Abstract
We previously demonstrated that nitric oxide (NO) contributes to compensatory vasodilation in the contracting human forearm subjected to acute hypoperfusion. We examined the potential role of an adenosine-NO interaction to this response in 17 male subjects (25 ± 2 yr). In separate protocols subjects performed rhythmic forearm exercise (20% of maximum) while hypoperfusion was evoked by balloon inflation in the brachial artery above the elbow. Each trial included exercise before inflation, exercise with inflation, and exercise after deflation (3 min each). Forearm blood flow (FBF; ultrasound) and local [brachial artery catheter pressure (BAP)] and systemic [mean arterial pressure (MAP); Finometer] arterial pressure were measured. In protocol 1 (n = 10), exercise was repeated during nitric oxide synthase inhibition [NG-monomethyl-l-arginine (l-NMMA)] alone and during l-NMMA-aminophylline (adenosine receptor blockade) administration. In protocol 2, exercise was repeated during aminophylline alone and during aminophylline-l-NMMA. Forearm vascular conductance (FVC; ml·min−1·100 mmHg−1) was calculated from blood flow (ml/min) and BAP (mmHg). Percent recovery in FVC during inflation was calculated as (steady-state inflation + exercise value − nadir)/[steady-state exercise (control) value − nadir]. In protocol 1, percent recovery in FVC was 108 ± 8% during the control (no drug) trial. Percent recovery in FVC was attenuated with inhibition of NO formation alone (78 ± 9%; P < 0.01 vs. control) and was attenuated further with combined inhibition of NO and adenosine (58 ± 9%; P < 0.01 vs. l-NMMA). In protocol 2, percent recovery was reduced with adenosine receptor blockade (74 ± 11% vs. 113 ± 6%, P < 0.01) compared with control drug trials. Percent recovery in FVC was attenuated further with combined inhibition of adenosine and NO (48 ± 11%; P < 0.05 vs. aminophylline). Our data indicate that adenosine contributes to compensatory vasodilation in an NO-independent manner during exercise with acute hypoperfusion.
Keywords: vasodilation, exercise, hypoperfusion
using a novel catheter-balloon system to partially occlude the brachial artery, we previously demonstrated (4) that skeletal muscle blood flow is restored in the exercising forearm during acute experimental hypoperfusion in the absence of a pressor response. This indicates that local vasodilator mechanisms and/or a myogenic response are critical to this response. In follow-up studies, we found that nitric oxide synthase (NOS) inhibition blunts and slows this response (3). However, there was still substantial forearm blood flow (FBF) recovery after NOS inhibition (77%), thus suggesting that additional vasodilator mechanisms are involved.
Along these lines, adenosine receptor antagonism blunts the recovery of hindlimb blood flow in dogs during high-intensity exercise in response to partial vascular occlusion (11). Furthermore, adenosine production has been shown to be correlated with the degree of hypoperfusion in the coronary circulation of dogs (7). Taken together, these findings suggest that adenosine may contribute to the blood flow recovery in contracting human muscle exposed to acute hypoperfusion. Since the potential exists for a strong nitric oxide (NO)-dependent component in adenosine-mediated vasodilation (15, 19, 27), our aim was to examine the potential role of adenosine and the interaction with NO in the blood flow restoration to the exercising human forearm subjected to acute hypoperfusion. Thus we tested the hypothesis that adenosine receptor-mediated vasodilation independent of NO contributes to the compensatory vasodilation seen during handgrip exercise with hypoperfusion.
METHODS
Subjects
A total of 17 young healthy male subjects volunteered to participate in two separate protocols (10 subjects in protocol 1; 7 subjects in protocol 2). Subjects gave written informed consent, were nonobese nonsmokers, and were not taking any medications. Studies were performed after an overnight fast and after the subjects refrained from exercise and caffeine for at least 24 h. All study protocols were approved by the Institutional Review Board and were in accordance with the Declaration of Helsinki.
Heart Rate and Systemic Blood Pressure
Heart rate (HR) was measured by three-lead electrocardiography (ECG). Systemic blood pressure was assessed (beat to beat) with a finger plethysmograph (Finometer) on the nonexercising hand and verified with an automated cuff on the same arm. The systemic pressure was used as an index of pressure proximal (upstream) from the balloon. Cardiac output (CO) was estimated with the Modelflow technique, which has been validated against other techniques and used in exercise studies (21, 29).
Arterial Catheterization and Balloon Placement
Brachial catheter placement and balloon insertion have been described in detail previously (4). Briefly, a 20-gauge, 5-cm catheter was placed in the brachial artery in the experimental arm under aseptic conditions after local anesthesia (2% lidocaine). A guide wire was then placed in the artery, which was then cannulated with a 4-Fr introducer (Cook, Bloomington, IN) that permitted insertion of a 2-Fr Fogarty balloon catheter into the brachial artery. A port and stopcock system allowed the measurement of arterial pressure, administration of study drugs, and drawing of arterial blood samples. The system was continuously flushed (3 ml/h) with heparinized saline. The configuration of the balloon upstream from the lumen of the introducer allowed measurement of the arterial pressure distal to the balloon that was perfusing the contracting forearm muscles.
Forearm Blood Flow
Brachial artery mean blood velocity (MBV) and brachial artery diameter were determined with a 12-MHz linear-array Doppler probe (model M12L, Vivid 7, General Electric, Milwaukee, WI). Brachial artery blood velocity was measured throughout each condition with a probe insonation angle previously calibrated to 60°. Brachial artery and balloon diameter measurements were obtained at end diastole and between contractions during steady-state conditions. Diameter measurement typically results in the loss of the pulse wave signal for 15–20 s. Velocity and diameter measurements were made 2–3 cm proximal to the balloon. FBF was calculated as the product of MBV (cm/s) and brachial artery cross-sectional area (cm2) and multiplied by 60 to present as milliliters per minute.
Forearm Exercise
Rhythmic forearm exercise was performed with a hand grip device by the nondominant arm at 20% of each subject's maximal voluntary contraction (MVC; mean 50 ± 2 kg, range 40–61 kg). The weight was lifted 4–5 cm over a pulley at a duty cycle of 1-s contraction and 2-s relaxation (20 contractions/min), with the use of a metronome to ensure correct timing. The average weights used for forearm exercise in protocols 1 and 2 were 9.9 ± 0.4 and 10.4 ± 0.3 kg, respectively.
Brachial Artery Balloon Inflation
To reduce FBF, the brachial artery was partially occluded via inflation of the Fogarty balloon catheter with saline and a calibrated microsyringe for tight control of balloon volume. Balloon inflations were targeted to reduce blood velocity by 40–50%.
Pharmacological Infusions
NG-monomethyl-l-arginine (l-NMMA; NOS inhibitor) was infused at a loading dose of 5 mg/min for 5 min and then at a maintenance dose of 1 mg/min for the remainder of the study. This dose of l-NMMA has been shown to effectively attenuate the forearm vasodilator response to exogenous acetylcholine administration (3, 5). Aminophylline (adenosine receptor antagonist) was administered to the forearm via the brachial artery catheter at a dose of 200 μg·dl forearm volume−1·min−1. Exogenous adenosine was administered at three doses (3.125, 6.25, and 12.5 μg·dl forearm volume−1·min−1) intra-arterially for 4 min at each dose before and after l-NMMA infusion alone and combined l-NMMA-aminophylline infusion. The purpose of the adenosine dose-response trials was to 1) determine the amount of adenosine-mediated vasodilation that is NO independent and 2) confirm adenosine receptor inhibition.
Experimental Protocol
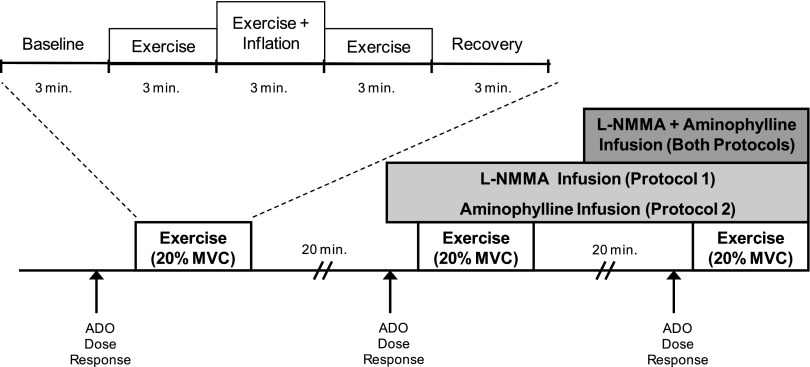
A schematic of the general experimental design is provided in Fig. 1. Each subject completed three exercise trials. Each exercise trial consisted of 3 min of rest, exercise, exercise with balloon inflation, exercise following balloon deflation, and recovery (15 min total; 9 min of total exercise). In protocol 1, exercise was performed during no drug administration, followed by l-NMMA alone and then combined l-NMMA and aminophylline. In protocol 2, exercise was performed during no drug administration, followed by aminophylline alone and then combined aminophylline and l-NMMA. Each trial was separated by 20 min of rest to allow FBF to return to baseline.
Fig. 1.
Schematic diagram of experimental protocol. Subjects completed 3 exercise trials. Each exercise trial consisted of baseline, exercise (control), exercise during inflation, exercise after deflation, and recovery measurements (3 min each). Exercise trials were performed during control (no drug), NG-monomethyl-l-arginine (l-NMMA; protocol 1), adenosine receptor inhibition (aminophylline; protocol 2), and combined l-NMMA-aminophylline infusions (both protocols). Each trial was separated by at least 20 min of rest to allow forearm blood flow (FBF) to return to baseline values. Adenosine (ADO) dose-response infusions were performed at the start of the study under control (no drug) conditions and repeated during l-NMMA (protocol 1), aminophylline (protocol 2), and combined l-NMMA-aminophylline (both protocols). MVC, maximal voluntary contraction.
Data Analysis and Statistics
Data were collected at 200 Hz, stored on a computer, and analyzed off-line with signal processing software (WinDaq, DATAQ Instruments, Akron, OH). Local mean arterial pressure [brachial artery catheter pressure (BAP)] was determined from the brachial artery pressure waveform measured distal to the balloon, systemic mean arterial pressure (MAP, e.g., pressure proximal to the balloon) was derived from the Finometer pressure waveform, and HR was determined from the electrocardiogram. FBF, BAP, MAP, CO, and HR were determined by averaging values during the last 30 s of rest, exercise, exercise with inflation, exercise after deflation, and recovery. In addition, all values were analyzed and averaged during the first 10 s of target balloon inflation (nadir) and the first 10 s immediately following balloon deflation. Forearm vascular conductance (FVC) was calculated as (FBF/BAP) × 100 and expressed as millimeters per minute per 100 mmHg.
All values are expressed as means ± SE. Within a given protocol, FBF, FVC, BAP, systemic MAP, HR, and CO during rest, exercise, the nadir after balloon inflation, exercise at the end of the balloon inflation, exercise after deflation, and recovery were analyzed by repeated-measures analysis of variance (ANOVA). When significance was detected, Tukey's post hoc test was used to identify individual differences and adjust P values to account for multiple comparisons, to preserve an overall type I error rate of 0.05.
Percent recovery in FBF and FVC were calculated [steady-state inflation + exercise value − nadir/steady-state exercise (control) value − nadir]. To investigate the role of NO and adenosine on percent recovery of blood flow and conductance one-way repeated-measures ANOVAs were performed between drug conditions. To further explore the contribution of local vasodilation to any restoration of flow, we analyzed balloon resistance and forearm vascular resistance and considered them individually and in series (3, 4, 20). Using systemic arterial pressure (SAP; Finometer), brachial artery pressure distal to the balloon (BAP; catheter), and brachial artery blood flow, we calculated the resistance of the balloon (SAP − BAP/flow) and vascular resistance (BAP/flow). The total resistance was calculated as the sum of these two resistors. Changes in vascular and balloon resistance were analyzed from the onset of balloon inflation (nadir) until the end of the inflation period and expressed as a percent change. One-way repeated-measures ANOVAs were used to compare the percent change in resistance between drug conditions. Statistical significance was set a priori at P < 0.05.
RESULTS
Nine subjects completed protocol 1. One subject did not complete the protocol because of technical difficulties associated with the balloon and was excluded from the analysis. Those subjects included in the group analysis for protocol 1 were 25 ± 2 yr of age and 180 ± 2 cm in height and weighed 79 ± 4 kg [body mass index (BMI): 24 ± 1 kg/m2]. Seven subjects completed protocol 2. The subjects were 25 ± 2 yr of age and 179 ± 1 cm in height and weighed 81 ± 2 kg (BMI: 25 ± 1 kg/m2).
Forearm Blood Flow and Vasodilation During Exercise with Balloon Inflation
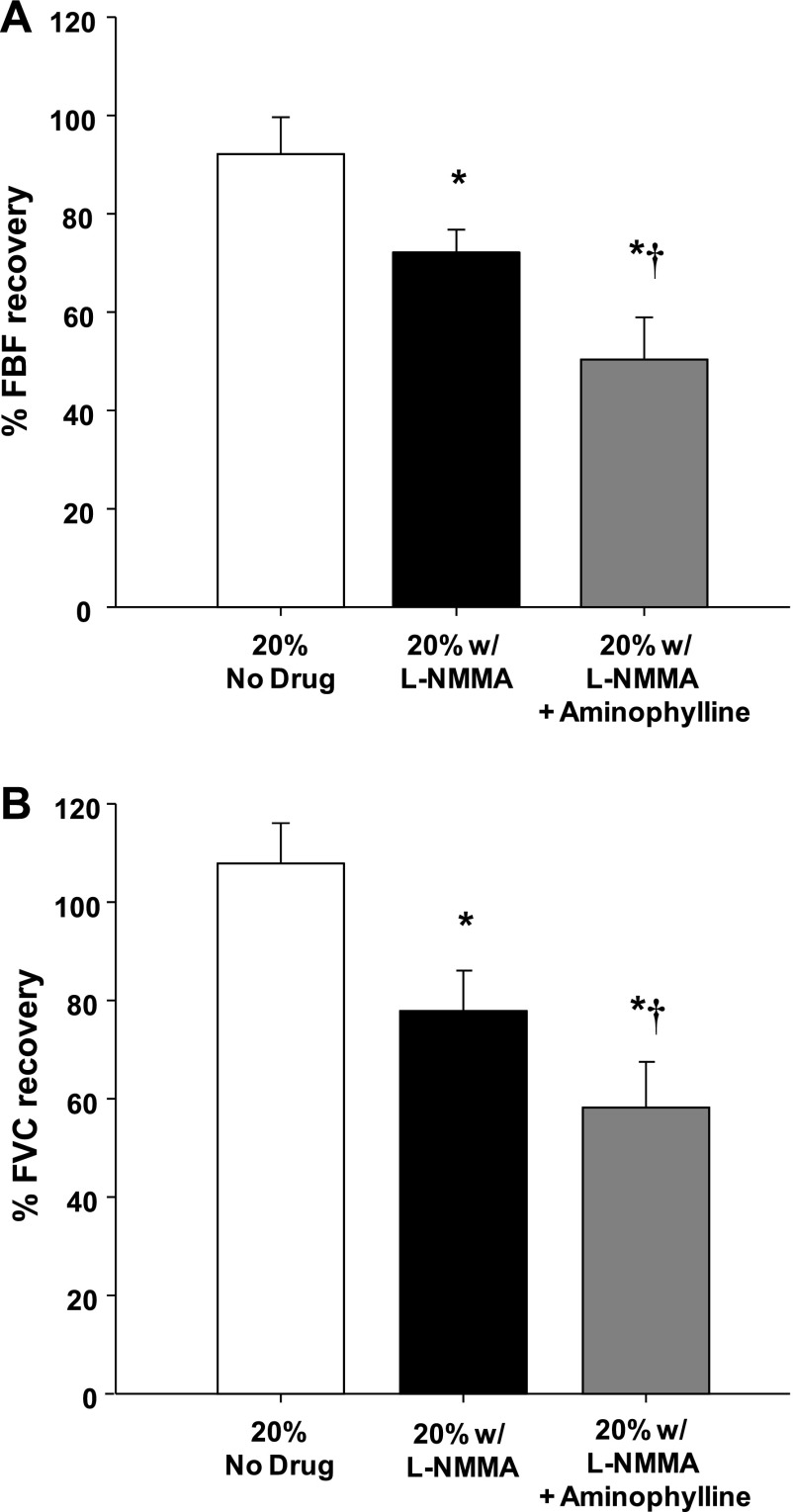
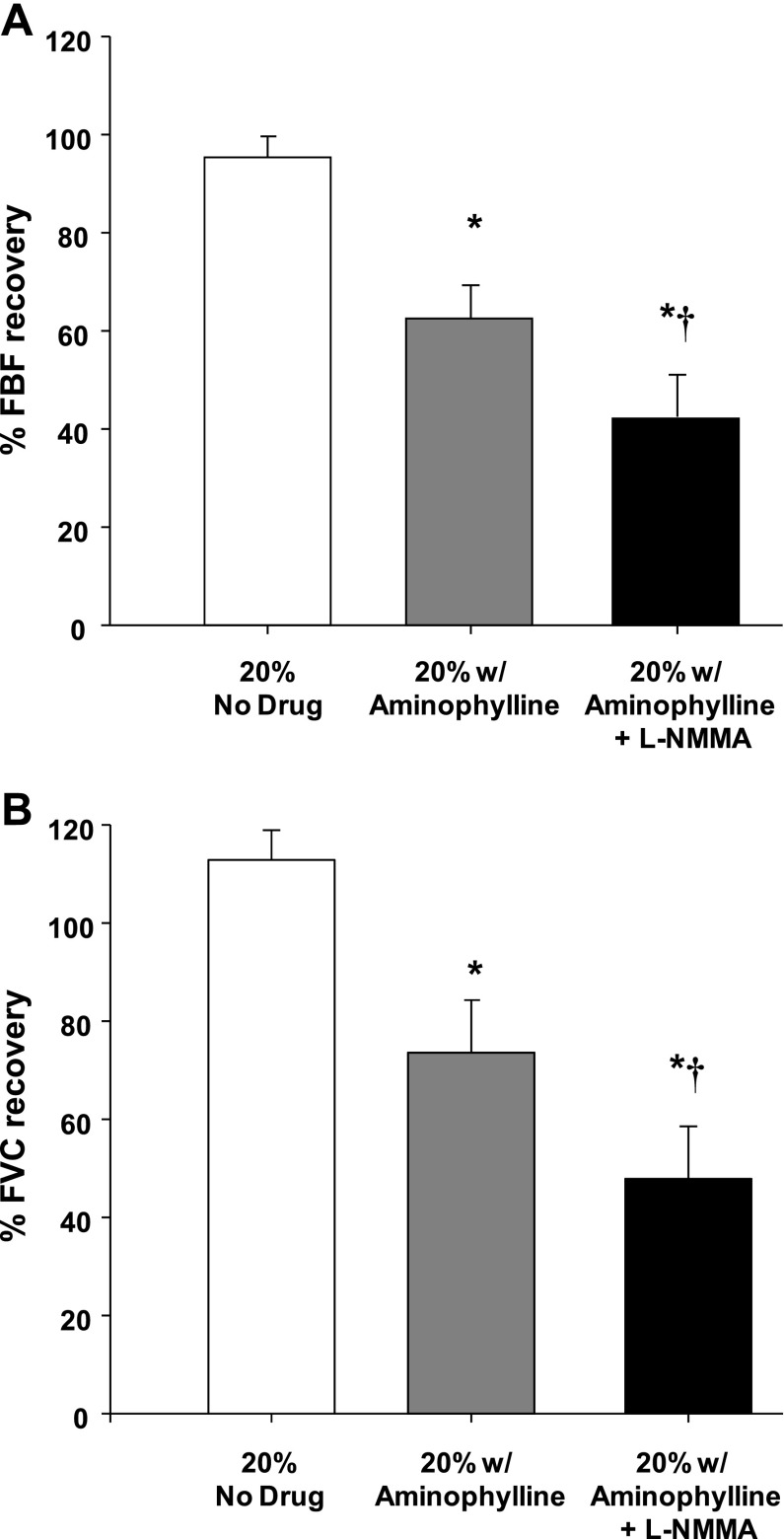
Group mean data for FBF and FVC responses are presented in Table 1. As expected, during both protocols exercise increased FBF and FVC in all three exercise trials (P < 0.001). In protocol 1, balloon inflation (nadir) during the exercise trial with no drug acutely reduced FBF by 51% and FVC by 35% (P < 0.001). In protocol 2, FBF and FVC were acutely reduced by 50% and 34%, respectively (P < 0.001). In both protocols, FBF and FVC at the end of inflation were restored to exercise (control) levels, which were substantially higher than their respective nadir values (P < 0.001). Percent recovery of FBF and FVC during the exercise trials are presented in Fig. 2, A and B (protocol 1), and Fig. 3, A and B (protocol 2), respectively.
Table 1.
Forearm blood flow and vasodilation during exercise with balloon inflation
| Baseline | Exercise (control) | Inflation (nadir) | Inflation (steady state) | Deflation (acute) | Deflation (steady state) | |
|---|---|---|---|---|---|---|
| Protocol 1 (n = 9) | ||||||
| 20% MVC (no drug) | ||||||
| FBF, ml/min | 79 ± 8 | 372 ± 37a | 181 ± 23b | 360 ± 40d | 481 ± 51c,d,f | 464 ± 44c,d,f |
| FVC, ml · min−1 · (100 mmHg)−1 | 89 ± 11 | 392 ± 33a | 253 ± 25b | 406 ± 38d | 503 ± 51c,d,f | 479 ± 41c,d |
| 20% MVC (l-NMMA) | ||||||
| FBF, ml/min | 61 ± 9 | 356 ± 36a | 159 ± 24b | 306 ± 34d,h | 416 ± 31d,f | 435 ± 38c,d,f |
| FVC, ml · min−1 · (100 mmHg)−1 | 66 ± 9 | 370 ± 33a | 242 ± 27b | 347 ± 34d,h | 433 ± 32d,f | 442 ± 36c,d,f |
| 20% MVC (l-NMMA + aminophylline) | ||||||
| FBF, ml/min | 123 ± 17g | 388 ± 32a | 185 ± 25b | 286 ± 40c,e,h | 467 ± 42d,f | 453 ± 39d,f |
| FVC, ml · min−1 · (100 mmHg)−1 | 133 ± 20g | 398 ± 30a | 251 ± 29b | 333 ± 36h | 481 ± 46d,f | 460 ± 40d,f |
| Protocol 2 (n = 7) | ||||||
| 20% MVC (no drug) | ||||||
| FBF, ml/min | 86 ± 13 | 545 ± 29a | 272 ± 24b | 532 ± 28d | 629 ± 53c,d,f | 614 ± 48d,f |
| FVC, ml · min−1 · (100 mmHg)−1 | 95 ± 13 | 583 ± 26a | 386 ± 27b | 605 ± 28d | 660 ± 49c,d | 639 ± 43d |
| 20% MVC (aminophylline) | ||||||
| FBF, ml/min | 120 ± 17h | 562 ± 35a | 289 ± 23b | 462 ± 30e,h | 693 ± 74c,df | 642 ± 48d,f |
| FVC, ml · min−1 · (100 mmHg)−1 | 130 ± 17h | 588 ± 42a | 424 ± 28b | 547 ± 32e,h | 710 ± 64c,d,f | 649 ± 44d |
| 20% MVC (aminophylline + l-NMMA) | ||||||
| FBF, ml/min | 112 ± 17 | 548 ± 39a | 245 ± 13b | 376 ± 41c,g | 714 ± 74c,d,f | 644 ± 57d,f |
| FVC, ml · min−1 · (100 mmHg)−1 | 120 ± 22 | 555 ± 38a | 361 ± 17b | 453 ± 31g | 734 ± 76c,d,f | 630 ± 45d,f |
Values are means ± SE for n subjects. FBF, forearm blood flow; FVC, forearm vascular conductance; l-NMMA, Ng-monomethyl-l-arginine; MVC, maximal voluntary contraction.
P < 0.001 vs. baseline;
P < 0.001 vs. exercise (control);
P < 0.05 vs. exercise;
P < 0.001 vs. nadir;
P < 0.05 vs. nadir;
P < 0.05 vs. inflation (steady state);
P < 0.05 vs. other trials;
P < 0.05 vs. no-drug trial.
Fig. 2.
Percent recovery in FBF (A) and forearm vascular conductance (FVC; B) during balloon inflation (protocol 1; n = 9). Nitric oxide synthase inhibition (l-NMMA) reduced the % recovery of FBF and FVC compared with the respective % recovery during the control (no drug) trial. Percent recovery of FBF and FVC was reduced even further with combined l-NMMA-aminophylline compared with the l-NMMA trial. *P < 0.01 vs. control (no drug); †P < 0.01 vs. l-NMMA alone.
Fig. 3.
Percent recovery in FBF (A) and FVC (B) during balloon inflation (protocol 2; n = 7). Adenosine receptor inhibition (aminophylline) reduced the % recovery of FBF and FVC compared with the respective % recovery during the control (no drug) trial. Percent recovery of FBF and FVC was reduced even further with combined aminophylline-l-NMMA compared with the aminophylline trial. *P < 0.01 vs. control (no drug); †P < 0.05 vs. aminophylline alone.
Contribution of NO and Adenosine to Compensatory Response
Protocol 1.
Although not significant, l-NMMA administration decreased baseline (resting) FBF by 23% (P = 0.14) and FVC by 26% (P = 0.12) below values observed during the control (no drug) trial. Balloon inflation (nadir) during the exercise trial with NOS inhibition acutely reduced FBF by 55% and FVC by 35% (P < 0.001). Similar to the control trials, the FBF and FVC at the end of inflation were partially restored to exercise (control) levels, which were substantially higher than their respective nadir values (P < 0.001). Percent recovery of FBF and FVC during the trial following l-NMMA were substantially lower than the percent recovery values observed during the control (no drug) trial (Fig. 2, A and B, respectively).
Combined infusion of l-NMMA and aminophylline increased baseline (resting) FBF and FVC compared with values observed during the control and l-NMMA-alone trials (P < 0.01). Balloon inflation (nadir) during the combined l-NMMA-aminophylline trial acutely reduced FBF by 52% and FVC by 37% (P < 0.001). Unlike the control and NOS inhibition trials, FBF at the end of the inflation period was lower compared with exercise (control) levels (P < 0.05). Consequently, the percent recovery of FBF and FVC with combined l-NMMA and aminophylline was substantially lower than the compensatory responses observed during the control (no drug) trial (P < 0.001) and the l-NMMA-alone trial (P < 0.01) (Fig. 2, A and B, respectively).
Vascular resistance during balloon inflation (from nadir to end of inflation) decreased during the control (no drug) trial (0.45 ± 0.08 vs. 0.26 ± 0.03 mmHg·ml−1·min; P < 0.01), with NOS inhibition alone (0.45 ± 0.09 vs. 0.31 ± 0.04 mmHg·ml−1·min; P < 0.05), and with combined l-NMMA and aminophylline (0.44 ± 0.06 vs. 0.35 ± 0.04 mmHg·ml−1·min; P < 0.01). Consequently, percent reduction in vascular resistance was less with NOS inhibition (−27 ± 4% vs. −38 ± 5%; P < 0.05) and attenuated even further with combined l-NMMA and aminophylline (−21 ± 3%; P < 0.05 vs. l-NMMA alone). Balloon resistance decreased (from nadir to end of inflation) in the no-drug trial (0.13 ± 0.05 vs. 0.04 ± 0.01 mmHg·ml−1·min; P < 0.001), with NOS inhibition (0.16 ± 0.03 vs. 0.07 ± 0.01 mmHg·ml−1·min; P < 0.001), and with combined l-NMMA and aminophylline (0.15 ± 0.03 vs. 0.07 ± 0.02 mmHg·ml−1·min; P < 0.001). However, the absolute (−0.08 ± 0.01 vs. −0.09 ± 0.02 vs. −0.08 ± 0.01 mmHg·ml−1·min; P = 0.11) and relative (−60 ± 6 vs. −58 ± 2% vs. −56 ± 3%; P = 0.77) changes in balloon resistance were not different between drug conditions.
Rapid deflation of the balloon during exercise resulted in an acute elevation (reactive hyperemia) in FBF and FVC compared with steady-state inflation values for all three trials (P < 0.05). However, the acute reactive hyperemia was only greater compared with exercise (control) values during the no-drug trial (P < 0.05) (Table 1). In other words, NOS inhibition alone and combined NOS-adenosine receptor inhibition blunted the acute reactive hyperemia after balloon deflation.
Protocol 2.
Infusion of aminophylline increased resting FBF and FVC compared with the control (no drug) trial (P < 0.05) but did not impact steady-state exercise (control) values (Table 1). Balloon inflation (nadir) during the exercise trial with aminophylline acutely reduced FBF by 48% and FVC by 27% (P < 0.001). Similar to the control trials, the FBF and FVC at the end of inflation were partially restored to exercise (control) levels, which were substantially higher than their respective nadir values (P < 0.001). Percent recovery of FBF and FVC during the trial following aminophylline were substantially lower than the percent recovery values observed during the control (no drug) trial (Fig. 3, A and B, respectively). Balloon inflation (nadir) during the combined aminophylline-l-NMMA trial acutely reduced FBF by 54% and FVC by 34% (P < 0.001). Unlike the control and aminophylline trials, FBF at the end of the inflation period was lower compared with exercise (control) levels (P < 0.05). Consequently, the percent recovery of FBF and FVC with combined aminophylline and l-NMMA were substantially lower than the compensatory responses observed during the control (no drug) trial (P < 0.001) and the aminophylline-alone trial (P <0.05) (Fig. 3 A and B, respectively).
Vascular resistance during balloon inflation (from nadir to end of inflation) decreased during the control (no drug) trial (0.27 ± 0.02 vs. 0.17 ± 0.01 mmHg·ml−1·min; P < 0.001), with aminophylline alone (0.24 ± 0.02 vs. 0.19 ± 0.01 mmHg·ml−1·min; P < 0.01), and with combined aminophylline and l-NMMA (0.28 ± 0.01 vs. 0.24 ± 0.01 mmHg·ml−1·min; P < 0.01). Consequently, the percent reduction in vascular resistance was less with aminophylline (−22 ± 3% vs. −36 ± 2%; P < 0.05) and attenuated even further with combined aminophylline and l-NMMA (−15 ± 2%; P < 0.05 vs. aminophylline alone). Balloon resistance decreased (from nadir to end of inflation) in the no-drug trial (0.08 ± 0.02 vs. 0.02 ± 0.01 mmHg·ml−1·min; P < 0.01), with aminophylline (0.10 ± 0.01 vs. 0.04 ± 0.01 mmHg·ml−1·min; P < 0.001), and with combined aminophylline and l-NMMA (0.13 ± 0.02 vs. 0.06 ± 0.02 mmHg·ml−1·min; P < 0.01). However, the absolute (−0.06 ± 0.02 vs. −0.06 ± 0.01 vs. −0.07 ± 0.01 mmHg·ml−1·min; P = 0.96) and relative (−63 ± 10% vs. −61 ± 8% vs. −58 ± 10%; P = 0.93) changes in balloon resistance were not different between drug conditions.
Rapid deflation of the balloon during exercise resulted in an acute elevation (reactive hyperemia) in FBF and FVC compared with steady-state inflation values for all three trials (P < 0.05). However, the acute reactive hyperemia was only greater compared with exercise (control) values during the no-drug and aminophylline-alone trials (P < 0.05) (Table 1).
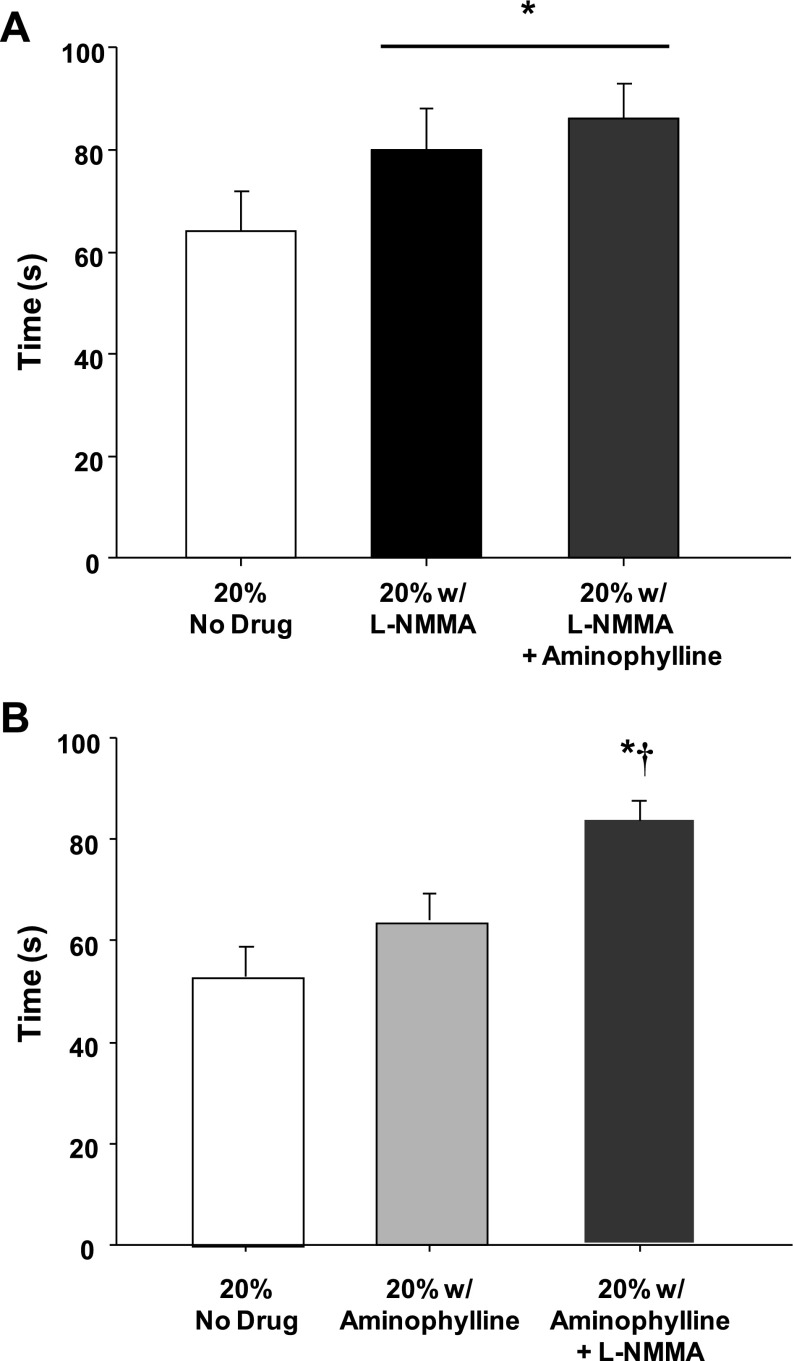
Timing of Compensatory Vasodilation
In protocol 1, NOS inhibition increased the time to reach steady-state FBF and FVC during balloon inflation by ∼25% compared with the no-drug trial (P < 0.05, Fig. 4A). The time to reach steady-state FBF and FVC during inflation during the combined l-NMMA-aminophylline trial was ∼33% longer compared with the no-drug trial (P < 0.01, Fig. 4A). There was no difference in the timing of compensation between the l-NMMA-alone and combined l-NMMA-aminophylline trials (P = 0.48 for FBF; P = 0.56 for FVC). In protocol 2, there was a nonsignificant trend for a delayed time to reach steady-state FBF (P = 0.07) and FVC (P = 0.13) during balloon inflation under adenosine receptor inhibition (aminophylline) (Fig. 4B). The combined infusion of aminophylline and l-NMMA delayed the timing of compensation compared with the no-drug and aminophylline-alone trials (Fig. 4B).
Fig. 4.
Timing of flow restoration. In protocol 1 (A), the time to reach steady-state blood flow during balloon inflation under nitric oxide synthase inhibition (l-NMMA) was increased. The combined inhibition of nitric oxide synthase and adenosine (l-NMMA-aminophylline) did not delay the restoration of flow beyond l-NMMA alone. In protocol 2 (B), the restoration of flow was not delayed during adenosine receptor inhibition (aminophylline) alone. Combined aminophylline-l-NMMA increased the time to reach steady-state blood flow compared with the no-drug and aminophylline-alone trials. *P < 0.05 vs. control (no drug); †P < 0.05 vs. aminophylline alone.
Effect of NOS Inhibition and Adenosine Receptor Blockade on Vasodilator Responses to Exogenous Adenosine
Protocol 1.
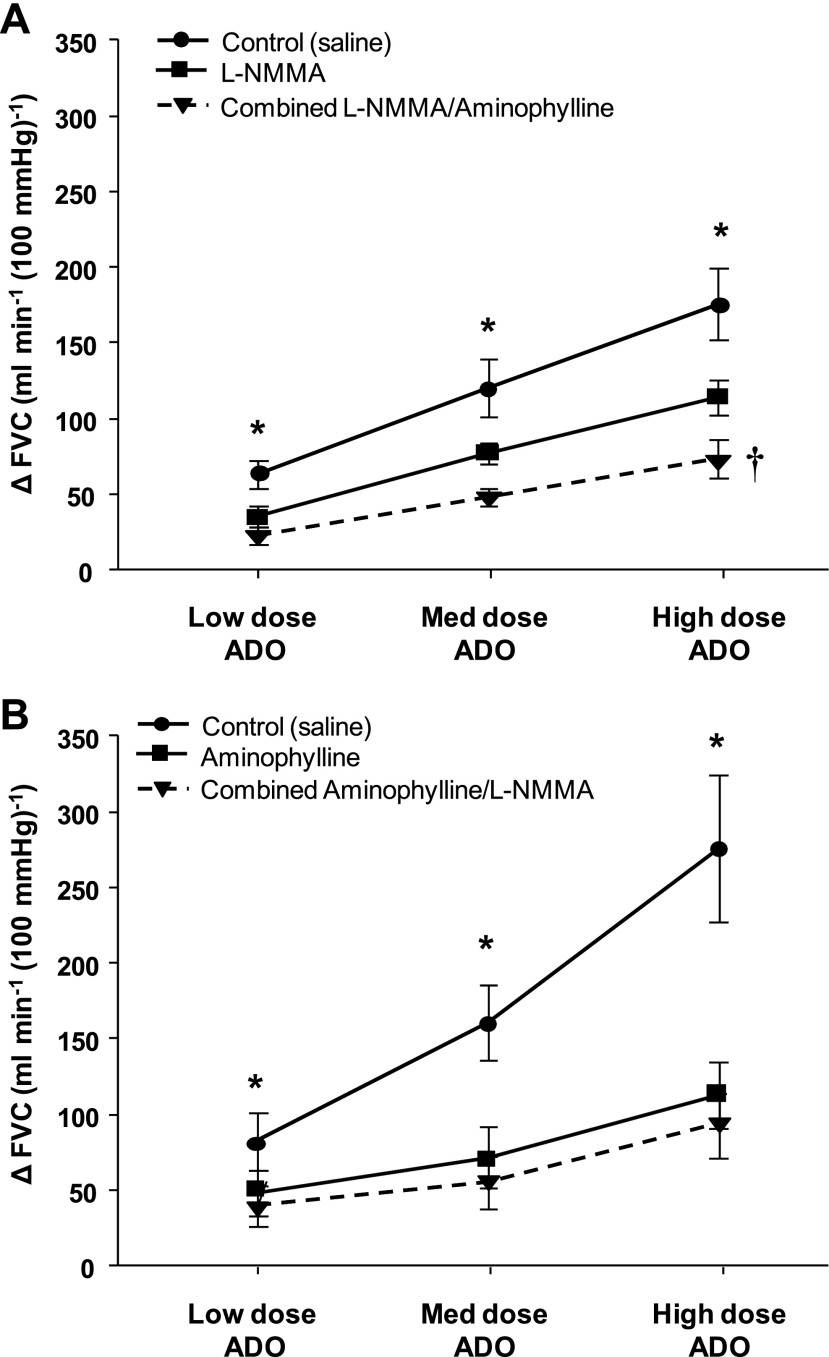
Repeated-measures ANOVA revealed significant time, drug, and time × drug effects during the adenosine dose-response trials (P < 0.001 for all). The vasodilator response (change in FVC from baseline) to exogenous adenosine infusion was significantly lower at all three doses (3.125, 6.25, and 12.5 μg·dl forearm volume−1·min−1) in the presence of l-NMMA and combined l-NMMA-aminophylline compared with no drug (saline) (Fig. 5A, P < 0.05). Furthermore, there was a significant main effect of drug during combined l-NMMA-aminophylline on the responsiveness to exogenous adenosine compared with l-NMMA alone (Fig. 5A, P < 0.01).
Fig. 5.
Vasodilator responses (ΔFVC) to incremental adenosine administration during saline, l-NMMA, aminophylline, and combined l-NMMA-aminophylline administration. In protocol 1 (A), nitric oxide synthase inhibition (l-NMMA) reduced ΔFVC at all doses of exogenous adenosine (ADO) compared with control (saline). Additionally, there was a significant main effect of drug during combined l-NMMA-aminophylline on the responsiveness to exogenous ADO compared with l-NMMA alone. In protocol 2 (B), adenosine receptor inhibition (aminophylline) reduced ΔFVC at all doses of exogenous ADO compared with control (saline). Combined aminophylline-l-NMMA did not reduce ΔFVC compared with aminophylline alone. *P < 0.01 vs. l-NMMA (protocol 1) or aminophylline (protocol 2) and combined l-NMMA-aminophylline; †P < 0.01 main effect combined l-NMMA-aminophylline vs. l-NMMA alone.
Protocol 2.
The vasodilator response to exogenous adenosine was substantially reduced in the presence of aminophylline and combined aminophylline and l-NMMA (Fig. 5B, P < 0.01). The combined infusion of aminophylline and l-NMMA did not reduce the responsiveness to exogenous adenosine compared with aminophylline alone (Fig. 5B, P = 0.84).
Hemodynamic Changes
Systemic hemodynamic responses during exercise are presented in Table 2. Exercise resulted in an increase in MAP in all trials (P < 0.05). MAP remained elevated above baseline values throughout each trial (P < 0.05). Estimated CO and HR did not change with exercise (control) in any of the trials. In protocol 1, MAP, HR, and CO did not change with balloon inflation compared with exercise (control) values. In protocol 2, MAP increased during balloon inflation compared with exercise (control) under combined aminophylline-l-NMMA conditions (P < 0.05).
Table 2.
Systemic hemodynamic responses
| Baseline | Exercise (control) | Inflation (nadir) | Inflation (steady state) | Deflation (acute) | Deflation (steady state) | |
|---|---|---|---|---|---|---|
| Protocol 1 (n = 9) | ||||||
| 20% MVC (no drug) | ||||||
| Mean arterial pressure, mmHg | 96 ± 4 | 102 ± 5* | 103 ± 6* | 105 ± 5* | 103 ± 5* | 105 ± 4* |
| Brachial artery pressure, mmHg | 91 ± 3† | 94 ± 4† | 70 ± 5*‡ | 88 ± 3†‡ | 96 ± 3† | 97 ± 4*† |
| Heart rate, beats/min | 63 ± 1 | 63 ± 2 | 64 ± 1 | 64 ± 1 | 63 ± 1 | 63 ± 2 |
| Cardiac output, l/min | 5.3 ± 0.6 | 5.7 ± 0.6 | 5.8 ± 0.7* | 5.9 ± 0.7* | 6.0 ± 0.7* | 5.9 ± 0.7* |
| 20% MVC (l-NMMA) | ||||||
| Mean arterial pressure, mmHg | 94 ± 4 | 102 ± 5* | 101 ± 4* | 105 ± 4* | 104 ± 4* | 105 ± 4* |
| Brachial artery pressure, mmHg | 92 ± 3† | 95 ± 3† | 66 ± 4*‡ | 87 ± 3†‡ | 95 ± 2† | 98 ± 3*† |
| Heart rate, beats/min | 63 ± 2 | 63 ± 2 | 63 ± 2 | 63 ± 2 | 63 ± 2 | 62 ± 2 |
| Cardiac output, l/min | 5.5 ± 0.5 | 5.6 ± 0.6 | 5.7 ± 0.5 | 5.9 ± 0.6 | 6.0 ± 0.6 | 6.1 ± 0.6* |
| 20% MVC (l-NMMA + aminophylline) | ||||||
| Mean arterial pressure, mmHg | 94 ± 4 | 101 ± 5* | 102 ± 5* | 103 ± 5* | 103 ± 5* | 103 ± 5* |
| Brachial artery pressure, mmHg | 94 ± 3† | 98 ± 4† | 71 ± 4*‡ | 85 ± 3*†‡ | 97 ± 3† | 99 ± 3† |
| Heart rate, beats/min | 62 ± 2 | 62 ± 2 | 62 ± 2 | 62 ± 2 | 62 ± 2 | 62 ± 2 |
| Cardiac output, l/min | 5.2 ± 0.5 | 5.5 ± 0.5 | 5.6 ± 0.6 | 5.6 ± 0.6 | 5.8 ± 0.6* | 5.9 ± 0.6* |
| Protocol 2 (n = 7) | ||||||
| 20% MVC (no drug) | ||||||
| Mean arterial pressure, mmHg | 86 ± 3 | 91 ± 3* | 89 ± 3* | 93 ± 3* | 95 ± 3*† | 93 ± 4* |
| Brachial artery pressure, mmHg | 86 ± 2† | 93 ± 3*† | 69 ± 3*‡ | 87 ± 3†‡ | 95 ± 3*† | 96 ± 4*† |
| Heart rate. beats/min | 64 ± 1 | 66 ± 1 | 66 ± 1 | 66 ± 1 | 66 ± 1 | 66 ± 1 |
| Cardiac output, l/min | 4.9 ± 0.3 | 5.4 ± 0.4 | 5.4 ± 0.4 | 5.5 ± 0.4* | 5.6 ± 0.4* | 5.6 ± 0.4* |
| 20% MVC (aminophylline) | ||||||
| Mean arterial pressure, mmHg | 89 ± 3 | 97 ± 2* | 97 ± 2* | 101 ± 3* | 100 ± 3* | 101 ± 4* |
| Brachial artery pressure, mmHg | 86 ± 2† | 96 ± 4*† | 68 ± 3*‡ | 85 ± 3†‡ | 97 ± 3*† | 99 ± 4*† |
| Heart rate, beats/min | 63 ± 1 | 65 ± 1 | 65 ± 1 | 65 ± 1 | 65 ± 1 | 65 ± 1 |
| Cardiac output, l/min | 4.8 ± 0.3 | 5.0 ± 0.2 | 5.2 ± 0.2 | 5.0 ± 0.3 | 5.0 ± 0.2 | 5.2 ± 0.3 |
| 20% MVC (aminophylline + l-NMMA) | ||||||
| Mean arterial pressure, mmHg | 90 ± 4 | 96 ± 4* | 98 ± 5* | 103 ± 5*‡ | 100 ± 4* | 102 ± 5* |
| Brachial artery pressure, mmHg | 89 ± 3† | 99 ± 4*† | 68 ± 4*‡ | 84 ± 6*†‡ | 97 ± 3*† | 100 ± 4*† |
| Heart rate, beats/min | 64 ± 2 | 65 ± 1 | 66 ± 1 | 66 ± 1 | 66 ± 1 | 66 ± 1 |
| Cardiac output, l/min | 5.0 ± 0.5 | 5.3 ± 0.6 | 5.3 ± 0.6 | 5.4 ± 0.7 | 5.6 ± 0.7* | 5.4 ± 0.6 |
Values are means ± SE for n subjects.
P < 0.05 vs. baseline;
P < 0.05 vs. nadir;
P < 0.05 vs. exercise (control).
DISCUSSION
The primary novel findings of this study are that 1) adenosine has an NO-independent role in the compensatory vasodilation during exercise with acute hypoperfusion, 2) combined inhibition of adenosine receptors and NO formation results in an additive reduction in the compensatory vasodilation in hypoperfused contracting muscles, and 3) although NOS inhibition slows the compensatory vasodilator response to hypoperfusion in the contracting human forearm (protocol 1), adenosine receptor inhibition did not appear to delay the time to reach steady-state FBF (protocol 2). These conclusions are supported by a substantial attenuation of the percent recovery of FBF and FVC with NOS inhibition alone (l-NMMA, protocol 1) and adenosine receptor inhibition alone (aminophylline, protocol 2) and a further attenuation with the combined administration of l-NMMA and aminophylline (protocols 1 and 2).
Role of Adenosine in Compensatory Vasodilation
In animals adenosine is thought to be a key mediator of skeletal muscle blood flow during exercise (17, 22–24). In humans, interstitial adenosine concentrations in skeletal muscle increase at a rate associated with intensity of muscle contraction and the magnitude of muscle blood flow (9). However, adenosine receptor blockade appears to only modestly attenuate the exercise-induced skeletal muscle vasodilation in human limbs, and these findings are not consistent (6, 16, 19, 25). One prevailing thought is that although adenosine is released from adequately perfused active muscle (12), it becomes a more important vasodilator signal during periods of reduced oxygen availability (10, 11). In this context, active hyperemia in dog skeletal muscle is potentiated during experimentally restricted flow in the presence of dipyridamole, thus suggesting a role of adenosine in the regulation of blood flow under conditions of arterial occlusion (10).
Of particular note for the present findings, inhibition of adenosine receptors via aminophylline has been shown to blunt the recovery of hindlimb blood flow in dogs during high-intensity treadmill exercise in response to partial vascular occlusion (11). Whether the blunted recovery of hindlimb blood flow during aminophylline administration was a result of less NO-mediated vasodilation was not determined. Our present data clearly demonstrate that adenosine contributes to the recovery of flow during acute hypoperfusion in an NO-independent manner. Interestingly, a substantial percentage of the vasodilatory effect of exogenous adenosine is mediated by the formation of prostaglandins (19). Therefore, in our present model adenosine may contribute to the compensatory vasodilator through either adenosine-specific or prostaglandin-mediated mechanisms.
The finding that adenosine is a key contributor to blood flow regulation under conditions of locally reduced oxygen availability (via hypoperfusion) is in contrast to our previous work related to compensatory vasodilation during exercise with systemic reductions in oxygen availability (via hypoxia) (5, 6). These previous findings demonstrated that adenosine receptor-mediated vasodilation was not obligatory to the compensatory vasodilation during hypoxic exercise in both the presence and the absence of NO (5, 6). Taken together, our findings may suggest that the role of adenosine in compensatory vasodilation is enhanced to a greater extent when perfusion pressure is reduced along with oxygen availability. Along these lines, adenosine production is positively correlated to the degree of hypoperfusion in the coronary circulation of dogs (7) and appears to contribute to vasodilation of the coronary resistance vessels during exercise in the presence of a flow-limiting stenosis (13).
Source and Action of Adenosine
The present study clearly demonstrates that inhibition of adenosine blunts the compensatory vasodilation (% recovery of FVC) during exercise with hypoperfusion as well as the vasodilator response to exogenous adenosine (ΔFVC). However, since we used the nonspecific adenosine receptor antagonist aminophylline (antagonizes all 4 adenosine receptor isoforms: A1, A2A, A2B, and A3) we cannot be certain which isoform is responsible for adenosine-mediated vasodilation in the human forearm. In the rat hindlimb adenosine is thought to play a role in skeletal muscle vasodilation during exercise via A2A receptors (26), whereas A1 receptors appear to be the dominant isoform in adenosine-mediated vasodilation under conditions of reduced oxygen availability (1). Whether these receptor isoforms are the key sites of adenosine-mediated vasodilation in human limbs needs further investigation. Moreover, it is unclear whether reductions in perfusion pressure alter the role of the adenosine receptors in regulating blood flow to the contracting muscle (2).
Theoretically, the adenosine that contributes to skeletal muscle blood flow can originate from a variety of sources including but not limited to the contracting muscle, interstitial space, endothelium, or degradation of intraluminal ATP. Under conditions of reduced oxygen availability the formation of adenosine from some if not all of these sources is likely enhanced. Along these lines, increased plasma adenosine from skeletal muscle of dogs during systemic hypoxia is formed intracellularly by the vascular tissue, whereas adenosine formation takes place extracellularly in the interstitial space of dogs during muscle contractions (18). Taken together, these findings suggest that the source of adenosine differs according to stimulus (hypoxia vs. muscle contraction) in dogs. In contrast, data in humans suggest that interstitial adenosine can be elevated during both systemic hypoxia (14) and muscle contraction (9). Furthermore, recent evidence in humans suggests a role of interstitial rather than intraluminal adenosine in the regulation of skeletal muscle blood flow under free-flow conditions (19). The contribution of interstitial versus intraluminal adenosine during combined exercise with hypoperfusion or hypoxia clearly needs further elucidation.
Vasodilator Response to Exogenous Adenosine
Our present findings demonstrate a strong NO-dependent component in adenosine-mediated vasodilation and are in agreement with previous findings in the human forearm (15, 27) and leg (19). To our knowledge, this is the first study to also examine the effect of adenosine receptor blockade during exercise in the absence of a fully functional NO signal in the same group of subjects. Our data revealed a significant main effect of drug during combined l-NMMA and aminophylline on the responsiveness to exogenous adenosine compared with l-NMMA alone (protocol 1; P < 0.01) but not compared with aminophylline alone (P = 0.84). Therefore, the reductions in ΔFVC with combined inhibition (l-NMMA-aminophylline) compared with l-NMMA alone clearly show that adenosine can elicit a significant vasodilator response independent of NO. The additional vasodilation beyond that which is regulated through a NO mechanism may also be partially mediated by prostaglandins (19).
Experimental Considerations
In the present study aminophylline administration resulted in significant increases in resting baseline FBF and FVC. Aminophylline is a known phosphodiesterase inhibitor thus increasing cAMP levels in the vascular smooth muscle, leading to increases in FBF (28). Theoretically, this elevated baseline FBF with aminophylline administration could alter the responsiveness of vascular smooth muscle during exercise with hypoperfusion. However, resting flow is not used in the calculation of percent recovery in FBF and FVC during exercise with hypoperfusion. Thus the aminophylline-induced increases in baseline FBF likely did not influence our results related to the role of adenosine in the compensatory vasodilator response to acute hypoperfusion in contracting muscles.
Perspectives
Our present data demonstrate that adenosine and/or NO are not obligatory to the exercise hyperemic response in skeletal muscle under normal inflow conditions (i.e., exercise before balloon inflation). However, in hypoperfused contracting skeletal muscle each of these vasodilator pathways becomes more important in regulating and maintaining flow. Interestingly, similar findings have been demonstrated in the coronary circulation of dogs. Inhibition of a single vasodilator pathway [i.e., adenosine, NO, prostaglandins, or ATP-sensitive potassium (KATP) channel opening] fails to blunt the increase in coronary blood flow in response to exercise in the normal heart. However, blockade of any one of these vasodilator pathways during exercise in the presence of a coronary artery stenosis can exacerbate myocardial hypoperfusion (8). In light of these findings, it is tempting to speculate that the mechanisms responsible for regulating blood flow to areas distal to a partial occlusion during exercise might be similar in skeletal and cardiac muscle in humans.
Conclusions
This study demonstrates that adenosine contributes to the overall magnitude of compensatory vasodilation during exercise with acute hypoperfusion in a manner that can be independent of NO-mediated mechanisms. However, inhibition of adenosine receptors did not prolong the timing of the vasodilator response, thus suggesting that adenosine may not be obligatory in regulating the kinetics of compensatory vasodilation during hypoperfusion in contracting skeletal muscle.
GRANTS
This study was supported by National Institutes of Health Research Grants HL-46493 (to M. J. Joyner) and AR-55819 (to D. P. Casey) and by Clinical and Translational Science Award (CTSA) RR-024150. The Caywood Professorship via the Mayo Foundation also supported this research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Branton Walker, Shelly Roberts, Jean Knutson, Karen Krucker, Chistopher Johnson, and Pam Engrav for their technical assistance. We also thank the volunteers for their time.
REFERENCES
- 1. Bryan PT, Marshall JM. Adenosine receptor subtypes and vasodilatation in rat skeletal muscle during systemic hypoxia: a role for A1 receptors. J Physiol 514: 151–162, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carlsson I, Sollevi A, Wennmalm A. The role of myogenic relaxation, adenosine and prostaglandins in human forearm reactive hyperaemia. J Physiol 389: 147–161, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Casey DP, Joyner MJ. NOS inhibition blunts and delays the compensatory dilation in hypoperfused contracting human muscles. J Appl Physiol 107: 1685–1692, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casey DP, Joyner MJ. Skeletal muscle blood flow responses to hypoperfusion at rest and during rhythmic exercise in humans. J Appl Physiol 107: 429–437, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Casey DP, Madery BD, Curry TB, Eisenach JH, Wilkins BW, Joyner MJ. Nitric oxide contributes to the augmented vasodilatation during hypoxic exercise. J Physiol 588: 373–385, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casey DP, Madery BD, Pike TL, Eisenach JH, Dietz NM, Joyner MJ, Wilkins BW. Adenosine receptor antagonist and augmented vasodilation during hypoxic exercise. J Appl Physiol 107: 1128–1137, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deussen A, Borst M, Kroll K, Schrader J. Formation of S-adenosylhomocysteine in the heart. II. A sensitive index for regional myocardial underperfusion. Circ Res 63: 250–261, 1988. [DOI] [PubMed] [Google Scholar]
- 8. Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev 88: 1009–1086, 2008. [DOI] [PubMed] [Google Scholar]
- 9. Hellsten Y, Maclean D, Radegran G, Saltin B, Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation 98: 6–8, 1998. [DOI] [PubMed] [Google Scholar]
- 10. Klabunde RE. Conditions for dipyridamole potentiation of skeletal muscle active hyperemia. Am J Physiol Heart Circ Physiol 250: H62–H67, 1986. [DOI] [PubMed] [Google Scholar]
- 11. Koch LG, Strick DM, Britton SL, Metting PJ. Reflex versus autoregulatory control of hindlimb blood flow during treadmill exercise in dogs. Am J Physiol Heart Circ Physiol 260: H436–H444, 1991. [DOI] [PubMed] [Google Scholar]
- 12. Laughlin MH, Klabunde RE, Delp MD, Armstrong RB. Effects of dipyridamole on muscle blood flow in exercising miniature swine. Am J Physiol Heart Circ Physiol 257: H1507–H1515, 1989. [DOI] [PubMed] [Google Scholar]
- 13. Laxson DD, Homans DC, Bache RJ. Inhibition of adenosine-mediated coronary vasodilation exacerbates myocardial ischemia during exercise. Am J Physiol Heart Circ Physiol 265: H1471–H1477, 1993. [DOI] [PubMed] [Google Scholar]
- 14. MacLean DA, Sinoway LI, Leuenberger U. Systemic hypoxia elevates skeletal muscle interstitial adenosine levels in humans. Circulation 98: 1990–1992, 1998. [DOI] [PubMed] [Google Scholar]
- 15. Martin EA, Nicholson WT, Eisenach JH, Charkoudian N, Joyner MJ. Bimodal distribution of vasodilator responsiveness to adenosine due to difference in nitric oxide contribution: implications for exercise hyperemia. J Appl Physiol 101: 492–499, 2006. [DOI] [PubMed] [Google Scholar]
- 16. Martin EA, Nicholson WT, Eisenach JH, Charkoudian N, Joyner MJ. Influences of adenosine receptor antagonism on vasodilator responses to adenosine and exercise in adenosine responders and nonresponders. J Appl Physiol 101: 1678–1684, 2006. [DOI] [PubMed] [Google Scholar]
- 17. Metting PJ, Weldy DL, Ronau TF, Britton SL. Effect of aminophylline on hindlimb blood flow autoregulation during increased metabolism in dogs. J Appl Physiol 60: 1857–1864, 1986. [DOI] [PubMed] [Google Scholar]
- 18. Mo FM, Ballard HJ. The effect of systemic hypoxia on interstitial and blood adenosine, AMP, ADP and ATP in dog skeletal muscle. J Physiol 536: 593–603, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mortensen SP, Nyberg M, Thaning P, Saltin B, Hellsten Y. Adenosine contributes to blood flow regulation in the exercising human leg by increasing prostaglandin and nitric oxide formation. Hypertension 53: 993–999, 2009. [DOI] [PubMed] [Google Scholar]
- 20. O'Leary DS, Sheriff DD. Is the muscle metaboreflex important in control of blood flow to ischemic active skeletal muscle in dogs? Am J Physiol Heart Circ Physiol 268: H980–H986, 1995. [DOI] [PubMed] [Google Scholar]
- 21. Ogoh S, Fadel PJ, Nissen P, Jans O, Selmer C, Secher NH, Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol 550: 317–324, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Persson MG, Ohlen A, Lindbom L, Hedqvist P, Gustafsson LE. Role of adenosine in functional hyperemia in skeletal muscle as indicated by pharmacological tools. Naunyn Schmiedebergs Arch Pharmacol 343: 52–57, 1991. [DOI] [PubMed] [Google Scholar]
- 23. Poucher SM. The role of the A2A adenosine receptor subtype in functional hyperaemia in the hindlimb of anaesthetized cats. J Physiol 492: 495–503, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poucher SM, Nowell CG, Collis MG. The role of adenosine in exercise hyperaemia of the gracilis muscle in anaesthetized cats. J Physiol 427: 19–29, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Radegran G, Calbet JA. Role of adenosine in exercise-induced human skeletal muscle vasodilatation. Acta Physiol Scand 171: 177–185, 2001. [DOI] [PubMed] [Google Scholar]
- 26. Ray CJ, Marshall JM. Elucidation in the rat of the role of adenosine and A2A-receptors in the hyperaemia of twitch and tetanic contractions. J Physiol 587: 1565–1578, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smits P, Williams SB, Lipson DE, Banitt P, Rongen GA, Creager MA. Endothelial release of nitric oxide contributes to the vasodilator effect of adenosine in humans. Circulation 92: 2135–2141, 1995. [DOI] [PubMed] [Google Scholar]
- 28. Taddei S, Pedrinelli R, Salvetti A. Theophylline is an antagonist of adenosine in human forearm arterioles. Am J Hypertens 4: 256–259, 1991. [DOI] [PubMed] [Google Scholar]
- 29. Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol 74: 2566–2573, 1993. [DOI] [PubMed] [Google Scholar]