Abstract
Previous attempts to detect global cerebral hemodynamic differences between those who develop headache, nausea, and fatigue following rapid exposure to hypoxia [acute mountain sickness (AMS)] and those who remain healthy have been inconclusive. In this study, we investigated the effects of two drugs known to reduce symptoms of AMS to determine if a common cerebral hemodynamic mechanism could explain the prophylactic effect within individuals. With the use of randomized, placebo-controlled, double-blind, crossover design, 20 healthy volunteers were given oral acetazolamide (250 mg), dexamethasone (4 mg), or placebo every 8 h for 24 h prior to and during a 10-h exposure to a simulated altitude of 4,875 m in a hypobaric chamber, which included 2 h of exercise at 50% of altitude-specific V̇o2max. Cerebral hemodynamic parameters derived from ultrasound assessments of dynamic cerebral autoregulation and vasomotor reactivity were recorded 15 h prior to and after 9 h of hypoxia. AMS symptoms were scored using the Lake Louise Questionnaire (LLQ). It was found that both drugs prevented AMS in those who became ill on placebo (∼70% decrease in LLQ), yet a common cerebral hemodynamic mechanism was not identified. Compared with placebo, acetazolamide reduced middle cerebral artery blood flow velocity (11%) and improved dynamic cerebral autoregulation after 9 h of hypoxia, but these effects appeared independent of AMS. Dexamethasone had no measureable cerebral hemodynamic effects in hypoxia. In conclusion, global cerebral hemodynamic changes resulting from hypoxia may not explain the development of AMS.
Keywords: acute mountain sickness, altitude, cerebral blood flow, autoregulation, vascular reactivity
people who travel to high altitude frequently suffer from headache, the central symptom of acute mountain sickness (AMS). Several million people experience symptoms of AMS annually (14). Unfortunately, the pathophysiology of headache in AMS remains unresolved. A prevalent hypothesis is that high-altitude headache results from changes in cerebral blood flow and oxygenation that disrupt the blood-brain barrier and lead to vasogenic cerebral edema (29). We recently tested the hypothesis that impairment of the ability to maintain cerebral blood flow as blood pressure fluctuates (cerebral autoregulation) is a key factor in the development of AMS. We showed that cerebral autoregulation was impaired by hypoxia, yet the degree of impairment was similar between subjects developing AMS and those remaining healthy, suggesting that autoregulation was not a causative factor in AMS (32). While these findings were robust, group comparisons are inherently less sensitive than intra-individual comparisons for detecting small, but potentially meaningful, changes in cerebral hemodynamics. We thus continued our investigation with longitudinal, intra-individual assessments. In addition, on subsequent visits, subjects were treated with drugs proven effective for AMS prophylaxis (16) to provide a more specific picture of cerebral hemodynamic adjustments that may offer protection against AMS.
We studied the effects of acetazolamide, a carbonic anhydrase enzyme inhibitor, and dexamethasone, a glucocorticoid steroid hormone, because they are the two most common and effective drugs used to prevent and treat AMS. Both drugs have been shown to alter cerebral hemodynamics, yet their specific mechanisms of action are unclear. Acetazolamide is thought to offset the stress of hypoxia by inducing metabolic acidosis (increased renal bicarbonate excretion) and cerebral tissue respiratory acidosis (inhibition of CO2 hydration), which together stimulate central chemoreceptors, increase ventilation, and thus improve arterial oxygenation (20). Additionally, acetazolamide has been shown to dilate cerebral blood vessels (10) and therefore may alleviate AMS by increasing cerebral oxygen delivery (4). Dexamethasone is believed to prevent cerebral edema by maintaining blood-brain barrier integrity (11), but has also been shown to affect cerebral blood flow in patients with brain tumors (35, 21, 3). To the best of our knowledge, no studies have evaluated effects of dexamethasone on cerebral blood flow in healthy individuals exposed to acute hypoxia. Whether these drugs have a common effect on the regulation of cerebral blood flow was the central question of this study.
We hypothesized that both acetazolamide and dexamethasone reduce symptoms of AMS by improving control of cerebral blood flow and oxygenation. We reasoned that if these parameters are responsible for the development of AMS, subjects who are susceptible to AMS may exhibit less favorable cerebral hemodynamic control than those who remain healthy. By treating AMS-susceptible subjects with acetazolamide and dexamethasone we expected to detect intraindividual improvements in cerebral hemodynamic regulation that could offer new insight into the pathophysiology of AMS.
MATERIALS AND METHODS
Recruitment and screening.
Following institutional ethics approval, healthy volunteers who had resided at 1,650 m for at least 1 yr were screened to exclude those with recent (<1 mo) exposure to altitudes above 2,500 m, medical conditions affected by hypoxia, or poor aerobic fitness, as previously described (32). All subjects voluntarily provided written consent and were treated in accordance with the Declaration of Helsinki.
General study design.
Using a randomized, double-blind, placebo controlled, crossover design, we evaluated the effects of acetazolamide (250 mg/8 h), dexamethasone (4 mg/8 h) and placebo on cerebral hemodynamics under baseline, normoxic conditions (Pb ∼625 mmHg, 1,650 m) and during 10 h of exposure to hypobaric hypoxia (Pb 425 mmHg, ∼4,875 m) in an environmental chamber. Medications were begun 24 h prior to chamber decompression and continued during hypoxia. A minimum of 3 wk was scheduled between trials to washout potential carryover effects from drugs and hypoxia.
Protocol.
Study participants were familiarized with all measurement techniques during an abbreviated practice trial in normoxia before receiving study medication. Six hours after starting respective medication, subjects' baseline physiological measurements were assessed in normoxia. Subjects were seated upright, with their back, shoulders, and arms supported. Sensors were placed to continuously monitor arterial blood pressure (finger plethysmography: Nexfin HD, BMeye), middle cerebral artery blood flow velocity (transcranial Doppler: ST3, Spencer Technologies), frontal cortex oxygenation (near infrared spectroscopy: Oxymon MKIII, Artinis), finger pulse oximetry (Nellcor N-595), pulmonary ventilation (spirometry: UVM, Vacumed), end-tidal gas concentrations (fast response gas analyzer: O2Cap, Oxigraf), and ECG (lead II: Bioamp, ADInstruments), as previously described (32). After steady-state, rhythmical breathing patterns were observed, resting data were recorded (200 Hz, Powerlab 16SP, ADInstruments) for 10 min to evaluate dynamic cerebral autoregulation, cerebral vascular resistance, and critical closing pressure. Additionally, spot measurements of basilar artery blood flow velocity were obtained during the last 2 min of rest with a second 2-MHz, handheld Doppler probe (ST3, Spencer Technologies) through the suboccipital window at penetration depths of ∼90 mm.
Next, subjects performed a modified rebreathing protocol to assess cerebral vasomotor reactivity to CO2 (9). Briefly, individuals were coached to hyperventilate for 2 min to reduce PetCO2 below 20 mmHg, then, following two deep inspirations of a gas mixture providing 250 mmHg PiO2 and 60 mmHg PiCO2, subjects were instructed to breathe normally while expired air was circulated through a rebreathing circuit to slowly raise PetCO2 to each person's limit of CO2 tolerance (>50 mmHg).
Subjects reported back to the lab on the following morning for testing in the hypobaric chamber. On reaching the target barometric pressure (425 mmHg), subjects performed four sets of 30-min cycling exercise (50% of altitude adjusted V̇o2max) with 15 min of rest between sets. This exercise protocol was designed to simulate physical activity that typically accompanies ascent to high terrestrial altitude and has been shown to increase the severity of AMS symptoms (30). For the remainder of the day, subjects rested inside the chamber. Cerebral hemodynamic assessments, as described above, were repeated after 4 and 9 h of hypobaric hypoxia. Self-reported sections (headache, gastrointestinal, dizziness, fatigue) of the Lake Louise AMS Questionnaire (LLQ) were used to evaluate AMS symptoms at corresponding time points (28). Subjects with LLQ ≥ 3, including headache, at 9 h were classified as ill (AMS susceptible) and those with LLQ ≤ 2, or without headache, were classified as healthy (AMS resistant).
Hemodynamic analyses.
To quantitatively describe the effects of spontaneous changes in resting blood pressure on cerebral blood flow velocity, a cerebral autoregulation index (ARI) was determined using transfer function analysis and subsequent step response (see appendix), as previously described (32, 31). Critical closing pressure and resistance area product of cerebral blood vessels were estimated using the x-intercept and inverse slope of the relationship between cerebral blood flow velocity (CBFv) vs. arterial blood pressure (ABP) across beats (27). A cerebral vascular resistance index was calculated as the quotient of mean ABP/mean CBFv. Arterial pulse-wave modeling was used to estimate cardiac output (36). Cerebral vasomotor reactivity (CVMR) was defined as the slope of the linear fit between percent resting CBFv and PetCO2 during the modified rebreathing segment of the protocol (25). Additionally, the cerebrovascular conductance index (CVCi), which corrected CVMR for changes in mean ABP, was calculated from the slope of the linear fit between percent resting CBFv/ABP and PetCO2 (8).
Statistics.
Only subjects completing all three drug trials were included in the statistical analysis. Each variable of interest was analyzed using a mixed factor ANOVA, with AMS classification after 9 h of hypoxia on placebo (AMS susceptible vs. AMS resistant) analyzed with respect to drug (placebo, acetazolamide, and dexamethasone) over time (baseline and 9 h). Criteria for significance were set at P < 0.05 for main and interaction effects. t-Tests were used for post hoc analyses of differences between AMS status (independent) and across drug and time (paired) using more stringent criteria (P < 0.01) to control for type I error. Additionally, we identified subjects with the best (top quartile) and worst (bottom quartile) cerebral autoregulation scores after 9 h of hypoxia during the placebo run. Additional nonparametric tests were run on these groups to determine if those with worse autoregulation responded differently to acetazolamide and dexamethasone than those with better autoregulation. Friedman's ANOVA by ranks was used to evaluate individual responses to the drugs (P < 0.05) and Mann-Whitney U tests were used to evaluate differences between groups using P < 0.05 as the criteria for significance. Data are presented as means ± SD.
RESULTS
Twenty-nine volunteers met the inclusion criteria and completed at least the placebo and one drug trial. Nine subjects dropped out of the study due to the large time commitment required to obtain an additional trial (∼20 h over 2 days). Twenty subjects (16 men, 4 women) participated in all three trials and were used in the analysis.
Effects of acetazolamide and dexamethasone in normoxia.
In normoxia, acetazolamide reduced PetCO2 by 5% (Table 1) and impaired cerebral autoregulation (Table 2), but had no other detectable effects compared with placebo. Dexamethasone elevated heart rate (12%) and cardiac output (15%) and lowered PetCO2 (4%). The drop in PetCO2 was associated with reductions in middle cerebral and basilar artery flow velocity (17 and 9%, respectively), increased cerebral vascular resistance (20%), and improved cerebral autoregulation (Tables 1–3).
Table 1.
Resting cardiopulmonary variables
| V̇e, min | Vt, liters | RR, beats/min | PetO2, mmHg | PetCO2, mmHg | SpO2,% | HR, beats/min | Q, l/min | MABP, mmHg | |
|---|---|---|---|---|---|---|---|---|---|
| Normoxia | |||||||||
| Placebo | |||||||||
| Mean | 8.7 | 0.62 | 16 | 79.2 | 36.2 | 95 | 63 | 6.0 | 92 |
| SD | 2.0 | 0.17 | 3 | 4.8 | 3.2 | 1 | 9 | 1.1 | 12 |
| Acetazolamide | |||||||||
| Mean | 9.6 | 0.66 | 15 | 81.8 | 34.5* | 96 | 63 | 5.9 | 90 |
| SD | 1.7 | 0.13 | 4 | 3.2 | 2.3 | 1 | 12 | 0.9 | 12 |
| Dexamethasone | |||||||||
| Mean | 10.2 | 0.66 | 16 | 81.1 | 34.8* | 95 | 71*‡ | 7.0*‡ | 90 |
| SD | 2.4 | 0.15 | 3 | 3.7 | 3.2 | 1 | 13 | 1.3 | 8 |
| Hypoxia | |||||||||
| Placebo | |||||||||
| Mean | 12.6† | 0.74† | 17 | 44.6† | 31.0† | 78† | 97† | 8.3† | 89 |
| SD | 3.3 | 0.19 | 4 | 5.1 | 3.3 | 8 | 11 | 1.6 | 13 |
| Acetazolamide | |||||||||
| Mean | 13.3† | 0.79† | 18† | 48.1*† | 27.6*† | 82*† | 94† | 7.5† | 84 |
| SD | 2.8 | 0.23 | 4 | 3.0 | 2.0 | 5 | 11 | 1.7 | 12 |
| Dexamethasone | |||||||||
| Mean | 13.1† | 0.84† | 17 | 43.1†‡ | 31.0†‡ | 77†‡ | 97† | 9.1†§ | 85† |
| SD | 2.6 | 0.22 | 4 | 4.6 | 2.3 | 8 | 10 | 1.9 | 12 |
Values are means ± SD, n =20. V̇e, minute ventilation; Vt, tidal volume; RR, respiratory rate; PetO2, partial pressure O2; PetCO2, partial pressure CO2; SpO2, arterial saturation; HR, heart rate; Q, cardiac output; MABP, mean arterial blood pressure.
Different from PL (P < 0.01 to control for type I error);
different from normoxia (P < 0.01);
different from Acetazolamide (P < 0.01).
Table 2.
Cerebral hemodynamic variables at rest and during rebreating
| Resting |
Rebreathing |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| MCA CBFv, cm/s | BA CBFv, cm/s | CVR mmHg · cm−1 · s−1 | TSI, % | ARI | CrCp, mmHg | RAP, cm · s · −1mmHg−1 | CVMR. %/mmHg | CVCi. %/mmHg2 | |
| Normoxia | |||||||||
| Placebo | |||||||||
| Mean | 56.5 | 37.8 | 1.64 | 83 | 4.8 | 27 | 1.16 | 2.93 | 1.19 |
| SD | 6.6 | 8.0 | 0.27 | 15 | 1.1 | 11 | 0.26 | 0.82 | 0.50 |
| Acetazolamide | |||||||||
| Mean | 52.4 | 37.3 | 1.77 | 84 | 4.2* | 22 | 1.34 | 2.73 | 1.23 |
| SD | 8.3 | 9.8 | 0.39 | 8 | 0.8 | 10 | 0.31 | 0.96 | 0.57 |
| Dexamethasone | |||||||||
| Mean | ‡ | 34.4* | 1.96* | 86 | 5.2*‡ | 27 | 1.36 | 3.21 | 1.50 |
| SD | 7.6 | 6.9 | 0.37 | 8 | 1.0 | 10 | 0.37 | 1.08 | 0.86 |
| Hypoxia | |||||||||
| Placebo | |||||||||
| Mean | 58.3 | 39.4 | 1.56 | 72† | 3.2† | 28 | 1.07 | 3.33 | 1.74† |
| SD | 8.6 | 12.3 | 0.32 | 14 | 1.4 | 13 | 0.29 | 1.40 | 1.00 |
| Acetazolamide | |||||||||
| Mean | 51.8* | 39.6 | 1.66 | 72† | 4.2* | 24 | 1.16 | 3.54 | 1.09 |
| SD | 7.6 | 10.3 | 0.35 | 12 | 0.8 | 12 | 0.28 | 1.42 | 0.65 |
| Dexamethasone | |||||||||
| Mean | 53.6† | 39.4† | 1.62† | 71† | 3.7† | 27 | 1.12 | 3.62 | 1.82§ |
| SD | 8.8 | 8.4 | 0.31 | 14 | 0.8 | 16 | 0.40 | 1.14 | 0.72 |
Values are means ± SD; n = 20. MCA CBFv, middle cerebral artery flow velocity; BA CBFv, basilar artery flow velocity; CVR, cerebral vascular resistance index; TSI, cerebral tissue oxygenation index (NIRS); ARI, autoregulation index; CrCP, critical closing pressure; RAP, resistance area product; CVMR, cerebral vasomotor reactivity to CO2; CVCi, cerebrovascular conductance index.
Different from Placebo (P < 0.01 to control for type I error);
different from normoxia (P < 0.01);
different from Acetazolamide (P < 0.01).
Table 3.
Transfer function analyses across low frequencies (<0.10 Hz)
| PSD CBFv, cm · s−1 · Hz−1 | PSD ABP, mmHg2/Hz | Coherence | Gain, %/% | Phase, rad | |
|---|---|---|---|---|---|
| Normoxia | |||||
| Placebo | |||||
| Mean | 6.29 | 10.57 | 0.56 | 0.55 | 0.59 |
| SD | 3.86 | 3.59 | 0.11 | 0.10 | 0.28 |
| Acetazolamide | |||||
| Mean | 5.50 | 9.56 | 0.56 | 0.54 | 0.43 |
| SD | 5.00 | 5.64 | 0.09 | 0.17 | 0.19 |
| Dexamethasone | |||||
| Mean | 4.53 | 10.56 | 0.52 | 0.46 | 0.65‡ |
| SD | 2.63 | 4.94 | 0.10 | 0.11 | 0.27 |
| Hypoxia | |||||
| Placebo | |||||
| Mean | 12.94 | 11.33 | 0.66 | 0.81† | 0.31† |
| SD | 12.14 | 7.67 | 0.14 | 0.20 | 0.17 |
| Acetazolamide | |||||
| Mean | 7.28 | 9.47 | 0.57* | 0.67 | 0.48 |
| SD | 4.08 | 5.92 | 0.13 | 0.25 | 0.26 |
| Dexamethasone | |||||
| Mean | 6.44† | 8.95 | 0.63† | 0.71† | 0.33† |
| SD | 3.33 | 5.63 | 0.10 | 0.26 | 0.16 |
Values are means ± SD; n = 20. PSD CBFv and PSD ABP, power spectal density of cerebral blood flow velocity and arterial blood pressure.
Different from PL (P < 0.01 to control for type I error);
different from normoxia (P < 0.01);
different from Acetazolamide (P < 0.01).
Effects of acetazolamide and dexamethasone in hypoxia.
During the placebo trial, 9 h of hypoxia elevated heart rate (54%), cardiac output (38%), and pulmonary ventilation (44%), lowered PetCO2 (44%), PetCO2 (14%), arterial oxygen saturation (18%) and cerebral oxygenation (4%), and impaired cerebral autoregulation (Tables 1–3). During CO2 rebreathing, CVCi was increased by 46% (Table 2). Six subjects (30%) were classified as AMS susceptible (mild to moderate AMS), while 14 were AMS resistant (Table 4). No differences were detected between AMS-susceptible and AMS-resistant subjects in any physiological parameters during the placebo trial. AMS susceptibility on placebo was used to classify data for subsequent statistical analysis to test whether the drugs had greater effects in AMS-susceptible than AMS-resistant subjects, thereby explaining potential prophylactic mechanisms of actions.
Table 4.
Lake Louise Questionnaire scores after 9 h of hypobaric hypoxia
| AMS Susceptible | AMS Resistant | |
|---|---|---|
| Placebo | 4.0 ± 0.6† | 1.1 ± 0.7 |
| Acetazolamide | 1.3 ± 1.2* | 1.1 ± 0.9 |
| Dexamethasone | 1.3 ± 0.8* | 0.7 ± 0.7 |
Values are means ± SD; n = 20. AMS susceptible (n = 6) had Lake Louise Questionnaire ≥3 with headache on placebo; AMS resistant (n = 14) had Lake Louise Questionnaire <3 or without headache on placebo.
Different from Placebo (P < 0.05);
different from AMS resistant.
Compared with placebo, both drugs reduced AMS symptoms after 9 h of hypoxia by ∼70% (Table 4). Acetazolamide increased PetCO2 (8%) and arterial oxygen saturation (4%), decreased PetCO2 (11%) and middle cerebral artery flow velocity (11%), and improved cerebral autoregulation compared with placebo (Tables 1–3). Effects of acetazolamide were similar between AMS-susceptible and AMS-resistant subjects (i.e., there were no drug-by-AMS status interactions). No physiological differences could be detected between placebo and dexamethasone trials in hypoxia.
Analysis of best vs. worst autoregulation quartiles.
Mean ARI scores for subjects with the best cerebral autoregulation after 9 h of hypoxia during the placebo trial (4.8 ± 0.8, n = 5) were higher than those with the worst autoregulation (1.5 ± 0.8, n = 5: P < 0.01). Mean LLQ scores for subjects with the best and worst ARI scores were not different during the placebo (2.4 ± 1.8 vs. 2.4 ± 2.1, P = 0.92), acetazolamide (1.6 ± 1.1 vs. 1.2 ± 1.1, P = 0.51), or dexamethasone (0.6 ± 0.5 vs. 1.4 ± 0.9, P = 0.12) trials. Intraindividual effects of the drugs on LLQ scores did not reach significance when data were grouped according to cerebral autoregulatory ability (P > 0.10).
DISCUSSION
Our results are the first to document the cerebral hemodynamic effects of acetazolamide and dexamethasone in healthy subjects exposed to prolonged periods of hypoxia. We found that both drugs effectively reduce symptoms of AMS, but their mechanism of action appeared unrelated to measures of dynamic cerebral autoregulation and vasomotor reactivity. These findings appear to support our previous assertion that AMS does not stem from global changes in the regulation of blood flow or oxygenation, but do not rule out the possibility that the drugs act via independent mechanisms that alleviate the symptoms of AMS (22).
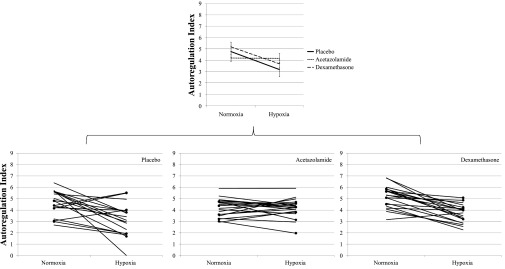
We specifically tested the hypothesis that acetazolamide and dexamethasone would improve cerebral autoregulation in hypoxia, thereby reducing the threat of vasogenic edema and AMS. The data demonstrated that acetazolamide reduced the cerebral autoregulation index in normoxia, but prevented further impairment in hypoxia (Fig. 1), resulting in better autoregulation scores compared with placebo after 9 h of hypoxia. However, since the effect was similar in AMS-susceptible and AMS-resistant subjects (Fig. 1) the degree of improvement could not explain the reduction in AMS symptoms with acetazolamide treatment. The lack of change in cerebral autoregulation across normoxia and hypoxia suggests that acetazolamide's effect on autoregulation is independent of changes in oxygen tension. Dexamethasone had no measureable effects on the impairment of cerebral autoregulation in hypoxia (Fig. 1), yet was highly effective at alleviating symptoms of AMS. These results argue against a common global cerebral hemodynamic pathway in AMS and provide further experimental evidence to support our previous claim that impairment of cerebral autoregulation may not be a key factor in the development of the illness (32).
Fig. 1.
Top: autoregulation index (ARI) responses from normoxic baseline to 9 h of hypoxia for all trials. On placebo, cerebral autoregulation was impaired after 9 h of hypobaric hypoxia. Acetazolamide reduced ARI scores in normoxia, but prevented further impairment in autoregulation after 9 h of hypoxia. Dexamethasone improved autoregulation in normoxia, but had no measureable effect in hypoxia. Bottom: circular markers indicate acute mountain sickness (AMS)-susceptible subjects (n = 6) identified during the placebo trial. Autoregulation was similar in AMS-susceptible and AMS-resistant subjects across all trials.
We tested an alternative hypothesis that acetazolamide would ameliorate AMS by stimulating vasodilation, thereby increasing cerebral blood flow and oxygenation in hypoxia (4). Our data provide evidence to the contrary. In hypoxia, acetazolamide reduced cerebral blood flow velocity in the middle cerebral artery and had no effect on cerebral oxygenation compared with placebo. These opposing findings may be related to dosage. While 250 mg is sufficient for AMS prophylaxis, higher doses (≥1,000 mg) may be needed to stimulate vasodilation and increase cerebral blood flow (12, 13, 15) and oxygenation (19, 33). The dosage may also have been insufficient to stimulate a measureable increase in overall pulmonary ventilation given the large variability in ventilatory responses between subjects, yet we consider the increase in PetO2 and decrease in PetCO2 evidence of mild hyperventilation. We believe this mild hyperventilation caused a CO2-mediated reduction of cerebral blood flow velocity in hypoxia that may have countered any small vasodilatory effects from the drug, but that cerebral oxygenation was preserved by the coinciding improvement in arterial oxygen saturation.
Such improvement in arterial oxygen saturation has long been suspected to provide the therapeutic effect of acetazolamide (4). While arterial saturation increased with acetazolamide, two observations are difficult to reconcile with the conventional therapeutic explanation. First, arterial oxygen saturation values in hypoxia were similar between AMS-susceptible and AMS-resistant subjects. Although this is contrary to some reports, the correlation between saturation and AMS is generally only moderate (r = −0.50 to −0.63) (2), indicating that other factors must influence the development of the illness (24). Second, while arterial oxygenation was improved with acetazolamide, cerebral oxygenation was not. As we stated above, improved arterial oxygenation may be offset by the reduction in cerebral blood flow accompanying hyperventilation. Nonetheless, if cerebral oxygenation is unaffected by acetazolamide, it is reasonable to question the conventional wisdom and explore alternative explanations.
We evaluated the effects of dexamethasone in the same subjects, believing that this glucocorticosteroid might reveal a cerebral hemodynamic link to AMS since dexamethasone is an effective prophylactic treatment (16, 17) with known cerebral hemodynamic effects (3, 21, 35). Dexamethasone did reduce cerebral flow in middle cerebral and basilar arteries and improved cerebral autoregulation in normoxia, yet these effects were likely results of reduced PetCO2, presumably from a mild increase in ventilation (26). No physiological effects of dexamethasone were detected in hypoxia. Importantly, symptoms of AMS were alleviated by dexamethasone without improvements in arterial oxygen saturation or cerebral oxygenation. These findings are similar to one previous MRI study showing no cerebrovascular effects of dexamethasone in hypoxia (22), but are in contrast to other reports showing that dexamethasone blunts sympathoadrenal responses to hypoxia (18, 23). Thus the physiological mechanism by which dexamethasone either prevents AMS or masks its symptoms (22) remains unclear.
Our results do not support the hypothesis that acetazolamide and dexamethasone share a common cerebral hemodynamic pathway in the prevention of AMS, yet it is important to stress that our measurements are reflective of global changes in brain blood flow. Since blood flow is markedly heterogeneous across the brain in hypoxia (7, 5), we cannot rule out the possibility that these drugs have specific regional effects that were not measurable with current technology. It is possible that both acetazolamide and dexamethasone may protect the integrity and permeability of the blood-brain barrier in local brain regions and therefore could prevent the development of cerebral edema in areas responsible for AMS. Alternatively, it has been suggested that neural effects from increased free radical production in hypoxia are the root cause of AMS symptoms and that mild cerebral edema is merely an ancillary effect (1). If so, acetazolamide and dexamethasone may share common antioxidant properties that have not yet been described.
We acknowledge that the stringent criteria used to define AMS resulted in a relatively low sample size of AMS-susceptible subjects. While this may be seen as a limitation, we believe it was necessary to isolate the most definitive cases of AMS to maximize our chances of finding significant drug effects. By using a repeated-measures design, in which all subjects were treated with placebo, acetazolamide, and dexamethasone, statistical power was sufficient to detect major hemodynamic effects of the drugs, yet a larger sample of AMS-susceptible subjects may still be needed to detect subtle differences from AMS-resistant subjects, particularly with respect to cerebral autoregulation measurements in which day-to-day variability might obscure small drug effects (6).
In summary, we report few cerebral hemodynamic effects of acetazolamide and dexamethasone and find no evidence to suggest that AMS stems from changes in the regulation of global cerebral blood flow and oxygenation.
GRANTS
Funding was provided by National Heart, Lung, and Blood Institute Grant HL-070362, The Maren Foundation, and the Altitude Research Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We express our gratitude to Alison Anderson, Jason Chapman, Ruth Johnson, Travis Pecha, and Nicholas Robbins for their efforts in study management and data collection. We also extend our appreciation to Vaughn Browne and Janet Uhde for their medical support throughout the study.
APPENDIX
Transfer function analysis (TFA), involving the relationship between ABP as input and CBFv as output has been the most widely used approach to quantify dynamic cerebral autoregulation (37). In general, the linear approximation has been justified by the relatively small changes in ABP and CBFv around a point of equilibrium. The cross-spectrum is defined as:
| (1) |
where P(f) and V(f) are the discrete Fourier transforms of ABP and CBFv beat-to-beat time series, obtained via the FFT algorithm. The expected value of the complex product, E[P(f)*.P(f)] is obtained by smoothing the spectra with a triangular moving average window and by averaging multiple segments of data. Similarly, the autospectra of ABP is computed as:
| (2) |
From the cross- and auto-spectra, the transfer function is calculated as:
| (3) |
From the real and imaginary parts of H(f), the amplitude and phase of the frequency response are calculated as:
| (4) |
| (5) |
The inverse Fourier transform of H(f) yields the CBFv impulse response as shown by Zhang et al. (37). Integration of the impulse response along time leads to the CBFv step response, which allows visualization of the speed of recovery of CBFv following a hypothetical step change in ABP. As proposed by Tiecks et al. (34), the temporal pattern of the CBFv step response can be classified with the ARI index, corresponding to 10 different template curves ranging from absence of autoregulation (ARI = 0) to best observed autoregulation (ARI = 9). The template curve with the best approximation to the CBFv step response derived by transfer function analysis is selected by least square fitting for the first 6 s of the response.
REFERENCES
- 1. Bailey DM, Bärtsch P, Knauth M, Baumgartner RW. Emerging concepts in acute mountain sickness and high-altitude cerebral edema: from the molecular to the morphological. Cell Mol Life Sci 66: 3583–3594, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bärtsch P, Swenson ER, Paul A, Jülg B, Hohenhaus E. Hypoxic ventilatory response, ventilation, gas exchange, and fluid balance in acute mountain sickness. High Alt Med Biol 3: 361–376, 2002. [DOI] [PubMed] [Google Scholar]
- 3. Behrens PF, Ostertag CB, Warnke PC. Regional cerebral blood flow in peritumoral brain edema during dexamethasone treatment: a xenon-enhanced computed tomographic study. Neurosurgery 43: 235–241, 1998. [DOI] [PubMed] [Google Scholar]
- 4. Bickler PE, Litt L, Severinghaus JW. Effects of acetazolamide on cerebrocortical NADH and blood volume. J Appl Physiol 65: 428–433, 1988. [DOI] [PubMed] [Google Scholar]
- 5. Binks AP, Cunningham VJ, Adams L, Banzett RB. Gray matter blood flow change is unevenly distributed during moderate isocapnic hypoxia in humans. J Appl Physiol 104: 212–217, 2008. [DOI] [PubMed] [Google Scholar]
- 6. Brodie FG, Atkins ER, Robinson TG, Panerai RB. Reliability of dynamic cerebral autoregulation measurement using spontaneous fluctuations in blood pressure. Clin Sci 116: 513–520, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Buck A, Schirlo C, Jasinksy V, Weber B, Burger C, von Schulthess GK, Koller EA, Pavlicek V. Changes of cerebral blood flow during short-term exposure to normobaric hypoxia. J Cereb Blood Flow Metab 18: 906–910, 1998. [DOI] [PubMed] [Google Scholar]
- 8. Claassen JAHR, Zhang R, Fu Q, Witkowski S, Levine BD. Transcranial Doppler estimation of cerebral blood flow and cerebrovascular conductance during modified rebreathing. J Appl Physiol 102: 870–877, 2007. [DOI] [PubMed] [Google Scholar]
- 9. Duffin J. Measuring the ventilatory response to hypoxia. J Physiol 584: 285–293, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ehrenreich DL, Burns RA, Alman RW, Fazekas JF. Influence of acetazolamide on cerebral blood flow. Arch Neurol 5: 227–232, 1961. [DOI] [PubMed] [Google Scholar]
- 11. Fischer S, Renz D, Schaper W, Karliczek GF. In vitro effects of dexamethasone on hypoxia-induced hyperpermeability and expression of vascular endothelial growth factor. Eur J Pharmacol 411: 231–243, 2001. [DOI] [PubMed] [Google Scholar]
- 12. Friberg L, Kastrup J, Rizzi D, Jensen JB, Lassen NA. Cerebral blood flow and end-tidal Pco2 during prolonged acetazolamide treatment in humans. Am J Physiol Heart Circ Physiol 258: H954–H959, 1990. [DOI] [PubMed] [Google Scholar]
- 13. Grossmann WM, Koeberle B. The dose-response relationship of acetazolamide on the cerebral blood flow in normal subjects. Cerebrovasc Dis 10: 65–69, 2000. [DOI] [PubMed] [Google Scholar]
- 14. Hackett PH, Roach RC. High-altitude illness. N Engl J Med 345: 107–114, 2001. [DOI] [PubMed] [Google Scholar]
- 15. Huang SY, McCullough RE, McCullough RG, Micco AJ, Manco-Johnson M, Weil JV, Reeves JT. Usual clinical dose of acetazolamide does not alter cerebral blood flow velocity. Respir Physiol 72: 315–326, 1988. [DOI] [PubMed] [Google Scholar]
- 16. Imray C, Wright A, Subudhi A, Roach R. Acute mountain sickness: pathophysiology, prevention, and treatment. Prog Cardiovasc Dis 52: 467–484, 2010. [DOI] [PubMed] [Google Scholar]
- 17. Johnson TS, Rock PB, Fulco CS, Trad LA, Spark RF, Maher JT. Prevention of acute mountain sickness by dexamethasone. N Engl J Med 310: 683–686, 1984. [DOI] [PubMed] [Google Scholar]
- 18. Johnson TS, Rock PB, Young JB, Fulco CS, Trad LA. Hemodynamic and sympathoadrenal responses to altitude in humans: effect of dexamethasone. Aviat Space Environ Med 59: 208–212, 1988. [PubMed] [Google Scholar]
- 19. Kaminogo M, Ichikura A, Shibata S, Toba T, Yonekura M. Effect of acetazolamide on regional cerebral oxygen saturation and regional cerebral blood flow. Stroke 26: 2358–2360, 1995. [DOI] [PubMed] [Google Scholar]
- 20. Leaf DE, Goldfarb DS. Mechanisms of action of acetazolamide in the prophylaxis and treatment of acute mountain sickness. J Appl Physiol 102: 1313–1322, 2007. [DOI] [PubMed] [Google Scholar]
- 21. Leenders KL, Beaney RP, Brooks DJ, Lammertsma AA, Heather JD, McKenzie CG. Dexamethasone treatment of brain tumor patients: effects on regional cerebral blood flow, blood volume, and oxygen utilization. Neurology 35: 1610–1616, 1985. [DOI] [PubMed] [Google Scholar]
- 22. Levine BD, Yoshimura K, Kobayashi T, Fukushima M, Shibamoto T, Ueda G. Dexamethasone in the treatment of acute mountain sickness. N Engl J Med 321: 1707–1713, 1989. [DOI] [PubMed] [Google Scholar]
- 23. Maggiorini M, Brunner-La Rocca H, Peth S, Fischler M, Böhm T, Bernheim A, Kiencke S, Bloch KE, Dehnert C, Naeije R, Lehmann T, Bärtsch P, Mairbäurl H. Both tadalafil and dexamethasone may reduce the incidence of high-altitude pulmonary edema: a randomized trial. Ann Intern Med 145: 497–506, 2006. [DOI] [PubMed] [Google Scholar]
- 24. Moore LG, Harrison GL, McCullough RE, McCullough RG, Micco AJ, Tucker A, Weil JV, Reeves JT. Low acute hypoxic ventilatory response and hypoxic depression in acute altitude sickness. J Appl Physiol 60: 1407–1412, 1986. [DOI] [PubMed] [Google Scholar]
- 25. Pandit JJ, Mohan RM, Paterson ND, Poulin MJ. Cerebral blood flow sensitivities to CO2 measured with steady-state and modified rebreathing methods. Respir Physiol Neurobiol 159: 34–44, 2007. [DOI] [PubMed] [Google Scholar]
- 26. Panerai RB, Deverson ST, Mahony P, Hayes P, Evans DH. Effects of CO2 on dynamic cerebral autoregulation measurement. Physiol Meas 20: 265–275, 1999. [DOI] [PubMed] [Google Scholar]
- 27. Panerai RB, Sammons EL, Smith SM, Rathbone WE, Bentley S, Potter JF, Evans DH, Samani NJ. Cerebral critical closing pressure estimation from Finapres and arterial blood pressure measurements in the aorta. Physiol Meas 27: 1387–1402, 2006. [DOI] [PubMed] [Google Scholar]
- 28. Roach RC, Bartsch P, Hackett P, Oelz O. The Lake Louise acute mountain sickness scoring system. In: Hypoxia and Molecular Medicine. Burlington, VT: Queen City Printers, 1993, p. 272–274. [Google Scholar]
- 29. Roach RC, Hackett PH. Frontiers of hypoxia research: acute mountain sickness. J Exp Biol 204: 3161–3170, 2001. [DOI] [PubMed] [Google Scholar]
- 30. Roach RC, Maes D, Sandoval D, Robergs RA, Icenogle M, Hinghofer-Szalkay H, Lium D, Loeppky JA. Exercise exacerbates acute mountain sickness at simulated high altitude. J Appl Physiol 88: 581–585, 2000. [DOI] [PubMed] [Google Scholar]
- 31. Subudhi AW, Panerai RB, Roach RC. Acute hypoxia impairs dynamic cerebral autoregulation: results from two independent techniques. J Appl Physiol 107: 1165–1171, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Subudhi AW, Panerai RB, Roach RC. Effects of hypobaric hypoxia on cerebral autoregulation. Stroke 41: 641–646, 2010. [DOI] [PubMed] [Google Scholar]
- 33. Tachtsidis I, Tisdall M, Delpy DT, Smith M, Elwell CE. Measurement of cerebral tissue oxygenation in young healthy volunteers during acetazolamide provocation: a transcranial Doppler and near-infrared spectroscopy investigation. Adv Exp Med Biol 614: 389–396, 2008. [DOI] [PubMed] [Google Scholar]
- 34. Tiecks FP, Lam AM, Aaslid R, Newell DW. Comparison of static and dynamic cerebral autoregulation measurements. Stroke 26: 1014–1019, 1995. [DOI] [PubMed] [Google Scholar]
- 35. Van Roost D, Hartmann A, Quade G. Changes of cerebral blood flow following dexamethasone treatment in brain tumour patients. A Xe/CT study. Acta Neurochir (Wien) 143: 37–44, 2001. [DOI] [PubMed] [Google Scholar]
- 36. Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol 74: 2566–2573, 1993. [DOI] [PubMed] [Google Scholar]
- 37. Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol Heart Circ Physiol 274: H233–H241, 1998. [DOI] [PubMed] [Google Scholar]