Abstract
A polyphasic identification approach was used to investigate the taxonomic position of Campylobacter-like isolates recovered from barnacle geese (Branta leucopsis) and Canada geese (Branta candensis). Seven strains were selected from a collection of 21 isolates and analyzed by extensive phenotypic testing; four strains were characterized by 16S rRNA gene sequence analysis. The results clearly identified the bird isolates as Helicobacter canadensis, recently described as an emerging human pathogen. This is the first report of an animal reservoir for this organism and of its presence in Europe and confirms the zoonotic potential of H. canadensis.
Helicobacter canadensis, a recently described species closely resembling the enterohepatic zoonotic agent Helicobacter pullorum (5), is one of many new enteropathogens isolated from humans (1, 16). The clinical importance of this bacterium is not fully established, but it has been isolated from fecal samples of patients with enteritis (5) and from a blood culture of a patient with bacteremia (19). It has been described as an emerging pathogen (5). No animal host has hitherto been identified for H. canadensis but its closest taxonomic relative, H. pullorum, is found in poultry and retail chicken products (3, 17). Here, the presence of H. canadensis in wild birds is reported for the first time and the zoonotic potential of this bacterium is discussed.
A study on the transmission, ecology, and epidemiology of Campylobacter spp. (in particular Campylobacter jejuni and Campylobacter lari) in cattle and wild birds on pastured meadows in Sweden has been conducted (J. Waldenström et al., unpublished data). Fieldwork was conducted at a shore meadow on southern Gotland, southeast Sweden, where a flock of approximately 1,000 barnacle geese (Branta leucopsis) settled for 4 weeks during spring migration in 2001. To obtain samples, the flock was disturbed and sterile cotton swabs were used to collect fecal material from fresh goose droppings. Care was taken not to touch the surrounding grass, and each of the 116 samples was placed in charcoal transport medium (Transwab; BioDisc, Solna, Sweden) and stored at 4 to 8°C in a refrigerator until cultivation. Samples were then plated onto Campylobacter selective blood-free medium (45.5 g of Campylobacter selective agar base LAB M/LAB 112/liter, 2 ampoules of cefoperazone-amphotericin supplement LAB M/X 112; IDG (UK) Limited, Bury, England) and incubated at 42°C in a microaerobic atmosphere (85% N2, 10% CO2, 5% O2) for 72 h, at which time the media were examined for bacterial growth. Presumptive identification as Campylobacter spp. was done by limited phenotypic characterization (cell morphology and oxidase, catalase, and hippurate hydrolysis).
For detailed genetic identification, the 23 Campylobacter-like isolates obtained were characterized by amplified fragment length polymorphism (AFLP)-based profiling. Chromosomal DNA was purified from bacterial cultures with the Puregene DNA isolation kit (Gentra Systems, Minneapolis, Minn.) according to the manufacturer's specifications. AFLP profiles were determined by digestion of ca. 125 ng of bacterial DNA with BglII and Csp6I, ligation of adapters, and subsequent PCR amplification and detection of fluorophore-labeled fragments by use of an ABI 377 GeneScan sequencer (Applied Biosystems, Foster City, Calif.), as described in detail previously (12). Strain identification was done by comparison of the bird isolate AFLP profiles with those in an existing database containing patterns from all extant species and subspecies of the genus Campylobacter (12). Numerical analysis was performed with the program BioNumerics, version 2.5 (Applied Maths, Kortrijk, Belgium). Of the 23 isolates examined, 6 were identified from the cluster analysis as C. jejuni but 17 formed a distinct cluster with AFLP profiles not resembling those of any extant Campylobacter species (data not shown).
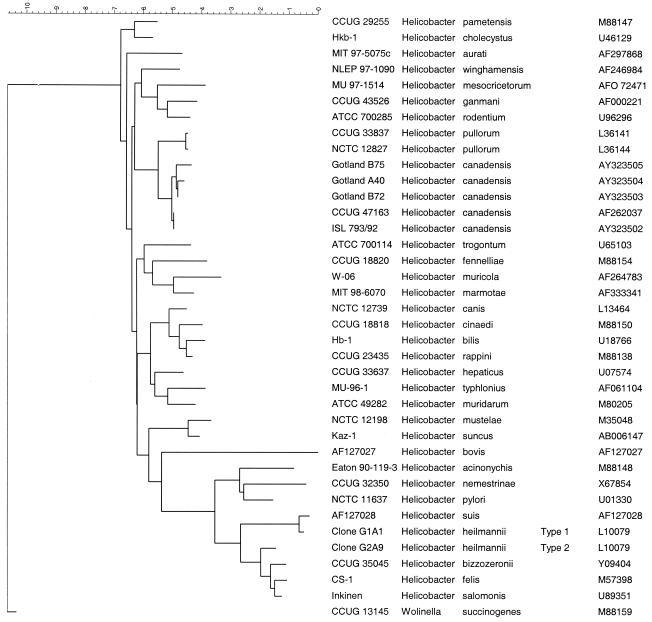
To further evaluate the taxonomic status of these 17 unidentified isolates, additional genotypic and phenotypic analyses were performed on Gotland strains A40, B72, and B75, which represented three distinct AFLP genotypes, i.e., the center and each of the two boundaries of the distinct AFLP cluster. The 16S ribosomal DNA (rDNA) sequences for these three strains were determined by methods described previously (2, 11). However, primer 1392r was replaced with primer 1509rx (5′-GTTACCTTGTTACGACTTACA-3′) in the initial PCR. Extensive phenotypic analysis was performed on these isolates and on the type strain of H. canadensis (CCUG 47163), with over 60 standardized tests included in a probabilistic identification system for Campylobacter, Arcobacter, and Helicobacter spp. (13). The 16S rDNA sequences of the selected bird isolates were compared with over 60 sequences representing all named Epsilobacteria and organisms that closely resemble them, by methods described previously (10). The bird isolate sequences demonstrated 99.1 to 99.9% similarity to each other and to that of the type strain of H. canadensis; these similarities were reflected in a neighbor-joining tree that clearly delineated all sequences to a clade related to, but distinct from, the closely related species H. pullorum (Fig. 1). Comparison of the phenotypic traits for H. canadensis (5; this study) confirmed the identity of the bird isolates (Table 1). Notably, all strains hydrolyzed indoxyl acetate, a key feature of H. canadensis (5).
FIG. 1.
Relationship of avian isolates from barnacle geese (Gotland B75, A40, and B72) and Canada geese (ISL 793/92) with Helicobacter species and the related Wolinella succinogenes as inferred by 16S rRNA gene sequence comparisons. The dendrogram is annotated with strain numbers, species designations, and GenBank accession numbers. Scale bar, 10.35% sequence dissimilarity, as determined by measuring the lengths of the horizontal lines connecting any two species.
TABLE 1.
Phenotypic characteristics of avian H. canadensis isolates compared with those of other enteric Helicobacter speciesa
| Taxon | Resultd of test for:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Catalase production | Nitrate reduction | Alkaline phosphatase hydrolysis | Urease | Indoxyl acetate hydrolysis | Growth at 42°C | Resistance to cephalothin | Tolerance of 0.5% NaF | |
| H. canadensis avian isolatesb | + (7) | + (7) | + (7) | − (0) | + (7) | + (7) | + (7) | + (7) |
| H. canadensis | + | V | −/w | − | + | + | + | +c |
| H. pullorum | + | + | − | − | − | + | + | − |
| H. pametensis | + | + | + | − | − | + | + | + |
| H. cholecystus | + | + | + | − | − | + | + | − |
| H. cinaedi | + | + | D | − | − | D | + | − |
| H. canis | − | − | + | − | + | + | D | − |
| H. fennelliae | + | − | D | − | + | D | − | V |
| H. marmotae | + | − | + | + | − | − | + | ND |
| H. typhlonius | − | V | − | − | − | + | + | ND |
| H. mesocricetorum | + | + | + | − | ND | + | + | ND |
| H. ganmani | − | + | − | − | − | − | + | + |
| H. rodentium | + | + | − | − | − | + | + | + |
| H. hepaticus | + | + | − | + | + | − | + | − |
| H. bilis | + | + | − | + | − | + | + | ND |
| H. trogontum | + | + | − | + | − | + | + | + |
| H. aurati | + | − | − | + | + | + | + | ND |
Data were obtained from references 5, 6, 13 to 15, and 17; this study (H. canadensis type strain); and unpublished data of S. L. W. On.
Results for strains Gotland A40, B72, and B75 (B. leucopsis isolates from Sweden) and ISL 787/92, ISL 790/92, ISL 792/92, and ISL 793/92 (B. canadensis isolates from the United Kingdom). Numbers in parentheses are numbers of positive strains.
Data are available for the type strain only, as determined in this study.
+, 80 to 100% of strains positive; V, 50 to 66% of strains positive; D, 20 to 43% of strains positive; −, 0 to 17% of strains positive; w, weak reaction; ND, not determined.
These results led us to reinvestigate the identity of four hitherto-unclassified isolates initially described as “Campylobacter hyointestinalis-like” that had been shown (by whole-cell protein and DNA macrorestriction profile analyses) to have two highly related genotypes (15). These isolates had been recovered in the United Kingdom from Branta candensis (Canada goose), a bird species closely related to B. leucopsis, from which the H. canadensis isolates described above had been recovered. The phenotypic test results for these four strains (Table 1) and 16S rDNA sequence analysis of a representative strain (ISL 793/92) concurred with those for the isolates from barnacle geese. Indeed, the 16S rDNA sequence of ISL 793/92 exhibited identity with that of the type H. canadensis strain (Fig. 1). The phenotypes of all seven avian isolates from barnacle geese and Canada geese were homogeneous, with the same result in 46 of 64 tests obtained for all strains. This level of variation is comparable to that observed in H. pylori and H. pullorum, where interstrain variation was seen in 14 and 20 of 67 phenotypic characters, respectively (13). No single strain proved atypical in all tests. These results show that H. canadensis naturally occurs in at least two wild bird species. Additionally, tolerance of 0.5% sodium fluoride appears to be a useful additional test for differentiating H. canadensis from H. pullorum (Table 1).
Most Helicobacter species have mammals as hosts (14). Only Helicobacter pametensis, H. pullorum, and two putative but as yet unnamed species designated Helicobacter sp. Bird-B and Helicobacter sp. Bird-C have birds as their natural reservoirs (4, 17). Although H. pametensis and the unnamed taxa have been found in wild birds, they have not as yet been isolated from cases of human infection. Conversely, H. pullorum has been found in poultry and associated products, and its association with enteritis and hepatic disease in humans and production of a cytolethal distending toxin clearly suggest that it is a zoonotic pathogen (1, 17-19, 21). H. canadensis appears highly related to H. pullorum in 16S rRNA gene sequence comparisons, and these species also share many biochemical properties (5; this study). Our finding of avian reservoirs of H. canadensis further illustrates the similarities between these two species; moreover, H. canadensis has, like H. pullorum, also been isolated from patients with diarrhea (5).
Our results indicate that H. canadensis should be considered a probable zoonotic agent. Wild birds are a recognized vector for transmission of zoonotic agents. Fecal contamination of surface water, grazing pastures for production animals, and park areas could all potentially expose humans to infection, as could the consumption of undercooked goose meat. The lifestyles of these birds support these possible routes of transmission. Both barnacle geese and Canada geese are herbivores that often feed on coastal meadows and agricultural fields, sometimes side by side with cattle and sheep, and fly over open seas and rivers during their migratory journey. In particular, Canada geese are frequently found in parks and are often fed by humans (notably children) to the extent that their natural migratory instinct is suppressed and thus these birds become residential in some urban areas, increasing the potential for exposure to fecal material. Both barnacle and Canada geese are hunted, and the meat is often regarded as a delicacy. Furthermore, C. lari, a close phylogenetic relative of H. canadensis, is an established human pathogen and a frequent colonizer of wild bird species, and its recovery from surface water, shellfish, and production animals illustrates a few of the routes by which it may infect humans (8, 20). Similarly, C. jejuni is extensively distributed in nature, and untreated drinking water has been found to be an important source of human infection (7). Finally, the possible natural presence of H. canadensis in common food animals must not be overlooked, since this species closely resembles H. pullorum, an organism that is naturally present in poultry (3, 17) and that is difficult to differentiate from zoonotic campylobacters such as C. lari and Campylobacter coli (18). Inadequate isolation and identification methods may contribute to an underestimation of the true prevalence and significance of such emerging pathogens, as suggested previously (5, 9). It is certainly important that the Swedish isolates in this study were recovered by fecal sampling, transport, and growth under conditions optimized for isolation of Campylobacter species, which may have led to an underestimation of H. canadensis in the barnacle geese examined. Many Helicobacter species cannot grow on blood-free culture media and are also sensitive to the cefoperazone antibiotic supplement that was used (13); the presence of hydrogen in the atmospheric gas mixture is also widely thought to substantially enhance culture of many species (8, 14). Thus, the true prevalence of H. canadensis (and indeed other enteric Helicobacter species) in human disease and animal or environmental sources can be determined only by appropriate detection methods. Nonetheless, it is evident that H. canadensis is widely distributed in nature, since the few isolates that have been documented originate from human disease in Canada (5) and Australia (19) and from barnacle geese in Sweden and Canada geese in the United Kingdom (this study).
In conclusion, our findings concerning a potential zoonotic pathway for the transmission of H. canadensis, the ecology and epidemiology of the bacterium, and the first documented isolates from Europe call for more attention to this emerging pathogen.
Acknowledgments
We are indebted to Enevold Falsen (Culture Collection of the University of Göteborg, Sweden) for his kind gift of the H. canadensis type strain, CCUG 47163.
REFERENCES
- 1.Andersen, L. P. 2001. New Helicobacter species in humans. Dig. Dis. 19:112-115. [DOI] [PubMed] [Google Scholar]
- 2.Angen, Ø., P. Ahrens, and C. Tegtmeier. 1998. Development of a species-specific PCR test for identification of [Haemophilus] somnus in pure and mixed cultures. Vet. Microbiol. 63:39-48. [DOI] [PubMed] [Google Scholar]
- 3.Atabay, H. I., J. E. L. Corry, and S. L. W. On. 1998. Identification of unusual Campylobacter-like organisms in poultry products as Helicobacter pullorum. J. Appl. Microbiol. 84:1017-1024. [DOI] [PubMed] [Google Scholar]
- 4.Dewhirst, F. E., C. Seymour, G. J. Fraser, B. J. Paster, and J. G. Fox. 1994. Phylogeny of Helicobacter isolates from bird and swine feces and description of Helicobacter pametensis sp. nov. Int. J. Syst. Bacteriol. 44:553-560. [DOI] [PubMed] [Google Scholar]
- 5.Fox, J. G., C. C. Chien, F. E. Dewhirst, B. J. Paster, Z. Shen, P. L. Melito, D. L. Woodward, and F. G. Rodgers. 2000. Helicobacter canadensis sp. nov. isolated from humans with diarrhea as an example of an emerging pathogen. J. Clin. Microbiol. 38:2546-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox, J. G., Z. Shen, S. Xu, Y. Feng, C. A. Dangler, F. E. Dewhirst, B. J. Paster, and J. M. Cullen. 2002. Helicobacter marmotae sp. nov. isolated from livers of woodchucks and intestines of cats. J. Clin. Microbiol. 40:2513-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanninen, M.-L., H. Haajanen, T. Pummi, K. Wermundsen, M. L. Katila, H. Sarkkinen, I. Miettinen, and H. Rautelin. 2003. Detection and typing of Campylobacter jejuni and Campylobacter coli and analysis of indicator organisms in three waterborne outbreaks in Finland. Appl. Environ. Microbiol. 69:1391-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lastovica, A. J., and M. B. Skirrow. 2000. Clinical significance of Campylobacter and related species other than Campylobacter jejuni and C. coli, p. 89-120. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 9.On, S. L. W. 1996. Identification methods for campylobacters, helicobacters, and related organisms. Clin. Microbiol. Rev. 9:405-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.On, S. L. W. 2001. Taxonomy of Campylobacter, Arcobacter, Helicobacter, and related bacteria: current status, future prospects, and immediate concerns. Symp. Ser. Soc. Appl. Microbiol. 90:1S-15S. [DOI] [PubMed] [Google Scholar]
- 11.On, S. L. W., H. I. Atabay, J. E. L. Corry, C. S. Harrington, and P. Vandamme. 1998. Emended description of Campylobacter sputorum and revision of its infrasubspecific (biovar) divisions, including C. sputorum bv. paraureolyticus, a urease-producing variant from cattle and humans. Int. J. Syst. Bacteriol. 48:195-206. [DOI] [PubMed] [Google Scholar]
- 12.On, S. L. W., and C. S. Harrington. 2000. Identification of taxonomic and epidemiological relationships among Campylobacter species by numerical analysis of AFLP profiles. FEMS Microbiol. Lett. 193:161-169. [DOI] [PubMed] [Google Scholar]
- 13.On, S. L. W., B. Holmes, and M. J. Sackin. 1996. A probability matrix for the identification of campylobacters, helicobacters, and allied taxa. J. Appl. Bacteriol. 81:425-432. [DOI] [PubMed] [Google Scholar]
- 14.On, S. L. W., A. Lee, J. O′Rourke, F. E. Dewhirst, B. J. Paster, J. G. Fox, and P. Vandamme. Genus Helicobacter. In G. M. Garrity (ed.), Bergey′s manual of systematic bacteriology, 2nd ed., in press. Springer-Verlag, New York, N.Y.
- 15.On, S. L. W., and P. Vandamme. 1997. Identification and epidemiological typing of Campylobacter hyointestinalis subspecies by phenotypic and genotypic methods and description of novel subgroups. Syst. Appl. Microbiol. 20:238-247. [Google Scholar]
- 16.Solnick, J. V., and D. B. Schauer. 2001. Emergence of diverse Helicobacter species in the pathogenesis of gastric and enterohepatic diseases. Clin. Microbiol. Rev. 14:59-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanley, J., D. Linton, A. P. Burnens, F. E. Dewhirst, S. L. W. On, A. Porter, R. J. Owen, and M. Costas. 1994. Helicobacter pullorum sp. nov.—genotype and phenotype of a new species isolated from poultry and from human patients with gastroenteritis. Microbiology 140:3441-3449. [DOI] [PubMed] [Google Scholar]
- 18.Steinbrueckner, B., G. Haerter, K. Pelz, A. Burnens, and M. Kist. 1998. Discrimination of Helicobacter pullorum and Campylobacter lari by analysis of whole cell fatty acid extracts. FEMS Microbiol. Lett. 168:209-212. [DOI] [PubMed] [Google Scholar]
- 19.Tee, W., J. Montgomery, and M. Dyall-Smith. 2001. Bacteremia caused by a Helicobacter pullorum-like organism. Clin. Infect. Dis. 33:1789-1791. [DOI] [PubMed] [Google Scholar]
- 20.Waldenström, J., T. Broman, I. Carlsson, D. Hasselquist, R. P. Achterberg, J. A. Wagenaar, and B. Olsen. 2002. Prevalence of Campylobacter jejuni, Campylobacter lari, and Campylobacter coli in different ecological guilds and taxa of migrating birds. Appl. Environ. Microbiol. 68:5911-5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young, V. B., C. C. Chien, K. A. Knox, N. S. Taylor, D. B. Schauer, and J. G. Fox. 2000. Cytolethal distending toxin in avian and human isolates of Helicobacter pullorum. J. Infect. Dis. 182:620-623. [DOI] [PubMed] [Google Scholar]