Abstract
A number of long coiled-coil proteins are present on the Golgi. Often referred to as “golgins,” they are well conserved in evolution and at least five are likely to have been present in the last common ancestor of all eukaryotes. Individual golgins are found in different parts of the Golgi stack, and they are typically anchored to the membrane at their carboxyl termini by a transmembrane domain or by binding a small GTPase. They appear to have roles in membrane traffic and Golgi structure, but their precise function is in most cases unclear. Many have binding sites for Rab family GTPases along their length, and this has led to the suggestion that the golgins act collectively to form a tentacular matrix that surrounds the Golgi to capture Rab-coated membranes in the vicinity of the stack. Such a collective role might explain the lack of cell lethality seen following loss of some of the genes in human familial conditions or mouse models.
Long coiled-coil proteins are present throughout Golgi stacks. These “golgins” are membrane anchored and may collectively form a matrix that is recognized by the Rab GTPases required for Golgi trafficking.
Coiled-coils are widely occurring protein structural motifs in which two or more α-helices wind around each other to form an extended rod-like structure. Proteins containing such structures are found in many parts of the cell, and play diverse roles including organizing centrosomes, chromatin, and synapses, or serving as molecular motors. As such there may seem little reason to consider them collectively beyond an interest in the structural and biophysical properties of the coiled-coil itself. However, the Golgi is unique amongst the cellular compartments in that several different large coiled-coil proteins are present on its cytoplasmic surface (Gillingham and Munro 2003; Lupashin and Sztul 2005; Short et al. 2005; Ramirez and Lowe 2009). A number of these share a similar organization in that most of the protein is predicted to form a coiled-coil, and that their carboxyl termini mediate attachment to Golgi membranes. They are generally ubiquitously expressed and well conserved in evolution, but their coiled-coil regions are relatively poorly conserved suggesting that much of their length serves as spacer. Given that 500 residues of coiled-coil is ∼75 nm in length then the proteins could extend for ∼100–400 nm. Some of the proteins have regions which appear likely to be unstructured and hence could serve as extensions or hinges to increase the proteins’ reach and flexibility (Oas and Endow 1994; Yamakawa et al. 1996). These shared features suggest that the proteins serve related functions on the Golgi. The term “golgin” is often applied to these proteins having been coined in early studies when several were found as human autoantigens (Fritzler et al. 1993), but the term lacks a clear definition. To provide a focus to this article, I will concentrate on “golgins” as defined by being a protein that is found primarily, if not exclusively, on the Golgi and is predicted to form a homodimeric parallel coiled-coil over most of its length. Proteins with shorter regions of coiled-coil are more likely to have roles distinct to the golgins, especially if further domains are present.
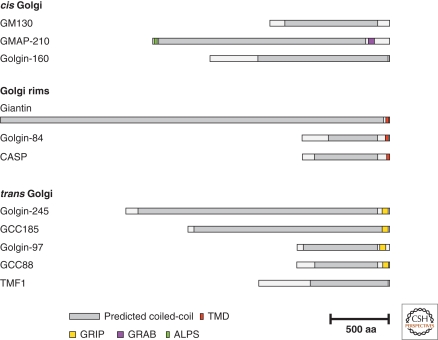
Golgin coiled-coil proteins are found on the cis-face of the Golgi, around the rims of the stack and on the trans-face of the Golgi (Fig. 1). The human golgins are summarized in Table 1, along with their orthologs in model organisms and the rather confusing gene names inflicted by the Human Gene Nomenclature Committee. I discuss what is known about the individual proteins from each of the parts of the Golgi, and mention briefly the Golgi coiled-coil proteins that are probably not golgins. I then discuss how the golgins are regulated and how their properties might reflect a shared function in Golgi organization and traffic.
Figure 1.
The golgin coiled-coil proteins of humans.
Schematic representations of known human golgins. Regions predicted to form coiled-coils are shown in gray, and known domains involved in protein function or subcellular targeting are indicated.
Table 1.
The canonical golgins of the human Golgi and their orthologs.
| Protein | Alternative names | Human gene symbol | D. melanogaster | C. elegans | S. cerevisiae | A. thaliana |
|---|---|---|---|---|---|---|
| GM130 | golgin-95 | GOLGA2 | CG11061 | F33G12.5 | BUG1 | |
| GMAP-210 | Trip230 CEV14 |
TRIP11 | CG7821 | Y111B2A.4 | RUD3 | At3g6157 At2g46180 |
| golgin-160 | Mea-2 IIGP165 GCP170 |
GOLGA3 | ||||
| golgin-84 | RFG5 | GOLGA5 | CG17785 | T24B1.1 | At1g18190 At2g19950 |
|
| CASP | CUX1 (alt) | Y54F10AM.4c (ceh-44) | COY1 | At3g18480 | ||
| giantin | macrogolgin GCP372 |
GOLGB1 | CG6450 (lva) |
|||
| golgin-97 | GOLGA1 | CG4840 (cbs) |
IMH1 | At5g66030 | ||
| golgin-245 | p230 tGolgin-1 |
GOLGA4 | CG3493 | F59A2.2/6 | ||
| GCC88 | GCC1 | CG10703 | C15C7.2.1 (klp-8) |
|||
| GCC185 | GCC2 | CG3532 | T05G5.9 | |||
| TMF | ARA160 | TMF1 | CG4557 | F39H12.1 | SGM1 | At1g79830 |
GOLGINS AT THE CIS-GOLGI: GM130, GMAP-210, AND GOLGIN-160
GM130 and its Relatives
Identified as a Golgi autoantigen, GM130 is a ubiquitously expressed protein of the cis-Golgi (Fritzler et al. 1993; Nakamura et al. 1995). The carboxyl terminus of the rat protein has been shown to bind the lipid-anchored PDZ domain protein GRASP65, and this region of the protein is required for Golgi localization (Barr et al. 1997; Barr et al. 1998). The protein is conserved in metazoans and fungi, and a diminutive yeast ortholog, Bug1, binds to the yeast ortholog of GRASP65 (Behnia et al. 2007). However the protein does not appear to have orthologs outside of opisthikonts, in contrast to GRASP65 which is conserved in protozoa, and has been reported to have roles in Golgi structure and the traffic of specific cargo beyond binding GM130 (D’Angelo et al. 2009; Xiang and Wang 2010).
GM130 has been shown to interact via its amino terminus with p115, a cytosolic protein that is comprised of armadillo repeats followed by a region of coiled-coil, and which has been shown to be involved in tethering ER-derived transport vesicles to the Golgi in mammals and yeast (Sapperstein et al. 1995; Nakamura et al. 1997; Cao et al. 1998; Satoh and Warren 2008; An et al. 2009; Striegl et al. 2010). GM130 is also an effector for the small GTPases Rab1, Rab30, and Rab33b (Moyer et al. 2001; Valsdottir et al. 2001; Weide et al. 2001; Sinka et al. 2008). However, it seems unlikely that GM130 is essential for membrane traffic as a mutant Chinese hamster cell line has been identified that lacks detectable GM130 and although temperature sensitive, it shows no defects in Golgi structure or protein transport at the permissive temperature (Vasile et al. 2003). GM130 is also the Golgi binding site of a protein kinase linked to directed cell migration, and is also suggested to have roles in SNARE assembly and regulation of centrosomes (reviewed in Nakamura 2010).
In addition to GM130, the human genome contains a family of over 30 genes that are related to GM130 and are referred to as GOLGA6A-J, GOLGA6AL1-10, and GOLGA8A-J (Jiang et al. 2008). Most are present in the repeats of an element that derived from GOLGA2, the gene encoding GM130 on chromosome 9, but has duplicated on the long arm of chromosome 15. These repeats appear to have been generated by genome rearrangements during primate evolution as similar repeats are found in the genome of the macaque, an old-world monkey, whereas nonprimate vertebrates such as mice only have the GOLGA2 gene (Zody et al. 2006). These genes are unlikely to all be pseudogenes as they are predicted to encode proteins and are well represented in EST databases. They are generally internally deleted versions of GM130 and one, golgin-67 (GOLGA8A), has been shown to be widely expressed and present on the Golgi (Eystathioy et al. 2000; Jakymiw et al. 2000). Their significance is unclear, and may be difficult to address, but at the very least, they are relevant to the study of GM130 function in primate cell lines.
GMAP-210
GMAP-210 was reported as one of several hits from a yeast-two hybrid screen with the thyroid hormone receptor and so the human gene is named TRIP11 for thyroid receptor interacting protein 11 (Lee et al. 1995). It has also been reported to bind microtubules, retinoblastoma protein, and centrosomes (Infante et al. 1999). However, the protein is located to the cis-face of the Golgi (Rios et al. 1994), and in mammals, yeast, and plants, the carboxy-terminal region is sufficient to confer a Golgi localization (Gillingham et al. 2004; Latijnhouwers et al. 2007). The carboxy-terminal region contains a well conserved GRAB domain which is related to the GRIP domain described below. The GRAB domain binds the small G protein Arf1, although in mammals binding of the GRAB domain to Golgi membranes appears to be stabilized by an adjacent amphipathic helix (Drin et al. 2008).
The protein is predicted to be coiled-coil over most of its length, but the amino-terminal 38 residues of the human protein have been shown to form an ALPS (amphipathic lipid-packing sensor) motif (Drin et al. 2008). This motif binds preferentially to highly curved membranes leading to the suggestion that the amino terminus could tether transport vesicles to the Golgi, although this is unlikely to be the only interaction made by the protein as the ALPS motif is not well conserved outside of vertebrates. GMAP-210 has also been reported to bind IFT20, a component of cilia, and removal of GMAP-210 causes IFT20 to be displaced from the Golgi (Follit et al. 2008).
A null mutant in mouse GMAP-210 results in impaired skeletal development, and loss-of-function mutations in the human gene have been found to underlie some cases of familial skeletal dysplasia (Smits et al. 2010). In the mutant mice there is a defect in secretion of extracellular matrix by chondrocytes, and Golgi structure appears perturbed, but effects on cilia are disputed (Follit et al. 2008; Smits et al. 2010). Genetic studies in Drosophila and yeast also show that the protein is not essential for bulk secretion, but the yeast ortholog shows several genetic interactions with known components of machinery for traffic through the early Golgi (Kim et al. 1999; VanRheenen et al. 1999; Gillingham et al. 2004; Friggi-Grelin et al. 2006).
Golgin-160
First identified in humans as an autoantigen, golgin-160 is located to the cis-Golgi, and orthologs are present only in deuterosomes (Fritzler et al. 1993). The amino-terminal 400 residues appear to be a conserved domain of unknown function, whilst the remaining 1100 residues are predicted to form a coiled-coil. Its mouse ortholog Mea2 was found as a gene expressed at high levels in testes with uniform low expression elsewhere (Su et al. 1992). A mouse mutant has been identified in which Mea2 is inadvertently disrupted by a transgene (Matsukuma et al. 1999). This results in loss of the first 488 residues and variable expression, with the lowest expression levels (about 5% of normal) correlating with defects in spermatogenesis (Banu et al. 2002).
The function of Golgin-160, and how it is recruited to the Golgi is unclear. It has been reported to interact with three cytoplasmic proteins of unknown function, GCP16 (GOLGA7), ACBD3 (GCP60), and GOPC (PIST) (Ohta et al. 2003; Hicks and Machamer 2005; Sbodio et al. 2006). The ACBD3 interaction is induced by apoptosis, but the GOPC interaction may be relevant to normal function as mice mutants lacking GOPC also show defects in spermatogenesis (Yao et al. 2002).
GOLGINS ON THE RIM OF THE GOLGI STACK: GIANTIN, GOLGIN-84, AND CASP
Three golgins have a transmembrane domain at their carboxyl terminus, and these transmembrane domains are related as they share conserved polar residues (Gillingham et al. 2002). In addition, all three proteins appear to be localized to the rim of the Golgi stack and in COPI vesicles, although the latter location would be obligatory for any integral membrane protein resident in the Golgi if, as is widely believed, COPI vesicles mediate the retrograde traffic of Golgi residents as cisternae mature (Martinez-Menárguez et al. 2001; Oka et al. 2004). For at least CASP and golgin-84, the carboxy-terminal region including the transmembrane domain is sufficient to confer a Golgi localization on a reporter (Bascom et al. 1999; Gillingham et al. 2002; Renna et al. 2005).
Giantin was found as the target of a monoclonal antibody raised against Golgi membranes, and also identified as a human autoantigen (Linstedt and Hauri 1993; Seelig et al. 1994). It appears to be present only in vertebrates, although Drosophila have a similarly large Golgi coiled-coil protein called Lava lamp (Sisson et al. 2000). This protein lacks a TMD but shares some sequence features with giantin and so may be a distant ortholog in insects. Giantin is predicted to be coiled-coil over most of its length, and so if fully extended would project at least 450 nm from the membrane. It has been found to interact with p115 and thus suggested to play a role in vesicle tethering in conjunction with GM130, although the details of this are disputed (Sönnichsen et al. 1998; Lesa et al. 2000; Linstedt et al. 2000). Like golgin-160, it has been reported to bind ACBD3/GCP60, a protein of unknown function (Sohda et al. 2001). The role of giantin itself may be redundant with other golgins as knockdown of the protein by antibody injection has no detectable effect on Golgi structure or traffic (Puthenveedu and Linstedt 2001).
Human golgin-84 was found as a probable false-positive in a yeast two-hybrid screen and is well conserved in evolution with orthologs in plants, but not fungi (Bascom et al. 1999). The third member of the family, CASP, was found by searching for proteins with a TMD related to those of giantin and golgin-84 (Gillingham et al. 2002). In metazoans it is produced by alternative splicing from a gene that also encodes a transcription factor called cut-like 1 (CDP/CUX1), but this seems to reflect a rearrangement in metazoan evolution because CASP is well conserved outside of metazoans being present in fungi, plants, and Trypanosomes where it is present as a single contiguous gene. The precise functions of CASP and golgin-84 are unclear, although they coprecipitate from mammalian cells and it has been suggested that this interaction could mediate tethering of COPI vesicles to the Golgi (Gillingham et al. 2002; Malsam et al. 2005). However, both proteins are not always conserved in the same species with fungi having CASP but not golgin-84 and Drosophila having golgin-84 but not CASP. Removal by RNAi of golgin-84 in cultured cells results in Golgi fragmentation, but forward transport continues (Diao et al. 2003; Sohda et al. 2010). In addition, the yeast ortholog of CASP, Coy1, shows genetic interactions with components of Golgi membrane traffic (Gillingham et al. 2002).
GOLGINS AT THE TRANS-GOLGI: THE GRIP DOMAIN GOLGINS AND TMF
GRIP Domain Golgins
In mammals there are four golgins that share a domain of about 80 residues at their carboxyl terminus called the GRIP domain (golgin-97, RanBP2α, Imh1p, and p230/golgin-245, (Barr 1999; Kjer-Nielsen et al. 1999; Munro and Nichols 1999)). These four proteins are also conserved in most invertebrates, whilst a single GRIP domain protein is present in the genome of many nonmetazoan organisms including fungi, plants, and protozoa. Golgin-97 and golgin-245 were identified as human autoantigens, and then GCC88 and GCC185 were identified by genome searches for further GRIP domain-containing proteins (Erlich et al. 1996; Griffith et al. 1997; Luke et al. 2003).
All the GRIP domain proteins appear to be on the trans-side of the Golgi and the GRIP domain has been shown to mediate Golgi targeting in mammals, yeast, plants, and Trypanosomes (McConville et al. 2002; Latijnhouwers et al. 2005). In all of these species the Golgi targeting of the GRIP domain has been found to depend on binding to a small G protein of the trans-Golgi called Arf-like 1 (Arl1) (Van Valkenburgh et al. 2001; Lu and Hong 2003; Panic et al. 2003b; Setty et al. 2003; Stefano et al. 2006). Structural studies of a complex between Arl1-GTP and the GRIP domain of human golgin-245 revealed that the GRIP domain is formed of three short α helixes, and dimerizes to allow simultaneous binding to two Arl1-GTP molecules (Panic et al. 2003a; Wu et al. 2004). There has been some debate as to whether or not mammalian Arl1 can bind the GRIP domain of GCC185, and whether an adjacent interaction with Rab6 is important for Golgi targeting, but as of yet no alternative interaction partner for the GCC185 GRIP domain has been identified (Panic et al. 2003a; Burguete et al. 2008; Houghton et al. 2009). In some cases, the GRIP domain is followed by a short amphipathic helix that may interact with the lipid bilayer to stabilize membrane association (Panic et al. 2003a).
The role of the mammalian GRIP domain proteins has been investigated by RNAi in cultured cells. Knockdown of individual proteins can result in fragmentation of the Golgi, and defects in the retrograde traffic of some cargo proteins from endosomes to the TGN (Lu et al. 2004; Yoshino et al. 2005; Reddy et al. 2006; Derby et al. 2007; Lieu et al. 2007; Hayes et al. 2009). It has also been suggested that at least some GRIP domain proteins are associated with carriers leaving the Golgi for the plasma membrane and thus they could help in the exit of specific cargo proteins from the TGN (Lieu et al. 2008). The single GRIP domain protein in yeast, Imh1, is not essential for secretion or viability but shows strong genetic interaction with other proteins involved in retrograde traffic from endosomes to Golgi (Tsukada et al. 1999).
TMF
Originally identified as a hit in screens for binding to a TATA element (hence TMF for TATA element modulatory factor) or to the androgen receptor, TMF was subsequently observed to be on the Golgi (Garcia et al. 1992; Hsiao and Chang 1999; Mori and Kato 2002). It is well conserved in evolution with a single ortholog in all metazoans so far examined as well as in fungi, plants and Dictyostelium (Fridmann-Sirkis et al. 2004; Latijnhouwers et al. 2007). Deletion of the S. cerevisiae ortholog Sgm1 results in no clear Golgi or trafficking defect, but the protein binds to Ypt6, the yeast ortholog of Rab6, via its carboxyl terminus, and requires Ypt6 for its Golgi localization (Siniossoglou and Pelham 2001). The mammalian protein also binds to Rab6 through its carboxyl terminus and this part of the protein is sufficient for Golgi targeting. Rab6 is found on the trans-side of the Golgi, but TMF has not been localized by immunoelectron microscopy, and at the light level appears more similar to giantin at the Golgi rims than to TGN markers (Fridmann-Sirkis et al. 2004; Yamane et al. 2007).
When TMF is knocked down by RNAi in mammalian cells, the Golgi becomes fragmented and there is a defect in retrograde transport from endosomes to the Golgi (Fridmann-Sirkis et al. 2004; Yamane et al. 2007). However, knockout mice lacking TMF develop normally and the females are fertile whilst the male mice show defects in sperm formation, but no defects in expression of an androgen receptor regulated gene (Lerer-Goldshtein et al. 2010). TMF is expressed ubiquitously, but at higher levels in the testes, and so may contribute to acrosome formation, as this is defective in the mutant mice.
NONGOLGIN GOLGI COILED-COIL PROTEINS
There are several other proteins on the Golgi which contain coiled-coil domains but whose properties suggest that their roles are likely to be distinct to those of the golgins, of which the most obvious are the SNARE proteins that form four helical bundles to drive membrane fusion. Others include p115 which acts in vesicle tethering and whose carboxy-terminal ∼300 residues are predicted to be coiled-coil (Barroso et al. 1995; Sapperstein et al. 1995; Sohda et al. 2005). However, most of the length of p115 encodes a large amino-terminal domain that is composed of armadillo repeats which are unrelated to the golgins (An et al. 2009; Striegl et al. 2010). The dynein adaptor Bicaudal-1/2 is coiled-coil over most of its length and binds Rab6 at the carboxyl terminus, but is also found in other parts of the cell (Matanis et al. 2002; Short et al. 2002; Kardon and Vale 2009). There are also some Golgi proteins of unknown function that have short regions of coiled such as SCOCO and the Rab2 effector golgin-45 (Short et al. 2001; Van Valkenburgh et al. 2001). In addition, the neuroendocrine coiled-coil proteins NECC1/2 were reported to be predominantly near the Golgi, but this has not been seen in subsequent studies (Cruz-Garcia et al. 2007; Vidal et al. 2009). Two further coiled-coil proteins, SCYLBP1 and ERC1, have been proposed to be golgins, but either lack extensive coiled-coils or are not exclusively on the Golgi. Nonetheless, they are worth more detailed consideration as they are of considerable current interest.
SCYL1BP1
Null mutations in the gene encoding SCYL1BP1 have recently been found to be the cause of gerodermia osteodysplastica, an autosomal recessive disorder resulting in osteoporosis and wrinkly skin (Hennies et al. 2008). The protein was found to be localized to the Golgi and to be an effector of Rab6 (and hence the human gene given the name GORAB). It is conserved in metazoans, with distant relatives in plants but not fungi. It contains a stretch of predicted coiled-coil that is only ∼150 residues long and is the most highly conserved part of the protein. SCYL1BP1 was first described as a hit from a yeast two-hybrid screen with the inactive protein kinase Scyl1 (aka NTKL and TEIF) (Di et al. 2003). Scyl1 has been proposed to have a variety of roles including an association with COPI vesicles (Burman et al. 2010), and its relative Scyl3/CVAK104 is associated with clathrin-coated vesicles (Conner and Schmid 2005; Borner et al. 2007). However the physiological significance of the interaction between Scyl1 and SCYL1BP1 is uncertain as mice lacking Scyl1 show a very different phenotype (neurodegeneration and muscle wasting) to that seen in patients with loss-of-function mutations in SCYL1BP1 (Schmidt et al. 2007). The function of SCYLBP1 itself is also unclear as fibroblasts from patients lacking the protein have no obvious defect in Golgi morphology (Hennies et al. 2008).
ERC1/RAB6IP2
Originally identified in a yeast two hybrid screen with Rab6, ERC1/RAB6IP2 is predicted to be mostly composed of a stretch of over 800 residues of coiled-coil (Monier et al. 2002). It exists in multiple alternatively spliced versions all of which have one of two different carboxyl termini depending on the inclusion of an optional exon (Nakata et al. 2002; Wang et al. 2002; Hida and Ohtsuka 2010). Inclusion of the exon results in the carboxyl terminus IWA, and such forms are found exclusively in the brain, whilst exclusion results in a carboxy-terminal extension that is ubiquitously present outside of the brain. This carboxy-terminal extension resembles the Rab-binding domain from the FIP family of Rab11 binding proteins, but the Rab6-binding site is in a region next to the carboxyl terminus that is shared by both carboxy-terminal variants (Monier et al. 2002). There is also a closely related paralog in vertebrates, ERC2, that is exclusively expressed in the brain and only exists in a form ending IWA (Hida and Ohtsuka 2010).
Although the proteins have a long coiled-coil region typical of a golgin, it is less clear that the Golgi is their principle location in the cell. ERC2 and the neuronal splice-variants of ERC1 are components of the presynaptic active zone, and bind via their carboxy-terminal IWA motifs to a PDZ domain in the presynaptic protein RIM (Wang et al. 2002). The role of the nonneuronal splice-variants is less clear, and although originally reported to be on the Golgi, more recent reports have suggested that they are located to vesicle docking sites on the plasma membrane (Ohara-Imaizumi et al. 2005; Grigoriev et al. 2007). Invertebrates have a single ortholog with the C. elegans protein (Elks-1) ending in IWA and being located to the active zone, but mutation causes no phenotype unless other active zone proteins are also missing (Deken et al. 2005; Dai et al. 2006). The Drosophila ortholog (Bruchpilot) is a widely used presynaptic marker whose deletion causes defects in synaptic transmission, although its carboxy-terminal region is different to that of either the mammalian proteins (Wagh et al. 2006).
REGULATION OF GOLGIN FUNCTION
An indication of the importance of golgins to the organization of the Golgi is that they are targets of modification during mitosis and apoptosis, two processes that involve Golgi fragmentation. During mitosis, golgin-84 and GM130 are phosphorylated, and it has been proposed that this alters their interactions and so induces Golgi fragmentation (Lowe et al. 1998; Diao et al. 2003; Diao et al. 2008). During apoptosis several golgins and associated proteins are cleaved by caspases, including GM130, giantin, golgin-97, and golgin-160 (Mancini et al. 2000; Chiu et al. 2002; Nozawa et al. 2002), which provides a potential mechanism for the associated fragmentation of the Golgi. It has also been found that one golgin, golgin-84, is cleaved during infection of cells by the bacterial pathogen Chlamydia, which replicates inside a membrane-bound vacuole (Heuer et al. 2009; Rejman Lipinski et al. 2009). It is thought that the resulting fragmentation of the Golgi is required for the diversion of lipids and other nutrients into the replicative vacuole.
INTEGRATING THE DIVERSE FUNCTIONS OF THE GOLGINS INTO A MODEL
Although the precise functions of the golgins remain unclear, several clear themes have emerged. At a phenotypic level, their removal can cause defects in membrane traffic either through the Golgi, or in recycling back from endosomes. However these phenotypes, if apparent at all, are usually not a complete block, and sometimes the only obvious phenotype is a fragmentation of the Golgi ribbon. In addition, studies with knockout mice or human genetic diseases have shown that loss of the apparently ubiquitously expressed golgins GMAP-210, TMF, or golgin-160 results in tissue-specific defects rather than cell lethality. At a molecular level, diverse binding partners have been found for particular golgins including kinases, cilia components, and microtubule organizers. However, one striking theme is that many golgins can bind to small GTPases. In some cases, these interactions are simply responsible for recruiting the golgin to membranes such as the binding of Arl1 to the GRIP domains or Rab6 to TMF. However, the first indication that there is potentially more to these interactions came from the finding that golgin-84 and giantin which have transmembrane domains are nonetheless Rab effectors (Diao et al. 2003; Satoh et al. 2003; Rosing et al. 2007). It has since emerged that the golgins that are recruited by G-proteins binding to their carboxyl termini also have Rab-binding sites along their length (Sinka et al. 2008). In some cases, multiple Rabs can bind to the same golgin, and one Rab can bind to multiple golgins including proteins from both cis and trans-ends of the stack.
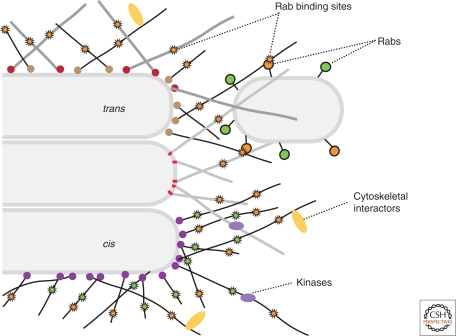
This has lead to the proposal that the individual golgins do not have clearly distinct roles, but rather act collectively to encase the Golgi in a tentacular cytomatrix studded with binding sites for molecules or structures that need to associate with the Golgi (Fig. 2). These would include specific proteins such as regulatory kinases, proteins that interact with the cytoskeleton, and also larger structures such as transport vesicles or even adjacent Golgi stacks. These membrane-bound structures would interact via associated Rab G proteins or by other vesicle-associated proteins such as p115, whilst other membranes or large structures such as ribosomes would be excluded. Electron microscopy studies have indicated that ribosomes are excluded from a zone around the Golgi, whilst the vesicles adjacent to the Golgi stack are emeshed in elongated structures (Orci et al. 1998; Mogelsvang et al. 2003; Kang and Staehelin 2008). Some of these different types of interactions may need to be dispersed over a large part of the stack and so would be shared with multiple golgins. It is noteworthy that in mammalian cells traffic arriving at the Golgi from both the ER and endosomal systems moves along microtubules and so is not directed to a particular part of the stack. Thus, dispersed Rab binding sites would allow capture of incoming carriers at first contact, and this could perhaps be refined by more restricted interactions leading to tethering at the correct cisternae. There have also been indications that individual golgins can bind each other and this may help hold the golgin cytomatrix together, especially if transmembrane domain golgins have to enter retrograde COPI-coated vesicles to remain in the Golgi (Malsam et al. 2005).
Figure 2.
A speculative model for golgin function.
The golgins surround the Golgi in an array of loosely associated tentacles. Golgin-specific interactions anchor the proteins via their carboxyl termini to particular parts of the Golgi, but the different golgins share at least some binding partners. This allows cytosolic proteins such as kinases or cytoskeletal interactors to bind to as much of the Golgi stack as is required. Transport vesicles or adjacent cisternae that display activated Rab G proteins are captured by binding directly to Rab binding motifs shared between subsets of golgins. In addition to the Rabs, other vesicle-associated proteins could also contribute to golgin recognition (Nakamura et al. 1997; Jing et al. 2010; Sohda et al. 2010). If the affinity or Rab specificity of these interactions varied through the stack it might direct vesicle movement from sites with a broad distribution (yellow) to those that are restricted to a particular part of the stack (green).
Thus, each golgin could be responsible for multiple types of interaction, of which at least some would be shared with other golgins. This would account not only for the variety of their interaction partners and of the phenotypes arising from their removal, but also for the relative mildness of such phenotypes. The golgins seem to have become longer during the evolution of higher eukaryotes, which may reflect the acquisition of more interaction partners. This model of golgins acting collectively is in some ways analogous to the nucleoporins, which have been proposed to collectively fill nuclear pores with long unstructured regions that contain FG repeats to bind importins but act to exclude other proteins (Wälde and Kehlenbach 2010). Nucleoporins are not coiled-coil proteins, but it is perhaps notable that similar very large coiled-coil proteins are also found around the centrosome and in the synapse, suggesting that these may also share principles of cytoplasmic organization with the golgins.
CONCLUDING REMARKS
The golgins are clearly important components of the Golgi but their precise functions are still not clear. Further biochemical and structural studies should reveal more binding partners and the mechanisms by which they recognize the golgins. If it is correct that each golgin can bind multiple partners then careful analysis will be required to dissect the precise role of each interaction. Determining the importance of the individual interactions made by a particular protein is likely to require the use of genetic systems and the combination of mutants to hopefully provide tractable phenotypes. There is clearly much work still to be performed, but the conservation of the proteins and their known properties suggest that such studies will reveal much about how the Golgi works.
ACKNOWLEDGMENTS
I would like to thank Francis Barr, Alison Gillingham, Martin Lowe, Franck Perez, Catherine Rabouille, Rita Sinka, and Graham Warren for stimulating discussions about golgin function.
Footnotes
Editors: Graham Warren and James Rothman
Additional Perspectives on The Golgi available at www.cshperspectives.org
REFERENCES
- An Y, Chen CY, Moyer B, Rotkiewicz P, Elsliger M-A, Godzik A, Wilson IA, Balch WE 2009. Structural and functional analysis of the globular head domain of p115 provides insight into membrane tethering. J Mol Biol 391: 26–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banu Y, Matsuda M, Yoshihara M, Kondo M, Sutou S, Matsukuma S 2002. Golgi matrix protein gene, Golga3/Mea2, rearranged and re-expressed in pachytene spermatocytes restores spermatogenesis in the mouse. Mol Reprod Dev 61: 288–301 [DOI] [PubMed] [Google Scholar]
- Barr FA 1999. A novel Rab6-interacting domain defines a family of Golgi-targeted coiled-coil proteins. Curr Biol 9: 381–384 [DOI] [PubMed] [Google Scholar]
- Barr FA, Nakamura N, Warren G 1998. Mapping the interaction between GRASP65 and GM130, components of a protein complex involved in the stacking of Golgi cisternae. EMBO J 17: 3258–3268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr FA, Puype M, Vandekerckhove J, Warren G 1997. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell 91: 253–262 [DOI] [PubMed] [Google Scholar]
- Barroso M, Nelson DS, Sztul E 1995. Transcytosis-associated protein (TAP)/p115 is a general fusion factor required for binding of vesicles to acceptor membranes. Proc Natl Acad Sci 92: 527–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascom RA, Srinivasan S, Nussbaum RL 1999. Identification and characterization of golgin-84, a novel Golgi integral membrane protein with a cytoplasmic coiled-coil domain. J Biol Chem 274: 2953–2962 [DOI] [PubMed] [Google Scholar]
- Behnia R, Barr FA, Flanagan JJ, Barlowe C, Munro S 2007. The yeast orthologue of GRASP65 forms a complex with a coiled-coil protein that contributes to ER to Golgi traffic. J Cell Biol 176: 255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner GHH, Rana AA, Forster R, Harbour M, Smith JC, Robinson MS 2007. CVAK104 is a novel regulator of clathrin-mediated SNARE sorting. Traffic 8: 893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burguete A, Fenn T, Brunger A, Pfeffer S 2008. Rab and Arl GTPase family members cooperate in the localization of the golgin GCC185. Cell 132: 286–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman JL, Hamlin JNR, McPherson PS 2010. Scyl1 regulates Golgi morphology. PLoS ONE 5: e9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Ballew N, Barlowe C 1998. Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J 17: 2156–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu R, Novikov L, Mukherjee S, Shields D 2002. A caspase cleavage fragment of p115 induces fragmentation of the Golgi apparatus and apoptosis. J Cell Biol 159: 637–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner SD, Schmid SL 2005. CVAK104 is a novel poly-L-lysine-stimulated kinase that targets the β2-subunit of AP2. J Biol Chem 280: 21539–21544 [DOI] [PubMed] [Google Scholar]
- Cruz-Garcia D, Vazquez-Martinez R, Peinado JR, Anouar Y, Tonon MC, Vaudry H, Castaño JP, Malagon MM 2007. Identification and characterization of two novel (neuro)endocrine long coiled-coil proteins. FEBS Lett 581: 3149–3156 [DOI] [PubMed] [Google Scholar]
- D’Angelo G, Prencipe L, Iodice L, Beznoussenko G, Savarese M, Marra P, Di Tullio G, Martire G, De Matteis MA, Bonatti S 2009. GRASP65 and GRASP55 sequentially promote the transport of C-terminal valine-bearing cargos to and through the Golgi complex. J Biol Chem 284: 34849–34860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Taru H, Deken SL, Grill B, Ackley B, Nonet ML, Jin Y 2006. SYD-2 Liprin-α organizes presynaptic active zone formation through ELKS. Nat Neurosci 9: 1479–1487 [DOI] [PubMed] [Google Scholar]
- Deken SL, Vincent R, Hadwiger G, Liu Q, Wang Z-W, Nonet ML 2005. Redundant localization mechanisms of RIM and ELKS in Caenorhabditis elegans. J Neurosci 25: 5975–5983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derby MC, Lieu ZZ, Brown D, Stow JL, Goud B, Gleeson PA 2007. The trans-Golgi network golgin, GCC185, is required for endosome-to-Golgi transport and maintenance of Golgi structure. Traffic 8: 758–773 [DOI] [PubMed] [Google Scholar]
- Di Y, Li J, Fang J, Xu Z, He X, Zhang F, Ling J, Li X, Xu D, Li L, et al. 2003. Cloning and characterization of a novel gene which encodes a protein interacting with the mitosis-associated kinase-like protein NTKL. J Hum Genet 48: 315–321 [DOI] [PubMed] [Google Scholar]
- Diao A, Frost L, Morohashi Y, Lowe M 2008. Coordination of golgin tethering and SNARE assembly: GM130 binds syntaxin 5 in a p115-regulated manner. J Biol Chem 283: 6957–6967 [DOI] [PubMed] [Google Scholar]
- Diao A, Rahman D, Pappin DJC, Lucocq J, Lowe M 2003. The coiled-coil membrane protein golgin-84 is a novel rab effector required for Golgi ribbon formation. J Cell Biol 160: 201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drin G, Morello V, Casella J-F, Gounon P, Antonny B 2008. Asymmetric tethering of flat and curved lipid membranes by a golgin. Science 320: 670–673 [DOI] [PubMed] [Google Scholar]
- Erlich R, Gleeson PA, Campbell P, Dietzsch E, Toh BH 1996. Molecular characterization of trans-Golgi p230. A human peripheral membrane protein encoded by a gene on chromosome 6p12-22 contains extensive coiled-coil α-helical domains and a granin motif. J Biol Chem 271: 8328–8337 [DOI] [PubMed] [Google Scholar]
- Eystathioy T, Jakymiw A, Fujita DJ, Fritzler MJ, Chan EK 2000. Human autoantibodies to a novel Golgi protein golgin-67: High similarity with golgin-95/gm 130 autoantigen. J Autoimmun 14: 179–187 [DOI] [PubMed] [Google Scholar]
- Follit JA, San Agustin JT, Xu F, Jonassen JA, Samtani R, Lo CW, Pazour GJ 2008. The Golgin GMAP210/TRIP11 anchors IFT20 to the Golgi complex. PLoS Genet 4: e1000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridmann-Sirkis Y, Siniossoglou S, Pelham HRB 2004. TMF is a golgin that binds Rab6 and influences Golgi morphology. BMC Cell Biol 5: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friggi-Grelin F, Rabouille C, Therond P 2006. The cis-Golgi Drosophila GMAP has a role in anterograde transport and Golgi organization in vivo, similar to its mammalian ortholog in tissue culture cells. Eur J Cell Biol 85: 1155–1166 [DOI] [PubMed] [Google Scholar]
- Fritzler MJ, Hamel JC, Ochs RL, Chan EK 1993. Molecular characterization of two human autoantigens: Unique cDNAs encoding 95- and 160-kD proteins of a putative family in the Golgi complex. J Exp Med 178: 49–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JA, Ou SH, Wu F, Lusis AJ, Sparkes RS, Gaynor RB 1992. Cloning and chromosomal mapping of a human immunodeficiency virus 1 “TATA” element modulatory factor. Proc Natl Acad Sci 89: 9372–9376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham AK, Munro S 2003. Long coiled-coil proteins and membrane traffic. Biochim Biophys Acta 1641: 71–85 [DOI] [PubMed] [Google Scholar]
- Gillingham AK, Pfeifer AC, Munro S 2002. CASP, the alternatively spliced product of the gene encoding the CCAAT-displacement protein transcription factor, is a Golgi membrane protein related to giantin. Mol Biol Cell 13: 3761–3774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham AK, Tong AHY, Boone C, Munro S 2004. The GTPase Arf1p and the ER to Golgi cargo receptor Erv14p cooperate to recruit the golgin Rud3p to the cis-Golgi. J Cell Biol 167: 281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith KJ, Chan EK, Lung CC, Hamel JC, Guo X, Miyachi K, Fritzler MJ 1997. Molecular cloning of a novel 97-kd Golgi complex autoantigen associated with Sjögren’s syndrome. Arthritis Rheum 40: 1693–1702 [DOI] [PubMed] [Google Scholar]
- Grigoriev I, Splinter D, Keijzer N, Wulf PS, Demmers J, Ohtsuka T, Modesti M, Maly IV, Grosveld F, Hoogenraad CC, et al. 2007. Rab6 regulates transport and targeting of exocytotic carriers. Dev Cell 13: 305–314 [DOI] [PubMed] [Google Scholar]
- Hayes GL, Brown FC, Haas AK, Nottingham RM, Barr FA, Pfeffer SR 2009. Multiple Rab GTPase binding sites in GCC185 suggest a model for vesicle tethering at the trans-Golgi. Mol Biol Cell 20: 209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennies H, Kornak U, Zhang H, Egerer J, Zhang X, Seifert W, Kühnisch J, Budde B, Nätebus M, Brancati F, et al. 2008. Gerodermia osteodysplastica is caused by mutations in SCYL1BP1, a Rab-6 interacting golgin. Nat Genet 40: 1410–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer D, Rejman Lipinski A, Machuy N, Karlas A, Wehrens A, Siedler F, Brinkmann V, Meyer TF 2009. Chlamydia causes fragmentation of the Golgi compartment to ensure reproduction. Nature 457: 731–735 [DOI] [PubMed] [Google Scholar]
- Hicks SW, Machamer CE 2005. Isoform-specific interaction of golgin-160 with the Golgi-associated protein PIST. J Biol Chem 280: 28944–28951 [DOI] [PubMed] [Google Scholar]
- Hida Y, Ohtsuka T 2010. CAST and ELKS proteins: Structural and functional determinants of the presynaptic active zone. J Biochem 148: 131–137 [DOI] [PubMed] [Google Scholar]
- Houghton FJ, Chew PL, Lodeho S, Goud B, Gleeson PA 2009. The localization of the Golgin GCC185 is independent of Rab6A/A’ and Arl1. Cell 138: 787–794 [DOI] [PubMed] [Google Scholar]
- Hsiao PW, Chang C 1999. Isolation and characterization of ARA160 as the first androgen receptor N-terminal-associated coactivator in human prostate cells. J Biol Chem 274: 22373–22379 [DOI] [PubMed] [Google Scholar]
- Infante C, Ramos-Morales F, Fedriani C, Bornens M, Rios RM 1999. GMAP-210, A cis-Golgi network-associated protein, is a minus end microtubule-binding protein. J Cell Biol 145: 83–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakymiw A, Raharjo E, Rattner JB, Eystathioy T, Chan EK, Fujita DJ 2000. Identification and characterization of a novel Golgi protein, golgin-67. J Biol Chem 275: 4137–4144 [DOI] [PubMed] [Google Scholar]
- Jiang Y-H, Wauki K, Liu Q, Bressler J, Pan Y, Kashork CD, Shaffer LG, Beaudet AL 2008. Genomic analysis of the chromosome 15q11-q13 Prader-Willi syndrome region and characterization of transcripts for GOLGA8E and WHCD1L1 from the proximal breakpoint region. BMC Genomics 9: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Junutula JR, Wu C, Burden J, Matern H, Peden AA, Prekeris R 2010. FIP1/RCP binding to Golgin-97 regulates retrograde transport from recycling endosomes to the trans-Golgi network. Mol Biol Cell 21: 3041–3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B, Staehelin L 2008. ER-to-Golgi transport by COPII vesicles in Arabidopsis involves a ribosome-excluding scaffold that is transferred with the vesicles to the Golgi matrix. Protoplasma 234: 51–64 [DOI] [PubMed] [Google Scholar]
- Kardon JR, Vale RD 2009. Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol 10: 854–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DW, Sacher M, Scarpa A, Quinn AM, Ferro-Novick S 1999. High-copy suppressor analysis reveals a physical interaction between Sec34p and Sec35p, a protein implicated in vesicle docking. Mol Biol Cell 10: 3317–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjer-Nielsen L, Teasdale RD, van Vliet C, Gleeson PA 1999. A novel Golgi-localisation domain shared by a class of coiled-coil peripheral membrane proteins. Curr Biol 9: 385–388 [DOI] [PubMed] [Google Scholar]
- Latijnhouwers M, Gillespie T, Boevink P, Kriechbaumer V, Hawes C, Carvalho CM 2007. Localization and domain characterization of Arabidopsis golgin candidates. J Exp Bot 58: 4373–4386 [DOI] [PubMed] [Google Scholar]
- Latijnhouwers M, Hawes C, Carvalho C, Oparka K, Gillingham AK, Boevink P 2005. An Arabidopsis GRIP domain protein locates to the trans-Golgi and binds the small GTPase ARL1. Plant J 44: 459–470 [DOI] [PubMed] [Google Scholar]
- Lee JW, Choi HS, Gyuris J, Brent R, Moore DD 1995. Two classes of proteins dependent on either the presence or absence of thyroid hormone for interaction with the thyroid hormone receptor. Mol Endocrinol 9: 243–254 [DOI] [PubMed] [Google Scholar]
- Lerer-Goldshtein T, Bel S, Shpungin S, Pery E, Motro B, Goldstein RS, Bar-Sheshet SI, Breitbart H, Nir U 2010. TMF/ARA160: A key regulator of sperm development. Dev Biol 348: 12–21 [DOI] [PubMed] [Google Scholar]
- Lesa GM, Seemann J, Shorter J, Vandekerckhove J, Warren G 2000. The amino-terminal domain of the Golgi protein giantin interacts directly with the vesicle-tethering protein p115. J Biol Chem 275: 2831–2836 [DOI] [PubMed] [Google Scholar]
- Lieu ZZ, Derby MC, Teasdale RD, Hart C, Gunn P, Gleeson PA 2007. The golgin GCC88 is required for efficient retrograde transport of cargo from the early endosomes to the trans-Golgi network. Mol Biol Cell 18: 4979–4991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu ZZ, Lock JG, Hammond LA, La Gruta NL, Stow JL, Gleeson PA 2008. A trans-Golgi network golgin is required for the regulated secretion of TNF in activated macrophages in vivo. Proc Natl Acad Sci 105: 3351–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linstedt AD, Hauri HP 1993. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol Biol Cell 4: 679–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linstedt AD, Jesch SA, Mehta A, Lee TH, Garcia-Mata R, Nelson DS, Sztul E 2000. Binding relationships of membrane tethering components. The giantin N terminus and the GM130 N terminus compete for binding to the p115 C terminus. J Biol Chem 275: 10196–10201 [DOI] [PubMed] [Google Scholar]
- Lowe M, Rabouille C, Nakamura N, Watson R, Jackman M, Jämsä E, Rahman D, Pappin DJ, Warren G 1998. Cdc2 kinase directly phosphorylates the cis-Golgi matrix protein GM130 and is required for Golgi fragmentation in mitosis. Cell 94: 783–793 [DOI] [PubMed] [Google Scholar]
- Lu L, Hong W 2003. Interaction of Arl1-GTP with GRIP domains recruits autoantigens Golgin-97 and Golgin-245/p230 onto the Golgi. Mol Biol Cell 14: 3767–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Tai G, Hong W 2004. Autoantigen Golgin-97, an effector of Arl1 GTPase, participates in traffic from the endosome to the trans-golgi network. Mol Biol Cell 15: 4426–4443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke MR, Kjer-Nielsen L, Brown DL, Stow JL, Gleeson PA 2003. GRIP domain-mediated targeting of two new coiled-coil proteins, GCC88 and GCC185, to subcompartments of the trans-Golgi network. J Biol Chem 278: 4216–4226 [DOI] [PubMed] [Google Scholar]
- Lupashin V, Sztul E 2005. Golgi tethering factors. Biochim Biophys Acta 1744: 325–339 [DOI] [PubMed] [Google Scholar]
- Malsam J, Satoh A, Pelletier L, Warren G 2005. Golgin tethers define subpopulations of COPI vesicles. Science 307: 1095–1098 [DOI] [PubMed] [Google Scholar]
- Mancini M, Machamer CE, Roy S, Nicholson DW, Thornberry NA, Casciola-Rosen LA, Rosen A 2000. Caspase-2 is localized at the Golgi complex and cleaves golgin-160 during apoptosis. J Cell Biol 149: 603–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Menárguez JA, Prekeris R, Oorschot VM, Scheller R, Slot JW, Geuze HJ, Klumperman J 2001. Peri-Golgi vesicles contain retrograde but not anterograde proteins consistent with the cisternal progression model of intra-Golgi transport. J Cell Biol 155: 1213–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matanis T, Akhmanova A, Wulf P, Del Nery E, Weide T, Stepanova T, Galjart N, Grosveld F, Goud B, De Zeeuw CI, et al. 2002. Bicaudal-D regulates COPI-independent Golgi-ER transport by recruiting the dynein-dynactin motor complex. Nat Cell Biol 4: 986–992 [DOI] [PubMed] [Google Scholar]
- Matsukuma S, Kondo M, Yoshihara M, Matsuda M, Utakoji T, Sutou S 1999. Mea2/Golga3 gene is disrupted in a line of transgenic mice with a reciprocal translocation between Chromosomes 5 and 19 and is responsible for a defective spermatogenesis in homozygotes. Mamm Genome 10: 1–5 [DOI] [PubMed] [Google Scholar]
- McConville MJ, Ilgoutz SC, Teasdale RD, Foth BJ, Matthews A, Mullin KA, Gleeson PA 2002. Targeting of the GRIP domain to the trans-Golgi network is conserved from protists to animals. Eur J Cell Biol 81: 485–495 [DOI] [PubMed] [Google Scholar]
- Mogelsvang S, Gomez-Ospina N, Soderholm J, Glick BS, Staehelin L 2003. Tomographic evidence for continuous turnover of Golgi cisternae in Pichia pastoris. Mol Biol Cell 14: 2277–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier S, Jollivet F, Janoueix-Lerosey I, Johannes L, Goud B 2002. Characterization of novel Rab6-interacting proteins involved in endosome-to-TGN transport. Traffic 3: 289–297 [DOI] [PubMed] [Google Scholar]
- Mori K, Kato H 2002. A putative nuclear receptor coactivator (TMF/ARA160) associates with hbrm/hSNF2α and BRG-1/hSNF2β and localizes in the Golgi apparatus. FEBS Lett 520: 127–132 [DOI] [PubMed] [Google Scholar]
- Moyer BD, Allan BB, Balch WE 2001. Rab1 interaction with a GM130 effector complex regulates COPII vesicle cis-Golgi tethering. Traffic 2: 268–276 [DOI] [PubMed] [Google Scholar]
- Munro S, Nichols BJ 1999. The GRIP domain—a novel Golgi-targeting domain found in several coiled-coil proteins. Curr Biol 9: 377–380 [DOI] [PubMed] [Google Scholar]
- Nakamura N 2010. Emerging new roles of GM130, a cis-Golgi matrix protein, in higher order cell functions. J Pharmacol Sci 112: 255–264 [DOI] [PubMed] [Google Scholar]
- Nakamura N, Lowe M, Levine TP, Rabouille C, Warren G 1997. The vesicle docking protein p115 binds GM130, a cis-Golgi matrix protein, in a mitotically regulated manner. Cell 89: 445–455 [DOI] [PubMed] [Google Scholar]
- Nakamura N, Rabouille C, Watson R, Nilsson T, Hui N, Slusarewicz P, Kreis TE, Warren G 1995. Characterization of a cis-Golgi matrix protein, GM130. J Cell Biol 131: 1715–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata T, Yokota T, Emi M, Minami S 2002. Differential expression of multiple isoforms of the ELKS mRNAs involved in a papillary thyroid carcinoma. Genes Chromosomes Cancer 35: 30–37 [DOI] [PubMed] [Google Scholar]
- Nozawa K, Casiano CA, Hamel JC, Molinaro C, Fritzler MJ, Chan EKL 2002. Fragmentation of Golgi complex and Golgi autoantigens during apoptosis and necrosis. Arthritis Res 4: R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oas TG, Endow SA 1994. Springs and hinges: Dynamic coiled coils and discontinuities. Trends Biochem Sci 19: 51–54 [DOI] [PubMed] [Google Scholar]
- Ohara-Imaizumi M, Ohtsuka T, Matsushima S, Akimoto Y, Nishiwaki C, Nakamichi Y, Kikuta T, Nagai S, Kawakami H, Watanabe T, et al. 2005. ELKS, a protein structurally related to the active zone-associated protein CAST, is expressed in pancreatic β cells and functions in insulin exocytosis: Interaction of ELKS with exocytotic machinery analyzed by total internal reflection fluorescence microscopy. Mol Biol Cell 16: 3289–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta E, Misumi Y, Sohda M, Fujiwara T, Yano A, Ikehara Y 2003. Identification and characterization of GCP16, a novel acylated Golgi protein that interacts with GCP170. J Biol Chem 278: 51957–51967 [DOI] [PubMed] [Google Scholar]
- Oka T, Ungar D, Hughson FM, Krieger M 2004. The COG and COPI complexes interact to control the abundance of GEARs, a subset of Golgi integral membrane proteins. Mol Biol Cell 15: 2423–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Perrelet A, Rothman JE 1998. Vesicles on strings: Morphological evidence for processive transport within the Golgi stack. Proc Natl Acad Sci 95: 2279–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panic B, Whyte JRC, Munro S 2003b. The ARF-like GTPases Arl1p and Arl3p act in a pathway that interacts with vesicle-tethering factors at the Golgi apparatus. Curr Biol 13: 405–410 [DOI] [PubMed] [Google Scholar]
- Panic B, Perisic O, Veprintsev DB, Williams RL, Munro S 2003a. Structural basis for Arl1-dependent targeting of homodimeric GRIP domains to the Golgi apparatus. Mol Cell 12: 863–874 [DOI] [PubMed] [Google Scholar]
- Puthenveedu MA, Linstedt AD 2001. Evidence that Golgi structure depends on a p115 activity that is independent of the vesicle tether components giantin and GM130. J Cell Biol 155: 227–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez IB-R, Lowe M 2009. Golgins and GRASPs: Holding the Golgi together. Semin Cell Dev Biol 20: 770–779 [DOI] [PubMed] [Google Scholar]
- Reddy JV, Burguete AS, Sridevi K, Ganley IG, Nottingham RM, Pfeffer SR 2006. A functional role for the GCC185 golgin in mannose 6-phosphate receptor recycling. Mol Biol Cell 17: 4353–4363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejman Lipinski A, Heymann J, Meissner C, Karlas A, Brinkmann V, Meyer TF, Heuer D 2009. Rab6 and Rab11 regulate Chlamydia trachomatis development and golgin-84-dependent Golgi fragmentation. PLoS Pathog 5: e1000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renna L, Hanton SL, Stefano G, Bortolotti L, Misra V, Brandizzi F 2005. Identification and characterization of AtCASP, a plant transmembrane Golgi matrix protein. Plant Mol Biol 58: 109–122 [DOI] [PubMed] [Google Scholar]
- Rios RM, Tassin AM, Celati C, Antony C, Boissier MC, Homberg JC, Bornens M 1994. A peripheral protein associated with the cis-Golgi network redistributes in the intermediate compartment upon brefeldin A treatment. J Cell Biol 125: 997–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosing M, Ossendorf E, Rak A, Barnekow A 2007. Giantin interacts with both the small GTPase Rab6 and Rab1. Exp Cell Res 313: 2318–2325 [DOI] [PubMed] [Google Scholar]
- Sapperstein SK, Walter DM, Grosvenor AR, Heuser JE, Waters MG 1995. p115 is a general vesicular transport factor related to the yeast endoplasmic reticulum to Golgi transport factor Uso1p. Proc Natl Acad Sci 92: 522–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A, Warren G 2008. In situ cleavage of the acidic domain from the p115 tether inhibits exocytic transport. Traffic 9: 1522–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A, Wang Y, Malsam J, Beard MB, Warren G 2003. Golgin-84 is a rab1 binding partner involved in Golgi structure. Traffic 4: 153–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbodio JI, Hicks SW, Simon D, Machamer CE 2006. GCP60 preferentially interacts with a caspase-generated golgin-160 fragment. J Biol Chem 281: 27924–27931 [DOI] [PubMed] [Google Scholar]
- Schmidt WM, Kraus C, Höger H, Hochmeister S, Oberndorfer F, Branka M, Bingemann S, Lassmann H, Müller M, Macedo-Souza LI, et al. 2007. Mutation in the Scyl1 gene encoding amino-terminal kinase-like protein causes a recessive form of spinocerebellar neurodegeneration. EMBO Rep 8: 691–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig HP, Schranz P, Schröter H, Wiemann C, Renz M 1994. Macrogolgin-a new 376 kD Golgi complex outer membrane protein as target of antibodies in patients with rheumatic diseases and HIV infections. J Autoimmun 7: 67–91 [DOI] [PubMed] [Google Scholar]
- Setty SRG, Shin ME, Yoshino A, Marks MS, Burd CG 2003. Golgi recruitment of GRIP domain proteins by Arf-like GTPase 1 is regulated by Arf-like GTPase 3. Curr Biol 13: 401–404 [DOI] [PubMed] [Google Scholar]
- Short B, Haas A, Barr FA 2005. Golgins and GTPases, giving identity and structure to the Golgi apparatus. Biochim Biophys Acta 1744: 383–395 [DOI] [PubMed] [Google Scholar]
- Short B, Preisinger C, Körner R, Kopajtich R, Byron O, Barr FA 2001. A GRASP55-rab2 effector complex linking Golgi structure to membrane traffic. J Cell Biol 155: 877–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short B, Preisinger C, Schaletzky J, Kopajtich R, Barr FA 2002. The Rab6 GTPase regulates recruitment of the dynactin complex to Golgi membranes. Curr Biol 12: 1792–1795 [DOI] [PubMed] [Google Scholar]
- Siniossoglou S, Pelham HR 2001. An effector of Ypt6p binds the SNARE Tlg1p and mediates selective fusion of vesicles with late Golgi membranes. EMBO J 20: 5991–5998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinka R, Gillingham A, Kondylis V, Munro S 2008. Golgi coiled-coil proteins contain multiple binding sites for Rab family G proteins. J Cell Biol 183: 607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson JC, Field C, Ventura R, Royou A, Sullivan W 2000. Lava lamp, a novel peripheral golgi protein, is required for Drosophila melanogaster cellularization. J Cell Biol 151: 905–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits P, Bolton AD, Funari V, Hong M, Boyden ED, Lu L, Manning DK, Dwyer ND, Moran JL, Prysak M, et al. 2010. Lethal skeletal dysplasia in mice and humans lacking the golgin GMAP-210. N Engl J Med 362: 206–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohda M, Misumi Y, Yamamoto A, Nakamura N, Ogata S, Sakisaka S, Hirose S, Ikehara Y, Oda K 2010. Interaction of Golgin-84 with the COG complex mediates the intra-Golgi retrograde transport. Traffic 11: 1552–1566 [DOI] [PubMed] [Google Scholar]
- Sohda M, Misumi Y, Yamamoto A, Yano A, Nakamura N, Ikehara Y 2001. Identification and characterization of a novel Golgi protein, GCP60, that interacts with the integral membrane protein giantin. J Biol Chem 276: 45298–45306 [DOI] [PubMed] [Google Scholar]
- Sohda M, Misumi Y, Yoshimura S-i, Nakamura N, Fusano T, Sakisaka S, Ogata S, Fujimoto J, Kiyokawa N, Ikehara Y 2005. Depletion of vesicle-tethering factor p115 causes mini-stacked Golgi fragments with delayed protein transport. Biochem Biophys Res Commun 338: 1268–1274 [DOI] [PubMed] [Google Scholar]
- Sönnichsen B, Lowe M, Levine T, Jämsä E, Dirac-Svejstrup B, Warren G 1998. A role for giantin in docking COPI vesicles to Golgi membranes. J Cell Biol 140: 1013–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano G, Renna L, Hanton SL, Chatre L, Haas TA, Brandizzi F 2006. ARL1 plays a role in the binding of the GRIP domain of a peripheral matrix protein to the Golgi apparatus in plant cells. Plant Mol Biol 61: 431–449 [DOI] [PubMed] [Google Scholar]
- Striegl H, Andrade-Navarro MA, Heinemann U 2010. Armadillo motifs involved in vesicular transport. PLoS ONE 5: e8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Kozak CA, Veerhuis R, Lau YF, Wiberg U 1992. Isolation of a phylogenetically conserved and testis-specific gene using a monoclonal antibody against the serological H-Y antigen. J Reprod Immunol 21: 275–291 [DOI] [PubMed] [Google Scholar]
- Tsukada M, Will E, Gallwitz D 1999. Structural and functional analysis of a novel coiled-coil protein involved in Ypt6 GTPase-regulated protein transport in yeast. Mol Biol Cell 10: 63–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsdottir R, Hashimoto H, Ashman K, Koda T, Storrie B, Nilsson T 2001. Identification of rabaptin-5, rabex-5, and GM130 as putative effectors of rab33b, a regulator of retrograde traffic between the Golgi apparatus and ER. FEBS Lett 508: 201–209 [DOI] [PubMed] [Google Scholar]
- Van Valkenburgh H, Shern JF, Sharer JD, Zhu X, Kahn RA 2001. ADP-ribosylation factors (ARFs) and ARF-like 1 (ARL1) have both specific and shared effectors: characterizing ARL1-binding proteins. J Biol Chem 276: 22826–22837 [DOI] [PubMed] [Google Scholar]
- VanRheenen SM, Cao X, Sapperstein SK, Chiang EC, Lupashin VV, Barlowe C, Waters MG 1999. Sec34p, a protein required for vesicle tethering to the yeast Golgi apparatus, is in a complex with Sec35p. J Cell Biol 147: 729–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasile E, Perez T, Nakamura N, Krieger M 2003. Structural integrity of the Golgi is temperature sensitive in conditional-lethal mutants with no detectable GM130. Traffic 4: 254–272 [DOI] [PubMed] [Google Scholar]
- Vidal RL, Valenzuela JI, Luján R, Couve A 2009. Cellular and subcellular localization of Marlin-1 in the brain. BMC Neurosci 10: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Dürrbeck H, Buchner S, Dabauvalle M-C, Schmidt M, Qin G, et al. 2006. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron 49: 833–844 [DOI] [PubMed] [Google Scholar]
- Wälde S, Kehlenbach RH 2010. The Part and the Whole: Functions of nucleoporins in nucleocytoplasmic transport. Trends Cell Biol 20: 461–469 [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu X, Biederer T, Südhof TC 2002. A family of RIM-binding proteins regulated by alternative splicing: Implications for the genesis of synaptic active zones. Proc Natl Acad Sci 99: 14464–14469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weide T, Bayer M, Köster M, Siebrasse JP, Peters R, Barnekow A 2001. The Golgi matrix protein GM130: a specific interacting partner of the small GTPase rab1b. EMBO Rep 2: 336–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Lu L, Hong W, Song H 2004. Structural basis for recruitment of GRIP domain golgin-245 by small GTPase Arl1. Nat Struct Mol Biol 11: 86–94 [DOI] [PubMed] [Google Scholar]
- Xiang Y, Wang Y 2010. GRASP55 and GRASP65 play complementary and essential roles in Golgi cisternal stacking. J Cell Biol 188: 237–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa H, Seog DH, Yoda K, Yamasaki M, Wakabayashi T 1996. Uso1 protein is a dimer with two globular heads and a long coiled-coil tail. J Struct Biol 116: 356–365 [DOI] [PubMed] [Google Scholar]
- Yamane J, Kubo A, Nakayama K, Yuba-Kubo A, Katsuno T, Tsukita S, Tsukita S 2007. Functional involvement of TMF/ARA160 in Rab6-dependent retrograde membrane traffic. Exp Cell Res 313: 3472–3485 [DOI] [PubMed] [Google Scholar]
- Yao R, Ito C, Natsume Y, Sugitani Y, Yamanaka H, Kuretake S, Yanagida K, Sato A, Toshimori K, Noda T 2002. Lack of acrosome formation in mice lacking a Golgi protein, GOPC. Proc Natl Acad Sci 99: 11211–11216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino A, Setty SRG, Poynton C, Whiteman EL, Saint-Pol A, Burd CG, Johannes L, Holzbaur EL, Koval M, McCaffery JM, et al. 2005. tGolgin-1 (p230, golgin-245) modulates Shiga-toxin transport to the Golgi and Golgi motility towards the microtubule-organizing centre. J Cell Sci 118: 2279–2293 [DOI] [PubMed] [Google Scholar]
- Zody MC, Garber M, Sharpe T, Young SK, Rowen L, O’Neill K, Whittaker CA, Kamal M, Chang JL, Cuomo CA, et al. 2006. Analysis of the DNA sequence and duplication history of human chromosome 15. Nature 440: 671–675 [DOI] [PubMed] [Google Scholar]