Abstract
Abstract
Skeletal muscle generates superoxide and nitric oxide at rest and this generation is increased by contractile activity. In young and adult animals and man, an increase in activities of these species and the secondary products derived from them (reactive oxygen species, ROS) stimulate redox-sensitive signalling pathways to modify the cellular content of cytoprotective regulatory proteins such as the superoxide dismutases, catalase and heat shock proteins that prevent oxidative damage to tissues. The mechanisms underlying these adaptive responses to contraction include activation of redox-sensitive transcription factors such as nuclear factor κB (NFκB), activator protein-1 (AP1) and heat shock factor 1 (HSF1). During ageing all tissues, including skeletal muscle, demonstrate an accumulation of oxidative damage that may contribute to loss of tissue homeostasis. The causes of this increased oxidative damage are uncertain, but substantial data now indicate that the ability of skeletal muscle from aged organisms to respond to an increase in ROS generation by increased expression of cytoprotective proteins through activation of redox-sensitive transcription factors is severely attenuated. This age-related lack of physiological adaptations to the ROS induced by contractile activity appears to contribute to a loss of ROS homeostasis and increased oxidative damage in skeletal muscle.
Malcolm Jackson is Head of the new Institute of Ageing and Chronic Disease at the University of Liverpool. He trained in Biochemistry and undertook a PhD at University College, London with the late Professor Richard H. T. Edwards. He sits on the MRC Population and Systems Medicine Board and the BBSRC Healthy Organism Panel. His major research area is the role of free radicals and reactive oxygen species in skeletal muscle. Anne McArdle's PhD was from the University of Liverpool and post-doc studies included training with Dr John Faulkner in the Muscle Mechanics Laboratory at the University of Michigan. She holds a personal Chair at Liverpool and her research interests are in muscle ageing with emphasis on stress responses (particularly heat shock proteins) in muscle and the cross-talk between muscle and inflammatory cells. She is a core member of BBSRC Grant Panel A.
 |
The generation and potential roles of free radicals, reactive oxygen species (ROS) and reactive nitrogen species in skeletal muscle has been studied since the late 1970s (Dillard et al. 1978; Davies et al. 1982), but despite extensive investigation there remains substantial controversy about the nature and sources of the specific species that are generated, the factors influencing their generation and their potential functions and effects in muscle and other cells. In the majority of studies to date there has been an assumption that these species are deleterious to cells and tissues, and even the earliest studies report attempts to scavenge the ROS generated and look for potential functional benefits of this intervention (Dillard et al. 1978). In part, this emphasis on the potential damaging effects of free radicals and ROS has derived from the techniques generally available to detect these species which have predominantly relied on monitoring markers of the oxidative modifications to lipids, DNA and proteins that are collectively described as oxidative damage (Halliwell & Gutteridge, 1989).
There is now increasing recognition that ROS also mediate many physiological processes. ROS are important signalling molecules with regulatory functions that modulate changes in cell and tissue homeostasis and gene expression (Droge, 2002; Jackson et al. 2002). Signalling by these reactive molecules is mainly achieved by targeted modifications of specific residues in proteins (Jansssen-Heinninger et al. 2008). The main physiological mechanism by which cells regulate ROS activities (and hence protect against oxidative damage) is modification of the expression and activities of the regulatory enzymes such as superoxide dismutases MnSOD and CuZnSOD, catalase, glutathione peroxidases (GPx), haem oxygenase-1 (HO-1) and by increases in other cytoprotective proteins, such as heat shock proteins (HSPs). Changes in the expression of these proteins are mediated by activation of signalling pathways by ROS (Powers & Jackson, 2008).
Nature of the reactive oxygen and nitrogen species that are generated by skeletal muscle
Skeletal muscle fibres respond to contractile activity by an increase in the intracellular generation of superoxide and nitric oxide (NO) with the formation of secondary ROS and reactive nitrogen species (Pye et al. 2007; Palomero et al. 2008; Powers & Jackson, 2008). In addition to the intracellular generation of ROS and NO by skeletal muscle, superoxide (Reid et al. 1992; McArdle et al. 2001), hydrogen peroxide (Vasilaki et al. 2006b) and NO (Balon & Nadler, 1994; Kobzik et al. 1994) are released into the interstitial space from muscle fibres (or generated on the extracellular side of the muscle plasma membrane). These species are increased in the interstitial space by contractile activity, but there are few data on the function(s) of these extracellular ROS derived from skeletal muscle fibres. The sources of the free radicals and ROS generated by muscle during contractions have been studied since the 1980s (Davies et al. 1982). Most authors have assumed that the ROS generated by contractions are predominantly generated by mitochondria, but recent data argue against this possibility (for discussion see Powers & Jackson, 2008). Non-mitochondrial sources for the generation of ROS within skeletal muscle have not been extensively studied, but NAD(P)H oxidase(s) have been described in skeletal muscle and localised to the plasma membrane (Javesghani et al. 2002), sarcoplasmic reticulum (Xia et al. 2003) and T-tubules (Espinosa et al. 2006). The activity of this enzyme has also been reported to be activated by contractions (Espinosa et al. 2006). These and other potential sources for ROS generation in skeletal muscle have recently been reviewed by Powers & Jackson (2008).
In order to evaluate the relative magnitude of the increase in ROS activity that occurs in skeletal muscle fibres in response to contractions, Palomero et al. (2008) applied a protocol of electrically stimulated, isometric contractions to single isolated fibres from the mouse flexor digitorum brevis (FDB) muscle. This contraction protocol has been extensively utilised by our research group and has been shown (i) to induce release of superoxide and nitric oxide from muscle cells in culture and muscles of mice in vivo (McArdle et al. 2001), (ii) to lead to a fall in muscle glutathione and protein thiol content (Vasilaki et al. 2006a) and (iii) to stimulate redox-regulated adaptive responses (Vasilaki et al. 2006b) when applied to intact muscles in vivo. The increase in intracellular 2′,7′-dichlorofluorescin (DCF) fluorescence induced by the contraction protocol was less than that following exposure of the fibres to 1 μm hydrogen peroxide. Palomero et al. (2008) calculated that the likely change in intracellular hydrogen peroxide following addition of 1 μm to the extracellular medium was ∼0.1 μm. Thus, it can be inferred that the absolute increase in cytosolic ROS activity in muscle fibres that was achieved following contractile activity was potentially equivalent to ∼0.1 μm hydrogen peroxide. Transient changes of this magnitude in hydrogen peroxide have generally been associated with a signalling role for the oxidant rather than with oxidative damage to tissues.
Adaptive responses of skeletal muscle to increased ROS activities
We and others have obtained evidence that changes in ROS activities modulate a number of physiological responses in skeletal muscle. A single period of contractile activity in mouse muscle was found to increase the activity of muscle antioxidant defense enzymes such as superoxide dismutase (SOD) and catalase together with HSP60 and HSP70 content (McArdle et al. 2001), changes that were replicated in human muscle studies (Khassaf et al. 2001). We also characterized the changes in gene expression that occur following an acute period of contractile activity in comparison with those induced by exposure of skeletal muscle cells to hydrogen peroxide and this identified a number of changes in gene expression that may be regulated directly by the hydrogen peroxide produced during contractile activity in vivo (McArdle et al. 2004b). In addition, other studies have implicated redox signalling in diverse processes in muscle such as maintenance of force production during contractions, glucose uptake and insulin signalling (Jackson, 2009).
ROS have become increasingly recognised as mediators of some adaptive responses of skeletal muscle to contractile activity through the activation of redox-sensitive transcription factors (TFs) (Jackson et al. 2002; Ji et al. 2004; Ristow et al. 2009). NFκB is one such factor, along with activator protein-1 (AP-1) and heat shock factor 1 (Cotto & Morimoto, 1999). NFκB is a redox-regulated factor and ROS have been proposed to be principal regulators of NFκB activation in many situations (Moran et al. 2001). NFκB family members expressed in skeletal muscle play critical roles in modulating the specificity of NFκB and NFκB modulates expression of a number of genes associated with myogenesis (Bakkar et al. 2008), catabolism-related genes (Van Gammeren et al. 2009) and cyto-protective proteins during adaptation to contractile activity (Vasilaki et al. 2006a). Moreover, skeletal muscle has been identified as an endocrine organ producing cytokines via NFκB activation following a number of stresses including systemic inflammation or physical strain (Lee et al. 2007).
Reactive oxygen species play a role in the fundamental processes of ageing
An increased activity of reactive oxygen species (ROS) has been implicated in the processes underlying ageing and in all species, tissues (including skeletal muscle) of aged organisms contain increased amounts of oxidative damage to lipids, DNA and proteins (Drew et al. 2003; Vasilaki et al. 2006b). The hypothesis that this increased oxidative damage plays a key role in age-related tissue dysfunction has been examined in a number of transgenic studies that have examined whether life span is altered, which is thought to be the gold standard for assessing an effect of manipulations on ageing (Salmon et al. 2010). In non-mammalian models, some interventions designed to reduce the activities of ROS, such as overexpression of CuZn, superoxide dismutase (CuZnSOD), catalase or both in Drosophila (Orr et al. 2003) or treatment with a MnSOD and catalase mimetic in C. elegans (Melov et al. 2000) extended lifespan and thus support the hypothesis, but these effects are not universally observed and are controversial (Gems and Doonan 2009). In mammals, only few genetic manipulations designed to reduce ROS activities have resulted in increased lifespan (Schriner et al. 2005; Yoshida et al. 2005). Salmon et al. (2010) conclude that oxidative stress is likely to play a limited role in ageing but that reduced oxidative stress retards pathology.
Much of the recent interest in ROS and ageing has concerned the potential role of mitochondria as a source and target for ROS (for recent reviews see Jang & van Remmen, 2009; Salmon et al. 2010). Miquel and co-workers (Miquel et al. 1980) originally suggested that accumulation of somatic mutations in mitochondrial DNA due to oxidative stress is the major contributor to ageing. Their theory indicates that ROS generated from the mitochondrial respiratory chain damages macromolecules, particularly mitochondrial DNA. This is thought to lead to defective mitochondrial respiration and a further increase in ROS generation (Jang & Van Remmen, 2009). There is considerable evidence that mitochondria isolated from tissues of aged individuals from different species do produce relatively increased amounts of ROS and that this occurs in association with impaired mitochondrial function and oxidative damage to mitochondrial components with ageing (e.g. see Vasilaki et al. 2006b). However, recent studies with transgenic and knockout mouse models have produced inconsistent results (Jang & van Remmen, 2009) and generally do not support the theory, although transgenic mice with catalase targeted to mitochondria have increased lifespan compared with control non-transgenic mice (Schriner et al. 2005). In summary therefore, the role of ROS, and particularly of mitochondria-derived ROS, in fundamental mechanisms of ageing remains controversial, but ROS generation and metabolism are clearly disrupted with increasing age. There is evidence that these changes in ROS contribute to the loss of muscle mass and function that occur with increasing age, but whether dysregulation of ROS is the prime cause of ageing, or a consequence of it, remains an open question.
Failure of oxidative signalling in muscle during ageing
The ability of cells and tissues from old mammals to respond to a variety of stresses by an increased content of HSPs and an increase in the activity of antioxidant defence enzymes is severely attenuated. The increase in HSP content and antioxidant defence enzyme activity that is evident in muscles of adult rodents following an acute period of isometric contractions was abolished in muscles of old rodents (Vasilaki et al. 2002, 2006) and this inability to adapt was shown to be due to the lack of complete activation of the appropriate transcription factors (Vasilaki et al. 2006a). Further studies from our laboratory have demonstrated that this age-related inability to produce HSPs plays a critical role in the development of functional deficits that occur with ageing in skeletal muscle. Studies using transgenic mice overexpressing HSP70 in skeletal muscle demonstrated that increased muscle content of this protein provided protection against the fall in specific force associated with ageing, and facilitated rapid and successful regeneration following contraction-induced damage in muscles of old mice compared with the impaired regeneration and recovery normally observed in old non-transgenic mice (McArdle et al. 2004a). This protection was associated with maintenance of the ability of muscles of old HSP70 overexpressor mice to activate NFκB following contractions (Broome et al. 2006). In recent studies, overexpression of a mitochondrial chaperone protein, HSP10, was also shown to preserve muscle function during ageing in mice (Kayani et al. 2010)
Activation of redox-responsive transcription factors is aberrant in muscles of old humans and mice and these muscles demonstrate both chronic constitutive activation of redox-sensitive TFs (Cuthbertson et al. 2005; Vasilaki et al. 2006a) and an inability to further activate these TFs following an acute non-damaging contraction protocol (Vasilaki et al. 2006a). The chronic activation of TFs such as NFκB in muscles of old mice is associated with chronic increases in the expression of a number of genes. For example, increased content and activities of antioxidant defence enzymes, such as the superoxide dismutases and catalase (Broome et al. 2006), increased content of HSPs (Vasilaki et al. 2006; Kayani et al. 2008) and increased production of pro- and anti-inflammatory cytokines and chemokines by muscle cells (Febbraio & Pedersen, 2005). The inability to further activate NFκB in response to an acute contraction protocol is associated with severe attenuation of normal changes in expression of cytoprotective genes (Demirel et al. 2003; Vasilaki et al. 2006a).
A diminished ability to respond to the stress of contractions has been reported to play an important role in other age-related defects in muscle function and adaptation. Ljubicic & Hood (2008) have reported a severe attenuation of the signalling pathways involved in mitochondrial biogenesis in fast muscle fibres of old rats following contractions compared with that seen in fibres from young rats. ROS are known to play an important role in the activation of signalling cascades (Irrcher et al. 2009). These authors suggest that ROS affect mitochondrial biogenesis via the up-regulation of transcriptional regulators such as peroxisome proliferator-activated receptor-gamma coactivator-1 protein-alpha (PGC-1α), suggesting that an aberrant activation of ROS generation following contractions may be responsible for the diminished mitochondrial biogenesis in muscles of old rats. This blunted or absent adaptation to stress in muscle of old humans and mice is not limited to the exercise response. Skeletal muscle of healthy elderly humans demonstrates a reduction in anabolic sensitivity and responsiveness of muscle protein synthesis pathways. Cuthbertson et al. (2005) demonstrated a reduction in the phosphorylation of mTOR and downstream translational regulators in response to essential amino acid (EAA) ingestion when compared with the young despite greater plasma EAA availability in elderly subjects. The authors concluded that the nutrient signal was not sensed or transduced as well by muscle in the elderly compared with muscle in the young, resulting in a lower protein synthesis response to the same nutrient stimulus.
Nature of the defect in redox signalling that occurs in skeletal muscle during ageing
The inability of skeletal muscle from old mice to activate redox-sensitive transcription factors such as NFκB or AP-1 in response to stress is characterised by chronic activation of transcription factors at rest and an inability to further activate these factors following an acute non-damaging muscle contraction protocol (Vasilaki et al. 2006a). This lack of activation of redox-sensitive transcription factors with contractile activity is associated with an inability to increase the expression of various cytoprotective proteins (Vasilaki et al. 2006) and influences the susceptibility of skeletal muscle from old animals to oxidative damage. The lack of activation of HSF can be overcome with the use of HSF1 activating drugs, suggesting no inherent defect in this process but supporting the theory of a failed signal for activation (Kayani et al. 2008). ROS play an important role in the activation of several signalling cascades (Irrcher et al. 2009) and in skeletal muscle, the increase in intracellular ROS is a key activator of NFκB and AP-1 in response to contractions, either through oxidation of upstream regulators or by oxidation of regulatory proteins for these transcription factors, such as thioredoxin 1 (Jackson, 2009). There are many potential sites at which the age-related defect in ROS-stimulated adaptive responses might occur (see Jackson, 2009 for a review), but paradoxically there is also evidence which indicates that attenuated transcriptional responses to contractions seen in aged mice are related to a lack of additional intracellular ROS generation in response to contractile activity. The contraction-induced increase in extracellular ROS derived from muscle is attenuated during ageing and the contraction-induced increase in extracellular superoxide seen in young mice is abolished in aged mice (Vasilaki et al. 2006b; Close et al. 2007). A consequence of this is that there is no transient increase in oxidation of both muscle and non-muscle tissues in old mice in response to muscle contractions (Vasilaki et al. 2006b; Close et al. 2007) and we speculate this may underlie the inability to further activate redox-regulated transcription factors. A schematic diagram illustrating this age-related defect in redox signalling in skeletal muscle is shown in Fig. 1.
Figure 1. Schematic representation of the processes by which ROS are generated in skeletal muscle in response to contractions and lead to the local oxidation of thiol (-SH) groups to activate redox-sensitive transcription factors including NFκB, AP1 and HSF1.
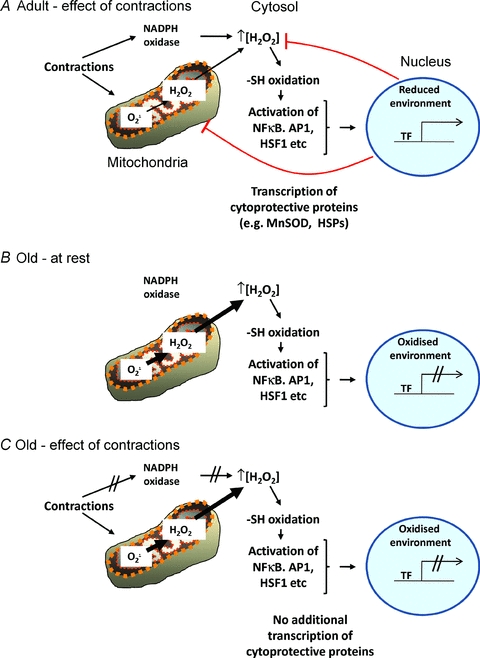
A, the activated transcription factors migrate to the nucleus and bind to DNA in a locally reduced environment to stimulate transcription of cytoprotective proteins that act to restore ROS homeostasis. B, during ageing there is evidence for a chronic increase in release of hydrogen peroxide from mitochondria at rest and this is hypothesised to cause chronic oxidation of the redox-sensitive transcription factors at rest, but an oxidation of the nuclear environment that prevents DNA binding of the transcription factor. C, during contractile activity in the elderly there appears to be a failure of the ability to further activate transcription factors and increase the expression for cytoprotective proteins that may occur at several levels including a lack of activation of local ROS generation, a failure of further transcription factor activation and defective nuclear transcription factor binding.
Conclusions
It is clear that the age-related loss of ability to adapt to free radicals and ROS induced in skeletal muscle by contractile activity is an important contributor to the loss of ROS homeostasis and oxidative damage that is seen in tissues from aged animals and man. Experimental manipulation of mice to overexpress specific protein products in muscle in order to by-pass the ageing-related block in adaptive responses has also demonstrated the importance of these responses in maintaining muscle function. Elucidation of the mechanisms by which ageing leads to defective adaptations to contractile activity may provide an insight into ways of reversing these defects in ageing humans and current data indicate this defect lies in a lack of signal for further activation of redox-sensitive transcription factors.
Acknowledgments
The authors would like to thank their many present and previous collaborators and colleagues and to acknowledge generous financial support from the BBSRC, MRC, Wellcome Trust, United States National Institute on Aging (Grant no. AG020591-06), Research into Ageing and the Dowager Countess Eleanor Peel Trust.
Glossary
Abbreviations
- AP
activator protein
- EAA
essential amino acid
- FDB
flexor digitorum brevis
- HSF
heat shock factor
- NFκB
nuclear factor κB
- ROS
reactive oxygen species
- TF
transcription factor
References
- Balon TW, Nadler JL. Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol. 1994;77:2519–2521. doi: 10.1152/jappl.1994.77.6.2519. [DOI] [PubMed] [Google Scholar]
- Bakkar J, Wang KJ, Ladner H, Wang JM, Dahlman M, Carathers S, Acharyya MA, Rudnicki AD, Hollenbach DC, Guttridge DC. IKK/NF-κB regulates skeletal myogenesis via a signaling switch to inhibit differentiation and promote mitochondrial biogenesis. J Cell Biol. 2008;180:787–802. doi: 10.1083/jcb.200707179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome CS, Kayani AC, Palomero J, Dillmann WH, Mestril R, Jackson MJ, McArdle A. Effect of lifelong overexpression of HSP70 in skeletal muscle on age-related oxidative stress and adaptation following non-damaging contractile activity. FASEB J. 2006;20:1549–1551. doi: 10.1096/fj.05-4935fje. [DOI] [PubMed] [Google Scholar]
- Close GL, Kayani AC, Ashton T, McArdle A, Jackson MJ. Release of superoxide from skeletal muscle of adult and old mice: An experimental test of the Reductive Hotspot Hypothesis. Aging Cell. 2007;6:189–195. doi: 10.1111/j.1474-9726.2007.00277.x. [DOI] [PubMed] [Google Scholar]
- Cotto JJ, Morimoto RI. Stress-induced activation of the heat-shock response: cell and molecular biology of heat-shock factors. Biochem Soc Symp. 1999;64:105–118. [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- Demirel HA, Hamilton KL, Shanely RA, Tümer N, Koroly MJ, Powers SK. Age and attenuation of exercise-induced myocardial HSP72 accumulation. Am J Physiol Heart Circ Physiol. 2003;285:H1609–H1615. doi: 10.1152/ajpheart.00982.2002. [DOI] [PubMed] [Google Scholar]
- Dillard CJ, Litov RE, Savin WM, Dumelin EE, Tappel AL. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J Appl Physiol. 1978;45:927–932. doi: 10.1152/jappl.1978.45.6.927. [DOI] [PubMed] [Google Scholar]
- Drew B, Phaneuf S, Dirks A, Selman C, Gredilla R, Lezza A, Barja G, Leeuwenburgh C. Effects of aging and caloric restriction on mitochondrial energy production in gastrocnemius muscle and heart. Am J Physiol Regul Integr Comp Physiol. 2003;284:R474–R480. doi: 10.1152/ajpregu.00455.2002. [DOI] [PubMed] [Google Scholar]
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Espinosa A, Leiva A, Pena M, Muller M, Debandi A, Hidalgo C, Carrasco MA, Jaimovich E. Myotube depolarization generates reactive oxygen species through NAD(P)H oxidase; ROS-elicited Ca2+ stimulates ERK, CREB, early genes. J Cell Physiol. 2006;209:379–388. doi: 10.1002/jcp.20745. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Pedersen BK. Contraction-induced myokine production and release: is skeletal muscle an endocrine organ? Exerc Sport Sci Rev. 2005;33:114–119. doi: 10.1097/00003677-200507000-00003. [DOI] [PubMed] [Google Scholar]
- Gems D, Doonan R. Antioxidant defense and aging in C. elegans: is the oxidative damage theory of aging wrong? Cell Cycle. 2009;8:1681–1687. doi: 10.4161/cc.8.11.8595. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radical Biology and Medicine. Oxford University Press; 1989. [Google Scholar]
- Irrcher R, Ljubicic V, Hood DA. Interactions between ROS and AMP kinase activity in the regulation of PGC-1α transcription in skeletal muscle cells. Am J Physiol Cell Physiol. 2009;296:C116–C123. doi: 10.1152/ajpcell.00267.2007. [DOI] [PubMed] [Google Scholar]
- Jackson MJ. Redox regulation of adaptive responses in skeletal muscle to contractile activity. Free Radic Biol Med. 2009;47:1267–1275. doi: 10.1016/j.freeradbiomed.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Jackson MJ, Papa S, Bolanos J, Bruckdorfer R, Carlsen H, Elliott RM, et al. Antioxidants, reactive oxygen and nitrogen species, gene induction and mitochondrial function. Mol Aspects Med. 2002;23:209–285. doi: 10.1016/s0098-2997(02)00018-3. [DOI] [PubMed] [Google Scholar]
- Jang YC, Van Remmen H. The mitochondrial theory of aging: insight from transgenic and knockout mouse models. Exp Gerontol. 2009;44:256–260. doi: 10.1016/j.exger.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, Van Der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javesghani D, Magder SA, Barreiro E, Quinn MT, Hussain SN. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am J Respir Crit Care Med. 2002;165:412–418. doi: 10.1164/ajrccm.165.3.2103028. [DOI] [PubMed] [Google Scholar]
- Ji LL, Gomez-Cabrera MC, Steinhafel N, Vina J. Acute exercise activates nuclear factor (NF)-κB signaling pathway in rat skeletal muscle. FASEB J. 2004;18:1499–1506. doi: 10.1096/fj.04-1846com. [DOI] [PubMed] [Google Scholar]
- Kayani AC, Close GL, Broome CS, Jackson MJ, McArdle A. Enhanced recovery from contraction-induced damage in skeletal muscles of old mice following treatment with the heat shock protein inducer 17-(allylamino)-17-demethoxygeldanamycin. Rejuvenation Res. 2008;11:1021–1030. doi: 10.1089/rej.2008.0795. [DOI] [PubMed] [Google Scholar]
- Kayani AC, Close GL, Dillmann WH, Mestril R, Jackson MJ, McArdle A. Overexpression of HSP10 in skeletal muscle of transgenic mice prevents the age-related fall in maximum tetanic force generation and muscle cross-sectional area. Am J Physiol Regul Integr Comp Physiol. 2010;299:R268–R276. doi: 10.1152/ajpregu.00334.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khassaf M, Child RB, McArdle A, Brodie DA, Esanu C, Jackson MJ. Time course of responses of human skeletal muscle to oxidative stress induced by non-damaging exercise. J App Physiol. 2001;90:1031–1036. doi: 10.1152/jappl.2001.90.3.1031. [DOI] [PubMed] [Google Scholar]
- Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- Lee CE, McArdle A, Griffiths RD. The role of hormones, cytokines and heat shock proteins during age-related muscle loss. Clin Nutr. 2007;26:524–534. doi: 10.1016/j.clnu.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Ljubicic V, Hood DA. Kinase-specific responsiveness to incremental contractile activity in skeletal muscle with low and high mitochondrial content. Am J Physiol Endocrinol Metab. 2008;295:E195–E204. doi: 10.1152/ajpendo.90276.2008. [DOI] [PubMed] [Google Scholar]
- McArdle A, Pattwell D, Vasilaki A, Griffiths RD, Jackson MJ. Contractile activity-induced oxidative stress: Cellular origin and adaptive responses. Am J Physiol Cell Physiol. 2001;280:C621–C627. doi: 10.1152/ajpcell.2001.280.3.C621. [DOI] [PubMed] [Google Scholar]
- McArdle A, Dillmann WH, Mestril R, Faulkner JA, Jackson MJ. Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB J. 2004a;18:355–357. doi: 10.1096/fj.03-0395fje. [DOI] [PubMed] [Google Scholar]
- McArdle F, Spiers S, Aldemir H, Vasilaki A, Beaver A, Iwanejko L, McArdle A, Jackson MJ. Preconditioning of skeletal muscle against contraction-induced damage: the role of adaptations to oxidants in mice. J Physiol. 2004b;561:233–244. doi: 10.1113/jphysiol.2004.069914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melov S, Ravenscroft J, Malik S, Gill MS, Walker DW, Clayton PE, Wallace DC, Malfroy B, Doctrow SR, Lithgow GJ. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567–1569. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- Miquel J, Economos AC, Fleming J, Johnson JE., Jr Mitochondrial role in cell aging. Exp Gerontol. 1980;15:575–591. doi: 10.1016/0531-5565(80)90010-8. [DOI] [PubMed] [Google Scholar]
- Moran LK, Gutteridge JM, Quinlan GJ. Thiols in cellular redox signalling and control. Curr Med Chem. 2001;8:763–772. doi: 10.2174/0929867013372904. [DOI] [PubMed] [Google Scholar]
- Orr WC, Mockett RC, Benes JJ, Sohal RS. Effects of overexpression of copper-zinc and manganese superoxide dismutases, catalase, and thioredoxin reductase genes on longevity in Drosophila melanogaster. J Biol Chem. 2003;278:26418–26422. doi: 10.1074/jbc.M303095200. [DOI] [PubMed] [Google Scholar]
- Palomero J, Pye D, Kabayo T, Spiller DG, Jackson MJ. In situ detection and measurement of intracellular reactive oxygen species in single isolated mature skeletal muscle fibers by real time fluorescence microscopy. Antioxid Redox Signal. 2008;10:1463–1474. doi: 10.1089/ars.2007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers S, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pye D, Palomero J, Kabayo T, Jackson MJ. Real-time measurement of nitric oxide in single mature mouse skeletal muscle fibres during contractions. J Physiol. 2007;581:309–318. doi: 10.1113/jphysiol.2006.125930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MB, Shoji T, Moody MR, Entman ML. Reactive oxygen in skeletal muscle. II. Extracellular release of free radicals. J Appl Physiol. 1992;73:1805–1809. doi: 10.1152/jappl.1992.73.5.1805. [DOI] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon AB, Richardson A, Pérez VI. Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med. 2010;48:642–655. doi: 10.1016/j.freeradbiomed.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Van Gammeren D, Damrauer JS, Jackman RW, Kandarian SC. The IκB kinases IKKα and IKKβ are necessary and sufficient for skeletal muscle atrophy. FASEB J. 2009;23:362–370. doi: 10.1096/fj.08-114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilaki A, Jackson MJ, McArdle A. Attenuated heat shock response of aged skeletal muscle to exercise. Muscle Nerve. 2002;25:902–905. doi: 10.1002/mus.10094. [DOI] [PubMed] [Google Scholar]
- Vasilaki A, McArdle F, Iwanejko LM, McArdle A. Adaptive responses of mouse skeletal muscle to contractile activity: the effect of age. Mech Ageing Dev. 2006a;127:830–839. doi: 10.1016/j.mad.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Vasilaki A, Mansouri A, Remmen H, Van Der Meulen JH, Larkin L, Richardson AG, McArdle A, Faulkner JA, Jackson MJ. Free radical generation by skeletal muscle of adult and old mice: effect of contractile activity. Aging Cell. 2006b;5:109–117. doi: 10.1111/j.1474-9726.2006.00198.x. [DOI] [PubMed] [Google Scholar]
- Xia R, Webb JA, Gnall LL, Cutler K, Abramson JJ. Skeletal muscle sarcoplasmic reticulum contains a NADH-dependent oxidase that generates superoxide. Am J Physiol Cell Physiol. 2003;285:C215–C221. doi: 10.1152/ajpcell.00034.2002. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Nakamura H, Masutani H, Yodoi J. The involvement of thioredoxin and thioredoxin binding protein-2 on cellular proliferation and aging process. Ann N Y Acad Sci. 2005;1055:1–12. doi: 10.1196/annals.1323.002. [DOI] [PubMed] [Google Scholar]


