Abstract
The extent to which heat stress compromises blood pressure control is variable among individuals, with some individuals becoming very intolerant to a hypotensive challenge, such as lower body negative pressure (LBNP) while heat stressed, while others are relatively tolerant. Heat stress itself reduces indexes of ventricular filling pressure, including central venous pressure, which may be reflective of reductions in tolerance in this thermal condition. This study tested the hypothesis that the magnitude of the reduction in central venous pressure in response to heat stress alone is related to the subsequent decrement in LBNP tolerance. In 19 subjects, central hypovolemia was imposed via LBNP to presyncope in both normothermic and heat-stress conditions. Tolerance to LBNP was quantified using a cumulative stress index (CSI), and the difference between normothermic CSI and heat-stress CSI was calculated for each individual. The eight individuals with the greatest CSI difference between normothermic and heat-stress tolerances (LargeDif), and the eight individuals with the smallest CSI difference (SmallDif), were grouped together. By design, the difference in CSI between thermal conditions was greater in the LargeDif group (969 vs. 382 mmHg × min; P < 0.001). Despite this profound difference in the effect of heat stress in decreasing LBNP tolerance between groups, coupled with no difference in the rise in core body temperatures to the heat stress (LargeDif, 1.4 ± 0.1°C vs. SmallDif, 1.4 ± 0.1°C; interaction P = 0.89), the reduction in central venous pressure during heat stress alone was similar between groups (LargeDif: 5.7 ± 1.9 mmHg vs. SmallDif: 5.2 ± 2.0 mmHg; interaction P = 0.85). Contrary to the proposed hypothesis, differences in blood pressure control during LBNP are not related to differences in the magnitude of the heat-stress-induced reductions in central venous pressure.
Keywords: hyperthermia, ventricular filling, blood pressure, hemorrhage
in humans, elevation in body (i.e., weighted average of core and skin temperature) temperature greatly compromises blood pressure control to an orthostatic challenge (25, 47), gravitational acceleration (1), and simulated hemorrhage via lower body negative pressure (LBNP) (10, 20, 46). The mechanisms resulting in this response are unclear, but may be related to marked cutaneous vasodilation (19), coupled with reduced sympathetically mediated cutaneous vasoconstrictor responsiveness to a hypotensive challenge (21, 45).
Heat stress causes a profound redistribution of cardiac output from the central to the cutaneous circulation (8). This response, in combination with increased cardiac output (38–40), leads to reductions in central blood volume (8) that are manifested by reductions in central venous and pulmonary capillary wedge pressures (7, 20, 31, 39, 48).
While blood pressure control to the aforementioned challenges is compromised in all individuals with elevated body temperatures (20, 25, 46), there is a large degree of intersubject variability in this response. For example, our laboratory recently reported that some subjects were not able to tolerate 20 mmHg of LNBP without experiencing syncopal symptoms following an increase in internal temperature of ∼1.5°C, whereas other subjects similarly heat stressed did not experience syncopal symptoms until LBNP of 60 mmHg (see Fig. 3B in Ref. 20). Acute volume infusion during heat stress, sufficient to restore central venous pressure (CVP), and presumably central blood volume and left ventricular filling pressures, to normothermic levels, completely restored LBNP tolerance relative to normothermic levels (20). These findings suggest that heat-stress-induced reductions in central venous and presumably central blood volume may be a key mechanism for the reduction in blood pressure control during a challenge such as LBNP or upright tilt while in this thermal condition. In this regard, in the aforementioned study (20), the individual with the highest LBNP tolerance while heat stressed (60 mmHg) had the smallest reduction in CVP (2 mmHg), and presumably the smallest reduction in central blood volume, in response to the heat stress.
The mechanisms that contribute to the interindividual variability of the reduction in blood pressure control while heat stressed remain elusive. Given that heat stress reduces central blood volume and ventricular filling pressures (7, 8, 20, 31, 39, 48) and restoration of CVP ameliorates heat-stress-induced orthostatic intolerance (20), the magnitude of the reduction in CVP in response to heat stress before the hypotensive challenge may be an important predictor of the magnitude of the ensuing compromised blood pressure control to that challenge. Therefore, the aim of this study was to test the hypothesis that the decrement in blood pressure control leading to compromised LBNP tolerance during heat stress is related to the magnitude of the reduction in CVP by the heat stress alone.
METHODS
Ethical Approval
The protocol and consent were approved by the Institutional Review Boards at the University of Texas Southwestern Medical Center at Dallas and Texas Health Presbyterian Hospital Dallas. Subjects were informed of the purpose and risks of the study before providing their informed, written consent.
Nineteen healthy, normotensive subjects (14 men and 5 women) participated in the study. Subjects were not taking medications and were free of any known cardiovascular, metabolic, or neurological diseases. The phase of the menstrual cycle was not controlled. Subjects refrained from alcohol, caffeine, and exercise for 24 h before the study. Some data from seven subjects have been previously published (20); however, the hypothesis investigated and data presented in this paper are unique relative to the prior publication.
The procedures were completed in a temperature-controlled laboratory (∼26°C) during two visits that were separated by a minimum of 1 day. One visit was used for normothermic data collection, while the other was used for data collection during a heat stress. These trials were performed at the same time of day, with the order counterbalanced and randomized.
Instrumentation and Measurements
Mean skin temperature was measured from the weighted average of six thermocouples attached to the skin (42). Each subject wore a water perfused tube-lined suit (Med-Eng, Ottawa, Canada) and was placed into a LBNP chamber, sealed at the iliac crest, while in the supine position. The suit covered the entire body, except for the head, face, hands, one arm, and feet. This suit permitted the control of skin and internal temperatures by adjusting the temperature of the water perfusing the suit. The temperature and flow rate of the water were controlled by a heating circulating bath (Lauda, model E106T) and an external centrifugal pump (Gorman-Rupp Industries, model 25501-006), respectively. Heart rate was continuously obtained from an electrocardiogram (HP Patient Monitor, Agilent, Santa Clara, CA) interfaced with a cardiotachometer (CWE, Ardmore, PA). Continuous beat-by-beat arterial blood pressure was recorded from a finger using the Penaz method (Finometer, Finapres Medical Systems, Amsterdam, the Netherlands). Intermittent blood pressure measurements were obtained by auscultation of the brachial artery via electrosphygmomanometry (SunTech, Raleigh, NC), with mean arterial blood pressure (MAP) being calculated as one-third pulse pressure plus diastolic blood pressure. Brachial artery blood pressures were used for data analysis, while continuous blood pressure measures from the Finometer were used for the detection of ensuing syncope during the experimental protocols (see below). Skin blood flow was indexed from the exposed forearm via laser-Doppler flowmetry using an integrating probe (MoorLab Laser Doppler Perfusion Monitor, Moor Instruments, Wilmington, DE). Cutaneous vascular conductance was calculated from the ratio of laser Doppler flux to MAP.
Experimental Protocol
Normothermic trial.
Following instrumentation, subjects rested on a patient table in the supine position for ∼80 min, while 34°C water circulated through the suit. An 80-min resting period was chosen to normalize the time that the subjects were in the supine position between study days (i.e., normothermia and heat stress) before measurements were obtained. After this resting period, baseline measures of hemodynamic data were obtained. Subjects were then exposed to central hypovolemia via graded LBNP to determine tolerance. The tolerance test began at 10-mmHg LBNP for 3 min, at which point the LBNP was decreased by 10 mmHg every 3 min (i.e., 20 mmHg, 30 mmHg, etc.) until the onset of syncopal symptoms. In a subset of subjects (N = 7), the starting LBNP was 20 mmHg. Despite these individuals beginning the test at a different LBNP, responses between these groups of subjects were indistinguishable. The criteria for LBNP test termination were as follows: subject expresses continued feelings of discomfort or dizziness, pallor, diaphoresis, rapid and progressive decrease in mean blood pressure associated with sustained systolic blood pressures <80 mmHg, and/or relative bradycardia accompanied with narrowing of pulse pressure (20). If 100-mmHg LBNP was achieved, this LBNP was kept constant until the onset of syncopal symptoms. The primary symptoms resulting in test termination were a rapid decrease in mean and systolic blood pressures, accompanied by bradycardia.
Heat stress trial.
Instrumentation was similar to the normothermic day. In addition, all subjects swallowed a telemetry pill, immediately on arrival at the laboratory (∼2–3 h before the start of the heat-stress data collection), for the measurement of intestinal temperature throughout the protocol (HQ, Palmetto, FL). Subjects were also instrumented with a peripherally inserted central venous catheter advanced into the superior vena cava via the basilic vein. Positioning of the central venous catheter was confirmed by the following: 1) the distance that the catheter was advanced; 2) observation of adequate pressure waveforms; and 3) an appropriate rapid rise and fall in pressure during a Valsalva and Mueller maneuver, respectively. The central venous catheter was connected to a pressure transducer and was zeroed at the position of the midaxillary line. This catheter was used for continuous measurement of CVP, as well as venous blood sampling, which allowed for subsequent determination of circulating catecholamines at predetermined time points (see below). Following instrumentation, subjects rested in the supine position for a minimum of 30 min, while 34°C water circulated through the suit, after which baseline normothermic thermal and hemodynamic data were obtained. A venous blood sample was also obtained for assessment of baseline plasma catecholamine concentrations. Subjects were then exposed to a heat stress by perfusing 49°C water through the suit until an increase in internal temperature of ∼1.4°C was obtained, at which time the temperature of the water circulating the suit was slightly decreased to attenuate the rate of rise in internal temperature during data collection. Baseline heat stress thermal and hemodynamic data were obtained, followed by another venous blood sample. Subjects were then exposed to the aforementioned graded LBNP challenge to determine tolerance with the same criteria used to terminate the test as was used during the normothermic day.
Measurement of plasma catecholamines.
Venous blood samples were obtained during the final minute of normothermic data collection, as well as during heat stress immediately before beginning LBNP. These samples were analyzed for plasma norepinephrine and epinephrine concentrations by an independent external laboratory (ARUP Laboratories, Salt Lake City, UT) using high-performance liquid chromatography, according to standardized procedures (33).
Quantification of orthostatic tolerance.
Tolerance to the LBNP challenge was quantified using a cumulative stress index (CSI) (20, 23, 24). This index was calculated by summing the product of the LBNP and the time at each level of LBNP across the entire trial (i.e., 10 mmHg × 3 min + 20 mmHg × 3 min + 30 mmHg × 3 min, etc.) until the test was terminated.
Data Analysis
Thermal and hemodynamic data were recorded via a data-acquisition system (Biopac System, Santa Barbara, CA). Steady-state data from the last 45 s of the 5-min normothermic baseline period, as well as 45 s of data immediately before the onset of LBNP during heat stress, were averaged.
All subjects were evaluated based on the difference in LBNP tolerance between normothermic and heat stress conditions, ranging from relatively small decrements in LBNP tolerance to heat stress to individuals with very large reductions in tolerance (both relative to normothermic tolerance testing). Data from the eight individuals with the largest difference in LBNP tolerance between normothermic and heat stress conditions (i.e., LargeDif) were grouped together, while data from the eight individuals with the smallest difference in LBNP tolerance between thermal conditions (i.e., SmallDif) were grouped together. In an effort to analyze the two extremes of the response spectrum, the “middle” group was not included in the analysis. The difference in the reduction of CSI between normothermic and heat-stress conditions, as well as the reduction in CVP during heat stress alone between the two groups, were compared using unpaired Student t-tests. A Pearson's correlation analysis was performed on all individuals (including the middle group) to further characterize the relationship between the difference in CSI between thermal conditions and the reduction in CVP to the heat stress. For the heat-stress day only, the effect of thermal condition (normothermia vs. heat stress) and subject group (LargeDif vs. SmallDif) on thermal and hemodynamic data was evaluated using a mixed-model two-way ANOVA. Last, hemodynamic data were averaged during the 10 s before LBNP test termination (i.e., presyncope). The change in hemodynamic responses (e.g., the magnitude of the reduction in measured variable) between control heat stress (i.e., pre-LBNP) relative to presyncope between the two groups was compared using unpaired Student t-tests. Statistical analyses were performed using a commercially available statistical software package (SigmaStat 3.11, Chicago, IL). The α-level for all analyses was set at 0.05. Results are reported as means ± SD.
RESULTS
Do Individuals With the Largest Reduction in LBNP Tolerance Between Normothermic and Heat-Stressed Conditions Have the Greatest Reduction in CVP During Heat Stress?
Average subject characteristics are as follows (mean ± SD): for the SmallDif group (6 men, 2 women), age, 38 ± 12 yr; height, 179 ± 6 cm; and weight, 78 ± 12 kg; and for the LargeDif group (5 men, 3 women), age, 39 ± 9 yr; height, 173 ± 11 cm; and weight, 75 ± 11 (P > 0.05 between groups for all variables).
Core temperature, MAP, and plasma epinephrine were higher, regardless of thermal condition in the SmallDif group relative to the LargeDif group (Table 1: main effect of subject group; P < 0.05 for all variables), whereas skin temperature, heart rate, cutaneous blood flow, cutaneous vascular conductance, and plasma norepinephrine were similar between groups (Table 1: main effect of subject group; P > 0.05 for all variables). In both groups, heat stress before LBNP had no effect on MAP or plasma epinephrine concentration (Table 1: main effect of thermal condition; P > 0.05 for both variables), while all other variables increased (Table 1: main effect of thermal condition; P < 0.05 for all variables). Regardless of the aforementioned variables, there were no interactions between subject group and thermal condition (Table 1, P > 0.05 for all variables).
Table 1.
Baseline (i.e., pre-LBNP) thermal and hemodynamic values during normothermia and heat stress for the individuals with the smallest difference and those with the largest difference in LBNP tolerance between normothermic and heat-stress conditions
| Normothermia |
Heat Stress |
Interaction (P Value) | |||
|---|---|---|---|---|---|
| SmallDif | LargeDif | SmallDif | LargeDif | ||
| CVP, mmHg | 6.7 ± 2.6 | 7.2 ± 2.5 | 1.0 ± 2.6* | 2.1 ± 2.4* | 0.77 |
| Core temperature, °C | 37.0 ± 0.3† | 36.8 ± 0.2 | 38.4 ± 0.2*† | 38.2 ± 0.2* | 0.89 |
| Skin temperature, °C | 34.3 ± 0.3 | 34.1 ± 0.2 | 38.6 ± 0.7* | 38.3 ± 0.5* | 0.82 |
| Heart rate, beats/min | 60 ± 7 | 59 ± 7 | 104 ± 14* | 98 ± 17* | 0.56 |
| MAP, mmHg | 89 ± 8† | 82 ± 10 | 85 ± 8† | 79 ± 7 | 0.97 |
| CBF, AU | 14 ± 7.0 | 14 ± 5 | 131 ± 45* | 106 ± 41* | 0.28 |
| CVC, AU/mmHg | 0.16 ± 0.08 | 0.16 ± 0.06 | 1.58 ± 0.63* | 1.33 ± 0.44* | 0.37 |
| Norepinephrine, pg/ml | 201 ± 79 | 196 ± 89 | 366 ± 114* | 382 ± 88* | 0.78 |
| Epinephrine, pg/ml | 30 ± 17† | 20 ± 6 | 46 ± 26† | 22 ± 11 | 0.34 |
Values are means ± SD. SmallDif, individuals with the smallest difference in lower body negative pressure (LBNP) tolerance between normothermic and heat-stress conditions; LargeDif, individuals with the largest difference in LBNP tolerance between normothermic and heat-stress conditions; CVP, central venous pressure; MAP, mean arterial pressure; CBF, cutaneous blood flow; AU, arbitrary units; CVC, cutaneous vascular conductance.
Main effect of subject group (P < 0.05).
Main effect of thermal condition (P < 0.001).
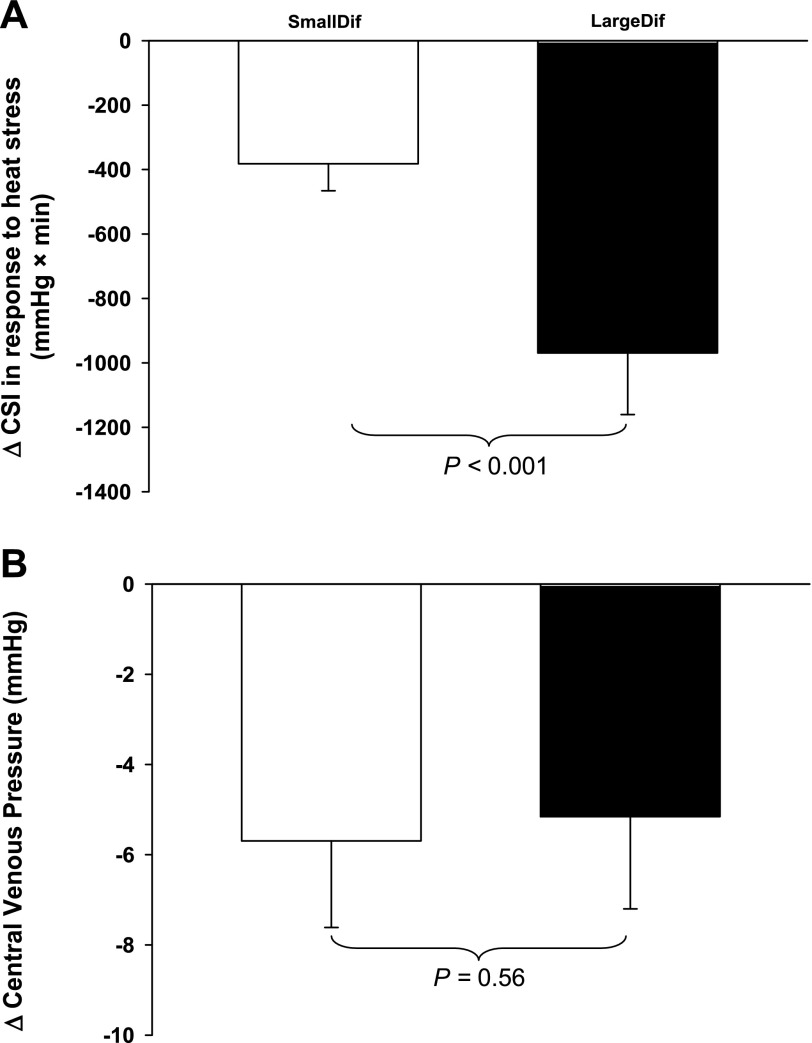
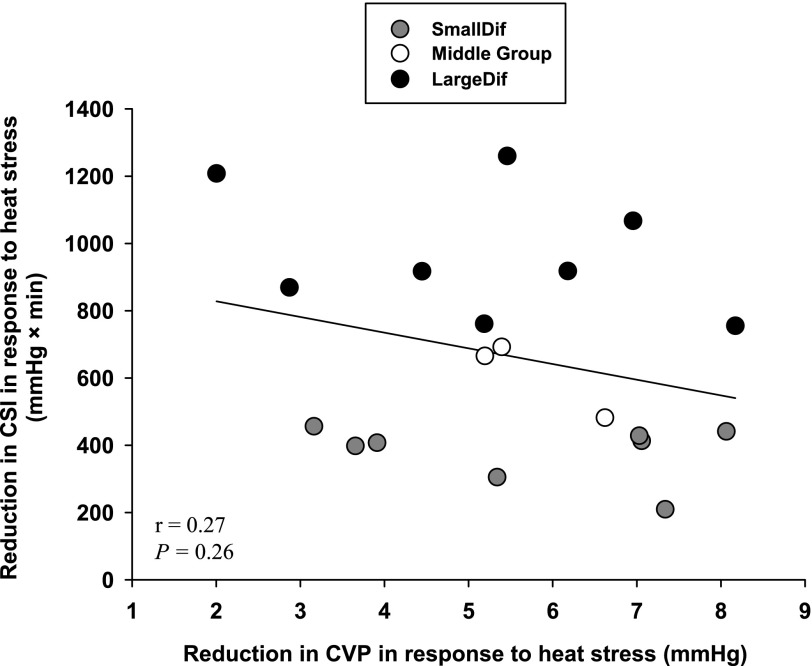
All subjects exhibited reduced LBNP tolerance while heat stressed. By design, the heat-stress-induced reduction in CSI of the SmallDif group (382 ± 83 mmHg × min) was smaller than the reduction in CSI of the LargeDif group (969 ± 191 mmHg × min; Fig. 1A, P < 0.001). CVP was similar between groups during normothermia (Table 1: main effect of subject group; P = 0.40). Heat stress alone (i.e., before LBNP) decreased CVP in both groups (Table 1: main effect of thermal condition; P < 0.001). However, both the magnitude of the reduction in CVP to heat stress alone (Table 1: interaction of subject group × thermal condition; P = 0.77) and the absolute CVP while heat stressed (P = 0.41) were not different between groups. When analyzed using a Student t-test, the reduction in CVP (i.e., ΔCVP) to heat stress was similar between groups (Fig. 1B: P = 0.56). Likewise, with the inclusion of the middle group, the correlation analysis did not reveal a significant correlation between the magnitude of the reduction in CSI to LBNP between thermal conditions and the reduction in CVP to heat stress alone (Fig. 2: r = 0.27, P = 0.26).
Fig. 1.
Effects of heat stress on the difference in lower body negative pressure (LBNP) tolerance between those with the smallest (SmallDif) and largest reduction in LBNP tolerance between normothermic and heat-stressed conditions (LargeDif; A), and the reduction (Δ) in central venous pressure (CVP) for the respective groups in response to the heat stress alone (B). Despite large differences in the reduction in LBNP tolerance between groups, indexed by the cumulative stress index (CSI; A), the reduction in CVP during heat stress alone was similar (P = 0.56; B). These findings strongly suggest that the reduction in CVP that accompanies heat stress is unlikely to be responsible for intersubject variability in heat-stress-induced reductions in LBNP tolerance. Values are means ± SD.
Fig. 2.
Relationship between the reduction in CVP to heat stress alone and the reduction in LBNP tolerance (defined as a CSI) from normothermic to heat-stressed conditions. Shaded circles represent responses for the SmallDif group, open circles represent the responses from the middle group, and the solid circles represent responses for the LargeDif group. There was no relationship between heat-stress-induced reductions in CVP and the reduction in LBNP tolerance between normothermic and heat-stress conditions (r = 0.27, P = 0.26).
There was no difference in the change of any hemodynamic variable from heat stress alone (i.e., pre-LBNP) to presyncope between groups (Table 2, P > 0.05 for all variables).
Table 2.
Change in hemodynamic values at LBNP termination (i.e., presyncope) relative to values during heat stress before LBNP for the individuals with the smallest difference and those with the largest difference in LBNP tolerance between normothermic and heat-stress conditions
| SmallDif | LargeDif | P Value | |
|---|---|---|---|
| CVP, mmHg | −3.0 ± 1.8 | −2.8 ± 1.9 | 0.88 |
| Heart rate, beats/min | 10 ± 30 | −8 ± 18 | 0.13 |
| MAP, mmHg | −20 ± 10 | −19 ± 8 | 0.86 |
| CBF, AU | −56 ± 24 | −56 ± 36 | 0.96 |
| CVC, AU/mmHg | −0.35 ± 0.20 | −0.17 ± 0.44 | 0.30 |
Values are means ± SD.
DISCUSSION
The aim of this study was to determine whether the large interindividual variability to tolerate a hypotensive challenge while heat stressed is related to the magnitude of the reduction in CVP in response to that heat stress before the hypotensive challenge. We tested the hypothesis that the individuals with the largest difference in LBNP tolerance between normothermic and heat-stress conditions (i.e., the largest reduction in LBNP tolerance to the heat stress) would be those with the largest decrease in CVP to heat stress alone. The primary findings are that the reduction in CVP during heat stress before LBNP was not different between high-tolerance and low-tolerance groups. Thus, contrary to the proposed hypotheses, these findings indicate that differences in the capacity to withstand a hypotensive challenge while heat stressed are not related to the heat-stress-induced reduction in CVP.
A positive correlation between plasma volume and orthostatic tolerance in normothermic individuals has been reported (27). Furthermore, restoration of plasma volume during dehydration (11, 16), space flight (4), and prolonged bed rest (17) is linked to improved normothermic orthostatic tolerance in these conditions. It is well established that heat stress increases the incidence of hypotension with pronounced reductions in cerebral perfusion (3, 26, 46, 47) and ultimately decreased tolerance during upright tilt (25, 47), angular acceleration (1), and simulated hemorrhage induced by LBNP (10, 20, 46). Heat stress itself, independent of orthostatic challenges, induces similar hemodynamic changes, including reductions in central blood volume (6, 8) and accompanied reductions in ventricular and central venous filling pressures (7, 31, 36, 48), which likely contribute to the impaired blood pressure regulation during the aforementioned challenges in this thermal condition.
A variety of countermeasures have been utilized to reverse heat-stress-induced decreases in orthostatic tolerance. Wilson et al. (47) demonstrated that acute skin surface cooling immediately before upright tilting in heat-stressed individuals improved orthostatic tolerance relative to heat stress without cooling. This effect is likely related to profound cutaneous vasoconstriction and associated redistribution of blood flow from the cutaneous circulation to the central circulation, resulting in increased CVP (9, 48), pulmonary capillary wedge pressure (48), and central blood volume (6) during the cold stimulus. Additionally, our laboratory recently reported that acute volume loading in heat-stressed individuals sufficient to restore CVP and presumably central blood volume to normothermic values completely reversed heat-stress-induced reductions in LBNP tolerance (20). While the physiological adjustments to skin surface cooling and acute volume loading during heat stress differ, they each restore CVP, and presumably central blood volume, toward the normothermic values, suggesting that these responses may be a primary mechanism for the observed improvements in tolerance. Furthermore, in the study by Keller et al. (20), the individual with the smallest reduction in CVP to the heat stress alone (∼2 mmHg) was the one with the highest LBNP tolerance to that heat stress (LBNP tolerance of 60 mmHg while heat stressed). Combined, these data raised the speculation that the magnitude of the decrease in CVP may contribute to heat-stress-induced reductions in blood pressure control, leading to orthostatic intolerance in this thermal condition. Contrary to this hypothesis, the present findings indicate that differences in the magnitude of the reduction in CVP do not explain interindividual variability in LBNP tolerance. These findings are consistent with a report in normothermic individuals, indicating that CVP was similar during supine rest and during various stages of LBNP in individuals with “higher” and “lower” orthostatic tolerance (5, 13).
A number of studies performed in normothermic individuals have attempted to identify differences in orthostatically “tolerant” and “nontolerant” individuals. Several factors have been implicated in reduced orthostatic tolerance, including reduced plasma renin activity and activation of the renin-angiotensin-aldosterone system (13, 18, 37, 41), higher supine parasympathetic responsiveness (14), reduced sympathetic activation upon assumption of the upright posture (14), and lower cardiac compliance (13, 27). The present study did not assess the aforementioned variables; however, an index of sympathetic activity (i.e., plasma catecholamine concentration) was similar between groups before the onset of the heat-stress LBNP challenge (Table 1). The exception was plasma epinephrine concentrations that were higher, regardless of the thermal condition, in the SmallDif group (Table 1). It is not clear why normothermic and heat-stress epinephrine concentrations were higher in this group, although it is doubtful that this response contributed to the relative preservation of LBNP tolerance while heat stressed in the SmallDif group, given that the change in epinephrine concentration in response to the heat stress alone was similar between groups (Table 1). These data suggest that the degree of sympathetic activation to heat stress alone was unlikely a contributing factor to the observed differences in orthostatic tolerance between groups.
Other factors that may influence LBNP tolerance during heat stress include pre-LBNP core temperature, cutaneous blood flow, cutaneous vascular conductance, and MAP. The increase in core temperature was similar between groups; however, the SmallDif group had a higher absolute core temperature (Table 1). If anything, however, a higher absolute core temperature would be expected to decrease LBNP tolerance, which is counter to that observed in the SmallDif group. Additionally, while the change in MAP during heat stress was similar between groups, the SmallDif group had a higher absolute MAP (Table 1). Such a response may be beneficial in improving blood pressure control during LBNP. Last, the increase in cutaneous blood flow and cutaneous vascular conductance to the heat stress, as well as the absolute values before LBNP, were similar between groups. Therefore, it is unlikely that the aforementioned variables contributed to the observed differences in orthostatic tolerance.
Heat stress shifts the operating point of a Frank-Starling curve to a steeper location on such a curve, leading to a greater decrease in stroke volume per decrease in pulmonary capillary wedge pressure (43, 44). We surmised that such a response may contribute to heat-stress-induced reductions in orthostatic tolerance. There was some degree of intersubject variability in the shift of the operating point in that study, and thus, while speculative, it is possible that, in the present study, the “less” tolerant individuals shifted their operating point on a Frank-Starling curve to a steeper location, despite similar absolute values and reductions in ventricular filling pressures (indexed from CVP) during heat stress. Likewise, despite reduced central blood volume (8) and ventricular filling pressures to heat stress alone (7, 20, 31, 39, 48), stroke volume is preserved during heat stress, in part, through increased left ventricular systolic function, while left ventricular diastolic function is maintained (2, 32). It is possible that the “less” tolerant individuals did not appropriately increase or had an attenuated increase in left ventricular systolic function and/or had impaired diastolic function during heat stress alone and perhaps during heat stress plus LBNP. Last, changes in CVP (22, 28) and pulmonary capillary wedge pressure (22) are not always reflective of changes in central blood volume. More specifically, while there is a positive directional relationship between CVP and central blood volume during an orthostatic challenge, this relationship can be nonlinear, given that the reduction in CVP begins to plateau at a time when central blood volume continues to decrease (5, 29, 34, 35). Thus it is possible that some individuals had a greater decrease in central blood volume, despite similar reductions in CVP while heat stressed, ultimately leading to reduced orthostatic tolerance.
Methodological Considerations
Comparison of hemodynamic responses at a fixed LBNP, but before LBNP tolerance, would provide valuable information regarding the effect of simulated hemorrhage on the measured cardiovascular variables. However, given the variability in LBNP tolerance within the subjects, this approach is not possible. Specifically, LBNP of 20 mmHg presents a much different cardiovascular challenge to an individual who becomes presyncopal at 30 mmHg LBNP, relative to one who becomes presyncopal at 70 mmHg. That said, the change in hemodynamic responses between heat stress before LBNP relative to that at presyncope was not different between groups (Table 2). This is not surprising, however, as it is expected that hemodynamic responses will be similar at each individual's respective “presyncopal level” of LBNP, regardless of the absolute LBNP at presyncope.
Women have reduced orthostatic tolerance relative to men in both normothermic and heat-stress conditions (15, 30), and the menstrual cycle affects sympathetic neural responses to orthostatic stress (12). However, orthostatic tolerance in heat-stressed women was equally impaired, regardless of the phase of the menstrual cycle (30). This latter finding, coupled with a relatively equal distribution of women in the high- and low-tolerant groups (regardless of analytic approach), minimizes the possibility that some of the variability between LBNP tolerances is related to the phase of the menstrual cycle of the subjects.
The relationship between the reduction in CVP to the heat stress and LBNP tolerance solely while heat stressed (i.e., independent of normothermic LBNP tolerance) was also evaluated (data not shown). Despite a lower CSI in the low-tolerant group (CSI: 86 ± 39 mmHg × min) compared with the high-tolerant group (439 ± 183 mmHg × min; P < 0.001) to LBNP while heat stressed, the reduction in CVP to the heat stress was not different between these groups (P = 0.22). Furthermore, there was a lack of a significant correlation (r = 0.03; P = 0.79) between the reduction in CVP to the heat stress and heat-stress LBNP tolerance across all subjects.
Conclusion
Counter to the proposed hypotheses, large intersubject variability in LBNP tolerance while heat stressed was not related to the magnitude of reduction in CVP by this thermal provocation. Thus other factors are responsible for the observed wide variations in LBNP tolerance while heat stressed. Further research is warranted to identify the source of this interindividual variability, leading to alterations in the control of blood pressure during whole body heat stress. Such findings will likely have important implications toward improved treatment for hemorrhage in hyperthermic individuals, which may occur in soldiers or firefighters.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants HL61388, HL84072, and HL092761 and by The Research and Education Institute of Texas Health Resources.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors express appreciation to Kimberly A. Hubing and Jena Porterfield for technical assistance, and the subjects for willing participation in this project.
REFERENCES
- 1. Allan JR, Crossley RJ. Effect of controlled elevation of body temperature on human tolerance to +Gz acceleration. J Appl Physiol 33: 418–420, 1972. [DOI] [PubMed] [Google Scholar]
- 2. Brothers RM, Bhella PS, Shibata S, Wingo JE, Levine BD, Crandall CG. Cardiac systolic and diastolic function during whole body heat stress. Am J Physiol Heart Circ Physiol 296: H1150–H1156, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brothers RM, Wingo JE, Hubing KA, Crandall CG. The effects of reduced end-tidal carbon dioxide tension on cerebral blood flow during heat stress. J Physiol 587: 3921–3927, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bungo MW, Charles JB, Johnson PC. Cardiovascular deconditioning during space flight and the use of saline as a countermeasure to orthostatic intolerance. Aviat Space Environ Med 56: 985–990, 1985. [PubMed] [Google Scholar]
- 5. Cai Y, Holm S, Jenstrup M, Stromstad M, Eigtved A, Warberg J, Hojgaard L, Friberg L, Secher NH. Electrical admittance for filling of the heart during lower body negative pressure in humans. J Appl Physiol 89: 1569–1576, 2000. [DOI] [PubMed] [Google Scholar]
- 6. Cai Y, Jenstrup M, Ide K, Perko M, Secher NH. Influence of temperature on the distribution of blood in humans as assessed by electrical impedance. Eur J Appl Physiol 81: 443–448, 2000. [DOI] [PubMed] [Google Scholar]
- 7. Crandall CG, Levine BD, Etzel RA. Effect of increasing central venous pressure during passive heating on skin blood flow. J Appl Physiol 86: 605–610, 1999. [DOI] [PubMed] [Google Scholar]
- 8. Crandall CG, Wilson TE, Marving J, Vongelsang TW, Kjaer A, Hesse B, Secher NH. Effects of passive heating on central blood volume and ventricular dimensions in humans. J Physiol 586: 293–301, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cui J, Durand S, Levine BD, Crandall CG. Effect of skin surface cooling on central venous pressure during orthostatic challenge. Am J Physiol Heart Circ Physiol 289: H2429–H2433, 2005. [DOI] [PubMed] [Google Scholar]
- 10. Cui J, Wilson TE, Crandall CG. Orthostatic challenge does not alter skin sympathetic nerve activity in heat-stressed humans. Auton Neurosci 116: 54–61, 2004. [DOI] [PubMed] [Google Scholar]
- 11. Davis JE, Fortney SM. Effect of fluid ingestion on orthostatic responses following acute exercise. Int J Sports Med 18: 174–178, 1997. [DOI] [PubMed] [Google Scholar]
- 12. Fu Q, Okazaki K, Shibata S, Shook RP, VanGunday TB, Galbreath MM, Reelick MF, Levine BD. Menstrual cycle effects on sympathetic neural responses to upright tilt. J Physiol 587: 2019–2031, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greenleaf JE, Petersen TW, Gabrielsen A, Pump B, Bie P, Christensen NJ, Warberg J, Videbaek R, Simonson SR, Norsk P. Low LBNP tolerance in men is associated with attenuated activation of the renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol 279: R822–R829, 2000. [DOI] [PubMed] [Google Scholar]
- 14. Grenon SM, Hurwitz S, Sheynberg N, Xiao X, Ramsdell CD, Mai CL, Kim C, Cohen RJ, Williams GH. Role of individual predisposition in orthostatic intolerance before and after simulated microgravity. J Appl Physiol 96: 1714–1722, 2004. [DOI] [PubMed] [Google Scholar]
- 15. Grenon SM, Xiao X, Hurwitz S, Sheynberg N, Kim C, Seely EW, Cohen RJ, Williams GH. Why is orthostatic tolerance lower in women than in men? Renal and cardiovascular responses to simulated microgravity and the role of midodrine. J Investig Med 54: 180–190, 2006. [DOI] [PubMed] [Google Scholar]
- 16. Harrison MH. Athletes, astronauts and orthostatic tolerance. Sports Med 3: 428–435, 1986. [DOI] [PubMed] [Google Scholar]
- 17. Haruna Y, Takenaka K, Suzuki Y, Kawakubo K, Gunji A. Effect of acute saline infusion on the cardiovascular deconditioning after 20-days head-down tilt bedrest. J Gravit Physiol 5: P45–P46, 1998. [PubMed] [Google Scholar]
- 18. Jacob G, Robertson D, Mosqueda-Garcia R, Ertl AC, Robertson RM, Biaggioni I. Hypovolemia in syncope and orthostatic intolerance role of the renin-angiotensin system. Am J Med 103: 128–133, 1997. [DOI] [PubMed] [Google Scholar]
- 19. Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Handbook of Physiology. Environmental Physiology. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 4, vol. I, chapt. 11, p. 215–243. [Google Scholar]
- 20. Keller DM, Low DA, Wingo JE, Brothers RM, Hastings J, Davis SL, Crandall CG. Acute volume expansion preserves orthostatic tolerance during whole-body heat stress in humans. J Physiol 587: 1131–1139, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kellogg DL, Jr, Johnson JM, Kosiba WA. Baroreflex control of the cutaneous active vasodilator system in humans. Circ Res 66: 1420–1426, 1990. [DOI] [PubMed] [Google Scholar]
- 22. Kumar A, Anel R, Bunnell E, Habet K, Zanotti S, Marshall S, Neumann A, Ali A, Cheang M, Kavinsky C, Parrillo JE. Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit Care Med 32: 691–699, 2004. [DOI] [PubMed] [Google Scholar]
- 23. Levine BD, Lane LD, Buckey JC, Friedman DB, Blomqvist CG. Left ventricular pressure/volume and Frank-Starling relations in endurance athletes: implications for orthostatic tolerance and exercise performance. Circ Res 84: 1016–1023, 1991. [DOI] [PubMed] [Google Scholar]
- 24. Levine BD, Zuckerman JH, Pawelczyk JA. Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation 96: 517–525, 1997. [DOI] [PubMed] [Google Scholar]
- 25. Lind AR, Leithead CS, McNicol GW. Cardiovascular changes during syncope induced by tilting men in the heat. J Appl Physiol 25: 268–276, 1968. [DOI] [PubMed] [Google Scholar]
- 26. Low DA, Wingo JE, Keller DM, Davis SL, Zhang R, Crandall CG. Cerebrovascular responsiveness to steady-state changes in end-tidal CO2 during passive heat stress. J Appl Physiol 104: 976–981, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ludwig DA, Convertino VA. Predicting orthostatic intolerance: physics or physiology? Aviat Space Environ Med 65: 404–411, 1994. [PubMed] [Google Scholar]
- 28. Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest 134: 172–178, 2008. [DOI] [PubMed] [Google Scholar]
- 29. Matzen S, Perko G, Groth S, Friedman DB, Secher NH. Blood volume distribution during head-up tilt induced central hypovolaemia in man. Clin Physiol 11: 411–422, 1991. [DOI] [PubMed] [Google Scholar]
- 30. Meendering JR, Torgrimson BN, Houghton BL, Halliwill JR, Minson CT. Menstrual cycle and sex affect hemodynamic responses to combined orthostatic and heat stress. Am J Physiol Heart Circ Physiol 289: H631–H642, 2005. [DOI] [PubMed] [Google Scholar]
- 31. Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters cardiovascular response to direct passive heating. J Appl Physiol 84: 1323–1332, 1998. [DOI] [PubMed] [Google Scholar]
- 32. Nelson MD, Haykowsky MJ, Petersen SR, DeLorey DS, Cheng-Baron J, Thompson RB. Increased left ventricular twist, untwisting rates, and suction maintain global diastolic function during passive heat stress in humans. Am J Physiol Heart Circ Physiol 298: H930–H937, 2010. [DOI] [PubMed] [Google Scholar]
- 33. Nyyssonen K, Parviainen MT. Practical observations and sources of error in assays of plasma catecholamines by “high-performance” liquid chromatography with electrochemical detection. Clin Chem 33: 1938–1939, 1987. [PubMed] [Google Scholar]
- 34. Pawelczyk JA, Matzen S, Friedman DB, Secher NH. Cardiovascular and hormonal responses to central hypovolaemia in humans. In: Blood Loss and Shock, edited by Secher NH, Pawelczyk JA, Ludbrook J. London: Arnold, 1993. [Google Scholar]
- 35. Pawelczyk JA, Pawelczyk RA, Matzen S, Secher NH. Thoracic impedance, not central venous pressure, predict changes in central blood volume during orthostatism (Abstract). FASEB J 6: A1771, 1992. [Google Scholar]
- 36. Peters JK, Nishiyasu T, Mack GW. Reflex control of the cutaneous circulation during passive body core heating in humans. J Appl Physiol 88: 1756–1764, 2000. [DOI] [PubMed] [Google Scholar]
- 37. Robertson D, Jacob G, Ertl A, Shannon J, Mosqueda-Garcia R, Robertson RM, Biaggioni I. Clinical models of cardiovascular regulation after weightlessness. Med Sci Sports Exerc 28: S80–S84, 1996. [DOI] [PubMed] [Google Scholar]
- 38. Rowell LB. Thermal stress. In: Human Circulation Regulation During Physical Stress. New York: Oxford University Press, 1986, p. 174–212. [Google Scholar]
- 39. Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol 27: 673–680, 1969. [DOI] [PubMed] [Google Scholar]
- 40. Rowell LB, Detry JR, Profant GR, Wyss C. Spanchnic vasoconstriction in hyperthermic man: role of falling blood pressure. J Appl Physiol 31: 864–869, 1971. [DOI] [PubMed] [Google Scholar]
- 41. Shvartz E, Convertino VA, Keil LC, Haines RF. Orthostatic fluid-electrolyte and endocrine responses in fainters and nonfainters. J Appl Physiol 51: 1404–1410, 1981. [DOI] [PubMed] [Google Scholar]
- 42. Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol 66: 1586–1592, 1989. [DOI] [PubMed] [Google Scholar]
- 43. Wilson TE, Brothers RM, Tollund C, Dawson EA, Nissen P, Yoshiga CC, Jons C, Secher NH, Crandall CG. Effect of thermal stress on Frank-Starling relations in humans. J Physiol 587: 3383–3392, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wilson TE, Crandall CG. Effect of thermal stress on cardiac function. Exerc Sport Sci Rev 39: 12–17, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wilson TE, Cui J, Crandall CG. Effect of whole-body and local heating on cutaneous vasoconstrictor responses in humans. Auton Neurosci 97: 122–128, 2002. [DOI] [PubMed] [Google Scholar]
- 46. Wilson TE, Cui J, Zhang R, Crandall CG. Heat stress reduces cerebral blood velocity and markedly impairs orthostatic tolerance in humans. Am J Physiol Regul Integr Comp Physiol 291: R1443–R1448, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wilson TE, Cui J, Zhang R, Witkowski S, Crandall CG. Skin cooling maintains cerebral blood flow velocity and orthostatic tolerance during tilting in heated humans. J Appl Physiol 93: 85–91, 2002. [DOI] [PubMed] [Google Scholar]
- 48. Wilson TE, Tollund C, Yoshiga CC, Dawson EA, Nissen P, Secher NH, Crandall CG. Effects of heat and cold stress on central vascular pressure relationships during orthostasis in humans. J Physiol 585: 279–285, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]