Abstract
We recently reported that reactive oxygen species (ROS) plays an excitatory role in modulation of the exercise pressor reflex (EPR) in normal rats. In this study, we further tested two independent hypotheses: 1) ROS interacts with EPR-related ionotropic receptors such as the purinergic receptors (P2) and transient receptor potential vanilloid 1 receptors (TRPV1) to indirectly modulate the EPR function; 2) ROS directly affects excitability of muscle afferents by modulating the voltage-gated sodium (Nav) channels. To test the first hypothesis, we performed animal experiments to investigate the effect of the SOD mimetic 4-hydroxy-2,2,6,6-tetramethyl piperidine 1-oxyl (Tempol) on the pressor response to hindlimb intra-arterial (IA) injection of either α,β-methylene ATP (a P2X agonist) or capsaicin (a TRPV1 agonist) in decerebrate rats. To test the second hypothesis, we used the patch-clamp technique to determine the effect of ROS on Nav channels on the soma of muscle afferents. We also performed local microinjection of a sodium channel blocker, tetrodotoxin (TTX), into ipsilateral L4/L5 dorsal root ganglia (DRGs) to investigate whether the blockade of Nav channels by TTX affects the EPR function. We found that Tempol did not affect the pressor response to injection of either capsaicin or α,β-methylene ATP but significantly decreased the Nav current in small and medium-sized 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI)-labeled DRG neurons. A membrane-permeant superoxide dismutase, polyethylene glycol (PEG)-SOD, had an effect on the Nav current in these neurons similar to that of Tempol. Microinjection of TTX into L4/L5 DRGs dramatically attenuated the pressor response to static contraction induced by electrical stimulation of L4/L5 ventral roots. These data suggest that ROS modulates the EPR by affecting the activity of the Nav channels on muscle afferents.
Keywords: exercise, muscle afferents, ion channels, free radicals
static contraction of skeletal muscle reflexively activates the sympathetic nervous system and increases arterial pressure and heart rate (HR). Two distinct neural control mechanisms have been postulated to explain the increases in cardiovascular function during exercise: central command and the exercise pressor reflex (EPR) (23, 26), the afferent arm of which consists of group III (thin myelinated Aδ fibers) and group IV (unmyelinated C fibers) muscle afferents (2, 12, 13, 19). To a large extent, activation of these afferents occurs in response to either muscle mechanical deformation (a stimulus to activate group III afferents) or local accumulation of metabolic by-products of muscle contraction (a stimulus to activate group IV afferents) (1, 4, 7, 12, 18, 20). Both mechanical and metabolic afferent signals are delivered to the dorsal horn of the spinal cord via dorsal root ganglia (DRGs), a collection of cell bodies of the afferent sensory fibers, which lie between adjacent vertebrae.
Skeletal muscle contraction-induced metabolic by-products such as K+, lactic acid, ATP, H+, and phosphate have been well documented to be involved in the modulation of the EPR (17, 18). Furthermore, in a recent study (31), we reported that hindlimb intra-arterial (IA) infusion of a reactive oxygen species (ROS) scavenger, 4-hydroxy-2,2,6,6-tetramethyl piperidine 1-oxyl (Tempol), attenuated the EPR induced by static contraction in decerebrate rats, indicating that ROS is also involved in the modulation of the EPR function in the normal state. However, the underlying cellular mechanisms by which ROS modulates the EPR in normal rats remain unknown. A potential possibility is that ROS indirectly affects muscle afferent function by acting on ionotropic channels such as purinergic receptors (P2) and transient receptor potential vanilloid 1 receptors (TRPV1), both of which have been reported to be involved in modulation of the EPR (14, 15, 24, 27). Therefore, the first goal of the present study was to determine whether there is a direct interaction between ROS and the P2X or TRPV1 receptor. In addition, ROS may also directly affect the excitability of muscle afferents by modulating primary ion channels such as sodium, potassium, and calcium channels. Since voltage-gated sodium (Nav) channels are critical for the initiation and propagation of action potentials and for the regulation of neuronal excitability (22, 35), the second goal of the present study was to investigate the effect of ROS on the Nav channels on the soma of muscle afferents (i.e., L4/L5 DRGs).
METHODS
Experiments were performed on male Sprague-Dawley rats weighing 350–420 g. These experiments were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center and were carried out under the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experiment I
To determine whether there is an interaction between ROS and the P2X or TRPV1 receptor, we performed experiments to investigate the effect of the SOD mimetic Tempol on the pressor response to hindlimb IA injection of either α,β-methylene ATP (a P2X agonist) or capsaicin (a TRPV1 agonist) in decerebrate rats.
General surgical preparation.
Rats were anesthetized with isoflurane (Halocarbon Labs, River Edge, NY; 5% in O2). A jugular vein and the trachea were cannulated. After tracheal cannulation, the lungs were ventilated with an anesthetic mixture of 2–3% isoflurane and O2. The right carotid artery was catheterized for measurement of arterial pressure (AP) and mean arterial pressure (MAP). HR was derived from the AP pulse by using the cardiotachometer function of the PowerLab (ADInstruments, Colorado Springs, CO). Body temperature was maintained between 37°C and 38°C by a heating pad.
Decerebration.
Previous studies have shown that the EPR is compromised by anesthesia, which is especially a concern in rats. However, after decerebration the effects of anesthesia on the EPR were largely abolished (26). Therefore, a decerebrate rat model was used in the present studies. The decerebration procedure was performed as described by us (31) and others (25). Briefly, rats were placed in a stereotaxic apparatus (Stoelting, Chicago, IL) and customized spinal frame. The head and pelvis were stabilized. Before decerebration, the lungs were ventilated with an isoflurane-oxygen mixture. Dexamethasone (0.2 mg iv) was given to reduce brain edema and inflammatory responses from the decerebration. The remaining intact carotid artery was isolated and ligated to reduce bleeding during decerebration. Subsequently, a portion of bone superior to the central sagittal sinus was removed. The dura mater was breached and reflected. The cerebral cortex was gently aspirated to visualize the superior and inferior colliculi. With a blunt instrument the brain was perpendicularly sectioned precollicularly, and the transected forebrain was aspirated. The cranial vault was filled with warm agar (37°C). After the decerebration had been completed, the lungs were ventilated with a mixture of room air and oxygen instead of the anesthetic gas. A minimum recovery period of 1.25 h was employed after decerebration before data collection began.
Hindlimb IA injection of α,β-methylene ATP and capsaicin.
A catheter was placed in the right iliac artery with its tip advanced to the abdominal aortic bifurcation, ensuring that α,β-methylene ATP (a P2X agonist; 10 and 40 μg/kg, 0.15 ml) or capsaicin (a TRPV1 agonist; 0.1 and 1.0 μg/kg, 0.15 ml) was delivered to the left hindlimb through the left iliac artery. Fifteen minutes after the first bolus of α,β-methylene ATP or capsaicin, the ROS scavenger Tempol (10 mg/kg, 0.2 ml IA, 10 min) was infused into the left hindlimb. After treatment with Tempol, a second IA injection of α,β-methylene ATP or capsaicin was performed to investigate whether or not pretreatment with Tempol affects the pressor response to hindlimb IA injection of α,β-methylene ATP or capsaicin.
Experiment II
In the second part of the present study, we took advantage of the patch-clamp technique to determine the effect of ROS on Nav channels on the soma of muscle afferents (i.e., L4/L5 DRGs).
1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate injection into hindlimb muscle.
We injected the fluorescent retrograde tracer 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI, 20 mg/ml; Molecular Probes, Eugene, OR) into hindlimb muscle in order to label muscle afferent DRG neurons. Male Sprague-Dawley rats were anesthetized by inhalation of an isoflurane-oxygen mixture (3–5% isoflurane in oxygen). The skin was incised and pulled away from underlying muscle tissue, and DiI was injected into the triceps surae muscles. The injection volume was 5 μl, and the injection was repeated three times at different locations (rostral, middle, and caudal). The injection needle was placed in the muscle for 5–10 min to prevent leakage of tracer. The skin overlying the muscle was then sutured. The animals were returned to their cages for 4–5 days to allow the retrograde tracer to be transported to DRG neurons.
Isolation of DRG neurons.
After anesthesia with pentobarbital sodium (80 mg/kg ip), rats were decapitated. DRGs at L4–L6 were removed on the side ipsilateral to the muscle injected with DiI and placed in ice-cold Ringer solution (mM: 137 NaCl, 25 NaHCO3, 3 KCl, 1.25 NaH2PO4, 1.2 CaCl2, 1.2 MgSO4, 10 glucose). The DRGs were dissected free and incubated for 30 min at 37°C in an enzymatic Ringer solution containing 0.1% collagenase-0.1% trypsin (Sigma-Aldrich, St Louis, MO). The tissues were mechanically triturated and then transferred to a Ringer solution containing 0.2% collagenase and 0.5% bovine serum albumin (BSA) and incubated for 30 min at 37°C. After digestion, dispersed DRG cells were washed in Dulbecco's modified Eagle's medium (DMEM; GIBCO, Carlsbad, CA) containing 6% BSA. The isolated cells were resuspended in culture medium and plated onto culture wells. The culture medium consisted of a 50/50 mixture of DMEM and Ham's F-12 medium supplemented with antibiotics and 10% fetal bovine serum. The DRG cells were cultured at 37°C in a humidified atmosphere of 95% air-5% CO2 for 4–24 h before the patch-clamp experiments.
Electrophysiological recordings.
Previous studies had reported that the DRG somata with Aδ (group III afferents) and C (group IV afferents) fibers were of a more uniform size and were restricted to the small (<25 μm) and medium-sized (25–35 μm) cells within the ganglia (5). Therefore, Nav currents were recorded by the whole cell patch-clamp technique with the Axopatch 200B patch-clamp amplifier (Axon Instruments, Burlingame, CA) only in small and medium DRG neurons. Only DiI-labeled DRG neurons were selected for the recording of Nav currents. In the voltage-clamp experiments, resistance of the patch pipette was 1–3 MΩ when filled with the following solution (in mM): 105 CsCl, 25 tetraethylammonium (TEA), 1 CaCl2, 10 HEPES, 10 EGTA, 5 MgATP, and 25 glucose (pH 7.3; 320 mosM). The extracellular solution consisted of (in mM) 70 NaCl, 10 CsCl, 60 choline-Cl, 0.1 CdCl2, 10 TEA, 4 MgCl2, 10 HEPES, and 10 glucose (pH 7.4; 330 mosM). Seventy millimolar Na+ in extracellular solution was used, because normal extracellular Na+ (140 mM) is sufficiently large to saturate the patch-clamp amplifier (10). Series resistance of 5–13 MΩ was electronically compensated 30–80%. Junction potential was calculated to be +9.9 mV with pCLAMP 10.2 software, and all values for membrane potential given throughout were corrected with this value. Current traces were sampled at 10 kHz and filtered at 5 kHz. The holding potential was −90 mV, and current-voltage relationships were elicited by 5-mV step increments to potentials between −90 and 40 mV for 40 ms. Peak currents were measured for each test potential, and current density was calculated by dividing peak current by cell membrane capacitance (Cm). Two doses of Tempol (1 mM and 5 mM) in the bath solution were used to investigate whether endogenous ROS dose-dependently modulates Nav currents in muscle afferent DRGs. Furthermore, a membrane-permeant superoxide dismutase, polyethylene glycol-superoxide dismutase (PEG-SOD, 50 U/ml) was used in the bath solution to investigate the specific effect of superoxide on the Nav current in muscle afferent DRG cells. In some of the patch-clamp experiments, in order to investigate which subclass of DRG neurons was targeted by ROS, we used Griffonia simplicifolia isolectin B4 (IB4, Alexa Fluor 488 conjugate; Invitrogen), a C fiber marker, to separate muscle afferent DRG neurons into two groups: IB4-positive and IB4-negative groups. Briefly, DRG neurons were incubated with IB4 for 20 min before recording. The IB4-stained neurons were easily recognized under epifluorescence illumination (Fig. 1). Previous studies (9, 28) have reported that IB4-positive DRG neurons are C-type neurons, which express receptor components for glial cell line-derived neurotrophic factor (GDNF) and preferentially transport GDNF. On the other hand, IB4-negative DRG neurons include three different types of DRG neurons, Aα/β-, Aδ-, and part of the C-type neurons, which depend on nerve growth factor (NGF).
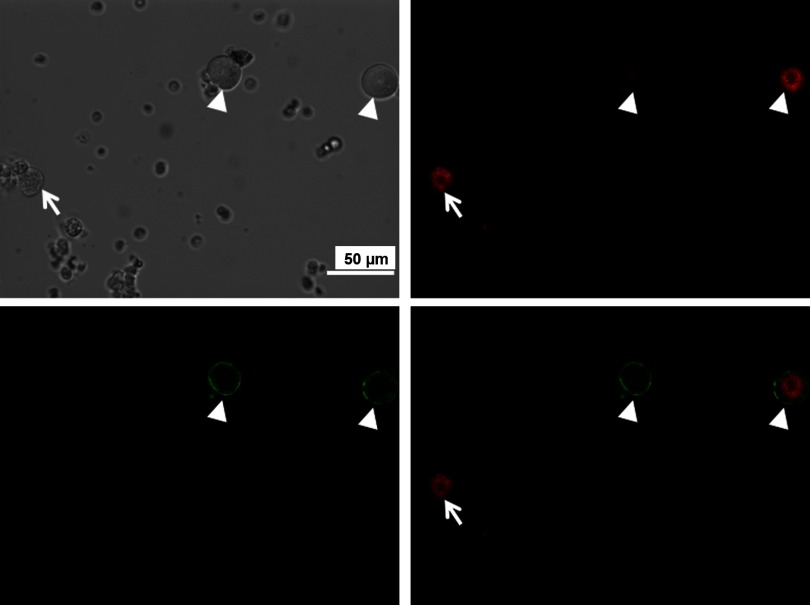
Fig. 1.
Representative images showing how to identify muscle afferent neurons in dorsal root ganglion (DRG) during patch-clamp experiments. Top: 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI)-labeled muscle afferent DRG neurons with red color in fluorescent light (right) and cells in the same field under regular light (left). Bottom: isolectin B4 (IB4) was used to further separate muscle afferent DRG neurons into 2 groups: IB4-positive neurons (indicated by white arrowhead) and IB4-negative neurons (indicated by white arrow). Digitally merged images from top right and bottom left panels are displayed in bottom right panel.
Measurement of superoxide level with lucigenin chemiluminescence method.
To confirm that Tempol and PEG-SOD do scavenge the superoxide as we expected, we performed lucigenin chemiluminescence experiments to assess the redox state in Tempol-, PEG-SOD-, or vehicle-treated L4/L5 DRGs. DRG samples were homogenized in PBS solution at 4°C. Total protein concentration was determined with a bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Superoxide level was measured by the lucigenin chemiluminescence method (30, 31). The homogenate (0.3 ml) was placed in 0.5-ml microfuge tubes containing dark-adapted lucigenin (5 μM), and then accumulative light emission was recorded for 5 min in a TD-20/20 Luminometer (Turner Designs, Sunnyvale, CA). The light emission-time curve was obtained. To investigate the acute effect of Tempol or PEG-SOD on superoxide production, the accumulative light emission was immediately measured after the addition of Tempol (1 mM) or PEG-SOD (50 U/ml).
Experiment III
To investigate whether Nav channels play a critical role in EPR function in the normal state, we also carried out additional experiments in which we performed local microinjection of a sodium channel blocker, tetrodotoxin (TTX; Sigma), into ipsilateral L4/L5 DRGs to investigate whether the blockade of Nav channels by TTX affects the EPR function in decerebrate rats. EPRs were evoked by a 30-s static contraction induced by electrical stimulation of L4/L5 ventral roots.
Procedures for static contraction.
To activate both mechanically and metabolically sensitive skeletal muscle afferent fibers, static hindlimb contraction was induced by electrical stimulation of ventral roots (31). A laminectomy exposing the lower lumbar portions of the spinal cord (L2–L6) was performed. The dura of the cord was cut and reflected, allowing visual identification of the L4–L6 spinal roots. The dorsal and ventral roots of L4 and L5 were carefully separated. The ventral roots were sectioned, and the cut peripheral ends were positioned on insulated bipolar platinum electrodes. The exposed neural tissue was covered in a pool of warm mineral oil (37°C). The animals were secured within the spinal adaptor (Stoelting, Wood Dale, IL) by clamps placed on rostral lumbar vertebrae. Afterwards, the pelvis was stabilized with steel posts within the frame, and the hindlimb containing the triceps surae muscles under study was fixed in one position with clamps. The angles of the hip and knee were 120° and 80°, respectively. The calcaneal bone was sectioned, and the Achilles tendon was connected to a force transducer (model FT-03, Grass Instruments, West Warwick, RI) for measurement of muscle tension. Electrical stimulation was performed with a Grass Instruments S88 stimulator. Electrically induced static muscle contraction of the triceps surae was performed by stimulating the L4/L5 ventral roots for 30–35 s. Constant-current stimulation was used at three times motor threshold (defined as the minimum current required to produce a muscle twitch) with a pulse duration of 0.1 ms at 40 Hz. In the experiments, all muscles of the hindlimb undergoing study were denervated except for the triceps surae muscle. At the end of this experiment, the neuromuscular blocking agent pancuronium bromide (200 μg/kg) was administered intravenously. Electrical activation of the ventral roots was repeated with the stimulus parameters described previously. This maneuver was instituted to eliminate the possibility that cardiovascular responses were mediated by direct activation of sensory afferent fibers during stimulation protocols.
DRG microinjection.
The left L4/L5 DRGs were exposed by removal of the posterior articular process of the L4/L5 vertebra. The exposed DRGs and other neural tissue were covered in a pool of warm mineral oil (37°C). The procedure of DRG microinjection was performed similarly to the procedure previously described by us in central microinjection (32). Microinjection was made from a single-barrel micropipette and performed by a four-channel pressure injector (PM2000B, World Precision Instruments). Injections of TTX (50 μM) were made over a 15-s period, and a 200-nl injection volume was measured by observing the movement of the fluid meniscus along a reticule in a microscope. The time interval between L4 and L5 DRG injections was within 2 min.
Data Acquisition and Statistical Analysis
In experiments I and III, MAP and HR responses to either hindlimb bolus IA injection of α,β-methylene ATP and capsaicin or a 30-s static contraction induced by electrical stimulation of L4/L5 ventral roots were acquired with PowerLab software (AD Instruments). Baseline values were determined by analyzing at least 30 s of the data before IA injection or muscle contraction. The peak response to either IA injection of chemicals or static contraction was determined in the period of the greatest change from baseline. The tension-time index (TTI) was calculated by integrating the area between the tension trace and the baseline level and is expressed in kilograms times second. Peak developed tension was calculated by subtracting the resting tension from the peak tension and is expressed in grams (experiment III). In experiment II, peak currents were measured for each test potential, and current density was calculated by dividing peak current by Cm. All values are expressed as means ± SE. Differences between groups were determined by a two-way ANOVA followed by the Tukey post hoc test. Changes in MAP, HR, TTI, and Nav current before and after IA administration (experiment I), DRG microinjection (experiment III), or bath solution (experiment II) of chemicals were determined by paired t-test. P < 0.05 was considered statistically significant.
RESULTS
Effect of Tempol on Pressor Response to Hindlimb IA Injection of α,β-Methylene ATP or Capsaicin
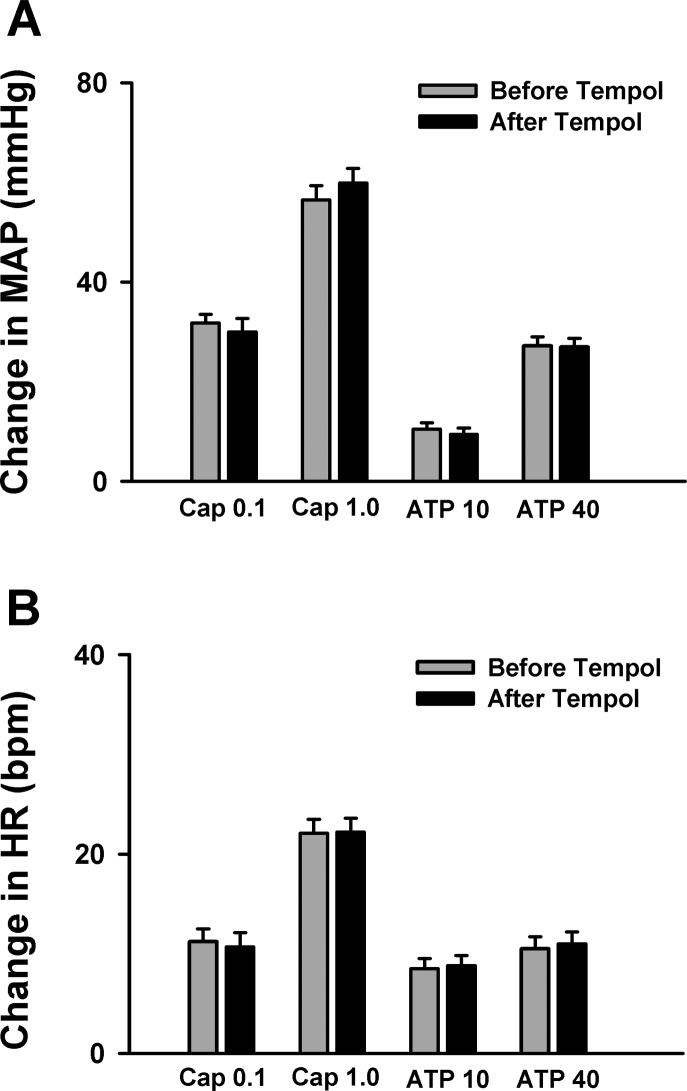
After decerebration, baseline MAP and HR were maintained at physiological levels in all animals (90.7 ± 3.4 mmHg, 380.4 ± 12.8 beats/min; n = 20). Capsaicin (0.1, 1.0 μg/kg, 0.15 ml) or α,β-methylene ATP (10, 40 μg/kg, 0.15 ml) was used to selectively activate the TRPV1 or P2X receptors on skeletal muscle afferent endings, respectively. As shown in Fig. 2, hindlimb IA injection of either capsaicin or α,β-methylene ATP caused significant pressor and cardioaccelerator responses in decerebrate rats. Pretreatment with Tempol (IA, 10 mg/kg, 10 min) slightly decreased baseline MAP (90.7 ± 3.4 vs. 83.3 ± 2.0 mmHg; n = 20, P < 0.05), which is consistent with our previous finding (31). Interestingly, Tempol did not affect the pressor response to injection of either capsaicin or α,β-methylene ATP (Fig. 2), indicating that Tempol has no effect on the TRPV1 or P2X receptor activity in skeletal muscle.
Fig. 2.
Mean data showing that hindlimb intra-arterial (IA) infusion of 4-hydroxy-2,2,6,6-tetramethyl piperidine 1-oxyl (Tempol) did not affect the pressor [mean arterial pressure (MAP); A] and cardioacceleration [heart rate (HR); B] responses to injection of either capsaicin [Cap, transient receptor potential vanilloid 1 (TRPV1) agonist; 0.1 or 1.0 μg/kg] or α,β-methylene ATP (ATP, P2X agonist; 10, 40 μg/kg) in decerebrate rats. Values are means ± SE; n = 10/group. bpm, Beats per minute.
Effect of Tempol on Total Nav Current in DiI-Labeled DRG of Normal Rats
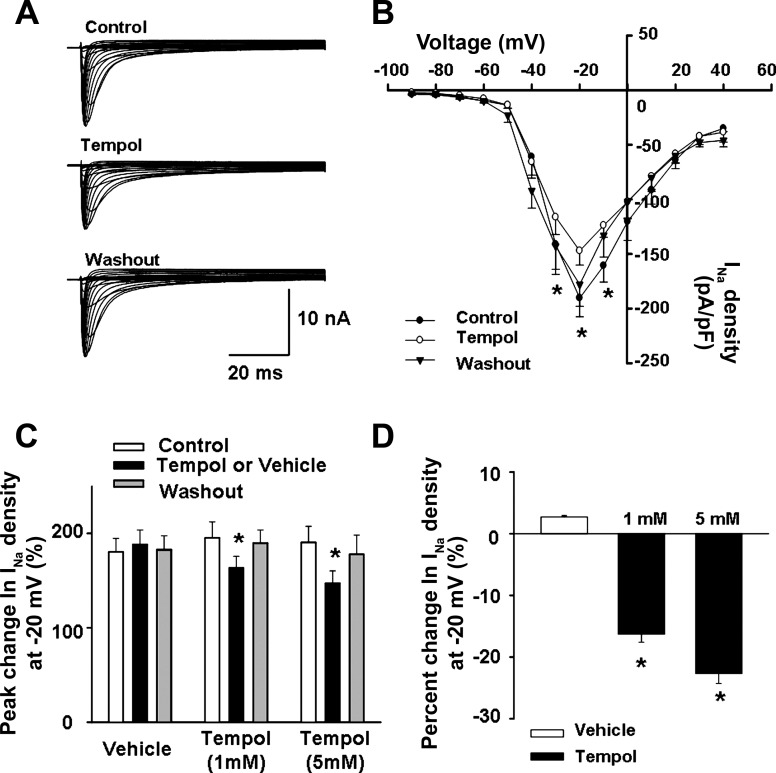
Next, we examined the possibility that ROS directly affects the excitability of muscle afferents by modulating Nav channels. Small (diameter <25 μm, n = 24) and medium-sized (25 μm < diameter <35 μm, n = 26) DRG neurons labeled with DiI (Fig. 1) were used to investigate the effect of the ROS scavenger Tempol on the total Nav current in normal rats. As shown in Fig. 3, Tempol decreased the total Nav current in DiI-labeled neurons in a dose-dependent manner (∼16% decrease after treatment with 1 mM Tempol, n = 26; ∼23% decrease after treatment with 5 mM Tempol, n = 24). The effect of Tempol on the Nav current was observed in both small and medium-sized DRG neurons (Fig. 4A).
Fig. 3.
Effect of Tempol on voltage-gated sodium (Nav) current density in muscle afferent DRG neurons from normal rats. A: representative Nav current recording showing that Tempol (5 mM) decreased the Nav current in a small-sized muscle afferent DRG neuron. B: current density-voltage curves showing the effect of Tempol (5 mM) on the Nav current in small and medium-sized muscle afferent DRG neurons (n = 24: 12 for small neurons and 12 for medium-sized neurons) from normal rats. C and D: dose-dependent effect of Tempol on the Nav current density in muscle afferent DRG neurons from normal rats [1 mM: n = 26 (12 for small neurons and 14 for medium-sized neurons); 5 mM: n = 24 (12 for small neurons and 12 for medium-sized neurons)]. Values are means ± SE. *P < 0.05 vs. Control.
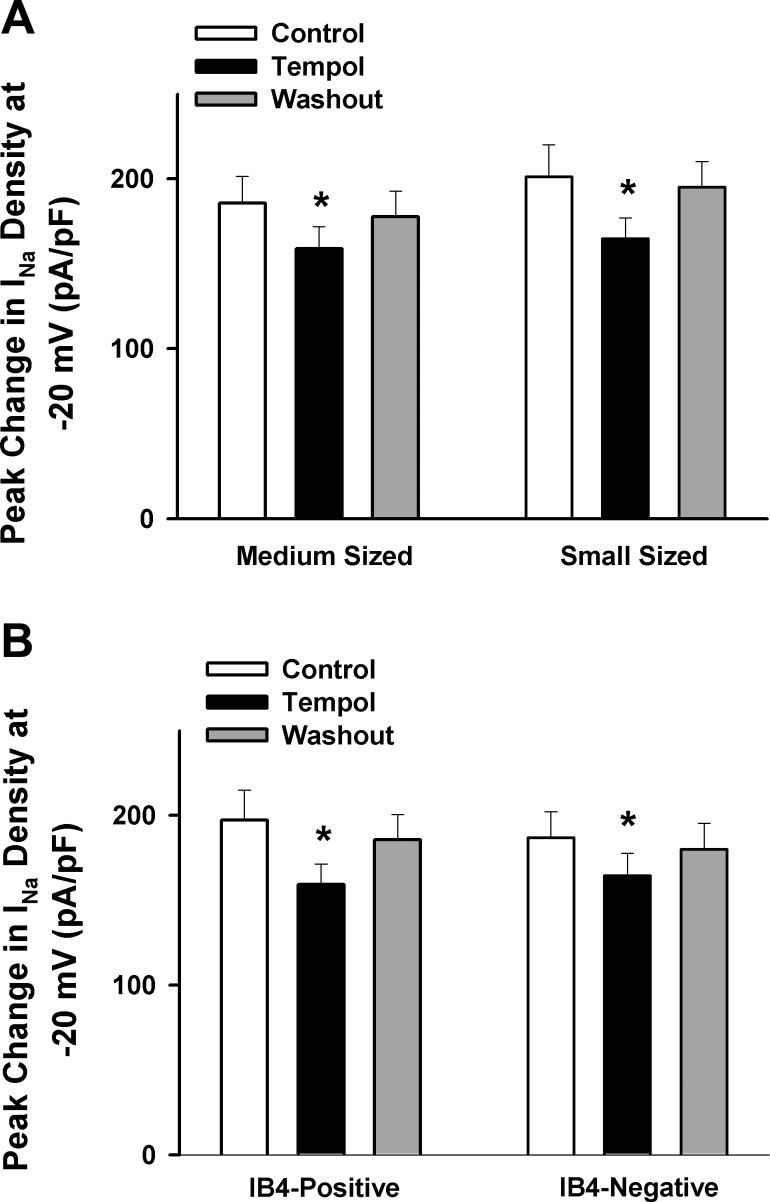
Fig. 4.
Mean data showing the effect of Tempol (1 mM) on the Nav current in different subtypes of muscle afferent DRG neurons (small vs. medium sized, n = 12 for small and 14 for medium-sized neurons; IB4-positive vs. IB4-negative, n = 11 for IB4-positive and 15 for IB4-negative neurons). Values are means ± SE. *P < 0.05 vs. Control.
Effect of Tempol on IB4-Negative and IB4-Positive DRG Labeling with DiI
To further determine whether the ROS scavenger Tempol affects the Nav current in all types of DRG neurons or only a certain type of DRG neuron, we evaluated the effect of Tempol on the total Nav current in IB4-negative and IB4-positive DRG cells labeled with DiI. We found that treatment with Tempol decreased the Nav current in both IB4-negative and IB4-positive DRG neurons (Fig. 4B), indicating that the effect of ROS on sodium channels was not limited to a certain type of DRG neurons.
Effect of PEG-SOD on Total Nav Current in DiI-Labeled DRG Neurons of Normal Rats
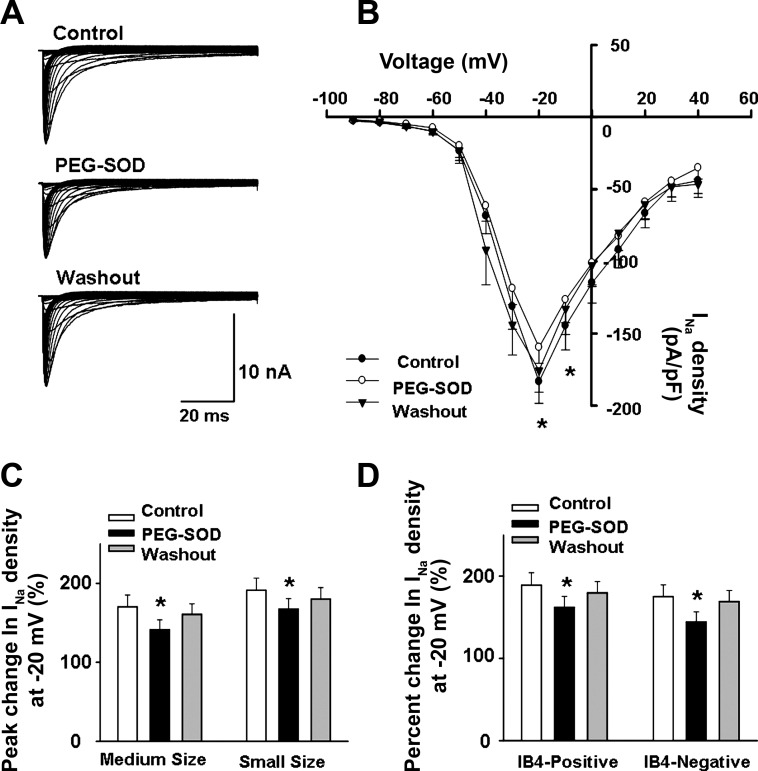
We also used a specific superoxide scavenger, PEG-SOD, to determine the role of superoxide anion in the ROS-mediated modulation of sodium channel function in DRG neurons of normal rats. As shown in Fig. 5, PEG-SOD (50 U/ml) significantly decreased the Nav current (∼15%) in small and medium-sized DRG neurons labeled with DiI. Similar to Tempol, the inhibitory effect of PEG-SOD on the Nav current was seen in both IB4-negative and IB4-positive DRG neurons. These data indicate that superoxide plays an important role in the ROS-mediated modulation of sodium channel function in DRG neurons.
Fig. 5.
Effect of polyethylene glycol (PEG)-SOD on Nav current density in muscle afferent DRG neurons. A: representative Nav current recording showing that PEG-SOD (50 U/ml) decreased the Nav current in a medium-sized muscle afferent DRG neuron. B: current density-voltage curves showing the effect of PEG-SOD (50 U/ml) on the Nav current in small and medium-sized muscle afferent DRG neurons (n = 14: 7 for small neurons and 7 for medium-sized neurons) from normal rats. C and D: mean data showing the effect of PEG-SOD (50 U/ml) on the Nav current in different subtypes of muscle afferent DRG neurons (small vs. medium-sized, n = 7 for small and 7 for medium-sized neurons; IB4-positive vs. IB4-negative, n = 8 for IB4-positive and 6 for IB4-negative neurons). Values are means ± SE. *P < 0.05 vs. Control.
Effects of Tempol and PEG-SOD on Superoxide Level in L4/L5 DRGs
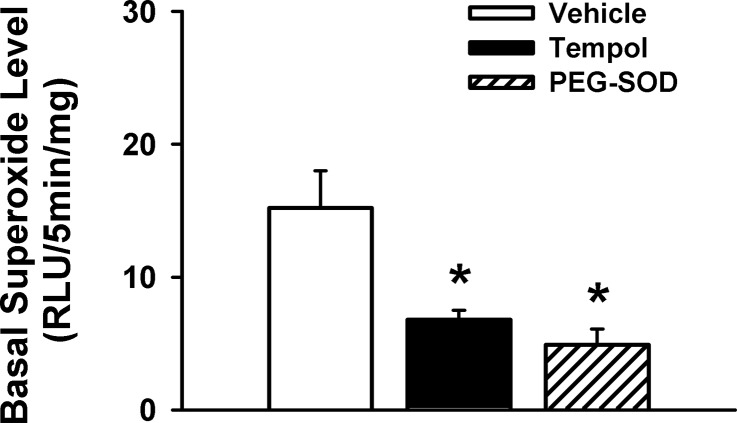
To confirm that Tempol and PEG-SOD do scavenge superoxide as expected, we measured the superoxide level between vehicle- and drug-treated DRGs by the lucigenin chemiluminescence technique. We found that there was a significantly lower superoxide level in either Tempol- or PEG-SOD-treated DRG samples compared with vehicle-treated DRG samples (Fig. 6), suggesting that these two drugs affected ROS metabolism as expected.
Fig. 6.
Influences of 2 redox agents (Tempol and PEG-SOD) on the superoxide level in the L4/L5 DRGs of normal rats. Values are means ± SE; n = 5. *P < 0.05 vs. vehicle. RLU, relative light units.
Effect of Microinjection of TTX into Ipsilateral L4/L5 DRGs on Pressor Response to Static Contraction Induced by Electrical Stimulation of L4/L5 Ventral Roots
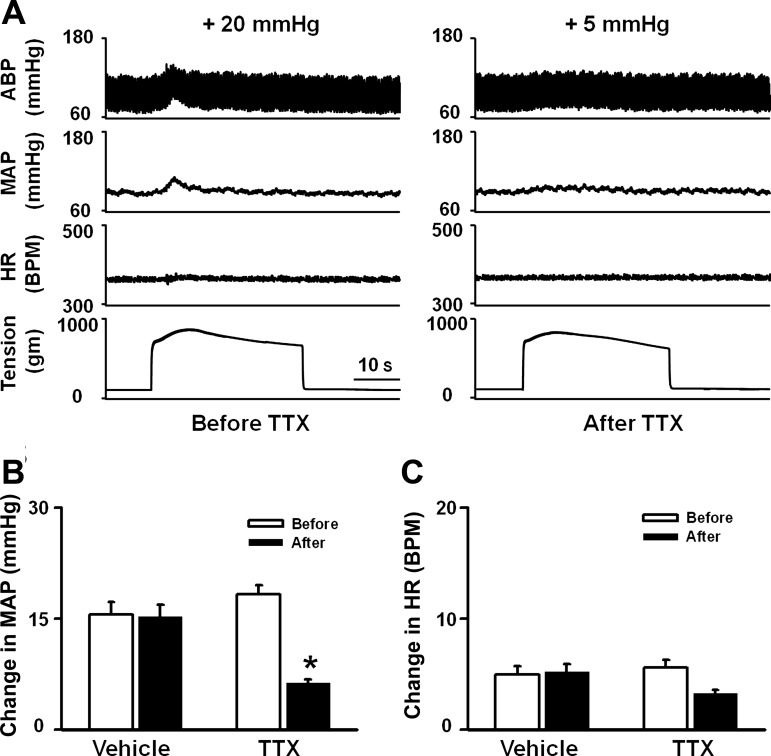
To investigate whether Nav channels play a critical role in EPR function in the normal state, we performed local microinjection of a sodium channel blocker, TTX, into ipsilateral L4/L5 DRGs to investigate whether the blockade of Nav channels by TTX affects EPR function. In decerebrate rats, static contraction induced by electrical stimulation of L4/L5 ventral roots caused significant increases in MAP and HR, which were dramatically attenuated by microinjection of TTX but not vehicle into the L4/L5 DRGs (Fig. 7). Microinjection of TTX into DRGs did not affect basal MAP and HR (MAP: 101.3 ± 3.8 vs. 101 ± 3.7 mmHg, before vs. after, n = 6, P > 0.05; HR: 370.1 ± 10.5 vs. 365 ± 10.3 beats/min, before vs. after, n = 6, P > 0.05). In addition, there was no significant difference in TTI before and after microinjection of TTX into DRGs (16.1 ± 3.0 vs. 15.8 ± 2.9 kg·s, before vs. after, n = 6, P > 0.05), indicating that the effect of TTX on the EPR is not due to a reduction of muscle tension. The data above suggested a critical role played by Nav channels in the EPR function in normal rats.
Fig. 7.
Effect of microinjection of tetrodotoxin (TTX) into ipsilateral L4/L5 DRGs on the pressor response to static contraction induced by electrical stimulation of L4/L5 ventral roots in decerebrate rats. A: representative recordings showing the pressor [arterial blood pressure (ABP) and MAP] and cardioacceleration (HR) responses to 30-s static contraction before and after microinjection of TTX (50 μM, 200 nl/DRG) into ipsilateral L4/L5 DRGs. B and C: mean data showing the effects of microinjection of TTX (50 μM, 200 nl/DRG) into the ipsilateral L4/L5 DRGs on the pressor (MAP; B) and cardioacceleration (HR; C) responses to 30-s static contraction. Values are means ± SE; n = 6/group. *P < 0.05 vs. before.
DISCUSSION
In a previous study (31), we reported that hindlimb IA infusion of several ROS scavengers such as Tempol and dimethylthiourea (DMTU) attenuated the pressor response to static contraction induced by electrical stimulation of L4/L5 ventral roots in normal rats, indicating that ROS plays an excitatory role in the modulation of the EPR. The present study further provided several new findings: 1) the ROS scavenger Tempol did not affect the pressor response to injection of either capsaicin or α,β-methylene ATP; 2) microinjection of a Nav channel blocker, TTX, into L4/L5 DRGs, but not vehicle treatment, dramatically attenuated the pressor response to static contraction induced by electrical stimulation of L4/L5 ventral roots, indicating a critical role played by Nav channels in the EPR function in normal rats; 3) endogenous ROS plays an excitatory role in the modulation of Nav channels on muscle afferent DRG neurons; 4) ROS affects the sodium channels in both IB4-positive and IB4-negative DRG neurons, indicating that the effect of ROS on the sodium channels is not limited to a specific class of DRG neurons; and 5) the superoxide scavenger PEG-SOD has a effect on voltage-gated sodium channels on DRG neurons similar to that of Tempol, indicating that superoxide plays an important role in the ROS-mediated modulation of sodium channel function in muscle afferent DRG neurons.
Skeletal muscle generates superoxide and its derivatives at rest and during contraction (3, 11). Despite the evidence above that ROS can be generated in skeletal muscle, the physiological roles of muscle ROS remain to be determined. Like other muscle metabolites such as bradykinin, K+, lactic acid, ATP, H+, and phosphate (17, 18), ROS may also modulate EPR function by stimulating or sensitizing the muscle afferent endings. This hypothesis is supported by a recent study from our laboratory (31) demonstrating that hindlimb IA infusion of ROS scavengers (i.e., Tempol and DMTU) decreased the EPR-induced pressor response to a 30-s static contraction in normal rats, indicating that ROS plays an excitatory role in modulation of EPR function in normal rats. However, the detailed mechanisms by which ROS modulates EPR function in the physiological state are unclear. Unlike other metabolites, ROS has no membrane receptors to bind. Therefore, we initially reasoned that ROS may interact with other EPR-dependent ionotropic receptors such as the TRPV1 and P2X receptors. In the present study, we found that Tempol did not affect the pressor response to injection of either capsaicin or α,β-methylene ATP, indicating that ROS has no direct interaction with TRPV1 and P2X receptors. However, we acknowledge the limitation that infusion of ATP or capsaicin may not mimic the EPR in a physiological way. Therefore, we cannot absolutely exclude the possibility that ROS interacts with TRPV1 and P2X receptors during the genesis of the EPR in the physiological state. We also tested the possibility that ROS may directly affect the excitability of muscle afferents by modulating ion channels such as Nav channels. We found that the current of Nav channels in muscle afferent DRG neurons can be modulated by endogenous ROS, especially superoxide, which supports our second hypothesis. Importantly, this is the first study to report that endogenous ROS is involved in the modulation of Nav channel function in muscle afferent DRG neurons in normal animals. Since the Nav channels are critical for the initiation and propagation of action potentials and for the regulation of neuronal excitability, a change in the electrophysiological property of the Nav channels on muscle afferents most likely affects the EPR function. In the present study, we provide direct evidence showing that microinjection of a Nav channel blocker, TTX, into L4/L5 DRGs dramatically attenuates the pressor response to static contraction induced by electrical stimulation of L4/L5 ventral roots, indicating a critical role played by Nav channels in the EPR function in normal rats. Therefore, an alteration in Nav channel function after ROS scavengers such as Tempol can ultimately affect the EPR function. Nevertheless, a detailed mechanism by which ROS modulates the Nav channels to affect the EPR function remains unknown. During muscle contraction, both mechanical deformation and metabolites can stimulate muscle afferents to evoke the EPR. At the cellular level, the genesis of the EPR needs action potential. For metabolic stimulus, metabolites can act on the ionotropic receptors of nerve endings such as P2X and TRPV receptors, which cause nonselective cation influx to either facilitate the generation of action potential or even directly produce action potential. However, for mechanical stimulus muscle deformation may not produce metabolites and activate these nonselective ionotropic receptors. In this condition, sodium channels may be more important in contributing to the transformation of mechanical signal (deformation) to electrical signal (action potential). However, these hypotheses remain to be tested by additional experiments.
The present finding that the ROS scavengers Tempol and PEG-SOD decreased Nav channel function in muscle afferent DRG neurons further supports a concept raised by our previous study (31) that ROS plays an excitatory role in the modulation of muscle afferent activity. Interestingly, a recent study by Koba et al. (16) reported that hindlimb IA administration of Tempol did not affect the pressor response to a 30-s static contraction in normal rats, indicating no effect of endogenous ROS on EPR function. A major difference in drug delivery methods between our study and that of Koba et al. is that in their study Tempol was trapped in the hindlimb circulation for 10 min and released for an additional 15–30 min, which might potentially cause ischemia-reperfusion problems. This, in turn, may affect the redox state in the muscle and interfere with the efficiency of Tempol (8, 21). This may explain why Tempol did not affect the EPR function in the study of Koba et al. (16). On the other hand, although hindlimb IA infusion of Tempol has the limitation that it may cause a potential central effect upon recirculation, in our previous study (31) we carried out additional control experiments to confirm the peripheral effect of Tempol on the EPR function. First, we compared the effects of IA and intravenous administration on EPR function at the same dose. We found that the effect of IA administration of Tempol on EPR function was greater than that of intravenous administration, supporting the view that IA administration of Tempol has a peripheral effect on the EPR. Second, in another control experiment the pressor response to stimulation of the central end of the dorsal roots was compared before and after drug administration. Stimulation of the central end of the dorsal roots directly activates the central nervous system but not the afferent endings. In our previous study (31), we found that IA infusion of Tempol as well as other redox drugs had no effect on the pressor response to stimulation of the central end of the dorsal roots, suggesting that IA infusion of these drugs affects the afferents. In the present study, we provide cellular evidence that Tempol attenuated Nav channel function in muscle afferent DRG neurons, which supports the concept that ROS can affect muscle afferent function in the normal state.
After identifying an excitatory effect of ROS on Nav channel function in muscle DRG neurons, we further determined which type(s) of DRG neurons was targeted by ROS. Previous studies have shown that conduction velocity of the dorsal root fibers has been associated with DRG cell size, classifying these neurons into four main groups: Aα (30–55 m/s), Aβ (14–30 m/s), Aδ (2.2–8 m/s), and C (<1.4 m/s) (6). Of all sized DRG neurons, the somata with Aδ and C fibers were of a more uniform size and were restricted to the small and medium-sized cells within the ganglia (5). Given that the EPR afferent arm consists of thin myelinated Aδ fibers (group III) and unmyelinated C fibers (group IV), we investigated the effect of ROS on the Nav channel function in small and medium-sized DiI-labeled DRG neurons. Furthermore, we used IB4 as a marker to separate these small and medium-sized DRG neurons into two groups: IB4-negative and IB4-positive neurons. Previous studies (9, 28) have reported that IB4-positive DRG neurons belong to a subtype of C-fiber neurons, which express receptor components for GDNF and preferentially transport GDNF. On the other hand, IB4-negative small and medium-sized DRG neurons include several different types of DRG neurons: very few Aα/β if any, Aδ, and another subtype of C-fiber peptidergic neurons that depend on NGF (9, 28). In the present study, we found that the effects of Tempol and PEG-SOD on Nav channel function were observed in both IB4-negative and IB4-positive small and medium-sized DRG neurons, indicating that endogenous ROS including superoxide modulates Nav channel function in different types of DRG neurons. The inhibitory effect of Tempol on Nav channel function in the IB4-positive DRG neurons confirmed that the GDNF-dependent C-fiber neurons are targeted by ROS. However, because of the lack of a marker for living DRG neurons to further isolate Aδ and NGF-dependent C-fiber neurons from the IB4-negative small and medium-sized neurons, we cannot definitively conclude that ROS affects Nav channel function in both Aδ and NGF-dependent C-fiber neurons. Nonetheless, in the present study, we noted that Tempol decreased the current of Nav channels in all IB4-negative neurons, indirectly suggesting that both Aδ and NGF-dependent C-fiber neurons might be targeted by ROS. This question needs to be clarified by additional experiments.
Although we identified an effect of ROS on Nav channel function in the muscle afferent neurons of normal rats, the possibility that ROS is also involved in the modulation of the K+ or Ca2+ channel function in these neurons cannot be excluded. As we know, both K+ and Ca2+ channels are also critical contributors to the neuronal excitability. Therefore, a potential effect of ROS on the K+ or Ca2+ channel function in muscle afferent neurons may also affect the sensitivity of muscle afferent limb. Several lines of evidence have shown that ROS, especially superoxide, affects neuronal excitability by modulating the electrophysiological properties of K+ or Ca2+ channels in either cultured neurons or in situ ganglion (29, 33, 34). However, whether ROS is also involved in the modulation of the K+ or Ca2+ channel function in muscle afferent DRG neurons remains unknown. Additional experiments need to be performed in order to address this issue.
In summary, the present study provides the first evidence that endogenous ROS, especially superoxide, is involved in the modulation of Nav channel function in muscle afferent DRG neurons in normal rats. These data help to clarify a cellular mechanism by which ROS modulates the EPR.
GRANTS
This work was supported, in part, by National Heart, Lung, and Blood Institute Grant PO1-HL-62222. H.-J. Wang was supported by a postdoctoral fellowship from the American Heart Association, Heartland Affiliate.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Kaye Talbitzer, Pamela Curry, and Richard Robinson for expert technical assistance.
REFERENCES
- 1. Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun 107: 1198–1205, 1982. [DOI] [PubMed] [Google Scholar]
- 4. Hanna RL, Kaufman MP. Activation of thin-fiber muscle afferents by a P2X agonist in cats. J Appl Physiol 96: 1166–1169, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol 359: 31–46, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harper AA, Lawson SN. Electrical properties of rat dorsal root ganglion neurones with different peripheral nerve conduction velocities. J Physiol 359: 47–63, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hayes SG, Kindig AE, Kaufman MP. Blockade of acid sensing ion channels attenuates the exercise pressor reflex in cats. J Physiol 581: 1271–1282, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huk I, Nanobashvili J, Neumayer C, Punz A, Mueller M, Afkhampour K, Mittlboeck M, Losert U, Polterauer P, Roth E, Patton S, Malinski T. l-Arginine treatment alters the kinetics of nitric oxide and superoxide release and reduces ischemia/reperfusion injury in skeletal muscle. Circulation 96: 667–675, 1997. [DOI] [PubMed] [Google Scholar]
- 9. Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci 2: 83–91, 2001. [DOI] [PubMed] [Google Scholar]
- 10. Ikeda SR, Schofield GG, Weight FF. Na+ and Ca2+ currents of acutely isolated adult rat nodose ganglion cells. J Neurophysiol 55: 527–539, 1986. [DOI] [PubMed] [Google Scholar]
- 11. Jackson MJ, Edwards RH, Symons MC. Electron spin resonance studies of intact mammalian skeletal muscle. Biochim Biophys Acta 847: 185–190, 1985. [DOI] [PubMed] [Google Scholar]
- 12. Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983. [DOI] [PubMed] [Google Scholar]
- 13. Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984. [DOI] [PubMed] [Google Scholar]
- 14. Kindig AE, Hayes SG, Hanna RL, Kaufman MP. P2 antagonist PPADS attenuates responses of thin fiber afferents to static contraction and tendon stretch. Am J Physiol Heart Circ Physiol 290: H1214–H1219, 2006. [DOI] [PubMed] [Google Scholar]
- 15. Kindig AE, Hayes SG, Kaufman MP. Blockade of purinergic 2 receptors attenuates the mechanoreceptor component of the exercise pressor reflex. Am J Physiol Heart Circ Physiol 293: H2995–H3000, 2007. [DOI] [PubMed] [Google Scholar]
- 16. Koba S, Gao Z, Sinoway LI. Oxidative stress and the muscle reflex in heart failure. J Physiol 587: 5227–5237, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li J, King NC, Sinoway LI. ATP concentrations and muscle tension increase linearly with muscle contraction. J Appl Physiol 95: 577–583, 2003. [DOI] [PubMed] [Google Scholar]
- 18. MacLean DA, Imadojemu VA, Sinoway LI. Interstitial pH, K+, lactate, and phosphate determined with MSNA during exercise in humans. Am J Physiol Regul Integr Comp Physiol 278: R563–R571, 2000. [DOI] [PubMed] [Google Scholar]
- 19. McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983. [DOI] [PubMed] [Google Scholar]
- 21. Ozyurt B, Iraz M, Koca K, Ozyurt H, Sahin S. Protective effects of caffeic acid phenethyl ester on skeletal muscle ischemia-reperfusion injury in rats. Mol Cell Biochem 292: 197–203, 2006. [DOI] [PubMed] [Google Scholar]
- 22. Ritter AM, Martin WJ, Thorneloe KS. The voltage-gated sodium channel Nav1.9 is required for inflammation-based urinary bladder dysfunction. Neurosci Lett 452: 28–32, 2009. [DOI] [PubMed] [Google Scholar]
- 23. Sinoway LI, Li J. A perspective on the muscle reflex: implications for congestive heart failure. J Appl Physiol 99: 5–22, 2005. [DOI] [PubMed] [Google Scholar]
- 24. Smith SA, Leal AK, Williams MA, Murphy MN, Mitchell JH, Garry MG. The TRPv1 receptor is a mediator of the exercise pressor reflex in rats. J Physiol 588: 1179–1189, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol 91: 89–102, 2006. [DOI] [PubMed] [Google Scholar]
- 27. Smith SA, Williams MA, Mitchell JH, Mammen PP, Garry MG. The capsaicin-sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation 111: 2056–2065, 2005. [DOI] [PubMed] [Google Scholar]
- 28. Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron 20: 629–632, 1998. [DOI] [PubMed] [Google Scholar]
- 29. Takeuchi K, Yoshii K. Superoxide modifies AMPA receptors and voltage-gated K+ channels of mouse hippocampal neurons. Brain Res 1236: 49–56, 2008. [DOI] [PubMed] [Google Scholar]
- 30. Tu H, Zhang L, Tran TP, Muelleman RL, Li YL. Reduced expression and activation of voltage-gated sodium channels contributes to blunted baroreflex sensitivity in heart failure rats. J Neurosci Res 88: 3337–3349, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang HJ, Pan YX, Wang WZ, Zucker IH, Wang W. NADPH oxidase-derived reactive oxygen species in skeletal muscle modulates the exercise pressor reflex. J Appl Physiol 107: 450–459, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang WZ, Gao L, Wang HJ, Zucker IH, Wang W. Interaction between cardiac sympathetic afferent reflex and chemoreflex is mediated by the NTS AT1 receptors in heart failure. Am J Physiol Heart Circ Physiol 295: H1216–H1226, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Whyte KA, Hogg RC, Dyavanapalli J, Harper AA, Adams DJ. Reactive oxygen species modulate neuronal excitability in rat intrinsic cardiac ganglia. Auton Neurosci 150: 45–52, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yin JX, Yang RF, Li S, Renshaw AO, Li YL, Schultz HD, Zimmerman MC. Mitochondria-produced superoxide mediates angiotensin II-induced inhibition of neuronal potassium current. Am J Physiol Cell Physiol 298: C857–C865, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu FH, Catterall WA. Overview of the voltage-gated sodium channel family. Genome Biol 4: 207, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]