Abstract
We hypothesized that nitric oxide activation of soluble guanylyl cyclase (sGC) participates in cutaneous vasodilation during whole body heat stress and local skin warming. We examined the effects of the sGC inhibitor, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), on reflex skin blood flow responses to whole body heat stress and on nonreflex responses to increased local skin temperature. Blood flow was monitored by laser-Doppler flowmetry, and blood pressure by Finapres to calculate cutaneous vascular conductance (CVC). Intradermal microdialysis was used to treat one site with 1 mM ODQ in 2% DMSO and Ringer, a second site with 2% DMSO in Ringer, and a third site received Ringer. In protocol 1, after a period of normothermia, whole body heat stress was induced. In protocol 2, local heating units warmed local skin temperature from 34 to 41°C to cause local vasodilation. In protocol 1, in normothermia, CVC did not differ among sites [ODQ, 15 ± 3% maximum CVC (CVCmax); DMSO, 14 ± 3% CVCmax; Ringer, 17 ± 6% CVCmax; P > 0.05]. During heat stress, ODQ attenuated CVC increases (ODQ, 54 ± 4% CVCmax; DMSO, 64 ± 4% CVCmax; Ringer, 63 ± 4% CVCmax; P < 0.05, ODQ vs. DMSO or Ringer). In protocol 2, at 34°C local temperature, CVC did not differ among sites (ODQ, 17 ± 2% CVCmax; DMSO, 18 ± 4% CVCmax; Ringer, 18 ± 3% CVCmax; P > 0.05). ODQ attenuated CVC increases at 41°C local temperature (ODQ, 54 ± 5% CVCmax; DMSO, 86 ± 4% CVCmax; Ringer, 90 ± 2% CVCmax; P < 0.05 ODQ vs. DMSO or Ringer). sGC participates in neurogenic active vasodilation during heat stress and in the local response to direct skin warming.
Keywords: nitric oxide; guanosine 3′,5′-cyclic monophosphate; microdialysis; skin; thermoregulation
in humans, the cutaneous circulation is a major effector of human thermoregulatory reflexes. During cold stress, reduced internal and skin temperatures (Tsk) lead to cutaneous vasoconstriction mediated by a sympathetic noradrenergic cotransmitter system, whereas, during heat stress, increases in these temperatures lead to reflex cutaneous vasodilation mediated largely by a neurogenic cholinergic cotransmitter system (21). Under normothermic conditions, skin blood flow (SkBF) averages ∼5% of cardiac output; however, the absolute flow of blood to the skin can vary from nearly zero during periods of cold stress to as much 8 l/min or 60% of cardiac output distributed over the body surface during maximal vasodilation in whole body heat stress (41).
The cutaneous active vasodilator system is responsible for 80–95% of the elevation in SkBF that accompanies heat stress (19, 42). Cutaneous active vasodilation, per se, is mediated by increased activity of sympathetic cholinergic nerves that release acetylcholine and one or more cotransmitters (6, 25). In addition to these “classical” neurotransmitters, nitric oxide (NO) production by NO synthase (NOS) is involved in the mechanism of cutaneous active vasodilation (22, 48). Approximately 30–45% of the increase in SkBF mediated by active vasodilation during heat stress is dependent on NO generation by NOS (22, 27, 47, 48, 57).
In addition to active vasodilation induced by whole body heating, local vasodilatory mechanisms respond to direct application of heat to skin, i.e., local warming can increase SkBF (21). The vasodilator response to local skin warming is biphasic, with sensory nerves mediating an initial transient vasodilatory “peak”, followed by a prolonged vasodilatory “plateau” that is mediated primarily by NOS generation of NO (24, 37) and has the potential of causing maximal vasodilation (53, 54).
While much work has addressed the role of NOS and NO generation in the control of SkBF, little work has examined how the NO generated mediates its vasodilator effects subsequent to its generation. Classically, the effects of NO are viewed as being dependent on activation of soluble guanylyl cyclase (sGC), although sGC-independent pathways for NO actions are extant (5, 33). NO-mediated activation of sGC is accomplished through the binding of the diatomic gas to the heme of sGC. Subsequent to activation, sGC effects the dephosphorylation of guanosine 5′-triphosphate to yield increased levels of guanosine 3′,5′-monophosphate (cGMP) at a rate of up to 400-fold that of basal enzyme activity (11, 17). Increases in cGMP lead to activation of specific protein kinases, in particular cGMP-dependent protein kinase (protein kinase G), which cause decreases in intracellular Ca2+ levels and Ca2+ sensitivity and thereby mediate vascular relaxation (7, 17, 40).
While the physiological significance of the sGC/cGMP pathway for NO is well established, there is increasing evidence that NO can affect additional physiological signaling pathways by sGC/cGMP-independent mechanisms (14). sGC-independent pathways have been found in a number of blood vessel types in several species, including humans. Such pathways can be demonstrated by incomplete antagonism of vasodilation by a selective sGC antagonist at doses that abolish NO-induced activation of sGC and cGMP production (56).
Whether the classical sGC-dependent mechanism is involved in transducing the effects of NO into increases in SkBF during cutaneous active vasodilation or local skin warming, or whether sGC/cGMP-independent mechanisms in either process are involved is unknown. We sought to test the hypothesis that NO mediates its vascular effects in cutaneous active vasodilation and local skin warming through activation of sGC-dependent mechanisms in humans. We tested that hypothesis by examining the effects of sGC inhibition on the cutaneous vasodilation evoked by whole body heat stress and on that induced by local skin warming.
MATERIALS
Antagonism of sGC activation by NO was achieved by administration of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), a potent and selective sGC antagonist that has been used to discriminate between cGMP-dependent and cGMP-independent effects of NO (11, 62). The agent competitively binds to the heme iron of sGC and inhibits NO-sensitive stimulation of cGMP production without altering basal sGC activity (31, 62). ODQ does not inhibit membrane-bound (particulate) guanylyl cyclases (62). Methylene blue and LY-83583 are also purported to be sGC antagonists; however, both of these agents additionally inhibit NO generation by NOS and enhance production of superoxide anion that can inactivate NO (1). These problematic aspects make methylene blue and LY-83583 unacceptable to define sGC-dependent and independent mechanisms and have led to the use of ODQ as the pharmacological agent of choice in defining such mechanisms (56).
Drug delivery was achieved by intradermal microdialysis to permit local administration of ODQ directly into the interstitial space of a small area of skin. This approach permitted monitoring of SkBF from untreated control areas and areas of high local drug concentration without potentially confounding systemic effects. SkBF was monitored by laser-Doppler flowmetry (LDF; MoorLab, Moor Instruments, Devon, UK) from the same small volume of skin (∼1 mm3) over the microdialysis probes. LDF measurements are specific to skin, being uninfluenced by blood flow in the underlying skeletal muscle tissue (44).
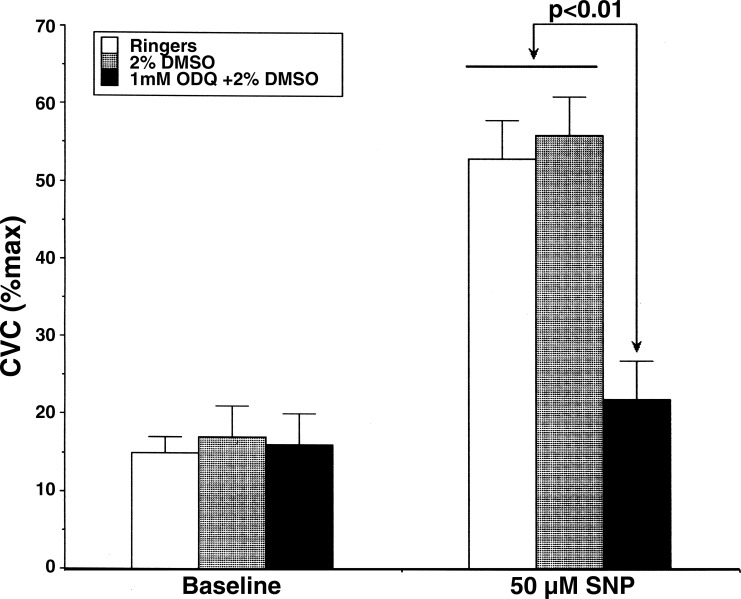
A series of preliminary experiments was performed to determine the appropriate ODQ concentration to inhibit sGC maximally. In these studies, we used intradermal microdialysis and tested the ability of varying concentrations of ODQ to antagonize the vasodilatory effect of 50 μM sodium nitroprusside (SNP), an NO donor and activator of sGC (16, 55). This concentration of SNP was found to increase cutaneous vascular conductance (CVC) to approximately one-half of maximal levels, and a concentration of 1 mM ODQ attenuated this response significantly (see Fig. 1).
Fig. 1.
Attenuation of sodium nitroprusside (SNP) induced vasodilation by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ). SNP (50 μM) increased cutaneous vascular conductance (CVC) to 53 ± 5% maximum CVC at microdialysis sites perfused with Ringer solution and to 56 ± 5% maximum CVC at sites perfused with 2% DMSO (P < 0.01 vs. baseline sites). The addition of 1 mM ODQ dissolved in 2% DMSO solution attenuated this vasodilation to 22 ± 5% maximum CVC. Values are means ± SE. P < 0.01, ODQ vs. Ringer or 2% DMSO with SNP. P > 0.05 vs. Ringer or 2% DMSO baseline sites.
Because LDF measurements provide a relative index of SkBF, these measurements are often normalized to basal or maximal CVC to facilitate comparisons between or among treatment sites (20, 23). For microdialysis studies, perfusion with high concentrations of SNP is often used to achieve maximal vasodilation for data normalization (22, 37, 51). Because ODQ is a competitive antagonist of sGC, which is involved in effecting vasodilation by SNP, we verified that 58 mM SNP would overcome competitive antagonism of 1 mM ODQ. Preliminary experiments showed that perfusion with 1 mM ODQ did not attenuate the vasodilation caused by 58 mM SNP compared with sites that received no ODQ (see Table 1). This was further verified by our finding that local heating of the skin to 42°C, with or without reactive hyperemia [an NO-independent process in skin (35, 59, 61)] induced by 10 min of arm blood flow occlusion, added no further vasodilation to that achieved by 58 mM SNP at ODQ-treated sites. Based on these findings, we chose to normalize our CVC measurements to the maximal values achieved by perfusion of microdialysis probes with 58 mM SNP.
Table 1.
Absolute values of baseline and maximal cutaneous vascular conductance in arbitrary units
| Ringer | 2% DMSO | 1 mM ODQ | |
|---|---|---|---|
| Baseline CVC | 5.5 ± 0.9 | 5.3 ± 0.6 | 5.4 ± 0.8 |
| Maximum CVC (58 mM SNP) | 44.1 ± 7.1 | 45.2 ± 5.9 | 46.4 ± 6.2 |
Values are means ± SE in arbitrary units. ODQ, 1H-{1,2,4}oxadiazolo{4,3-a}quinoxalin-1-one; CVC, cutaneous vascular conductance; SNP, sodium nitroprusside.
ODQ has limited aqueous solubility; therefore, the use of DMSO was required to increase the ODQ concentration achievable in Ringer solution. This approach has been used before in similar studies with other agents of poor aqueous solubility (29, 46). A concentration of 1 mM ODQ could be achieved in a solution of 2% DMSO in Ringer. Greater ODQ concentrations could only be achieved with higher concentrations of DMSO (2.5 mM ODQ required 10% DMSO in Ringer), but provided no greater attenuation of vasodilatory responses to SNP. Based on the foregoing findings and to avoid any unanticipated effects of DMSO, we chose to use 1 mM ODQ in 2% DMSO in Ringer for our subsequent studies, because this concentration maximally inhibited the sGC-mediated vasodilation induced by 50 μM SNP, yet required a minimal concentration of DMSO. In the study protocols, one microdialysis site was perfused with 2% DMSO in Ringer solution to serve as a control for any effects of this agent (46).
Microdialysis probes were made in our laboratory from polyimide tubing and a 1-cm length of capillary microdialysis membrane (200-μm diameter, molecular cutoff 20 kDa) reinforced by a 51-μm-diameter coated stainless steel wire placed in the lumen of the membrane and tubing. On arrival in the laboratory, each subject had three probes placed at separate sites on the ventral aspect of one forearm as follows. Ice was applied to the chosen skin site to achieve temporary local anesthesia (15). A 25-gauge needle was inserted through the dermis using sterile technique, with entry and exit points 2.5 to 3 cm apart. The microdialysis probe was then threaded through the internal lumen of the needle, the needle was withdrawn, and the probe left in place. The microdialysis membrane remained entirely within the dermis connected from the skin to the perfusion pump by the polyimide tubing. After probe insertion and before additional instrumentation, subjects waited for at least 2 h to permit resolution of insertion trauma (3).
All subjects who volunteered for the protocols were in good health, were nonsmokers, and were taking no medications. Before participation, written, informed consent was obtained from all subjects. The studies conformed to the standards set by the Declaration of Helsinki, and institutional ethics committees approved all procedures. Subjects were instructed to forego caffeinated products on the day of the study. The menstrual phase of the female subjects was not assessed. All studies were performed in an air-conditioned laboratory with a constant ambient temperature of 24°C. Studies were done at the same time of day to control for any circadian variations in responses (4). Two subjects participated in both protocols.
Protocol 1: whole body heat stress.
Eight healthy subjects (3 men and 5 women) participated in this part of the study. The subjects' average age (±SE) was 39 ± 3 yr, average weight was 61 ± 4 kg, and average height was 164 ± 2 cm.
To induce thermoregulatory reflexes, subjects wore a tube-lined suit. The suit was used to control Tsk by perfusion with water of different temperatures to produce periods of normothermia, cold stress, and heat stress (43). The suit was perfused with cold water to decrease Tsk and induce cold stress and warm water to raise Tsk to 38–39°C during heating periods. Over the suit, subjects wore a water-impermeable plastic garment to insulate them from the environment and prevent sweat evaporation. The suit and garment covered the entire body, with the exception of the head, hands, feet, and the forearm, from which blood flow measurements were made.
Internal temperature was monitored with a thermocouple held in the sublingual sulcus [oral temperature (Tor)]. Tsk was recorded as the weighted electrical average from six thermocouples taped on the skin surface (18, 52). Pulse rate (PR) and mean arterial pressure (MAP) were recorded continuously from a finger (Finapres BP Monitor, Ohmeda, Madison, WI) (39).
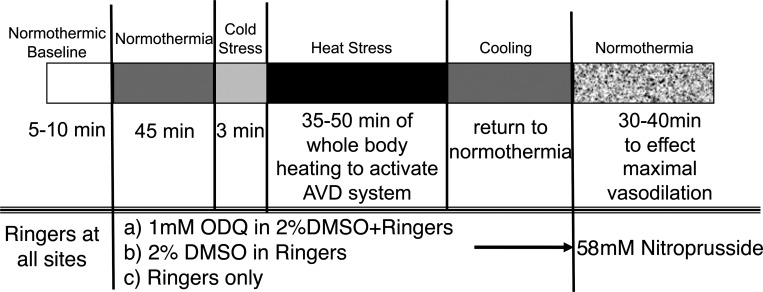
For the study, subjects were placed in the supine position and instrumented. Tsk was maintained at 34°C. Data collection began 2 h following microdialysis probe placement with a 5- to 10-min baseline control period during which the microdialysis probes were perfused with Ringer solution at a rate of 2 μl/min using a micro-infusion pump. After this control period, the perfusate at one site was changed to 2% DMSO in Ringer and to 1 mM ODQ in the 2% DMSO-Ringer solution at another site. Perfusion was maintained with Ringer only at the third site. Perfusion was maintained with these solutions for 45 min under normothermic conditions to allow ODQ to reach steady state in the intradermal space. Following this normothermic period, Tsk was decreased to induce cold stress for 3 min. Tsk was then raised to 38–39°C and maintained at that level for 35–50 min to induce heat stress and thereby stimulate the active vasodilator system. Body heating was maintained until LDF values at all sites had achieved stable elevated plateaus. Subjects were then cooled and returned to a normothermic Tsk of 34°C. All microdialysis sites were then perfused with 58 mM SNP to effect maximal vasodilation at each site. CVC values were normalized to these maximal levels for data analysis. The protocol is illustrated in Fig. 2.
Fig. 2.
Whole body heat stress protocol. This protocol was designed to examine the effects of antagonism of soluble guanylyl cyclase by ODQ on the reflex vasodilation induced by whole body heat stress. Three intradermal microdialysis sites were perfused with either 1 mM ODQ in 2% DMSO, 2% DMSO, or Ringer solution alone. Maximal cutaneous vasodilation was achieved at all sites at the end of the study by perfusion with 58 mM SNP. The perfusion rate at all microdialysis sites was 2 μl/min. AVD, active vasodilator.
Data are presented as means ± SE. For data analysis, CVC values were indexed as LDF/MAP and were normalized to their respective maxima, as elicited by 58 mM SNP to facilitate comparisons among sites, both within and among subjects, after verifying that there were no statistically significant differences among the absolute values achieved with SNP perfusion (P > 0.05 among sites). The vasomotor responses at the different microdialysis sites were analyzed by comparing the Tor thresholds at which CVC increases started during whole body heating. The internal temperature threshold for the onset of vasodilation for each site was defined as the level of Tor at which a sustained increase in CVC began during whole body heating and were chosen from graphs of CVC vs. Tor by an investigator blinded as to the conditions, subjects, and antagonist treatment. The thresholds for cutaneous vasodilation were compared by ANOVA for repeated measures. Normothermic baseline CVC values, CVC levels during the final minute of drug infusion in normothermia, CVC during the final minute of cold stress, and CVC from the final 3 min of heat stress were also compared among sites by ANOVA for repeated measures, followed by specific means comparisons. MAP and PR changes from normothermia to the end of heat stress were compared by paired t-tests. The level of statistical significance was defined as P < 0.05.
Protocol 2: local skin warming.
Seven healthy subjects (3 men and 4 women) participated in this part of the study. The subjects' average age (±SE) was 33 ± 6 yr, average weight 61 ± 3 kg, and average height 161 ± 3 cm.
Subjects were placed in the supine position and instrumented to measure LDF at all microdialysis sites. Each LDF probe was equipped with a special holder that incorporated both heating elements and thermocouples to permit simultaneous LDF measurements and control of local skin temperature (Tloc) (24). A Finapres device was used for continuous monitoring of PR and MAP.
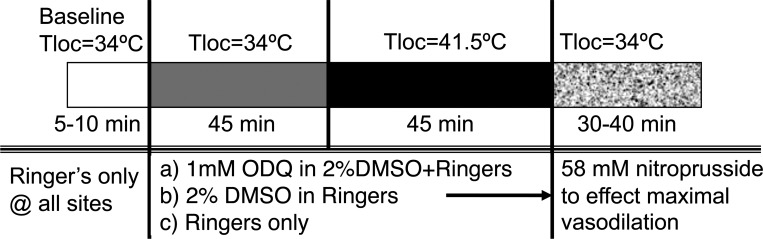
Data collection began with a 5- to 10-min control period with Tloc maintained at 34°C. Subsequently, the perfusate of one microdialysis site was maintained with Ringer solution, whereas the perfusate at a second microdialysis site was changed to 1 mM ODQ in 2% DMSO/Ringer solution. The perfusate at a third microdialysis site was changed to 2% DMSO in Ringer solution. Perfusion rate at all sites was 2 μl/min.
Tloc was maintained at 34°C for 45 min, after which Tloc was increased slowly over a period of 20 min to 41°C at all sites to evoke vasodilation. A slow increase in temperature to 41°C was chosen to avoid pain fiber activation, which evokes skin vasodilation by NO-independent mechanisms (24). Finally, the perfusates at all sites were changed to 58 mM SNP for data normalization (26, 32). The protocol is illustrated in Fig. 3.
Fig. 3.
Local skin warming protocol. This protocol was designed to examine the effects of antagonism of soluble guanylyl cyclase by ODQ on the vasodilation induced by local warming of the skin. Three intradermal microdialysis sites were perfused with 1 mM ODQ in 2% DMSO, 2% DMSO, or Ringer solution. Maximal cutaneous vasodilation was achieved at all sites at the end of the study by perfusion with 58 mM SNP. The perfusion rate at all microdialysis sites was 2 μl/min. Tloc, local skin temperature.
Data are presented as means ± SE. For data analysis, CVC was calculated (CVC = LDF/MAP) and normalized to maximum for each site (22). Normalized CVC responses were analyzed by comparing the mean levels achieved during initial peaks (when observed) and the final 3 min of the two thermal periods by repeated-measures ANOVA, with the level of statistical significance defined as 0.05.
RESULTS
Protocol 1: whole body heat stress.
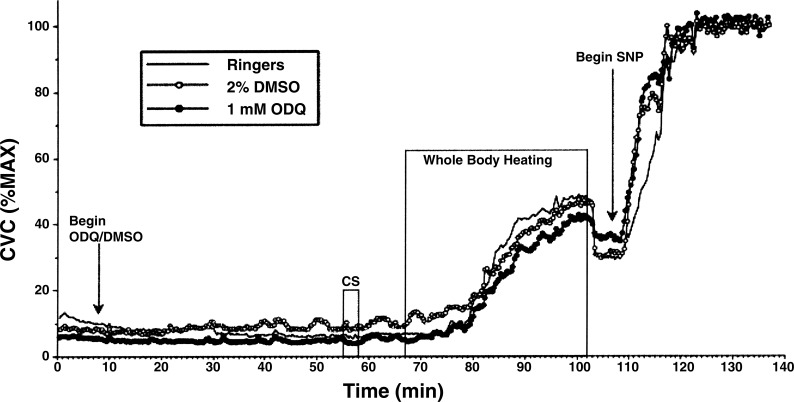
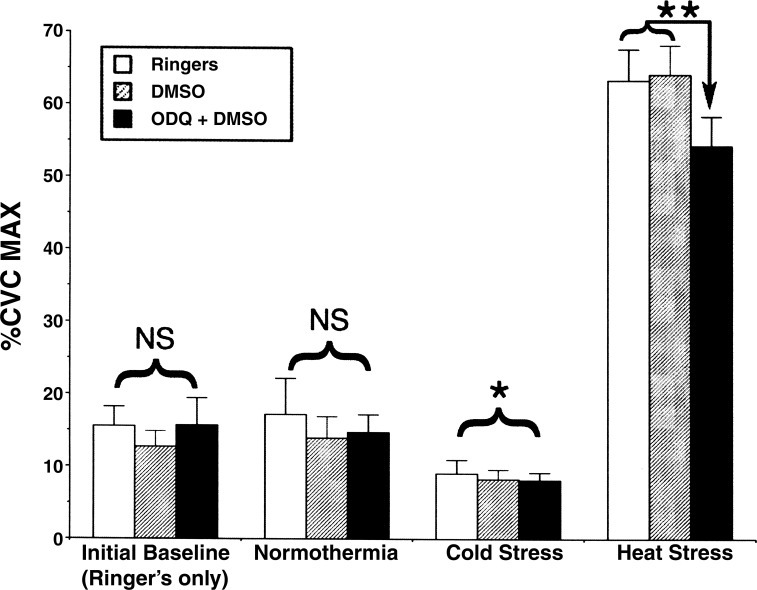
A representative result from one subject is illustrated in Fig. 4. CVC responses at each thermal condition of protocol 1 are summarized in Fig. 5. Under normothermic conditions, during perfusion of all microdialysis sites with Ringer solution only, there were no significant differences for the entire group among CVC values at the different sites (P > 0.05). CVC values in normothermia were unaltered when the perfusate at one site was changed to 1 mM ODQ in 2% DMSO and to 2% DMSO at a second site (P > 0.05 among sites). In response to cold stress, CVC fell at all sites (P < 0.05 vs. normothermia). These responses did not differ among treatment sites (P > 0.05 among sites).
Fig. 4.
CVC responses to the whole body heating protocol in one subject. Perfusion with 1 mM ODQ and 2% DMSO began 8 min into the study. Periods of whole body cooling [cold stress (CS)] and whole body heating are indicated. Perfusion with 58 mM SNP began at 108 min.
Fig. 5.
Summary of CVC responses to whole body heat stress. Values are means ± SE. Under normothermic conditions during perfusion of all sites with Ringer solution, CVC values, normalized to their respective maxima, did not differ significantly among sites (P > 0.05). Also during normothermia, perfusion of one site with 2% DMSO and another with 1 mM ODQ did not produce any significant differences among sites (P > 0.05). In response to CS, CVC fell at all sites compared with normothermia (*P < 0.05), but responses did not differ among sites (P > 0.05). During heat stress, CVC increased at all sites compared with normothermia (P < 0.05). In heat stress, CVC increased to similar levels at sites that received Ringer only and 2% DMSO (P > 0.05), but was significantly attenuated at sites that received 1 mM ODQ (**P < 0.05, ODQ vs. Ringer or 2% DMSO). NS, nonsignificant.
At the peak of heat stress, CVC at sites perfused with Ringer reached 63 ± 4% maximum and 64 ± 4% maximum at sites that received 2% DMSO. These values did not significantly differ (P > 0.05). CVC at sites perfused with ODQ reached 54 ± 4% maximum, a value that was significantly less than those at the other two sites (P < 0.05).
During whole body heating, CVC at the sites perfused with Ringer began to rise when Tor reached values of 36.9 ± 0.1°C, at 36.9 ± 0.1°C for sites perfused with ODQ, and at 36.9 ± 0.1°C for sites perfused with 2% DMSO. There was no statistical difference among these Tor threshold values (P > 0.05).
Under normothermic conditions, MAP averaged 85 ± 3 mmHg and fell to 78 ± 4 mmHg at the peak of heat stress (P < 0.05, see Fig. 5). PR averaged 60 ± 2 beats/min in normothermia and increased to 93 ± 3 beats/min at the peak of heat stress (P > 0.01). Tor averaged 36.9 ± 0.1°C in normothermia and increased to 37.6 ± 0.1°C at the end of heat stress (P < 0.01).
Protocol 2: local skin warming.
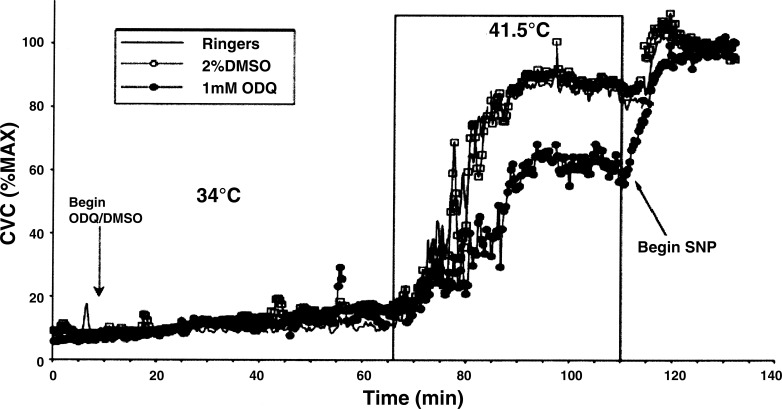
A representative result from one subject is illustrated in Fig. 6. During perfusion with Ringer only, there were no statistical differences in CVC among sites (P > 0.05). At Tloc = 34°C, CVC averaged 18 ± 3% maximum at untreated sites, 18 ± 3% maximum at sites that received 2% DMSO, and 14 ± 2% maximum at sites that were treated with 1 mM ODQ. These CVC values did not differ significantly among sites (P > 0.05 among sites).
Fig. 6.
CVC responses to the local skin warming protocol in one subject. In the first part of the protocol, Tloc was controlled at 34°C. Perfusion with 1 mM ODQ and 2% DMSO began 9 min into the study. At 66 min into the study, Tloc was increased to 41.5°C. Perfusion with 58 mM SNP began at 111 min.
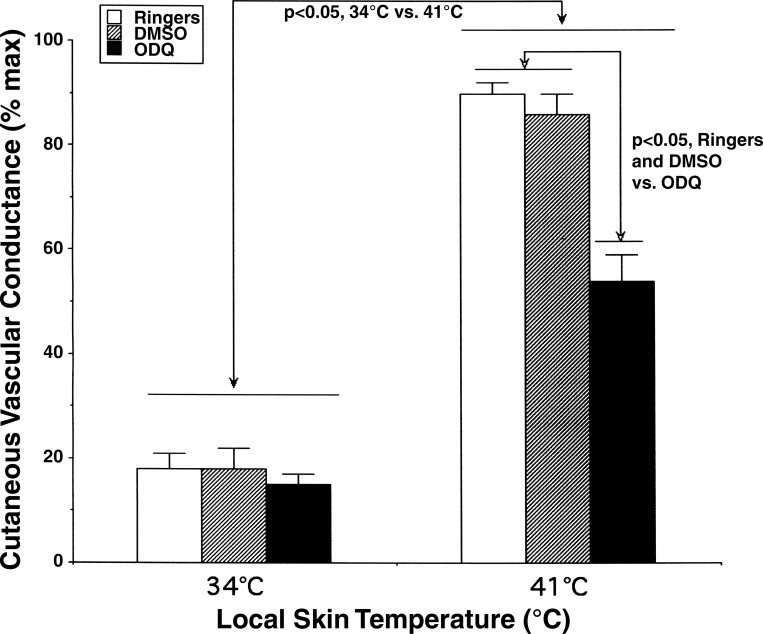
When Tloc was slowly increased to 41°C, small initial peaks were observed in four of seven subjects and did not differ among sites (P > 0.05). Thereafter, CVC at all sites increased significantly to stable plateau levels (P < 0.05 vs. 34°C). These CVC values were 90 ± 2% maximum at untreated sites, 86 ± 4% maximum at 2% DMSO sites, and 54 ± 5% maximum at sites that were treated with 1 mM ODQ. The CVC values at sites treated with ODQ were attenuated compared with those attained at untreated and 2% DMSO sites (P < 0.05, ODQ vs. untreated or DMSO). CVC increases at untreated and DMSO-treated sites did not differ (P > 0.05 between sites). These results are summarized in Fig. 7.
Fig. 7.
Summary of CVC responses to local skin warming. Values are means ± SE. At Tloc = 34°C during perfusion with Ringer, 2% DMSO, and 1 mM ODQ, there were no statistical differences among sites (P > 0.05). Increasing Tloc from 34 to 41°C increased CVC significantly at all sites (P < 0.05, 34 vs. 41°C). CVC values were significantly less at sites treated with 1 mM ODQ (54 ± 5% maximum) than at either Ringer (90 ± 2% maximum) or 2% DMSO (86 ± 4% maximum) sites when Tloc = 41°C (P < 0.05, ODQ vs. Ringer or DMSO).
DISCUSSION
The important new findings of our study are that the specific sGC antagonist ODQ attenuated the increases in CVC induced by the cutaneous active vasodilator system during whole body heat stress and the nonreflex cutaneous vasodilator response to local skin warming compared with responses at sites perfused with Ringer only or perfused with 2% DMSO. Responses at sites perfused with 2% DMSO did not differ from those perfused with Ringer only, showing that control responses were not due to DMSO. Our findings show that the classical pathway of heme-dependent activation of sGC by NO effects at least part of the vasodilator responses to both whole body heat stress and to local skin warming.
While the above result was not unexpected, the relatively small degree to which ODQ attenuated cutaneous active vasodilation relative to the vasodilation induced by local warming was unanticipated. Based on studies with NOS antagonists, it is generally accepted that ∼30–45% of the increase in SkBF mediated by active vasodilation during heat stress is dependent on NO generation by NOS (22, 27, 47, 48, 57). The remainder of the overall active vasodilator response is presumably through vasoactive intestinal peptide or other cotransmitters acting through pathways independent of NO (6, 34, 47, 58, 60). If NO acted to effect cutaneous active vasodilation solely through the NO-sGC-cGMP pathway, a similar amount should be due to activation of sGC. Based on the present findings with sGC antagonism, only ∼15% of the increase in SkBF during heat stress was attributable to sGC activation and suggests the novel conclusion that a significant portion of the heat stress-induced active cutaneous vasodilation effected by NO is not mediated by sGC-dependent mechanisms, but rather by sGC-independent mechanisms.
During the initiation of responses to heat stress, we found that the Tor threshold for the initiation of active vasodilation was not altered by sGC antagonism. If NO activation of sGC were a determinant of the threshold for vasodilation, the threshold would have been altered by ODQ, but this was not the case. This lack of effect is consistent with the lack of effect reported with many (10, 22, 29, 49), but not all (60), NOS antagonist-based studies. Our observation that sGC antagonism does not alter the Tor threshold for vasodilation reinforces the view that the threshold for initiation of active vasodilation is not dependent on the generation of NO by the NOS system, but instead is more likely dependent on the vesicular release of classical neurotransmitters (28, 29).
In contrast to the relatively small attenuation of cutaneous active vasodilation during whole body heat stress, antagonism of sGC by 1 mM ODQ caused a much greater attenuation of the vasodilation induced by local warming. Compared with the responses at sites perfused with 2% DMSO, 1 mM ODQ attenuated the local warming vasodilation by 37 ± 6%, in contrast to the 15 ± 5% attenuation during whole body heat stress. This suggests a greater role for the NO-sGC-cGMP pathway in the vasodilator response to local heating. These results are consistent with studies with NOS antagonists that show ∼50% of the increase in SkBF during local skin warming to be dependent on NOS generation of NO (28, 30, 37, 50).
ODQ, the antagonist used in our study, is highly selective for NO-sensitive sGC, which it inhibits by oxidizing the ferrous form of the enzyme's heme to the ferric form that has poor NO sensitivity, thus antagonizing heme-dependent activation of sGC by NO (12, 45). ODQ was chosen because of its higher specificity for sGC relative to methylene blue or LY-83538, which also inhibit sGC, but which also inhibit NOS and generate superoxide (16). ODQ does not inhibit particulate guanylyl cyclases, which are stimulated by peptides, such as atrial natriuretic peptide (62), nor does it inhibit adenylyl cyclases (11). ODQ has been found to have an in vitro IC50 as low as 50 nM in studies in which sGC activity was stimulated with SNP, and that 10 μM concentrations achieve maximal inhibition of sGC (56). ODQ is thus an important tool that has been used extensively to successfully elucidate roles of NO-sensitive sGC in a wide variety of physiological processes (11, 13, 62).
ODQ did not alter SkBF under normothermic conditions, nor did this agent alter the cutaneous vasoconstrictor response to cold stress relative to responses at sites that were perfused with Ringer or 2% DMSO in Ringer. This result shows that ODQ did not alter the vasoconstrictor system and supports the assumption of specificity of ODQ for sGC inhibition in our protocol.
SNP is a NO donor and causes vasodilation largely through heme-dependent activation of sGC (16, 55). The concentration of 1 mM ODQ used in our protocols produced the same inhibition of SNP-induced, sGC-dependent vasodilation that we observed in preliminary studies with ODQ concentrations of up to 2.5 mM. This result indicates that maximal inhibition of heme-dependent NO activation of sGC was achieved with 1 mM ODQ; however, we cannot exclude the possibility that 1 mM ODQ achieved only partial blockade.
While sGC has usually been viewed as the mediator of NO vasodilator effects, NO can also effect physiological signaling through sGC-independent mechanisms, such as S-nitrosation (14, 64). This sGC-independent process involves posttranslational modification of proteins at the thiol side chains of cysteine residues directly by NO and creates an S-nitrosothiol modification of the thiol side chain. Protein S-nitrosation is a reversible covalent modification and can affect proteins of all types in a fashion analogous to protein phosphorylation or acetylation (63). This process generally occurs at higher concentrations of NO than required for sGC activation alone and also occurs on a longer time course of up to a few minutes rather than the seconds typically required for sGC activation (2, 13, 64). S-nitrosation of proteins is involved in the modulation of neurotransmitter release (36, 38). NO-mediated S-nitrosation specifically facilitates soluble N-ethylmaleimide-sensitive factor attachment protein receptor complex formation and subsequent exocytosis (9, 38). Our findings clearly show a role for heme-dependent sGC activation by NO in the control of skin blood vessels and raise the possibility that S-nitrosation could also play a role. Involvement of such a mechanism in cutaneous active vasodilation would be consistent with the finding by Wilkins et al. (57) that exogenous NO caused greater vasodilation during heat stress than in normothermia, and their proposal that NO interacts with other neurotransmitters to synergistically augment cutaneous active vasodilation during heat stress. Given that we cannot totally exclude incomplete sGC antagonism by 1 mM ODQ in our studies, establishing such a role for S-nitration will require additional studies.
In contrast to our findings with whole body heat stress, our results show that the NO portion of the vasodilation due to local skin warming is mainly through heme-dependent sGC activation, and that the roles for other mechanisms are minor. Because increases in SkBF effected by local warming appear to be mainly due to NO binding to sGC heme to cause activation, our results support the clinical use of local skin warming to assess this NO-mediated sGC aspect of endothelial function (8).
In summary, we found that specific and maximal antagonism of sGC with ODQ attenuated both cutaneous active vasodilation in heat stress and the vasodilation induced by local skin warming. These results indicate that NO-sensitive, heme-dependent sGC activation is involved in both vasodilator responses. In addition, our results suggest that NO mediates cutaneous active vasodilation in heat stress both by sGC-independent mechanisms, as well as by sGC-dependent mechanisms, in approximately equal amounts. Our finding that ODQ antagonism of sGC activation had a much greater effect on the vasodilation induced by local skin warming suggests that NO mediation of the local warming response is more reliant on heme-dependent sGC activation.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant HL-065599.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Abi-Gerges N, Hove-Madsen Fischmeister R, Mery PF. A comparative study of the effects of three guanylyl cyclase inhibitors on the L-type Ca2+ and muscarinic K+ currents in frog cardiac myocytes. Br J Pharmacol 121: 1369–1377, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahern GP, Klyachko VA, Jackson MB. cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. Trends Neurosci 25: 510–517, 2002. [DOI] [PubMed] [Google Scholar]
- 3. Anderson C, Andersson T, Wardell K. Changes in skin circulation after insertion of a microdialysis probe visualized by laser-Doppler perfusion imaging. J Invest Dermatol 102: 808–811, 1994. [DOI] [PubMed] [Google Scholar]
- 4. Aoki K, Stephens DP, Johnson JM. Diurnal variation in cutaneous vasodilator and vasoconstrictor systems during heat stress. Am J Physiol Regul Integr Comp Physiol 281: R591–R595, 2001. [DOI] [PubMed] [Google Scholar]
- 5. Bellamy TC, Wood J, Garthwaite J. On the activation of soluble guanylyl cyclase by nitric oxide. Proc Natl Acad Sci U S A 99: 507–510, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bennett LA, Johnson JM, Stephens DP, Saad AR, Kellogg DL., Jr Evidence for a role for vasoactive intestinal peptide in active vasodilation in the cutaneous vasculature in humans. J Physiol 552: 223–232, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carvajal JA, Germain AM, Huidobro-Toro JP, Weiner CP. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J Cell Physiol 184: 409–420, 2000. [DOI] [PubMed] [Google Scholar]
- 8. Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci 27: 503–508, 2006. [DOI] [PubMed] [Google Scholar]
- 9. Deak F, Xu Y, Chang W, Khvotchev M, Liu X, Sudhof TC, Rizo J. Munc18-1 binding to the neuronal SNARE complex controls synaptic vesicle priming. J Cell Biol 184: 751–764, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dietz NM, Rivera JM, Warner DO, Joyner MJ. Is nitric oxide involved in cutaneous vasodilation during body heating in humans? J Appl Physiol 76: 2047–2053, 1994. [DOI] [PubMed] [Google Scholar]
- 11. Friebe A, Koesling D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circ Res 93: 96–105, 2003. [DOI] [PubMed] [Google Scholar]
- 12. Garthwaite J, Southan GJ, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. J Pharmacol Exp Ther 48: 184–188, 1995. [PubMed] [Google Scholar]
- 13. Gonzalez DR, Fernandez IC, Ordenes PP, Treuer AV, Eller G, Boric MP. Differential role of S-nitrosylation and the NO-cGMP-PKG pathway in cardiac contractility. Nitric Oxide 18: 157–167, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Hill BG, Dranka BP, Bailey SM, Lancaster JR, Jr, Darley-Usmar VM. What part of NO don't you understand? Some answers to the cardinal questions in nitric oxide biology. J Biol Chem 285: 19699–19704, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hodges GJ, Chu C, Kosiba WA, Zhao K, Johnson JM. The effect of microdialysis needle trauma on cutaneous vascular responses in humans. J Appl Physiol 106: 1112–1118, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hwang T, Wu C, Teng C. Comparison of two soluble guanylyl cyclase inhibitors, methylene blue and ODQ, of sodium nitroprusside-induced relaxation in guinea-pig trachea. Br J Pharmacol 125: 1158–1163, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jackson EB, Jr, Mukhopadhyay S, Tulis DA. Pharmacologic modulators of soluble guanylate cyclase/cyclic guanosine monophosphate in the vascular system–from bench top to bedside. Curr Vasc Pharmacol 5: 1–14, 2007. [PubMed] [Google Scholar]
- 18. Johnson JM, Park MK. Effect of upright exercise on threshold for cutaneous vasodilation and sweating. J Appl Physiol 50: 814–818, 1981. [DOI] [PubMed] [Google Scholar]
- 19. Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Handbook of physiology. Environmental Physiology. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 4, vol. I, chapt. 11, p. 215–243. [Google Scholar]
- 20. Johnson JM, Taylor WF, Shepherd AP, Park MK. Laser-Doppler measurement of skin blood flow: comparison with plethysmography. J Appl Physiol 56: 798–803, 1984. [DOI] [PubMed] [Google Scholar]
- 21. Kellogg DL., Jr In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans. J Appl Physiol 100: 1709–1718, 2006. [DOI] [PubMed] [Google Scholar]
- 22. Kellogg DL, Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol 85: 824–829, 1998. [DOI] [PubMed] [Google Scholar]
- 23. Kellogg DL, Jr, Johnson JM, Kenney WL, Pergola PE, Kosiba WA. Mechanisms of control of skin blood flow during prolonged exercise in humans. Am J Physiol Heart Circ Physiol 265: H562–H568, 1993. [DOI] [PubMed] [Google Scholar]
- 24. Kellogg DL, Jr, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol 86: 1185–1190, 1999. [DOI] [PubMed] [Google Scholar]
- 25. Kellogg DL, Jr, Pérgola PE, Kosiba WA, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve co-transmission. Circ Res 77: 1222–1228, 1995. [DOI] [PubMed] [Google Scholar]
- 26. Kellogg DL, Jr, Zhao JL, Coey U, Green JV. Acetylcholine induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J Appl Physiol 98: 629–632, 2005. [DOI] [PubMed] [Google Scholar]
- 27. Kellogg DL, Jr, Zhao JL, Friel C, Roman LJ. Nitric oxide concentration increases in the cutaneous interstitial space during heat stress in humans. J Appl Physiol 94: 1971–1977, 2003. [DOI] [PubMed] [Google Scholar]
- 28. Kellogg DL, Jr, Zhao JL, Wu Y. Endothelial nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. Am J Physiol Heart Circ Physiol 295: H123–H129, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kellogg DL, Jr, Zhao JL, Wu Y. Neuronal nitric oxide synthase mechanisms in the cutaneous vasculature of humans in vivo. J Physiol 586: 847–857, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kellogg DL, Jr, Zhao JL, Wu Y. Roles of nitric oxide synthase isoforms in cutaneous vasodilation induced by local warming of the skin and whole body heat stress in humans. J Appl Physiol 107: 1438–1444, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koesling D, Russwurm M, Mergia E, Mullershausen F, Friebe A. Nitric oxide-sensitive guanylyl cyclase: structure and regulation. Neurochem Int 45: 813–819, 2004. [DOI] [PubMed] [Google Scholar]
- 32. Kreidstein ML, Pang CY, Carlsen LN, Xu N. Evidence for endothelium-dependent and endothelium-independent vasodilation in human skin flaps. Can J Physiol Pharmacol 70: 1208–1216, 1992. [DOI] [PubMed] [Google Scholar]
- 33. Lamas S, Lowenstein CJ, Michel T. Nitric oxide signaling comes of age: 20 years and thriving. Cardiovasc Res 75: 207–209, 2007. [DOI] [PubMed] [Google Scholar]
- 34. McCord GR, Cracowski JL, Minson CT. Prostanoids contribute to cutaneous active vasodilation in humans. Am J Physiol Regul Integr Comp Physiol 291: R596–R602, 2006. [DOI] [PubMed] [Google Scholar]
- 35. Medow MS, Taneja I, Stewart JM. Cyclooxygenase and nitric oxide synthase dependence of cutaneous reactive hyperemia in humans. Am J Physiol Heart Circ Physiol 293: H425–H432, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meffert MK, Calakos NC, Scheller RH, Schulman H. Nitric oxide modulates synaptic vesicle docking/fusion reactions. Neuron 16: 1229–1236, 1996. [DOI] [PubMed] [Google Scholar]
- 37. Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 91: 1619–1626, 2001. [DOI] [PubMed] [Google Scholar]
- 38. Palmer ZJ, Duncan RR, Johnson JR, Lian L, Mello LV, Booth D, Barclay JW, Graham ME, Burgoyne RD, Prior IA, Morgan A. S-nitrosylation of syntaxin 1 at Cys145 is a regulatory switch controlling Munc18-1 binding. Biochem J 413: 479–491, 2008. [DOI] [PubMed] [Google Scholar]
- 39. Parati G, Casadei R, Groppelli A, Di Rienzo M, Mancia G. Comparison of finger and intra arterial blood pressure monitoring at rest and during laboratory testing. Hypertension 13: 647–655, 1989. [DOI] [PubMed] [Google Scholar]
- 40. Poulos TL. Soluble guanylate cyclase. Curr Opin Struct Biol 16: 736–743, 2006. [DOI] [PubMed] [Google Scholar]
- 41. Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev 54: 75–159, 1974. [DOI] [PubMed] [Google Scholar]
- 42. Rowell LB. Human Circulation: Regulation During Physical Stress. New York: Oxford Univ. Press, 1986. [Google Scholar]
- 43. Rowell LB, Murray JA, Brengelmann GL, Kraning KK., II Human cardiovascular adjustments to rapid changes in skin temperature during exercise. Cir Res 24: 711–724, 1969. [DOI] [PubMed] [Google Scholar]
- 44. Saumet JL, Kellogg DL, Jr, Taylor WF, Johnson JM. Cutaneous laser-Doppler flowmetry: influence of underlying muscle blood flow. J Appl Physiol 65: 478–481, 1988. [DOI] [PubMed] [Google Scholar]
- 45. Schrammel A, Behrends S, Schmidt K, Koesling D, Mayer B. Characterization of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. J Pharmacol Exp Ther 50: 1–5, 1996. [PubMed] [Google Scholar]
- 46. Shastry S, Joyner MJ. Geldanamycin attenuates NO-mediated dilation in human skin. Am J Physiol Heart Circ Physiol 282: H232–H236, 2002. [DOI] [PubMed] [Google Scholar]
- 47. Shastry S, Minson CT, Wilson SA, Dietz NK, Joyner MJ. Effects of atropine and l-NAME on cutaneous blood flow during body heating in humans. J Appl Physiol 88: 467–472, 2000. [DOI] [PubMed] [Google Scholar]
- 48. Shastry S, Reed AS, Halliwill JR, Dietz NM, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol 85: 830–834, 1998. [DOI] [PubMed] [Google Scholar]
- 49. Shibasaki M, Wilson TE, Cui J, Crandall CG. Acetylcholine released from cholinergic nerves contributes to cutaneous vasodilation during heat stress. J Appl Physiol 93: 1947–1951, 2002. [DOI] [PubMed] [Google Scholar]
- 50. Stewart JM, Medow MS, Minson CT, Taneja I. Cutaneous neuronal nitric oxide is specifically decreased in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 293: H2161–H2167, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stewart JM, Taneja I, Raghunath N, Clarke D, Medow MS. Intradermal angiotensin II administration attenuates the local cutaneous vasodilator heating response. Am J Physiol Heart Circ Physiol 295: H237–H334, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol 66: 1586–1592, 1989. [DOI] [PubMed] [Google Scholar]
- 53. Taylor WF, Johnson JM, O'Leary D, Park MK. Effect of high local temperature on reflex cutaneous vasodilation. J Appl Physiol 57: 191–196, 1984. [DOI] [PubMed] [Google Scholar]
- 54. Taylor WF, Johnson JM, O'Leary D, Park MK. Modification of the cutaneous vascular response to exercise by local skin temperature. J Appl Physiol 57: 1878–1884, 1984. [DOI] [PubMed] [Google Scholar]
- 55. Tseng CL, Tabrizi-Fard M, Fung H. Differential sensitivity among nitric oxide donors toward odq-mediated inhibition of vascular relaxation. J Pharmacol Exp Ther 292: 737–742, 2000. [PubMed] [Google Scholar]
- 56. Wanstall JC, Homer KL, Doggrell Evidence for, and importance of, cGMP-independent mechanisms with NO and NO donors on blood vessels and platelets. Curr Vasc Pharmacol 3: 41–53, 2005. [DOI] [PubMed] [Google Scholar]
- 57. Wilkins BW, Holowatz LA, Wong BJ, Minson CT. Nitric oxide is not permissive for cutaneous active vasodilation in humans. J Physiol 548: 963–969, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wong BJ, Minson CT. Neurokinin-1 receptor desensitization attenuates cutaneous active vasodilation in humans. J Physiol 577: 1043–1051, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wong BJ, Wilkins BW, Holowatz LA, Minson CT. Nitric oxide synthase inhibition does not alter the reactive hyperemic response in the cutaneous circulation. J Appl Physiol 95: 504–510, 2003. [DOI] [PubMed] [Google Scholar]
- 60. Wong BJ, Wilkins BW, Minson CT. H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J Physiol 560: 941–948, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhao JL, Pergola PE, Roman LJ, Kellogg DL., Jr. Bioactive nitric oxide concentration does not increase during reactive hyperemia in humans. J Appl Physiol 96: 628–632, 2004. [DOI] [PubMed] [Google Scholar]
- 62. Zhao Y, Brandish PE, DiValentin M, Schelvis JPM, Babcock GT, MAM Inhibition of soluble guanylate cyclase by ODQ. Biochemistry 39: 10848–10854, 2000. [DOI] [PubMed] [Google Scholar]
- 63. Zimmet JM, Hare JM. Nitroso-redox interactions in the cardiovascular system. Circulation 114: 1531–1544, 2006. [DOI] [PubMed] [Google Scholar]
- 64. Ziolo MT. The fork in the nitric oxide road: cyclic GMP or nitrosylation. Nitric Oxide 18: 153–156, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]