Abstract
Acute intermittent hypoxia (AIH) elicits a form of respiratory plasticity known as long-term facilitation (LTF). Here, we tested four hypotheses in unanesthetized, spontaneously breathing rats using radiotelemetry for EEG and diaphragm electromyography (Dia EMG) activity: 1) AIH induces LTF in Dia EMG activity; 2) diaphragm LTF (Dia LTF) is more robust during sleep vs. wakefulness; 3) AIH (or repetitive AIH) disrupts natural sleep-wake architecture; and 4) preconditioning with daily AIH (dAIH) for 7 days enhances Dia LTF. Sleep-wake states and Dia EMG were monitored before (60 min), during, and after (60 min) AIH (10, 5-min hypoxic episodes, 5-min normoxic intervals; n = 9), time control (continuous normoxia, n = 8), and AIH following dAIH preconditioning for 7 days (n = 7). Dia EMG activities during quiet wakefulness (QW), rapid eye movement (REM), and non-REM (NREM) sleep were analyzed and normalized to pre-AIH values in the same state. During NREM sleep, diaphragm amplitude (25.1 ± 4.6%), frequency (16.4 ± 4.7%), and minute diaphragm activity (amplitude × frequency; 45.2 ± 6.6%) increased above baseline 0–60 min post-AIH (all P < 0.05). This Dia LTF was less robust during QW and insignificant during REM sleep. dAIH preconditioning had no effect on LTF (P > 0.05). We conclude that 1) AIH induces Dia LTF during NREM sleep and wakefulness; 2) Dia LTF is greater in NREM sleep vs. QW and is abolished during REM sleep; 3) AIH and repetitive AIH disrupt natural sleep patterns; and 4) Dia LTF is unaffected by dAIH. The capacity for plasticity in spinal pump muscles during sleep and wakefulness suggests an important role in the neural control of breathing.
Keywords: respiratory plasticity, respiratory muscle, breathing, metaplasticity, vigilance state
plasticity is an important property of neural systems, including respiratory motor control (41, 49). Respiratory plasticity enables adjustments to longer-lasting physiological changes, such as altitude, aging, pregnancy, and the onset of disease. One of the most comprehensively studied models of respiratory plasticity is long-term facilitation (LTF) (12, 31, 40). LTF is a progressive (>1 h) increase in respiratory motor output [e.g., phrenic (pLTF) and/or hypoglossal nerve activity] following acute intermittent hypoxia (AIH). Cellular mechanisms of pLTF have been explored largely in reduced in vivo or in vitro preparations (5, 7, 29, 30, 45). Although a considerable mechanistic understanding has been gained from these studies (9), the biological relevance of LTF is not yet clear. Therefore, there is need for intact animal models to further our understanding.
Ventilatory LTF has been demonstrated in unanesthetized, spontaneously breathing dogs (8), goats (57), ducks (42), rats (37, 38, 44, 46), mice (26, 55), and humans (22, 47). However, whether ventilatory and pLTF share a common mechanism remains unclear, as ventilatory LTF exhibits more variability in magnitude, duration, and breathing pattern, depending on experimental preparation and/or protocol (31, 40, 49). In unanesthetized, spontaneously breathing mammals, LTF is often expressed as increased breathing frequency with little change in tidal volume (37, 38, 46). In contrast, LTF in anesthetized animals is predominantly expressed as increased inspiratory nerve burst amplitude (tidal volume equivalent) (6). This discrepancy raises the question of whether the magnitude of diaphragm activity is a major contributor to ventilatory LTF in unanesthetized, spontaneously breathing, poikilocapnic animals.
Vigilance state (i.e., wakefulness vs. sleep) also plays a critical role in respiratory plasticity including respiratory LTF. LTF is more often observed during sleep in healthy humans (47) and snorers or sleep apnea patients (1–3) vs. awake subjects (25, 35). Similarly, sleep-wake state dependence of ventilatory LTF has been demonstrated in unanesthetized rats, with the greatest effect during deep non-rapid eye movement (NREM) sleep (44). Ventilatory LTF during NREM sleep in these rats was expressed predominantly in tidal volume (44), similar to pLTF in anesthetized, vagotomized, and ventilated rats (12, 40). Although LTF is reported during wakefulness in rats (37, 38, 46) and mice (26, 55), most studies did not assess sleep-wake state via EEG, leaving some doubt as to the animal's vigilance state.
Apart from the prevailing vigilance state, sleep is important for neural plasticity in other ways. For example, sleep deprivation impairs hippocampal long-term potentiation (a form of synaptic plasticity thought to underlie learning and memory) (53) and ventilatory LTF (36) via mechanisms associated with adenosine A1 receptor activation. Thus, sufficient sleep is necessary to express some forms of neural plasticity. Several studies have assessed the impact of chronic intermittent hypoxia (CIH; e.g., 12 h/day for days to weeks) on aspects of polysomnography, such as REM sleep periods and NREM sleep intensity (21, 48, 58). However, despite the impact of sleep state and quality on respiratory plasticity, the impact of AIH (3–10 hypoxic episodes <2 h) on sleep architecture has not been explored. Therefore, detailed information on sleep architecture before, during, and after AIH is of considerable interest to LTF research.
Respiratory LTF can exhibit metaplasticity, as preconditioning with CIH (∼8–12 h/day over days to weeks) enhances AIH-induced LTF in anesthetized (28, 64) and unanesthetized preparations (37). However, CIH also elicits pathologies such as systemic hypertension (13), hippocampal cell death, learning deficits (21, 50), and metabolic syndrome (54). On the other hand, daily exposure to AIH (dAIH) enhances hypoglossal LTF in anesthetized rats, without evidence of weight loss, hypertension (62), or hippocampal cell death (I. Satriotomo and G. S. Mitchell, unpublished observations). Thus modest protocols of intermittent hypoxia (e.g., repetitive AIH) may elicit beneficial respiratory plasticity without adverse side effects caused by more severe protocols, such as CIH. Thus repetitive AIH represents a possible therapeutic tool in the treatment of breathing disorders (39), including cervical spinal injuries or amyotrophic lateral sclerosis (10, 60).
One limitation in studies of ventilatory LTF (vs. pLTF) is that we cannot be sure which respiratory muscles contribute to the plasticity. There would be considerable benefit in developing experimental preparations that enable studies of targeted respiratory muscle activation (e.g., diaphragm vs. inspiratory intercostal muscle) during LTF in unanesthetized, spontaneously breathing animals in different vigilance states. Such models would help us understand the impact of AIH, repetitive AIH, and CIH on respiratory motor control and the potential of repetitive AIH as a therapeutic tool in the treatment of respiratory insufficiency.
Here, we report a chronically instrumented rat preparation with a telemetry system that allows simultaneous assessment of sleep-wake state and diaphragm electromyography (Dia EMG) signals following AIH (and dAIH). We tested four specific hypotheses: 1) AIH induces LTF in Dia EMG activity during sleep and wakefulness; 2) diaphragm LTF (Dia LTF) is sleep-wake state dependent; 3) AIH (and/or dAIH) disrupts natural sleep-wake architecture; and 4) preconditioning with 7 days of AIH exposure enhances AIH-induced Dia LTF. This is the first study demonstrating AIH-induced Dia LTF during sleep and wakefulness in unanesthetized rats. This model forms the basis for further studies testing the significance of these potentially important forms of respiratory plasticity.
METHODS
Animals
All experiments were performed on 3- to 5-mo-old male Sprague-Dawley rats (312–445 g, colony 218a, Harlan, Indianapolis, IN). Animals were individually housed in a controlled environment (12-h light/dark cycle). The Animal Care and Use Committee at the School of Veterinary Medicine, University of Wisconsin (Madison, WI), approved all experimental procedures.
Experimental Preparation
Surgical preparation.
Sterile surgery was performed under anesthesia/sedation with isoflurane in 100% O2 and medetomidine (0.1 mg/kg sc). The rats were injected with buprenorphine (0.03 mg/kg), carprofen (Rimadyl, 5 mg/kg), and enrofloxacin (Baytril, 4 mg/kg sc) to minimize potential postoperative pain and infection. Catheters were placed in the tail vein for fluid administration (lactated Ringer; 0.7 ml·100 g−1·h−1). Body temperature was maintained at 36.5–37.5°C using a rectal probe and external heating pad. An endotracheal tube was placed to enable artificial ventilation (tidal volume, 2.0–2.5 ml; rodent ventilator, model 683; Harvard Apparatus, Holliston, MA) with 1.5–2.5% isoflurane in 100% O2 throughout the surgery. Effective anesthesia was judged by abolition of pedal withdrawal to a toe pinch and elimination of corneal blink reflexes. Oxygen saturation was monitored by pulse oximetry (model 8600; Nonin Medical, Plymouth, MN) during the surgical procedures. With the rats supine, the ventral surface of the abdominal muscle was exposed, and a sterilized telemetry transmitter body [model 4ET-S1/2; Data Sciences International (DSI), St. Paul, MN] was inserted into the peritoneal cavity. The transmitter allowed simultaneous and continuous monitoring of electrical biopotentials, body temperature, and general locomotor activity. In the present study, the three biopotential channels were used to record EEG and EMG of diaphragm and neck muscle. All biopotential lead pairs (bipolar) were tunneled subcutaneously from the body of the transmitter in the ventral abdomen to the site of implantation (i.e., neck, diaphragm, and cranium). EEG and EMG leads were placed on the basis of previous reports (33, 44, 55, 61). Briefly, the right costal part of the diaphragm was exposed, and two leads were implanted on the right side of the midcostal diaphragm using a 23-gauge syringe needle guide and tissue adhesive (Vetbond 1469SB; 3M Animal Care Products, St. Paul, MN). Next, nuchal muscles were exposed and displaced to allow skull access. With the use of a sterilized micro drill bit (1.2 mm) and hand-held drill, two holes in the cranium (2 mm bilateral to the midline and 2 mm rostral to Lambda) were made for EEG lead implantation. After placement of the EEG leads, the leads were securely covered in dental acrylic and fixed to the cranium. Neck EMG leads were sutured onto the dorsal neck muscles using a 20-gauge syringe needle guide. Before extubation, atipamezole (Antisedan; 0.4 mg/kg, intramuscularly) was given for reversing medetomidine sedation. At the end of surgery, buprenorphine, carprofen, and enrofloxacin, at the same dose rates received prior to the surgery, were administered at 12-h intervals for 48 h postsurgery. Rats were visually monitored and weighed daily. Experiments were not initiated until rats resumed normal weight gain.
Telemetry signal assessment.
The rats were placed in custom-made Plexiglas chambers (see Normoxic and hypoxic control in chambers) positioned on receivers (model RPC-2; DSI). Signals from the implanted radiotelemetry transmitter were detected by the receivers and sent to a data exchange matrix (model ACQ-7700; DSI). Two channels of EMG, one channel of EEG, body temperature, and general locomotor activity in unanesthetized, freely moving rats were monitored during the experimental protocol on a laboratory computer (data acquisition system: PONEMAH Physiology Platform; DSI). EMG and EEG signals were sampled at 500 samples/s, and then EMGs were amplified and filtered (100–3,000 Hz). An analysis of all parameters was performed with NeuroScore software (DSI) as (see Data Analyses).
Normoxic and hypoxic control in chambers.
Normoxic [fraction of inspired oxygen (FiO2) = 0.210] and hypoxic (FiO2 = 0.105) conditions were established in custom-made chambers (Plexiglas cylinder, 12 × 4 in. internal diameter; 1 rat/chamber) by mixing O2 and N2 gas via a custom-made, computer-controlled system to obtain the desired, inspired oxygen concentrations. Within the chambers, CO2 and O2 levels were continuously monitored (O2 Analyzer, model 17518; CO2 Analyzer, model 17515; VacuMed, Ventura, CA). Gas flowed through the chamber at a rate of 4 l/min, keeping CO2 concentration in the chamber <0.5% at all times. Ninety-five percent of the change in O2 levels within the chamber (i.e., intermittent hypoxia) was achieved in 25 ± 5 s. The rats were poikilocapnic (i.e., arterial CO2 levels were not controlled).
Protocols.
Once normal body mass increases resumed following surgery (7–11 days postsurgery), the rats were acclimated to the chambers for 2 h at least 1 day before an experiment. At 8:00 AM on the experimental day, the rats were placed in the chamber above the signal receivers. At 10:00 AM, after the 2-h acclimation for each rat, baseline recording during normoxia for 1 h began (see Fig. 1). At 11:00 AM, after the baseline recording, one-half of the rats were administered AIH (10, 5-min hypoxic episodes with 5-min normoxic intervals; see Fig. 1, middle), and the other half were administered time control (TC) exposures (time-matched continuous normoxia; see Fig. 1, top). Post-treatment recordings during normoxia for 1 h were made until 1:35 PM. TC experiments demonstrated a lack of time-dependent Dia EMG changes during experiments (P > 0.05).
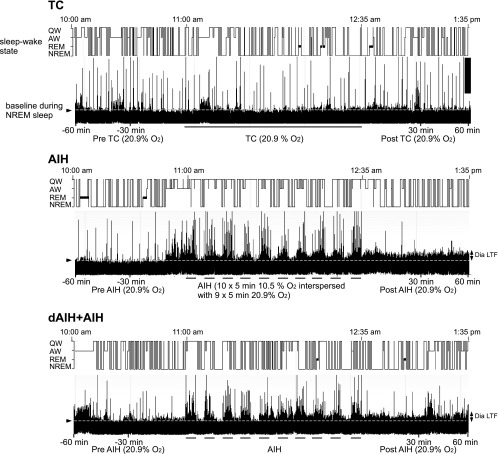
Fig. 1.
Representative traces of vigilance-state change and diaphragm electromyography (Dia EMG) activity in unanesthetized rats depicting each experimental protocol. Sleep-wake state (top traces) and integrated Dia EMG activity (bottom traces) are shown before, during, and after normoxia [top: time control (TC)], acute intermittent hypoxia (AIH; middle), and AIH following preconditioning with daily AIH (dAIH) + AIH (bottom), shown on the last day of AIH exposure. Note: 1) long-lasting increase in diaphragm amplitude above baseline (dotted white line) is diaphragm long-term facilitation (Dia LTF; arrows on right) following AIH and dAIH + AIH. No Dia LTF was observed in TC experiments; 2) sleep-wake states changed fairly rapidly in all groups; and 3) during each hypoxic episode in AIH exposures, non-rapid eye movement (NREM) sleep periods were decreased. AW, active wakefulness; QW, quiet wakefulness.
To investigate the effect of dAIH preconditioning on AIH-induced LTF, rats, which had undergone the AIH/LTF protocols the day before, were placed daily into Plexiglas chambers and exposed to AIH for 7 consecutive days. One day post-dAIH preconditioning, an AIH/LTF protocol with EMG and EEG recordings was repeated (dAIH + AIH protocol, total 8 days; see Fig. 1, bottom). At least 7 days after the initial TC, AIH, or dAIH + AIH study, rats with functional telemeters underwent a second (or third) study, switching protocols. The number of rats in each experimental group was: TC = 8; AIH = 9; dAIH + AIH = 7. All three experimental protocols (TC, AIH, and dAIH + AIH) were successfully performed on six of the rats. The remaining rats were excluded from subsequent studies, mainly because the quality of EEG signals had deteriorated. This exclusion did not affect the conclusions from the present study (data not shown). Rats had ad libitum access to food and water throughout experiments. Chamber temperature was 22.5–24.5°C. Chamber O2 and CO2 concentrations were continuously monitored during experiments.
Data Analyses
Assessment of sleep-wake states.
Sleep-wake (vigilance) states were determined by visual inspection of EEG, neck EMG, and locomotor activity signals on the basis of many previous studies in rodents (43, 52, 55, 59). The vigilance state was classified into active wakefulness (AW), quiet wakefulness (QW), REM sleep, and NREM sleep for every 10-s epoch. To minimize bias in selecting sleep-wake states, the EEG and neck EMG were scored without reference to diaphragm signals. When multiple vigilance states were observed during the time window (10 s), a dominant sleep-wake state (>50%) was determined as the state in the period. With the use of NeuroScore software, Fast Fourier transformation, periodogram (Hamming window), and autoregressive spectrum analysis were used to estimate spread of the EEG power spectrum. The band limits were δ (0.5–4 Hz), θ (4–7.5 Hz), α (7.5–13.5 Hz), and β (13.5–30). Delta power of NREM sleep was expressed as a percentage of the total EEG power. Time spent in each vigilance state was shown as percentage of total time before, during, and after treatments as described below. The percentage of sleep-wake state time was always AW + QW + slow-wave sleep + REM = 100.
Analysis of variables.
All variables were analyzed with NeuroScore software. Dia EMG signals were rectified, integrated (100 ms), and quantified in arbitrary units. Mean peak amplitude, respiratory frequency, and calculated values of minute diaphragm activity (amplitude × frequency) and peritoneal temperature in 10-s epochs (i.e., each sleep-wake state) were averaged in all rats. These values were divided into sleep-wake states (AW, QW, NREM, and REM) and averaged (grouped) in each 20-min period before (from −60 to −40, −40 to −20, and −20 to 0 min) and after (0–20, 20–40, and 40–60 min) treatment and every 5-min period during treatments [i.e, the first hypoxic (normoxic) period, described as h1 (n1) in results]. EMG and EEG values during the AW period (e.g., sniffing and grooming) were excluded in the analysis, because Dia EMG and EEG signals were distorted during this period (see Fig. 2A). Data obtained during the pretreatment period (1 h) in each vigilance state were used to calculate their respective baseline values. All respiratory values before, during, and after treatment were expressed as a percent change from normalized baseline (i.e., pretreatment) values. Additionally, to investigate the sleep architecture during and after AIH, the number, duration, and delta power of NREM sleep episodes were averaged and grouped in the periods before (1 h), after (1 h), and during treatment (hypoxia: h1–10, total 50 min; normoxia: n1–9, total 45 min).
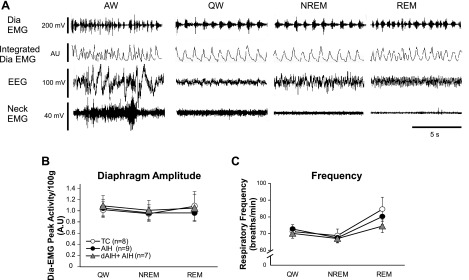
Fig. 2.
A: representative resting diaphragm activity during normoxia across sleep-wake states. Pretreatment-raw and integrated Dia EMG, neck EMG, and EEG in each sleep-wake state are shown. B and C: absolute values of mean Dia EMG peak activity and respiratory frequency during pretreatment normoxic period (1 h) across vigilance states in all treatment groups (TC, AIH, and dAIH + AIH). Note: baselines during normoxia in all groups show similar values in diaphragm amplitude and frequency. Values are means ± SE. All variables in the dAIH + AIH group were measured on the last day of AIH exposure (day 8). AU, arbitrary units.
Statistical analysis.
Statistical comparisons were made for time (pre-, during, and post-treatments) and treatment groups (TC, AIH, and dAIH + AIH) using two-way repeated measures ANOVA with Bonferroni's post hoc tests (SigmaStat version 2.03, Systat Software, San Jose, CA). Respiratory variables (diaphragm amplitude, frequency, and minute diaphragm activity) were compared among vigilance states (QW, REM sleep, and NREM sleep) during and post-treatment condition by one-way repeated measures ANOVA with Bonferroni's post hoc tests. Differences indicated as statistically significant were P < 0.05. All values are expressed as means ± SE.
RESULTS
Baseline Diaphragm Muscle Activity Across Sleep-Wake States
Resting Dia EMG signals during the normoxic pretreatment period differed among vigilance states (Fig. 2A). The following general patterns were observed in each sleep-wake state. First, there were immediate changes in diaphragm activity after each vigilance-state change. Second, the Dia EMG signals during NREM sleep and QW were regular and stable, but the Dia EMG signals during REM showed irregular activity, higher burst frequency, and occasional cessation of breathing (i.e., spontaneous apnea). Finally, the Dia EMG and EEG signals during AW were distorted due to the rats' activities, such as sniffing, grooming, and eating. Averaged diaphragm amplitude and frequency during pretreatment (i.e., normoxia) across vigilance states were similar in all experimental groups (P > 0.05; Fig. 2, B and C). These results indicate that preconditioning with dAIH for 7 days (i.e., dAIH) had no effect on baseline diaphragm amplitude or frequency (on day 8); therefore, Dia LTF comparisons relative to baseline values in different treatment groups were appropriate. The value of pretreatment diaphragm amplitude and frequency in each treatment group (TC, AIH, and dAIH + AIH) showed no significant differences among sleep-wake states (P > 0.05; see Experimental considerations in discussion).
Short-Term Hypoxic Responses
Averaged diaphragm amplitude, frequency, and calculated values of minute diaphragm activity (amplitude × frequency) during hypoxia (e.g., h1–10) were increased from baseline similarly in the AIH- and dAIH + AIH-treated rats (all P < 0.05; Table 1) in both NREM sleep and QW periods. Minute diaphragm activities during hypoxia in the QW period in both AIH- and dAIH + AIH-treated rats (148.4 ± 16.1% and 150.3 ± 12.5% above baseline, respectively) tended to be greater than the activities during NREM sleep (109.5 ± 23.5% and 123.3 ± 16.9% above baseline, respectively), but these apparent differences were not significant (P > 0.05). Frequency increases contributed more than diaphragm amplitude to increased minute activity in both AIH and dAIH + AIH rats. There was no difference in the hypoxic response between AIH- and dAIH + AIH-treated rats during NREM sleep or QW (P > 0.05). The same time course of normoxia (h1–10) in TC-treated rats did not show changes in diaphragm activity, frequency, or minute activity during QW or NREM sleep (P > 0.05).
Table 1.
Short-term hypoxic responses in diaphragm EMG amplitude, respiratory frequency, and minute diaphragm activity during NREM sleep and quiet wakefulness
| Short-term hypoxic responses (% above baseline) |
||||
|---|---|---|---|---|
| Treatment | ΔDia | ΔFrequency | ΔMinute activity | |
| NREM | TC (n = 8) | 4.6 ± 3.8 | 1.7 ± 3.6 | 6.7 ± 5.9 |
| AIH (n = 9) | 29.4 ± 7.9* | 57.7 ± 13.5* | 109.5 ± 23.5* | |
| dAIH + AIH (n = 7) | 29.7 ± 3.9* | 70.9 ± 10.0* | 123.3 ± 16.9* | |
| QW | TC | 2.5 ± 3.4 | 1.3 ± 3.9 | 1.6 ± 5.7 |
| AIH | 39.9 ± 5.9* | 78.0 ± 9.0* | 148.4 ± 16.1* | |
| dAIH + AIH | 34.0 ± 2.9* | 83.6 ± 8.2* | 150.3 ± 12.5* | |
Values are means ± 1 SE. Dia [diaphragm electromyography (EMG) amplitude], frequency, and calculated values of minute activity (amplitude × frequency) during hypoxia (h1–h10: 1st–10th episode of hypoxia) were averaged and expressed as percent change above baseline. TC, time control; AIH, acute intermittent hypoxia; dAIH, daily AIH; NREM, non-rapid eye movement sleep; QW, quiet wakefulness.
Significantly different from baseline value (P < 0.05).
Sleep-Wake Architecture
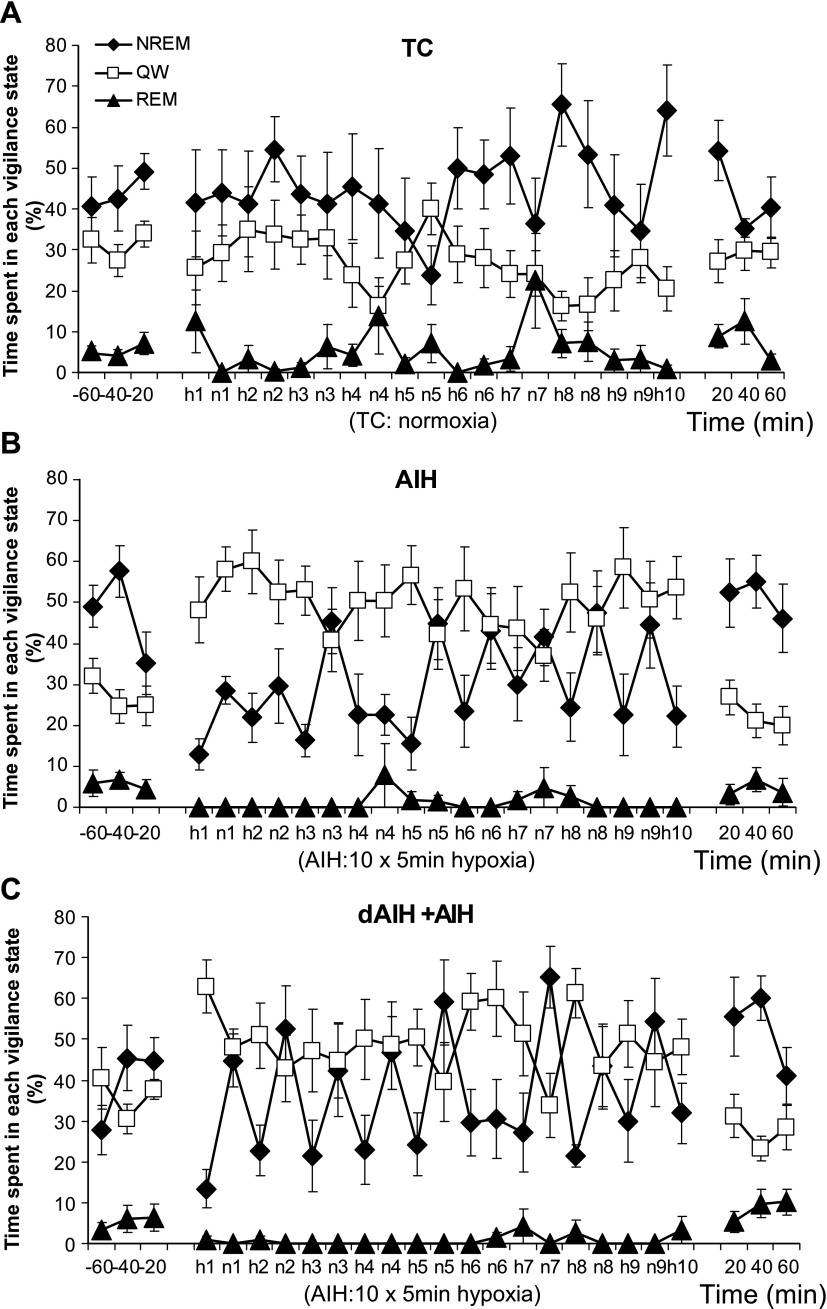
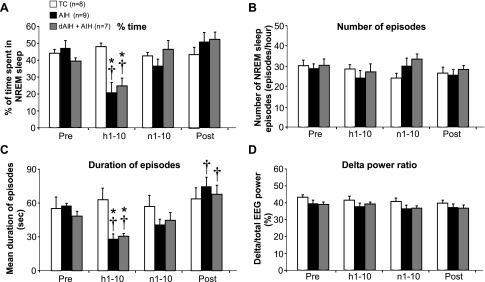
Figure 3 shows the relative time spent in each vigilance state before, during, and after treatment protocols (TC, AIH, and dAIH + AIH). During hypoxia (h1–10), the fraction of time in NREM sleep decreased from the beginning of the AIH stimulation in AIH and dAIH + AIH groups, whereas QW time increased (both P < 0.05; statistical summary of time spent in all vigilance states is provided in online Supplement 1). The sleep-architecture during AIH was mainly disrupted by a decrease in the duration of NREM sleep episodes in AIH- and dAIH + AIH-treated rats (Fig. 4, A and C; P < 0.05). However, neither the number nor the intensity (i.e., delta power) of NREM sleep episodes was significantly affected by AIH (P > 0.05; Fig. 4, B and D). After AIH, the time spent in each vigilance state returned to the pre-AIH level (P > 0.05; Figs. 3 and 4) in both AIH and dAIH + AIH rats. Only duration of NREM sleep was affected (extended) after AIH exposure in AIH- and dAIH + AIH-treated rats (both P < 0.05 relative to baseline and h1–10), indicative of sleep rebound. There was no significant difference in sleep-wake variables between the AIH- and dAIH + AIH-treated groups at any period (P > 0.05). Normoxia (i.e., TC) did not affect time spent in each vigilance state or the duration, number, or delta power of NREM sleep episodes (P > 0.05).
Fig. 3.
Percentage of time spent in each vigilance state before, during, and after TC (A), AIH (B), and dAIH + AIH (C) treatments. Time spent in each vigilance state was averaged in every 20-min period (before and after the treatment) or every 5-min period (during treatment). Note (B and C): 1) the time spent in NREM and QW during AIH groups switched (QW > NREM) relative to the pre- and post-treatment periods (NREM > QW); 2) the time spent in NREM sleep during hypoxia (h1–10) gradually increased toward the end of hypoxic episodes; and 3) the amount of time spent in REM sleep was suppressed during AIH. Values are means ± SE. All variables in the dAIH + AIH group were measured on the last day of AIH exposure (day 8). Although AW is not shown, the percentage of sleep-wake state time is always AW + QW + slow-wave sleep + REM = 100.
Fig. 4.
Effect of AIH on NREM sleep. Percentage of time (A), number (B), duration (C), and delta power (D) of NREM sleep pre (1 h)-, during (hypoxia, h1–10, total 50 min; normoxia, n1–10, total 45 min), and post (1 h)-treatments (TC, AIH, and dAIH + AIH) are expressed as averaged values. Note: 1) time spent in NREM sleep was significantly decreased during hypoxia (h1–10) in AIH- and dAIH + AIH-treated rats (A); 2) duration of NREM sleep episode during hypoxia (h1–10) was decreased but was significantly extended post-AIH treatment in both AIH- and dAIH + AIH-treated rats (C). Values are means ± SE. All variables in the dAIH + AIH group were measured on the last day of AIH exposure (day 8). *Significantly different from TC; †significantly different from baseline; P < 0.05.
Although not statistically significant, apparent differences in REM sleep periods among TC, AIH, and dAIH + AIH groups are interesting. The AIH and dAIH + AIH groups exhibited almost no REM periods during AIH stimulation (AIH group, 0.6 ± 0.6%; dAIH + AIH group, 1.2 ± 0.8%; only one rat in the AIH group and two rats in the dAIH + AIH group had a short period of REM sleep during hypoxia; see online Supplement 1), but the TC group exhibited REM sleep as seen during the pretreatment period (seven of eight rats had REM sleep episodes during the same time course of normoxia). The AIH and dAIH + AIH groups grew accustomed to the intermittent hypoxia, as seen by the increase in NREM sleep states over time (see Fig. 3, B and C).
Body Temperature Regulation
Body temperatures during the 1-h baseline period were similar in all experimental groups (AIH, 37.4 ± 0.1°C; dAIH + AIH, 37.6 ± 0.1°C; TC, 37.4 ± 0.1°C; P > 0.05). During AIH exposures, body temperatures progressively declined in both AIH- and dAIH + AIH-treated rats (Fig. 5). Peritoneal temperature reached a nadir of about 1.0°C below baseline values during the last episode (h10) of hypoxia. By 20-min post-hypoxia, peritoneal temperature began to return toward baseline values, but recovery was not complete at 40–60 min post-hypoxia. TC rats did not exhibit any changes in body temperature at any time (P > 0.05).
Fig. 5.
Time course of rat peritoneal temperature before, during, and after treatment (TC, AIH, and dAIH + AIH). Absolute values of average baseline temperature (1 h) were similar in all treatment groups: AIH, 37.4 ± 0.1°C; dAIH + AIH, 37.6 ± 0.1°C; TC, 37.4 ± 0.1°C. Peritoneal temperatures prior to the 6th episode of hypoxia and 40–60 min after treatment were not significantly different among groups. After the 6th hypoxic episode, peritoneal temperature in AIH and dAIH + AIH groups had significantly decreased relative to TC rats, but these values had returned to TC levels by 40–60 min post-AIH. All variables in the dAIH + AIH group were obtained on the last day of AIH exposure (day 8). Values are means ± SE. *Significantly different from TC; †significantly different from baseline; P < 0.05.
An important implication of these data is that failure to account for changes in body temperature during and (continuously) following AIH will introduce systematic calibration errors when ventilation is measured using whole body plethysmography, and many previous studies examining ventilatory LTF do not account for this body temperature change during or immediately post-AIH.
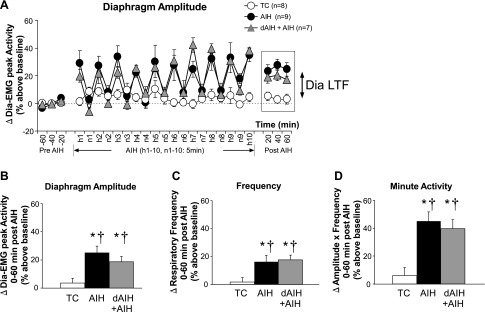
Dia LTF During Sleep
AIH and dAIH + AIH caused a sustained increase in Dia EMG amplitude during NREM sleep 0–60 min post-AIH (AIH group, 25.1 ± 4.6% above baseline; dAIH + AIH group, 18.7 ± 3.7% above baseline; both P < 0.05 vs. baseline and TC; Fig. 6, A and B), indicating robust Dia LTF during NREM sleep. Frequency (AIH group, 16.4 ± 4.7% above baseline; dAIH + AIH group, 17.6 ± 3.6% above baseline; both P < 0.05; Fig. 6C) and minute diaphragm activity (AIH group, 45.2 ± 6.6% above baseline; dAIH + AIH group, 39.7 ± 6.4% above baseline; both P < 0.05; Fig. 6D) during NREM sleep were also significantly increased 0–60 min post-AIH in both AIH- and dAIH + AIH-treated rats (i.e., LTF of frequency and minute daphragm activity). On the other hand, TC experiments revealed no changes in Dia EMG activity, frequency, or minute diaphragm activity during NREM sleep (all P > 0.05). There was no difference in the amplitude of LTF (any respiratory variable) during NREM sleep between AIH- and dAIH + AIH-treated rats (P > 0.05), demonstrating that preconditioning with dAIH did not enhance AIH-induced Dia LTF during NREM sleep. Since about one-third of the rats in AIH and dAIH + AIH did not have a REM sleep period, either pre- or post-AIH (see online Supplement 2), values in these rats were not represented in the analysis. However, post-AIH diaphragm amplitude during REM sleep tended to increase following AIH and dAIH (not significant, P > 0.05).
Fig. 6.
A: changes in diaphragm activity expressed as percent change from baseline during NREM sleep before, during, and after treatments (TC, AIH, and dAIH + AIH). Significant increases above baseline were observed in diaphragm amplitude (B), frequency (C), and minute activity (amplitude × frequency; D) during NREM sleep, 0–60 min post-treatment in AIH- and dAIH + AIH-treated rats. Similar changes were not observed in TC rats. Thus, AIH induced robust Dia LTF during NREM sleep. There was no significant difference in any variable between AIH and dAIH + AIH rats. All variables in the dAIH + AIH group were obtained on the last day of AIH exposure (day 8). Values are means ± SE. *Significantly different from TC; †significantly different from baseline; P < 0.05.
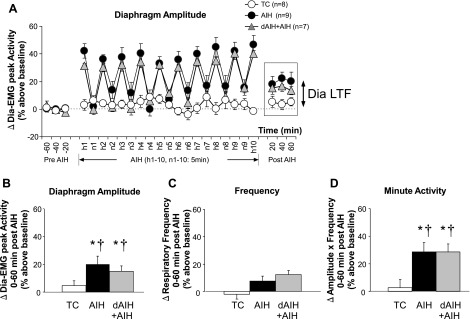
Dia LTF During Wakefulness
Similar to NREM sleep, diaphragm amplitude and minute diaphragm activity during QW were significantly increased 0–60 min post-AIH in AIH (20.1 ± 5.8%, 28.9 ± 6.6% above baseline; both P < 0.05; Fig. 7, A, B, and D)- and dAIH + AIH-treated rats (15.1 ± 3.8%, 28.6 ± 5.9% above baseline; both P < 0.05), indicative of Dia LTF during QW. However, frequency 0–60 min post-treatments was unaffected by AIH in either AIH- and dAIH + AIH-treated rats (i.e., no frequency LTF was observed during QW, P > 0.05; Fig. 7C). Additionally, LTF in minute diaphragm activity during QW was smaller than LTF during NREM sleep in both AIH- and dAIH + AIH-treated rats (both P < 0.05), indicating sleep-state dependency of Dia LTF. TC rats did not exhibit time-dependent changes in diaphragm activity, frequency, or minute diaphragm activity during QW (P > 0.05). dAIH preconditioning did not affect the amplitude of LTF in diaphragm activity during QW (P > 0.05) similar to NREM sleep.
Fig. 7.
A: changes in diaphragm activity expressed as percent change from baseline during QW before, during, and after treatment (TC, AIH, and dAIH + AIH) in unanesthetized rats. Significant increases above baseline were observed in diaphragm amplitude (B) and minute activity (amplitude × frequency) (D) during QW, 0–60 min post-treatment in AIH- and dAIH + AIH-treated rats. Similar effects were not observed in TC rats. Thus AIH elicits Dia LTF during QW, although frequency LTF was not observed during QW (C). There was no significant difference in any variable between the AIH and dAIH + AIH groups. All variables in the dAIH + AIH group were measured on the last day of AIH exposure (day 8). Values are means ± SE. *Significantly different from TC; †significantly different from baseline; P < 0.05.
DISCUSSION
To our knowledge, this is the first report showing Dia LTF during sleep and wakefulness in unanesthetized, spontaneously breathing (and poikilocapnic) rats. Based on this finding, we conclude that Dia LTF (reflecting pLTF) contributes to increased tidal volume following AIH [i.e., ventilatory LTF (44)]. Similar to ventilatory LTF, Dia LTF exhibited vigilance-state dependence, with the greatest response during NREM sleep. Another finding of interest is that AIH initially disrupted natural sleep patterns, but the rats acclimated toward the end of AIH exposure periods. Following AIH, the sleep-wake pattern returned to pre-AIH levels, except for the duration of NREM sleep episodes. Finally, since preconditioning with dAIH did not affect AIH-induced Dia LTF, there was no evidence for metaplasticity in Dia LTF.
Respiratory Patterns During LTF
Dia LTF during NREM sleep and QW in Sprague-Dawley rats was primarily manifested as changes in integrated diaphragm amplitude, suggesting mechanisms linked to tidal volume vs. respiratory timing. This finding coincides with our recent study, demonstrating increased tidal volume during ventilatory LTF in unanesthetized, sleeping Lewis rats (44). The larger contribution of integrated diaphragm amplitude suggests greater plasticity in neural mechanisms associated with burst pattern formation vs. rhythm generation. Given abundant evidence that pLTF arises largely from spinal mechanisms (7, 12, 31), we suggest that similar mechanisms underlie Dia LTF of burst amplitude in the present study.
Earlier literature suggested that AIH elicits predominantly frequency LTF in unanesthetized animals, with less (or even no) apparent tidal volume LTF (6). Several possibilities may explain this. First, since earlier studies did not account for sleep-wake state using EEG recordings (26, 37, 38, 46), increased frequency during ventilatory LTF may reflect combined values from REM sleep, QW, and/or AW, in addition to NREM sleep (see Fig. 2A; irregular and/or higher frequency in these states relative to NREM sleep). Second, genetic differences (species or strain) may account for differences in the regulation of respiratory frequency (4); ventilatory LTF in mice is mainly expressed during sleep and wakefulness as changes in frequency, with smaller changes in tidal volume (55). Even within the same species, respiratory LTF expression under anesthesia differs among rat strains and even substrains (16). Third, vagal feedback may influence frequency vs. amplitude contributions to ventilatory LTF. Spontaneously breathing rats have intact vagus nerves, whereas studies of anesthetized and ventilated rats are most often vagotomized to prevent entrainment of respiratory motor output with the ventilator (6). Fourth, differential PaCO2 regulation among studies (poikilocapnia vs. isocapnia) in unanesthetized preparations may contribute to differential LTF expression; Olson and colleagues (46) demonstrated differential contributions of frequency vs. tidal volume to ventilatory LTF, depending on whether poikilocapnic or isocapnic conditions were maintained. Although factors distinguishing LTF in frequency and amplitude remain unexplored, we suggest that Dia LTF during sleep and wakefulness is due, at least in part, to cellular/synaptic mechanisms, similar to pLTF in anesthetized rats.
Diaphragm Amplitude (vs. Tidal Volume) LTF
Increased diaphragm amplitude presumably reflects increased tidal volume, since integrated phrenic nerve amplitude correlates strongly with tidal volume in spontaneously breathing cats (11). Thus, since LTF in Dia minute activity during NREM sleep was 45%, there was presumably a similar increase in pulmonary ventilation (Fig. 6D). This increased ventilation represents a powerful hyperventilation that would decrease PaCO2 by nearly 12 or 13 mmHg (assumptions: steady-state, constant dead space-to-tidal volume ratio, no change in metabolic rate, and a baseline PaCO2 of 40 mmHg). Such profound hypocapnia would restrain LTF expression due to inhibitory chemoreceptor feedback (44, 46). The underlying neural LTF without chemofeedback must, therefore, have been considerably greater. It should be pointed out that the Dia LTF magnitude found here was considerably greater than that of ventilatory LTF reported previously in unanesthetized rats (37, 38, 44, 46), suggesting the possibility that the diaphragm expresses greater LTF than other respiratory muscles under the current experimental conditions and that Dia LTF overestimates concurrent ventilatory LTF in these same animals.
The magnitude of LTF varies considerably among respiratory motoneuron pools. For example, in anesthetized cats, inspiratory, intercostal LTF is considerably larger than pLTF following carotid sinus nerve stimulation (15). Similarly, in vitro LTF using a neonatal rat brain stem-spinal cord preparation shows differential pattern sensitivity between the cervical and thoracic regions (29). Thus other respiratory muscles are likely to be involved in ventilatory LTF, making differential contributions to the overall response. Thus the increase in tidal volume during ventilatory LTF may not accurately reflect any single respiratory muscle.
Mantilla and colleagues (33) showed differences in diaphragm muscle EMG activity across different ventilatory (e.g., eupnea, hypoxia-hypercapnia) and nonventilatory (e.g., sneezing) behaviors in rats via four individual types of motor units. Motor force generated during muscle contraction appears to reflect the number and type of motor units recruited. These authors suggested that relative EMG measurement is the most appropriate means of expression, particularly if normalized to a maximal activity, such as sneezing. Similar suggestions concerning normalization to a maximal value were made by Fregosi and Mitchell (15). Although we expressed Dia EMG amplitude as a percent change from baseline, it reflects many studies of pLTF in anesthetized rats, where normalization to baseline is standard (63).
Specific respiratory muscles contributing to ventilatory LTF or diaphragm motor unit types that contribute to Dia LTF remain unknown. Thus there is a need to study LTF in multiple inspiratory and expiratory muscles, as well as motor unit types within a muscle group. Only then will we understand how neural mechanisms of LTF in individual motor pools contribute to the overall increase in breathing during ventilatory LTF.
Impact of Sleep-Wake States on LTF
The present results are consistent with our previous study on ventilatory LTF (44), demonstrating profound vigilance-state dependence with the greatest response during NREM sleep. Changes in sleep-wake architecture, such as time spent in sleep (vs. wakefulness) and the duration of NREM sleep episodes, were observed during AIH exposures. Although little is known concerning mechanisms whereby sleep-wake state affects LTF, accounting for changes in vigilance state is important in any study of LTF. Furthermore, since many studies have speculated that LTF may mitigate (or worsen) sleep apnea (31, 32, 34), detailed information concerning sleep-state dependence of LTF is essential in both respiratory pump muscles and muscles regulating upper airway resistance. Although our results show LTF during NREM sleep in a major respiratory pump muscle, more understanding of upper airway muscle LTF during sleep is necessary to appreciate the potential role of LTF in obstructive sleep apnea.
AIH disrupted sleep architecture via a decrease in the duration of NREM sleep (Figs. 3 and 4). Recent studies suggest that sleep architecture affects neural plasticity, including LTF (36). For example, sleep fragmentation for 24 h impairs ventilatory LTF via adenosine and A1 receptor-dependent mechanisms (36). Adenosine is a homeostatic sleep regulator that promotes the transition from wakefulness to sleep by inhibiting specific basal forebrain neurons (56). On the other hand, other studies have shown that adenosine A2A agonists elicit phrenic motor facilitation (19), whereas A2A antagonists potentiate AIH-induced pLTF (23). Therefore, the degree of Dia LTF in the present study may be influenced by changes in sleep-wake architecture via adenosinergic mechanisms. Although our results during REM sleep were not conclusive (see online Supplemental Material), LTF during REM is of considerable interest, since ventilatory instability is a frequent problem during REM sleep. Further investigation of LTF during REM in unanesthetized preparations is needed, particularly in an animal model with a greater portion time spent in REM sleep.
Finally, particular neurochemicals (e.g., serotonin, noradrenaline, and orexin) may contribute to vigilance-state differences in respiratory control (24, 27, 31). The activity of neurons containing these neurochemicals decreases in the progression from wakefulness to NREM to REM sleep. Thus AIH may stimulate hypothalamic neurons, brain stem neurons, and/or phrenic motor neurons to a greater relative extent during sleep vs. wakefulness. Collectively, we propose that altered sleep architecture during AIH may affect Dia and ventilatory LTF expression.
dAIH Preconditioning and AIH-Induced Dia LTF
dAIH preconditioning had no effect on resting diaphragm activity, the short-term hypoxic Dia response, sleep-wake architecture, or body temperature, 0–60 min post-AIH, in unanesthetized and poikilocapnic Sprague-Dawley rats. Contrary to expectations, 7 days of dAIH preconditioning did not enhance AIH-induced Dia LTF during sleep or wakefulness. We had anticipated that repetitive AIH would induce LTF metaplasticity, since our view has been that modest intermittent hypoxia protocols elicit plasticity without many of the adverse side effects attendant to severe protocols of CIH (10, 60, 62). However, several points should be considered when interpreting these results. First, lack of dAIH effects on Dia LTF could relate to changes in CO2 over the 8 days of exposure. Second, dAIH (i.e., 7 consecutive days, 10 episodes/day) may not be sufficient to induce metaplasticity of Dia LTF vs. other, more robust protocols of intermittent hypoxia (longer, more frequent exposures, more severe hypoxia, etc.). Consistent with this idea, dAIH enhances hypoglossal LTF, but not pLTF, in anesthetized Brown Norway rats, a strain that normally exhibits minimal LTF (62). On the other hand, CIH (7 consecutive days of 72 hypoxic episodes/day) enhances pLTF in anesthetized (28, 64) and ventilatory LTF in unanesthetized Sprague Dawley rats (37).
On the other hand, repetitive AIH may have greater impact in limited conditions, such as following spinal injury. For example, both dAIH (M. R. Lovett-Barr and G. S. Mitchell, unpublished observations) and CIH (17) strengthen spinal synaptic pathways to phrenic motoneurons in spinally injured rats, whereas CIH has no similar effect when applied to uninjured rats (17). Modified protocols of repetitive AIH and/or investigations in spinally injured rats may reveal metaplasticity in AIH-induced Dia LTF (10). Alternately, repetitive AIH preconditioning may have a greater impact on other respiratory motor pools/muscles (62).
Experimental Considerations
Several limitations should be considered for proper interpretation of our findings. First, Dia EMG amplitude may have differed among rats due to methodological limitations (e.g., inconsistency of diaphragm EMG lead implantation). Although this issue may have caused variability in absolute values of diaphragm amplitude (see Fig. 2B), Dia LTF was clearly evident in all rats following AIH when expressed as a percentage of baseline. Another limitation is the lack of information concerning arterial PCO2 and/or CO2 production; assessment of PaCO2 and/or CO2 production in each sleep-wake state is problematic due to rapid changes in sleep-wake state and other factors (see Fig. 1). Indeed, body temperature was decreased during and following AIH (see Fig. 5), presumably due to a combination of hypoxia-induced decreases in the temperature set point, hypometabolism, and exposure at an ambient temperature below the lower critical temperature of the thermal neutral zone in rats (14, 20, 51). Our previous studies showed that AIH-induced changes in CO2 production mask ventilatory LTF to some extent (44). Third, since data recordings in all experiments were made at the same time of day (light phase at 10:00 AM–1:35 PM; see Fig. 1), the potential impact of circadian rhythms could not be assessed; however, a recent study examined this question in humans (18). Fourth, we observed central (but not obstructive) apneas during sleep, both pre- and post-AIH. However, we cannot make statements concerning the impact of AIH on ventilatory stability, since the limited 1-h pre/post-treatment periods did not permit sufficient time in REM sleep, which is the state when central apneas are most likely to occur in rodents. Fifth, our protocol differed in several respects from our recent study of ventilatory LTF (44), possibly limiting comparisons between studies: 1) Sprague-Dawley (here) vs. Lewis rats (44); 2) 10 (here) vs. 5 (44) hypoxic episodes during AIH; 3) standard criteria of sleep-wake state determination in rodents (43, 52, 55, 59) (i.e., AW, QW, NREM, and REM sleep) were used here to minimize experimental bias, whereas a transition between deep-NREM sleep and QW was defined as light-NREM sleep in our previous report; and 4) although AIH was induced during deep NREM sleep in the previous study, we began automatically after 1 h of baseline (pretreatment) recordings in the present study, regardless of the prevailing sleep-wake state. Differences between these studies may cause slightly different LTF values during sleep and wakefulness, although the fundamental conclusion that ventilatory and Dia LTF share similar state dependence is robust regardless of these differences.
Conclusions
This study provides new and important information on LTF expression in diaphragm activity of unanesthetized, spontaneously breathing rats. The capacity for plasticity in spinal respiratory pump muscle activity during wakefulness and sleep suggests an important role for LTF in the neural control of breathing. An understanding of basic mechanisms giving rise to such plasticity may aid in the development of new therapeutic strategies in the treatment of sleep-disordered breathing or other conditions that cause respiratory insufficiency, such as motor neuron disease, spinal cord injuries, or the onset of chronic lung disease.
GRANTS
This work was supported by the National Institutes of Health (R01 HL-080209).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank G. Sieck and W. Z. Zhan for excellent technical advice concerning diaphragm EMG implantation; B. Wathen, S. Vinit, and I. Satriotomo for expert technical advice/support; and C. Guenther, E. Dale-Nagle, and P. MacFarlane for thoughtful critiques of the manuscript.
REFERENCES
- 1. Aboubakr SE, Taylor A, Ford R, Siddiqi S, Badr MS. Long-term facilitation in obstructive sleep apnea patients during NREM sleep. J Appl Physiol 91: 2751–2757, 2001. [DOI] [PubMed] [Google Scholar]
- 2. Babcock M, Shkoukani M, Aboubakr SE, Badr MS. Determinants of long-term facilitation in humans during NREM sleep. J Appl Physiol 94: 53–59, 2003. [DOI] [PubMed] [Google Scholar]
- 3. Babcock MA, Badr MS. Long-term facilitation of ventilation in humans during NREM sleep. Sleep 21: 709–716, 1998. [PubMed] [Google Scholar]
- 4. Baker-Herman TL, Bavis RW, Dahlberg JM, Mitchell AZ, Wilkerson JE, Golder FJ, Macfarlane PM, Watters JJ, Behan M, Mitchell GS. Differential expression of respiratory long-term facilitation among inbred rat strains. Respir Physiol Neurobiol 170: 260–267, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7: 48–55, 2004. [DOI] [PubMed] [Google Scholar]
- 6. Baker-Herman TL, Mitchell GS. Determinants of frequency long-term facilitation following acute intermittent hypoxia in vagotomized rats. Respir Physiol Neurobiol 162: 8–17, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22: 6239–6246, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cao KY, Zwillich CW, Berthon-Jones M, Sullivan CE. Increased normoxic ventilation induced by repetitive hypoxia in conscious dogs. J Appl Physiol 73: 2083–2088, 1992. [DOI] [PubMed] [Google Scholar]
- 9. Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol 669: 225–230, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dale-Nagle EA, Hoffman MS, MacFarlane PM, Satriotomo I, Lovett-Barr MR, Vinit S, Mitchell GS. Spinal plasticity following intermittent hypoxia: implications for spinal injury. Ann NY Acad Sci 1198: 252–259, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eldridge FL. Relationship between phrenic nerve activity and ventilation. Am J Physiol 221: 535–543, 1971. [DOI] [PubMed] [Google Scholar]
- 12. Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fletcher EC, Lesske J, Qian W, Miller CC, 3rd, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension 19: 555–561, 1992. [DOI] [PubMed] [Google Scholar]
- 14. Frappell P, Lanthier C, Baudinette RV, Mortola JP. Metabolism and ventilation in acute hypoxia: a comparative analysis in small mammalian species. Am J Physiol Regul Integr Comp Physiol 262: R1040–R1046, 1992. [DOI] [PubMed] [Google Scholar]
- 15. Fregosi RF, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J Physiol 477 (Pt 3): 469–479, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fuller DD, Baker TL, Behan M, Mitchell GS. Expression of hypoglossal long-term facilitation differs between substrains of Sprague-Dawley rat. Physiol Genomics 4: 175–181, 2001. [DOI] [PubMed] [Google Scholar]
- 17. Fuller DD, Johnson SM, Olson EB, Jr., Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci 23: 2993–3000, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gerst DG, 3rd, Yokhana SS, Carney LM, Lee DS, Badr MS, Qureshi T, Anthouard MN, Mateika JH. The hypoxic ventilatory response and ventilatory long term facilitation are altered by time of day and repeated daily exposure to intermittent hypoxia. J Appl Physiol 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci 28: 2033–2042, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gordon CJ, Fogelson L. Comparative effects of hypoxia on behavioral thermoregulation in rats, hamsters, and mice. Am J Physiol Regul Integr Comp Physiol 260: R120–R125, 1991. [DOI] [PubMed] [Google Scholar]
- 21. Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci 21: 2442–2450, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harris DP, Balasubramaniam A, Badr MS, Mateika JH. Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am J Physiol Regul Integr Comp Physiol 291: R1111–R1119, 2006. [DOI] [PubMed] [Google Scholar]
- 23. Hoffman MS, Golder FJ, Mahamed S, Mitchell GS. Spinal adenosine A2(A) receptor inhibition enhances phrenic long term facilitation following acute intermittent hypoxia. J Physiol 588: 255–266, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Horner RL. Neuromodulation of hypoglossal motoneurons during sleep. Respir Physiol Neurobiol 164: 179–196, 2008. [DOI] [PubMed] [Google Scholar]
- 25. Jordan AS, Catcheside PG, O'Donoghue FJ, McEvoy RD. Long-term facilitation of ventilation is not present during wakefulness in healthy men or women. J Appl Physiol 93: 2129–2136, 2002. [DOI] [PubMed] [Google Scholar]
- 26. Kline DD, Overholt JL, Prabhakar NR. Mutant mice deficient in NOS-1 exhibit attenuated long-term facilitation and short-term potentiation in breathing. J Physiol 539: 309–315, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuwaki T. Orexinergic modulation of breathing across vigilance states. Respir Physiol Neurobiol 164: 204–212, 2008. [DOI] [PubMed] [Google Scholar]
- 28. Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr., Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci 21: 5381–5388, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lovett-Barr MR, Mitchell GS, Satriotomo I, Johnson SM. Serotonin-induced in vitro long-term facilitation exhibits differential pattern sensitivity in cervical and thoracic inspiratory motor output. Neuroscience 142: 885–892, 2006. [DOI] [PubMed] [Google Scholar]
- 30. MacFarlane PM, Mitchell GS. Respiratory long-term facilitation following intermittent hypoxia requires reactive oxygen species formation. Neuroscience 152: 189–197, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol 92: 27–37, 2007. [DOI] [PubMed] [Google Scholar]
- 32. Mahamed S, Mitchell GS. Respiratory long-term facilitation: too much or too little of a good thing? Adv Exp Med Biol 605: 224–227, 2008. [DOI] [PubMed] [Google Scholar]
- 33. Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol 173: 101–106, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mateika JH, Narwani G. Intermittent hypoxia and respiratory plasticity in humans and other animals: does exposure to intermittent hypoxia promote or mitigate sleep apnoea? Exp Physiol 94: 279–296, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McEvoy RD, Popovic RM, Saunders NA, White DP. Effects of sustained and repetitive isocapnic hypoxia on ventilation and genioglossal and diaphragmatic EMGs. J Appl Physiol 81: 866–875, 1996. [DOI] [PubMed] [Google Scholar]
- 36. McGuire M, Tartar JL, Cao Y, McCarley RW, White DP, Strecker RE, Ling L. Sleep fragmentation impairs ventilatory long-term facilitation via adenosine A1 receptors. J Physiol 586: 5215–5229, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McGuire M, Zhang Y, White DP, Ling L. Chronic intermittent hypoxia enhances ventilatory long-term facilitation in awake rats. J Appl Physiol 95: 1499–1508, 2003. [DOI] [PubMed] [Google Scholar]
- 38. McGuire M, Zhang Y, White DP, Ling L. Effect of hypoxic episode number and severity on ventilatory long-term facilitation in awake rats. J Appl Physiol 93: 2155–2161, 2002. [DOI] [PubMed] [Google Scholar]
- 39. Mitchell GS. Respiratory plasticity following intermittent hypoxia: guide for novel therapeutic approaches to ventilatory control disorders. In Genetic Basis for Respiratory Control Disorders, edited by Gaultier C. New York: Springer, 2007. [Google Scholar]
- 40. Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol 90: 2466–2475, 2001. [DOI] [PubMed] [Google Scholar]
- 41. Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol 94: 358–374, 2003. [DOI] [PubMed] [Google Scholar]
- 42. Mitchell GS, Powell FL, Hopkins SR, Milsom WK. Time domains of the hypoxic ventilatory response in awake ducks: episodic and continuous hypoxia. Respir Physiol 124: 117–128, 2001. [DOI] [PubMed] [Google Scholar]
- 43. Nakamura A, Fukuda Y, Kuwaki T. Sleep apnea and effect of chemostimulation on breathing instability in mice. J Appl Physiol 94: 525–532, 2003. [DOI] [PubMed] [Google Scholar]
- 44. Nakamura A, Olson EB, Jr., Terada J, Wenninger JM, Bisgard GE, Mitchell GS. Sleep state dependence of ventilatory long-term facilitation following acute intermittent hypoxia in Lewis rats. J Appl Physiol 109: 323–331, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Neverova NV, Saywell SA, Nashold LJ, Mitchell GS, Feldman JL. Episodic stimulation of α1-adrenoreceptors induces protein kinase C-dependent persistent changes in motoneuronal excitability. J Neurosci 27: 4435–4442, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Olson EB, Jr., Bohne CJ, Dwinell MR, Podolsky A, Vidruk EH, Fuller DD, Powell FL, Mitchel GS. Ventilatory long-term facilitation in unanesthetized rats. J Appl Physiol 91: 709–716, 2001. [DOI] [PubMed] [Google Scholar]
- 47. Pierchala LA, Mohammed AS, Grullon K, Mateika JH, Badr MS. Ventilatory long-term facilitation in nonsnoring subjects during NREM sleep. Respir Physiol Neurobiol 160: 259–266, 2008. [DOI] [PubMed] [Google Scholar]
- 48. Polotsky VY, Rubin AE, Balbir A, Dean T, Smith PL, Schwartz AR, O'Donnell CP. Intermittent hypoxia causes REM sleep deficits and decreases EEG delta power in NREM sleep in the C57BL/6J mouse. Sleep Med 7: 7–16, 2006. [DOI] [PubMed] [Google Scholar]
- 49. Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134, 1998. [DOI] [PubMed] [Google Scholar]
- 50. Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med 167: 1548–1553, 2003. [DOI] [PubMed] [Google Scholar]
- 51. Saiki C, Matsuoka T, Mortola JP. Metabolic-ventilatory interaction in conscious rats: effect of hypoxia and ambient temperature. J Appl Physiol 76: 1594–1599, 1994. [DOI] [PubMed] [Google Scholar]
- 52. Sood S, Morrison JL, Liu H, Horner RL. Role of endogenous serotonin in modulating genioglossus muscle activity in awake and sleeping rats. Am J Respir Crit Care Med 172: 1338–1347, 2005. [DOI] [PubMed] [Google Scholar]
- 53. Tartar JL, Ward CP, McKenna JT, Thakkar M, Arrigoni E, McCarley RW, Brown RE, Strecker RE. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci 23: 2739–2748, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thorac Soc 5: 207–217, 2008. [DOI] [PubMed] [Google Scholar]
- 55. Terada J, Nakamura A, Zhang W, Yanagisawa M, Kuriyama T, Fukuda Y, Kuwaki T. Ventilatory long-term facilitation in mice can be observed during both sleep and wake periods and depends on orexin. J Appl Physiol 104: 499–507, 2008. [DOI] [PubMed] [Google Scholar]
- 56. Thakkar MM, Winston S, McCarley RW. A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic basal forebrain. J Neurosci 23: 4278–4287, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Turner DL, Mitchell GS. Long-term facilitation of ventilation following repeated hypoxic episodes in awake goats. J Physiol 499 (Pt 2): 543–550, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 27: 194–201, 2004. [DOI] [PubMed] [Google Scholar]
- 59. Vecchio LM, Grace KP, Liu H, Harding S, Le AD, Horner RL. State-dependent vs. central motor effects of ethanol on breathing. J Appl Physiol 108: 387–400, 2010. [DOI] [PubMed] [Google Scholar]
- 60. Vinit S, Lovett-Barr MR, Mitchell GS. Intermittent hypoxia induces functional recovery following cervical spinal injury. Respir Physiol Neurobiol 169: 210–217, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wenninger JM, Olson EB, Wang Z, Keith IM, Mitchell GS, Bisgard GE. Carotid sinus nerve responses and ventilatory acclimatization to hypoxia in adult rats following 2 wk of postnatal hyperoxia. Respir Physiol Neurobiol 150: 155–164, 2006. [DOI] [PubMed] [Google Scholar]
- 62. Wilkerson JE, Mitchell GS. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp Neurol 217: 116–123, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zabka AG, Behan M, Mitchell GS. Selected contribution: Time-dependent hypoxic respiratory responses in female rats are influenced by age and by the estrus cycle. J Appl Physiol 91: 2831–2838, 2001. [DOI] [PubMed] [Google Scholar]
- 64. Zabka AG, Mitchell GS, Olson EB, Jr., Behan M. Selected contribution: chronic intermittent hypoxia enhances respiratory long-term facilitation in geriatric female rats. J Appl Physiol 95: 2614–2623; discussion 2604, 2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.