Creation of inhibitors targeted to a gene of interest is key both to the study of gene function and to drug development, and RNA interference (RNAi) is an attractive approach that complements the development of standard small-molecule drugs. A key factor for the successful application of this technology is the design of potent triggers of RNAi that can be employed at sufficiently low concentrations to avoid or minimize off-target or toxic effects. In an article that appeared recently in Molecular Cell,1 Fellmann et al. report the development of a library selection screen for the generation of potent RNAi triggers that is likely to work for virtually any target gene transcript. The selection method screens every possible overlapping sequence stretch for a particular target gene, thereby providing complete coverage of a given target to identify the most efficacious RNAi triggers.
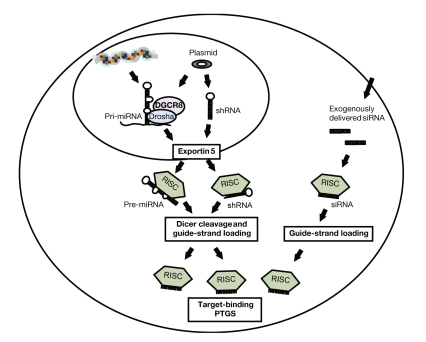
RNAi is based on a ubiquitous mechanism of gene regulation in eukaryotic cells mediated by endogenous microRNAs (miRNAs). The process triggers sequence-specific RNA degradation resulting in posttranscriptional silencing of targeted genes. RNAi can also be triggered by the delivery of small interfering RNAs (siRNAs) directly to cells or tissues or by a gene therapy approach that expresses short hairpin RNAs (shRNAs) from plasmid or viral vectors. Primary endogenous miRNA transcripts are processed to precursor miRNAs of approximately 70 nucleotides (nt), which in turn are processed by the enzyme Dicer into double-stranded 21- to 23-nt miRNAs2, whereas shRNAs expressed from gene vectors are directly converted into siRNAs by Dicer processing. The functional, or “guide,” strand of these siRNAs is then loaded into the RNA-induced silencing complex (RISC), which guides it to the complementary target site and catalyzes the cleavage and degradation of the target messenger RNA (mRNA; Figure 1).
Figure 1.
Schematic representation of RNA interference mechanisms. Primary endogenous or recombinant miRNAs (pri-miRNAs) are processed by Drosha and its partner DGCR8 to ~70-nt precursors, which are transported to the cytoplasm by Exportin 5. Plasmid-expressed short hairpin RNAs (shRNAs) do not require Drosha processing and use the same export pathway to reach the cytoplasm. Following Dicer cleavage, which yields short 21- to 23-nt double-stranded RNA molecules, the functional or “guide” strand is loaded into the RNA-induced silencing complex (RISC). Exogenous 21- to 23-nt small interfering RNAs (siRNAs) are delivered into the cytoplasm and directly loaded into the RISC. The RISC-bound guide strand is directed to bind to its target and triggers posttranscriptional gene silencing (PTGS).
Rules for the generation of effective shRNAs and siRNAs have emerged in the past several years3 and have been incorporated into algorithms that take into account secondary structural features of the target RNAs and the thermodynamic stability of the siRNAs' ends. Although these algorithms can help identify effective synthetic siRNAs,4,5 they are often not useful for identifying optimal shRNAs because we still do not fully understand the biology of RNAi guide-strand selection.6,7 Another important consideration when designing RNAi triggers is that exogenously delivered siRNAs or shRNAs act via the same cellular pathways as endogenous miRNAs, posing the risk of saturating essential components of the RNAi machinery. Indeed, competition for incorporation into the RISC can lead to cellular toxicity.8 Thus, it is important to identify the most potent siRNA or shRNA that can be effective at the lowest possible concentration. shRNA or siRNA asymmetry is another important parameter because the relative ability of the guide strand to be selected and incorporated into the RISC over the so-called “passenger” strand is a key consideration in avoiding the risk of off-target effects. Some modifications of the passenger strand can be incorporated to preferentially facilitate incorporation of the guide sequence,9,10 but this is feasible only for the design of synthetic siRNAs. Ultimately, the best siRNA or shRNA will be determined by a combination of factors, including specific nucleotides at set positions, asymmetry, and target accessibility. However, it is not currently possible to predict the efficacy of a designed siRNA or shRNA without empirical testing. Thus, the identification of truly potent shRNAs is not a trivial task, and the development of a robust approach for the design of expressed shRNAs is an important advance in the field.
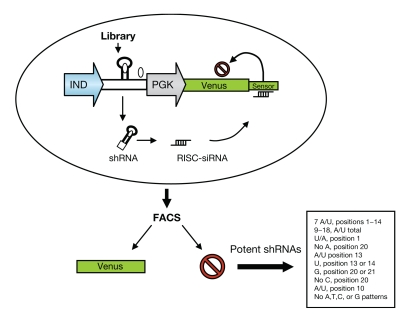
Genome-wide siRNA and shRNA libraries, those focused on subsets of genes involved in specific cellular pathways, and custom libraries are commercially available for use in screening. However, they carry the inherent downside of a possible lack of target specificity because not all siRNAs or shRNAs that favor incorporation of the passenger strand are excluded. Moreover, libraries usually do not include every possible siRNA or shRNA design and carry the risk of missing a potent inhibitor of some cellular targets. To address these issues, Fellmann and colleagues1 tiled nine different mammalian transcripts with a library of 20,000 shRNAs directed against every possible target site in these mRNAs, using siRNAs that cover bases 121, 222, 323, etc. The shRNAs were inserted into a miRNA scaffold so as to undergo miRNA pathway processing, which has been shown to minimize competition with endogenous miRNAs by the exogenous sequences.11 To express the shRNA sequences, they were placed under the control of a doxycycline-inducible promoter in a viral vector that also contained the target (or sensor) gene of the shRNA fused to a green fluorescent reporter gene, Venus. Thus, shRNAs that effectively inhibited the expression of their cognate targets in the sensor would also inhibit expression of the reporter gene, resulting in loss of fluorescence (Figure 2). Repetitive rounds of selection involving induction and withdrawal of doxycycline, and hence both up- and downregulation of the shRNAs, were accompanied by fluorescence-activated cell sorting. This “ping-pong” strategy allowed the gating and identification of the best shRNA and target site combinations for each gene. The most potent shRNAs showed a strong bias for incorporation of the intended guide strand (target complementary sequence) into the RISC. An interesting result of these analyses is that potent, single-copy shRNAs were found to be rather uncommon, representing only 2.4% of all the possible shRNAs.
Figure 2.
Sensor assay to select potent shRNAs. A library of 20,000 short hairpin RNAs (shRNAs) was cloned into an miR30 scaffold under the control of a Tet-inducible promoter (IND) in a vector (pSensor) expressing a green fluorescent protein (GFP) marker gene (Venus) linked to a “sensor” target region containing the cognate 50-nt target region from nine different genes targeted for silencing. Following induction, the shRNAs that are able to inhibit their target will in turn reduce or block GFP expression. The most potent shRNAs can be then isolated following fluorescence-activated cell sorting (FACS) of the cells that lose GFP expression. Nucleotides found at high frequency in the most effective shRNAs are indicated. PGK, phosphoglycerate kinase promoter; RISC, RNA-induced silencing complex; siRNA, small interfering RNA.
The analyses of the selected shRNAguide sequence combinations are consistent with previously established rules for siRNA design.3 The most effective siRNAs and shRNAs contained 918 A/U nt and a low G/C content. As has been found previously for the design of siRNAs, the authors determined the advantage of an A/U-rich 5′ end of the antisense strand, an A/U at position 10, thermodynamic asymmetry, and a lack of internal repeats.3 An A/U at position 13 and a U at position 14 were also consistent with previous findings.3 Fellmann et al. also showed that an A/U at position 13 or 14, a G at position 20 or 21, and a C at position 20 affected the accuracy of cleavage of primary or recombinant endogenous miRNAs (pri-miRNAs). Including these newly identified rules in the design of miRNA scaffolds for shRNA expression will ensure more efficient processing and thus greater potency of the mature siRNA sequence. Moreover, the effect of asymmetry that has previously been implicated in RISC loading seems instead to be more important at a later step—when the siRNA guide sequence interacts with its mRNA target—perhaps to facilitate unwinding and RISC turnover.12,13 Finally, although it has long been thought that noncoding or regulatory regions should be avoided because protein binding or secondary structures within these regions might impair siRNA target pairing, the authors showed that, with the exception of G/C-rich regions, targets for potent shRNAs seem to be evenly distributed throughout the transcripts.
Although a portion of the shRNA library was not processed according to the previously known structure and processing requirements for miRNAs, which is important in the context that Dicer and RISC association with processed siRNAs can differ dramatically between two siRNAs differing by only a single nucleotide,14 this scheme represents the most powerful shRNA selection approach to date because it is able to functionally identify the most optimal shRNA for a given target. Thus, this strategy should become the method of choice for identifying and selecting shRNAs for therapeutic applications, because these must be potent and highly target-specific at the lowest possible concentration. Such a screening system should also be appealing to companies as a high-throughput method for identifying potent shRNAs for virtually any gene of interest. The Venus fluorescence marker could also potentially be replaced with a “suicide” gene such as thymidine kinase or cytosine deaminase. Strong downregulation of the target would result in positive selection in the presence of the pro-drugs ganciclovir or 5-fluorocytosine, which could simplify the selection screen, perhaps also rendering it a more facile approach for use in small academic gene therapy labs.
RNAi continues to show great potential for therapeutic applications, including treatment of cancer as well as cardiovascular, neurological, and metabolic diseases and viral infections.15,16 However, for it to achieve therapeutic success, its potency and safety must be increased by eliminating the unwanted outcomes of suboptimal RNAi trigger designs. The work by Fellmann and colleagues has brought us one step closer to this ultimate aim.
REFERENCES
- Fellmann C, Zuber J, McJunkin K, Chang K, Malone CD, Dickins RA.et al. (2011Functional identification of optimized RNAi triggers using a massively parallel sensor assay Mol Cell 41733–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MA., and, Hammond SM. Emerging paradigms of regulated microRNA processing. Genes Dev. 2010;24:1086–1092. doi: 10.1101/gad.1919710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajeri PB., and, Singh SK. siRNAs: their potential as therapeutic agent—Part I. Designing of siRNAs. Drug Discov Today. 2009;14:851–858. doi: 10.1016/j.drudis.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Huesken D, Lange J, Mickanin C, Weiler J, Asselbergs F, Warner J.et al. (2005Design of a genome-wide siRNA library using an artificial neural network Nat Biotechnol 23995–1001. [DOI] [PubMed] [Google Scholar]
- Vert JP, Foveau N, Lajaunie C., and, Vandenbrouck Y. An accurate and interpretable model for siRNA efficacy prediction. BMC Bioinformatics. 2006;7:520. doi: 10.1186/1471-2105-7-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassik MC, Lebbink RJ, Churchman LS, Ingolia NT, Patena W, LeProust EM.et al. (2009Rapid creation and quantitative monitoring of high coverage shRNA libraries Nat Methods 6443–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Lin X, Khvorova A, Fesik SW., and, Shen Y. Defining the optimal parameters for hairpin-based knockdown constructs. RNA. 2007;13:1765–1774. doi: 10.1261/rna.599107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR.et al. (2006Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways Nature 441537–541. [DOI] [PubMed] [Google Scholar]
- Chen PY, Weinmann L, Gaidatzis D, Pei Y, Zavolan M, Tuschi T.et al. (2008Strand-specific 5ʹ-O-methylation of siRNA duplexes controls guide strand selection and targeting specificity RNA 14263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernolovskaya EL., and, Zenkova MA. Chemical modification of siRNA. Curr Opin Mol Ther. 2010;12:158–167. [PubMed] [Google Scholar]
- Castanotto D, Sakurai K, Lingeman R, Li H, Shively L, Aagaard L.et al. (2007Combinatorial delivery of small interfering RNAs reduces RNAi efficacy by selective incorporation into RISC Nucleic Acids Res 355154–5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley B., and, Zamore PD. Kinetic analysis of the RNAi enzyme complex. Nat Struct Mol Biol. 2004;11:599–606. doi: 10.1038/nsmb780. [DOI] [PubMed] [Google Scholar]
- Leuschner PJ, Ameres SL, Kueng S., and, Martinez J. Cleavage of the siRNA passenger strand during RISC assembly in human cells. EMBO Rep. 2006;7:314–320. doi: 10.1038/sj.embor.7400637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai K, Amarzguioui M, Kim DH, Alluin J, Heale B, Song MS.et al. (2011A role for human Dicer in pre-RISC loading of siRNAs Nucleic Acids Res 391510–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanotto D., and, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457:426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lares MR, Rossi JJ., and, Ouellet DL. RNAi and small interfering RNAs in human disease therapeutic applications. Trends Biotechnol. 2010;28:570–579. doi: 10.1016/j.tibtech.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]