Abstract
Abstract
A pivotal role has been ascribed to oxidative stress in determining the imbalance between protein synthesis and degradation leading to muscle atrophy in many pathological conditions and in disuse. However, a large variability in disuse-induced alteration of redox homeostasis through muscles, models and species emerges from the literature. Whereas the causal role of oxidative stress appears well established in the mechanical ventilation model, findings are less compelling in the hindlimb unloaded mice and very limited in humans. The mere coexistence of muscle atrophy, indirect indexes of increased reactive oxygen species (ROS) production and impairment of antioxidant defence systems, in fact, does not unequivocally support a causal role of oxidative stress in the phenomenon. We hypothesise that in some muscles, models and species only, due to a large redox imbalance, the leading phenomena are activation of proteolysis and massive oxidation of proteins, which would become more susceptible to degradation. In other conditions, due to a lower extent and variable time course of ROS production, different ROS-dependent, but also -independent intracellular pathways might dominate determining the variable extent of atrophy and even dispensable protein oxidation. The ROS production and removal are complex and finely tuned phenomena. They are indeed important intracellular signals and redox balance maintains normal muscle homeostasis and can underlie either positive or negative adaptations to exercise. A precise approach to determine the levels of ROS in living cells in various conditions appears to be of paramount importance to define and support such hypotheses.
Roberto Bottinelli teaches Physiology to medical students at the University of Pavia, Italy. He devoted most of his research work to the study of the mechanisms underlying the large heterogeneity and plasticity of skeletal muscles. His research goal was pursued through the analysis of structure and function of skinned muscle fibres containing different myosin isoforms and of pure myosin isoforms in both biochemical assays and reconstituted contractile systems in vitro. Maria Antonietta Pellegrino teaches human physiology at the University of Pavia, Italy. She has devoted most of her research activity to the study of skeletal muscle plasticity in humans and small mammals. She has mainly studied the role of myofibrillar protein isoforms in muscle adaptations in physiological and pathological conditions using electrophoretic techniques and Western blot. More recently, she has extended the analysis of muscle phenotype by developing proteomic techniques.
 |
Introduction
Skeletal muscle is well known to undergo atrophy and a slower to faster shift in contractile and metabolic properties in several animal and human models of disuse (Thomason & Booth, 1990; Baldwin, 1996; Desplanches, 1997; Baldwin & Haddad, 2001).
Muscle atrophy, which is the major determinant of disuse muscle weakness, is due to an imbalance between protein synthesis and degradation. At least in the most studied animal models of disuse, it is believed that an initial decrease in protein synthesis is followed by a sustained and likely predominant increase in protein breakdown (Thomason & Booth, 1990; Powers et al. 2005). Most studies have focused on protein degradation and have shown a complex and still developing picture in which the ubiquitin–proteasome pathway and the activation of caspase-3 and calpains are likely to play a major role (Powers et al. 2005, 2010). Importantly, myofibrillar proteins have been shown to be lost at a higher rate that other muscle fibre proteins (Thomason & Booth, 1990; Jackman & Kandarian, 2004).
Oxidative stress has been shown to occur in disuse and in many pathological conditions, and is now widely considered a major trigger of the imbalance between protein synthesis and degradation leading to muscle atrophy (Powers et al. 2005, 2010; Moylan & Reid, 2007). However, the causal role of oxidative stress in determining disuse atrophy has not been definitely established yet. Some contradictory results have been reported, suggesting that care should be taken over generalizing conclusions through different species, disuse models and muscles. We will focus on the most widely studied models of disuse in animals (mechanical ventilation, limb immobilization and hindlimb unloading), and in humans (bed rest, unilateral lower limb suspension, limb immobilization), and we will consider how conclusive the evidence for a causal role of oxidative stress is.
Oxidant antioxidant balance and oxidative stress
It has been known for a long time that reactive oxygen species (ROS) are present in skeletal muscle (Commoner et al. 1954) and can be generated during exercise (Dillard et al. 1978; Davies et al. 1982). As ROS can damage cell proteins, DNA and lipids through oxidation, they have been considered to just be damaging agents, and antioxidants are used to scavenge them (Dillard et al. 1978; Davies et al. 1982). The term oxidative stress has been consequently defined as the ‘imbalance between oxidants and antioxidants in favor of the oxidants, potentially leading to damage’ (Sies, 1997). Importantly, more recently it has been understood that ROS are major signals involved in muscle homeostasis, i.e. in maintaining normal skeletal muscle structure and function (Droge, 2002; Smith & Reid, 2006; Brigelius-Flohe, 2009; Musaro et al. 2010). ROS production due to heavy exercise training (Sastre et al. 1992; Vina et al. 2000; Palazzetti et al. 2003; Aguilo et al. 2005; Silva et al. 2010) has been shown to determine muscle damage, documented by increased lipid peroxidation, protein carbonylation, increase in serum creatine kinase and altered glutathione redox status. On the contrary, ROS production during moderate exercise caused positive adaptations among which are increases in insulin sensitivity, mitochondria biogenesis and antioxidant defence systems (Powers & Jackson, 2008; Jackson, 2009; Ristow et al. 2009; Silva et al. 2009; Strobel et al. 2011). Consequently, antioxidant administration may counter muscle damage following heavy exercise (Sastre et al. 1992; Vina et al. 2000; Palazzetti et al. 2004; Silva et al. 2010), but also positive adaptations following moderate exercise (Ristow et al. 2009).
The mechanisms underlying the opposite effects of ROS on muscle homeostasis in different conditions are still unclear. It could be that small, compartmentalized, or transient (minutes) increases in ROS mostly modulate intracellular signals by reversible oxidation of specific protein residues (Ghezzi, 2005; Janssen-Heininger et al. 2008) and consequently affect gene expression (Jackson et al. 2002; Ji et al. 2004; Powers et al. 2005, 2010). The latter phenomenon could occur in response to moderate exercise. In heavy exercise, disuse and pathological conditions, sustained (hours, days), large increases in ROS could (i) have a direct, non-specific, large scale oxidative effect on proteins, which would become more susceptible to proteolysis; (ii) damage plasma membrane and sarcoplasmic reticulum altering calcium homeostasis and activating proteases (e.g. calpains), enhancing proteolysis, (iii) damage lysosome and cause a leakage of catabolic enzymes in the cytosol. Oxidized proteins could be more susceptible to proteolysis because they are more easily targeted by the ubiquitin–proteasome system, which is up-regulated by ROS (Davies, 1987; Shang et al. 1997), because their recognition by calpain and caspase is enhanced (Smuder et al. 2010), or because they could be directly degraded by proteasome without being ubiquitinated (Grune et al. 2003), or for all the above causes.
As ROS are short lived and the direct determination of their concentration is complex and exposed to error (Smith & Reid, 2006; Palomero et al. 2008), in most studies on disuse atrophy, ROS activity has been studied ‘indirectly’, namely by measuring protein oxidation and lipid peroxidation (Lawler et al. 2003; Urso & Clarkson, 2003). The latter approaches have been mostly combined with another indirect index of ROS activity, namely the activity or content of antioxidant defence systems among them superoxide dismutase, catalase and glutathione peroxidase. The correlation between muscle atrophy, increase in protein and lipid peroxidation and impairment of antioxidant defence systems has been taken as a strong indication that oxidative stress occurred and was involved in muscle wasting (Lawler et al. 2003; Powers et al. 2005). The administration of antioxidants has been used to counteract oxidative stress with the goal of confirming the role of ROS through amelioration of muscle atrophy (Kondo et al. 1992; Appell et al. 1997; Arbogast et al. 2007).
Animal models of disuse
Disuse atrophy due to mechanical ventilation
Studies on the mechanical ventilation model can be considered a paradigm of the relevance of studying a disuse model for clinical practice and of how an analysis can successfully develop providing strong evidence of the role of oxidative stress. The topic has been recently reviewed in detail (Powers et al. 2009). Table 1, presented as online Supplemental Material, summarizes findings of the major references cited regarding human and animal models of disuse in order of appearance.
Mechanical ventilation (MV) is extremely important in clinical practice, as it save lives of patients with critical respiratory problems due to a variety of conditions, e.g. respiratory diseases, spinal cord injuries, neuromuscular diseases, coma, general anaesthesia, drug overdoses.
In the MV model, as animals are tracheostomized and a ventilator delivers all breaths, the diaphragm is completely inactive (McClung et al. 2007). As early as 2002 available data suggested that MV induces an extremely rapid (12–18 h) force loss and diaphragm atrophy, and that oxidative stress plays a major role in the phenomenon (Shanely et al. 2002). Lately, the picture of the role of oxidative stress in MV was further refined showing that MV cause a very early depression of protein synthesis (Shanely et al. 2004), besides increasing protein degradation; that insoluble proteins, likely myosin and actin, are oxidized as early as 6 h into MV (Zergeroglu et al. 2003); and that the increase in ROS depends on both an increase in production and a decrease in removal due to down-regulation of antioxidant defence systems (e.g. glutathione, glutathione peroxidase, superoxide dismutase) (Falk et al. 2006).
Finally, a very recent work has brilliantly confirmed in humans undergoing MV some conclusions obtained in the rat model of MV: (i) muscle fibre atrophy, (ii) decrease in an antioxidant defence system (glutathione), and (iii) increase in caspase-3 and increase in expression of two key ubiquitin ligases (MurF-1 and atrogin-1) of the ubiquitin–proteasome pathways (Levine et al. 2008).
Importantly, administration of an antioxidant, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), a vitamin E analogue with strong and specific antioxidant properties, blunted diaphragm atrophy in mechanically ventilated rats (Betters et al. 2004; McClung et al. 2007; Whidden et al. 2010) (Fig. 1). Even more importantly, Trolox prevented activation of calpain and caspase-3 indicating that oxidative stress is a requirement for the activation of a major proteolytic pathway underlying MV muscle atrophy (Whidden et al. 2010). Such an example illustrates how a direct link between oxidative stress and proteolysis can be established, whereas most evidence of the role of oxidative stress in other models relies just on correlations, e.g. on the presence at the same time of atrophy and signs of oxidative stress.
Figure 1. The impact of mechanical ventilation and antioxidant administration (Trolox) on cross-sectional areas (CSA) of different muscle fibres (type I, IIa and IIb/x).
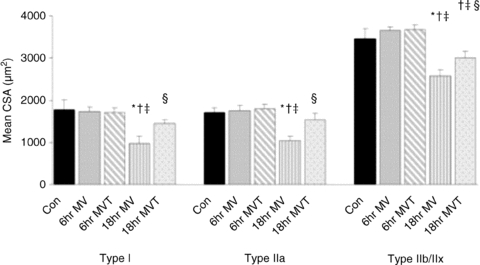
Five groups of rats were studied: controls (Con), mechanically ventilated for 6 and 18 h without and with Trolox treatment (6 h MV, 6 h MVT, 18 h MV, 18 h MVT). (Reprinted from McClung et al. 2007 with permission from Wiley-Blackwell.) *Significantly (P < 0.05) different from control values. †Significantly (P < 0.05) different from 6 h MV values. ‡Significantly (P < 0.05) different from 6 h MVT values. §Significantly (P < 0.05) different from 18 h MV values.
Disuse atrophy due to hindlimb unloading and limb immobilization
Several models of disuse have been used in small mammals, among which the most used have been hindlimb unloading (HU) and limb immobilization.
The potential role of oxidative stress in determining disuse atrophy was studied by Kondo et al. (1992, 1993) in pioneering works using the limb immobilization model. No change in two antioxidant enzymes, glutathione peroxidase and catalase, but an increase in the Cu,Zn cytoplasmic isoform of superoxide dismutase and in several indicators of oxidative stress (free iron, xanthine oxidase activity, lipid peroxidation and oxidized glutathione/reduced glutathione ratio) suggested, for the first time, a major role of oxidative stress in disuse atrophy.
The issue was reconsidered in detail 10 years later by Lawler et al. (2003, 2006) using the HU model. The data confirmed Kondo's hypothesis showing increase in ROS, alterations in antioxidant defence systems (increase in Cu,Zn superoxide dismutase (SOD), decrease in catalase and glutathione peroxidase activities), decrease in non-enzymatic antioxidant scavenging capacity (Lawler et al. 2003) and decrease in heat shock proteins, Hsp25 and Hsp70 (Lawler et al. 2006), which can play relevant roles in protecting cells against oxidative damage (Senf et al. 2008).
As the presence in the same muscle of atrophy, of indirect signs of ROS increase, and of an impairment of antioxidant defence systems cannot be considered a direct demonstration of the causal role of oxidative stress, antioxidants have been used in an attempt to prevent muscle atrophy, thereby proving the role of ROS. Whereas early experiments in the limb immobilization model suggested that antioxidant administration (vitamin E) can blunt soleus atrophy (Kondo et al. 1992; Appell et al. 1997), contradictory results were obtained in HU. No effect either in soleus or in gastrocnemius was observed in the first study on HU using vitamin E (Koesterer et al. 2002), and using allopurinol, an xanthine oxidase inhibitor (Matuszczak et al. 2004). The Bowman–Birk inhibitor, a soy protein extract which directly buffers ROS, ameliorated soleus atrophy (Arbogast et al. 2007), but as the drug also inhibits serine protease activity (Arbogast et al. 2007), its effect could be independent from its antioxidant activity. In another study, cysteine administration was shown to partially prevent unweighting-induced ubiquitination and degradation of proteins in parallel with redox system normalization, but the same group further showed no benefit of vitamin E (Ikemoto et al. 2002a,b;). Interestingly, in a more recent study, a moderate effect of vitamin E on soleus atrophy was observed, but was accounted for by vitamin E inhibition of proteolytic enzymes, rather than its antioxidant capacity (Servais et al. 2007).
We very recently applied the proteomic approach (Brocca et al. 2010) and electrophysiological measurements (Desaphy et al. 2010) to mice following HU in the presence and absence of Trolox administration. Soleus data recapitulated what was previously observed (Lawler et al. 2003): muscle fibre atrophy (Fig. 2A), increase in lipid peroxidation and protein oxidation (Fig. 2B), and impairment of several antioxidant defence systems (Fig. 2C). In an electrophysiological study on the same animals, a slow to fast shift in muscle phenotype and an increase in chloride conductance and ClC-1 chloride channel expression were found, which are early markers of disuse atrophy (Pierno et al. 2002; Desaphy et al. 2005). Therefore, soleus data seemingly supported the idea that oxidative stress could be a major trigger of disuse atrophy. Interestingly, the level of lipid peroxidation was studied in soleus, gastrocnemius, tibialis anterior and EDL muscle and was found to be linearly related to percentage muscle atrophy (Fig. 2D) (Desaphy et al. 2010).
Figure 2. The impact of hindlimb unloading (HU) and antioxidant administration (Trolox) in a slow, soleus (Sol), and a fast, gastrocnemius (Gas), muscle of the mouse.
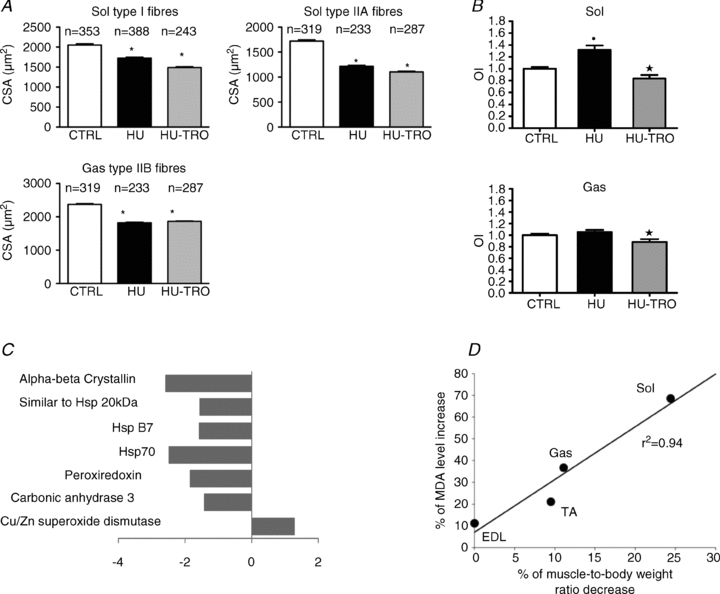
A, cross-sectional area of type I and IIA fibres from Sol and of type IIB fibres from Gas in control mice (CTRL), hindlimb unloaded mice for 14 days (HU-14) and hindlimb unloaded mice for 14 days treated by Trolox (HU-TRO). *Significantly different from CTRL (P < 0.05). (Redrawn from Brocca et al. (2010).) B, protein oxidation index (OI). The height of each vertical bar represents the mean ± SEM. *Significantly different from all groups (P < 0.05). (Reprinted from Brocca et al. (2010) with permission from Wiley-Blackwell.) C, differentially expressed proteins belonging to antioxidant defence systems in soleus muscles of HU mice, identified by proteomic analysis. (Redrawn from Brocca et al. 2010.) D, malondialdeyde (MDA) levels following 14 days HU plotted against the mean percentage of muscle-to-body weight ratio decrease for EDL, tibialis anterior (TA), Gas and Sol muscles of the mice. The points were linearly correlated (r2= 0.94). (Reprinted from Desaphy et al. 2010 with permission from Elsevier.)
The analysis of HU gastrocnemius and of Trolox treated HU soleus and gastrocnemius casted doubts about the role of oxidative stress in determining muscle atrophy. HU gastrocnemius, a fast muscle rarely analysed in disuse models as it is considered to be less susceptible to atrophy, did show atrophy (11%), but surprisingly no increase in lipid peroxidation and protein oxidation, and an increase, rather than a decrease, of antioxidant defence systems (Brocca et al. 2010). No change in antioxidant defence systems was observed in the only previous work on HU gastrocnemius, although, based on increased lipid peroxidation, a pathogenetic role of oxidative stress was suggested (Siu et al. 2008). Moreover, Trolox administration, which increased antioxidant defence systems in both soleus and gastrocnemius muscles, fully prevented lipid peroxidation and protein oxidation, counteracted the increase in chloride conductance and partially counteracted the slow to fast shift in muscle phenotype, but did not have any impact on muscle and muscle fibre atrophy (Brocca et al. 2010; Desaphy et al. 2010).
Interestingly, these data suggest that the existence of a correlation between an indirect index of ROS production and muscle atrophy (Fig. 2D) does not necessarily imply a causal role for ROS. Indeed, protein oxidation might follow muscle atrophy or occur in parallel and the relationship still hold. In neurons, it has been shown that ROS production by mitochondria depends on Bax, a member of the Bcl-2 family of apoptotic regulators, and that caspase 3, a major enzyme involved in disuse induced proteolysis, mediates part of Bax induced ROS production (Kirkland et al. 2010). In principle, the latter finding opens the possibility that protein oxidation could, at least to some extent, follow proteolysis.
We reasoned that, notwithstanding Trolox administration, oxidative stress could have occurred at early times during HU, still determining muscle atrophy but dying away by the time of our analysis, i.e. 14 days of unloading. Therefore, we analysed four animals of the same strain and age as those used by Brocca et al. (2010) following 3 days of HU. Although muscle fibres (type 1 and 2A in soleus and type 2B in gastrocnemius) were significantly atrophic (Fig. 3A) (16% on average), no protein oxidation was detectable (Fig. 3B) and no impairment was observed regarding three components of the antioxidant defence systems previously found to be differentially expressed after 14 days of HU, namely superoxide dismutase, Hsp70 and α,β-crystallin. The latter results, consistent with the lack of benefits from antioxidant administration in mice, support the hypothesis of a marginal role of oxidative stress in muscle atrophy induced by HU, which is clearly in contrast with the causal role played in diaphragm atrophy following mechanical ventilation.
Figure 3. The impact of 3 days hindlimb unloading (HU-3) on cross-sectional area (CSA) of identified types of muscle fibres (A) and on protein oxidation index (OI) (B) of soleus (Sol) and gastrocnemius (Gas) muscles of the mouse.
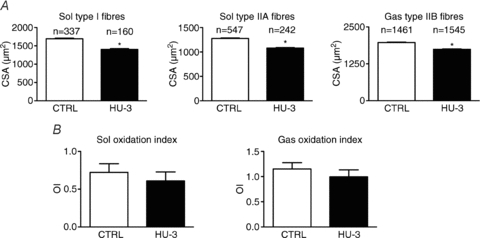
The number (n) of fibres measured is indicated above each bar. Both CSA and OI were determined exactly as by Brocca et al. (2010). *Significantly different from CTRL (P < 0.05). Four mice were used.
Human models of disuse
Notwithstanding the large amount of work on structure and function of skeletal muscle in several disuse models in humans (Fitts et al. 2000; Trappe et al. 2001; Narici et al. 2003; Hortobagyi & Devita, 2006; de Boer et al. 2007a; Pavy-Le Traon et al. 2007; Rittweger et al. 2009), information on oxidative stress and on the potential mechanisms involved in increased proteolysis is scanty. The first of such studies on bed rest suggested a potential role of oxidative stress in human disuse in line with what was previously observed in small mammals (Dalla Libera et al. 2009). Following 35 days (T35), but not 8 days (T8), of bed rest, vastus lateralis muscle samples showed muscle fibre atrophy (18%) (Fig. 4A) and increased protein carbonylation (Fig. 4B). Interestingly, an inverse linear relationship was found between normalised levels of protein oxidation and CSA of muscle fibres of biopsy samples (Fig. 4C), consistent with what was reported for mice muscles in Fig. 3D (Desaphy et al. 2010). At T8 the transient increase in two heat shock proteins known to be up-regulated in response to ROS production (Motterlini, 2005) (Ryter et al. 2006), haem oxygenase-1 (HO-1) and mitochondrial Hsp-70 isoform (Grp75), suggested the occurrence of oxidative stress. A very recent global analysis of gene expression (Reich et al. 2010) has shown an up-regulation of the pathways involved in oxidative stress response following 48 h of unilateral lower leg suspension (ULLS), consistent with the increase in HO-1 observed by Dalla Libera et al. (2009). However, some inconsistencies emerged, as discussed by the authors. For instance the content of both heat shock proteins was normal at T35, suggesting that some mechanism had blunted the stress response after T8, although increased carbonylation was actually observed at T35.
Figure 4. The impact of 8 (T8) and 35 (T35) days of bed rest on cross-sectional area (CSA) of muscle fibres and on protein oxidation (Oxy/RP) of muscle samples from the vastus lateralis muscle of humans.
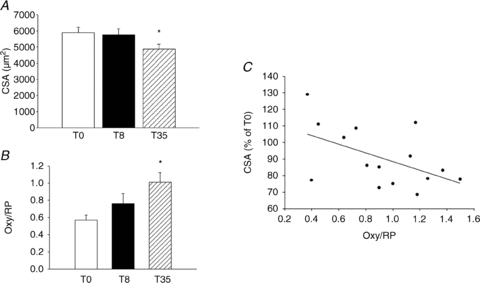
A, mean values of CSA of muscle fibres before bed rest (T0) and at T8 and T35. B, protein oxidation index (Oxy/RP). *Significantly different from T0 (P < 0.05). C, regression analysis of normalized values of muscle protein oxidation (Oxy/RP) plotted against the percentage change of fibre CSA of the same muscles, determined at T8 and T35; the slope of the line was significantly different from zero (P < 0.05), reprinted from Dalla Libera et al 2009 used with permission from The American Physiological Society.
More recently, in a limb immobilisation human model, muscle (5.7%) and muscle fibre (5.6–11.8%) atrophy were observed at the end of 14 days of immobilisation (T14) in the absence of lipid peroxidation (Fig. 5A) and protein oxidation (Fig. 5B) in vastus lateralis muscle. Ubiquitin conjugates were higher at day 2, but normal at T14 (Fig. 5C), and caspase 3/7 activity was normal at both 2 and 14 days (Fig. 5D) (Glover et al. 2010). Based on their own data and on previously published findings, which did not show increased protein breakdown in human disuse (Paddon-Jones et al. 2004; Symons et al. 2009), the authors suggested that muscle atrophy was likely to be independent of oxidative stress and increased proteolysis, although the rate of protein breakdown was not determined (Glover et al. 2010).
Figure 5. The impact of 2 days (2 d) and 14 days (14 d) leg immobilization on lipid peroxidation (4-HNE-ponceau) (A), protein carbonylation (oxyblot-ponceau) (B), ubiquitin protein conjugates content (ubiquitin-ponceau) (C) and caspase 3/7 activity (D).
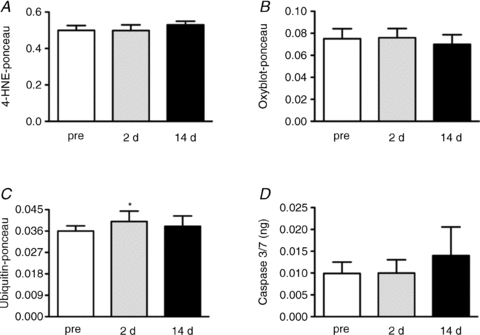
Redrawn from Glover et al. 2010.
The lack of increased proteolysis in muscle atrophy following immobilisation is consistent with a number of studies strongly suggesting that, contrary to what observed in small mammals, the major phenomenon involved in humans is a decrease in protein synthesis exacerbated by anabolic resistance, namely by a decreased stimulation of protein synthesis by exogenous amino acids (Rennie et al. 2010). In muscle atrophy following ULLS, rates of myofibrillar protein synthesis fell and no clear signs of increased expression of MuRF-1 and atrogin-1, used as markers of proteolysis, were observed (de Boer et al. 2007b). The increase in whole-body protein synthesis following amino acid feeding was blunted in bed rest (Biolo et al. 2004) and the infusion of amino acids increased proteins synthesis less in the immobilised leg than in the non-immobilised leg (Glover et al. 2008) suggesting anabolic resistance.
Although most evidence points to a decreased protein synthesis as the dominant phenomenon in disuse atrophy in humans, some findings suggested an early and transient increase in protein breakdown (Tesch et al. 2008; Glover et al. 2010; Gustafsson et al. 2010). A very recent global gene expression analysis showed up-regulation of pathways involved in protein ubiquitination and oxidative stress response following 48 h ULLS (Reich et al. 2010). Moreover, a pivotal role of oxidative stress and increased proteolysis in human chronic disease (e.g. respiratory, kidney and cardiac disease, and muscular dystrophy) has been suggested by a number of studies (Moylan & Reid, 2007) in agreement with what was previously observed in many disuse conditions in small mammals (Powers et al. 2007). In humans following 2–5 days of spinal cord injury, muscle atrophy was associated with an increased expression, although not with an increased content of key components of the ubiquitin proteasome pathway (Urso et al. 2007). However, it has been strongly argued that, in the absence of inflammation or other phenomena occurring in chronic diseases, disuse muscle atrophy can be fully explained by a decreased protein synthesis with little evidence of a possible role of increased proteolysis and of oxidative damage or protein carbonylation (Murton et al. 2008; Glover et al. 2010; Rennie et al. 2010). Consequently, caution has been suggested in transferring the results obtained on small mammals to humans (Rennie et al. 2010).
To the best of our knowledge, there are no more studies which assessed the presence of oxidative stress in human limb muscles in disuse. The lack of a major role for proteolysis, the key phenomenon supposedly triggered by oxidative stress, suggests that the latter may not be a major phenomenon in disuse atrophy of limb muscles in humans. However, studies on oxidative stress and disuse atrophy in humans are still limited. Moreover, ROS might still play a role, but not through an increase in proteolysis. Indeed, it has been recently suggested that a ROS induced increase in atrogin-1 following 20 days of bed rest could cause atrophy by impairing protein synthesis (Ogawa et al. 2006), reconciling the strong evidence of a predominating decrease in protein synthesis in humans with the observation of a transient increase in atrogin-1, a well established index of proteolysis. The latter hypothesis, which is still to be confirmed, finds some support in several observations. Atrogin-1 is up-regulated in disuse atrophy through a potentially ROS-dependent mechanism (Li et al. 2003; Powers et al. 2010). It is a key component of the ubiquitin–proteasome proteolytic pathway, but has been also shown to have a modulatory role on eukaryotic initiation factor 3 subunit 5 (eIF3-f), a component of the AKT/mTor pathway (Lagirand-Cantaloube et al. 2008), which controls protein synthesis (Sandri, 2008).
Table 1 (online Supplemental Material) summarizes findings of the major references cited regarding human and animal models of disuse in order of appearance.
Why oxidative stress may not be equally relevant in all disuse conditions
The role of an alteration of redox homeostasis in disuse atrophy appears to widely vary through muscles, models and species. Oxidative stress very likely plays a causal role in diaphragm following MV. It is less clear whether it is a cause or consequence of disuse atrophy in soleus and even more so in gastrocnemius of HU mice, and very limited evidence exists that it could play a determinant role in humans. The discrepancy between the strong and rapid oxidative stress-dependent atrophy of human diaphragm following mechanical ventilation (Levine et al. 2008) and the smaller and slower oxidative stress-independent atrophy of human limb muscles (Glover et al. 2010) strengthens the relevance of variability among different muscles and experimental conditions.
ROS acutely affects muscle force and are necessary for normal muscle homeostasis (Reid et al. 1993; Smith & Reid, 2006; Brigelius-Flohe, 2009). They play a significant role in positive adaptations following moderate exercise, being intracellular signals modulating gene expression (Jackson et al. 2002; Ji et al. 2004; Brigelius-Flohe, 2009; Jackson, 2009; Ristow et al. 2009); they cause muscle damage in heavy exercise; they modulate intracellular signalling pathway involved in disuse atrophy (Powers et al. 2010); and they can directly oxidize proteins on a large scale enhancing their degradation rate (Smuder et al. 2010). Consequently, it is very likely that differences in the time course of ROS production, in the levels of ROS, in the cellular locations of ROS production and possibly in the nature of the ROS can occur in different tissues and conditions supporting different responses. Interestingly, NADPH oxidases, a major source of ROS, are a family of enzymes whose members have different tissue localizations (Geiszt & Leto, 2004) and are present in different subcellular compartments (Ushio-Fukai, 2006).
Given the large spectrum of ROS potential effects it is not surprising that their role in disuse atrophy can vary according to muscles, species and models. The difficulties in differentiating the role of ROS in different disuse conditions might depend on the experimental approach used and on technical limitations. The analysis of correlations between atrophy and indirect indexes of ROS activity might be misleading. It can be argued that such correlations cannot definitely discriminate between oxidative stress being a cause or a consequence of muscle atrophy (Fig. 3D) (Desaphy et al. 2010). Moreover, determination of carbonyls and lipid peroxidation is likely to be sensitive to large scale oxidative phenomena missing more subtle, but still potentially modulating, levels of ROS.
The precise determination of ROS levels in living cells is, therefore, of paramount importance and its lack is a major drawback in all attempts to differentiate ROS effects in different conditions. Recent advances in this respect may open important opportunities (Palomero et al. 2008). Notwithstanding the latter problems, some phenomena potentially responsible for the variability in the response to disuse and in the ascertained roles of ROS can be put forward.
The major muscles studied have different fibre type composition. Soleus has the highest percentage of slow fibres, gastrocnemius the lowest and diaphragm is intermediate (Pellegrino et al. 2004; Desaphy et al. 2010). Interestingly, diaphragm muscle has the higher capillary and mitochondrial density than any other skeletal muscle (Hoppeler et al. 1981) and soleus is well known to be much more oxidative than gastrocnemius. Considering that mitochondria are among the major potential sources of ROS and that a proportion of electron flow (0.15%, which is small in relative terms, but relevant in absolute values) gives rise to hydrogen peroxide (Chance et al. 1979; St-Pierre et al. 2002), soleus and especially diaphragm could be more exposed to ROS production.
The decrease in load could have different effects on diaphragm, which is chronically active, and on soleus, which is a postural muscle, than on gastrocnemius, which is a fast, phasic muscle. MV, limb immobilisation, and HU could decrease neuromuscular activity to different extents. In MV diaphragmatic fibres are totally inactive (Powers et al. 2002) and go through passive shortening during mechanical expansion of the lungs (Froese & Bryan, 1974). Inactivity in the shortened position is known to favour atrophy (Loughna et al. 1987). Immobilisation (Fischbach & Robbins, 1969) might decrease neuromuscular activity more than HU, in which some authors have shown no significant decrease in integrated EMG (Alford et al. 1987), and others have shown an initial decrease in the first 6 days followed by recovery towards normal levels (De-Doncker et al. 2005).
Therefore, diaphragm might go through higher ROS production then soleus and soleus than gastrocnemius. In diaphragm all ROS inducible pathways could be activated, whereas in soleus and especially in gastrocnemius protein oxidation could not be an earlier (Fig. 3B) and major (Fig. 2B) phenomenon and other mechanisms independent from ROS or induced by a more subtle increase in ROS could play a role.
Interestingly, the possibility that ROS production occurs at different extents and rates and that different mechanisms prevail in different models and muscles is consistent with the very variable rate of muscle atrophy. The rate of muscle atrophy is, in fact, extremely fast in rat diaphragm following MV reaching 15–30% in 18 h, very fast in rat soleus following limb immobilisation (∼50% in 8 days) and much slower in soleus (24%) and in gastrocnemius (11%) of mice following 14 days HU (Brocca et al. 2010; Desaphy et al. 2010).
The discrepancies between animal and human models appear even larger than within the different animal models. It has been known for a long time that oxidative metabolism and heat production per unit body weight are inversely related to body weight (Kleiber, 1947), and that small animals are more exposed to ROS production and, possibly for this reason, have shorter lifespan (Demetrius, 2005). Interestingly, it has been observed that the higher the metabolic rate the higher muscle atrophy and that small mammals, having a smaller percentage of muscle mass, have less metabolic resilience than large mammals (Demetrius, 2005). Moreover, disuse models in small mammals could determine some stress and this might make muscle more prone to an increase in protein breakdown (Paddon-Jones, 2006; Paddon-Jones et al. 2006). Finally, xanthine oxidase, a major source of ROS, might be less expressed in human than in rat muscle (Linder et al. 1999). Indeed, the rate of disuse atrophy is much higher in small mammals (∼3% a day) (Thomason & Booth, 1990) than in humans (5–25% in 23 weeks) (de Boer et al. 2007b).
Figure 6 reports a scheme summarizing the major factors potentially involved in determining variable alterations of redox homeostasis through muscles, species and models.
Figure 6. A scheme of the factors potentially involved in determining variable alterations of redox homeostasis through muscles, species and models reported in the first three rows.
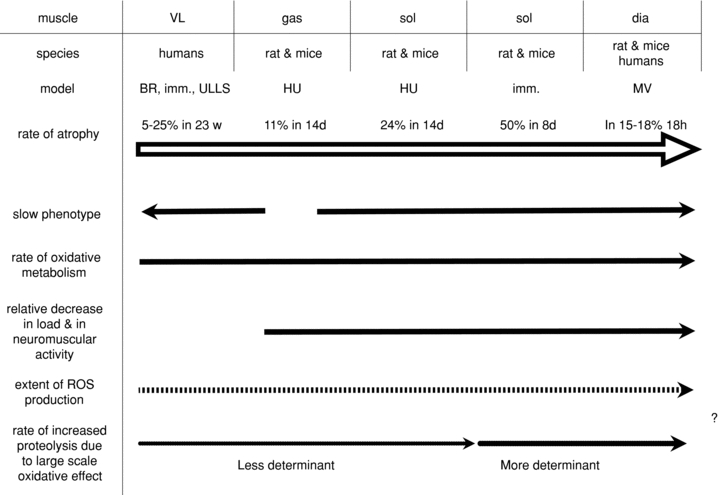
Arrows point to the direction of an increase in the parameter. The large open arrow refers to the rate of atrophy, which increases from left to right. The dashed arrow hypothesizes an increase in the extent of ROS production, from left to right, depending (i) on the increase in the rate of oxidative metabolism due to small species having higher metabolic rate and to the progressively slower phenotype (i.e. relative content of slow, type 1 fibres) of muscles and (ii) on the increase in the relative extent of unloading at least from HU gastrocnemius towards diaphragm subjected to MV. Consequently, the dotted arrow hypothesizes an increase in the rate of proteolysis, from left to right, which is less determinant (or minor) in humans, and in HU gastrocnemius and soleus of rat and mice, but more determinant (or major) in immobilized soleus and in diaphragm following MV due to a progressively more evident large scale oxidation of proteins. Abbreviations: BR, bed rest; imm., immobilization; HU, hindlimb unloading; MV, mechanical ventilation; d, days; w, weeks; h, hours.
Conclusions
The observation that ROS play a major role in muscle homeostasis, but can cause muscle wasting as well, indicated that ROS production can be finely tuned and controlled. It is thus very likely that the extent and time course of ROS production, the ROS-dependent intracellular pathways activated and, therefore, roles of ROS widely vary through different muscles, species and disuse models.
It could be wise to apply the term ‘oxidative stress’ only to conditions in which oxidative damage is documented to avoid misunderstanding by pooling phenomena in which ROS modulate intracellular pathways and gene expression contributing to muscle homeostasis and plasticity and possibly to muscle atrophy, and those in which ROS enhance proteolysis acting directly and non-specifically on proteins.
In the absence of readily available approaches to determine ROS levels in living cells, the evaluation of the time course of changes in muscle mass, antioxidant defence systems and signals involved in protein synthesis and breakdown in both the absence and presence of antioxidants should be the more correct approach to clearly define the cause and effect relationship between such phenomena. The simple correlation between muscle atrophy and indirect indexes of enhanced ROS production at any given time during the process does not seem to be able to provide definitive conclusions (Fig. 2D and Fig. 4C).
Acknowledgments
The authors wish to thank the Italian Space Agency for the grants to R.B. and D.C.C. (project OSMA ‘Osteoporosis and Muscle Atrophy’) and the European Commission for MYOAGE (no. 223576) funded under FP7 to R.B. We thank Prof. Carlo Reggiani for stimulating discussions. The authors wish to thank Mr. Luigi Guidotti for excellent technical help during all experiments related to muscle disuse.
Glossary
Abbreviations
- eIF3-f
eukaryotic initiation factor 3 subunit 5
- Grp75
Hsp-70 isoform
- Hsp
heat shock protein
- HU
hindlimb unloading
- HO-1
haem oxygenase-1
- MV
mechanical ventilation
- SOD
superoxide dismutase
- ROS
reactive oxygen species
Supplementary material
Suppl. Table 1.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Aguilo A, Tauler P, Fuentespina E, Tur JA, Cordova A, Pons A. Antioxidant response to oxidative stress induced by exhaustive exercise. Physiol Behav. 2005;84:1–7. doi: 10.1016/j.physbeh.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Alford EK, Roy RR, Hodgson JA, Edgerton VR. Electromyography of rat soleus, medial gastrocnemius, and tibialis anterior during hind limb suspension. Exp Neurol. 1987;96:635–649. doi: 10.1016/0014-4886(87)90225-1. [DOI] [PubMed] [Google Scholar]
- Appell HJ, Duarte JA, Soares JM. Supplementation of vitamin E may attenuate skeletal muscle immobilization atrophy. Int J Sports Med. 1997;18:157–160. doi: 10.1055/s-2007-972612. [DOI] [PubMed] [Google Scholar]
- Arbogast S, Smith J, Matuszczak Y, Hardin BJ, Moylan JS, Smith JD, Ware J, Kennedy AR, Reid MB. Bowman-Birk inhibitor concentrate prevents atrophy, weakness, and oxidative stress in soleus muscle of hindlimb-unloaded mice. J Appl Physiol. 2007;102:956–964. doi: 10.1152/japplphysiol.00538.2006. [DOI] [PubMed] [Google Scholar]
- Baldwin KM. Effect of spaceflight on the functional, biochemical, and metabolic properties of skeletal muscle. Med Sci Sports Exerc. 1996;28:983–987. doi: 10.1097/00005768-199608000-00008. [DOI] [PubMed] [Google Scholar]
- Baldwin KM, Haddad F. Effects of different activity and inactivity paradigms on myosin heavy chain gene expression in striated muscle. J Appl Physiol. 2001;90:345–357. doi: 10.1152/jappl.2001.90.1.345. [DOI] [PubMed] [Google Scholar]
- Betters JL, Criswell DS, Shanely RA, Van Gammeren D, Falk D, Deruisseau KC, Deering M, Yimlamai T, Powers SK. Trolox attenuates mechanical ventilation-induced diaphragmatic dysfunction and proteolysis. Am J Respir Crit Care Med. 2004;170:1179–1184. doi: 10.1164/rccm.200407-939OC. [DOI] [PubMed] [Google Scholar]
- Biolo G, Ciocchi B, Lebenstedt M, Barazzoni R, Zanetti M, Platen P, Heer M, Guarnieri G. Short-term bed rest impairs amino acid-induced protein anabolism in humans. J Physiol. 2004;558:381–388. doi: 10.1113/jphysiol.2004.066365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigelius-Flohe R. Commentary: oxidative stress reconsidered. Genes Nutr. 2009;4:161–163. doi: 10.1007/s12263-009-0131-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocca L, Pellegrino MA, Desaphy JF, Pierno S, Camerino DC, Bottinelli R. Is oxidative stress a cause or consequence of disuse muscle atrophy in mice? A proteomic approach in hindlimb-unloaded mice. Exp Physiol. 2010;95:331–350. doi: 10.1113/expphysiol.2009.050245. [DOI] [PubMed] [Google Scholar]
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Commoner B, Townsend J, Pake GE. Free radicals in biological materials. Nature. 1954;174:689–691. doi: 10.1038/174689a0. [DOI] [PubMed] [Google Scholar]
- Dalla Libera L, Ravara B, Gobbo V, Tarricone E, Vitadello M, Biolo G, Vescovo G, Gorza L. A transient antioxidant stress response accompanies the onset of disuse atrophy in human skeletal muscle. J Appl Physiol. 2009;107:549–557. doi: 10.1152/japplphysiol.00280.2009. [DOI] [PubMed] [Google Scholar]
- Davies KJ. Protein damage and degradation by oxygen radicals. I. general aspects. J Biol Chem. 1987;262:9895–9901. [PubMed] [Google Scholar]
- Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- de Boer MD, Maganaris CN, Seynnes OR, Rennie MJ, Narici MV. Time course of muscular, neural and tendinous adaptations to 23 day unilateral lower-limb suspension in young men. J Physiol. 2007a;583:1079–1091. doi: 10.1113/jphysiol.2007.135392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer MD, Selby A, Atherton P, Smith K, Seynnes OR, Maganaris CN, Maffulli N, Movin T, Narici MV, Rennie MJ. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol. 2007b;585:241–251. doi: 10.1113/jphysiol.2007.142828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Doncker L, Kasri M, Picquet F, Falempin M. Physiologically adaptive changes of the L5 afferent neurogram and of the rat soleus EMG activity during 14 days of hindlimb unloading and recovery. J Exp Biol. 2005;208:4585–4592. doi: 10.1242/jeb.01931. [DOI] [PubMed] [Google Scholar]
- Demetrius L. Of mice and men. When it comes to studying ageing and the means to slow it down, mice are not just small humans. EMBO Rep. 2005;6(Spec No):S39–44. doi: 10.1038/sj.embor.7400422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaphy JF, Pierno S, Liantonio A, De Luca A, Didonna MP, Frigeri A, Nicchia GP, Svelto M, Camerino C, Zallone A, Camerino DC. Recovery of the soleus muscle after short- and long-term disuse induced by hindlimb unloading: effects on the electrical properties and myosin heavy chain profile. Neurobiol Dis. 2005;18:356–365. doi: 10.1016/j.nbd.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Desaphy JF, Pierno S, Liantonio A, Giannuzzi V, Digennaro C, Dinardo MM, Camerino GM, Ricciuti P, Brocca L, Pellegrino MA, Bottinelli R, Camerino DC. Antioxidant treatment of hindlimb-unloaded mouse counteracts fiber type transition but not atrophy of disused muscles. Pharmacol Res. 2010;61:553–563. doi: 10.1016/j.phrs.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Desplanches D. Structural and functional adaptations of skeletal muscle to weightlessness. Int J Sports Med. 1997;18(Suppl 4):S259–264. doi: 10.1055/s-2007-972722. [DOI] [PubMed] [Google Scholar]
- Dillard CJ, Litov RE, Savin WM, Dumelin EE, Tappel AL. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J Appl Physiol. 1978;45:927–932. doi: 10.1152/jappl.1978.45.6.927. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Falk DJ, Deruisseau KC, Van Gammeren DL, Deering MA, Kavazis AN, Powers SK. Mechanical ventilation promotes redox status alterations in the diaphragm. J Appl Physiol. 2006;101:1017–1024. doi: 10.1152/japplphysiol.00104.2006. [DOI] [PubMed] [Google Scholar]
- Fischbach GD, Robbins N. Changes in contractile properties of disused soleus muscles. J Physiol. 1969;201:305–320. doi: 10.1113/jphysiol.1969.sp008757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts RH, Riley DR, Widrick JJ. Physiology of a microgravity environment invited review: microgravity and skeletal muscle. J Appl Physiol. 2000;89:823–839. doi: 10.1152/jappl.2000.89.2.823. [DOI] [PubMed] [Google Scholar]
- Froese AB, Bryan AC. Effects of anesthesia and paralysis on diaphragmatic mechanics in man. Anesthesiology. 1974;41:242–255. doi: 10.1097/00000542-197409000-00006. [DOI] [PubMed] [Google Scholar]
- Geiszt M, Leto TL. The Nox family of NAD(P)H oxidases: host defense and beyond. J Biol Chem. 2004;279:51715–51718. doi: 10.1074/jbc.R400024200. [DOI] [PubMed] [Google Scholar]
- Ghezzi P. Oxidoreduction of protein thiols in redox regulation. Biochem Soc Trans. 2005;33:1378–1381. doi: 10.1042/BST0331378. [DOI] [PubMed] [Google Scholar]
- Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol. 2008;586:6049–6061. doi: 10.1113/jphysiol.2008.160333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EI, Yasuda N, Tarnopolsky MA, Abadi A, Phillips SM. Little change in markers of protein breakdown and oxidative stress in humans in immobilization-induced skeletal muscle atrophy. Appl Physiol Nutr Metab. 2010;35:125–133. doi: 10.1139/H09-137. [DOI] [PubMed] [Google Scholar]
- Grune T, Merker K, Sandig G, Davies KJ. Selective degradation of oxidatively modified protein substrates by the proteasome. Biochem Biophys Res Commun. 2003;305(3):709–718. doi: 10.1016/s0006-291x(03)00809-x. [DOI] [PubMed] [Google Scholar]
- Gustafsson T, Osterlund T, Flanagan JN, von Walden F, Trappe TA, Linnehan RM, Tesch PA. Effects of 3 days unloading on molecular regulators of muscle size in humans. J Appl Physiol. 2010;109:721–727. doi: 10.1152/japplphysiol.00110.2009. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Mathieu O, Krauer R, Claassen H, Armstrong RB, Weibel ER. Design of the mammalian respiratory system. VI Distribution of mitochondria and capillaries in various muscles. Respir Physiol. 1981;44(1):87–111. doi: 10.1016/0034-5687(81)90078-5. [DOI] [PubMed] [Google Scholar]
- Hortobagyi T, Devita P. Mechanisms responsible for the age-associated increase in coactivation of antagonist muscles. Exerc Sport Sci Rev. 2006;34:29–35. doi: 10.1097/00003677-200601000-00007. [DOI] [PubMed] [Google Scholar]
- Ikemoto M, Nikawa T, Kano M, Hirasaka K, Kitano T, Watanabe C, Tanaka R, Yamamoto T, Kamada M, Kishi K. Cysteine supplementation prevents unweighting-induced ubiquitination in association with redox regulation in rat skeletal muscle. Biol Chem. 2002a;383:715–721. doi: 10.1515/BC.2002.074. [DOI] [PubMed] [Google Scholar]
- Ikemoto M, Okamura Y, Kano M, Hirasaka K, Tanaka R, Yamamoto T, Sasa T, Ogawa T, Sairyo K, Kishi K, Nikawa T. A relative high dose of vitamin E does not attenuate unweighting-induced oxidative stress and ubiquitination in rat skeletal muscle. J Physiol Anthropol Appl Human Sci. 2002b;21:257–263. doi: 10.2114/jpa.21.257. [DOI] [PubMed] [Google Scholar]
- Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol. 2004;287:C834–843. doi: 10.1152/ajpcell.00579.2003. [DOI] [PubMed] [Google Scholar]
- Jackson MJ. Redox regulation of adaptive responses in skeletal muscle to contractile activity. Free Radic Biol Med. 2009;47:1267–1275. doi: 10.1016/j.freeradbiomed.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Jackson MJ, Papa S, Bolanos J, Bruckdorfer R, Carlsen H, Elliott RM, Flier J, Griffiths HR, Heales S, Holst B, Lorusso M, Lund E, Oivind Moskaug J, Moser U, Di Paola M, Polidori MC, Signorile A, Stahl W, Vina-Ribes J, Astley SB. Antioxidants, reactive oxygen and nitrogen species, gene induction and mitochondrial function. Mol Aspects Med. 2002;23:209–285. doi: 10.1016/s0098-2997(02)00018-3. [DOI] [PubMed] [Google Scholar]
- Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, Van Der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji LL, Gomez-Cabrera MC, Steinhafel N, Vina J. Acute exercise activates nuclear factor (NF)-κB signaling pathway in rat skeletal muscle. FASEB J. 2004;18:1499–1506. doi: 10.1096/fj.04-1846com. [DOI] [PubMed] [Google Scholar]
- Kirkland RA, Saavedra GM, Cummings BS, Franklin JL. Bax regulates production of superoxide in both apoptotic and nonapoptotic neurons: role of caspases. J Neurosci. 2010;30:16114–16127. doi: 10.1523/JNEUROSCI.2862-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiber M. Body size and metabolic rate. Physiol Rev. 1947;27:511–541. doi: 10.1152/physrev.1947.27.4.511. [DOI] [PubMed] [Google Scholar]
- Koesterer TJ, Dodd SL, Powers S. Increased antioxidant capacity does not attenuate muscle atrophy caused by unweighting. J Appl Physiol. 2002;93:1959–1965. doi: 10.1152/japplphysiol.00511.2002. [DOI] [PubMed] [Google Scholar]
- Kondo H, Miura M, Itokawa Y. Antioxidant enzyme systems in skeletal muscle atrophied by immobilization. Pflugers Arch. 1993;422:404–406. doi: 10.1007/BF00374299. [DOI] [PubMed] [Google Scholar]
- Kondo H, Miura M, Nakagaki I, Sasaki S, Itokawa Y. Trace element movement and oxidative stress in skeletal muscle atrophied by immobilization. Am J Physiol Endocrinol Metab. 1992;262:E583–590. doi: 10.1152/ajpendo.1992.262.5.E583. [DOI] [PubMed] [Google Scholar]
- Lagirand-Cantaloube J, Offner N, Csibi A, Leibovitch MP, Batonnet-Pichon S, Tintignac LA, Segura CT, Leibovitch SA. The initiation factor eIF3-f is a major target for atrogin1/MAFbx function in skeletal muscle atrophy. EMBO J. 2008;27:1266–1276. doi: 10.1038/emboj.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic Biol Med. 2003;35:9–16. doi: 10.1016/s0891-5849(03)00186-2. [DOI] [PubMed] [Google Scholar]
- Lawler JM, Song W, Kwak HB. Differential response of heat shock proteins to hindlimb unloading and reloading in the soleus. Muscle Nerve. 2006;33:200–207. doi: 10.1002/mus.20454. [DOI] [PubMed] [Google Scholar]
- Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- Li YP, Chen Y, Li AS, Reid MB. Hydrogen peroxide stimulates ubiquitin-conjugating activity and expression of genes for specific E2 and E3 proteins in skeletal muscle myotubes. Am J Physiol Cell Physiol. 2003;285:C806–812. doi: 10.1152/ajpcell.00129.2003. [DOI] [PubMed] [Google Scholar]
- Linder N, Rapola J, Raivio KO. Cellular expression of xanthine oxidoreductase protein in normal human tissues. Lab Invest. 1999;79:967–974. [PubMed] [Google Scholar]
- Loughna PT, Goldspink DF, Goldspink G. Effects of hypokinesia and hypodynamia upon protein turnover in hindlimb muscles of the rat. Aviat Space Environ Med. 1987;58:A133–138. [PubMed] [Google Scholar]
- Matuszczak Y, Arbogast S, Reid MB. Allopurinol mitigates muscle contractile dysfunction caused by hindlimb unloading in mice. Aviat Space Environ Med. 2004;75:581–588. [PubMed] [Google Scholar]
- McClung JM, Kavazis AN, Whidden MA, DeRuisseau KC, Falk DJ, Criswell DS, Powers SK. Antioxidant administration attenuates mechanical ventilation-induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling. J Physiol. 2007;585:203–215. doi: 10.1113/jphysiol.2007.141119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motterlini R. Heme oxygenase-1: a key step in counteracting cellular dysfunction. Cell Mol Biol (Noisy-le-grand) 2005;51:343–346. [PubMed] [Google Scholar]
- Moylan JS, Reid MB. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve. 2007;35:411–429. doi: 10.1002/mus.20743. [DOI] [PubMed] [Google Scholar]
- Murton AJ, Constantin D, Greenhaff PL. The involvement of the ubiquitin proteasome system in human skeletal muscle remodelling and atrophy. Biochim Biophys Acta. 2008;1782:730–743. doi: 10.1016/j.bbadis.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Musaro A, Fulle S, Fano G. Oxidative stress and muscle homeostasis. Curr Opin Clin Nutr Metab Care. 2010;13:236–242. doi: 10.1097/MCO.0b013e3283368188. [DOI] [PubMed] [Google Scholar]
- Narici M, Kayser B, Barattini P, Cerretelli P. Effects of 17-day spaceflight on electrically evoked torque and cross-sectional area of the human triceps surae. Eur J Appl Physiol. 2003;90:275–282. doi: 10.1007/s00421-003-0955-7. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Furochi H, Mameoka M, Hirasaka K, Onishi Y, Suzue N, Oarada M, Akamatsu M, Akima H, Fukunaga T, Kishi K, Yasui N, Ishidoh K, Fukuoka H, Nikawa T. Ubiquitin ligase gene expression in healthy volunteers with 20-day bedrest. Muscle Nerve. 2006;34:463–469. doi: 10.1002/mus.20611. [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D, Sheffield-Moore M, Urban RJ, Sanford AP, Aarsland A, Wolfe RR, Ferrando AA. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab. 2004;89:4351–4358. doi: 10.1210/jc.2003-032159. [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D. Interplay of stress and physical inactivity on muscle loss: Nutritional countermeasures. J Nutr. 2006;136(8):2123–2126. doi: 10.1093/jn/136.8.2123. [DOI] [PubMed] [Google Scholar]
- Palazzetti S, Richard MJ, Favier A, Margaritis I. Overloaded training increases exercise-induced oxidative stress and damage. Can J Appl Physiol. 2003;28:588–604. doi: 10.1139/h03-045. [DOI] [PubMed] [Google Scholar]
- Palazzetti S, Rousseau AS, Richard MJ, Favier A, Margaritis I. Antioxidant supplementation preserves antioxidant response in physical training and low antioxidant intake. Br J Nutr. 2004;91:91–100. doi: 10.1079/bjn20031027. [DOI] [PubMed] [Google Scholar]
- Palomero J, Pye D, Kabayo T, Spiller DG, Jackson MJ. In situ detection and measurement of intracellular reactive oxygen species in single isolated mature skeletal muscle fibers by real time fluorescence microscopy. Antioxid Redox Signal. 2008;10:1463–1474. doi: 10.1089/ars.2007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavy-Le Traon A, Heer M, Narici MV, Rittweger J, Vernikos J. From space to Earth: advances in human physiology from 20 years of bed rest studies (1986–2006) Eur J Appl Physiol. 2007;101:143–194. doi: 10.1007/s00421-007-0474-z. [DOI] [PubMed] [Google Scholar]
- Pellegrino MA, D'Antona G, Bortolotto S, Boschi F, Pastoris O, Bottinelli R, Polla B, Reggiani C. Clenbuterol antagonizes glucocorticoid-induced atrophy and fibre type transformation in mice. Exp Physiol. 2004;89(1):89–100. doi: 10.1113/expphysiol.2003.002609. [DOI] [PubMed] [Google Scholar]
- Pierno S, Desaphy JF, Liantonio A, De Bellis M, Bianco G, De Luca A, Frigeri A, Nicchia GP, Svelto M, Leoty C, George AL, Jr, Camerino DC. Change of chloride ion channel conductance is an early event of slow-to-fast fibre type transition during unloading-induced muscle disuse. Brain. 2002;125:1510–1521. doi: 10.1093/brain/awf162. [DOI] [PubMed] [Google Scholar]
- Powers SK, Duarte J, Kavazis AN, Talbert EE. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol. 2010;95:1–9. doi: 10.1113/expphysiol.2009.050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2005;288:R337–344. doi: 10.1152/ajpregu.00469.2004. [DOI] [PubMed] [Google Scholar]
- Powers SK, Kavazis AN, Levine S. Prolonged mechanical ventilation alters diaphragmatic structure and function. Crit Care Med. 2009;37:S347–353. doi: 10.1097/CCM.0b013e3181b6e760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. J Appl Physiol. 2007;102:2389–2397. doi: 10.1152/japplphysiol.01202.2006. [DOI] [PubMed] [Google Scholar]
- Powers SK, Shanely RA, Coombes JS, Koesterer TJ, McKenzie M, Van Gammeren D, Cicale M, Dodd SL. Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. J Appl Physiol. 2002;92:1851–1858. doi: 10.1152/japplphysiol.00881.2001. [DOI] [PubMed] [Google Scholar]
- Reich KA, Chen YW, Thompson PD, Hoffman EP, Clarkson PM. Forty-eight hours of unloading and 24 h of reloading lead to changes in global gene expression patterns related to ubiquitination and oxidative stress in humans. J Appl Physiol. 2010;109:1404–1415. doi: 10.1152/japplphysiol.00444.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MB, Khawli FA, Moody MR. Reactive oxygen in skeletal muscle. III. Contractility of unfatigued muscle. J Appl Physiol. 1993;75:1081–1087. doi: 10.1152/jappl.1993.75.3.1081. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Selby A, Atherton P, Smith K, Kumar V, Glover EL, Philips SM. Facts, noise and wishful thinking: muscle protein turnover in aging and human disuse atrophy. Scand J Med Sci Sports. 2010;20:5–9. doi: 10.1111/j.1600-0838.2009.00967.x. [DOI] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittweger J, Simunic B, Bilancio G, De Santo NG, Cirillo M, Biolo G, Pisot R, Eiken O, Mekjavic IB, Narici M. Bone loss in the lower leg during 35 days of bed rest is predominantly from the cortical compartment. Bone. 2009;44:612–618. doi: 10.1016/j.bone.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 2008;23:160–170. doi: 10.1152/physiol.00041.2007. [DOI] [PubMed] [Google Scholar]
- Sastre J, Asensi M, Gasco E, Pallardo FV, Ferrero JA, Furukawa T, Vina J. Exhaustive physical exercise causes oxidation of glutathione status in blood: prevention by antioxidant administration. Am J Physiol Regul Integr Comp Physiol. 1992;263:R992–995. doi: 10.1152/ajpregu.1992.263.5.R992. [DOI] [PubMed] [Google Scholar]
- Senf SM, Dodd SL, McClung JM, Judge AR. Hsp70 overexpression inhibits NF-κB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB J. 2008;22:3836–3845. doi: 10.1096/fj.08-110163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servais S, Letexier D, Favier R, Duchamp C, Desplanches D. Prevention of unloading-induced atrophy by vitamin E supplementation: links between oxidative stress and soleus muscle proteolysis? Free Radic Biol Med. 2007;42:627–635. doi: 10.1016/j.freeradbiomed.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanely RA, Van Gammeren D, Deruisseau KC, Zergeroglu AM, McKenzie MJ, Yarasheski KE, Powers SK. Mechanical ventilation depresses protein synthesis in the rat diaphragm. Am J Respir Crit Care Med. 2004;170:994–999. doi: 10.1164/rccm.200304-575OC. [DOI] [PubMed] [Google Scholar]
- Shanely RA, Zergeroglu MA, Lennon SL, Sugiura T, Yimlamai T, Enns D, Belcastro A, Powers SK. Mechanical ventilation-induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. Am J Respir Crit Care Med. 2002;166:1369–1374. doi: 10.1164/rccm.200202-088OC. [DOI] [PubMed] [Google Scholar]
- Shang F, Gong X, Taylor A. Activity of ubiquitin-dependent pathway in response to oxidative stress. Ubiquitin-activating enzyme is transiently up-regulated. J Biol Chem. 1997;272:23086–23093. doi: 10.1074/jbc.272.37.23086. [DOI] [PubMed] [Google Scholar]
- Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- Silva LA, Pinho CA, Scarabelot KS, Fraga DB, Volpato AM, Boeck CR, De Souza CT, Streck EL, Pinho RA. Physical exercise increases mitochondrial function and reduces oxidative damage in skeletal muscle. Eur J Appl Physiol. 2009;105:861–867. doi: 10.1007/s00421-008-0971-8. [DOI] [PubMed] [Google Scholar]
- Silva LA, Pinho CA, Silveira PC, Tuon T, De Souza CT, Dal-Pizzol F, Pinho RA. Vitamin E supplementation decreases muscular and oxidative damage but not inflammatory response induced by eccentric contraction. J Physiol Sci. 2010;60:51–57. doi: 10.1007/s12576-009-0065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu PM, Pistilli EE, Alway SE. Age-dependent increase in oxidative stress in gastrocnemius muscle with unloading. J Appl Physiol. 2008;105:1695–1705. doi: 10.1152/japplphysiol.90800.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Reid MB. Redox modulation of contractile function in respiratory and limb skeletal muscle. Respir Physiol Neurobiol. 2006;151:229–241. doi: 10.1016/j.resp.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Smuder AJ, Kavazis AN, Hudson MB, Nelson WB, Powers SK. Oxidation enhances myofibrillar protein degradation via calpain and caspase-3. Free Radic Biol Med. 2010;49:1152–1160. doi: 10.1016/j.freeradbiomed.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- Strobel NA, Peake JM, Matsumoto A, Marsh SA, Coombes JS, Wadley GD. Antioxidant supplementation reduces skeletal muscle mitochondrial biogenesis. Med Sci Sports Exerc. 2011 doi: 10.1249/MSS.0b013e318203afa3. (in press) [DOI] [PubMed] [Google Scholar]
- Symons TB, Sheffield-Moore M, Chinkes DL, Ferrando AA, Paddon-Jones D. Artificial gravity maintains skeletal muscle protein synthesis during 21 days of simulated microgravity. J Appl Physiol. 2009;107:34–38. doi: 10.1152/japplphysiol.91137.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesch PA, von Walden F, Gustafsson T, Linnehan RM, Trappe TA. Skeletal muscle proteolysis in response to short-term unloading in humans. J Appl Physiol. 2008;105:902–906. doi: 10.1152/japplphysiol.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol. 1990;68:1–12. doi: 10.1152/jappl.1990.68.1.1. [DOI] [PubMed] [Google Scholar]
- Trappe SW, Trappe TA, Lee GA, Widrick JJ, Costill DL, Fitts RH. Comparison of a space shuttle flight (STS-78) and bed rest on human muscle function. J Appl Physiol. 2001;91:57–64. doi: 10.1152/jappl.2001.91.1.57. [DOI] [PubMed] [Google Scholar]
- Urso ML, Chen YW, Scrimgeour AG, Lee PC, Lee KF, Clarkson PM. Alterations in mRNA expression and protein products following spinal cord injury in humans. J Physiol. 2007;579:877–892. doi: 10.1113/jphysiol.2006.118042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189:41–54. doi: 10.1016/s0300-483x(03)00151-3. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci STKE. 2006;2006:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- Vina J, Gimeno A, Sastre J, Desco C, Asensi M, Pallardo FV, Cuesta A, Ferrero JA, Terada LS, Repine JE. Mechanism of free radical production in exhaustive exercise in humans and rats; role of xanthine oxidase and protection by allopurinol. IUBMB Life. 2000;49:539–544. doi: 10.1080/15216540050167098. [DOI] [PubMed] [Google Scholar]
- Whidden MA, Smuder AJ, Wu M, Hudson MB, Nelson WB, Powers SK. Oxidative stress is required for mechanical ventilation-induced protease activation in the diaphragm. J Appl Physiol. 2010;108:1376–1382. doi: 10.1152/japplphysiol.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zergeroglu MA, McKenzie MJ, Shanely RA, Van Gammeren D, DeRuisseau KC, Powers SK. Mechanical ventilation-induced oxidative stress in the diaphragm. J Appl Physiol. 2003;95:1116–1124. doi: 10.1152/japplphysiol.00824.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


