Non-technical summary
Repeated activity of muscle fibres generates reactive oxygen species (ROS), and these may affect many intracellular processes, possibly modifying force production both in the short-term and the long-term. ROS applied to muscle fibres can cause increased or decreased force responses, but the mechanisms and sites involved are not well understood. In the experiments here, the surface membrane was removed from individual muscle fibres and various ROS applied. In this way it was possible to determine the effects on each of the steps involved in muscle contraction. The various ROS were found to affect force responses primarily by increasing or decreasing the sensitivity of the contractile proteins to calcium ions. The ROS did not appreciably affect electrical excitability, calcium release from the internal stores or the maximum force producing capacity of the contractile proteins unless applied at very high concentration for a prolonged period. Interestingly, one particular ROS known to be generated inside muscle fibres did disrupt signalling and contraction, and this might account for the ability of ROS to cause long-lasting muscle fatigue in certain circumstances.
Abstract
Abstract
S-Nitrosoglutathione (GSNO) is generated in muscle and may S-glutathionylate and/or S-nitrosylate various proteins involved in excitation–contraction (EC) coupling, such as Na+-K+-ATPases, voltage-sensors (VSs) and Ca2+ release channels (ryanodine receptors, RyRs), possibly changing their properties. Using mechanically skinned fibres from rat extensor digitorum longus muscle, we sought to identify which EC coupling processes are most susceptible to GSNO-modulated changes and whether these changes could be important in muscle function and fatigue. For comparison, we examined the effect of other oxidation, nitrosylation, or glutathionylation treatments (S-nitroso-N-acetyl-penicillamine (SNAP), hydrogen peroxide, 2,2′-dithiodipyridine and reduced glutathione) on twitch and tetanic force, action potential (AP) repriming, sarcoplasmic reticulum (SR) Ca2+ loading and leakage, and contractile apparatus properties. None of the treatments detectably altered AP repriming, indicating that t-system excitability was relatively insensitive to such oxidative modification. Importantly, the overall effect on twitch and tetanic force of a given treatment was determined primarily by its action on Ca2+ sensitivity of the contractile apparatus. For example, S-nitrosylation with the NO• donor, SNAP, caused matching decreases in the contractile Ca2+ sensitivity and twitch response, and GSNO applied ∼10 min after preparation had very similar effects. The only exception was when GSNO was applied immediately after preparation, which resulted in irreversible decreases in twitch and tetanic responses even though it concomitantly increased Ca2+ sensitivity by ∼0.1 pCa units, the latter evidently due to S-glutathionylation of the contractile apparatus. This decrease in AP-mediated force responses was due to impaired VS–RyR coupling and was accompanied by increased Ca2+ leakage through RyRs. Such oxidation-related impairment of coupling could be responsible for prolonged low frequency fatigue in certain circumstances.
Introduction
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are generated in skeletal muscle with normal activity and also in pathological conditions, and are thought to affect muscle function both acutely and over the long term (Smith & Reid, 2006; Supinski & Callahan, 2007; Allen et al. 2008; Powers & Jackson, 2008). To identify such actions, exogenous ROS and RNS have been applied in a range of preparations, including in whole muscles, isolated intact fibres, skinned fibres, and various isolated protein preparations, each type of study having advantages and disadvantages. ROS and RNS might affect muscle function by oxidation, S-nitrosylation or S-glutathionylation of one or many different possible target proteins, the primary ones likely to acutely affect excitation–contraction (EC) coupling being the Na+/K+-ATPases in the transverse-tubular (t-) system, the t-system voltage-sensor molecules (dihydropyridine receptors), the Ca2+ release channels (ryanodine receptors, RyRs) in the sarcoplasmic reticulum (SR), the SERCA pumps that return Ca2+ to the SR, and of course various elements of the contractile apparatus itself.
Two cysteine residues on the myosin heads (called SH1 and SH2) are reactive to many different ROS, RNS and alkylating agents, and such reactions are said to result in decreased myosin ATPase activity, velocity of shortening and maximum force (Reisler et al. 1974; Srivastava & Wikman-Coffelt, 1980; Tiago et al. 2006), though other sites on myosin may also be involved (Prochniewicz et al. 2008; Nogueira et al. 2009). It was noted in one early study (Crowder & Cooke, 1984), however, that such oxidation effects occurred far less readily with myosin in situ in the contractile apparatus than in isolated myosin preparations, making it difficult to be certain about the extent and circumstances in which such myosin dysfunction occurs. Experiments on intact fast-twitch fibres, in which force and intracellular Ca2+ were simultaneously measured, found that application of hydrogen peroxide (H2O2, 300 μm and lower) (Andrade et al. 1998a, 2001) or nitric oxide donors (Andrade et al. 1998b) in fact had little if any effect on maximum force production and affected submaximal tetanic force primarily by increasing or decreasing the Ca2+ sensitivity of the contractile apparatus. In those experiments, however, the applied agents are likely to have reacted with various intracellular constituents, in particular glutathione (GSH), and so the actual reactive species and reactions involved in the observed force changes is uncertain. Some further insight was provided by experiments in skinned fibres comparing the effects on the contractile apparatus of applying H2O2 alone, or H2O2 in various combinations with myoglobin and GSH (Lamb & Posterino, 2003; Murphy et al. 2008). Those experiments indicated that the decreased Ca2+ sensitivity of the contractile apparatus was probably due not to H2O2 itself but rather to hydroxyl (OH•) or related radicals generated in the cytoplasm, and that the increased Ca2+ sensitivity likely involved S-glutathionylation of contractile apparatus proteins (i.e. formation of protein-SSG), presumably due either to GSH reacting with oxidized cysteine residues and/or generation of GSH-derived species (e.g. GS• or GSNO) that then S-glutathionylated cysteine residues (Halliwell & Gutteridge, 2007; Lamb & Westerblad, 2011). The case of GSNO is particularly interesting, as it potentially could cause either or both S-glutathionylation and S-nitrosylation, with these two actions expected to have opposite effects on Ca2+ sensitivity. Recent experiments using skinned fibres reported that GSNO caused only decreases in contractile Ca2+ sensitivity, with no change in maximum force, much like another NO• donor S-nitroso-N-acetyl-penicillamine (SNAP) (Spencer & Posterino, 2009). On the other hand, another recent study (Nogueira et al. 2009) found that a more prolonged GSNO treatment caused S-nitrosylation of the myosin heads in both skinned fibres and isolated myosin preparations, resulting in major inhibition of the myosin Mg-ATPase activity, which presumably would result in greatly decreased maximum force production.
ROS and RNS also readily act on the Ca2+ release channel, and were variously found to potentiate or inhibit channel opening in SR vesicle and isolated channel preparations (Hart & Dulhunty, 2000; Pessah et al. 2002; Hidalgo et al. 2005). Nevertheless, skinned fibre experiments showed that even though H2O2-induced oxidation of the release channels sensitized their activation by caffeine and Ca2+-induced Ca2+ release (CICR), it did not appreciably alter the amount of Ca2+ released to physiological stimulation where action potentials trigger the voltage sensors to activate the release channels (Posterino et al. 2003), in agreement with findings with tetanic stimulation in intact fibres (Andrade et al. 1998a). GSNO causes both S-glutathionylation and S-nitrosylation of the release channels, acting on the different cysteine residues in different ways, and stimulating channel opening by two distinctly different mechanisms (Aracena et al. 2003; Aracena-Parks et al. 2006). Whether such modifications affect voltage-sensor activation of Ca2+ release however is less clear. Treatment of intact fibres with NO• donors increased tetanic Ca2+ transients in one study (Andrade et al. 1998b) whereas it decreased Ca2+ release to voltage-step stimuli in another study (Pouvreau et al. 2004); the extent of S-nitrosylation and S-glutathionylation occurrence is not known in either case. One further finding of note is that fatiguing stimulation caused a long-lasting decrease in SR Ca2+ release in fibres from wild-type mice but not from mice over-expressing superoxide dismutase 2, indicating that excessive production of superoxide can lead to ROS-mediated impairment of Ca2+ release and prolonged low frequency force depression (Bruton et al. 2008). The reactive species underlying those effects however were not identified.
Here we use mechanically skinned muscle fibres with functional EC coupling to examine the effects of GSNO not only on the twitch and tetanic force responses, but also on each of the major steps in the EC coupling sequence that underlie those force responses. In this way it was possible to identify which steps were most affected by a given treatment and how this affected the overall response. It was hypothesized that GSNO would affect twitch and tetanic force primarily by altering the Ca2+ sensitivity of the contractile apparatus, rather than by affecting Ca2+ release, fibre excitability or maximum Ca2+-activated force production, but that these processes might be also affected if the agent was applied at higher concentration for a longer period. We investigated whether the effects of GSNO treatment were due primarily to glutathionylation or to nitrosylation by comparing its effects to those of treatments that specifically exert either one or other action. Finally, as GSNO is known to undergo transformations in aqueous solutions (Huang & Huang, 2002), and that its production of NO• varies over time, we examined whether its effects varied with time after its preparation.
Methods
Preparations and force recording
Male Long–Evans hooded rats (≥5 months old) were killed by overdose of isoflurane (4% v/v) in a glass chamber. The experiments were carried out in accordance with the Australian National Health and Medical Research Council's ‘Australian code of practice for the care and use of animals for scientific purposes’, and with approval of the La Trobe University Animal Ethics Committee. EDL muscles were rapidly excised and pinned at their resting length under paraffin oil (Ajax Chemicals, Sydney, Australia) in a Petri dish. The muscles were kept cool (∼10°C) on an icepack. Individual fibre segments were mechanically skinned with jeweller's forceps and then mounted at 120% of resting length on a force transducer (AME801, SensoNor, Horten, Norway) with a resonance frequency >2 kHz. The skinned fibre segment was then equilibrated for 2 min in a perspex bath containing 2 ml of a K-HDTA solution or relaxing solution depending on the experiment being conducted (see below). Force responses were recorded using a Bioamp pod and Powerlab 4/20 series hardware (ADInstruments, Sydney, Australia). All experiments were performed at room temperature (∼23 ± 2°C), and values are presented as mean ± standard error of the mean (s.e.m.), with n denoting the number of fibres examined. Statistical significance (P < 0.05) was determined with Student's two-tailed paired t test.
Skinned fibre solutions
All chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA) unless otherwise stated. As previously described (Lamb & Stephenson, 1994), the ‘standard’ (K-HDTA) solution used for depolarization and caffeine experiments contained (in mm): hexa-methylene-diamine-tetraacetate (HDTA2−; Fluka, Buchs, Switzerland), 50; total ATP, 8; creatine phosphate (CrP), 10; Na+, 36; K+, 126; total Mg2+, 8.5; total EGTA, 0.050; Hepes, 90; pH 7.1 and pCa (−log10[Ca2+]) 6.9, except where stated. For examination of contractile apparatus properties, solutions similar to the standard K-HDTA solution were made in which all HDTA was replaced with EGTA or CaEGTA for very strong Ca2+ buffering. The maximum Ca2+-activating solution ‘max’ contained 50 mm CaEGTA and had a pCa ∼4.5, and the ‘relaxing’ solution containing 50 mm free EGTA had a pCa >10, with total Mg2+ adjusted to maintain 1 mm free (see Stephenson & Williams, 1981 for apparent affinity constants). These two solutions were mixed in an appropriate ratio to produce solutions with pCa in the range 6.7–4.5. All solutions had an osmolality of 295 ± 5 mosmol kg−1.
Oxidizing and reducing solutions and [NO•]
S-Nitrosoglutathione (GSNO) might cause either S-nitrosylation or S-glutathionylation of a protein thiol (Fig. 1). GSNO was dissolved in solution and either applied immediately to the fibre (i.e. within 30 s, termed ‘GSNOimm’) or applied ∼10 min later (i.e. a 10 min delay, termed ‘GSNOdel’), always with an exposure period of 2 min; it was found that the effects of the GSNO treatment was quite different in the two cases. The commonly used NO• donor compound SNAP was used to produce S-nitrosylation (Fig. 1). SNAP was always applied immediately after addition to solution (i.e. within 30 s), and the exposure period was either 2 min (denoted as ‘SNAP’ in Tables 1 and 2) or 10 min (SNAP10 min). The amount of NO• liberated from 1 mm GSNO and 1 mm SNAP in standard solution at 23 °C was measured using an Apollo 4000 NO• analyser system (World Precision Instruments). The [NO•] on dissolving 1 mm GSNO in standard solution was initially ∼0.5 μm and rose steadily to a peak [NO•] of ∼6 μm after 6 min and then declined with a half-life of ∼10 min. When SNAP (1 mm) was dissolved in standard solution, [NO•] was initially ∼0.4 μm and then rose approximately linearly to a peak of ∼1.2 μm after 25 min and then decayed with a half-life of ∼20 min. Based on these experiments, the average [NO•] during the 2 min exposure periods was estimated to be ∼0.15 and 3 μm for 0.1 and 2 mm GSNOimm, respectively, and ∼4 μm for 2 mm GSNOdel. The average [NO•] estimates for the SNAP treatments were ∼0.05, 1 and 5 μm for the 2 min exposures to 0.1, 2 and 10 mm SNAP, and ∼1.6 and 8 μm for the 10 min exposures to 2 and 10 mm SNAP, respectively.
Figure 1. S-Nitrosylation or S-glutathionylation of a protein thiol by different treatments.
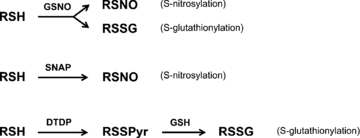
Protein thiol denoted as ‘RSH’. GSNO reacting with the thiol can produce either S-nitrosylation (RSNO) or S-glutathionylation (RSSG), depending on the conditions and the particular thiol involved. Treatment with NO• donor, SNAP, produces only S-nitrosylation. Successive treatments with dithiodipyridine (DTDP) and GSH produces glutathionylation in a two step reaction: DTDP oxidizes the thiol, leaving a pyridine group (Pyr) linked to the protein by a disulphide bond (RSSPyr), and subsequent treatment with GSH produces RSSG by a thiol-disulphide exchange reaction. DTT reduces both RSNO and RSSG back to RSH (not shown).
Table 1.
Summarised data of twitch, tetanus and repriming period following various treatments
| Treatment set | Twitch peak (%) | Tetanus peak (%) | RP (ms) | |
|---|---|---|---|---|
| 1. | Control | 100 (3) | 100 (3) | 4.7 ± 0.3 (3) |
| 100 μm SNAP | 98.4 ± 2.2 | 99.7 ± 0.9 | 4.3 ± 0.3 | |
| 2. | Control | 100 (6) | 100 (6) | 4.6 ± 0.3 (6) |
| 2 mm SNAP | 58.5 ± 5.9* | 82.4 ± 4.8* | 4.2 ± 0.2 | |
| 10 mm DTT | 79.4 ± 10.1*† | 91.6 ± 3.5*† | (not examined) | |
| 3. | Control | 100 (7) | 100 (6) | 4.2 ± 0.2 (6) |
| 10 mm SNAP | 54.2 ± 9.2* | 88.3 ± 4.3* | 4.3 ± 0.2 | |
| 10 mm DTT | 86.9 ± 7.8† | 101.9 ± 3.0† | 4.0 ± 0.3 | |
| 4. | Control | 100 (3) | 100 (3) | 4.3 ± 0.3 (3) |
| 100 μm DTDP | 69.8 ± 5.5* | 95.8 ± 0.4* | 4.0 ± 0.0 | |
| 5 mm GSH | 157.7 ± 5.8* | 111.4 ± 5.6 | 4.0 ± 0.0 | |
| 10 mm DTT | 99.0 ± 2.4† | 91.2 ± 14.9 | 4.3 ± 0.3 | |
| 5. | Control | 100 (5) | 4.2 ± 0.2 (5) | |
| 10 mm H2O2 (5 min) | 108.2 ± 4.6 | (not examined) | 4.2 ± 0.2 | |
| 6. | Control | 100 (9) | 100 (9) | 4.6 ± 0.3 (5) |
| 2 mm GSNOdel. | 65.2 ± 4.7* | 91.2 ± 2.1* | 4.4 ± 0.3 | |
| 10 mm DTT | 89.7 ± 5.4† | 96.5 ± 4.5 | 4.2 ± 0.2 | |
| 7. | Control | 100 (4) | 100 (4) | 4.5 ± 0.5 (4) |
| 100 μm GSNOimm. | 111.0 ± 6.9 | 102.3 ± 6.0 | 4.3 ± 0.6 | |
| 8. | Control | 100 (20) | 100 (17) | 3.5 ± 0.6 (9) |
| 2 mm GSNOimm. | 81.3 ± 8.7* | 84.8 ± 5.5* | 3.2 ± 0.6 |
Data are mean ±s.e.m. values of given parameter after the indicated treatment expressed relative to initial control level prior to any treatment. Fibres given up to three different successive treatments, as indicated in the given group; number of fibres (n) shown in brackets. Twitch and tetanic force in control conditions were 27 ± 4% (n = 57) and 92 ± 2% (n = 48), respectively, of maximum Ca2+-activated force (at pCa 4.5). The repriming period (RP), a measure of the AP refractory period, was measured as the minimum interpulse interval between a pair of pulses needed to elicit >50% of maximal incremental increase in twitch size (see Fig. 3C).
Significant difference (P < 0.05) between value for indicated treatment relative to initial control level.
Value following DTT treatment significantly different from preceding value.
Table 2.
Summary of contractile apparatus properties following various treatments
| Treatment | Δmax, % | Δmax, % (After DTT) | ΔpCa50 | ΔpCa50 (After DTT) | Δh | Δh (After DTT) | n |
|---|---|---|---|---|---|---|---|
| 1. 100 μm SNAP | −1.5 ± 0.9 | +1.5 ± 0.4† | +0.001 ± 0.005 | −0.003 ± 0.005 | +2.1 ± 0.3* | +2.5 ± 0.5* | 3 |
| 2. 2 mm SNAP | −1.4 ± 0.9 | +1.3 ± 0.3† | −0.055 ± 0.013* | −0.012 ± 0.005† | −0.7 ± 1.0 | −0.4 ± 0.3 | 3 |
| 3. 2 mm SNAP10min | −1.1 ± 0.8 | +2.1 ± 2.0 | −0.131 ± 0.007* | −0.038 ± 0.012*† | +0.9 ± 1.3 | +0.2 ± 0.3 | 4 |
| 4. 10 mm SNAP | −0.4 ± 0.3 | −1.0 ± 1.0 | −0.072 ± 0.014* | −0.027 ± 0.005*† | +0.3 ± 0.7 | +0.3 ± 0.7 | 5 |
| 5. 10 mm SNAP10min | +0.3 ± 0.6 | +0.3 ± 0.9 | −0.104 ± 0.006* | −0.033 ± 0.005*† | +0.9 ± 0.8 | −0.4 ± 0.3 | 4 |
| 6. 2 mm GSNOdel | 0.0 ± 0.2 | +1.1 ± 0.3† | −0.059 ± 0.012* | −0.014 ± 0.004*† | −0.8 ± 0.7 | +0.2 ± 0.3 | 4 |
| 7. 100 μm GSNOimm | −1.6 ± 0.5 | +1.0 ± 1.1 | +0.057 ± 0.026 | −0.001 ± 0.008 | −2.2 ± 1.1 | +0.7 ± 0.9 | 3 |
| 8. 2 mm GSNOimm | −0.3 ± 0.7 | +1.6 ± 0.2† | +0.136 ± 0.022* | +0.042 ± 0.013*† | −0.2 ± 0.1* | −0.1 ± 0.5 | 6 |
Mean (±s.e.m.) change in maximum Ca2+-activated force (max), pCa50 and h following indicated treatment relative to pre-treatment (control) level. After DTT: response after 10 min with 10 mm DTT. Exposure time 2 min, except where indicated as 10 min. n, number of fibres. Mean control pCa50 and h (before any treatment): 5.802 ± 0.011 and 5.8 ± 0.3 (n = 32), respectively.
Significant difference between value for indicated treatment relative to initial control level.
Value following DTT treatment significantly different from before DTT treatment. The apparent increase in h (steepness) in three fibres after 100 μm SNAP (treatment 1), which was not reversed with DTT, arose due to only small changes at the foot of force–pCa relationships and possibly just reflects minor variability in the measurements.
GSNO (2 and 10 mm) and SNAP (2 and 10 mm) were dissolved directly into K-HDTA or K-EGTA solution at the desired final concentration, and the 100 μm GSNO and SNAP solutions were made by 20-fold dilution of the respective 2 mm stock. Hydrogen peroxide (H2O2) was added from a 30% aqueous stock solution to the experimental solutions at a final concentration of 10 mm. A 100 mm stock of reduced glutathione (GSH) was made in standard solution with pH re-adjusted to 7.10 with KOH, and then diluted 20-fold to give 5 mm in the final solution. A 100 mm stock solution of 2,2′-dithiodipyridine (DTDP) was made in absolute ethanol and diluted 1000-fold in the final solution to 100 μm; matching control solutions with the same amount of ethanol (0.1%) had no noticeably different effect than controls without ethanol. To assess the reversibility of effects, dithiothreitol (DTT) was added to K-HDTA or relaxing solution at 10 mm final concentration from a 1 m stock made in double distilled water. Fibres were never activated in the presence of any of the treatments and were simply exposed to each treatment, washed in standard solution/relaxing solution as appropriate, and then transferred back into the solutions in which the force responses were elicited.
t-system excitability experiments
After being pre-equilibrated in standard K-HDTA solution (2 min) the mounted fibre segment was positioned parallel to, and midway between, two platinum electrodes in a stimulating chamber containing 135 μl of the standard solution. An in-house stimulator was used to apply brief electric field pulses (duration, 1 ms; 75 V cm−1) to generate APs in the sealed t-system synchronously along the entire length of the fibre segment. Supramaximal electric field pulses were used that were ∼2.5-fold greater than that required to elicit maximum twitch force (see Dutka et al. 2008). Twitch (single pulse) and tetani (50 Hz) were elicited in standard K-HDTA solution before and after each treatment, with the fibre washed in the K-HDTA solution for >10 s before stimulation. Additionally, the repriming period (RP) of APs in the t-system (i.e. the recovery time needed to elicit a second AP) was determined by applying pairs of supra-maximal pulses with various interpulse intervals (i.e. 1–20 ms) as described previously (Dutka & Lamb, 2007b); the RP was measured as the minimum interpulse interval needed to elicit >50% of the maximum incremental increase in twitch size to a pulse pair (see Fig. 3C).
Figure 3. Effects of DTDP and GSH on twitch, tetani and t-system excitability.
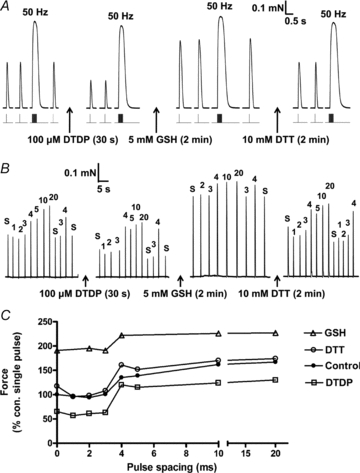
A, twitch and tetanic (50 Hz) responses in an EDL fibre elicited before treatment (control) and after successive treatments with DTDP (100 μm, 30 s), GSH (5 mm, 2 min) and DTT (10 mm, 2 min). Twitch force was decreased by oxidation with DTDP and greatly potentiated by glutathionylation with GSH. These changes were explicable by alterations in Ca2+ sensitivity of the contractile apparatus, and were fully reversed by DTT treatment. B, twitch response to single electrical pulses (‘s’) or to pairs of pulses with interpulse intervals of 1–20 ms (shown above responses), before and after each treatment; twitch force increased substantially with interpulse intervals of 4 ms or longer in all cases, indicative of the repriming period required for the second pulse to also trigger an AP. C, twitch responses from B, all expressed relative to the response to a single pulse under control conditions.
SR Ca2+-release experiments using caffeine and low [Mg2+]
After 2 min equilibration in the ‘standard’ (K-HDTA based) solution, the SR was depleted of all releasable Ca2+ by first pre-equilibrating the fibre segment in standard solution with 0.25 or 0.5 mm EGTA (for 10 s) and then transferring the fibre into the full release solution (FRS) containing 30 mm caffeine, 50 μm free Mg2+ and the same EGTA (i.e. 0.25 or 0.5 mm). The time integral (i.e. area) of the force response is indicative of the relative amount of Ca2+ loaded into the SR (Lamb & Cellini, 1999). The endogenous level of SR Ca2+ was gauged from the response elicited when emptying the SR of the freshly skinned fibre in the FRS, and then the SR was reloaded with Ca2+ (in standard solution at pCa 6.7 or in some cases at pCa 6.4 with 1 mm total EGTA) for the period needed to elicit a similar sized response when emptying the SR again. Additionally, the SR was loaded with Ca2+ for various set times up to 3 min at pCa 6.7 or pCa 6.4, the 3 min load time considered to produce close to maximal Ca2+ loading of the SR for the given conditions (Fryer & Stephenson, 1996; Murphy et al. 2009). Such assessment of the relationship between load time and SR Ca2+ content over its full range provided information about the balance between SR Ca2+ uptake and SR Ca2+ leak (see Fig. 9). After each exposure to the FRS and after treatments, the fibre was washed for 1 min in standard solution with 0.5 mm EGTA before reloading. The load–release–wash cycle was repeated twice before and after each treatment (e.g. exposure to 2 mm GSNOimm). This procedure has been extensively described elsewhere (Lamb et al. 2001). See figure legends for the specific protocol used for the examples shown. In some experiments the free [Mg2+] in the load solution was raised from the normal level of 1 mm to 10 mm by increasing the total Mg2+ to 22.9 mm (Lamb & Stephenson, 1994).
Figure 9. Mean data on effect of GSNO and SNAP on SR Ca2+ accumulation.
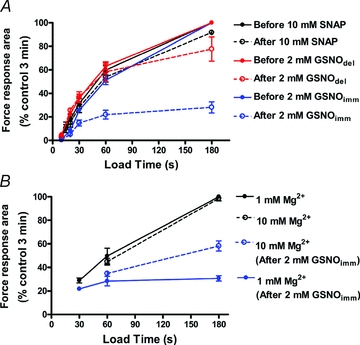
A, mean data showing the effects of GSNOimm (blue, n = 8 fibres), GSNOdel (red, n = 3) and SNAP (black, n = 3) treatments on SR Ca2+ content after loading at pCa 6.7 for indicated times (as in Fig. 8); all force response areas expressed relative to that found with 3 min loading before treatment in same fibre. Fibres treated with DTT after indicated treatment, in order to reverse any change in contractile apparatus sensitivity. Only GSNOimm treatment decreased net Ca2+ uptake by the SR. Different fibres used for each of the three treatments. B, mean data as in panel A, showing that raising the free [Mg2+] in the load solution from the normal level (1 mm) to 10 mm (broken lines), in order to reduce Ca2+-induced Ca2+ release and leak through RyRs, increased net Ca2+ uptake at long load times in GSNOimm-treated fibres (values obtained in each of 5 EDL fibres, both with 1 mm and 10 mm Mg2+ and both before and after treatment).
Contractile apparatus experiments and analysis
The force–Ca2+ relationship was determined in each fibre as previously described (Trinh & Lamb, 2006; Murphy et al. 2008) by exposing the skinned fibre segment to a sequence of solutions heavily buffered at progressively higher free [Ca2+] (pCa 10 to 4.5) until maximum force was elicited, and then the fibre was fully relaxed again in the relaxing solution. This procedure was performed twice before (‘control’) and twice after each treatment to verify reproducibility and also gauge any small changes occurring with repeated activation and over time. Force produced at each [Ca2+] within a given sequence was expressed relative to maximum force generated in that same sequence, and analysed by individually fitting a Hill curve to each sequence, for each fibre segment, using GraphPad Prism 4 software, yielding separate values of pCa at half-maximum force (pCa50) and the Hill coefficient (h) for every case. Maximum force reached in each force–[Ca2+] sequence was expressed relative to the control level before any treatment in the given fibre, after correcting for the small decline occurring with each repetition of the force staircase (typically ∼2 to 3% in EDL fibres), as gauged from the initial control repetitions in the given fibre (see also Murphy et al. 2008).
Results
Effects of nitrosylation and glutathionylation on twitch and tetanic responses and t-system excitability
As GSNO treatment can result in either or both S-nitrosylation or S-glutathionylation, we first investigated the effects of treatments that should have only one or other such action (Fig. 1). Application of the S-nitrosylating agent SNAP at 2 mm for 2 min, which generates an average [NO•] of ∼1 μm (see Methods), decreased the twitch response to ∼59% of control, with much less effect on the tetanic (50 Hz) response (Fig. 2; mean data in Table 1 set 2); these changes were largely reversed by treatment with the reducing agent, DTT (10 mm, 2 min). These effects of the SNAP treatment could be entirely accounted for by its action directly on the contractile apparatus (see Discussion), which displayed a reversible decrease in Ca2+ sensitivity (mean ΔpCa50 of −0.055 pCa units, which corresponds to an increase in Ca50 of ∼14%) with no change in maximum force (see mean data in Table 2, set 2). Application of SNAP at a 5-fold higher concentration (10 mm) for 2 min (estimated [NO•]∼5 μm) caused similar or only slightly greater decrease in twitch force (Table 1, set 3) and Ca2+ sensitivity (Table 2, set 4). Prolonging the SNAP exposure from 2 to 10 min resulted in ∼1.5- to 2-fold greater decrease in Ca2+ sensitivity, again with no change in maximum force (Table 2, sets 3 & 5); the effects on twitch and tetanic force of these comparatively long exposures was not investigated. Application of a low concentration of SNAP (100 μm) for 2 min, which generates very little NO• (estimated as only ∼0.05 μm), had little or no effect on twitches, tetani or the contractile properties (set 1 in both Tables 1 and 2).
Figure 2. SNAP treatment decreases twitch size in an EDL fibre.
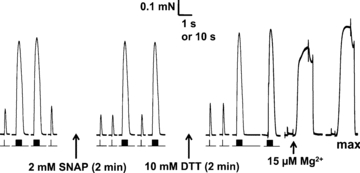
Twitch (single pulse) and tetanic responses (50 Hz, 20 pulses) elicited in a skinned EDL fibre before and after exposure to NO• donor SNAP (2 mm for 2 min), and also after treatment with DTT. Stimulus timing shown under force trace. Responses elicited in standard K-HDTA solution. Lowering free [Mg2+] from 1 mm to 15 μm triggered a large and prolonged release of Ca2+ from SR. Maximum Ca2+-activated force (‘max’) measured at pCa 4.5 (and 1 mm free Mg2+). Time scale: 1 s for twitches and tetani, and 10 s for 15 μm free Mg2+ and max responses.
Our laboratory has previously shown that treatment of skinned EDL fibres with the oxidant DTDP (100 μm) causes a decrease in Ca2+ sensitivity of the contractile apparatus by ∼−0.06 pCa units (with near maximal effects with 15 s exposure), and that a subsequent 2 min application of 5 mm GSH to the same fibre causes a very large increase in Ca2+ sensitivity (∼0.23 pCa units), evidently due to glutathionylation of site(s) on the contractile apparatus (Lamb & Posterino, 2003) (see Fig. 1). Consistent with this and extending our previous findings (Posterino et al. 2003), Fig. 3A demonstrates that 30 s treatment of an EDL fibre with 100 μm DTDP caused a marked reduction in the twitch response and that a subsequent 2 min exposure to GSH (5 mm) greatly potentiated the twitch response, with little change in 50 Hz tetanic force, and with the effects being fully reversed by 2 min treatment with 10 mm DTT (mean data in Table 1, set 4). As shown in the expanded traces in Fig. 4, when the fibre had been subjected to the sequential DTDP–GSH treatments (i.e. glutathionylation), not only was the peak of the twitch force increased, but also the rate of force development was faster (mean of 2.1 ± 0.3 times faster in rising from 0 to 50% of peak force; control: 0.65 ± 0.23 % of Fmax per ms; after DTDP–GSH: 1.25 ± 0.31 % of Fmax per ms, 3 EDL fibres, P < 0.05 paired t test; Fmax is maximum Ca2+-activated force under control conditions).
Figure 4. Glutathionylation treatment increases the rate of twitch force development.
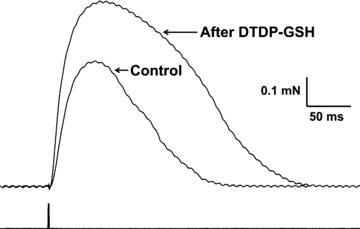
Superimposed traces of twitch responses from Fig. 3A, obtained under initial control conditions (smaller force trace) and following sequential treatments with DTDP and GSH (larger force trace). Time of stimulation pulses shown underneath force traces.
In addition, the t-system repriming period (RP), a measure reflecting the refractory behaviour of the APs in the t-system, was examined. The RP is a sensitive indicator of whether the t-system is partially depolarized (Dutka & Lamb, 2007b) and was assessed by applying pairs of supramaximal pulses with various delays between the first and the second pulse (see Methods). Twitch force is markedly potentiated when the second pulse in a pair is able to elicit an AP and hence additional SR Ca2+ release, and in control conditions with the t-system well polarized this occurs with interpulse intervals of ≥4 ms (e.g. Fig. 3B). The repriming period (RP) was not significantly altered by the DTDP treatment, nor by the subsequent treatments with either GSH or DTT (Fig. 3B and C; mean data in Table 1, set 4). Similarly, repriming was not affected by any of the SNAP treatments examined (Table 1, sets 1 to 3), nor by application of even 10 mm of the oxidizing agent H2O2 (Table 1, set 5). Thus, none of these various oxidization, nitrosylation or glutathionylation treatments noticeably affected t-system polarization and excitability, and the reductions in the twitch response seen with SNAP and DTDP treatments were not attributable to inadequate AP stimulation.
Effects of GSNO on contractile apparatus properties and twitch responses
It has been reported previously that GSNO appears to act as a nitrosylating agent on the contractile apparatus of muscle fibres (Spencer & Posterino, 2009). In partial agreement with this, it was found here that when EDL fibres were exposed for 2 min to a solution in which 2 mm GSNO had been added 10–20 min beforehand (denoted as ‘GSNOdel’), the effects were very similar to those of SNAP treatment, there being decreases in both the twitch response (Table 1, set 6) and Ca2+ sensitivity of the contractile apparatus (Fig. 5A right-hand side; mean data in Table 2, set 6). Moreover, the magnitude of the changes in both the twitch and the Ca2+ sensitivity seemed consistent with those found with SNAP treatment, given that the estimated [NO•] was ∼4 μm for GSNOdel which fell between the levels for 2 and 10 mm SNAP (∼1 and 5 μm respectively). Again both effects were reversible with DTT (Fig. 5A and Tables 1 and 2) and there was no change to t-system repriming (Table 1, set 6). In addition, in two cases the contractile properties were examined after subjecting a fibre first to the GSNOdel treatment and then to SNAP treatment (10 mm, 2 min), which resulted in mean successive decreases in Ca2+ sensitivity of −0.079 (± 0.027 s.d.) and −0.039 (± 0.029 s.d.) pCa units respectively, the total decrease being similar to the largest decrease achievable with extensive SNAP treatment alone (∼−0.12 pCa units, see sets 3 and 5 in Table 2), which is consistent with the GSNOdel and SNAP treatments having similar modes of actions.
Figure 5. GSNOimm increases and GSNOdel decreases Ca2+ sensitivity of the contractile apparatus.
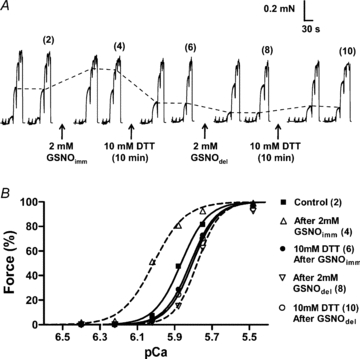
A, two force–pCa staircases elicited before and after successive treatments with 2 mm GSNOimm (for 2 min), DTT, 2 mm GSNOdel (for 2 min), and finally DTT again. pCa of solutions in each staircase: >10, 6.40, 6.22, 6.02, 5.88, 5.75, 5.48, with maximum force at pCa 4.5. Dashed lines indicate force at pCa 5.88, to highlight Ca2+ sensitivity changes. B, Hill fits to force–pCa staircases 2, 4, 6, 8 and 10 from panel A. See Table 2 for mean changes in contractile apparatus properties.
In contrast, when 2 mm GSNO was applied to an EDL fibre immediately (i.e. <30 s) after being dissolved (denoted as ‘GSNOimm’), the Ca2+ sensitivity of the contractile apparatus was very substantially increased (Fig. 5A left-hand side, and Fig. 5B), much like that occurring with glutathionylation treatment with DTDP–GSH, and likewise this increase was largely or fully reversed with DTT (Fig. 5 and Table 2, set 8). In three additional experiments, an EDL fibre was first subjected to maximal DTDP–GSH treatment (5 min with 100 μm DTDP, followed by 2 min with 5 mm GSH; see Lamb & Posterino (2003)), which caused a mean Ca2+ sensitivity increase of +0.234 ± 0.006 pCa units, and then to the GSNOimm treatment (2 mm, 2 min), which produced no further change in sensitivity (–0.001 ± 0.003 pCa units). In three further cases, a fibre was first given the GSNOimm treatment, increasing sensitivity by +0.101 ± 0.030 pCa units, and then the maximal DTDP–GSH treatment, which caused a further increase of only +0.070 ± 0.028 pCa units; subsequent reversal with DTT treatment reduced Ca2+ sensivity by –0.221 ± 0.006 pCa units and then repeat of the DTDP–GSH treatment increased Ca2+ sensitivity by +0.208 ± 0.005 pCa units. These data strongly suggest that the GSNOimm and DTDP–GSH treatments both increased the Ca2+ sensitivity by the same mechanism, that is, by glutathionylation of the contractile apparatus.
Neither the immediate nor the delayed treatments with 2 mm GSNO for 2 min detectably altered maximum force production of the contractile apparatus (Table 2, sets 6 to 8). Given that a much longer (15 min) treatment with 3 mm GSNO has been reported to cause S-nitrosylation of myosin heads and reduce myosin ATPase activity by ∼30% (Nogueira et al. 2009), we also investigated the effects of a more stringent and prolonged GSNO treatment. In the three EDL fibres exposed to 4 mm GSNOimm for 20 min, maximum Ca2+-activated force decreased by 45 ± 12%; on average the Ca2+ sensitivity was not significantly altered (ΔpCa50+0.022 ± 0.085 and Δh−1.4 ± 0.8), but this was the result of one fibre undergoing a large increase in sensitivity and the other two fibres showing a moderate decrease, which is likely to reflect different combinations of the opposing effects of glutathionylation and nitrosylation on Ca2+ sensitivity that can both occur with GSNO treatment.
Effects of GSNOimm on twitch, tetanus and t-system excitability
Even though treatment with 2 mm GSNOimm for 2 min markedly increased the Ca2+ sensitivity of the contractile apparatus (Fig. 5), somewhat surprisingly the twitch and tetanic responses were actually decreased by ∼19% and 15%, respectively, immediately following treatment (Fig. 6A; and Table 1, set 8), and then became even smaller at later times (Figs 6 and 7). This inhibitory effect was not reversed by DTT treatment (Fig. 6A). The decreases in twitch and tetani evidently were not due to chronic t-system depolarization and consequent loss of excitability, because the repriming time was not altered (Fig. 6A and Table 1, set 8). Furthermore, it was not caused by depletion of the Ca2+ levels in the SR nor block of Ca2+ release through the RyRs, because (i) direct stimulation of the RyRs by lowering cytoplasmic free [Mg2+] (Lamb & Stephenson, 1994) still triggered a large and prolonged release of Ca2+ (e.g. Figs 6A and 7A), and (ii) a tetanic response on occasion could be immediately followed by a prolonged release of Ca2+, indicative that it had triggered substantial Ca2+-induced Ca2+ release (e.g. Fig. 7A). Furthermore, after the GSNOimm treatment had resulted in substantial decrease in the tetani (to 48.2 ± 4.4% of pre-treatment level, n = 10), the responses were not significantly altered (52.7 ± 4.2%, paired t test P > 0.05) by bathing the fibres in a SR loading solution (pCa 6.7, 1 mm total EGTA) for ≥10 s, a procedure that normally increases the SR Ca2+ content of an EDL fibre by an extra ∼50% of resting content (Lamb et al. 2001; Trinh & Lamb, 2006; Murphy et al. 2009). Thus, it appears that the decrease in the twitch and tetanic responses reflects some irreversible impairment of the coupling between the DHPRs and the RyRs.
Figure 6. Effect of GSNOimm on twitch and tetanic responses.
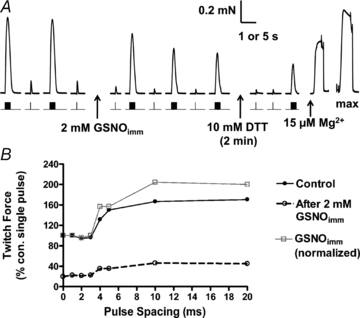
A, amplitude of twitch and tetanic responses became progressively smaller following a 2 min exposure to 2 mm GSNOimm, and this was not ameliorated by DTT treatment. Time scale: 1 s for twitches and tetani, 5 s for 15 μm Mg2+ and max responses. B, twitch responses to single and paired pulses (as in Fig. 3B), before and after 2 mm GSNOimm. Control (filled circles), and post-GSNOimm responses expressed relative to control single pulse response (open circles) or normalized to post-treatment single pulse response (grey squares). Exposure to GSNOimm did not alter t-system repriming period (∼4 ms).
Figure 7. Progressive decrease in tetanic responses following GSNOimm treatment.
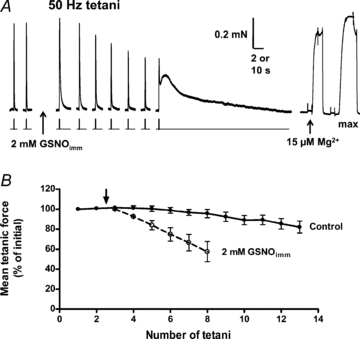
A, tetani obtained before and after exposure to 2 mm GSNOimm. The last post-treatment tetanus triggered a prolonged contracture indicative of Ca2+-induced Ca2+ release, which is never seen in control conditions. The large, prolonged force response to 15 μm free Mg2+ (slow time scale) indicates the SR still contained considerable Ca2+. Time scale: 10 s during responses to 15 μm Mg2+ and max, and 2 s elsewhere. B, mean tetanic force (relative to initial) in five EDL fibres treated for 2 min with 2 mm GSNOimm (open circles, arrow indicates time of GSNOimm exposure) and five control fibres (filled circles) examined in alternating order with treated fibres.
GSNOimm treatment increases SR Ca2+ leak through RyRs
In view of the very prominent Ca2+-induced Ca2+ release evident following GSNOimm treatment in some fibres (e.g. Fig. 7A), we investigated whether the ability of the SR to load and retain Ca2+ was compromised. The relative amount of Ca2+ present in the SR of a skinned fibre was gauged from the area of the force response when fully depleting the SR by exposing the fibre to a caffeine–low [Mg2+] solution with a set amount of EGTA present to partially buffer the released Ca2+, as previously described (Lamb & Cellini, 1999; Lamb et al. 2001) (see Methods). Each fibre was subjected to repeated cycles of emptying and reloading the SR, both before and after the given treatment. In EDL fibres the endogenous Ca2+ content of the SR is only ∼25 to 30% of the maximum level that it can hold (see Fryer & Stephenson, 1996; Murphy et al. 2009) and normally it can be reloaded to that endogenous level in ∼20 s in the standard loading solution used in the present experiments (containing 1 mm total EGTA at pCa 6.7, 1 mm free Mg2+), and the SR reaches the maximum Ca2+ content with ∼3 min loading. However, when fibres were treated with GSNOimm for 2 min, followed immediately by DTT treatment to reverse changes in contractile apparatus Ca2+ sensitivity (see Fig. 5 & Table 2), it was evident that the ability of the SR to load and retain Ca2+ was markedly reduced (Fig. 8). When loading was carried out in the presence of 1 mm free Mg2+ on average the SR accumulated only about half as much Ca2+ after 1 min as it did before treatment, and increasing the loading time to 3 min resulted in almost no further increase in Ca2+ content (Fig. 9A). In contrast, in fibres given 2 min ‘nitrosylation’ treatment with either 10 mm SNAP or 2 mm GSNOdel (each followed by DTT treatment), the ability of the SR to load and retain Ca2+ was unaltered (Fig. 9A).
Figure 8. Effect of GSNOimm treatment on SR Ca2+ accumulation.
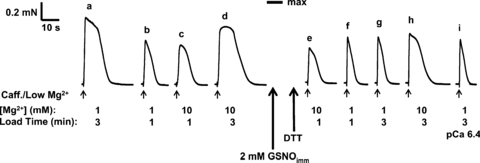
Force responses elicited in a skinned EDL fibre upon fully emptying the SR of all Ca2+ by exposure to caffeine–low [Mg2+] release solution (small arrows) (see Methods). Time integral (‘area’) of force responses indicative of SR Ca2+ content reached after having loaded the SR for the indicated time (1 or 3 min) in a load solution with indicated free [Mg2+] (1 or 10 mm) at pCa 6.7, or at pCa 6.4 (response ‘i’). Following the 2 min treatment with GSNOimm, the fibre was treated with DTT (10 mm, 10 min) to restore the normal Ca2+ sensitivity of contractile apparatus. After GSNOimm treatment, the ability of the SR to accumulate Ca2+ during a 3 min loading period in 1 mm Mg2+ was greatly depressed (area of response is much smaller in ‘g’ than in ‘a’). Raising the [Mg2+] in the load solution to 10 mm markedly increased the amount accumulated with 3 min loading (compare ‘h’ with ‘g’). Mean data shown in Fig. 9B.
As the GSNOimm treatment had relatively little effect on the amount of SR Ca2+ accumulated when the loading period was only 30 s (see Fig. 9B), it was apparent that the SR still could take up Ca2+ at close to the normal rate, and hence that the decrease in maximum content was not primarily due to decreased SERCA pumping but instead to increased leakage of Ca2+ out of the SR. Such leakage could be expected to increase with increased SR content, and evidently it matched the Ca2+ uptake by the SERCA when the content reached just 30% or so of the original maximum level (Fig. 9). The net load could not be increased by raising the free [Ca2+] in the load solution (from pCa 6.7 to 6.4), as seen in Fig. 8 (comparing responses ‘g’ and ‘i’). In order to determine whether the increased Ca2+ leakage out of the SR was through the RyRs, or through the SERCA itself as occurs with raised cytoplasmic [ADP] (Macdonald & Stephenson, 2001), we investigated the effect of raising the free [Mg2+] in the load solution from 1 to 10 mm, as the Mg2+ would be expected to antagonize opening of the RyRs, particularly any Ca2+-induced Ca2+ release (Meissner et al. 1986; Laver et al. 1997), but not to prevent back flux through the SERCA. Raising the [Mg2+] in the load solution, which had little or no effect on net Ca2+ accumulation by the SR before treatment, resulted in a striking increase in net accumulation at longer load times after GSNOimm treatment (compare ‘g’ with ‘h’ in Fig. 8; mean data in Fig. 9B), indicating that much if not all of the increased Ca2+ leakage was through the RyRs.
Discussion
The first main finding of this study is that, with the exception of the GSNOimm treatment, the overall effect of all of the various nitrosylation, oxidation and glutathionylation treatments on twitch and tetanic force in the EDL fibres was evidently determined primarily by the effect of the given treatment on the contractile apparatus, not on excitability or Ca2+ release and uptake. The ‘expected’ effect on twitch and tetanic responses of some given change in contractile sensitivity can be roughly estimated by considering how that particular shift in the force–pCa relationship (e.g. Fig. 5B) would affect the relevant force response. For example, treatment with 2 mm SNAP for 2 min decreased Ca2+ sensitivity by a mean pCa50 shift of −0.055 units (Table 2, set 2), and given that the twitch response before treatment reached on average ∼27% of maximum Ca2+-activated force, such a rightward shift of the Ca2+ sensitivity curve would be ‘expected’ to reduce the twitch to ∼59% of its original level if the [Ca2+] were unchanged (e.g. see change in relative force at constant pCa in Fig. 5B), close to the value actually observed (Table 1, set 2). Similarly, the decrease Ca2+ sensitivity with 10 mm SNAP, 2 mm GSNOdel and 100 μm DTDP (ΔpCa50 of −0.072, −0.059 and −0.065, respectively) would be expected to cause large reductions in the twitch response (to ∼46%, 56% and 52% of initial), values similar to or slightly greater than the actual observed reductions (Table 1). In further accord, H2O2 treatment, which gives no shift in pCa50 (Lamb & Posterino, 2003), had no significant effect on twitch force (Table 1 set 5), and glutathionylation treatment, by 2 min application of GSH after DTDP, which causes a very large increase in Ca2+ sensitivity (∼+0.23), greatly potentiated twitch force (Fig. 3 and Table 1, set 4). In all cases tetanic force, which reached a high percentage of maximum Ca2+-activated force (∼92%) before any treatment, was likewise decreased or increased by the various treatments but only to a much smaller extent, which is to be expected given that the force–pCa relationship was near saturation. Despite the simplifications and inherent limitations involved in these considerations, it is apparent that the twitch force was increased or decreased in a manner that could be accounted for by the effect of the given treatment on the Ca2+ sensitivity of the contractile apparatus.
Thus, it is apparent that the amount of Ca2+ released from the SR by AP stimulation was little altered by any of the treatments (except GSNOimm, as discussed below). During an AP the voltage sensors in the t-system normally very potently activate the adjacent RyRs in the SR, triggering Ca2+ release at a peak rate of ∼200 μm ms−1 and releasing ∼20% of the total SR Ca2+ within ∼2 ms (Baylor & Hollingworth, 2003; Posterino & Lamb, 2003). This AP-induced Ca2+ release is evidently little affected by oxidation or nitrosylation of the RyRs, even though such treatments increase the sensitivity of RyR opening to other much less potent stimuli such as caffeine and Ca2+ itself, as observed with isolated RyRs in bilayers and in SR vesicles (Marengo et al. 1998; Oba et al. 2002; Aracena et al. 2003). Such increase in responsiveness of RyRs to caffeine and Ca2+ (and submaximal depolarization) upon oxidative treatment with DTDP or H2O2 can even be seen in skinned fibres with the voltage sensors and RyRs remaining juxtaposed in their normal configuration (Posterino et al. 2003; see also Pouvreau & Jacquemond, 2005). Nevertheless, the amount of AP-induced Ca2+ release remains unchanged after oxidative treatment, consistent with results in intact fibres (Andrade et al. 1998a).
Both ‘nitrosylation’ treatments used here, application of SNAP or GSNOdel, caused a decrease in twitch response and contractile sensitivity (Fig. 2; Tables 1 and 2). In intact fibres, application of NO• donors caused little change in submaximal tetanic force, because even though Ca2+ sensitivity was decreased the effect was approximately countered by an increase in the peak of the Ca2+ transients (Andrade et al. 1998b). The latter might reflect increased Ca2+ release, though it may instead have resulted simply from greater initial occupancy of Ca2+ binding sites in the cytoplasm that could have arisen from a small increase in SR Ca2+ leakage through nitrosylated RyRs (Aracena et al. 2003; Bellinger et al. 2009) or from decreased SR Ca2+ uptake. Such small changes in SR Ca2+ leakage or uptake would not appreciably affect the binding site occupancy in the skinned fibre preparation used here, where the cytoplasm is open to the bathing solution. A study using NOS inhibitors in voltage-clamped muscle fibres on the other hand concluded that NO• depresses voltage-sensor induced SR Ca2+ release (Pouvreau & Jacquemond, 2005). However, this is not necessarily inconsistent with the results found with AP stimulation, because as discussed above, modulation of the properties of the RyRs can alter their response to various submaximal stimuli and nevertheless not alter their quantal Ca2+ release behaviour to AP stimulation.
If the t-system is chronically depolarized ∼10 to 15 mV below its normal resting level, the rate of AP repriming is greatly slowed (Dutka & Lamb, 2007b). As neither the amount of AP-induced Ca2+ release (see above) nor the AP repriming time (Table 1) was noticeably altered by any of the treatments, it is evident that t-system membrane potential and excitability were little affected. The t-system potential in the skinned fibre preparation used here is highly dependent on continual functioning of the Na+–K+ pump in the t-system membrane (Dutka & Lamb, 2007a; Dutka et al. 2008), and so it can be concluded that the various treatments examined did not substantially hinder Na+–K+ pump function, even though these would be considered by many measures to be very stringent treatments (e.g. application of 10 mm H2O2, or ∼5 μm NO•, or 100 μm of the highly reactive sulphydryl reagent, DTDP). Similarly, it can also be concluded that the SR Ca2+ pumps (SERCA) were not deleteriously affected to any great extent by the treatments because the rates of decline of the twitch and tetanic force were not appreciably slowed (e.g. Figs 2 and 3A), as occurs prominently when uptake by the SERCA is substantially inhibited with SERCA blockers (Posterino & Lamb, 2003).
Deleterious effects of oxidative treatments
Whilst in general qualitative agreement with the present study, our previous investigation of the effects of combined DTDP–GSH treatment (Posterino et al. 2003) found less marked potentiation of the twitch as well as a substantial overall deterioration of the responses following DTT reversal. This was because, in contrast to the present study, the intracellular solutions in that earlier study contained the mitochondrial inhibitor azide (1 mm), which subsequently was shown to cause chronic partial depolarization of the t-system in skinned fibres (Ortenblad & Stephenson, 2003). When the problem was avoided here by omitting azide, it was apparent that the changes to the twitch response were dictated primarily by the changes in contractile apparatus Ca2+ sensitivity, as discussed above. Nevertheless, those earlier findings do serve to show that oxidative treatments can have deleterious actions on particular steps in EC coupling, such as exacerbating the inhibitory effects of partial depolarization on excitability and/or DHPR to RyR coupling. Of particular note was the finding here that a 2 min treatment with GSNO increased or decreased Ca2+ sensitivity without any effect on the maximum force level (Fig. 5A) whereas a more stringent and prolonged treatment (4 mm for 20 min) caused a large decrease in maximum force, likely to have been due to nitrosylation of the myosin heads and consequent inhibition of the myosin ATPase, as was found to occur when purified myosin was subjected to similar stringent GSNO treatment (Nogueira et al. 2009). This vividly illustrates that relatively ‘mild’ ROS or RNS treatments can have major ‘modulatory’ effects on muscle function which differ substantially from the likely ‘deleterious’ effects occuring with more stringent treatments, the latter possibly being more relevant to pathological rather than physiological changes in muscle function. Such findings also highlight the value of the skinned fibre preparation used here, in which it is possible to investigate the effects of a particular level of oxidative treatment on each of the individual steps in the EC coupling sequence, as well on the overall response of the whole process, that is, on twitch and tetanic force.
Uncoupling with GSNOimm treatment and RyR Ca2+ leak
An important further finding was that when GSNO was applied to a skinned fibre soon after being dissolved in solution (denoted GSNOimm treatment), it caused increased Ca2+ sensitivity of the contractile apparatus (Fig. 5) but paradoxically caused irreversible inhibition of the twitch and tetanic responses (Figs 6 and 7), evidently due to disruption of the coupling between the voltage-sensors and the RyRs. This disruption in coupling was not due to general dysfunction of the RyRs, but in fact was accompanied by heightened CICR by the RyRs (Figs 7–9). Significantly, the disruption to coupling occurred only in the conditions where the GSNO caused S-glutathionylation of the contractile apparatus and consequent increased Ca2+ sensitivity, and not in the conditions where GSNO caused only S-nitrosylation and consequent decreased Ca2+ sensitivity. This was also verified by Western blotting of the contractile proteins with antibodies to detect S-glutathionylation and S-nitrosylation, which is the subject of a separate publication (G. D. Lamb and colleagues, unpublished data). The propensity of the GSNOimm treatment to cause S-gluthathionylation of proteins is quite possibly explained by the finding that GSNO readily undergoes spontaneous chemical transformation in aqueous solution, generating several glutathione derivatives including glutathione thiosulfinate (GS(O)SG), which is very reactive to any protein thiol and produces S-gluthathiolyation (Huang & Huang, 2002). The overall action of the GSNO solution changed over time from glutathionylation to nitrosylation, which presumably resulted from a change in proportion of GSNO and its various derivatives over time. It is not possible to say, however, whether the same progressive change also occurs when GSNO is generated in the cytoplasm of a cell, nor even whether the GSNO species itself will cause glutathionylation or nitrosylation (or both) of a given target protein. For example, it was found that when GSNO was applied to SR vesicles in vitro, particular cysteine residues on the RyRs underwent only S-glutathionylation whereas others underwent either S-glutathionylation or S-nitrosylation or formed disulphide bonds (Aracena-Parks et al. 2006).
Interestingly, the same group has used specific treatments to cause one or other reaction and showed that S-glutathionylation of the RyRs greatly decreases the inhibitory effect of cytoplasmic Mg2+ on the RyRs (increasing the K0.5 from ∼60 μm to >2 mm Mg2+), whereas S-nitrosylation increases the RyR sensitivity to cytoplasmic Ca2+ when [Mg2+] is low but not in the presence of physiological (mm) Mg2+ levels (Aracena et al. 2003). The former effect is likely to be due to decreased Mg2+ affinity of the so-called ‘I’ site on the RyR, which is normally occupied by Mg2+, causing resting inhibition of the channels, whereas the latter effect is likely to reflect increased Ca2+ sensitivity of the so-called ‘Ca2+-activation’ or ‘A’ site, where Ca2+ binding stimulates and Mg2+ binding inhibits channel opening (Laver et al. 1997; Laver et al. 2004). The heightened CICR seen here in the presence of 1 mm Mg2+ following GSNOimm treatment (e.g. Fig. 7A), and the ability of 10 mm Mg2+ to inhibit CICR through the RyR when loading at pCa 6.7 (Figs 8 and 9), might thus be explained by the GSNOimm treatment causing S-glutathionylation of the RyRs, which also seems in accord with the S-glutathionylation of the contractile apparatus that occurs in the same circumstances. In contrast, S-nitrosylation of the RyRs alone would not readily account for the heightened CICR in the presence of 1 mm Mg2+, and this is consistent with the other nitrosylating treatments (SNAP or GSNOdel) also not causing evident CICR in the fibres here. It is possible nevertheless that the GSNOimm treatment caused both S-glutathionylation and S-nitrosylation of the RyRs. It was not possible however to investigate this further using Western blotting, because the RyR content of single fibre segments was too low to enable antibody detection of S-glutathionylation and S-nitrosylation of the RyRs.
The cause of the VS–RyR uncoupling, however, remains uncertain. It is possible that it resulted from S-glutathionylation or some other oxidative action on the voltage-sensor, and that this was unrelated to the effects on the RyRs. Alternatively, it is possible that the glutathionylation or other actions on the RyR, or on the voltage-sensor, led to both the disrupted VS–RyR coupling and the heightened CICR. It can be noted that the physical coupling of the voltage sensors to the RyRs in mature mammalian skeletal muscle normally exerts an inhibitory action on the RyRs and reduces their sensitivity to CICR and propensity to produce Ca2+ sparks (Shirokova et al. 1999; Weiss et al. 2004; Zhou et al. 2006; Brown et al. 2007). Thus, disrupting the VS–RyR coupling by an oxidation-related process, or by any other means, might be expected to also cause increased CICR through the RyRs.
Finally, the disruption of VS–RyR coupling occurring with GSNOimm treatment may play a role in prolonged low frequency force depression (PLFFD) occurring in certain circumstances. Oxidation-related processes can cause PLFFD in isolated muscle fibres either by reducing Ca2+ sensitivity of the contractile apparatus or by reducing SR Ca2+ release to tetanic stimulation (Bruton et al. 2008). This latter disruption of SR Ca2+ release, which occurred in fibres from wild-type mice but not in fibres from mice over-expressing superoxide dismutase 2, persisted for >30 min after the original fatiguing stimulation and, like the GSNOimm uncoupling effect reported here, could not be reversed by application of DTT. The underlying action was thought to be linked in some way to elevated levels of superoxide, but the exact reactants and targets were not identified (Bruton et al. 2008). Given that ROS readily react with the GSH present endogenously in muscle fibres, it seems quite possible that long-term disruption of Ca2+ release was caused by similar glutathionylation or related processes as occurred with the GSNOimm treatment reported here.
Consideration of experimental limitations
We note two issues in regard to possible limitations on the conclusions that can be drawn from the findings presented here. Firstly, the results here were all obtained in skinned fibres, where various diffusible intracellular constituents present in vivo would have been lost from the fibre, possibly including redox-sensitive kinases or phosphatases or other co-factors. Consequently, it is possible that ROS and RNS generated within, or applied to, an intact muscle fibre might exert different or additional effects to those found here. We point out, however, that the findings here and in our earlier skinned fibre studies (Lamb & Posterino, 2003; Posterino et al. 2003; Murphy et al. 2008) do seem in generally good accord with those found when applying ROS and RNS in intact fibres (Andrade et al. 1998a,b;), such as in regard to the increases or decreases in contractile sensitivity occurring without change in maximum force, and the lack of major changes in fibre excitability and Ca2+ release. This suggests that the major acute effects reported here probably do generally reflect those happening in intact fibres, perhaps either because the ROS and RNS exert their main actions directly on the principal proteins (e.g. on myosin, RyRs, Na+-K+ pumps, etc.) or because sufficient amounts of the ancillary proteins that the ROS/RNS target (e.g. particular kinases or phosphatases) remain bound in appropriate position in the skinned fibres and still functional, as is known to be the case for various kinases (e.g. creatine kinase near both the SERCA and contractile proteins). The second caveat on the conclusions is that in the present experiments the various oxidative agents were applied only when the fibre was in a resting state, that is, without stimulation or direct contractile activation. Consequently, it is possible that the some of the treatments might cause additional or different effects in actively contracting fibres. We note however that whilst we have reported previously that application of oxidants to contracting skinned fibres does cause a reversible increase in Ca2+ sensitivity (Lamb & Posterino, 2003), this effect is (i) quite small in fast-twitch fibres, (ii) only happens with comparatively prolonged activation, and (iii) appears to occur quite independently of glutathionylation effects in the same fibres. Furthermore, when skinned fibres have been electrically stimulated in the presence of an oxidant (e.g. 10 mm H2O2) (Posterino et al. 2003) the effects on fibre excitability, Ca2+ release and twitch force were not obviously different from that found here with similar exposure in the absence of stimulation (Table 1).
Concluding remarks
In agreement with the conclusions of single fibre studies (Andrade et al. 1998a,b;), the present results clearly demonstrate that the most prominent acute effects of various oxidation, nitrosylation and glutathionylation treatments is on the Ca2+ sensitivity of the contractile apparatus, either increasing or decreasing it, and as a result significantly altering submaximal force production. These acute modulatory changes in sensitivity are likely to be important in normal muscle function, such as in aiding or reducing the rate of muscle contraction and also in influencing the rates of onset and recovery of muscle fatigue. On the other hand, more excessive acute oxidative insult, and perhaps also prolonged exposure even to low ROS levels, can lead to dysfunction in a variety of steps in EC coupling, such as interruption to voltage-sensor activation of Ca2+ release, chronic SR Ca2+ leakage, and long-term reduction in maximum force production and Ca2+ sensitivity of the contractile apparatus, which separately or together may underlie muscle weakness in a variety of conditions and diseases.
Acknowledgments
We thank Maria Cellini for technical assistance and the National Health & Medical Research Council of Australia for financial support (Grant number 541938).
Glossary
Abbreviations
- AP
action potential
- CaEGTA
calcium bound to EGTA
- CICR
calcium-induced calcium release
- CrP
creatine phosphate
- DTDP
2,2′-dithiodipyridine
- EC coupling
excitation–contraction coupling
- EDL
extensor digitorum longus
- FRS
full release solution
- GSH
reduced glutathione
- GSNO
S-nitrosoglutathione
- GS(O)SG
glutathione thiosulfinate
- h
Hill coefficient
- HDTA
hexa-methylene-diamine-tetraacetate
- H2O2
hydrogen peroxide
- K-HDTA
potassium hexa-methylene-diamine-tetraacetate
- n
number of fibres
- NO•
nitric oxide
- OH•
hydroxyl
- pCa
–log10[Ca2+]
- PLFFD
prolonged low frequency force depression
- RP
repriming period
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RyR
ryanodine receptor
- SERCA
SR Ca2+ pump
- SNAP
S-nitroso-N-acetyl-penicillamine
- SR
sarcoplasmic reticulum
- t-system
transverse tubular system
- VS
voltage-sensor
Author contributions
T.L.D. performed all physiological experiments, analysed data and drafted various sections of the manuscript. J.P.M. performed all biochemical experiments and related analysis and interpretation. G.S.P. determined the [NO•] in the test solutions, and helped conceive and design experiments. G.D.L. conceived and designed and helped analyse most experiments and wrote/critically revised most of the manuscript. All authors have read the final version of the submitted manuscript. This study was performed entirely at La Trobe University (Melbourne, Victoria, Australia).
References
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol. 1998a;509:565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of nitric oxide on single skeletal muscle fibres from the mouse. J Physiol. 1998b;509:577–586. doi: 10.1111/j.1469-7793.1998.577bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade FH, Reid MB, Westerblad H. Contractile response of skeletal muscle to low peroxide concentrations: myofibrillar calcium sensitivity as a likely target for redox-modulation. FASEB J. 2001;15:309–311. doi: 10.1096/fj.00-0507fje. [DOI] [PubMed] [Google Scholar]
- Aracena-Parks P, Goonasekera SA, Gilman CP, Dirksen RT, Hidalgo C, Hamilton SL. Identification of cysteines involved in S-nitrosylation, S-glutathionylation, and oxidation to disulfides in ryanodine receptor type 1. J Biol Chem. 2006;281:40354–40368. doi: 10.1074/jbc.M600876200. [DOI] [PubMed] [Google Scholar]
- Aracena P, Sanchez G, Donoso P, Hamilton SL, Hidalgo C. S-Glutathionylation decreases Mg2+ inhibition and S-nitrosylation enhances Ca2+ activation of RyR1 channels. J Biol Chem. 2003;278:42927–42935. doi: 10.1074/jbc.M306969200. [DOI] [PubMed] [Google Scholar]
- Baylor SM, Hollingworth S. Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibres of mouse muscle. J Physiol. 2003;551:125–138. doi: 10.1113/jphysiol.2003.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, Matecki S, Lacampagne A, Marks AR. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med. 2009;15:325–330. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LD, Rodney GG, Hernandez-Ochoa E, Ward CW, Schneider MF. Ca2+ sparks and T tubule reorganization in dedifferentiating adult mouse skeletal muscle fibers. Am J Physiol Cell Physiol. 2007;292:C1156–1166. doi: 10.1152/ajpcell.00397.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton JD, Place N, Yamada T, Silva JP, Andrade FH, Dahlstedt AJ, Zhang SJ, Katz A, Larsson NG, Westerblad H. Reactive oxygen species and fatigue-induced prolonged low-frequency force depression in skeletal muscle fibres of rats, mice and SOD2 overexpressing mice. J Physiol. 2008;586:175–184. doi: 10.1113/jphysiol.2007.147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder MS, Cooke R. The effect of myosin sulphydryl modification on the mechanics of fibre contraction. J Muscle Res Cell Motil. 1984;5:131–146. doi: 10.1007/BF00712152. [DOI] [PubMed] [Google Scholar]
- Dutka TL, Lamb GD. Na+-K+ pumps in the transverse tubular system of skeletal muscle fibers preferentially use ATP from glycolysis. Am J Physiol Cell Physiol. 2007a;293:C967–977. doi: 10.1152/ajpcell.00132.2007. [DOI] [PubMed] [Google Scholar]
- Dutka TL, Lamb GD. Transverse tubular system depolarization reduces tetanic force in rat skeletal muscle fibers by impairing action potential repriming. Am J Physiol Cell Physiol. 2007b;292:C2112–2121. doi: 10.1152/ajpcell.00006.2007. [DOI] [PubMed] [Google Scholar]
- Dutka TL, Murphy RM, Stephenson DG, Lamb GD. Chloride conductance in the transverse tubular system of rat skeletal muscle fibres: importance in excitation–contraction coupling and fatigue. J Physiol. 2008;586:875–887. doi: 10.1113/jphysiol.2007.144667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MW, Stephenson DG. Total and sarcoplasmic reticulum calcium contents of skinned fibres from rat skeletal muscle. J Physiol. 1996;493:357–370. doi: 10.1113/jphysiol.1996.sp021388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford: Oxford University Press; 2007. [Google Scholar]
- Hart JD, Dulhunty AF. Nitric oxide activates or inhibits skeletal muscle ryanodine receptors depending on its concentration, membrane potential and ligand binding. J Membr Biol. 2000;173:227–236. doi: 10.1007/s002320001022. [DOI] [PubMed] [Google Scholar]
- Hidalgo C, Donoso P, Carrasco MA. The ryanodine receptors Ca2+ release channels: cellular redox sensors? IUBMB Life. 2005;57:315–322. doi: 10.1080/15216540500092328. [DOI] [PubMed] [Google Scholar]
- Huang KP, Huang FL. Glutathionylation of proteins by glutathione disulfide S-oxide. Biochem Pharmacol. 2002;64:1049–1056. doi: 10.1016/s0006-2952(02)01175-9. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Cellini MA. High intracellular [Ca2+] alters sarcoplasmic reticulum function in skinned skeletal muscle fibres of the rat. J Physiol. 1999;519:815–827. doi: 10.1111/j.1469-7793.1999.0815n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Cellini MA, Stephenson DG. Different Ca2+ releasing action of caffeine and depolarisation in skeletal muscle fibres of the rat. J Physiol. 2001;531:715–728. doi: 10.1111/j.1469-7793.2001.0715h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Posterino GS. Effects of oxidation and reduction on contractile function in skeletal muscle fibres of the rat. J Physiol. 2003;546:149–163. doi: 10.1113/jphysiol.2002.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Effects of intracellular pH and [Mg2+] on excitation–contraction coupling in skeletal muscle fibres of the rat. J Physiol. 1994;478:331–339. doi: 10.1113/jphysiol.1994.sp020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Westerblad H. Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J Physiol. 2011;589:2119–2127. doi: 10.1113/jphysiol.2010.199059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver DR, Baynes TM, Dulhunty AF. Magnesium inhibition of ryanodine-receptor calcium channels: evidence for two independent mechanisms. J Membr Biol. 1997;156:213–229. doi: 10.1007/s002329900202. [DOI] [PubMed] [Google Scholar]
- Laver DR, O'Neill ER, Lamb GD. Luminal Ca2+-regulated Mg2+ inhibition of skeletal RyRs reconstituted as isolated channels or coupled clusters. J Gen Physiol. 2004;124:741–758. doi: 10.1085/jgp.200409092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald WA, Stephenson DG. Effects of ADP on sarcoplasmic reticulum function in mechanically skinned skeletal muscle fibres of the rat. J Physiol. 2001;532:499–508. doi: 10.1111/j.1469-7793.2001.0499f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengo JJ, Hidalgo C, Bull R. Sulfhydryl oxidation modifies the calcium dependence of ryanodine-sensitive calcium channels of excitable cells. Biophys J. 1998;74:1263–1277. doi: 10.1016/S0006-3495(98)77840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G, Darling E, Eveleth J. Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry. 1986;25:236–244. doi: 10.1021/bi00349a033. [DOI] [PubMed] [Google Scholar]
- Murphy RM, Dutka TL, Lamb GD. Hydroxyl radical and glutathione interactions alter calcium sensitivity and maximum force of the contractile apparatus in rat skeletal muscle fibres. J Physiol. 2008;586:2203–2216. doi: 10.1113/jphysiol.2007.150516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RM, Larkins NT, Mollica JP, Beard NA, Lamb GD. Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast- and slow-twitch fibres of rat. J Physiol. 2009;587:443–460. doi: 10.1113/jphysiol.2008.163162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira L, Figueiredo-Freitas C, Casimiro-Lopes G, Magdesian MH, Assreuy J, Sorenson MM. Myosin is reversibly inhibited by S-nitrosylation. Biochem J. 2009;424:221–231. doi: 10.1042/BJ20091144. [DOI] [PubMed] [Google Scholar]
- Oba T, Murayama T, Ogawa Y. Redox states of type 1 ryanodine receptor alter Ca2+ release channel response to modulators. Am J Physiol Cell Physiol. 2002;282:C684–692. doi: 10.1152/ajpcell.01273.2000. [DOI] [PubMed] [Google Scholar]
- Ortenblad N, Stephenson GD. A novel signalling pathway originating in mitochondria modulates rat skeletal muscle membrane excitability. J Physiol. 2003;548:139–145. doi: 10.1113/jphysiol.2002.036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Kim KH, Feng W. Redox sensing properties of the ryanodine receptor complex. Front Biosci. 2002;7:a72–79. doi: 10.2741/A741. [DOI] [PubMed] [Google Scholar]
- Posterino GS, Cellini MA, Lamb GD. Effects of oxidation and cytosolic redox conditions on excitation-contraction coupling in rat skeletal muscle. J Physiol. 2003;547:807–823. doi: 10.1113/jphysiol.2002.035204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posterino GS, Lamb GD. Effect of sarcoplasmic reticulum Ca2+ content on action potential-induced Ca2+ release in rat skeletal muscle fibres. J Physiol. 2003;551:219–237. doi: 10.1113/jphysiol.2003.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouvreau S, Allard B, Berthier C, Jacquemond V. Control of intracellular calcium in the presence of nitric oxide donors in isolated skeletal muscle fibres from mouse. J Physiol. 2004;560:779–794. doi: 10.1113/jphysiol.2004.072397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouvreau S, Jacquemond V. Nitric oxide synthase inhibition affects sarcoplasmic reticulum Ca2+ release in skeletal muscle fibres from mouse. J Physiol. 2005;567:815–828. doi: 10.1113/jphysiol.2005.089599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochniewicz E, Lowe DA, Spakowicz DJ, Higgins L, O'Conor K, Thompson LV, Ferrington DA, Thomas DD. Functional, structural, and chemical changes in myosin associated with hydrogen peroxide treatment of skeletal muscle fibers. Am J Physiol Cell Physiol. 2008;294:C613–626. doi: 10.1152/ajpcell.00232.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisler E, Burke M, Harrington WF. Cooperative role of two sulfhydryl groups in myosin adenosine triphosphatase. Biochemistry. 1974;13:2014–2022. doi: 10.1021/bi00707a003. [DOI] [PubMed] [Google Scholar]
- Shirokova N, Shirokov R, Rossi D, Gonzalez A, Kirsch WG, Garcia J, Sorrentino V, Rios E. Spatially segregated control of Ca2+ release in developing skeletal muscle of mice. J Physiol. 1999;521:483–495. doi: 10.1111/j.1469-7793.1999.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Reid MB. Redox modulation of contractile function in respiratory and limb skeletal muscle. Respir Physiol Neurobiol. 2006;151:229–241. doi: 10.1016/j.resp.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Spencer T, Posterino GS. Sequential effects of GSNO and H2O2 on the Ca2+ sensitivity of the contractile apparatus of fast- and slow-twitch skeletal muscle fibers from the rat. Am J Physiol Cell Physiol. 2009;296:C1015–1023. doi: 10.1152/ajpcell.00251.2008. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Wikman-Coffelt J. An investigation into the role of SH1 and SH2 groups of myosin in calcium binding and tension generation. Biochem Biophys Res Commun. 1980;92:1383–1388. doi: 10.1016/0006-291x(80)90439-8. [DOI] [PubMed] [Google Scholar]
- Stephenson DG, Williams DA. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supinski GS, Callahan LA. Free radical-mediated skeletal muscle dysfunction in inflammatory conditions. J Appl Physiol. 2007;102:2056–2063. doi: 10.1152/japplphysiol.01138.2006. [DOI] [PubMed] [Google Scholar]
- Tiago T, Simao S, Aureliano M, Martin-Romero FJ, Gutierrez-Merino C. Inhibition of skeletal muscle S1-myosin ATPase by peroxynitrite. Biochemistry. 2006;45:3794–3804. doi: 10.1021/bi0518500. [DOI] [PubMed] [Google Scholar]
- Trinh HH, Lamb GD. Matching of sarcoplasmic reticulum and contractile properties in rat fast- and slow-twitch muscle fibres. Clin Exp Pharmacol Physiol. 2006;33:591–600. doi: 10.1111/j.1440-1681.2006.04412.x. [DOI] [PubMed] [Google Scholar]
- Weiss RG, O'Connell KM, Flucher BE, Allen PD, Grabner M, Dirksen RT. Functional analysis of the R1086H malignant hyperthermia mutation in the DHPR reveals an unexpected influence of the III–IV loop on skeletal muscle EC coupling. Am J Physiol Cell Physiol. 2004;287:C1094–1102. doi: 10.1152/ajpcell.00173.2004. [DOI] [PubMed] [Google Scholar]
- Zhou J, Yi J, Royer L, Launikonis BS, Gonzalez A, Garcia J, Rios E. A probable role of dihydropyridine receptors in repression of Ca2+ sparks demonstrated in cultured mammalian muscle. Am J Physiol Cell Physiol. 2006;290:C539–553. doi: 10.1152/ajpcell.00592.2004. [DOI] [PubMed] [Google Scholar]


