP1eGFP at doses of 5 × 1010 vg/mouse. This latter dose had previously been shown to correct orotic aciduria in spfash mice,8 while the enhanced green fluorescent protein (eGFP) vector was included as a negative control to exclude possible competition effects that might nonspecifically ameliorate the effect of shRNA-OTC delivery. As expected, all mice receiving shRNA-OTC in combination with the eGFP-encoding vector developed neurological signs within 13–15 days and were found to have significantly elevated plasma ammonia levels (Figure 3a) and reduced OTC enzymatic activity in the liver (Figure 3b). In contrast, all mice receiving shRNA-OTC in combination with the mOTC-encoding vector remained clinically well with normal plasma ammonia levels (Figure 3a) and restoration of OTC enzymatic activity from the levels seen in untreated spfash mice to approximately twofold above wild-type levels (Figure 3b).
Figure 3.
Protection of hyperammonemia by treatment with a therapeutic vector. Adult spfash male mice were simultaneously injected with recombinant adeno-associated virus (rAAV) 2/8-shRNA-OTC (1 × 1011 vg) and either rAAV2/8-LSP1mOTC or control vector rAAV2/8-LSP1eGFP (5 × 1010 vg), and observed for 1 month. At signs of neurological damage, or 1 month postinjection, blood, and liver were collected for analysis. (a) Plasma ammonia levels and (b) OTC enzymatic activity in liver lysates from spfash mice injected with shRNA-OTC and either rAAV2/8-LSP1eGFP (n = 4) or rAAV2/8-LSP1mOTC (n = 4). Error bars represent mean ± SEM. eGFP, enhanced green fluorescent protein; LSP1, liver-specific promoter; OTC, ornithine transcarbamylase; shRNA, short hairpin RNA; vg, vector genomes.
Hyperammonemia can be prevented with a fivefold lower dose of therapeutic vector than is required to normalize orotic aciduria
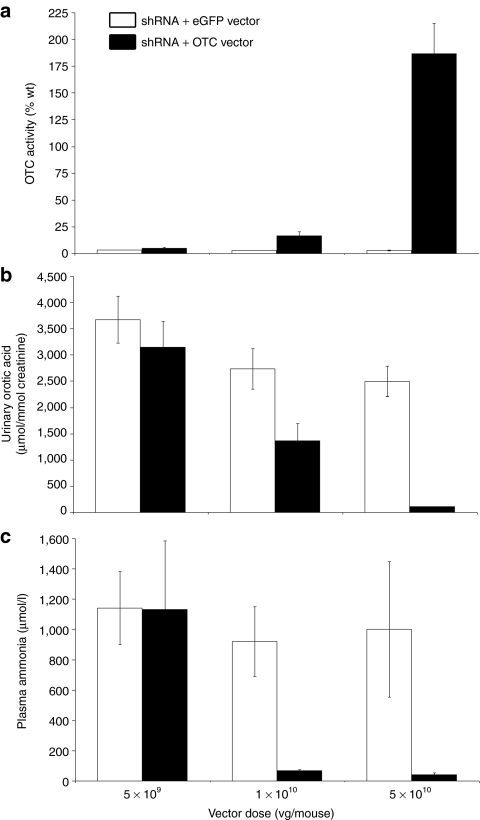
In a further experiment, we set out to establish the minimum dose of mOTC-encoding vector required to prevent the development of hyperammonemia. Four young adult male spfash mice received simultaneous intraperitoneal injections of vector encoding shRNA-OTC at a dose of 1 × 1011 vg/mouse and rAAV2/8-LSP1mOTC or rAAV2/8-LSP1eGFP at decreasing doses of 5 × 1010, 1 × 1010, and 5 × 109 vg/mouse. Mice receiving shRNA-OTC and the eGFP-encoding vector exhibited persistent orotic aciduria and developed neurological signs within 9–17 days at all doses tested, with hyperammonemia and reduced OTC enzymatic activity (Figure 4). In the cohort of mice that received the lowest dose of rescue vector (5 × 109 vg/mouse) all exhibited persistent orotic aciduria and developed hyperammonemia, with 3 of 4 mice showing neurological signs. Interestingly, OTC activity in these mice (4.9 ± 1.1% wild-type) was similar to that observed in untreated spfash mice. In mice receiving the intermediate dose of rescue vector (1 × 1010 vg/mouse) all were protected from hyperammonemia, with reduction, but not normalization, of orotic aciduria. Mean OTC activity in these mice was approximately threefold above the levels observed in untreated spfash mice and 16.6 ± 3.6% wild-type levels. At the highest dose of rescue vector examined (5 × 1010 vg/mouse) all mice were protected from hyperammonemia and urinary orotic acid levels were normalized. Taken together, these results show that the dose of OTC-encoding vector required to correct hyperammonemia, the therapeutically relevant end point, is fivefold lower than the dose required to correct orotic aciduria.
Figure 4.
Dose titration of therapeutic vector. Adult spfash male mice were injected with shRNA-OTC and different doses of either rAAV2/8-LSP1mOTC or rAAV2/8-LSP1eGFP, down to 5 × 109 At signs of neurological damage, or 1 month postinjection, blood, and liver were collected for analysis. (a) OTC activity in liver lysate, (b) urinary orotic acid levels, and (c) plasma ammonia levels were analyzed from spfash mice injected with shRNA-OTC and either rAAV2/8-LSP1eGFP (white) or rAAV2/8-LSP1mOTC (black) over a range of doses (n = 4 for each cohort). Error bars represent mean ± SEM. eGFP, enhanced green fluorescent protein; LSP1, liver-specific promoter; mOTC, murine ornithine transcarbamylase; shRNA, short hairpin RNA; vg, vector genomes.
Induction of an OTC-deficient phenotype in wild-type mice
In a final experiment, we set out to establish whether an OTC-deficient phenotype could be induced in wild-type mice using the OTC knockdown vector. Ten adult wild-type C57BL/6 male mice were injected with either knockdown vector or the negative control vector at a dose of 1 × 1011 vg/mouse, and monitored up to 6 weeks postinjection. All mice receiving the knockdown vector developed marked orotic acidura, with a slight but not clinically significant elevation in ammonia levels, consistent with the successful induction of a mild OTC-deficient phenotype (Table 3). The residual OTC enzyme levels observed were similar to those in untreated OTC-deficient spfash mice.
Table 3. Knockdown of OTC activity in adult wild-type C57BL/6 male mice.
Discussion
Urea cycle defects are attractive targets for gene therapy and have the potential to serve as a paradigm for the broader application of gene therapy to genetic/metabolic liver disease. The central function of the urea cycle is to convert ammonia, a highly neurotoxic product of protein catabolism, to urea for excretion in the urine.2 Among urea cycle defects OTC deficiency is the most prevalent, and commonly presents in the newborn period with life-threatening hyperammonemia requiring aggressive medical management and liver transplantation as early as surgically feasible.12,13 More mild phenotypes can be managed medically, but control and prevention of hyperammonemic episodes precipitated by excess protein intake, fasting, and intercurrent illness remain the key therapeutic challenge.
Existing mouse models have proven useful in the evaluation of gene therapy approaches to the treatment of urea cycle defects,5,14 but are inherently limited by the neurotoxicity of ammonia. In gene knockout models, such as for argininosuccinate synthetase deficiency (citrullinaemia),15 affected pups die from hyperammonemia within the first 24 hours of life, making experimental intervention difficult. Milder phenotypes, such as the OTC-deficient spfash mouse model used in this study,9 survive to adulthood but do not exhibit clinically significant hyperammonemia. As a consequence, biochemical end points such as normalization of urinary orotic acid levels and restoration of liver ureagenic capacity are used as measures of therapeutic efficacy.7,8 While capable of definitively demonstrating full phenotype correction, these end point measures do not allow determination of the minimal levels of gene transfer that would be required to control of hyperammonemia. For human clinical translation this is a critical question, the answer to which will provide important insights into the levels of gene transfer that are likely to be required for clinical benefit.
To address this question we have developed a model system in which the mild OTC-deficient phenotype of spfash mice is converted to a severe hyperammonemic phenotype by shRNA-mediated knockdown of residual endogenous OTC mRNA. Highly efficient liver-directed delivery of shRNA was achieved using an AAV2/8 vector, and specificity for knockdown of endogenous OTC mRNA by targeting sequences in the 3′ UTR. This strategy allows rescue of the induced hyperammonemic phenotype by vectors encoding the mOTC cDNA open reading frame, but lacking the 3′UTR. At all vector doses tested, down to 1 × 1011 vg/mouse, adult spfash mice reliably developed severe hyperammonemia with associated ataxia and rapid deterioration within 4–14 days of vector delivery by simple intraperitoneal injection. Even at the highest vector doses tested, 1.5 × 1012 vg/mouse, there was no evidence of nonspecific liver toxicity as judged by the presence of normal plasma alanine transaminase levels. Associated OTC enzymatic activity in hyperammonemic mice ranged from 1 to 2.5% wild-type levels, consistent with residual enzymatic activities observed in humans presenting early in life with life-threatening hyperammonemia.16
Using a previously described mOTC-encoding AAV2/8 vector,8 this model system was then exploited to define the minimal levels of gene transfer required to control hyperammonemia. Several important observations emerged. First, the dose of OTC-encoding AAV2/8 vector required to control shRNA-OTC-induced hyperammonemia was found to be fivefold lower than the dose required to normalize urinary orotic acid levels. Second, after knockdown of endogenous residual OTC activity in spfash mice, the level of vector-encoded (exogenous) OTC enzymatic activity required to prevent hyperammonemia was approximately threefold above the enzymatic activity present in untreated spfash mice. Put simply, vector-encoded OTC activity proved less efficacious than equivalent levels of endogenous activity. This is likely to be the consequence of the nonphysiological levels and patterns of exogenous OTC expression across the hepatic lobule following AAV-mediated gene transfer. We have previously shown that the OTC-encoding vector used in this study, while capable of transducing up to 100% of hepatocytes in the mouse liver, achieves higher levels of OTC expression around the central vein of the hepatic lobule.11 Endogenously expressed urea cycle enzymes, including OTC, exhibit metabolic zonation17,18 with maximal expression in the periportal regions of the hepatic lobule and progressively lower levels toward the central vein. Exogenous OTC activity expressed in excess toward the centre of the hepatic lobule would therefore be expected to contribute relatively less to ammonia clearance and ureagenesis. Similarly, exogenous OTC activity in excess of physiological levels in individual hepatocytes, irrespective of location within the hepatic lobule, is likely to contribute little to the overall ureagenic capacity of the liver. Taken together, these observations show that while the levels of gene transfer required to control hyperammonemia are considerably lower than those required to control orotic aciduria, optimal therapeutic effectiveness will require the development of vector expression cassettes capable of delivering more physiological patterns of gene expression.
In summary, we have successfully developed a hyperammonemic mouse model of OTC deficiency that better represents the challenge of gene therapy for urea cycle defects than do the currently available mouse models with mild OTC-deficient phenotypes. The model also avoids the challenges of working with knockout models of urea cycle defects where hyperammonemia is neonatal lethal, by allowing programmed induction of a severe hyperammonemic phenotype in adulthood. In addition to the development of gene therapy strategies for treating metabolic liver disease, the model should also prove useful for the study of the neuropathology caused by hyperammonemia and the underlying mechanisms of toxicity.
Materials and Methods
Cell culture. Human embryonic kidney 293 cells19 were maintained in Dulbecco's modified Eagle medium (Gibco, Invitrogen, Grand Island, NY) supplemented with 10% (v/v) fetal bovine serum (JRH Biosciences, Lenexa, KS) and 1% (w/v) -glutamine (Gibco, Invitrogen), and maintained at 37 °C in a humidified 5% CO2-air atmosphere.
shRNA expression constructs and virus production. Complementary shRNA-encoding DNA sequences, designed using commercially available algorithms, were synthesized, annealed and inserted into PPT.CGH110 via BamHI and PacI restriction sites to generate shRNA expression cassettes. The shRNA constructs consisted of sense and antisense sequences 19–21 nucleotides long separated by a short spacer sequence enabling formation of the hairpin structure, under the control of the H1 RNA polymerase III promoter. The mOTC coding sequence and part of the 3′ UTR (Accession number NM008769) were inserted into the pTarget expression vector (Promega, Madison, WI) (pTarget-mOTC-3′UTR) and used to screen for knockdown. Selected shRNA expression cassettes were removed by KpnI digestion and inserted into the AAV vector plasmid pLSP1eGFP.11 Recombinant AAV vectors pseudo-serotyped with the AAV8 capsid (p5E18-VD2/8; courtesy of James M Wilson, University of Pennsylvania) were produced in human embryonic kidney 293 cells as previously described.11 Vector genomes were titred by real-time quantitative polymerase chain reaction using a primer/probe set specific for the woodchuck post-transcriptional regulatory element20 using the Maxima Probe Master Mix (Fermentas, Glen Burnie, MD).
Animals. All animal care and experimental procedures were evaluated and approved by the CMRI and CHW Animal Care and Ethics Committee (ACEC). Breeding pairs of spfash mice (C57BL/6/C3H-F1 background) were obtained from The Jackson Laboratory (Bar Harbor, ME). All injections were administered via the intraperitoneal route at 8–12 weeks of age.
Measurement of urinary orotic acid. Urine was collected for orotic acid analysis as previously described.8 Briefly, urine was collected over a 24 hour period on Whatman filter paper, eluted, and analyzed for orotic acid levels using Liquid Chromatography/Tandem Mass Spectrometry. Results were standardized against creatinine levels, measured by the modified Jaffe reaction.
Plasma ammonia analysis. Blood was collected by cardiac puncture and immediately centrifuged at 7,500g for 5 minutes at 4 °C. The plasma was snap-frozen in liquid Nitrogen, and stored at −80 °C. Ammonia was measured using the Ammonia Assay Kit (Sigma-Aldrich, St Louis, MO).
OTC enzyme activity. OTC enzyme activity was assayed in liver lysate as described previously.21
Western blot analysis. Proteins in liver lysates (30 µg per lane) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.22 The proteins were transferred to nitrocellulose membrane, blocked in 5% (w/v) skim milk and 0.05% (v/v) Tween-20 in phosphate-buffered saline, and probed with rabbit antihuman OTC antibody (Abcam, Cambridge, UK) and rabbit anti-actin antibody (1/250 dilution; Sigma-Aldrich). Bound primary antibody was detected with goat anti-rabbit IgG (1/20,000 dilution; Bio-Rad, Hercules, CA) and SuperSignal West Pico Chemiluminescence Substrate (Pierce, Rockford, IL).
Acknowledgments
We thank Margot Latham (The Children's Hospital at Westmead) for assistance in manuscript preparation. E.K. is supported by a Fellowship from the Sydney Medical School Foundation. This work was supported by a grant (423400) from the National Health and Medical Research Council of Australia.
REFERENCES
- Meyburg J., and, Hoffmann GF. Liver transplantation for inborn errors of metabolism. Transplantation. 2005;80 1 Suppl:S135–S137. doi: 10.1097/01.tp.0000186905.10088.e5. [DOI] [PubMed] [Google Scholar]
- Braissant O. Current concepts in the pathogenesis of urea cycle disorders. Mol Genet Metab. 2010;100 Suppl 1:S3–S12. doi: 10.1016/j.ymgme.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Lee B., and, Goss J. Long-term correction of urea cycle disorders. J Pediatr. 2001;138 1 Suppl:S62–S71. doi: 10.1067/mpd.2001.111838. [DOI] [PubMed] [Google Scholar]
- Leonard JV., and, McKiernan PJ. The role of liver transplantation in urea cycle disorders. Mol Genet Metab. 2004;81 Suppl 1:S74–S78. doi: 10.1016/j.ymgme.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Alexander IE, Cunningham SC, Logan GJ., and, Christodoulou J. Potential of AAV vectors in the treatment of metabolic disease. Gene Ther. 2008;15:831–839. doi: 10.1038/gt.2008.64. [DOI] [PubMed] [Google Scholar]
- Brusilow SW, Horwich AL.1995Urea cycle enzymes Scriver CR, Beaudet AL, Sly WS, Valle D.eds). The Metabolic and Molecular Bases of Inherited Disease7th edn. McGraw-Hill Inc.: New York; pp 1187–1232. [Google Scholar]
- Moscioni D, Morizono H, McCarter RJ, Stern A, Cabrera-Luque J, Hoang A.et al. (2006Long-term correction of ammonia metabolism and prolonged survival in ornithine transcarbamylase-deficient mice following liver-directed treatment with adeno-associated viral vectors Mol Ther 1425–33. [DOI] [PubMed] [Google Scholar]
- Cunningham SC, Spinoulas A, Carpenter KH, Wilcken B, Kuchel PW., and, Alexander IE. AAV2/8-mediated correction of OTC deficiency is robust in adult but not neonatal Spf (ash) mice. Mol Ther. 2009;17:1340–1346. doi: 10.1038/mt.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle DP, Hulbert LL., and, Cordy C. A new allele of the sparse fur gene in the mouse. J Hered. 1974;65:194–195. doi: 10.1093/oxfordjournals.jhered.a108500. [DOI] [PubMed] [Google Scholar]
- Cingolani E, Ramirez Correa GA, Kizana E, Murata M, Cho HC., and, Marbán E. Gene therapy to inhibit the calcium channel beta subunit: physiological consequences and pathophysiological effects in models of cardiac hypertrophy. Circ Res. 2007;101:166–175. doi: 10.1161/CIRCRESAHA.107.155721. [DOI] [PubMed] [Google Scholar]
- Cunningham SC, Dane AP, Spinoulas A, Logan GJ., and, Alexander IE. Gene delivery to the juvenile mouse liver using AAV2/8 vectors. Mol Ther. 2008;16:1081–1088. doi: 10.1038/mt.2008.72. [DOI] [PubMed] [Google Scholar]
- Fernandes J, Saudubray J-M, van den Berghe G, Walter JH.2006Inborn Metabolic Diseases-Diagnosis and Treatment4th edn. Springer Medizin Verlag: Heidelberg [Google Scholar]
- Sokal EM. Liver transplantation for inborn errors of liver metabolism. J Inherit Metab Dis. 2006;29:426–430. doi: 10.1007/s10545-006-0288-x. [DOI] [PubMed] [Google Scholar]
- Deignan JL, Cederbaum SD., and, Grody WW. Contrasting features of urea cycle disorders in human patients and knockout mouse models. Mol Genet Metab. 2008;93:7–14. doi: 10.1016/j.ymgme.2007.08.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patejunas G, Bradley A, Beaudet AL., and, O'Brien WE. Generation of a mouse model for citrullinemia by targeted disruption of the argininosuccinate synthetase gene. Somat Cell Mol Genet. 1994;20:55–60. doi: 10.1007/BF02257486. [DOI] [PubMed] [Google Scholar]
- Tuchman M, Jaleel N, Morizono H, Sheehy L., and, Lynch MG. Mutations and polymorphisms in the human ornithine transcarbamylase gene. Hum Mutat. 2002;19:93–107. doi: 10.1002/humu.10035. [DOI] [PubMed] [Google Scholar]
- Morris SM., Jr Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr. 2002;22:87–105. doi: 10.1146/annurev.nutr.22.110801.140547. [DOI] [PubMed] [Google Scholar]
- Jungermann K., and, Katz N. Functional specialization of different hepatocyte populations. Physiol Rev. 1989;69:708–764. doi: 10.1152/physrev.1989.69.3.708. [DOI] [PubMed] [Google Scholar]
- Graham FL, Smiley J, Russell WC., and, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Lizée G, Aerts JL, Gonzales MI, Chinnasamy N, Morgan RA., and, Topalian SL. Real-time quantitative reverse transcriptase-polymerase chain reaction as a method for determining lentiviral vector titers and measuring transgene expression. Hum Gene Ther. 2003;14:497–507. doi: 10.1089/104303403764539387. [DOI] [PubMed] [Google Scholar]
- Ye X, Robinson MB, Batshaw ML, Furth EE, Smith I., and, Wilson JM. Prolonged metabolic correction in adult ornithine transcarbamylase-deficient mice with adenoviral vectors. J Biol Chem. 1996;271:3639–3646. doi: 10.1074/jbc.271.7.3639. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ogino S, Gulley ML, den Dunnen JT., and, Wilson RB, Association for Molecular Pathology Training and Education Committtee Standard mutation nomenclature in molecular diagnostics: practical and educational challenges. J Mol Diagn. 2007;9:1–6. doi: 10.2353/jmoldx.2007.060081. [DOI] [PMC free article] [PubMed] [Google Scholar]