Abstract
The endoplasmic reticulum (ER) as an intracellular Ca2+ store not only sets up cytosolic Ca2+ signals, but, among other functions, also assembles and folds newly synthesized proteins. Alterations in ER homeostasis, including severe Ca2+ depletion, are an upstream event in the pathophysiology of many diseases. On the one hand, insufficient release of activator Ca2+ may no longer sustain essential cell functions. On the other hand, loss of luminal Ca2+ causes ER stress and activates an unfolded protein response, which, depending on the duration and severity of the stress, can reestablish normal ER function or lead to cell death. We will review these various diseases by mainly focusing on the mechanisms that cause ER Ca2+ depletion.
Loss of Ca2+ from the ER is associated with diabetes and other conditions. It disturbs the function of chaperones such as calreticulin and triggers stress responses that can lead to cell death.
Cytosolic [Ca2+] ([Ca2+]cyt) is precisely regulated in time and space because Ca2+ controls essential cell functions like proliferation, differentiation, secretion, contraction, metabolism, trafficking, gene transcription and apoptosis, and in this way controls complex processes like development or learning behavior (Berridge et al. 2000). An abnormal [Ca2+]cyt caused by disturbances of Ca2+ channels, Ca2+ transporters, Ca2+ pumps, and Ca2+-binding proteins can induce multiple pathologies (Missiaen et al. 2000). Ca2+ channelopathies in the nervous system leading to paralysis, ataxia, or migraine can be caused by mutations in subunits of voltage-operated Ca2+ channels in the plasma membrane (Bidaud et al. 2006; Lorenzon and Beam 2008). Other channelopathies like malignant hyperthermia and central core disease in skeletal muscle, and some tachycardias and tachyarrhythmias in the heart are because of mutations in Ca2+-release channels or Ca2+-binding proteins of the sarcoplasmic reticulum (SR) (Durham et al. 2007; Lorenzon and Beam 2008; Blayney and Lai 2009; Gyorke 2009). Deafness and skin diseases can also be because of mutations in Ca2+ pumps (Foggia and Hovnanian 2004; Van Baelen et al. 2004; Brini and Carafoli 2009). Ca2+ dysregulation may also lead to more complex diseases like Alzheimer and other neurodegenerative diseases (Bezprozvanny 2009; Berridge 2010; Supnet and Bezprozvanny 2010).
Disease states associated with a decreased [Ca2+] in the lumen of the ER ([Ca2+]ER) have thus far received less attention. The ER controls the synthesis, modification, folding, and export of proteins. An imbalance between the demand for protein synthesis and the capacity to handle them leads to the accumulation of misfolded or unfolded proteins, which is referred to as ER stress. An unfolded protein response (UPR) is initiated to reestablish normal ER function (Schroder and Kaufman 2005; Ron and Walter 2007). If the stress is too prolonged or severe to be corrected, the adaptive response triggered by the UPR will not overcome the ER stress and a cell-death program is triggered to eliminate the damaged cell. Many diseases affect the ER environment leading to ER stress, a UPR, and apoptosis (Xu et al. 2005; Lindholm et al. 2006; Kim et al. 2008). Some of them first deplete ER Ca2+, with disturbed function of luminal proteins (Michalak et al. 2002). The decreased [Ca2+]ER, rather than the increased [Ca2+]cyt, then triggers apoptosis (Nakano et al. 2006; Yoshida et al. 2006).
We will review the diseases in which a decreased [Ca2+]ER is an upstream event in the pathophysiology and show that ER stress often plays an essential role. We will first briefly review the mechanisms controlling the [Ca2+]ER, then focus on how ER stress leads to apoptosis, and finally review the mechanisms of ER Ca2+ depletion in the various diseases.
Ca2+ HOMEOSTASIS IN THE ER/SR
To function as an intracellular Ca2+ store, the ER/SR needs to express at least three different types of proteins (Pozzan et al. 1994): (1) Ca2+ pumps for uphill transport of Ca2+ from the cytosol to the lumen; (2) luminal Ca2+-binding proteins for storing Ca2+; and (3) Ca2+ channels for the controlled release of Ca2+ to the cytosol along its electrochemical gradient. Although the ER is generally assumed to form a continuous compartment, it can be heterogeneous at the level of its Ca2+-handling proteins. A heterogeneous distribution allows on the one hand localized Ca2+ pumping and release, and on the other hand, the setting up of Ca2+ signals without disturbing Ca2+-dependent processes within the ER lumen (Petersen et al. 2001; Berridge 2002; Papp et al. 2003).
Ca2+ pumps of the SERCA type (sarco/endoplasmic-reticulum Ca2+-ATPase) actively pump Ca2+ into the store (Fig. 1). They are encoded by three different genes, whereby each of them exists as various splice variants. SERCA2b has the highest Ca2+ affinity and is the most ubiquitous pump. Other isoforms have a more restricted expression pattern. Thapsigargin is a much-used specific inhibitor of the SERCA pumps. This sesquiterpene lactone irreversibly interacts with their M3-transmembrane helix. Phospholamban is the major endogenous regulator of SERCA pumps (at least for isoforms 1a, 2a, and 2b), but it is only expressed in muscle cells. This small protein decreases their Ca2+ affinity (Brini and Carafoli 2009; Vangheluwe et al. 2009).
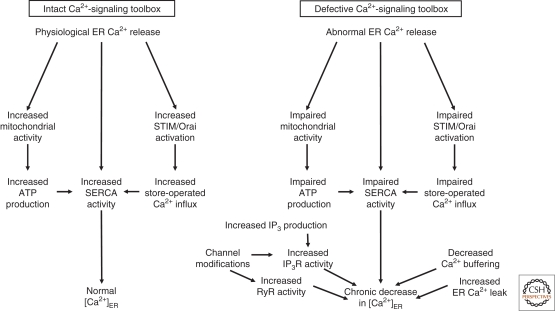
Figure 1.
Normal and abnormal [Ca2+]ER. A tight coordination between ER Ca2+-release and -refilling mechanisms enables proper Ca2+ signaling in response to physiological stimuli. Under these conditions, Ca2+ released from the ER stimulates mitochondrial activity and bioenergetics, leading to more ATP production. The initial decline in [Ca2+]ER activates STIM, allowing for store-operated Ca2+ influx. Ca2+ is recycled via the SERCA pumps. Normal [Ca2+]ER is restored and ER-related processes continue. In contrast, in pathological conditions, stress responses will occur and affect the Ca2+-signaling toolbox in various ways. Impaired mitochondrial activity, store-operated Ca2+ influx, or SERCA activity may all cause failure in restoring normal [Ca2+]ER in response to ER Ca2+-signaling processes, and lead to a decreased [Ca2+]ER. A chronic decrease in [Ca2+]ER may also be because of an imbalance between the Ca2+-on and -off mechanisms as a result of increased IP3R or RyR activity, decreased Ca2+ buffering, or increased ER Ca2+ leak.
Ca2+ in the lumen of the ER/SR is buffered by Ca2+-binding proteins. Calsequestrin is the main Ca2+-binding protein in skeletal and cardiac muscle (Beard et al. 2004). In other tissues Ca2+ binds to calreticulin (Michalak et al. 2002) and other Ca2+-dependent chaperones like calnexin, 78-kDa glucose-regulated protein/immunoglobulin heavy chain binding protein (GRP78/BiP), GRP94, and various protein-disulfide isomerases (PDI) (Papp et al. 2003). All these proteins combine at least two of the following three properties: Ca2+ binding, regulation of Ca2+ pumps or Ca2+-release channels, and chaperone function (Berridge 2002; Papp et al. 2003), emphasizing the close interrelation between the [Ca2+]ER and ER function.
The main Ca2+-release channels in the ER/SR belong to either the ryanodine-receptor (RyR) (Zalk et al. 2007) or the inositol 1,4,5-trisphosphate (IP3)-receptor (IP3R) (Foskett et al. 2007) families. In each family, three genes code for receptor subunits, which assemble to produce very large tetrameric Ca2+-release channels (∼2.2 MDa for the RyRs, ∼1.2 MDa for the IP3Rs). Further diversity occurs by alternative splicing and by the formation of both homo- and, at least for the IP3R, heterotetramers. The differences in channel and regulatory properties, and in subcellular localization, allow highly specific Ca2+ signals propagating through the cell. RyRs are predominantly expressed in muscles and neurons although they can also be present at low levels in other cells. Skeletal muscle expresses mainly RyR1, which is activated by direct interaction with L-type voltage-operated Ca2+ channels, whereas the RyR2 in cardiac tissue and the RyR3 are activated by Ca2+ itself (Endo 2009). IP3Rs on the other hand are expressed in all cell types. They generally become active when IP3 is produced on cell stimulation by extracellular agonists. IP3 binding at the amino terminus of the receptor induces channel opening at its carboxyl terminus (Bosanac et al. 2004). The further regulation of channel opening by cytosolic factors including Ca2+, by regulatory proteins, and by phosphorylation/dephosphorylation, as well as their subcellular localization allow them to set up highly specific spatio-temporal Ca2+ signals (Vermassen et al. 2004; Foskett et al. 2007; Mikoshiba 2007; Vanderheyden et al. 2009).
In normal conditions, several mechanisms are operative to prevent ER Ca2+ depletion or overload, e.g., both Ca2+ channels and Ca2+ pumps are sensitive to luminal [Ca2+]. The IP3R becomes more sensitive to IP3 when the [Ca2+]ER increases (Irvine 1990; Missiaen et al. 1992) and also the RyR is stimulated by luminal Ca2+ (Nelson and Nelson 1990; Gyorke and Terentyev 2008). SERCA-mediated Ca2+ uptake into the ER is sensitive to [Ca2+]ER (Takenaka et al. 1982). The release of Ca2+ from the ER during the generation of cytosolic Ca2+ signals should not decrease the [Ca2+]ER to a level at which ER function and Ca2+ signaling become compromised (Sammels et al. 2010). A mechanism has evolved that couples ER Ca2+ depletion to an increase of Ca2+ entry into the cell. This phenomenon is known as “capacitative” (Putney 1986) or “store-operated” Ca2+ entry. STIM1 and STIM2 are ubiquitously expressed single-pass transmembrane ER and, to some extent, plasma-membrane proteins with a luminal Ca2+ sensor (Stathopulos et al. 2008). Depending on the extent of ER depletion, either STIM1 or STIM2 oligomerize and interact with Orai1 proteins (Brandman et al. 2007). These tetrameric Ca2+ channels in the plasma membrane are then responsible for an increased Ca2+ entry (Cahalan 2009; Deng et al. 2009; Schindl et al. 2009).
ER STRESS AND APOPTOSIS
The ER not only fulfills a crucial role in Ca2+ signaling, but also provides a quality-control system for the proper folding of proteins and for sensing stress (Fig. 2). A plethora of ER-resident chaperones including calreticulin, calnexin, PDI, and GRP78/BiP bind unfolded or misfolded proteins via inappropriately exposed hydrophobic or hypo-glycosylated residues (Austin 2009). Calreticulin and calnexin bind to polypeptide chains entering the ER lumen through glycosylated residues, whereas PDI mediates the correct formation of disulfide bonds. GRP78/BiP undergoes cycles of binding and release of unfolded proteins until they are properly folded and hydrophobic residues are inaccessible. ER-resident chaperones like calreticulin, GRP78/BiP, and GRP94 need a high [Ca2+]ER for their activity (Ma and Hendershot 2004) with Ca2+ binding to paired anionic amino acids (Lucero and Kaminer 1999). Moreover, several of the ER chaperones also act as Ca2+ buffers (Lievremont et al. 1997; Papp et al. 2003). Determination of the Ca2+ affinities suggests up to millimolar levels in the ER (Sambrook 1990), and depletion of ER Ca2+ by treating cells with a Ca2+ ionophore or thapsigargin can lead to inappropriate secretion, aggregation, and degradation of unassembled proteins (Gaut and Hendershot 1993).
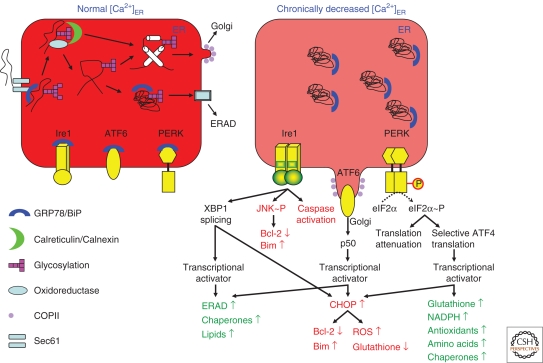
Figure 2.
The UPR. At a normal [Ca2+]ER the ER-stress sensors are scaffolded and inactivated by GRP78/BiP. Protein trafficking and quality-control mechanisms work normally. Polypeptides are translocated through Sec61 and glycosylated. This transport is facilitated by the molecular chaperone GRP78/BiP. Glucosidases then prepare the glycoprotein for binding to the ER lectins, calreticulin, and calnexin, whereas oxidoreductases catalyze disulfide-bond formation. ER-resident chaperones facilitate the proper folding of the nascent protein and prevent its aggregation. Further deglucosidation releases the ER lectins and once the protein is correctly folded and processed, the protein leaves the ER via the coat protein (COPII)-coated vesicles to the secretory pathway. Misfolded proteins, in contrast, associate with various chaperones, including GRP78/BiP, and are removed from the ER through ERAD. In contrast, when the [Ca2+]ER is chronically decreased, the function of chaperones becomes disturbed and unfolded proteins accumulate and act as a sponge for luminal GRP78/BiP. As a consequence, ER-stress sensors are devoid of GRP78/BiP and become activated, yielding early adaptive responses promoting survival (indicated in green) or late responses promoting apoptosis under conditions of severe or on-going ER stress (indicated in red). Ire1 undergoes dimerization and activation of its kinase and endoribonuclease activity, thereby splicing XBP1 mRNA and yielding a potent transcriptional activator that induces the expression of genes involved in ERAD, protein folding (like GRP78/BiP), and lipid synthesis. ATF6 goes to the Golgi compartment, where it is proteolytically cleaved to yield a cytosolic fragment (p50) that migrates to the nucleus and activates the transcription of UPR genes, like GRP78/BiP and CHOP. PERK dimerizes, autophosphorylates, and phosphorylates eIF2α, thereby suppressing its activity and reducing the rate of translation initiation, while increasing the rate of translation of ATF4, a potent transcription factor that augments the expression of genes involved in antioxidative stress, amino acid metabolism, and protein chaperoning. During on-going ER stress or irreparable ER damage, apoptotic pathways are activated. Ire1 phosphorylates JNK, leading to inhibition of Bcl-2 activity and activation of Bim, and recruits, releases, and activates procaspases in the cytosol. Induction of CHOP via XBP1, ATF6 or ATF4, down-regulates prosurvival Bcl-2-family members, increases prodeath proteins (like Bim) and ROS, and decreases the levels of glutathione, a ROS scavenger. In the presence of ROS, Ca2+ transfer to the mitochondria leads to the release of cytochrome c. The balance between proapoptotic and antiapoptotic Bcl-2-family members is disturbed, with activation of the intrinsic apoptotic pathway.
The [Ca2+]ER must be maintained in an environment of continuous intracellular Ca2+ signaling. Failure of this homeostatic mechanism, for example, by inhibition of SERCA with thapsigargin, triggers a UPR to either reestablish normal ER function or to eliminate the cell (Xu et al. 2005). The adaptive mechanisms initiated by the UPR involve reduced translation of misfolded proteins, enhanced translation of ER chaperones to increase the folding capacity of the ER, and degradation of misfolded proteins through ER-assisted degradation (ERAD) (Schroder and Kaufman 2005; Malhotra and Kaufman 2007). Global mRNA translation is inhibited for a few hours to reduce the influx of new proteins into the ER, whereas alarm signals involving the activation of mitogen-activated protein kinases (MAPK) are induced (Kim et al. 2008). The UPR involves three signaling pathways: PERK (PKR-like ER kinase), Ire1 (inositol-requiring enzyme 1), and ATF6 (activating transcription factor 6).
The recognition of misfolded proteins by the Ser/Thr kinase PERK leads to phosphorylation and inactivation of the eukaryotic initiation factor 2α (eIF2α). This shuts off mRNA translation, thereby preventing the accumulation of newly synthesized proteins in the ER (Harding et al. 1999), activates the transcription factor ATF4, which increases the level of chaperones such as GRP78/BiP and GRP94, and helps to restore the cellular redox homeostasis (Harding et al. 2000, 2003).
Ire1 has endoribonuclease and Ser/Thr-kinase activity. Its endoribonuclease activity degrades many mRNAs to reduce the protein load on the ER (Hollien and Weissman 2006). Ire1 removes an intron from the mRNA of X-box-binding protein 1 (XBP1), leading to the expression of XBP1. This transcription factor is involved in the expression of several UPR and ERAD genes (Rao and Bredesen 2004). The kinase activity of Ire1 is involved in apoptotic signaling via ASK1 (apoptosis signal-regulating kinase 1) and JNK (c-Jun N-terminal kinase). JNK activates the proapoptotic BH3-only protein Bim (Lei and Davis 2003; Putcha et al. 2003), and inactivates the antiapoptotic Bcl-2 protein (Yamamoto et al. 1999). Ire1 also recruits caspase 12 (Yoneda et al. 2001), which may play a role in ER stress-induced apoptosis (Szegezdi et al. 2003). However, caspase 12 is not present in humans, and although caspase 4, its close paralogue, may perform such function, it remains uncertain whether caspase 4 is vital for ER stress-induced apoptosis (Egger et al. 2003).
The transcription factor ATF6 is translocated to the Golgi during ER stress and is proteolytically activated. ATF6 stimulates ER-stress genes as a homodimer or as a heterodimer with other transcription factors like XBP1, whose transcription is also induced by ATF6 (Yoshida et al. 2001; Malhotra and Kaufman 2007). ATF6 is cytoprotective, possibly mediated by RCAN1 (regulator of calcineurin-1), an endogenous inhibitor of calcineurin (Belmont et al. 2008). This enzyme dephosphorylates the proapoptotic Bad (Bcl-2 antagonist of cell death), which then dimerizes and inhibits antiapoptotic family members such as Bcl-2 and Bcl-Xl (Wang et al. 1999).
ATF4, ATF6, and XBP1 all induce the transcription of the gene encoding CHOP (C/EBP homologous protein) (Kim et al. 2008). The Ire1-ASK1-p38-MAPK pathway enhances CHOP activity at a posttranscriptional level (Wang and Ron 1996). CHOP is involved in ER stress-induced apoptosis by down-regulating the antiapoptotic Bcl-2 (McCullough et al. 2001), and by inducing expression of the proapoptotic Bim (Puthalakath et al. 2007) and of ER oxidase 1α, thereby rendering the ER more oxidative and exacerbating ER stress (Marciniak et al. 2004). Misfolded proteins are eventually eliminated via proteins involved in the ERAD pathway, which are induced and controlled by both Ire1-XBP1 and ATF6 pathways (Yoshida et al. 2003).
No trigger for ER stress selectively elicits either adaptive responses or apoptosis. The switch between life and death is regulated by the complex interdependent UPR-signaling pathways that each may result in prosurvival or prodeath responses. The different time courses of the three main UPR branches may influence the cell fate (Lin et al. 2007a). The early termination of Ire1α activity is needed for cell death. Differential activation of PERK and Ire1α may lead to life or death (Lin et al. 2009). Cell death is induced by apoptosis and by caspase-independent necrosis. ER stress also induces autophagy (Ogata et al. 2006; Bernales et al. 2006; Hoyer-Hansen and Jaattela 2007). The PERK-ATF4 branch stimulates the expression of ATG12, an autophagy gene (Kouroku et al. 2007). This catabolic process removes unfolded proteins and their aggregates independently of the ubiquitin/proteasome system, thereby promoting cell survival. Ultimately, however, enhanced autophagic vacuolization may lead to non-apoptotic cell death (Levine and Kroemer 2008).
ER stress and cell death involve many Ca2+-dependent processes (Kim et al. 2008) including phospholipases, scramblases, nitric-oxide (NO) synthases, calpains, calcineurin, FKBP38, fortilin, a putative modulator of Mcl-1 (myeloid cell leukemia sequence 1), death-associated protein kinase 1, mitochondrial fission, and Ca2+-dependent pathways triggering autophagy. Some pathways require interplay between mitochondria and the ER in zones of close contact (Giorgi et al. 2008, 2009). These microdomains involve the close proximity of ER Ca2+-release channels like the IP3R and mitochondrial Ca2+-transport mechanisms, like the voltage-dependent anion channel (VDAC) and the Ca2+ uniporter (Giorgi et al. 2009). Changes in ER Ca2+ homeostasis in this way affect mitochondrial Ca2+ signaling. Lowering of the [Ca2+]ER by antiapoptotic proteins such as Bcl-2 has been described (Scorrano et al. 2003) and is expected to lower the sensitivity to apoptotic Ca2+ transfer from the ER to the mitochondria (Rizzuto et al. 2009). Bcl-Xl, a related antiapoptotic protein was found to induce prosurvival ER-to-mitochondria Ca2+ signaling by sensitizing the IP3R to basal levels of IP3 (White et al. 2005). ER-to-mitochondria Ca2+ signals can regulate cell survival by enhancing mitochondrial bioenergetics. Mitochondrial Ca2+ overload, on the other hand, by a larger or more persistent [Ca2+] rise was found to induce cell death (Rong and Distelhorst 2008). Cell death is characterized by mitochondrial outer membrane permeabilization (MOMP) and the loss of the mitochondrial transmembrane potential ΔΨm (Kroemer et al. 2007). Mitochondrial Ca2+ overload can cause breakdown of ΔΨm by activating the permeability transition pore (PTP). Loss of ΔΨm, however, seems to be a secondary event and not required for MOMP and the release of cytochrome c (Chipuk and Green 2008). Accordingly, PTP opening probably plays a role in necrosis but not apoptosis. Deficiency of Bax and Bak confers resistance to apoptotic cell death induced by conventional anticancer therapies. SERCA inhibitors like thapsigargin, however, can efficiently kill Bax/Bak−/− MEFs by inducing mitochondrial Ca2+ overload, PTP opening and necrotic cell death (Janssen et al. 2009). In addition to PTP opening, the activation and oligomerization of the executioner proapoptotic Bcl-2-family members Bax and Bak induce MOMP in response to a variety of apoptotic triggers (Chipuk and Green 2008; Brunelle and Letai 2009). The activity of Bax/Bak is tightly controlled by proteins of the Bcl-2 family. The antiapoptotic Bcl-2-family members, including Bcl-2, Bcl-Xl and Mcl-1, neutralize and prevent oligomerization of Bax/Bak, whereas activator proapoptotic BH3-only proteins, including Bim, cleaved Bid and cytosolic p53, directly bind to Bax/Bak, causing a conformational change, membrane insertion, and oligomerization. In addition, sensitizer BH3-only proteins, including Bad, Noxa, and Puma, bind to the antiapoptotic Bcl-2-family members, neutralizing their antiapoptotic activity. Many of these proteins affect ER Ca2+ homeostasis by binding to the IP3R and/or changing its phosphorylation, resulting in altered Ca2+-flux properties of the channel (Oakes et al. 2005; White et al. 2005; Rong and Distelhorst 2008).
DIABETES MELLITUS
Intracellular Ca2+ signaling is perturbed in this chronic metabolic disease with hyperglycemia. Resting [Ca2+]cyt increases, and stimulus-induced [Ca2+]cyt increases in many tissues decrease (Levy 1999; Verkhratsky and Fernyhough 2008). The [Ca2+]ER and SR [Ca2+] ([Ca2+]SR) decrease in the pancreatic ß-cell and in tissues affected by diabetic complications.
Pancreatic β-Cell
The progressive reduction in cell mass and eventually failure of the ß-cell is because of apoptotic cell death. ER stress is an important mechanism of apoptosis (Eizirik et al. 2008), at least in some types of diabetes (Akerfeldt et al. 2008). The [Ca2+]ER in the ß-cell is decreased, but the mechanism involved depends on the type of diabetes. The low [Ca2+]ER impairs proinsulin processing and transport (Guest et al. 1997). The subsequently activated UPR can lead to apoptosis resulting in insufficient insulin secretion (Oyadomari and Mori 2004; Eizirik et al. 2008). The very high secretion rate of ß-cells makes them very sensitive to apoptosis induced by ER Ca2+ depletion (Araki et al. 2003; Cardozo et al. 2005; Tonnesen et al. 2009).
Type-1 diabetes is characterized by an autoimmune ß-cell destruction caused by overproduction of NO (Gotoh and Mori 2006). Cytokines released from infiltrating T-cells and macrophages up-regulate inducible NO synthase in an NF-kB- and STAT-1-dependent manner (Eizirik et al. 2008). NO depletes ER Ca2+ in ß-cells by acting on SERCA and on the Ca2+-release channels (Oyadomari et al. 2001). NO down-regulates SERCA2b expression (Cardozo et al. 2005), perhaps through inhibition of the Sp1 transcription factor (Pirot et al. 2008). NO also reacts with superoxide anion to form peroxynitrite, which inhibits SERCA by reacting with two tyrosine residues in the channel-like domain (Viner et al. 1999; Grover et al. 2003). Peroxynitrite also activates RyR2 by poly-S-nitrosylation of the channel (Xu et al. 1998). The cytokines also up-regulate death protein 5, a BH3-only protein that contributes to Ca2+ depletion and ER stress (Gurzov et al. 2009). This depletion mainly occurs when IP3Rs and RyRs are stimulated (Luciani et al. 2009). Type-1 diabetes was furthermore associated with the single-nucleotide polymorphism rs2296336 in the gene encoding IP3R3 (Roach et al. 2006). Increased cholinergic tone with more acetylcholine-induced IP3 production, and up-regulation of the IP3R during hyperglycemia (Lee et al. 1999) therefore promote ER Ca2+ depletion. NO-induced ER stress in ß-cells does not activate the ATF6 branch of the UPR (Cardozo et al. 2005; Tonnesen et al. 2009). It is still debated whether cytokine-induced ER stress is a direct cause of ß-cell apoptosis or a parallel and/or downstream event (Akerfeldt et al. 2008).
Nonautoimmune type-1 diabetes in Wolfram syndrome is caused by mutations in the gene encoding the ER glycoprotein wolframin (Inoue et al. 1998; Strom et al. 1998). This genetic defect lowers [Ca2+]ER in ß-cells (Takei et al. 2006) and activates the UPR and triggers the apoptotic pathway (Yamada et al. 2006). Reconstitution of this ER-resident transmembrane protein into planar lipid bilayers induces a cation-selective ion channel (Osman et al. 2003). Wolframin also prevents ER stress via other mechanisms, e.g., by negatively regulating ATF6α through the ubiquitin-proteasome pathway (Fonseca et al. 2010).
Type-2 diabetes is characterized by insulin resistance in liver, skeletal muscle and adipose tissue, and a failure of the ß-cell to compensate for the increasing demand. Insulin resistance in liver and adipose tissue may be because of ER stress (van der Kallen et al. 2009). ER Ca2+ depletion with thapsigargin leads to insulin resistance (Ozcan et al. 2004), but so far there are no studies linking peripheral insulin resistance to a decreased [Ca2+]ER. A high-fat diet or obesity often leads to the development of type-2 diabetes (Eizirik et al. 2008). Free fatty acids trigger ß-cell loss (Leonardi et al. 2003). One model of lipotoxicity proposes that palmitate activates the UPR in ß-cells (Eizirik et al. 2008). The mechanism may again involve ER Ca2+ depletion (Cunha et al. 2008; Gwiazda et al. 2009) by decreased expression (Roe et al. 1994; Evans-Molina et al. 2009) or activity of SERCA (Cunha et al. 2008). Peroxisome proliferator-activated receptor-γ (PPAR-γ) agonists, which improve sensitivity to insulin, also restore SERCA expression and attenuate ER stress in the ß-cell (Evans-Molina et al. 2009). Despite variation in the gene encoding SERCA3 in type-2 diabetic patients (Varadi et al. 1999), insulin secretion and blood glucose levels were normal in SERCA3−/− mice (Arredouani et al. 2002), probably because of compensatory mechanisms.
Polymorphisms in the gene for insulin receptor substrate 1 (IRS-1) have been linked to type-2 diabetes (Almind et al. 1993). IRS-1 directly interacts with SERCA3 (Borge and Wolf 2003). Mice with deleted IRS-1 have reduced SERCA2b and 3 levels, more transient increases in [Ca2+]cyt and less insulin secretion (Kulkarni et al. 2004).
Diabetic Cardiomyopathy
The remodeling of the SR resulting in a slower Ca2+ uptake, a lower [Ca2+]SR, and release of less activator Ca2+, slows relaxation kinetics of the ventricle and eventually leads to systolic dysfunction, independently of vascular or valve disease (Rubler et al. 1972). Hyperglycemia causes these effects (Ren et al. 1997). The changes in SR Ca2+ handling depend on the type of diabetes, the experimental model, the degree of hyperglycemia, and the extent of disease progression. SR function is already abnormal at an insulin-resistant stage before the manifestation of overt type-2 diabetes (Dutta et al. 2002; Wold et al. 2005; Vasanji et al. 2006; Reuter et al. 2008).
Phospholamban, which inhibits SERCA2a, becomes up-regulated (Kim et al. 2001; Choi et al. 2002; Belke et al. 2004; Zhou et al. 2006) at an early stage of the disease (Zhong et al. 2001). Its phosphorylation by protein kinase A and Ca2+/calmodulin-dependent protein kinase, which regulates the interaction with SERCA2a, and therefore stimulates Ca2+ uptake, decreases (Choi et al. 2002; Belke et al. 2004; Vasanji et al. 2004, 2006).
The activity and expression of SERCA2a decrease in the diabetic heart (Teshima et al. 2000; Kim et al. 2001; Trost et al. 2002; Choi et al. 2002; Belke et al. 2004; Vasanji et al. 2004; Wold et al. 2005; Zhang et al. 2008; Stolen et al. 2009; Wang et al. 2010). Reduced activity of SERCA2a is not only because of the effect on phospholamban, but also to increased formation of advanced glycation end products of SERCA2a (Bidasee et al. 2004), depressed activity of protein kinase A (Dutta et al. 2002), and sensory denervation leading to diminished production of NO and peroxynitrite, which at basal concentrations activate SERCA2a through S-nitrosylation of Cys-349 (Bencsik et al. 2008). The fact that peroxynitrite both stimulates (Adachi et al. 2004) and inhibits SERCA (Viner et al. 1999; Schmidt et al. 2003b) seems to indicate that the effect is very much dependent on the experimental conditions or perhaps on the isoform studied. SERCA2a expression decreases in a later stage of the disease (Zhong et al. 2001), perhaps by increased O-glycosylation of the transcription factor Sp1 with β-N-acetylglucosamine because of the hyperglycemia (Clark et al. 2003), or by reduced expression and activity of SIRT1, a histone deacetylase (Sulaiman et al. 2010). RyR2 function changes by formation of disulfide bonds between adjacent sulfhydryl groups (Bidasee et al. 2003a), by increased glycation (Bidasee et al. 2003b), by decreased FKBP12.6 expression and binding (Belke et al. 2004; Shao et al. 2007), by hyperphosphorylation (Shao et al. 2007; Stolen et al. 2009), and by a reduced density of T-tubules (Stolen et al. 2009). Two RyR populations appear: one with enhanced responsiveness to Ca2+ and another being unresponsive (Shao et al. 2007). Dysfunctional RyR2 can cause dyssynchronous and diastolic Ca2+ releases, sometimes with ventricular arrhythmia (Shao et al. 2007). Spontaneous Ca2+ sparks representing aberrant RyR2 activation increase in frequency (Yaras et al. 2005; Shao et al. 2007). SR Ca2+ leak increases (Belke et al. 2004; Stolen et al. 2009). Expression of RyR2 decreases (Teshima et al. 2000; Choi et al. 2002; Guner et al. 2004; Pereira et al. 2006; Zhou et al. 2006; Reuter et al. 2008; Wang et al. 2010) at later stages of the disease (Zhong et al. 2001). IP3R1, 2, and 3 become down-regulated, but these effects may be species related (Guner et al. 2004; Zhou et al. 2006). The roles of IP3Rs in the heart are furthermore not entirely clear.
Some treatments directly affect the Ca2+ signal. Overexpression of SERCA2a protects the heart from contractile dysfunction (Trost et al. 2002; Vetter et al. 2002; Sakata et al. 2007) and reverses established cardiomyopathy (Suarez et al. 2008) and the transcriptional profile induced by diabetes (Karakikes et al. 2009). SERCA2a expression and cardiac function can be normalized by PPAR-γ agonists (Shah et al. 2005), total triterpene acids from Cornus officinalis Sieb. (Qi et al. 2008), and the SIRT1 activator resveratrol (Sulaiman et al. 2010). Breviscapine in Chinese medicine decreases phospholamban expression and increases that of SERCA2a and RyR2 (Wang et al. 2010). Exercise training also normalizes abnormal Ca2+ signaling (Shao et al. 2009; Stolen et al. 2009).
Vascular Disease
Diabetes lowers [Ca2+]ER in the smooth-muscle cells, macrophages and platelets. These changes contribute to the vascular complications including atherosclerosis (Cooper et al. 2001).
In healthy smooth-muscle cells, basal levels of NO react with superoxide anion to form peroxynitrite, which together with glutathione reacts with Cys-674 of SERCA and increases its activity (Adachi et al. 2004). The hyperglycemia of diabetes induces high levels of oxidants that irreversibly oxidize Cys-674 leading to less S-glutathionylation-induced stimulation of SERCA2 (Adachi et al. 2004) and faster degradation (Ying et al. 2008). Insulin also inhibits SERCA via enhanced nitrotyrosine formation (Kobayashi et al. 2007). SERCA2 is also redistributed to a peri-nuclear pattern (Searls et al. 2010). The subsequently decreased [Ca2+]ER stimulates plasma-membrane Ca2+ influx and induces migration of the smooth-muscle cell, which contributes to neointimal hyperplasia and atherosclerosis (Tong et al. 2008). Dedifferentiation of smooth-muscle cells precedes their migration from the media to the intima. The up-regulation of the secretory-pathway Ca2+-ATPase 1 (SPCA1) in diabetes (Lai and Michelangeli 2009) probably reflects the change from a contractile to a secretory cell. Sp1 and YY1, transcription factors controlling SPCA1 transcription (Kawada et al. 2005), become more active in high glucose (Han and Kudlow 1997). The expression of IP3R and RyR decreases (Ma et al. 2008; Searls et al. 2010).
Macrophages in type-2 diabetes express more CHOP and are therefore more susceptible to ER stress-induced apoptosis. CHOP induces ER oxidase 1α, with hyperoxidation of the ER lumen and disulfide-bond formation between two cysteines in IP3R1. This causes dissociation of the disulfide isomerase-like protein ERp44 (Kang et al. 2008) and more IP3-induced Ca2+ release (Li et al. 2009). These changes favor plaque necrosis.
Altered Ca2+ signaling in platelets makes them hyperreactive. Their increased adhesiveness and aggregability contribute to the development of the angiopathy (Knobler et al. 1998). The hyperglycemia causes oxidant stress in platelets (Vericel et al. 2004), which enhances tyrosine nitration of SERCA2 and in this way decreases SERCA2 function and, at least at high HbA1C levels, expression in type-2 diabetic patients (Randriamboavonjy et al. 2008). PPAR-γ agonists decrease tyrosine nitration of SERCA and increase its expression. Increased levels of homocysteine in type-2 diabetic patients also release Ca2+ from agonist-sensitive Ca2+ stores (Zbidi et al. 2010). The direct stimulatory interaction of STIM1 with SERCA3 is impaired in type-2 diabetes (Lopez et al. 2008), which can explain the increased plasma-membrane Ca2+ entry, and the higher [Ca2+]cyt at rest and during thrombin stimulation (Saavedra et al. 2004). SERCA3b was up-regulated in type-1 diabetes (Chaabane et al. 2007). This isoform is involved in cell adhesion (Chaabane et al. 2006) and its up-regulation can thus explain the increased adhesiveness in diabetic patients.
Diabetic Nephropathy
This complication is an important cause of end-stage renal disease. Apoptosis induced by ER stress also occurs in the diabetic kidney (Liu et al. 2008). In podocytes, advanced glycation end products release ER Ca2+ and trigger a UPR leading to apoptosis during the early stage of the nephropathy (Chen et al. 2008). The loss of podocytes is an important determinant in the progression of the disease. Tubulointerstitial cells also show ER stress (Lindenmeyer et al. 2008), probably induced by the hyperglycemia and the massive protein reabsorption as a result of the proteinuria, but possible changes in [Ca2+]ER were not investigated. The activated UPR selectively enhances the prosurvival pathway of the response, suggesting that diabetic damage may occur independently of any terminal UPR process (Brosius and Kaufman 2008). Decreased IP3R1 expression in the afferent arteriole and mesangial cell leads to smaller [Ca2+]cyt increases in response to vasoconstrictors, resulting in renal hyperfiltration and glomerular damage (Sharma et al. 1999).
Sensory Neuropathy
Diabetic neuropathy can produce prolonged changes in the nervous system, with pain, sensory loss, food ulceration, infection, gangrene and poor wound healing (Huang et al. 2002; Verkhratsky and Fernyhough 2008). The [Ca2+]ER was decreased because of a decreased SERCA expression by a so far unidentified mechanism and by a decreased activity of the pump (Verkhratsky and Fernyhough 2008). The decreased activity of SERCA may be caused by impaired mitochondrial ATP production in diabetes because of reduced stimulation of insulin receptors (Fernyhough and Calcutt 2010). This effect on the mitochondria seems to be independent of the hyperglycemia. The decreased [Ca2+]ER then affects protein synthesis, posttranslational modification and trafficking, which in turn diminish the supply of voltage-gated Ca2+ channels to the axons, thus resulting in the decrease of nerve-conductance velocity (Verkhratsky and Fernyhough 2008). Stimulus-induced [Ca2+]cyt increases decrease (Kruglikov et al. 2004) as a result of the decreased [Ca2+]ER and probably also as a result of decreased IP3R function. Protein glycosylation with β-N-acetylglucosamine is increased in diabetes (Hu et al. 2005). Glycosylation of IP3R1 by β-N-acetylglucosamine decreases its function (Rengifo et al. 2007).
Salivary Glands
Abnormal Ca2+ signaling in the salivary glands leads to dryness of the mouth, loss of taste sensation, sialosis, and other disorders of the oral cavity (Nicolau et al. 2009). SERCA is inhibited in the submandibular gland of streptozotocin-induced diabetic rats and therefore [Ca2+]ER and IP3-induced [Ca2+]cyt increases decrease (Fedirko et al. 2006). The decreased [Ca2+]ER results in improper posttranslational processing, folding, and exit of ER proteins. This could explain the decreased saliva protein content and amylase activity.
NEUROLOGICAL DISEASES
Neural Ischemia
Ischemia depletes ER Ca2+. Both the decreased [Ca2+]ER (Paschen and Mengesdorf 2005) and the increased [Ca2+]cyt (Verkhratsky 2005) contribute to cell death. The induced UPR may lead to apoptosis in the peri-infarct area (DeGracia et al. 2002). The release of Ca2+ amplifies the [Ca2+]cyt increase evoked by ischemia-induced Ca2+ entry (Xiong et al. 2007). Inhibition of this release with dantrolene reduces cell injury (Wei and Perry 1996). The mechanism of ER Ca2+ depletion remains unclear. Cytosolic Ca2+ enhances NO synthesis, which inhibits mitochondrial electron transport, and augments the generation of reactive oxygen species (ROS) (Moncada and Erusalimsky 2002). SERCA becomes inhibited by excessive NO production (Doutheil et al. 2000), by ischemia-induced inhibition of the coupling of ATP hydrolysis to Ca2+ transport (Parsons et al. 1999), and by activated calpain by the increased [Ca2+]cyt (French et al. 2006; Bevers and Neumar 2008). ER Ca2+-release channels are affected during neural ischemia. RyR2 is activated by S-glutathionylation by NO and ROS (Bull et al. 2008), and by calpain-induced proteolysis (Rardon et al. 1990). Calpain causes proteolysis of the IP3R resulting in decreased IP3 binding, suggesting that site-specific cleavage decreases the affinity of the remaining protein species for IP3 (Nagata et al. 1994; Dahl et al. 2000). Although this would indicate that calpain prevents Ca2+ release, it is also possible that the proteolysis simply removes the ligand regulation of the channel and leads to baseline Ca2+ release from the ER, contributing to Ca2+ overload (Bevers and Neumar 2008). Calpain also inhibits IP3 metabolism by cleaving IP3 kinase B (Pattni et al. 2003), allowing it to act longer on the IP3R, thereby potentiating Ca2+ efflux from the ER (Bevers and Neumar 2008). Enhanced activity of phospholipase C and A2 during ischemia liberates free fatty acids, which release ER Ca2+ (O'Neil et al. 1999).
Neurodegeneration
Ca2+ signaling is often abnormal in neurodegenerative diseases (Mattson 2007). Diseases with an increased [Ca2+]ER, like Alzheimer disease (Tu et al. 2006; Berridge 2010), fall outside the scope of this review. ER stress and the UPR also occur in Parkinson disease (Ryu et al. 2002), amyotrophic lateral sclerosis (Kanekura et al. 2009), and polyglutamate diseases (Lindholm et al. 2006), but the effects on [Ca2+]ER are not well documented. We will focus on diseases with a decreased [Ca2+]ER.
Some lysosomal storage diseases lead to a decreased [Ca2+]ER. In neurons of GM1-gangliosidosis, GM1 accumulates at the ER membrane and depletes ER Ca2+ stores (Tessitore et al. 2004) by interacting with the phosphorylated form of the IP3R (Sano et al. 2009). The subsequent activation of the UPR leads to apoptosis (Sano et al. 2009). Silencing of IP3R1 with siRNA reduces the number of apoptotic cells (Sano et al. 2009). Increased Ca2+ release from the ER in Gaucher disease is because of overactivation of the RyR (Korkotian et al. 1999; Pelled et al. 2005), because glucosylceramide, the lipid that accumulates in this disease, directly modulates the RyR (Lloyd-Evans et al. 2003). In Sandhoff disease, SERCA activity is inhibited by the accumulation of GM2-ganglioside (Pelled et al. 2003), which depends on an exposed sialic-acid residue on GM2 (Ginzburg et al. 2008). The UPR is also activated by the accumulation of palmitoylated proteins in the infantile form of Batten disease, but ER Ca2+ handling was not investigated (Zhang et al. 2007). The decreased SERCA2 and IP3R1 expression in Niemann-Pick A disease did not activate a UPR (Ginzburg and Futerman 2005). The suggestion that the UPR is a common mediator of apoptosis in neurodegenerative lysosomal storage diseases (Wei et al. 2008a) can therefore be questioned (Farfel-Becker et al. 2009). Increasing [Ca2+]ER by inhibiting the RyR or by SERCA2b overexpression partially restored mutant-enzyme homeostasis in several lysosomal storage diseases (Ong et al. 2010).
Transmissible spongiform encephalopathies include Creutzfeldt-Jakob disease in humans, and bovine spongiform encephalopathy and scrapie in animals (Prusiner 1998). These diseases are associated with extracellular accumulation of a conformationally modified abnormal isoform of the prion protein, a widely expressed plasma membrane-associated glycoprotein with highest levels of expression on neurons and glia. This protein binds to the cell surface and sends a signal to the ER to release Ca2+ through the IP3R and RyR (Hetz et al. 2003; Ferreiro et al. 2006, 2008). The subsequent decreased [Ca2+]ER leads to a UPR and activates the ER-stress-induced apoptosis pathway. Dantrolene and xestospongin C, which are inhibitors of the RyR and IP3R respectively, prevent neuronal death (Ferreiro et al. 2006, 2008).
Neuropathic Pain
Neuropathic pain is pain arising from nerve injury. The soma of sensory neurons is affected by injuring the peripheral axons. Spinal-nerve ligation depletes ER Ca2+ (Rigaud et al. 2009) by a loss of ER and therefore of SERCA (Gemes et al. 2009). Rigaud et al. (2009) suggested that this may trigger a UPR, but this was not directly shown. Depletion of ER Ca2+ stores thus contributes to the pathogenesis of neuropathic pain.
Anesthesia
General anesthesia may cause cognitive deficits after surgery (Moller et al. 1998). Inhalation anesthetics can overactivate the IP3R, with excessive ER Ca2+ release leading to apoptosis (Wei et al. 2008b; Yang et al. 2008). Neurons with enhanced IP3R activity, for example, in familial Alzheimer or Huntington disease, may be especially vulnerable.
CARDIOVASCULAR DISEASES
Atherosclerosis
Macrophages play a critical role in this chronic inflammatory disease (Fan and Watanabe 2003). They accumulate unesterified cholesterol in advanced lesions, which changes the fluidity of the ER membrane and in this way inhibits SERCA (Li et al. 2004). Depletion of ER Ca2+ stores induces a UPR and apoptosis (Feng et al. 2003). Excessive apoptosis plays a key role in the progression of atherosclerosis. The UPR also sets up a positive feedback loop with more Ca2+ release via induction of ER oxidase 1α. Hyperoxidation of the ER lumen activates Ca2+ release (Li et al. 2009) by disulfide-bond formation between two cysteines in IP3R1 and dissociation of the inhibitory ERp44 (Kang et al. 2008). This mechanism complements the increased ER Ca2+ leak through induction of a truncated variant of SERCA1 through the PERK pathway (Chami et al. 2008).
Homocysteine, a risk factor for cardiovascular disease, depletes ER Ca2+ in aortic smooth muscle, induces ER stress and in this way accelerates atherosclerosis (Dickhout et al. 2007). Also increased production of superoxide anion inhibits SERCA in blood vessels (Tong et al. 2009).
Endothelial dysfunction already occurs early during atherogenesis. Increased peroxynitrite formation in the endothelium inhibits SERCA, depletes ER Ca2+ and induces a UPR (Dickhout et al. 2005).
Chronic Heart Failure
Reduced [Ca2+]cyt increases caused by a decreased SR Ca2+ content make the heart muscle too weak to pump sufficient blood through the body (Bers et al. 2003). Ca2+ pumping is reduced because of a decreased ratio of SERCA2a relative to phospholamban expression (Hasenfuss and Pieske 2002), or because phospholamban is either mutated with more inhibition of SERCA2a (Franz et al. 2001; Schmitt et al. 2003; Haghighi et al. 2006; Kranias and Bers 2007) or less phosphorylated (Frank et al. 2002; Bers et al. 2003; Yano et al. 2008) because of a more active protein phosphatase 1 (del Monte and Hajjar 2008). SERCA2 mutations have not been linked to heart failure (Schmidt et al. 2003a). SERCA3f, an isoform with a specific role in ER stress, becomes up-regulated (Dally et al. 2009). Enhanced Na+-Ca2+ exchange leading to more extrusion of Ca2+ from the cell also depletes SR Ca2+ (O'Rourke et al. 1999). Subconductance states of the RyR2 and decreased coupled gating of RyR2-channel clusters can increase SR Ca2+ leak during diastole (Reiken et al. 2003; Wehrens et al. 2003, 2005a, 2006; Lehnart et al. 2005, 2008; Huang et al. 2006; Zalk et al. 2007). Hyperactivation of RyR2 may arise from activated protein kinase A by sympathetic neurons and increased levels of catecholamines, and subsequent hyperphosphorylation of RyR2 at Ser-2809 and dissociation of FKBP12.6 (Marx et al. 2000) (but see Bers et al. 2003; Seidler et al. 2007; Yano et al. 2008). Enhanced Ca2+/calmodulin-dependent protein kinase δ-dependent phosphorylation of RyR2 at Ser-2815 also increases diastolic Ca2+ leak and reduces SR Ca2+ load (Ai et al. 2005).
ß-blockers prevent the hyperphosphorylation of RyR2 by protein kinase A, normalize channel function and improve cardiac function (Reiken et al. 2001; Doi et al. 2002). Heart failure can also be prevented by JTV519, which inhibits the dissociation of FKBP12.6 from RyR2; thereby stabilizing the channel, enhancing cooperativity among the subunits, and promoting coupled gating (Yano et al. 2003; Wehrens et al. 2005b). Overexpression of SERCA2 (Inesi et al. 2008; Kawase and Hajjar 2008), of pseudophosphorylated phospholamban (Hoshijima et al. 2002), or of FKBP12.6 (Huang et al. 2006), gene transfer of a phospholamban-targeted antibody (Dieterle et al. 2005), and down-regulation of phospholamban (Andino et al. 2008) can correct in vivo cardiac function. Modification of SERCA/phospholamban activity/expression is a promising target for remediation of cardiac disease. Indeed, a clinical trial of SERCA2a-gene therapy is initiated (Jaski et al. 2009).
VIRUS INFECTION
Complete virions or viral proteins can decrease the [Ca2+]ER. Some viruses stimulate Ca2+ release via the IP3R, often by increasing the [IP3] (Table 1). Other viruses release Ca2+ via an increased expression or function of the RyR. They may also decrease SERCA activity or expression, enhance the passive Ca2+ leak from the ER, or form pores in the ER membrane. Nef of human immunodeficiency virus type 1 directly interacts with the IP3R and activates Ca2+ entry, without however inducing Ca2+ release (Foti et al. 1999; Manninen and Saksela 2002).
Table 1.
Effects of complete virions or viral proteins on the [Ca2+]ER.
| Target | Effect | Virus or viral protein | Reference |
|---|---|---|---|
| IP3R | Increased IP3R activity | p12I of human T-cell lymphotropic virus type 1 | Ding et al. 2002 |
| glycoproteins of human herpes simplex virus type 1 and type 2 | Cheshenko et al. 2003 | ||
| Increased IP3R activity because of increased IP3 production | influenza A virus | Hartshorn et al. 1988 | |
| Poliovirus | Guinea et al. 1989 | ||
| gp120 and Tat of human immunodeficiency virus type 1 | Dayanithi et al. 1995 | ||
| Mayne et al. 2000 | |||
| Haughey and Mattson 2002 | |||
| nonstructural protein 4 of rotavirus | Tian et al. 1995 | ||
| Dong et al. 1997 | |||
| Seo et al. 2008 | |||
| gp86 of human cytomegalovirus | Keay et al. 1995 | ||
| G-protein coupled receptor and viral macrophage inflammatory protein-I and -II of human herpes virus 8 | Arvanitakis et al. 1997 | ||
| Nakano et al. 2003 | |||
| RyR | Increased RyR activity | Tat of human immunodeficiency virus type 1 | Norman et al. 2008 |
| Poliovirus | Brisac et al. 2010 | ||
| Increased RyR expression | Borna disease virus | Williams and Lipkin 2006 | |
| SERCA | Decreased SERCA activity | core protein of hepatitis C virus | Benali-Furet et al. 2005 |
| Decreased SERCA expression | Borna disease virus | Williams and Lipkin 2006 | |
| latent membrane protein-1 of Epstein-Barr virus | Dellis et al. 2009 | ||
| Passive Ca2+ leak | Enhanced passive Ca2+ leak | nonstructural protein 5A of hepatitis C virus | Robinson and Marchant 2008 |
| ER membrane | Pore formation | p7 and core protein of hepatitis C virus | Griffin et al. 2003 |
| Bergqvist et al. 2003 | |||
| 2B and 2BC proteins of entero- and rhinoviruses | Aldabe et al. 1997 | ||
| de Jong et al. 2008 | |||
| nonstructural protein 4 of rotavirus | Zhou et al. 2009 | ||
| pUL37x1 protein of human cytomegalovirus | Sharon-Friling et al. 2006 | ||
| Zhou et al. 2009 | |||
| 6K protein of alphavirus | Antoine et al. 2007 |
ER Ca2+ depletion may be apoptotic or antiapoptotic, depending on the virus, its life cycle, and the induced pathology (Chami et al. 2006; Zhou et al. 2009). Ca2+ depletion by, for example, enteroviruses and human cytomegalovirus, delays apoptosis, giving the virus more time for replication. These viruses reduce ER-mitochondrial Ca2+ fluxes and prevent opening of the PTP with less release of cytochrome c and less caspase activation (van Kuppeveld et al. 2005; Sharon-Friling et al. 2006). ER and also Golgi Ca2+ depletion by e.g., enteroviruses leads to the accumulation of ER/Golgi-derived vesicles, where viral RNA replication takes place (van Kuppeveld et al. 2005), and inhibits vesicular protein trafficking and so down-regulates immune responses of the ghost (de Jong et al. 2006). In contrast, ER Ca2+ depletion by hepatitis C virus in liver promotes apoptosis and facilitates virion release because of translocation of Bax to the mitochondria, depolarization of the mitochondrial membrane, release of cytochrome c, and activation of caspase 3 (Benali-Furet et al. 2005). Abnormal Ca2+ signaling by Gp120 and Tat causes neuronal apoptosis and dysfunction and eventually AIDS dementia (Haughey and Mattson 2002). The decreased SERCA expression and increased RyR expression in Borna disease lead to ER stress, activation of the UPR and apoptotic degeneration of the cerebellum and hippocampus (Williams and Lipkin 2006). ER Ca2+ depletion also increases [Ca2+]cyt and therefore activates Ca2+-dependent enzymatic processes and transcription factors, promoting virus replication and the induction of a variety of responses.
Some antiviral drugs directly affect the [Ca2+]ER. Human immunodeficiency virus-protease inhibitors induce the accumulation of free cholesterol in the ER of macrophages, deplete ER Ca2+, and induce ER stress and apoptosis (Zhou et al. 2005). This may explain the increased incidence of atherosclerosis and cardiovascular disease in patients treated with protease inhibitors. Lopinavir and ritonavir also deplete ER Ca2+ and activate the UPR in intestinal epithelial cells, thus disrupting the epithelial barrier integrity with drug-induced diarrhea as a frequent side effect (Wu et al. 2010).
Bacteria can also cause ER stress. For example, Shiga toxins of Shigella dysenteriae serotype 1 and some serotypes of Escherichia coli deplete ER Ca2+ and trigger a UPR with apoptosis (Lee et al. 2008).
LUNG DISEASES
Asthma
SERCA2 in airway smooth muscle is down-regulated in this chronic inflammatory disease with airway remodeling, leading to more sustained [Ca2+]cyt increases and enhanced cell motility, proliferation and secretion (Mahn et al. 2009). Decreased SERCA expression might be caused by enhanced cytokine production during airway inflammation (Sathish et al. 2009).
ORMDL3 is a genetic risk factor associated with asthma (Moffatt et al. 2007). The gene encodes an ER protein (Hjelmqvist et al. 2002) that binds to and inhibits SERCA, leading to a decreased [Ca2+]ER and an UPR (Cantero-Recasens et al. 2010).
Toxicity
Chronic exposure to cadmium in humans is associated with lung, but also bone and renal damage. Cadmium stimulates the IP3R through IP3 production, and inhibits SERCA (Biagioli et al. 2008). The reduced [Ca2+]ER leads to ER stress and ER-mediated apoptosis.
LIVER DISEASES
Nonalcoholic Fatty Liver
Triglycerides and free fatty acids accumulate in the liver of obese individuals. Palmitate and stearate deplete ER Ca2+ stores and activate the UPR leading to cell death (Wei et al. 2009).
Cholestatic Liver Disease
Intrahepatic accumulation of bile acids induces hepatocellular injury. Glycochenodeoxycholic acid depletes ER Ca2+ and induces a UPR and apoptosis (Tsuchiya et al. 2006). It is unclear to what extent the decreasing [Ca2+]ER directly contributes to the pathology.
Burn Injury
Severe burn injury impairs liver function. Thermal skin injury in rats depletes ER Ca2+ in the liver (Jeschke et al. 2010). This effect is because of an activation of the IP3R by released cytochrome c and an increased IP3R expression. ER Ca2+ depletion activates the UPR leading to apoptosis.
SKELETAL-MUSCLE DISEASES
Brody Disease
Mutations in the gene of SERCA1 (Odermatt et al. 1996) leading to reduced Ca2+-pump expression or activity and hence a prolonged [Ca2+]cyt elevation cause muscle cramping and impaired relaxation during exercise (Brody 1969). Chianina cattle congenital pseudomyotonia (Drogemuller et al. 2008) and Belgian Blue cattle congenital muscular dystony (Charlier et al. 2008) are related pathologies. Until now, no evidence for a UPR leading to apoptosis has been provided, but the ongoing contracture in cattle may induce rhabdomyolysis (Sacchetto et al. 2009).
Autosomal Centronuclear Myopathy
Centronuclear myopathies are characterized by small myofibers with centrally placed nuclei. Mutations of the muscle-specific inositol phosphatase MIP/MTMR14 cause the dominant form of the disease (Tosch et al. 2006; Shen et al. 2009). Mice deficient in this phosphatase produce less contractile force, have prolonged relaxation, and show exacerbated fatigue. PtdIns(3,5)P2 and PtdIns(3,4)P2 accumulate and directly activate RyR1, resulting in an increased Ca2+ leak, a lower [Ca2+]SR and a higher [Ca2+]cyt. This proposed effect of PtdIns(3,5)P2 and PtdIns(3,4)P2 on RyR1 still needs confirmation.
Central Core Disease
Some mutations in the gene for RyR1 lead to hypotonia, proximal-muscle weakness, and central cores on muscle biopsy (Zhang et al. 1993). They can lead to a leaky channel and a reduced [Ca2+]SR, with deleterious consequences for contractions (Brini et al. 2005).
SKIN DISEASE
Darier disease is an inherited skin disorder with less adhesion between epidermal cells and abnormal keratinization. Mutations in the gene encoding SERCA2 (Sakuntabhai et al. 1999) lower the [Ca2+]ER in keratinocytes (Foggia et al. 2006). ER stress may occur (Onozuka et al. 2006).
The fruit hull of mangosteen is used in Southeast Asia to treat skin infections and wounds (Mahabusarakam et al. 1987). α-mangostin inhibits SERCA, leading to a UPR and apoptosis (Sato et al. 2004).
CANCER
Malignant Transformation
Altered Ca2+ signaling may be involved in malignant transformation (Monteith et al. 2007). [Ca2+]ER is often decreased, making the cell resistant to apoptosis. Subsequent Ca2+ entry increases [Ca2+]cyt and changes gene expression, DNA repair, and cell-cycle regulation, resulting in cancer development (Korosec et al. 2006; Monteith et al. 2007; Lipskaia et al. 2009).
Human hepatitis B virus, an etiologic factor of hepatocellular carcinoma, integrates with its DNA into the gene for SERCA1 and cis-activates chimeric transcripts producing inactive proteins that deplete ER Ca2+ stores (Chami et al. 2000). Mice with a heterozygous deletion of the gene encoding SERCA2 (Liu et al. 2001) and some patients with Darier disease (Burge and Wilkinson 1992) develop squamous cell carcinomas. Neoplastic transformation has been linked to a down-regulated SERCA2 (Pacifico et al. 2003; Vanoverberghe et al. 2004; Bergner et al. 2009) or SERCA3 (Gelebart et al. 2002; Brouland et al. 2005), e.g., by somatic or germ-like mutations or epigenetic mechanisms involving promotor methylation (Endo et al. 2004; Korosec et al. 2006, 2008). ER Ca2+ depletion can also result from overexpression of Ca2+-release channels. IP3R3 is overexpressed in disseminated gastric cancer (Sakakura et al. 2003). The amplification of the gene for IP3R2 increases in some tumors (Heighway et al. 1996). Increased IP3R expression does not occur in all cancers (Bergner et al. 2009).
Anticancer Drugs
Most chemotherapeutic approaches kill tumor cells via the induction of MOMP. However, drugs that compromise the normal function and homeostasis of the ER may also induce programmed cell death or improve the therapeutic efficacy of existing anticancer drugs (Boelens et al. 2007). Some drugs primarily reduce the [Ca2+]ER and in this way induce a UPR leading to apoptosis. Known SERCA blockers like thapsigargin and curcumin have anticancer activity (Denmeade et al. 2003; Anand et al. 2008; Bakhshi et al. 2008). Anticancer drugs like the stable analogue of the Bcl-2 antagonist HA 14-1 (Hermanson et al. 2009), artemisinin (Stockwin et al. 2009), amiloride analogues (Park et al. 2009), and 2,5-dimethyl-celecoxib (Johnson et al. 2002; Pyrko et al. 2007) also inhibit SERCA with ER stress as a result. SERCA2 expression decreases after photodynamic therapy with hypericin (Buytaert et al. 2006). ER Ca2+ stores are depleted by euplotin C through activated RyRs (Cervia et al. 2006), by paclitaxel through formation of Bax dimers in the ER (Liao et al. 2008), and by epigallocatechin gallate through inhibited protein processing at the level of glucosidase II (Magyar et al. 2009) and GRP78/BiP (Ermakova et al. 2006). Ca2+ depletion and ER stress are also induced by cisplatin (Nawrocki et al. 2005), dehydrocostuslactone (Hsu et al. 2009; Hung et al. 2010), honokiol (Chen et al. 2010), diaryl- and triarylmethanes (Abdelrahim et al. 2006), inhibitors of heat shock protein 90 (Taiyab et al. 2009), n-3 long-chain polyunsaturated fatty acids (Jakobsen et al. 2008), rhein (Lai et al. 2009), cardiotoxin III (Chien et al. 2008), homoharringtonine (Jie et al. 2007), berberine (Lin et al. 2007b), diindolylmethane (Savino et al. 2006), the multi-kinase inhibitor sorafenib (Rahmani et al. 2007), the p210 bcr-abl tyrosine-kinase inhibitor STI571 (Pattacini et al. 2004), parthenolide (Zhang et al. 2004), photodynamic therapy with tetra-S-glycosylated porphyrin (Thompson et al. 2008), and by many other drugs. Edelfosine leads to Bax/Bak-mediated ER Ca2+ depletion and apoptosis, without inducing a UPR (Nieto-Miguel et al. 2007).
Ca2+ depletion-induced ER stress can also lead to autophagy, for example, in response to the tyrosine-kinase inhibitor imatinib (Bellodi et al. 2009), or to necrosis, for example, in therapy-resistant tumors with down-regulated Bax or Bak (Janssen et al. 2009).
CONCLUDING REMARKS
Depletion of ER Ca2+ occurs in many diseases. The accompanying ER stress often triggers a UPR leading to apoptosis. The release of insufficient activator Ca2+ may compromise essential cell functions. We now begin to understand the molecular mechanisms that reduce the ER Ca2+ content. Some therapies already directly target the Ca2+-signaling pathway. A better understanding of the defective Ca2+ signal and the development of better drugs targeting the proteins involved will eventually result in better treatments for these various diseases.
ACKNOWLEDGMENTS
Work performed in our laboratory was supported by grants from the Research Foundation—Flanders, the Concerted Actions of the K.U.Leuven, and the Interuniversity Attraction Poles Programme.
Footnotes
Editors: Martin D. Bootman, Michael J. Berridge, James W. Putney, and H. Llewelyn Roderick
Additional Perspectives on Calcium Signaling available at www.cshperspectives.org
REFERENCES
- Abdelrahim M, Newman K, Vanderlaag K, Samudio I, Safe S 2006. 3,3'-diindolylmethane (DIM) and its derivatives induce apoptosis in pancreatic cancer cells through endoplasmic reticulum stress-dependent upregulation of DR5. Carcinogenesis 27: 717–728 [DOI] [PubMed] [Google Scholar]
- Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, Cohen RA 2004. S-glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med 10: 1200–1207 [DOI] [PubMed] [Google Scholar]
- Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM 2005. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res 97: 1314–1322 [DOI] [PubMed] [Google Scholar]
- Akerfeldt MC, Howes J, Chan JY, Stevens VA, Boubenna N, McGuire HM, King C, Biden TJ, Laybutt DR 2008. Cytokine-induced β-cell death is independent of endoplasmic reticulum stress signaling. Diabetes 57: 3034–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldabe R, Irurzun A, Carrasco L 1997. Poliovirus protein 2BC increases cytosolic free calcium concentrations. J Virol 71: 6214–6217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almind K, Bjorbaek C, Vestergaard H, Hansen T, Echwald S, Pedersen O 1993. Aminoacid polymorphisms of insulin receptor substrate-1 in non-insulin-dependent diabetes mellitus. Lancet 342: 828–832 [DOI] [PubMed] [Google Scholar]
- Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB 2008. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett 267: 133–164 [DOI] [PubMed] [Google Scholar]
- Andino LM, Takeda M, Kasahara H, Jakymiw A, Byrne BJ, Lewin AS 2008. AAV-mediated knockdown of phospholamban leads to improved contractility and calcium handling in cardiomyocytes. J Gene Med 10: 132–142 [DOI] [PubMed] [Google Scholar]
- Antoine AF, Montpellier C, Cailliau K, Browaeys-Poly E, Vilain JP, Dubuisson J 2007. The αvirus 6K protein activates endogenous ionic conductances when expressed in Xenopus oocytes. J Membr Biol 215: 37–48 [DOI] [PubMed] [Google Scholar]
- Araki E, Oyadomari S, Mori M 2003. Impact of endoplasmic reticulum stress pathway on pancreatic β-cells and diabetes mellitus. Exp Biol Med 228: 1213–1217 [DOI] [PubMed] [Google Scholar]
- Arredouani A, Guiot Y, Jonas JC, Liu LH, Nenquin M, Pertusa JA, Rahier J, Rolland JF, Shull GE, Stevens M, et al. 2002. SERCA3 ablation does not impair insulin secretion but suggests distinct roles of different sarcoendoplasmic reticulum Ca2+ pumps for Ca2+ homeostasis in pancreatic β-cells. Diabetes 51: 3245–3253 [DOI] [PubMed] [Google Scholar]
- Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn MC, Cesarman E 1997. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature 385: 347–350 [DOI] [PubMed] [Google Scholar]
- Austin RC 2009. The unfolded protein response in health and disease. Antioxid Redox Signal 11: 2279–2287 [DOI] [PubMed] [Google Scholar]
- Bakhshi J, Weinstein L, Poksay KS, Nishinaga B, Bredesen DE, Rao RV 2008. Coupling endoplasmic reticulum stress to the cell death program in mouse melanoma cells: effect of curcumin. Apoptosis 13: 904–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard NA, Laver DR, Dulhunty AF 2004. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog Biophys Mol Biol 85: 33–69 [DOI] [PubMed] [Google Scholar]
- Belke DD, Swanson EA, Dillmann WH 2004. Decreased sarcoplasmic reticulum activity and contractility in diabetic db/db mouse heart. Diabetes 53: 3201–3208 [DOI] [PubMed] [Google Scholar]
- Bellodi C, Lidonnici MR, Hamilton A, Helgason GV, Soliera AR, Ronchetti M, Galavotti S, Young KW, Selmi T, Yacobi R, et al. 2009. Targeting autophagy potentiates tyrosine kinase inhibitor-induced cell death in Philadelphia chromosome-positive cells, including primary CML stem cells. J Clin Invest 119: 1109–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont PJ, Tadimalla A, Chen WJ, Martindale JJ, Thuerauf DJ, Marcinko M, Gude N, Sussman MA, Glembotski CC 2008. Coordination of growth and endoplasmic reticulum stress signaling by regulator of calcineurin 1 (RCAN1), a novel ATF6-inducible gene. J Biol Chem 283: 14012–14021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benali-Furet NL, Chami M, Houel L, De Giorgi F, Vernejoul F, Lagorce D, Buscail L, Bartenschlager R, Ichas F, Rizzuto R, et al. 2005. Hepatitis C virus core triggers apoptosis in liver cells by inducing ER stress and ER calcium depletion. Oncogene 24: 4921–4933 [DOI] [PubMed] [Google Scholar]
- Bencsik P, Kupai K, Giricz Z, Gorbe A, Huliak I, Furst S, Dux L, Csont T, Jancso G, Ferdinandy P 2008. Cardiac capsaicin-sensitive sensory nerves regulate myocardial relaxation via S-nitrosylation of SERCA: role of peroxynitrite. Br J Pharmacol 153: 488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergner A, Kellner J, Tufman A, Huber RM 2009. Endoplasmic reticulum Ca2+-homeostasis is altered in small and non-small cell lung cancer cell lines. J Exp Clin Cancer Res 28: 25 doi:101186/1756-9966-28-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergqvist A, Sundstrom S, Dimberg LY, Gylfe E, Masucci MG 2003. The hepatitis C virus core protein modulates T cell responses by inducing spontaneous and altering T-cell receptor-triggered Ca2+ oscillations. J Biol Chem 278: 18877–18883 [DOI] [PubMed] [Google Scholar]
- Bernales S, McDonald KL, Walter P 2006. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol 4: e423 doi:101371/journalpbio0040423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ 2002. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium 32: 235–249 [DOI] [PubMed] [Google Scholar]
- Berridge MJ 2010. Calcium hypothesis of Alzheimer's disease. Pflugers Arch 459: 441–449 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD 2000. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21 [DOI] [PubMed] [Google Scholar]
- Bers DM, Eisner DA, Valdivia HH 2003. Sarcoplasmic reticulum Ca2+ and heart failure: roles of diastolic leak and Ca2+ transport. Circ Res 93: 487–490 [DOI] [PubMed] [Google Scholar]
- Bevers MB, Neumar RW 2008. Mechanistic role of calpains in postischemic neurodegeneration. J Cereb Blood Flow Metab 28: 655–673 [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I 2009. Calcium signaling and neurodegenerative diseases. Trends Mol Med 15: 89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagioli M, Pifferi S, Ragghianti M, Bucci S, Rizzuto R, Pinton P 2008. Endoplasmic reticulum stress and alteration in calcium homeostasis are involved in cadmium-induced apoptosis. Cell Calcium 43: 184–195 [DOI] [PubMed] [Google Scholar]
- Bidasee KR, Nallani K, Besch HR Jr, Dincer UD 2003a. Streptozotocin-induced diabetes increases disulfide bond formation on cardiac ryanodine receptor (RyR2). J Pharmacol Exp Ther 305: 989–998 [DOI] [PubMed] [Google Scholar]
- Bidasee KR, Nallani K, Yu Y, Cocklin RR, Zhang Y, Wang M, Dincer UD, Besch HR Jr 2003b. Chronic diabetes increases advanced glycation end products on cardiac ryanodine receptors/calcium-release channels. Diabetes 52: 1825–1836 [DOI] [PubMed] [Google Scholar]
- Bidasee KR, Zhang Y, Shao CH, Wang M, Patel KP, Dincer UD, Besch HR Jr 2004. Diabetes increases formation of advanced glycation end products on sarco(endo)plasmic reticulum Ca2+-ATPase. Diabetes 53: 463–473 [DOI] [PubMed] [Google Scholar]
- Bidaud I, Mezghrani A, Swayne LA, Monteil A, Lory P 2006. Voltage-gated calcium channels in genetic diseases. Biochim Biophys Acta 1763: 1169–1174 [DOI] [PubMed] [Google Scholar]
- Blayney LM, Lai FA 2009. Ryanodine receptor-mediated arrhythmias and sudden cardiac death. Pharmacol Ther 123: 151–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelens J, Lust S, Offner F, Bracke ME, Vanhoecke BW 2007. The endoplasmic reticulum: a target for new anticancer drugs. In Vivo 21: 215–226 [PubMed] [Google Scholar]
- Borge PD Jr, Wolf BA 2003. Insulin receptor substrate 1 regulation of sarco-endoplasmic reticulum calcium ATPase 3 in insulin-secreting β-cells. J Biol Chem 278: 11359–11368 [DOI] [PubMed] [Google Scholar]
- Bosanac I, Michikawa T, Mikoshiba K, Ikura M 2004. Structural insights into the regulatory mechanism of IP3 receptor. Biochim Biophys Acta 1742: 89–102 [DOI] [PubMed] [Google Scholar]
- Brandman O, Liou J, Park WS, Meyer T 2007. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell 131: 1327–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brini M, Carafoli E 2009. Calcium pumps in health and disease. Physiol Rev 89: 1341–1378 [DOI] [PubMed] [Google Scholar]
- Brini M, Manni S, Pierobon N, Du GG, Sharma P, MacLennan DH, Carafoli E 2005. Ca2+ signaling in HEK-293 and skeletal muscle cells expressing recombinant ryanodine receptors harboring malignant hyperthermia and central core disease mutations. J Biol Chem 280: 15380–15389 [DOI] [PubMed] [Google Scholar]
- Brisac C, Teoule F, Autret A, Pelletier I, Colbere-Garapin F, Brenner C, Lemaire C, Blondel B 2010. Calcium flux between the endoplasmic reticulum and mitochondria contributes to poliovirus-induced apoptosis. J Virol 84: 12226–12235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody IA 1969. Muscle contracture induced by exercise: a syndrome attributable to decreased relaxing factor. N Engl J Med 281: 187–192 [DOI] [PubMed] [Google Scholar]
- Brosius FC III, Kaufman RJ 2008. Is the ER stressed out in diabetic kidney disease? J Am Soc Nephrol 19: 2040–2042 [DOI] [PubMed] [Google Scholar]
- Brouland JP, Gelebart P, Kovacs T, Enouf J, Grossmann J, Papp B 2005. The loss of sarco/endoplasmic reticulum calcium transport ATPase 3 expression is an early event during the multistep process of colon carcinogenesis. Am J Pathol 167: 233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelle JK, Letai A 2009. Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci 122: 437–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull R, Finkelstein JP, Galvez J, Sanchez G, Donoso P, Behrens MI, Hidalgo C 2008. Ischemia enhances activation by Ca2+ and redox modification of ryanodine receptor channels from rat brain cortex. J Neurosci 28: 9463–9472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge SM, Wilkinson JD 1992. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol 27: 40–50 [DOI] [PubMed] [Google Scholar]
- Buytaert E, Callewaert G, Hendrickx N, Scorrano L, Hartmann D, Missiaen L, Vandenheede JR, Heirman I, Grooten J, Agostinis P 2006. Role of endoplasmic reticulum depletion and multidomain proapoptotic BAX and BAK proteins in shaping cell death after hypericin-mediated photodynamic therapy. FASEB J 20: 756–758 [DOI] [PubMed] [Google Scholar]
- Cahalan MD 2009. STIMulating store-operated Ca2+ entry. Nat Cell Biol 11: 669–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantero-Recasens G, Fandos C, Rubio-Moscardo F, Valverde MA, Vicente R 2010. The asthma-associated ORMDL3 gene product regulates endoplasmic reticulum-mediated calcium signaling and cellular stress. Hum Mol Genet 19: 111–121 [DOI] [PubMed] [Google Scholar]
- Cardozo AK, Ortis F, Storling J, Feng YM, Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz A, Eizirik DL 2005. Cytokines down-regulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic β-cells. Diabetes 54: 452–461 [DOI] [PubMed] [Google Scholar]
- Cervia D, Martini D, Garcia-Gil M, Di Giuseppe G, Guella G, Dini F, Bagnoli P 2006. Cytotoxic effects and apoptotic signalling mechanisms of the sesquiterpenoid euplotin C, a secondary metabolite of the marine ciliate Euplotes crassus, in tumour cells. Apoptosis 11: 829–843 [DOI] [PubMed] [Google Scholar]
- Chaabane C, Corvazier E, Bredoux R, Dally S, Raies A, Villemain A, Dupuy E, Enouf J, Bobe R 2006. Sarco/endoplasmic reticulum Ca2+ATPase type 3 isoforms (SERCA3b and SERCA3f): distinct roles in cell adhesion and ER stress. Biochem Biophys Res Commun 345: 1377–1385 [DOI] [PubMed] [Google Scholar]
- Chaabane C, Dally S, Corvazier E, Bredoux R, Bobe R, Ftouhi B, Raies A, Enouf J 2007. Platelet PMCA- and SERCA-type Ca2+-ATPase expression in diabetes: a novel signature of abnormal megakaryocytopoiesis. J Thromb Haemost 5: 2127–2135 [DOI] [PubMed] [Google Scholar]
- Chami M, Oules B, Paterlini-Brechot P 2006. Cytobiological consequences of calcium-signaling alterations induced by human viral proteins. Biochim Biophys Acta 1763: 1344–1362 [DOI] [PubMed] [Google Scholar]
- Chami M, Gozuacik D, Saigo K, Capiod T, Falson P, Lecoeur H, Urashima T, Beckmann J, Gougeon ML, Claret M, et al. 2000. Hepatitis B virus-related insertional mutagenesis implicates SERCA1 gene in the control of apoptosis. Oncogene 19: 2877–2886 [DOI] [PubMed] [Google Scholar]
- Chami M, Oules B, Szabadkai G, Tacine R, Rizzuto R, Paterlini-Brechot P 2008. Role of SERCA1 truncated isoform in the proapoptotic calcium transfer from ER to mitochondria during ER stress. Mol Cell 32: 641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier C, Coppieters W, Rollin F, Desmecht D, Agerholm JS, Cambisano N, Carta E, Dardano S, Dive M, Fasquelle C, et al. 2008. Highly effective SNP-based association mapping and management of recessive defects in livestock. Nat Genet 40: 449–454 [DOI] [PubMed] [Google Scholar]
- Chen Y, Liu CP, Xu KF, Mao XD, Lu YB, Fang L, Yang JW, Liu C 2008. Effect of taurine-conjugated ursodeoxycholic acid on endoplasmic reticulum stress and apoptosis induced by advanced glycation end products in cultured mouse podocytes. Am J Nephrol 28: 1014–1022 [DOI] [PubMed] [Google Scholar]
- Chen YJ, Wu CL, Liu JF, Fong YC, Hsu SF, Li TM, Su YC, Liu SH, Tang CH 2010. Honokiol induces cell apoptosis in human chondrosarcoma cells through mitochondrial dysfunction and endoplasmic reticulum stress. Cancer Lett 291: 20–30 [DOI] [PubMed] [Google Scholar]
- Cheshenko N, Del Rosario B, Woda C, Marcellino D, Satlin LM, Herold BC 2003. Herpes simplex virus triggers activation of calcium-signaling pathways. J Cell Biol 163: 283–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien CM, Yang SH, Chang LS, Lin SR 2008. Involvement of both endoplasmic reticulum- and mitochondria-dependent pathways in cardiotoxin III-induced apoptosis in HL-60 cells. Clin Exp Pharmacol Physiol 35: 1059–1064 [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Green DR 2008. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol 18: 157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KM, Zhong Y, Hoit BD, Grupp IL, Hahn H, Dilly KW, Guatimosim S, Lederer WJ, Matlib MA 2002. Defective intracellular Ca2+ signaling contributes to cardiomyopathy in type 1 diabetic rats. Am J Physiol 283: H1398–H1408 [DOI] [PubMed] [Google Scholar]
- Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH 2003. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem 278: 44230–44237 [DOI] [PubMed] [Google Scholar]
- Cooper ME, Bonnet F, Oldfield M, Jandeleit-Dahm K 2001. Mechanisms of diabetic vasculopathy: an overview. Am J Hypert 14: 475–486 [DOI] [PubMed] [Google Scholar]
- Cunha DA, Hekerman P, Ladriere L, Bazarra-Castro A, Ortis F, Wakeham MC, Moore F, Rasschaert J, Cardozo AK, Bellomo E, et al. 2008. Initiation and execution of lipotoxic ER stress in pancreatic β-cells. J Cell Sci 121: 2308–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl C, Haug LS, Spilsberg B, Johansen J, Ostvold AC, Diemer NH 2000. Reduced [3H]IP3 binding but unchanged IP3 receptor levels in the rat hippocampus CA1 region following transient global ischemia and tolerance induction. Neurochem Int 36: 379–388 [DOI] [PubMed] [Google Scholar]
- Dally S, Monceau V, Corvazier E, Bredoux R, Raies A, Bobe R, del Monte F, Enouf J 2009. Compartmentalized expression of three novel sarco/endoplasmic reticulum Ca2+ATPase 3 isoforms including the switch to ER stress, SERCA3f, in non-failing and failing human heart. Cell Calcium 45: 144–154 [DOI] [PubMed] [Google Scholar]
- Dayanithi G, Yahi N, Baghdiguian S, Fantini J 1995. Intracellular calcium release induced by human immunodeficiency virus type 1 (HIV-1) surface envelope glycoprotein in human intestinal epithelial cells: a putative mechanism for HIV-1 enteropathy. Cell Calcium 18: 9–18 [DOI] [PubMed] [Google Scholar]
- DeGracia DJ, Kumar R, Owen CR, Krause GS, White BC 2002. Molecular pathways of protein synthesis inhibition during brain reperfusion: implications for neuronal survival or death. J Cereb Blood Flow Metab 22: 127–141 [DOI] [PubMed] [Google Scholar]
- de Jong AS, de Mattia F, Van Dommelen MM, Lanke K, Melchers WJ, Willems PH, van Kuppeveld FJ 2008. Functional analysis of picornavirus 2B proteins: effects on calcium homeostasis and intracellular protein trafficking. J Virol 82: 3782–3790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong AS, Visch HJ, de Mattia F, van Dommelen MM, Swarts HG, Luyten T, Callewaert G, Melchers WJ, Willems PH, van Kuppeveld FJ 2006. The coxsackievirus 2B protein increases efflux of ions from the endoplasmic reticulum and Golgi, thereby inhibiting protein trafficking through the Golgi. J Biol Chem 281: 14144–14150 [DOI] [PubMed] [Google Scholar]
- Dellis O, Arbabian A, Brouland JP, Kovacs T, Rowe M, Chomienne C, Joab I, Papp B 2009. Modulation of B-cell endoplasmic reticulum calcium homeostasis by Epstein-Barr virus latent membrane protein-1. Mol Cancer 8: 59 doi:101186/1476-4598-8-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Monte F, Hajjar RJ 2008. Intracellular devastation in heart failure. Heart Fail Rev 13: 151–162 [DOI] [PubMed] [Google Scholar]
- Deng X, Wang Y, Zhou Y, Soboloff J, Gill DL 2009. STIM and Orai: dynamic intermembrane coupling to control cellular calcium signals. J Biol Chem 284: 22501–22505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denmeade SR, Jakobsen CM, Janssen S, Khan SR, Garrett ES, Lilja H, Christensen SB, Isaacs JT 2003. Prostate-specific antigen-activated thapsigargin prodrug as targeted therapy for prostate cancer. J Natl Cancer Inst 95: 990–1000 [DOI] [PubMed] [Google Scholar]
- Dickhout JG, Sood SK, Austin RC 2007. Role of endoplasmic reticulum calcium disequilibria in the mechanism of homocysteine-induced ER stress. Antioxid Redox Signal 9: 1863–1873 [DOI] [PubMed] [Google Scholar]
- Dickhout JG, Hossain GS, Pozza LM, Zhou J, Lhotak S, Austin RC 2005. Peroxynitrite causes endoplasmic reticulum stress and apoptosis in human vascular endothelium: implications in atherogenesis. Arterioscler Thromb Vasc Biol 25: 2623–2629 [DOI] [PubMed] [Google Scholar]
- Dieterle T, Meyer M, Gu Y, Belke DD, Swanson E, Iwatate M, Hollander J, Peterson KL, Ross J Jr, Dillmann WH 2005. Gene transfer of a phospholamban-targeted antibody improves calcium handling and cardiac function in heart failure. Cardiovasc Res 67: 678–688 [DOI] [PubMed] [Google Scholar]
- Ding W, Albrecht B, Kelley RE, Muthusamy N, Kim SJ, Altschuld RA, Lairmore MD 2002. Human T-cell lymphotropic virus type 1 p12I expression increases cytoplasmic calcium to enhance the activation of nuclear factor of activated T cells. J Virol 76: 10374–10382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M, Yano M, Kobayashi S, Kohno M, Tokuhisa T, Okuda S, Suetsugu M, Hisamatsu Y, Ohkusa T, Kohno M, et al. 2002. Propranolol prevents the development of heart failure by restoring FKBP12.6-mediated stabilization of ryanodine receptor. Circulation 105: 1374–1379 [DOI] [PubMed] [Google Scholar]
- Dong Y, Zeng CQ, Ball JM, Estes MK, Morris AP 1997. The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5-trisphosphate production. Proc Natl Acad Sci 94: 3960–3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doutheil J, Althausen S, Treiman M, Paschen W 2000. Effect of nitric oxide on endoplasmic reticulum calcium homeostasis, protein synthesis and energy metabolism. Cell Calcium 27: 107–115 [DOI] [PubMed] [Google Scholar]
- Drogemuller C, Drogemuller M, Leeb T, Mascarello F, Testoni S, Rossi M, Gentile A, Damiani E, Sacchetto R 2008. Identification of a missense mutation in the bovine ATP2A1 gene in congenital pseudomyotonia of Chianina cattle: an animal model of human Brody disease. Genomics 92: 474–477 [DOI] [PubMed] [Google Scholar]
- Durham WJ, Wehrens XH, Sood S, Hamilton SL 2007. Diseases associated with altered ryanodine receptor activity. Subcell Biochem 45: 273–321 [DOI] [PubMed] [Google Scholar]
- Dutta K, Carmody MW, Cala SE, Davidoff AJ 2002. Depressed PKA activity contributes to impaired SERCA function and is linked to the pathogenesis of glucose-induced cardiomyopathy. J Mol Cell Cardiol 34: 985–996 [DOI] [PubMed] [Google Scholar]
- Egger L, Schneider J, Rheme C, Tapernoux M, Hacki J, Borner C 2003. Serine proteases mediate apoptosis-like cell death and phagocytosis under caspase-inhibiting conditions. Cell Death Differ 10: 1188–1203 [DOI] [PubMed] [Google Scholar]
- Eizirik DL, Cardozo AK, Cnop M 2008. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 29: 42–61 [DOI] [PubMed] [Google Scholar]
- Endo M 2009. Calcium-induced calcium release in skeletal muscle. Physiol Rev 89: 1153–1176 [DOI] [PubMed] [Google Scholar]
- Endo Y, Uzawa K, Mochida Y, Shiiba M, Bukawa H, Yokoe H, Tanzawa H 2004. Sarcoendoplasmic reticulum Ca2+ ATPase type 2 downregulated in human oral squamous cell carcinoma. Int J Cancer 110: 225–231 [DOI] [PubMed] [Google Scholar]
- Ermakova SP, Kang BS, Choi BY, Choi HS, Schuster TF, Ma WY, Bode AM, Dong Z 2006. (-)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res 66: 9260–9269 [DOI] [PubMed] [Google Scholar]
- Evans-Molina C, Robbins RD, Kono T, Tersey SA, Vestermark GL, Nunemaker CS, Garmey JC, Deering TG, Keller SR, Maier B, et al. 2009. Peroxisome proliferator-activated receptor γ activation restores islet function in diabetic mice through reduction of endoplasmic reticulum stress and maintenance of euchromatin structure. Mol Cell Biol 29: 2053–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Watanabe T 2003. Inflammatory reactions in the pathogenesis of atherosclerosis. J Atheroscler Thromb 10: 63–71 [DOI] [PubMed] [Google Scholar]
- Farfel-Becker T, Vitner E, Dekel H, Leshem N, Enquist IB, Karlsson S, Futerman AH 2009. No evidence for activation of the unfolded protein response in neuronopathic models of Gaucher disease. Hum Mol Genet 18: 1482–1488 [DOI] [PubMed] [Google Scholar]
- Fedirko NV, Kruglikov IA, Kopach OV, Vats JA, Kostyuk PG, Voitenko NV 2006. Changes in functioning of rat submandibular salivary gland under streptozotocin-induced diabetes are associated with alterations of Ca2+ signaling and Ca2+ transporting pumps. Biochim Biophys Acta 1762: 294–303 [DOI] [PubMed] [Google Scholar]
- Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, et al. 2003. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol 5: 781–792 [DOI] [PubMed] [Google Scholar]
- Fernyhough P, Calcutt NA 2010. Abnormal calcium homeostasis in peripheral neuropathies. Cell Calcium 47: 130–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreiro E, Resende R, Costa R, Oliveira CR, Pereira CM 2006. An endoplasmic-reticulum-specific apoptotic pathway is involved in prion and amyloid-β peptides neurotoxicity. Neurobiol Dis 23: 669–678 [DOI] [PubMed] [Google Scholar]
- Ferreiro E, Costa R, Marques S, Cardoso SM, Oliveira CR, Pereira CM 2008. Involvement of mitochondria in endoplasmic reticulum stress-induced apoptotic cell death pathway triggered by the prion peptide PrP(106-126). J Neurochem 104: 766–776 [DOI] [PubMed] [Google Scholar]
- Foggia L, Hovnanian A 2004. Calcium pump disorders of the skin. Am J Med Genet C 131C: 20–31 [DOI] [PubMed] [Google Scholar]
- Foggia L, Aronchik I, Aberg K, Brown B, Hovnanian A, Mauro TM 2006. Activity of the hSPCA1 Golgi Ca2+ pump is essential for Ca2+-mediated Ca2+ response and cell viability in Darier disease. J Cell Sci 119: 671–679 [DOI] [PubMed] [Google Scholar]
- Fonseca SG, Ishigaki S, Oslowski CM, Lu S, Lipson KL, Ghosh R, Hayashi E, Ishihara H, Oka Y, Permutt MA, et al. 2010. Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J Clin Invest 120: 744–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett JK, White C, Cheung KH, Mak DO 2007. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev 87: 593–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti M, Cartier L, Piguet V, Lew DP, Carpentier JL, Trono D, Krause KH 1999. The HIV Nef protein alters Ca2+ signaling in myelomonocytic cells through SH3-mediated protein-protein interactions. J Biol Chem 274: 34765–34772 [DOI] [PubMed] [Google Scholar]
- Frank KF, Bolck B, Brixius K, Kranias EG, Schwinger RH 2002. Modulation of SERCA: implications for the failing human heart. Basic Res Cardiol 97: I72–I78 [DOI] [PubMed] [Google Scholar]
- Franz WM, Muller OJ, Katus HA 2001. Cardiomyopathies: from genetics to the prospect of treatment. Lancet 358: 1627–1637 [DOI] [PubMed] [Google Scholar]
- French JP, Quindry JC, Falk DJ, Staib JL, Lee Y, Wang KK, Powers SK 2006. Ischemia-reperfusion-induced calpain activation and SERCA2a degradation are attenuated by exercise training and calpain inhibition. Am J Physiol 290: H128–H136 [DOI] [PubMed] [Google Scholar]
- Gaut JR, Hendershot LM 1993. The modification and assembly of proteins in the endoplasmic reticulum. Curr Opin Cell Biol 5: 589–595 [DOI] [PubMed] [Google Scholar]
- Gelebart P, Kovacs T, Brouland JP, van Gorp R, Grossmann J, Rivard N, Panis Y, Martin V, Bredoux R, Enouf J, et al. 2002. Expression of endomembrane calcium pumps in colon and gastric cancer cells. Induction of SERCA3 expression during differentiation. J Biol Chem 277: 26310–26320 [DOI] [PubMed] [Google Scholar]
- Gemes G, Rigaud M, Weyker PD, Abram SE, Weihrauch D, Poroli M, Zoga V, Hogan QH 2009. Depletion of calcium stores in injured sensory neurons: anatomic and functional correlates. Anesthesiology 111: 393–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzburg L, Futerman AH 2005. Defective calcium homeostasis in the cerebellum in a mouse model of Niemann-Pick A disease. J Neurochem 95: 1619–1628 [DOI] [PubMed] [Google Scholar]
- Ginzburg L, Li SC, Li YT, Futerman AH 2008. An exposed carboxyl group on sialic acid is essential for gangliosides to inhibit calcium uptake via the sarco/endoplasmic reticulum Ca2+-ATPase: relevance to gangliosidoses. J Neurochem 104: 140–146 [DOI] [PubMed] [Google Scholar]
- Giorgi C, De Stefani D, Bononi A, Rizzuto R, Pinton P 2009. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell Biol 41: 1817–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C, Romagnoli A, Pinton P, Rizzuto R 2008. Ca2+ signaling, mitochondria and cell death. Curr Mol Med 8: 119–130 [DOI] [PubMed] [Google Scholar]
- Gotoh T, Mori M 2006. Nitric oxide and endoplasmic reticulum stress. Arterioscler Thromb Vasc Biol 26: 1439–1446 [DOI] [PubMed] [Google Scholar]
- Griffin SD, Beales LP, Clarke DS, Worsfold O, Evans SD, Jaeger J, Harris MP, Rowlands DJ 2003. The p7 protein of hepatitis C virus forms an ion channel that is blocked by the antiviral drug, Amantadine. FEBS Lett 535: 34–38 [DOI] [PubMed] [Google Scholar]
- Grover AK, Kwan CY, Samson SE 2003. Effects of peroxynitrite on sarco/endoplasmic reticulum Ca2+ pump isoforms SERCA2b and SERCA3a. Am J Physiol 285: C1537–C1543 [DOI] [PubMed] [Google Scholar]
- Guest PC, Bailyes EM, Hutton JC 1997. Endoplasmic reticulum Ca2+ is important for the proteolytic processing and intracellular transport of proinsulin in the pancreatic β-cell. Biochem J 323: 445–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinea R, Lopez-Rivas A, Carrasco L 1989. Modification of phospholipase C and phospholipase A2 activities during poliovirus infection. J Biol Chem 264: 21923–21927 [PubMed] [Google Scholar]
- Guner S, Arioglu E, Tay A, Tasdelen A, Aslamaci S, Bidasee KR, Dincer UD 2004. Diabetes decreases mRNA levels of calcium-release channels in human atrial appendage. Mol Cell Biochem 263: 143–150 [DOI] [PubMed] [Google Scholar]
- Gurzov EN, Ortis F, Cunha DA, Gosset G, Li M, Cardozo AK, Eizirik DL 2009. Signaling by IL-1β+IFN-γ and ER stress converge on DP5/Hrk activation: a novel mechanism for pancreatic β-cell apoptosis. Cell Death Differ 16: 1539–1550 [DOI] [PubMed] [Google Scholar]
- Gwiazda KS, Yang TL, Lin Y, Johnson JD 2009. Effects of palmitate on ER and cytosolic Ca2+ homeostasis in β-cells. Am J Physiol 296: E690–E701 [DOI] [PubMed] [Google Scholar]
- Gyorke S 2009. Molecular basis of catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 6: 123–129 [DOI] [PubMed] [Google Scholar]
- Gyorke S, Terentyev D 2008. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res 77: 245–255 [DOI] [PubMed] [Google Scholar]
- Haghighi K, Kolokathis F, Gramolini AO, Waggoner JR, Pater L, Lynch RA, Fan GC, Tsiapras D, Parekh RR, Dorn GW II, et al. 2006. A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc Natl Acad Sci 103: 1388–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han I, Kudlow JE 1997. Reduced O glycosylation of Sp1 is associated with increased proteasome susceptibility. Mol Cell Biol 17: 2550–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D 1999. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397: 271–274 [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D 2000. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5: 897–904 [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, et al. 2003. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11: 619–633 [DOI] [PubMed] [Google Scholar]
- Hartshorn KL, Collamer M, Auerbach M, Myers JB, Pavlotsky N, Tauber AI 1988. Effects of influenza A virus on human neutrophil calcium metabolism. J Immunol 141: 1295–1301 [PubMed] [Google Scholar]
- Hasenfuss G, Pieske B 2002. Calcium cycling in congestive heart failure. J Mol Cell Cardiol 34: 951–969 [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Mattson MP 2002. Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J Acquir Immune Defic Syndr 31 Suppl 2: S55–S61 [DOI] [PubMed] [Google Scholar]
- Heighway J, Betticher DC, Hoban PR, Altermatt HJ, Cowen R 1996. Coamplification in tumors of KRAS2, type 2 inositol 1,4,5 triphosphate receptor gene, and a novel human gene, KRAG. Genomics 35: 207–214 [DOI] [PubMed] [Google Scholar]
- Hermanson D, Addo SN, Bajer AA, Marchant JS, Das SG, Srinivasan B, Al-Mousa F, Michelangeli F, Thomas DD, Lebien TW, et al. 2009. Dual mechanisms of sHA 14-1 in inducing cell death through endoplasmic reticulum and mitochondria. Mol Pharmacol 76: 667–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C, Russelakis-Carneiro M, Maundrell K, Castilla J, Soto C 2003. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J 22: 5435–5445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmqvist L, Tuson M, Marfany G, Herrero E, Balcells S, Gonzalez-Duarte R 2002. ORMDL proteins are a conserved new family of endoplasmic reticulum membrane proteins. Genome Biol 3: RESEARCH0027 doi:101186/gb-2002-3-6-research0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollien J, Weissman JS 2006. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313: 104–107 [DOI] [PubMed] [Google Scholar]
- Hoshijima M, Ikeda Y, Iwanaga Y, Minamisawa S, Date MO, Gu Y, Iwatate M, Li M, Wang L, Wilson JM, et al. 2002. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat Med 8: 864–871 [DOI] [PubMed] [Google Scholar]
- Hoyer-Hansen M, Jaattela M 2007. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ 14: 1576–1582 [DOI] [PubMed] [Google Scholar]
- Hsu YL, Wu LY, Kuo PL 2009. Dehydrocostuslactone, a medicinal plant-derived sesquiterpene lactone, induces apoptosis coupled to endoplasmic reticulum stress in liver cancer cells. J Pharmacol Exp Ther 329: 808–819 [DOI] [PubMed] [Google Scholar]
- Hu Y, Belke D, Suarez J, Swanson E, Clark R, Hoshijima M, Dillmann WH 2005. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ Res 96: 1006–1013 [DOI] [PubMed] [Google Scholar]
- Huang TJ, Sayers NM, Fernyhough P, Verkhratsky A 2002. Diabetes-induced alterations in calcium homeostasis in sensory neurones of streptozotocin-diabetic rats are restricted to lumbar ganglia and are prevented by neurotrophin-3. Diabetologia 45: 560–570 [DOI] [PubMed] [Google Scholar]
- Huang F, Shan J, Reiken S, Wehrens XH, Marks AR 2006. Analysis of calstabin2 (FKBP12.6)-ryanodine receptor interactions: rescue of heart failure by calstabin2 in mice. Proc Natl Acad Sci 103: 3456–3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung JY, Hsu YL, Ni WC, Tsai YM, Yang CJ, Kuo PL, Huang MS 2010. Oxidative and endoplasmic reticulum stress signaling are involved in dehydrocostuslactone-mediated apoptosis in human non-small cell lung cancer cells. Lung Cancer 68: 355–365 [DOI] [PubMed] [Google Scholar]
- Inesi G, Prasad AM, Pilankatta R 2008. The Ca2+ ATPase of cardiac sarcoplasmic reticulum: Physiological role and relevance to diseases. Biochem Biophys Res Commun 369: 182–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Tanizawa Y, Wasson J, Behn P, Kalidas K, Bernal-Mizrachi E, Mueckler M, Marshall H, Donis-Keller H, Crock P, et al. 1998. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome). Nat Genet 20: 143–148 [DOI] [PubMed] [Google Scholar]
- Irvine RF 1990. Quantal Ca2+ release and the control of Ca2+ entry by inositol phosphates–a possible mechanism. FEBS Lett 263: 5–9 [DOI] [PubMed] [Google Scholar]
- Jakobsen CH, Storvold GL, Bremseth H, Follestad T, Sand K, Mack M, Olsen KS, Lundemo AG, Iversen JG, Krokan HE, et al. 2008. DHA induces ER stress and growth arrest in human colon cancer cells: associations with cholesterol and calcium homeostasis. J Lipid Res 49: 2089–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen K, Horn S, Niemann MT, Daniel PT, Schulze-Osthoff K, Fischer U 2009. Inhibition of the ER Ca2+ pump forces multidrug-resistant cells deficient in Bak and Bax into necrosis. J Cell Sci 122: 4481–4491 [DOI] [PubMed] [Google Scholar]
- Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, Borow K, Dittrich H, Zsebo KM, Hajjar RJ 2009. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail 15: 171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke MG, Gauglitz GG, Song J, Kulp GA, Finnerty CC, Cox RA, Barral JM, Herndon DN, Boehning D 2010. Calcium and ER stress mediate hepatic apoptosis after burn injury. J Cell Mol Med 13: 1857–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie H, Donghua H, Xingkui X, Liang G, Wenjun W, Xiaoyan H, Zhen C 2007. Homoharringtonine-induced apoptosis of MDS cell line MUTZ-1 cells is mediated by the endoplasmic reticulum stress pathway. Leuk Lymphoma 48: 964–977 [DOI] [PubMed] [Google Scholar]
- Johnson AJ, Hsu AL, Lin HP, Song X, Chen CS 2002. The cyclo-oxygenase-2 inhibitor celecoxib perturbs intracellular calcium by inhibiting endoplasmic reticulum Ca2+-ATPases: a plausible link with its anti-tumour effect and cardiovascular risks. Biochem J 366: 831–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekura K, Suzuki H, Aiso S, Matsuoka M 2009. ER stress and unfolded protein response in amyotrophic lateral sclerosis. Mol Neurobiol 39: 81–89 [DOI] [PubMed] [Google Scholar]
- Kang S, Kang J, Kwon H, Frueh D, Yoo SH, Wagner G, Park S 2008. Effects of redox potential and Ca2+ on the inositol 1,4,5-trisphosphate receptor L3-1 loop region: implications for receptor regulation. J Biol Chem 283: 25567–25575 [DOI] [PubMed] [Google Scholar]
- Karakikes I, Kim M, Hadri L, Sakata S, Sun Y, Zhang W, Chemaly ER, Hajjar RJ, Lebeche D 2009. Gene remodeling in type 2 diabetic cardiomyopathy and its phenotypic rescue with SERCA2a. PLoS One 4: e6474 doi:101371/journalpone0006474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada H, Nishiyama C, Takagi A, Tokura T, Nakano N, Maeda K, Mayuzumi N, Ikeda S, Okumura K, Ogawa H 2005. Transcriptional regulation of ATP2C1 gene by Sp1 and YY1 and reduced function of its promoter in Hailey-Hailey disease keratinocytes. J Invest Dermatol 124: 1206–1214 [DOI] [PubMed] [Google Scholar]
- Kawase Y, Hajjar RJ 2008. The cardiac sarcoplasmic/endoplasmic reticulum calcium ATPase: a potent target for cardiovascular diseases. Nat Clin Pract Cardiovasc Med 5: 554–565 [DOI] [PubMed] [Google Scholar]
- Keay S, Baldwin BR, Smith MW, Wasserman SS, Goldman WF 1995. Increases in [Ca2+]i mediated by the 92.5-kDa putative cell membrane receptor for HCMV gp86. Am J Physiol 269: C11–C21 [DOI] [PubMed] [Google Scholar]
- Kim I, Xu W, Reed JC 2008. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 7: 1013–1030 [DOI] [PubMed] [Google Scholar]
- Kim HW, Ch YS, Lee HR, Park SY, Kim YH 2001. Diabetic alterations in cardiac sarcoplasmic reticulum Ca2+-ATPase and phospholamban protein expression. Life Sci 70: 367–379 [DOI] [PubMed] [Google Scholar]
- Knobler H, Savion N, Shenkman B, Kotev-Emeth S, Varon D 1998. Shear-induced platelet adhesion and aggregation on subendothelium are increased in diabetic patients. Thromb Res 90: 181–190 [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Taguchi K, Takenouchi Y, Matsumoto T, Kamata K 2007. Insulin-induced impairment via peroxynitrite production of endothelium-dependent relaxation and sarco/endoplasmic reticulum Ca2+-ATPase function in aortas from diabetic rats. Free Radic Biol Med 43: 431–443 [DOI] [PubMed] [Google Scholar]
- Korkotian E, Schwarz A, Pelled D, Schwarzmann G, Segal M, Futerman AH 1999. Elevation of intracellular glucosylceramide levels results in an increase in endoplasmic reticulum density and in functional calcium stores in cultured neurons. J Biol Chem 274: 21673–21678 [DOI] [PubMed] [Google Scholar]
- Korosec B, Glavac D, Rott T, Ravnik-Glavac M 2006. Alterations in the ATP2A2 gene in correlation with colon and lung cancer. Cancer Genet Cytogenet 171: 105–111 [DOI] [PubMed] [Google Scholar]
- Korosec B, Glavac D, Volavsek M, Ravnik-Glavac M 2008. Alterations in genes encoding sarcoplasmic-endoplasmic reticulum Ca2+ pumps in association with head and neck squamous cell carcinoma. Cancer Genet Cytogenet 181: 112–118 [DOI] [PubMed] [Google Scholar]
- Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, Ogawa S, Kaufman RJ, Kominami E, Momoi T 2007. ER stress (PERK/eIF2α phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ 14: 230–239 [DOI] [PubMed] [Google Scholar]
- Kranias EG, Bers DM 2007. Calcium and cardiomyopathies. Subcell Biochem 45: 523–537 [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Brenner C 2007. Mitochondrial membrane permeabilization in cell death. Physiol Rev 87: 99–163 [DOI] [PubMed] [Google Scholar]
- Kruglikov I, Gryshchenko O, Shutov L, Kostyuk E, Kostyuk P, Voitenko N 2004. Diabetes-induced abnormalities in ER calcium mobilization in primary and secondary nociceptive neurons. Pflugers Arch 448: 395–401 [DOI] [PubMed] [Google Scholar]
- Kulkarni RN, Roper MG, Dahlgren G, Shih DQ, Kauri LM, Peters JL, Stoffel M, Kennedy RT 2004. Islet secretory defect in insulin receptor substrate 1 null mice is linked with reduced calcium signaling and expression of sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)-2b and -3. Diabetes 53: 1517–1525 [DOI] [PubMed] [Google Scholar]
- Lai P, Michelangeli F 2009. Changes in expression and activity of the secretory pathway Ca2+ ATPase 1 (SPCA1) in A7r5 vascular smooth muscle cells cultured at different glucose concentrations. Biosci Rep 29: 397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai WW, Yang JS, Lai KC, Kuo CL, Hsu CK, Wang CK, Chang CY, Lin JJ, Tang NY, Chen PY, et al. 2009. Rhein induced apoptosis through the endoplasmic reticulum stress, caspase- and mitochondria-dependent pathways in SCC-4 human tongue squamous cancer cells. In Vivo 23: 309–316 [PubMed] [Google Scholar]
- Lee B, Jonas JC, Weir GC, Laychock SG 1999. Glucose regulates expression of inositol 1,4,5-trisphosphate receptor isoforms in isolated rat pancreatic islets. Endocrinology 140: 2173–2182 [DOI] [PubMed] [Google Scholar]
- Lee SY, Lee MS, Cherla RP, Tesh VL 2008. Shiga toxin 1 induces apoptosis through the endoplasmic reticulum stress response in human monocytic cells. Cell Microbiol 10: 770–780 [DOI] [PubMed] [Google Scholar]
- Lehnart SE, Wehrens XH, Marks AR 2005. Defective ryanodine receptor interdomain interactions may contribute to intracellular Ca2+ leak: a novel therapeutic target in heart failure. Circulation 111: 3342–3346 [DOI] [PubMed] [Google Scholar]
- Lehnart SE, Mongillo M, Bellinger A, Lindegger N, Chen BX, Hsueh W, Reiken S, Wronska A, Drew LJ, Ward CW, et al. 2008. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest 118: 2230–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Davis RJ 2003. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci 100: 2432–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi O, Mints G, Hussain MA 2003. β-cell apoptosis in the pathogenesis of human type 2 diabetes mellitus. Eur J Endocrinol 149: 99–102 [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G 2008. Autophagy in the pathogenesis of disease. Cell 132: 27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J 1999. Abnormal cell calcium homeostasis in type 2 diabetes mellitus: a new look on old disease. Endocrine 10: 1–6 [DOI] [PubMed] [Google Scholar]
- Li Y, Ge M, Ciani L, Kuriakose G, Westover EJ, Dura M, Covey DF, Freed JH, Maxfield FR, Lytton J, et al. 2004. Enrichment of endoplasmic reticulum with cholesterol inhibits sarcoplasmic-endoplasmic reticulum calcium ATPase-2b activity in parallel with increased order of membrane lipids: implications for depletion of endoplasmic reticulum calcium stores and apoptosis in cholesterol-loaded macrophages. J Biol Chem 279: 37030–37039 [DOI] [PubMed] [Google Scholar]
- Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, Tabas I 2009. Role of ERO1-α-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol 186: 783–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao PC, Tan SK, Lieu CH, Jung HK 2008. Involvement of endoplasmic reticulum in paclitaxel-induced apoptosis. J Cell Biochem 104: 1509–1523 [DOI] [PubMed] [Google Scholar]
- Lievremont JP, Rizzuto R, Hendershot L, Meldolesi J 1997. BiP, a major chaperone protein of the endoplasmic reticulum lumen, plays a direct and important role in the storage of the rapidly exchanging pool of Ca2+. J Biol Chem 272: 30873–30879 [DOI] [PubMed] [Google Scholar]
- Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P 2007a. IRE1 signaling affects cell fate during the unfolded protein response. Science 318: 944–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Li H, Zhang Y, Ron D, Walter P 2009. Divergent effects of PERK and IRE1 signaling on cell viability. PLoS One 4: e4170 doi:101371/journalpone0004170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JP, Yang JS, Chang NW, Chiu TH, Su CC, Lu KW, Ho YT, Yeh CC, Yang MD, Lin HJ, et al. 2007b. GADD153 mediates berberine-induced apoptosis in human cervical cancer Ca ski cells. Anticancer Res 27: 3379–3386 [PubMed] [Google Scholar]
- Lindenmeyer MT, Rastaldi MP, Ikehata M, Neusser MA, Kretzler M, Cohen CD, Schlondorff D 2008. Proteinuria and hyperglycemia induce endoplasmic reticulum stress. J Am Soc Nephrol 19: 2225–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D, Wootz H, Korhonen L 2006. ER stress and neurodegenerative diseases. Cell Death Differ 13: 385–392 [DOI] [PubMed] [Google Scholar]
- Lipskaia L, Hulot JS, Lompre AM 2009. Role of sarco/endoplasmic reticulum calcium content and calcium ATPase activity in the control of cell growth and proliferation. Pflugers Arch 457: 673–685 [DOI] [PubMed] [Google Scholar]
- Liu LH, Boivin GP, Prasad V, Periasamy M, Shull GE 2001. Squamous cell tumors in mice heterozygous for a null allele of Atp2a2, encoding the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 Ca2+ pump. J Biol Chem 276: 26737–26740 [DOI] [PubMed] [Google Scholar]
- Liu G, Sun Y, Li Z, Song T, Wang H, Zhang Y, Ge Z 2008. Apoptosis induced by endoplasmic reticulum stress involved in diabetic kidney disease. Biochem Biophys Res Commun 370: 651–656 [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E, Pelled D, Riebeling C, Bodennec J, de-Morgan A, Waller H, Schiffmann R, Futerman AH 2003. Glucosylceramide and glucosylsphingosine modulate calcium mobilization from brain microsomes via different mechanisms. J Biol Chem 278: 23594–23599 [DOI] [PubMed] [Google Scholar]
- Lopez JJ, Jardin I, Bobe R, Pariente JA, Enouf J, Salido GM, Rosado JA 2008. STIM1 regulates acidic Ca2+ store refilling by interaction with SERCA3 in human platelets. Biochem Pharmacol 75: 2157–2164 [DOI] [PubMed] [Google Scholar]
- Lorenzon NM, Beam KG 2008. Disease causing mutations of calcium channels. Channels (Austin) 2: 163–179 [DOI] [PubMed] [Google Scholar]
- Lucero HA, Kaminer B 1999. The role of calcium on the activity of ERcalcistorin/protein-disulfide isomerase and the significance of the C-terminal and its calcium binding. A comparison with mammalian protein-disulfide isomerase. J Biol Chem 274: 3243–3251 [DOI] [PubMed] [Google Scholar]
- Luciani DS, Gwiazda KS, Yang TL, Kalynyak TB, Bychkivska Y, Frey MH, Jeffrey KD, Sampaio AV, Underhill TM, Johnson JD 2009. Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and β-cell death. Diabetes 58: 422–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM 2004. ER chaperone functions during normal and stress conditions. J Chem Neuroanat 28: 51–65 [DOI] [PubMed] [Google Scholar]
- Ma L, Zhu B, Chen X, Liu J, Guan Y, Ren J 2008. Abnormalities of sarcoplasmic reticulum Ca2+ mobilization in aortic smooth muscle cells from streptozotocin-induced diabetic rats. Clin Exp Pharmacol Physiol 35: 568–573 [DOI] [PubMed] [Google Scholar]
- Magyar JE, Gamberucci A, Konta L, Margittai E, Mandl J, Banhegyi G, Benedetti A, Csala M 2009. Endoplasmic reticulum stress underlying the pro-apoptotic effect of epigallocatechin gallate in mouse hepatoma cells. Int J Biochem Cell Biol 41: 694–700 [DOI] [PubMed] [Google Scholar]
- Mahabusarakam W, Iriyachitra P, Taylor WC 1987. Chemical constituents of garcinia mangostana. J Nat Prod 50: 474–478 [Google Scholar]
- Mahn K, Hirst SJ, Ying S, Holt MR, Lavender P, Ojo OO, Siew L, Simcock DE, McVicker CG, Kanabar V, et al. 2009. Diminished sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) expression contributes to airway remodelling in bronchial asthma. Proc Natl Acad Sci 106: 10775–10780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ 2007. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol 18: 716–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manninen A, Saksela K 2002. HIV-1 Nef interacts with inositol trisphosphate receptor to activate calcium signaling in T cells. J Exp Med 195: 1023–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D 2004. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18: 3066–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR 2000. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101: 365–376 [DOI] [PubMed] [Google Scholar]
- Mattson MP 2007. Calcium and neurodegeneration. Aging Cell 6: 337–350 [DOI] [PubMed] [Google Scholar]
- Mayne M, Holden CP, Nath A, Geiger JD 2000. Release of calcium from inositol 1,4,5-trisphosphate receptor-regulated stores by HIV-1 Tat regulates TNF-α production in human macrophages. J Immunol 164: 6538–6542 [DOI] [PubMed] [Google Scholar]
- McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ 2001. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21: 1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak M, Robert Parker JM, Opas M 2002. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium 32: 269–278 [DOI] [PubMed] [Google Scholar]
- Mikoshiba K 2007. IP3 receptor/Ca2+ channel: from discovery to new signaling concepts. J Neurochem 102: 1426–1446 [DOI] [PubMed] [Google Scholar]
- Missiaen L, De Smedt H, Droogmans G, Casteels R 1992. Ca2+ release induced by inositol 1,4,5-trisphosphate is a steady-state phenomenon controlled by luminal Ca2+ in permeabilized cells. Nature 357: 599–602 [DOI] [PubMed] [Google Scholar]
- Missiaen L, Robberecht W, Van Den Bosch L, Callewaert G, Parys JB, Wuytack F, Raeymaekers L, Nilius B, Eggermont J, De Smedt H 2000. Abnormal intracellular Ca2+ homeostasis and disease. Cell Calcium 28: 1–21 [DOI] [PubMed] [Google Scholar]
- Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, Depner M, von Berg A, Bufe A, Rietschel E, et al. 2007. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature 448: 470–473 [DOI] [PubMed] [Google Scholar]
- Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, et al. 1998. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 351: 857–861 [DOI] [PubMed] [Google Scholar]
- Moncada S, Erusalimsky JD 2002. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol 3: 214–220 [DOI] [PubMed] [Google Scholar]
- Monteith GR, McAndrew D, Faddy HM, Roberts-Thomson SJ 2007. Calcium and cancer: targeting Ca2+ transport. Nat Rev Cancer 7: 519–530 [DOI] [PubMed] [Google Scholar]
- Nagata E, Tanaka K, Gomi S, Mihara B, Shirai T, Nogawa S, Nozaki H, Mikoshiba K, Fukuuchi Y 1994. Alteration of inositol 1,4,5-trisphosphate receptor after six-hour hemispheric ischemia in the gerbil brain. Neuroscience 61: 983–990 [DOI] [PubMed] [Google Scholar]
- Nakano K, Isegawa Y, Zou P, Tadagaki K, Inagi R, Yamanishi K 2003. Kaposi's sarcoma-associated herpesvirus (KSHV)-encoded vMIP-I and vMIP-II induce signal transduction and chemotaxis in monocytic cells. Arch Virol 148: 871–890 [DOI] [PubMed] [Google Scholar]
- Nakano T, Watanabe H, Ozeki M, Asai M, Katoh H, Satoh H, Hayashi H 2006. Endoplasmic reticulum Ca2+ depletion induces endothelial cell apoptosis independently of caspase-12. Cardiovasc Res 69: 908–915 [DOI] [PubMed] [Google Scholar]
- Nawrocki ST, Carew JS, Pino MS, Highshaw RA, Dunner K Jr, Huang P, Abbruzzese JL, McConkey DJ 2005. Bortezomib sensitizes pancreatic cancer cells to endoplasmic reticulum stress-mediated apoptosis. Cancer Res 65: 11658–11666 [DOI] [PubMed] [Google Scholar]
- Nelson TE, Nelson KE 1990. Intra- and extraluminal sarcoplasmic reticulum membrane regulatory sites for Ca2+-induced Ca2+ release. FEBS Lett 263: 292–294 [DOI] [PubMed] [Google Scholar]
- Nicolau J, De Souza DN, Simoes A 2009. Alteration of Ca2+-ATPase activity in the homogenate, plasma membrane and microsomes of the salivary glands of streptozotocin-induced diabetic rats. Cell Biochem Funct 27: 128–134 [DOI] [PubMed] [Google Scholar]
- Nieto-Miguel T, Fonteriz RI, Vay L, Gajate C, Lopez-Hernandez S, Mollinedo F 2007. Endoplasmic reticulum stress in the proapoptotic action of edelfosine in solid tumor cells. Cancer Res 67: 10368–10378 [DOI] [PubMed] [Google Scholar]
- Norman JP, Perry SW, Reynolds HM, Kiebala M, De Mesy Bentley KL, Trejo M, Volsky DJ, Maggirwar SB, Dewhurst S, Masliah E, et al. 2008. HIV-1 Tat activates neuronal ryanodine receptors with rapid induction of the unfolded protein response and mitochondrial hyperpolarization. PLoS One 3: e3731 101371/journalpone0003731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, Korsmeyer SJ 2005. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci 102: 105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt A, Taschner PE, Khanna VK, Busch HF, Karpati G, Jablecki CK, Breuning MH, MacLennan DH 1996. Mutations in the gene-encoding SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+ ATPase, are associated with Brody disease. Nat Genet 14: 191–194 [DOI] [PubMed] [Google Scholar]
- Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, et al. 2006. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26: 9220–9231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil BJ, McKeown TR, DeGracia DJ, Alousi SS, Rafols JA, White BC 1999. Cell death, calcium mobilization, and immunostaining for phosphorylated eukaryotic initiation factor 2-α (eIF2α) in neuronally differentiated NB-104 cells: arachidonate and radical-mediated injury mechanisms. Resuscitation 41: 71–83 [DOI] [PubMed] [Google Scholar]
- Ong DS, Mu TW, Palmer AE, Kelly JW 2010. Endoplasmic reticulum Ca2+ increases enhance mutant glucocerebrosidase proteostasis. Nat Chem Biol 6: 424–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onozuka T, Sawamura D, Goto M, Yokota K, Shimizu H 2006. Possible role of endoplasmic reticulum stress in the pathogenesis of Darier's disease. J Dermatol Sci 41: 217–220 [DOI] [PubMed] [Google Scholar]
- O'Rourke B, Kass DA, Tomaselli GF, Kaab S, Tunin R, Marban E 1999. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, I: experimental studies. Circ Res 84: 562–570 [DOI] [PubMed] [Google Scholar]
- Osman AA, Saito M, Makepeace C, Permutt MA, Schlesinger P, Mueckler M 2003. Wolframin expression induces novel ion channel activity in endoplasmic reticulum membranes and increases intracellular calcium. J Biol Chem 278: 52755–52762 [DOI] [PubMed] [Google Scholar]
- Oyadomari S, Mori M 2004. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11: 381–389 [DOI] [PubMed] [Google Scholar]
- Oyadomari S, Takeda K, Takiguchi M, Gotoh T, Matsumoto M, Wada I, Akira S, Araki E, Mori M 2001. Nitric oxide-induced apoptosis in pancreatic β cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci 98: 10845–10850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS 2004. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306: 457–461 [DOI] [PubMed] [Google Scholar]
- Pacifico F, Ulianich L, De Micheli S, Treglia S, Leonardi A, Vito P, Formisano S, Consiglio E, Di Jeso B 2003. The expression of the sarco/endoplasmic reticulum Ca2+-ATPases in thyroid and its down-regulation following neoplastic transformation. J Mol Endocrinol 30: 399–409 [DOI] [PubMed] [Google Scholar]
- Papp S, Dziak E, Michalak M, Opas M 2003. Is all of the endoplasmic reticulum created equal? The effects of the heterogeneous distribution of endoplasmic reticulum Ca2+-handling proteins. J Cell Biol 160: 475–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KS, Poburko D, Wollheim CB, Demaurex N 2009. Amiloride derivatives induce apoptosis by depleting ER Ca2+ stores in vascular endothelial cells. Br J Pharmacol 156: 1296–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Churn SB, DeLorenzo RJ 1999. Global ischemia-induced inhibition of the coupling ratio of calcium uptake and ATP hydrolysis by rat whole brain microsomal Mg2+/Ca2+ ATPase. Brain Res 834: 32–41 [DOI] [PubMed] [Google Scholar]
- Paschen W, Mengesdorf T 2005. Endoplasmic reticulum stress response and neurodegeneration. Cell Calcium 38: 409–415 [DOI] [PubMed] [Google Scholar]
- Pattacini L, Mancini M, Mazzacurati L, Brusa G, Benvenuti M, Martinelli G, Baccarani M, Santucci MA 2004. Endoplasmic reticulum stress initiates apoptotic death induced by STI571 inhibition of p210 bcr-abl tyrosine kinase. Leuk Res 28: 191–202 [DOI] [PubMed] [Google Scholar]
- Pattni K, Millard TH, Banting G 2003. Calpain cleavage of the B isoform of Ins(1,4,5)P3 3-kinase separates the catalytic domain from the membrane anchoring domain. Biochem J 375: 643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelled D, Lloyd-Evans E, Riebeling C, Jeyakumar M, Platt FM, Futerman AH 2003. Inhibition of calcium uptake via the sarco/endoplasmic reticulum Ca2+-ATPase in a mouse model of Sandhoff disease and prevention by treatment with N-butyldeoxynojirimycin. J Biol Chem 278: 29496–29501 [DOI] [PubMed] [Google Scholar]
- Pelled D, Trajkovic-Bodennec S, Lloyd-Evans E, Sidransky E, Schiffmann R, Futerman AH 2005. Enhanced calcium release in the acute neuronopathic form of Gaucher disease. Neurobiol Dis 18: 83–88 [DOI] [PubMed] [Google Scholar]
- Pereira L, Matthes J, Schuster I, Valdivia HH, Herzig S, Richard S, Gomez AM 2006. Mechanisms of [Ca2+]i transient decrease in cardiomyopathy of db/db type 2 diabetic mice. Diabetes 55: 608–615 [DOI] [PubMed] [Google Scholar]
- Petersen OH, Tepikin A, Park MK 2001. The endoplasmic reticulum: one continuous or several separate Ca2+ stores? Trends Neurosci 24: 271–276 [DOI] [PubMed] [Google Scholar]
- Pirot P, Cardozo AK, Eizirik DL 2008. Mediators and mechanisms of pancreatic β-cell death in type 1 diabetes. Arq Bras Endocrinol Metabol 52: 156–165 [DOI] [PubMed] [Google Scholar]
- Pozzan T, Rizzuto R, Volpe P, Meldolesi J 1994. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev 74: 595–636 [DOI] [PubMed] [Google Scholar]
- Prusiner SB 1998. Prions. Proc Natl Acad Sci 95: 13363–13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putcha GV, Le S, Frank S, Besirli CG, Clark K, Chu B, Alix S, Youle RJ, LaMarche A, Maroney AC, et al. 2003. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron 38: 899–914 [DOI] [PubMed] [Google Scholar]
- Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, et al. 2007. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129: 1337–1349 [DOI] [PubMed] [Google Scholar]
- Putney JW Jr 1986. A model for receptor-regulated calcium entry. Cell Calcium 7: 1–12 [DOI] [PubMed] [Google Scholar]
- Pyrko P, Kardosh A, Liu YT, Soriano N, Xiong W, Chow RH, Uddin J, Petasis NA, Mircheff AK, Farley RA, et al. 2007. Calcium-activated endoplasmic reticulum stress as a major component of tumor cell death induced by 2,5-dimethyl-celecoxib, a non-coxib analogue of celecoxib. Mol Cancer Ther 6: 1262–1275 [DOI] [PubMed] [Google Scholar]
- Qi MY, Liu HR, Dai DZ, Li N, Dai Y 2008. Total triterpene acids, active ingredients from Fructus Corni, attenuate diabetic cardiomyopathy by normalizing ET pathway and expression of FKBP12.6 and SERCA2a in streptozotocin-rats. J Pharm Pharmacol 60: 1687–1694 [DOI] [PubMed] [Google Scholar]
- Rahmani M, Davis EM, Crabtree TR, Habibi JR, Nguyen TK, Dent P, Grant S 2007. The kinase inhibitor sorafenib induces cell death through a process involving induction of endoplasmic reticulum stress. Mol Cell Biol 27: 5499–5513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randriamboavonjy V, Pistrosch F, Bolck B, Schwinger RH, Dixit M, Badenhoop K, Cohen RA, Busse R, Fleming I 2008. Platelet sarcoplasmic endoplasmic reticulum Ca2+-ATPase and mu-calpain activity are altered in type 2 diabetes mellitus and restored by rosiglitazone. Circulation 117: 52–60 [DOI] [PubMed] [Google Scholar]
- Rao RV, Bredesen DE 2004. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr Opin Cell Biol 16: 653–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rardon DP, Cefali DC, Mitchell RD, Seiler SM, Hathaway DR, Jones LR 1990. Digestion of cardiac and skeletal muscle junctional sarcoplasmic reticulum vesicles with calpain II. Effects on the Ca2+ release channel. Circ Res 67: 84–96 [DOI] [PubMed] [Google Scholar]
- Reiken S, Gaburjakova M, Gaburjakova J, He KL, Prieto A, Becker E, Yi GH, Wang J, Burkhoff D, Marks AR 2001. ß-adrenergic receptor blockers restore cardiac calcium release channel (ryanodine receptor) structure and function in heart failure. Circulation 104: 2843–2848 [DOI] [PubMed] [Google Scholar]
- Reiken S, Lacampagne A, Zhou H, Kherani A, Lehnart SE, Ward C, Huang F, Gaburjakova M, Gaburjakova J, Rosemblit N, et al. 2003. PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle: defective regulation in heart failure. J Cell Biol 160: 919–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Gintant GA, Miller RE, Davidoff AJ 1997. High extracellular glucose impairs cardiac E-C coupling in a glycosylation-dependent manner. Am J Physiol 273: H2876–H2883 [DOI] [PubMed] [Google Scholar]
- Rengifo J, Gibson CJ, Winkler E, Collin T, Ehrlich BE 2007. Regulation of the inositol 1,4,5-trisphosphate receptor type I by O-GlcNAc glycosylation. J Neurosci 27: 13813–13821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H, Gronke S, Adam C, Ribati M, Brabender J, Zobel C, Frank KF, Wippermann J, Schwinger RH, Brixius K, et al. 2008. Sarcoplasmic Ca2+ release is prolonged in nonfailing myocardium of diabetic patients. Mol Cell Biochem 308: 141–149 [DOI] [PubMed] [Google Scholar]
- Rigaud M, Gemes G, Weyker PD, Cruikshank JM, Kawano T, Wu HE, Hogan QH 2009. Axotomy depletes intracellular calcium stores in primary sensory neurons. Anesthesiology 111: 381–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Marchi S, Bonora M, Aguiari P, Bononi A, De Stefani D, Giorgi C, Leo S, Rimessi A, Siviero R, et al. 2009. Ca2+ transfer from the ER to mitochondria: when, how and why. Biochim Biophys Acta 1787: 1342–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach JC, Deutsch K, Li S, Siegel AF, Bekris LM, Einhaus DC, Sheridan CM, Glusman G, Hood L, Lernmark A, et al. 2006. Genetic mapping at 3-kilobase resolution reveals inositol 1,4,5-triphosphate receptor 3 as a risk factor for type 1 diabetes in Sweden. Am J Hum Genet 79: 614–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LC, Marchant JS 2008. Enhanced Ca2+ leak from ER Ca2+ stores induced by hepatitis C NS5A protein. Biochem Biophys Res Commun 368: 593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe MW, Philipson LH, Frangakis CJ, Kuznetsov A, Mertz RJ, Lancaster ME, Spencer B, Worley JF III, Dukes ID 1994. Defective glucose-dependent endoplasmic reticulum Ca2+ sequestration in diabetic mouse islets of Langerhans. J Biol Chem 269: 18279–18282 [PubMed] [Google Scholar]
- Ron D, Walter P 2007. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8: 519–529 [DOI] [PubMed] [Google Scholar]
- Rong Y, Distelhorst CW 2008. Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annu Rev Physiol 70: 73–91 [DOI] [PubMed] [Google Scholar]
- Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A 1972. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30: 595–602 [DOI] [PubMed] [Google Scholar]
- Ryu EJ, Harding HP, Angelastro JM, Vitolo OV, Ron D, Greene LA 2002. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson's disease. J Neurosci 22: 10690–10698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra FR, Redondo PC, Hernandez-Cruz JM, Salido GM, Pariente JA, Rosado JA 2004. Store-operated Ca2+ entry and tyrosine kinase pp60src hyperactivity are modulated by hyperglycemia in platelets from patients with non insulin-dependent diabetes mellitus. Arch Biochem Biophys 432: 261–268 [DOI] [PubMed] [Google Scholar]
- Sacchetto R, Testoni S, Gentile A, Damiani E, Rossi M, Liguori R, Drogemuller C, Mascarello F 2009. A defective SERCA1 protein is responsible for congenital pseudomyotonia in Chianina cattle. Am J Pathol 174: 565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakura C, Hagiwara A, Fukuda K, Shimomura K, Takagi T, Kin S, Nakase Y, Fujiyama J, Mikoshiba K, Okazaki Y, et al. 2003. Possible involvement of inositol 1,4,5-trisphosphate receptor type 3 (IP3R3) in the peritoneal dissemination of gastric cancers. Anticancer Res 23: 3691–3697 [PubMed] [Google Scholar]
- Sakata S, Lebeche D, Sakata Y, Sakata N, Chemaly ER, Liang L, Nakajima-Takenaka C, Tsuji T, Konishi N, del Monte F, et al. 2007. Transcoronary gene transfer of SERCA2a increases coronary blood flow and decreases cardiomyocyte size in a type 2 diabetic rat model. Am J Physiol 292: H1204–H1207 [DOI] [PubMed] [Google Scholar]
- Sakuntabhai A, Ruiz-Perez V, Carter S, Jacobsen N, Burge S, Monk S, Smith M, Munro CS, O'Donovan M, Craddock N, et al. 1999. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat Genet 21: 271–277 [DOI] [PubMed] [Google Scholar]
- Sambrook JF 1990. The involvement of calcium in transport of secretory proteins from the endoplasmic reticulum. Cell 61: 197–199 [DOI] [PubMed] [Google Scholar]
- Sammels E, Parys JB, Missiaen L, De Smedt H, Bultynck G 2010. Intracellular Ca2+ storage in health and disease: a dynamic equilibrium. Cell Calcium 47: 297–314 [DOI] [PubMed] [Google Scholar]
- Sano R, Annunziata I, Patterson A, Moshiach S, Gomero E, Opferman J, Forte M, d'Azzo A 2009. GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca2+-dependent mitochondrial apoptosis. Mol Cell 36: 500–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish V, Thompson MA, Bailey JP, Pabelick CM, Prakash YS, Sieck GC 2009. Effect of proinflammatory cytokines on regulation of sarcoplasmic reticulum Ca2+ reuptake in human airway smooth muscle. Am J Physiol 297: L26–L34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Fujiwara H, Oku H, Ishiguro K, Ohizumi Y 2004. α-mangostin induces Ca2+-ATPase-dependent apoptosis via mitochondrial pathway in PC12 cells. J Pharmacol Sci 95: 33–40 [DOI] [PubMed] [Google Scholar]
- Savino JA III, Evans JF, Rabinowitz D, Auborn KJ, Carter TH 2006. Multiple, disparate roles for calcium signaling in apoptosis of human prostate and cervical cancer cells exposed to diindolylmethane. Mol Cancer Ther 5: 556–563 [DOI] [PubMed] [Google Scholar]
- Schindl R, Muik M, Fahrner M, Derler I, Fritsch R, Bergsmann J, Romanin C 2009. Recent progress on STIM1 domains controlling Orai activation. Cell Calcium 46: 227–232 [DOI] [PubMed] [Google Scholar]
- Schmidt AG, Haghighi K, Frank B, Pater L, Dorn GW, Walsh RA, Kranias EG 2003a. Polymorphic SERCA2a variants do not account for inter-individual differences in phospholamban-SERCA2a interactions in human heart failure. J Mol Cell Cardiol 35: 867–870 [DOI] [PubMed] [Google Scholar]
- Schmidt T, Zaib F, Samson SE, Kwan CY, Grover AK 2003b. Peroxynitrite resistance of sarco/endoplasmic reticulum Ca2+ pump in pig coronary artery endothelium and smooth muscle. Cell Calcium 36: 77–82 [DOI] [PubMed] [Google Scholar]
- Schmitt JP, Kamisago M, Asahi M, Li GH, Ahmad F, Mende U, Kranias EG, MacLennan DH, Seidman JG, Seidman CE 2003. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science 299: 1410–1413 [DOI] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ 2005. The mammalian unfolded protein response. Annu Rev Biochem 74: 739–789 [DOI] [PubMed] [Google Scholar]
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ 2003. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 300: 135–139 [DOI] [PubMed] [Google Scholar]
- Searls YM, Loganathan R, Smirnova IV, Stehno-Bittel L 2010. Intracellular Ca2+ regulating proteins in vascular smooth muscle cells are altered with type 1 diabetes due to the direct effects of hyperglycemia. Cardiovasc Diabetol 9: 8 2010 101186/1475-2840-9-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler T, Hasenfuss G, Maier LS 2007. Targeting altered calcium physiology in the heart: translational approaches to excitation, contraction, and transcription. Physiology 22: 328–334 [DOI] [PubMed] [Google Scholar]
- Seo NS, Zeng CQ, Hyser JM, Utama B, Crawford SE, Kim KJ, Hook M, Estes MK 2008. Inaugural article: integrins α1β1 and α2β1 are receptors for the rotavirus enterotoxin. Proc Natl Acad Sci 105: 8811–8818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah RD, Gonzales F, Golez E, Augustin D, Caudillo S, Abbott A, Morello J, McDonough PM, Paolini PJ, Shubeita HE 2005. The antidiabetic agent rosiglitazone upregulates SERCA2 and enhances TNF-α- and LPS-induced NF-kB-dependent transcription and TNF-α-induced IL-6 secretion in ventricular myocytes. Cell Physiol Biochem 15: 41–50 [DOI] [PubMed] [Google Scholar]
- Shao CH, Rozanski GJ, Patel KP, Bidasee KR 2007. Dyssynchronous (non-uniform) Ca2+ release in myocytes from streptozotocin-induced diabetic rats. J Mol Cell Cardiol 42: 234–246 [DOI] [PubMed] [Google Scholar]
- Shao CH, Wehrens XH, Wyatt TA, Parbhu S, Rozanski GJ, Patel KP, Bidasee KR 2009. Exercise training during diabetes attenuates cardiac ryanodine receptor dysregulation. J Appl Physiol 106: 1280–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Wang L, Zhu Y, DeGuzman A, Cao GY, Lynn RB, Joseph SK 1999. Renal type I inositol 1,4,5-trisphosphate receptor is reduced in streptozotocin-induced diabetic rats and mice. Am J Physiol 276: F54–F61 [DOI] [PubMed] [Google Scholar]
- Sharon-Friling R, Goodhouse J, Colberg-Poley AM, Shenk T 2006. Human cytomegalovirus pUL37x1 induces the release of endoplasmic reticulum calcium stores. Proc Natl Acad Sci 103: 19117–19122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Yu WM, Brotto M, Scherman JA, Guo C, Stoddard C, Nosek TM, Valdivia HH, Qu CK 2009. Deficiency of MIP/MTMR14 phosphatase induces a muscle disorder by disrupting Ca2+ homeostasis. Nat Cell Biol 11: 769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M 2008. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell 135: 110–122 [DOI] [PubMed] [Google Scholar]
- Stockwin LH, Han B, Yu SX, Hollingshead MG, ElSohly MA, Gul W, Slade D, Galal AM, Newton DL 2009. Artemisinin dimer anticancer activity correlates with heme-catalyzed reactive oxygen species generation and endoplasmic reticulum stress induction. Int J Cancer 125: 1266–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolen TO, Hoydal MA, Kemi OJ, Catalucci D, Ceci M, Aasum E, Larsen T, Rolim N, Condorelli G, Smith GL, et al. 2009. Interval training normalizes cardiomyocyte function, diastolic Ca2+ control, and SR Ca2+ release synchronicity in a mouse model of diabetic cardiomyopathy. Circ Res 105: 527–536 [DOI] [PubMed] [Google Scholar]
- Strom TM, Hortnagel K, Hofmann S, Gekeler F, Scharfe C, Rabl W, Gerbitz KD, Meitinger T 1998. Diabetes insipidus, diabetes mellitus, optic atrophy and deafness (DIDMOAD) caused by mutations in a novel gene (wolframin) coding for a predicted transmembrane protein. Hum Mol Genet 7: 2021–2028 [DOI] [PubMed] [Google Scholar]
- Suarez J, Scott B, Dillmann WH 2008. Conditional increase in SERCA2a protein is able to reverse contractile dysfunction and abnormal calcium flux in established diabetic cardiomyopathy. Am J Physiol 295: R1439–R1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman M, Matta MJ, Sunderesan NR, Gupta MP, Periasamy M, Gupta M 2010. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. Am J Physiol 298: H833–H843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supnet C, Bezprozvanny I 2010. The dysregulation of intracellular calcium in Alzheimer disease. Cell Calcium 47: 183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegezdi E, Fitzgerald U, Samali A 2003. Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann N Y Acad Sci 1010: 186–194 [DOI] [PubMed] [Google Scholar]
- Taiyab A, Sreedhar AS, Rao ChM 2009. Hsp90 inhibitors, GA and 17AAG, lead to ER stress-induced apoptosis in rat histiocytoma. Biochem Pharmacol 78: 142–152 [DOI] [PubMed] [Google Scholar]
- Takei D, Ishihara H, Yamaguchi S, Yamada T, Tamura A, Katagiri H, Maruyama Y, Oka Y 2006. WFS1 protein modulates the free Ca2+ concentration in the endoplasmic reticulum. FEBS Lett 580: 5635–5640 [DOI] [PubMed] [Google Scholar]
- Takenaka H, Adler PN, Katz AM 1982. Calcium fluxes across the membrane of sarcoplasmic reticulum vesicles. J Biol Chem 257: 12649–12656 [PubMed] [Google Scholar]
- Teshima Y, Takahashi N, Saikawa T, Hara M, Yasunaga S, Hidaka S, Sakata T 2000. Diminished expression of sarcoplasmic reticulum Ca2+-ATPase and ryanodine sensitive Ca2+ channel mRNA in streptozotocin-induced diabetic rat heart. J Mol Cell Cardiol 32: 655–664 [DOI] [PubMed] [Google Scholar]
- Tessitore A, del P Martin M, Sano R, Ma Y, Mann L, Ingrassia A, Laywell ED, Steindler DA, Hendershot LM, d'Azzo A 2004. GM1-ganglioside-mediated activation of the unfolded protein response causes neuronal death in a neurodegenerative gangliosidosis. Mol Cell 15: 753–766 [DOI] [PubMed] [Google Scholar]
- Thompson S, Chen X, Hui L, Toschi A, Foster DA, Drain CM 2008. Low concentrations of a non-hydrolysable tetra-S-glycosylated porphyrin and low light induces apoptosis in human breast cancer cells via stress of the endoplasmic reticulum. Photochem Photobiol Sci 7: 1415–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian P, Estes MK, Hu Y, Ball JM, Zeng CQ, Schilling WP 1995. The rotavirus nonstructural glycoprotein NSP4 mobilizes Ca2+ from the endoplasmic reticulum. J Virol 69: 5763–5772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Evangelista A, Cohen RA 2009. Targeting the redox regulation of SERCA in vascular physiology and disease. Curr Opin Pharmacol 10: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Ying J, Pimentel DR, Trucillo M, Adachi T, Cohen RA 2008. High glucose oxidizes SERCA cysteine-674 and prevents inhibition by nitric oxide of smooth muscle cell migration. J Mol Cell Cardiol 44: 361–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnesen MF, Grunnet LG, Friberg J, Cardozo AK, Billestrup N, Eizirik DL, Storling J, Mandrup-Poulsen T 2009. Inhibition of Nuclear Factor-kB or Bax prevents endoplasmic reticulum stress- but not nitric oxide-mediated apoptosis in INS-1E cells. Endocrinology 150: 4094–4103 [DOI] [PubMed] [Google Scholar]
- Tosch V, Rohde HM, Tronchere H, Zanoteli E, Monroy N, Kretz C, Dondaine N, Payrastre B, Mandel JL, Laporte J 2006. A novel PtdIns3P and PtdIns(3,5)P2 phosphatase with an inactivating variant in centronuclear myopathy. Hum Mol Genet 15: 3098–3106 [DOI] [PubMed] [Google Scholar]
- Trost SU, Belke DD, Bluhm WF, Meyer M, Swanson E, Dillmann WH 2002. Overexpression of the sarcoplasmic reticulum Ca2+-ATPase improves myocardial contractility in diabetic cardiomyopathy. Diabetes 51: 1166–1171 [DOI] [PubMed] [Google Scholar]
- Tsuchiya S, Tsuji M, Morio Y, Oguchi K 2006. Involvement of endoplasmic reticulum in glycochenodeoxycholic acid-induced apoptosis in rat hepatocytes. Toxicol Lett 166: 140–149 [DOI] [PubMed] [Google Scholar]
- Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I 2006. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell 126: 981–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Baelen K, Dode L, Vanoevelen J, Callewaert G, De Smedt H, Missiaen L, Parys JB, Raeymaekers L, Wuytack F 2004. The Ca2+/Mn2+ pumps in the Golgi apparatus. Biochim Biophys Acta 1742: 103–112 [DOI] [PubMed] [Google Scholar]
- Vanderheyden V, Devogelaere B, Missiaen L, De Smedt H, Bultynck G, Parys JB 2009. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochim Biophys Acta 1793: 959–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kallen CJ, van Greevenbroek MM, Stehouwer CD, Schalkwijk CG 2009. Endoplasmic reticulum stress-induced apoptosis in the development of diabetes: is there a role for adipose tissue and liver? Apoptosis 14: 1424–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangheluwe P, Sepulveda MR, Missiaen L, Raeymaekers L, Wuytack F, Vanoevelen J 2009. Intracellular Ca2+- and Mn2+-transport ATPases. Chem Rev 109: 4733–4759 [DOI] [PubMed] [Google Scholar]
- van Kuppeveld FJ, de Jong AS, Melchers WJ, Willems PH 2005. Enterovirus protein 2B po(u)res out the calcium: a viral strategy to survive? Trends Microbiol 13: 41–44 [DOI] [PubMed] [Google Scholar]
- Vanoverberghe K, Vanden Abeele F, Mariot P, Lepage G, Roudbaraki M, Bonnal JL, Mauroy B, Shuba Y, Skryma R, Prevarskaya N 2004. Ca2+ homeostasis and apoptotic resistance of neuroendocrine-differentiated prostate cancer cells. Cell Death Differ 11: 321–330 [DOI] [PubMed] [Google Scholar]
- Varadi A, Lebel L, Hashim Y, Mehta Z, Ashcroft SJ, Turner R 1999. Sequence variants of the sarco(endo)plasmic reticulum Ca2+-transport ATPase 3 gene (SERCA3) in Caucasian type II diabetic patients (UK Prospective Diabetes Study 48). Diabetologia 42: 1240–1243 [DOI] [PubMed] [Google Scholar]
- Vasanji Z, Cantor EJ, Juric D, Moyen M, Netticadan T 2006. Alterations in cardiac contractile performance and sarcoplasmic reticulum function in sucrose-fed rats is associated with insulin resistance. Am J Physiol 291: C772–C780 [DOI] [PubMed] [Google Scholar]
- Vasanji Z, Dhalla NS, Netticadan T 2004. Increased inhibition of SERCA2 by phospholamban in the type I diabetic heart. Mol Cell Biochem 261: 245–249 [DOI] [PubMed] [Google Scholar]
- Vericel E, Januel C, Carreras M, Moulin P, Lagarde M 2004. Diabetic patients without vascular complications display enhanced basal platelet activation and decreased antioxidant status. Diabetes 53: 1046–1051 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A 2005. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev 85: 201–279 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Fernyhough P 2008. Mitochondrial malfunction and Ca2+ dyshomeostasis drive neuronal pathology in diabetes. Cell Calcium 44: 112–122 [DOI] [PubMed] [Google Scholar]
- Vermassen E, Parys JB, Mauger JP 2004. Subcellular distribution of the inositol 1,4,5-trisphosphate receptors: functional relevance and molecular determinants. Biol Cell 96: 3–17 [DOI] [PubMed] [Google Scholar]
- Vetter R, Rehfeld U, Reissfelder C, Weiss W, Wagner KD, Gunther J, Hammes A, Tschope C, Dillmann W, Paul M 2002. Transgenic overexpression of the sarcoplasmic reticulum Ca2+ATPase improves reticular Ca2+ handling in normal and diabetic rat hearts. FASEB J 16: 1657–1659 [DOI] [PubMed] [Google Scholar]
- Viner RI, Ferrington DA, Williams TD, Bigelow DJ, Schoneich C 1999. Protein modification during biological aging: selective tyrosine nitration of the SERCA2a isoform of the sarcoplasmic reticulum Ca2+-ATPase in skeletal muscle. Biochem J 340: 657–669 [PMC free article] [PubMed] [Google Scholar]
- Wang XZ, Ron D 1996. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP Kinase. Science 272: 1347–1349 [DOI] [PubMed] [Google Scholar]
- Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC 1999. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science 284: 339–343 [DOI] [PubMed] [Google Scholar]
- Wang M, Zhang WB, Zhu JH, Fu GS, Zhou BQ 2010. Breviscapine ameliorates cardiac dysfunction and regulates the myocardial Ca2+-cycling proteins in streptozotocin-induced diabetic rats. Acta Diabetol doi:101007/s00592-009-0164-x [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, et al. 2003. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell 113: 829–840 [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Marks AR 2005a. Intracellular calcium release and cardiac disease. Annu Rev Physiol 67: 69–98 [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken S, van der Nagel R, Morales R, Sun J, Cheng Z, Deng SX, de Windt LJ, Landry DW, et al. 2005b. Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function in heart failure. Proc Natl Acad Sci 102: 9607–9612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR 2006. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci 103: 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Perry DC 1996. Dantrolene is cytoprotective in two models of neuronal cell death. J Neurochem 67: 2390–2398 [DOI] [PubMed] [Google Scholar]
- Wei H, Kim SJ, Zhang Z, Tsai PC, Wisniewski KE, Mukherjee AB 2008a. ER and oxidative stresses are common mediators of apoptosis in both neurodegenerative and non-neurodegenerative lysosomal storage disorders and are alleviated by chemical chaperones. Hum Mol Genet 17: 469–477 [DOI] [PubMed] [Google Scholar]
- Wei H, Liang G, Yang H, Wang Q, Hawkins B, Madesh M, Wang S, Eckenhoff RG 2008b. The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology 108: 251–260 [DOI] [PubMed] [Google Scholar]
- Wei Y, Wang D, Gentile CL, Pagliassotti MJ 2009. Reduced endoplasmic reticulum luminal calcium links saturated fatty acid-mediated endoplasmic reticulum stress and cell death in liver cells. Mol Cell Biochem 331: 31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB, Foskett JK 2005. The endoplasmic reticulum gateway to apoptosis by Bcl-XL modulation of the InsP3R. Nat Cell Biol 7: 1021–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BL, Lipkin WI 2006. Endoplasmic reticulum stress and neurodegeneration in rats neonatally infected with borna disease virus. J Virol 80: 8613–8626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold LE, Dutta K, Mason MM, Ren J, Cala SE, Schwanke ML, Davidoff AJ 2005. Impaired SERCA function contributes to cardiomyocyte dysfunction in insulin resistant rats. J Mol Cell Cardiol 39: 297–307 [DOI] [PubMed] [Google Scholar]
- Wu X, Sun L, Zha W, Studer E, Gurley E, Chen L, Wang X, Hylemon PB, Pandak WM Jr, Sanyal AJ, et al. 2010. HIV protease inhibitors induce ER stress and disrupt barrier integrity in intestinal epithelial cells. Gastroenterology 138: 197–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong ZG, Chu XP, Simon RP 2007. Acid sensing ion channels–novel therapeutic targets for ischemic brain injury. Front Biosci 12: 1376–1386 [DOI] [PubMed] [Google Scholar]
- Xu C, Bailly-Maitre B, Reed JC 2005. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115: 2656–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Eu JP, Meissner G, Stamler JS 1998. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science 279: 234–237 [DOI] [PubMed] [Google Scholar]
- Yamada T, Ishihara H, Tamura A, Takahashi R, Yamaguchi S, Takei D, Tokita A, Satake C, Tashiro F, Katagiri H, et al. 2006. WFS1-deficiency increases endoplasmic reticulum stress, impairs cell cycle progression and triggers the apoptotic pathway specifically in pancreatic β-cells. Hum Mol Genet 15: 1600–1609 [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Ichijo H, Korsmeyer SJ 1999. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G2/M. Mol Cell Biol 19: 8469–8478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei H 2008. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology 109: 243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Kobayashi S, Kohno M, Doi M, Tokuhisa T, Okuda S, Suetsugu M, Hisaoka T, Obayashi M, Ohkusa T, et al. 2003. FKBP12.6-mediated stabilization of calcium-release channel (ryanodine receptor) as a novel therapeutic strategy against heart failure. Circulation 107: 477–484 [DOI] [PubMed] [Google Scholar]
- Yano M, Yamamoto T, Kobayashi S, Ikeda Y, Matsuzaki M 2008. Defective Ca2+ cycling as a key pathogenic mechanism of heart failure. Circ J 72: A22–A30 [DOI] [PubMed] [Google Scholar]
- Yaras N, Ugur M, Ozdemir S, Gurdal H, Purali N, Lacampagne A, Vassort G, Turan B 2005. Effects of diabetes on ryanodine receptor Ca release channel (RyR2) and Ca2+ homeostasis in rat heart. Diabetes 54: 3082–3088 [DOI] [PubMed] [Google Scholar]
- Ying J, Sharov V, Xu S, Jiang B, Gerrity R, Schoneich C, Cohen RA 2008. Cysteine-674 oxidation and degradation of sarcoplasmic reticulum Ca2+ ATPase in diabetic pig aorta. Free Radic Biol Med 45: 756–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T, Tohyama M 2001. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem 276: 13935–13940 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Hosokawa N, Kaufman RJ, Nagata K, Mori K 2003. A time-dependent phase shift in the mammalian unfolded protein response. Dev Cell 4: 265–271 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K 2001. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107: 881–891 [DOI] [PubMed] [Google Scholar]
- Yoshida I, Monji A, Tashiro K, Nakamura K, Inoue R, Kanba S 2006. Depletion of intracellular Ca2+ store itself may be a major factor in thapsigargin-induced ER stress and apoptosis in PC12 cells. Neurochem Int 48: 696–702 [DOI] [PubMed] [Google Scholar]
- Zalk R, Lehnart SE, Marks AR 2007. Modulation of the ryanodine receptor and intracellular calcium. Annu Rev Biochem 76: 367–385 [DOI] [PubMed] [Google Scholar]
- Zbidi H, Redondo PC, Lopez JJ, Bartegi A, Salido GM, Rosado JA 2010. Homocysteine induces caspase activation by endoplasmic reticulum stress in platelets from type 2 diabetics and healthy donors. Thromb Haemost 103: 1022–1032 [DOI] [PubMed] [Google Scholar]
- Zhang S, Ong CN, Shen HM 2004. Critical roles of intracellular thiols and calcium in parthenolide-induced apoptosis in human colorectal cancer cells. Cancer Lett 208: 143–153 [DOI] [PubMed] [Google Scholar]
- Zhang L, Cannell MB, Phillips AR, Cooper GJ, Ward ML 2008. Altered calcium homeostasis does not explain the contractile deficit of diabetic cardiomyopathy. Diabetes 57: 2158–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen HS, Khanna VK, De Leon S, Phillips MS, Schappert K, Britt BA, Browell AK, MacLennan DH 1993. A mutation in the human ryanodine receptor gene associated with central core disease. Nat Genet 5: 46–50 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lee YC, Kim SJ, Choi MS, Tsai PC, Xu Y, Xiao YJ, Zhang P, Heffer A, Mukherjee AB 2007. Palmitoyl-protein thioesterase-1 deficiency mediates the activation of the unfolded protein response and neuronal apoptosis in INCL. Hum Mol Genet 15: 337–346 [DOI] [PubMed] [Google Scholar]
- Zhong Y, Ahmed S, Grupp IL, Matlib MA 2001. Altered SR protein expression associated with contractile dysfunction in diabetic rat hearts. Am J Physiol 281: H1137–H1147 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Frey TK, Yang JJ 2009. Viral calciomics: interplays between Ca2+ and virus. Cell Calcium 46: 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BQ, Hu SJ, Wang GB 2006. The analysis of ultrastructure and gene expression of sarco/endoplasmic reticulum calcium handling proteins in alloxan-induced diabetic rat myocardium. Acta Cardiol 61: 21–27 [DOI] [PubMed] [Google Scholar]
- Zhou H, Pandak WM Jr, Lyall V, Natarajan R, Hylemon PB 2005. HIV protease inhibitors activate the unfolded protein response in macrophages: implication for atherosclerosis and cardiovascular disease. Mol Pharmacol 68: 690–700 [DOI] [PubMed] [Google Scholar]