Non-technical summary
Odorants are transported into the nasal cavity upon air inhalation where they are detected by olfactory receptor neurons (ORNs), which transduce the odorant molecules into action potentials. The rate of stimulation thus depends on the chosen breathing frequency, which in mice ranges from 2 to 10 Hz. This poses the question how ORNs respond to rapidly changing stimulation rates. Individual mouse ORNs respond reliably to repetitive 2 Hz stimulations resembling normal breathing, but actually perform much poorer when the stimulation rate is increased to 5 Hz, which is more akin to sniffing. In this case, rarely more than 50% of the stimulations elicit any response, with an increase in odorant concentration further reducing the response rate, becoming zero at high concentrations. This counterintuitive observation can be understood in the framework of an adaptive filter, which allows the animal to selectively alter its ORN output depending on the chosen breathing rate.
Abstract
Abstract
Vertebrate olfactory receptor neurons (ORNs) are stimulated in a rhythmic manner in vivo, driven by delivery of odorants to the nasal cavity carried by the inhaled air, making olfaction a sense where animals can control the frequency of stimulus delivery. How ORNs encode repeated stimulation at resting, low breathing frequencies and at increased sniffing frequencies is not known, nor is it known if the olfactory transduction cascade is accurate and fast enough to follow high frequency stimulation. We investigated mouse olfactory responses to stimulus frequencies mimicking odorant exposure during low (2 Hz) and high (5 Hz) frequency sniffing. ORNs reliably follow low frequency stimulations with high fidelity by generating bursts of action potentials at each stimulation at intermediate odorant concentrations, but fail to do so at high odorant concentrations. Higher stimulus frequencies across all odorant concentrations reduced the likelihood of action potential generation, increased the latency of response, and decreased the reliability of encoding the onset of stimulation. Thus an increase in stimulus frequency degrades and at high odorant concentrations entirely prevents action potential generation in individual ORNs, causing reduced signalling to the olfactory bulb. These results demonstrate that ORNs do not simply relay timing and concentration of an odorous stimulus, but also process and modulate the stimulus in a frequency-dependent manner which is controlled by the chosen sniffing rate.
Introduction
Olfaction in rodents begins with active odorant delivery, driven by the respiratory cycle, to the olfactory mucosa. Rhythmogenesis of breathing can be controlled voluntarily by afferent CNS input (Bianchi et al. 1995) and is also influenced by both odorant concentration and the behavioural context in which odorants are presented (see for example Laing, 1983; Youngentob et al. 1987; Sobel et al. 1998; Kepecs et al. 2005; Mainland & Sobel, 2006; Verhagen et al. 2007). Active olfactory exploration is often accompanied by an increase in breathing frequency from ∼3 Hz at rest to 5–10 Hz (‘sniffing’) in mice and rats, with sniffing implicated in such diverse functions as directing odorant flow to different parts of the olfactory epithelium, increasing odorant flux to the olfactory epithelium, promoting behavioural discrimination, enhancing discovery of new odorants, adaptive filtering of olfactory information and coordination of the olfactory system with other brain areas (Youngentob et al. 1987; Sobel & Tank, 1993; Kepecs et al. 2005; Schoenfeld & Cleland, 2005; Kepecs et al. 2007; Verhagen et al. 2007; Wesson et al. 2008).
Once odorants reach the olfactory epithelium they enter the mucus layer that lines the nasal cavity and bind to odorant receptors located on ORN cilia to activate a cAMP-based second messenger cascade. Odorant receptor (OR) activation triggers the activation of the G protein Golf, which in turn activates adenylyl cyclase III, and ciliary cAMP increases. This leads to opening of the olfactory cyclic nucleotide-gated (CNG) channel, Ca2+ influx and subsequent increased excitatory drive due to opening a Cl− channel (for review see Kleene, 2008; Kaupp, 2010). The ORN response is terminated by degradation of cAMP by phosphodiesterase 1C (PDE1C) (Boccaccio et al. 2006; Cygnar & Zhao, 2009) and removal of Ca2+ by a Na+-dependent Ca2+ extrusion mechanism (Reisert & Matthews, 1998) to close the Cl− channel. The odorant-induced depolarization triggers a train of action potentials (APs) which propagate to glomerular targets in the olfactory bulb, with ORNs expressing the same OR targeting to the same glomerulus in the bulb. An increase in odorant concentration increases the AP firing rate but also causes a shortening of the spike train due to a progressive and finally complete decline of the AP amplitude (Shibuya & Shibuya, 1963; Gesteland et al. 1965; Getchell & Shepherd, 1978; van Drongelen, 1978; Reisert & Matthews, 1999; Rospars et al. 2003). High odorant concentrations generate only a few APs at the onset of odorant exposure. This phenomenon has been attributed to progressive inactivation of voltage-gated Na+ and Ca2+ channels (Trotier, 1994; Kawai et al. 1997) due to strong odorant-induced depolarization.
Two critically important questions remain unresolved. First, is olfactory transduction fast enough to reliably respond to normal and sniff-like stimulation paradigms? Second, can ORNs transduce odorant information into AP firing to be conveyed to the olfactory bulb during both low- and high-frequency respiration cycles? We show that both an increase in odorant concentration and, more interestingly, an increase in stimulus frequency cause a significant reduction in the probability of an ORN generating APs in response to each stimulus. AP generation fails entirely at high odorant concentrations. Thus ‘sniffing’ paradoxically brings about a degradation of information flow to the olfactory bulb from the perspective of a single ORN and the glomerulus that is targeted by axons of ORNs expressing a given OR, but might enable mice to control olfactory information flow by regulating their breathing rates.
Methods
Adult mice were killed using CO2 followed by decapitation, following a protocol approved by the Monell Chemical Senses Center Institutional Animal Care and Use Committee, conforming to NIH guidelines. Mice used were either 129SVEV (for suction pipette recordings from ORNs when cineole was the olfactory stimulus or for experiments using the whole epithelium on-cell recording approach), or mice which were genetically altered to co-express GFP with either the I7 (Bozza et al. 2002) or the mOR-EG (Oka et al. 2006) odorant receptor. Olfactory turbinates and septal tissue were removed from the nasal cavity and stored in oxygenated Ringer solution at 4°C until use. A small piece of olfactory epithelium was placed in an Eppendorf tube containing 200 μl of Ringer solution and briefly vortexed (Reisert & Matthews, 2001a). The resulting cell suspension containing isolated ORNs was transferred to a recording chamber on an inverted microscope equipped with phase-contrast optics, and allowed to settle for 20 min before bath perfusion began. ORNs were recognized by their morphology or, for ORNs isolated from I7-GFP or mOR-EG-GFP mice, using fluorescence optics.
The suction-pipette technique was used to record from isolated ORNs (Lowe & Gold, 1991; Reisert & Matthews, 2001a). The cell body of an isolated mouse ORN was drawn into the tip of the recording pipette, leaving the cilia exposed to the bath solution and accessible to solution changes. In this recording configuration, the recorded current (suction pipette current) represents the transduction current which enters at the cilia and exits at the cell body. In addition, since the intracellular voltage is free to vary, ORNs can generate action potentials, which are also recorded as typically biphasic, fast current transients. The suction current was filtered at DC–5000 Hz (−3 dB, 8-pole Bessel filter) or DC–50 Hz (−3 dB, 8-pole Bessel filter) to only record the slow receptor current. The sampling frequency was 10 kHz. Currents were recorded with a Warner PC-501A patch clamp amplifier (Warner Instruments, LLC, Hamden, CT, USA), and digitized using a Mikro1401 A/D converter and Signal acquisition software (Cambridge Electronic Design, Cambridge, UK).
ORNs were stimulated by rapidly transferring the tip of the recording pipette (holding the ORN) across the interface of neighbouring streams of solutions emerging from a three-barrelled glass pipette using the Perfusion Fast-Step solution changer (Warner Instruments). The speed of solution exchange was determined by recording the junction current arising from stepping the recording pipette from normal into 10% diluted Ringer solution. A delay of 28 ms (measured at half-height) occurred between the command pulse to initiate the solution exchange at t = 0 and the actual solution exchange itself and was subtracted from all timing data for example spike delays or time to peak. Solution exchange was complete within 7 ms as determined from the 10–90% rise- or fall time of the junction current. Single cell experiments were performed at mammalian body temperature by heating the solutions just prior to entering the recording chamber to 37°C using a flow heater modified from Matthews (1999).
For single cell recordings from ORNs still situated in the intact epithelium the olfactory epithelium was peeled off the septum and turbinates and stored in mammalian Ringer solution at 4°C. Pieces of epithelium up to 2–3 mm long were transferred to a custom-built recording chamber mounted on the stage of a Nikon E600FN upright microscope. The tissue was held down with a platinum wire harp (a ∼1.5 cm long wire bent in a horseshoe shape with 3 strings across) while the recording chamber was continuously perfused with mammalian Ringer solution (composition below) at room temperature. Dendritic knobs were visualized with a 60×/1.0 NA Nikon Fluor water immersion objective along with DIC optics and a camera connected to a TV monitor. Whole epithelium experiments were performed at room temperature.
Action potentials were recorded as capacitatively coupled currents in loose patch (30–50 MΩ seal resistance) cell-attached voltage clamp recordings from dendritic knobs using 7–8 MΩ patch pipettes. The pipettes were filled with mammalian Ringer solution and knobs were patched under visual guidance. Knobs were approached with positive pressure in the pipette. Upon touching the knob the positive pressure was released usually resulting in an increase in resistance. Spikes were clearly distinguished as biphasic current deflections. The voltage-clamp recordings were performed with a HEKA EPC10USB double patch clamp (with built-in LiH8 + 8 AD/DA converter) and the data, sampled at 10 kHz and filtered at 3 kHz using the built-in 4-pole Bessel filter, were stored on a PC running PatchMaster software (HEKA Elektronik, Lambrecht/Pfalz, Germany). The PatchMaster also provided synchronized voltage pulses that triggered a valve driver (General Valve Corp. Fairfield, NJ, USA) that, in turn, opened a three-way solenoid valve connected to a puffer pipette. With the valve open the pipette was pressurized to approx. 3–4 p.s.i. for 1 s. The puffer pipette was a patch pipette filled with 1 mm 3-isobutyl-1-methylxanthine (IBMX) in Ringer solution. Double puffs with varying inter-puff interval were delivered.
Mammalian Ringer solution contained (in mm): 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 0.01 EDTA, 10 Hepes, and 10 glucose. The pH was adjusted to 7.5 with NaOH. Cineole solutions were made from a 1 mm stock. In the low Na+ solution, 85 mm NaCl was replaced with equimolar choline chloride. Heptanal and eugenol solutions were prepared daily from a 20 mm dimethyl sulphoxide (DMSO) stock. All chemicals, including IBMX, were purchased from Sigma (St Louis, MO, USA).
Results
Olfactory receptor neuron processing of stimulation patterns resembling breathing
To study ORN function in response to repetitive stimulation we used the suction pipette technique. ORNs were exposed to odorants for 0.1 s and returned to normal Ringer solution for 0.4 s for 30 cycles to approximate a baseline breathing pattern of 2 Hz. Judging from breathing records in rats (Kepecs et al. 2007; Verhagen et al. 2007) and the average breathing rate of 2.3 Hz for 129SVEV mice (Tankersley et al. 1994), this pattern was considered a realistic approximation. ORNs were not exposed to odorants for more than 30 times per trial to avoid overstimulation and quick rundown. When stimulated at 2 Hz at the low cineole concentration of 30 μm an ORN responded to the first exposure with a large receptor current with associated action potential (AP) firing, and thereafter quite reliably with a short burst of APs driven by small underlying increases in receptor current (Fig. 1A and see also Fig. 2A with an expanded time scale to see individual APs). This ORN failed to generate APs to two of the 29 stimuli at 2 Hz (7%‘misses’). The first response at t = 0 was excluded from this analysis, since it did not have a preceding stimulation.
Figure 1. Olfactory receptor neuron action potential generation depends on frequency of stimulation.
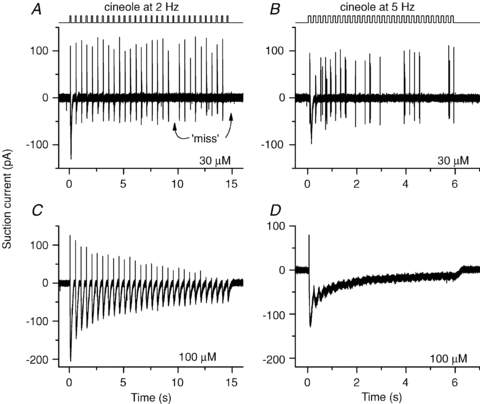
An isolated olfactory receptor neuron was stimulated repeatedly 30 times with the odorant cineole for 100 ms as indicated by the solution monitor at the top. The response was recorded using the suction pipette technique. The interstimulation interval was either 400 or 100 ms, which gave stimulation frequencies of 2 Hz (A and C) or 5 Hz (B and D), respectively. Cineole concentrations were 30 μm (A and B) or 100 μm (C and D) as noted next to each recording. All recordings from the same neuron.
Figure 2. Changes in action potential patterns with increased stimulation frequency and odorant concentration.
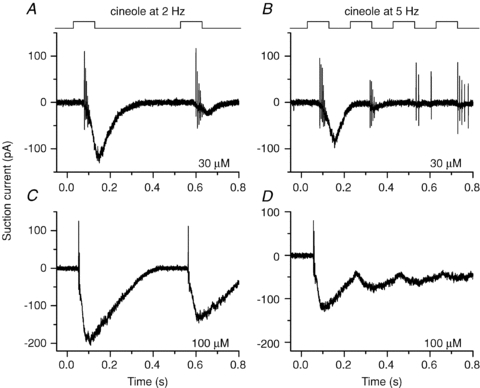
Beginning of the same recordings as in Fig. 1, on an expanded time scale to resolve individual action potentials.
A sniff rate of 5 Hz was simulated by shortening the inter-odorant recovery phase to 0.1 s, in accordance with the shortened exhalation (but not inhalation) phase during high-frequency sniffing (Kepecs et al. 2007). 129SVEV mice can maintain breathing rates over 5 Hz (Tankersley et al. 1994). During a 5 Hz stimulation sequence the ORN did not respond to and generate APs at each stimulation and (Figs 1B and 2B) the failure rate increased greatly (40% misses). Additionally, a phase shift was introduced, since the delay for the first spike generated at each cineole exposure increased from 96 ms at 2 Hz to 110 ms at 5 Hz. Therefore, when stimulated at 5 Hz the ORN, on average, only began to fire after each stimulation ceased. Overall, the cell became less reliable in tracking the odour changes when stimulated at 5 Hz. At 100 μm the ORN generated quite large receptor currents in response to every 2 Hz stimulation and still fired APs at every exposure (Figs 1C and 2C), unlike its response when stimulated at 5 Hz (Figs 1D and 2D). At 5 Hz the receptor current responses of individual responses began to merge to form a continuous standing current, preventing the cell from hyperpolarizing to de-inactivate voltage-gated Na+ and Ca2+ channels (Trotier, 1994; Kawai et al. 1997) and thus no APs were triggered; hence this ORN failed entirely to code for the changes in odorant concentration at the AP level. Similar results have been observed in 12 ORNs. In some ORNs, stimulation with even higher odorant concentrations abolished spike firing at the 2 Hz stimulation rate. We also tried to stimulate at 10 Hz with an odorant exposure shortened to 50 ms. In rare cases where the receptor current responses stayed small and did not merge between individual stimulations, APs were generated throughout, but spike patterns began not to be phase-locked to the stimulations, and it became impossible to determine which of the odorant exposures triggered which burst of APs.
Effects of changes in stimulus frequency
Since a change from low- to high-sniff frequency is often achieved in a single respiratory cycle (Kepecs et al. 2007; Verhagen et al. 2007), we exposed ORNs first to odorants at 2 Hz, followed by a ‘sniff’ bout at 5 Hz (Fig. 3), and again the stimulus duration was kept at 0.1 s in both cases. We chose a mouse line which has been genetically engineered to carry GFP in ORNs which express the eugenol receptor mOR-EG (Oka et al. 2006). This allowed us to record from ORNs with a known receptor and ligand (eugenol) instead of randomly picked ORNs expressing unknown ORs (with greatly varying sensitivity to cineole) as done above. As these identified ORNs have similar odorant sensitivity (Oka et al. 2006), this also enabled us to subsequently average results obtained from several mOR-EG ORNs (see Fig. 5). At 0.3 μm and 1 μm eugenol the ORN generated occasional APs phase-locked with the stimulus, and showed little AP firing once switched to 5 Hz. At 3 μm up to 100 μm the ORN reliably generated bursts of APs and large receptor currents of roughly equal size at every 2 Hz exposure. At 5 Hz APs were generated at ∼50% of stimulations at 3 μm but at 10 μm fewer APs were generated and at 30 and 100 μm firing ceased entirely. We conclude that the switch to the higher frequency stimulation at higher odorant concentrations actually leads to a reduction of information conveyed to the olfactory bulb since AP firing was abolished at high concentrations.
Figure 3. Change in stimulation frequency alters action potential firing in olfactory receptor neurons expressing the mOR-EG odorant receptor.
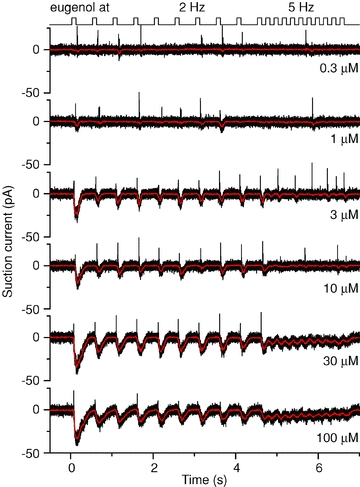
Suction pipette recordings from an olfactory receptor neuron expressing the mOR-EG odorant receptor, stimulated at increasing concentrations of eugenol, the mOR-EG agonist. A stimulus train at 2 Hz was followed by 5 Hz stimulations; each odorant exposure was 100 ms. Recording bandwidth 0–5 kHz (black) to display action potentials and 0–50 Hz (red) to show the underlying receptor current.
Figure 5. Action potential firing rate modulation and response delay during low and high frequency stimulation.
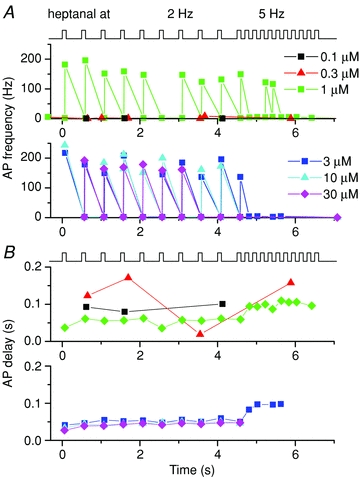
A, action potential firing rate of an olfactory receptor neuron that expresses the I7 odorant receptor during 2 and 5 Hz stimulations at the six heptanal concentrations as indicated. B, delay of the first AP generated in response to each stimulus. Analysis of the data shown in Fig. 4.
The same set of experiments was also performed on a mouse line which expressed GFP with the I7 odorant receptor (Bozza et al. 2002) and ORNs showing GFP fluorescence were stimulated with the I7 ligand heptanal. In general, I7-expressing ORNs showed a similar pattern to mOR-EG ORNs: an increase in stimulation frequency and an increase in heptanal concentration led to the generation of fewer bursts of APs with each stimulation (Fig. 4), suggesting that the observed phenomena are not restricted to individual ORNs expressing a given OR, but are a general effect.
Figure 4. Change in stimulation frequency alters spike firing in olfactory receptor neurons expressing the I7 odorant receptor.
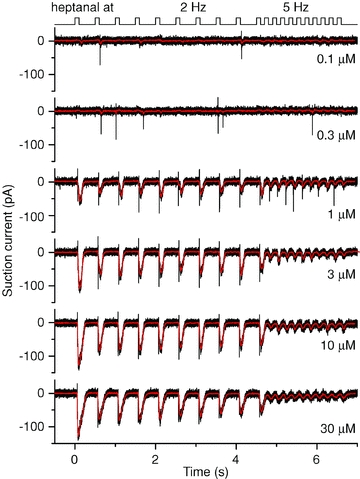
Suction pipette recordings from an olfactory receptor neuron expressing the I7 odorant receptor stimulated at increasing concentrations of heptanal, the I7 agonist. A stimulus train at 2 Hz was followed by 5 Hz stimulations; each odorant exposure was 100 ms. Recording bandwidth 0–5 kHz (black) and 0–50 Hz (red).
AP patterns were investigated by calculating the AP firing rate as the inverse of the inter-spike interval: response (AP) delay as the time difference between the arrival of odorant solution and the generation of the first AP (Reisert & Matthews, 1999). Figure 5 shows such an analysis for the data in Fig. 4 obtained from an I7 ORN. The maximal AP firing rate increases in a dose-dependent manner for the first stimulation, and stays relatively stable over subsequent 2 Hz stimulations (Fig. 5A). The AP delay (Fig. 5B) is, as shown previously (Reisert & Matthews, 1999), reduced with an increase in odorant concentration, but increased upon switching to 5 Hz stimulation.
Summarized data from cohorts of mOR-EG or I7 odorant receptor expressing ORNs are shown in Fig. 6. The latency for the first AP to be generated in response to the first odorant exposure during the stimulus sequence decreased progressively when the odorant concentration was increased (Fig. 6A and B for mOR-EG and I7 ORNs stimulated with eugenol or heptanal, respectively). For low odorant concentrations ORNs typically responded only after the 100 ms stimulus pulse had ceased and declined to ∼40 ms for mOR-EG ORNs and even further to as short as ∼25 ms for I7 ORNs. The smaller minimal latency for I7 ORNs is possibly due to the higher odorant concentration (300 μm for I7 ORNs vs. 100 μm for mOR-EG ORNs) used.
Figure 6. Response delay and reliability of action potential generation.
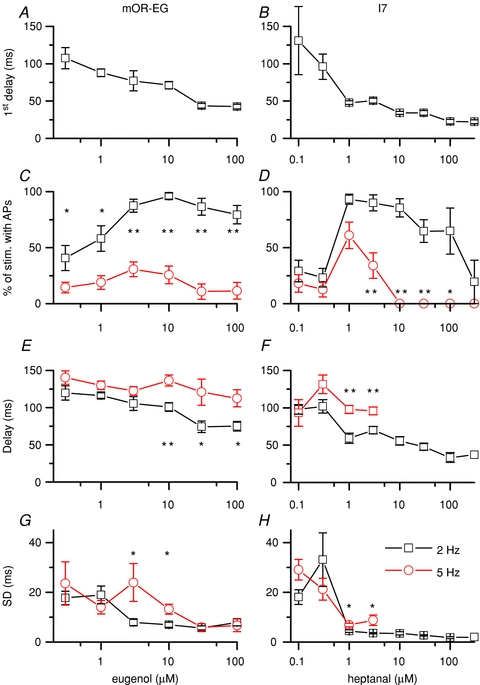
Analysis of the recordings from mOR-EG and I7 ORNs. A and B, the delay of the first action potential generated in response to the first eugenol or heptanal stimulus at t = 0, respectively. C and D, percentage of stimulations that elicited action potentials at 2 and 5 Hz. E and F, delay of action potential firing averaged over all 2 and 5 Hz odorant stimulations in a given sweep. G and H, average of the standard deviations obtained from each cell for the AP delay. Data points are means ± SEM of 9–15 ORNs for mOR-EG ORNs and 3–17 for I7 ORNs. Statistical significance: *P < 0.05 and **P < 0.005, two sample t test.
The data were further analysed by calculating the percentage chance that APs were generated during either a 2 or a 5 Hz stimulation train. For all subsequent analysis the first stimulation at the beginning of the train of stimuli (t = 0 s) was excluded. At 2 Hz the chance of APs being fired increased with odorant concentration (fewer ‘misses’) and ORNs generated APs with near 100% fidelity at intermediate concentrations (Fig. 6C and D). At high concentration the chance of AP generation decreased and began to fail nearly entirely at very high concentrations for I7 ORNs. At 5 Hz stimulation rate a similar overall increase and subsequent decrease in probability to generate APs was observed but at a reduced level, demonstrating that an increase in stimulation frequency actually leads to reduced AP output. For mOR-EG ORNs AP generation never improved to more than ∼25% while for I7 ORNs it at least reached 50% at 1 μm heptanal, but decreased precipitously thereafter, and at concentrations higher than 3 μm, I7 ORNs fell silent and no APs were generated.
Two parameters were extracted from the delay of APs being generated during a stimulus train: the average delay for all responses during 2 and 5 Hz at a given concentration and the standard deviation of this average, which is a measure of the preciseness with which ORNs can phase-lock their responses to a stimulus. The delay during stimulus trains, similar to the delay to the first stimulation, decreased with an increase in odorant concentration, but was longer during 5 Hz than during 2 Hz stimulation (Fig. 6E and F). Thus a higher stimulus frequency introduces a phase shift to longer delays by around 50 ms. The jitter (SD) also decreased quickly with an increase in odorant concentration at 2 Hz (Fig. 6G and H) but remained elevated for intermediate concentrations at 5 Hz compared to 2 Hz. Hence at higher odorant concentrations, ORNs encode the time of odorant arrival more precisely, while higher stimulus frequencies degrade the phase locking between stimulus and response. In conclusion, an increase in stimulus frequency leads to less precise coding of the arrival time of the stimulus at intermediate concentrations.
ORNs lose most of their axon during the isolation process, which could alter their ability to generate APs, as could the isolation procedure itself. Thus we investigated if an increase in stimulus frequency can also lead to a failure to elicit APs in ORNs still situated in the intact epithelium to control for the possibility of cell isolation-induced artifacts. On-cell loose patch recordings from dendritic knobs of ORNs in the olfactory epithelium were performed (Ma et al. 1999). In this configuration APs are recoded as capacitatively coupled current transients (Fig. 7). Responses were elicited using the phosphodiesterase inhibitor IBMX, a feasible alternative to odorant stimulation since it elicits responses with similar time courses (Reisert et al. 2007). ORNs were stimulated for 1 s twice in succession, with decreasing interpulse intervals as done before using isolated ORNs and the suction pipette technique (Song et al. 2008) where it was found that the recovery time course in a double pulse protocol was similar to odorant stimulation. A short train of APs was observed during the first IBMX stimulation (Fig. 7). The spike amplitude quickly declined and AP firing ceased. When a stimulus interval of 1.5 s was used (Fig. 7, top), the ORN failed to generate APs in response to the second IBMX exposure, while after the longer interpulse interval of 2.5 s (bottom), APs were generated again at full amplitude with subsequent amplitude decline. Note that APs reappeared at full spike height and did not re-emerge progressively from the recording noise after the end of stimulation. Similar results were obtained in a total of five ORNs. These results are qualitatively similar to the results obtained with isolated ORNs: an increase in stimulus frequency (shorter interpulse interval) abolishes AP generation. Thus, the observation that AP firing is prevented by high frequency stimulation persists in the intact epithelium and is not an artifact of the ORN isolation. It should be noted that in the previous isolated ORN recordings using IBMX, spiking typically occurred in nearly all ORNs after the short interpulse interval of 1 s (Song et al. 2008). It is not surprising that longer interpulse periods are required for APs to be generated again under the experimental conditions here compared to the suction pipette recordings since the experiments in Fig. 7 were performed at room temperature and solution exchange is considerably slower in the whole epithelium preparation, around 150 ms (Reisert, 1998).
Figure 7. Failure to generate action potentials at higher stimulus frequencies persists in olfactory receptor neurons in the intact epithelium.
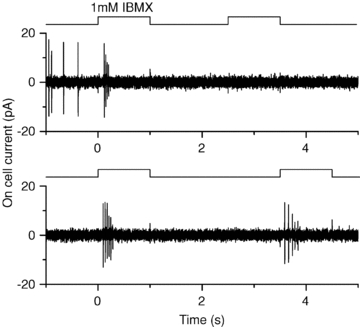
On-cell loose seal recording from a dendritic knob of an olfactory receptor neuron situated in the intact epithelium. The ORN was stimulated with the phosphodiesterase inhibitor IBMX twice for 1 s with an interpulse interval of 1.5 s (top) and 2.5 s (bottom) respectively. First IBMX exposure in both cases at t = 0 s. Experiments were performed at room temperature. Recordings filtered 5–3000 Hz.
Fast response termination is essential to maintain high fidelity
Which aspect of the odorant response is important to enable ORNs to fire APs reliably during repeated stimulation? We investigated the rate of response termination. A major accelerator of response termination is the ciliary Na+/Ca2+ exchanger. Prevention of Ca2+ extrusion by lowering external Na+ leads to a prolonged Ca2+-activated Cl− current, prolonged depolarization and continued adaptation (Reisert & Matthews, 1998, 2001a). The rat mucosal Na+ concentration has been reported to be 55 mm (Reuter et al. 1998), low compared to interstitial Na+. This is just slightly less than the reported Kd of 62 mm external Na+ for the amphibian ciliary Na+/Ca2+ exchanger (Antolin & Matthews, 2007) and thus could begin to slow Na+/Ca2+ exchange and fast response termination which could greatly influence the AP patterns in response to high-frequency stimulation. Na+ was lowered to 55 mm in the solution superfusing the cilia (equimolar replacement with choline, which does not support Na+/Ca2+ exchange in ORNs; Reisert & Matthews, 2001a). Responses to repetitive odorant pulses were compared to the results obtained in normal Ringer solution to investigate the contribution of an altered, more mucus-like ionic environment to odorant-elicited AP patterns. A eugenol concentration of 10 μm was used, which causes mOR-EG-GFP ORNs to fire most reliably when stimulated at 2 Hz (see Figs 3 and 6). When the external solution was normal Ringer solution (containing 140 mm Na+), ORNs reliably generated APs at every odour exposure (Fig. 8) and the receptor current fell with a time course of 0.16 ± 0.01 s (mean ± SEM, average of 6 ORNs, decay of the first response was used to determine the fall in current, which was fitted with a single exponential). But when the Na+ concentration was reduced to 55 mm, the response recovery slowed slightly to 0.19 ± 0.02 s (n = 6). This slowing was sufficient to reduce the likelihood of AP generation on average from 87 ± 8% in normal Ringer solution to only 4 ± 2% in 55 mm Na+ (6 cells, statistically different, t test at 0.005 level). When the external Na+ concentration was reduced to 0 mm, AP generation failed completely after the first odorant exposure (not shown, decay time constant 0.26 ± 0.01 s, n = 6). The lack of AP firing is not due to the lack of a Na+ gradient, since only the cilia were exposed to low Na+ solutions and not the cell body, which resided inside the suction pipette, containing normal Ringer solution. Also, APs were generated during the first odorant exposure. In conclusion, even only subtle prolongations of the response recovery time course drastically reduce ORNs’ ability to follow rapid stimulations.
Figure 8. The contribution of external Na+ concentration to the likelihood of action potential generation.
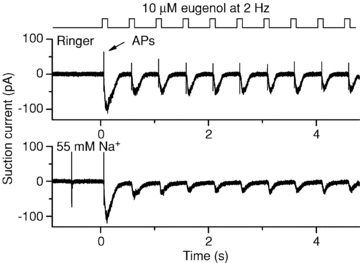
A mOR-EG ORN was exposed to its ligand eugenol (10 μm) at a stimulation frequency of 2 Hz in normal Ringer solution (top) or in reduced external Na+ Ringer solution (55 mm, replaced with choline+, bottom). Low Na+ exposure began 1 s before the first odorant exposure. Note the complete lack of action potentials after the first stimulation.
Discussion
We investigated the interplay between the frequency of odorant stimulation and action potential generation in ORNs. The first fundamental question is whether or not olfactory transduction can generate a receptor current with kinetics fast enough to follow stimulation patterns reflecting the natural stimulation of ORNs in situ during resting breathing and exploratory sniffing. Second, what kind of AP output does the receptor current generate and how does it represent the stimulus? In other words, do ORNs simply convert and relay odorous information or do they contribute to the processing of the signal as well?
We show here that isolated ORNs can, with high fidelity, respond to repeated odorant stimulation with AP generation at stimulation frequencies which resemble resting respiratory patterns, particularly at intermediate (1–30 μm for the I7 and mOR-EG expressing ORNs) odour concentrations. Individual ORNs begin to fail to reliably encode odorant stimuli at higher odorant concentrations where the termination of the receptor current response is not fast enough to allow ORNs to be hyperpolarized before the onset of the next stimulation, leading to failure to generate APs. An increase in stimulus frequency from 2 to 5 Hz caused ORNs to respond less reliably to each stimulation even at intermediate odorant concentrations. ORNs showed near complete inability to generate APs at higher odorant concentrations in response to the still present recurring increases in receptor current. As such, individual ORNs filter out stimuli at high odour concentration and/or low-pass filter stimuli at higher (5 Hz) stimulus frequencies. Furthermore, an increase in stimulus frequency also phase-shifted the AP response to later times compared to the stimulus onset, as well as increasing the timing uncertainty (jitter) with which APs are generated. But it should be pointed out that while for example I7-expressing ORNs fail to reliably signal at 5 Hz at heptanal concentrations above 3 μm, ORNs which express a less sensitive hepatanal receptor might follow the same stimulation with reasonable accuracy. This could ensure that the odorant can still be perceived, although by a different set of ORNs.
Do the response patterns observed in isolated ORNs resemble those of ORNs in the intact epithelium? First, the loss of most of the axon could alter ORN AP generation, but we believe that this is not likely to be the case, since we observed qualitatively similar response patterns from ORNs in the intact epithelium (see Fig. 7). Also, in vivo work done in rats at mammalian body temperature (Rospars et al. 2008) shows similar properties compared to isolated ORNs in term of maximal spike rate, dose–response relation and shortening of the AP train and cessation of AP firing at higher odorant concentrations. Second, once the AP amplitude declines during a spike train, does spiking truly cease or is, for example, the spike initiation zone simply moved further along the axon and APs continue to be generated and send to the olfactory bulb? If that were the case the AP amplitude (during in vivo recordings) should progressively re-emerge at the end of a stimulus-induced depolarization when the AP initiation zone moves back closer to the cell body. While this is observed (Getchell & Shepherd, 1978; van Drongelen, 1978) it is also observed that APs reappear at full amplitude (see e.g. Sicard & Holley, 1984; Reisert & Matthews, 2001b and this publication). This suggests that after rapid AP-amplitude collapse, AP firing truly ceases.
How will the mucus that lines the nasal cavity contribute to odorant response kinetics and AP coding? Compared to the rapid application and removal of odorant in the isolated ORN experiments, odorant application and removal from the complex structure of the intact nose is probably slower as odorant has to diffuse into and clear from the mucus. This notion is supported by mathematical modelling of temporal odorant deposition in the olfactory mucosa (Zhao & Jiang, 2008). This suggests that our results represent a ‘best case’ scenario and that, as the odorant concentration is likely to change more slowly in the mucus, ORNs in the intact nose might be even more susceptible to suppression of AP generation during rapid sniffing. On the other hand bulbar recordings of presynaptic Ca2+ signals suggest that odorants clear within one breathing cycle from the olfactory epithelium during resting rates of breathing (Carey et al. 2009).
A second issue related to the mucus layer is the different mucosal ionic environment surrounding the cilia compared to interstitial ion concentrations. A limiting factor for ORNs to fire APs in response to high frequency stimulation is the time required for response termination after the end of odour application. A major contributor to response termination is Na+-dependent Ca2+ extrusion, which leads to rapid Cl− channel closure and receptor current termination (Reisert & Matthews, 1998, 2001a). Lowering external Na+ to the reported mucosal Na+ concentration of 55 mm (Reuter et al. 1998), which is also close to the Kd of the amphibian Na+/Ca2+ exchanger (Antolin & Matthews, 2007), almost entirely abolished spike generation even during 2 Hz stimulation (Fig. 8). Furthermore, the olfactory Na+/Ca2+ exchanger has recently been identified as being NCKX4 and hence also K+ dependent (Stephan et al. 2010). The mucosal K+ concentration has been reported to be high compared to interstitial fluid and around 69 mm (Reuter et al. 1998). This could potentially further slow Ca2+ extrusion and therefore response termination. Nevertheless, in an air-phase electroolfactogram, where the natural mucosal ion concentration is maintained, knocking out NCKX4 greatly prolongs the odorant-induced response compared to wild-type, demonstrating that NCKX4 still functions under the ionic gradients across the ciliary membrane (Stephan et al. 2010). How response termination precisely affects AP generation during repetitive odorant stimulation in situ in the nasal cavity during air-phase stimulation remains to be investigated.
Besides Na+/Ca2+ exchange what other transduction components could be important for controlling the response time course? Surprisingly, both the odorant receptor, which has a very short life time (Bhandawat et al. 2005), and phosphodiesterase (PDE) contribute little to controlling the overall time course of the response (Cygnar & Zhao, 2009). We recently investigated a subtle response prolongation (50 ms) caused by the lack of a calmodulin binding site in the B1b subunit of the CNG channel (Song et al. 2008). This prolongation led to a reduced ability to generate APs in response to the second odour stimulus in a double pulse stimulation protocol for short (high frequency) interpulse intervals. Importantly, we observed equivalent reductions in bulbar odorant-induced responses in a double pulse stimulation protocol in this mouse line (Song et al. 2008). These results also suggest that ‘low-pass filtering’ properties of the mucus do not slow the odorant response to such an extent as to make it the dominant time constant during sniffing.
How is odorous information represented in the olfactory bulb and is it modulated by odorant stimulation frequency? Often recordings from mitral cells in the bulb are done under anaesthetized conditions when breathing frequencies are typically low and constant, thus representing breathing at rest. Odorant-induced AP firing of mitral cells in anaesthetized animals typically occurs in bursts synchronized with the breathing cycle and mitral cells fire quite reliably during each breathing cycle (Chaput et al. 1992; Imamura et al. 1992; Sobel & Tank, 1993; Cang & Isaacson, 2003, Macribes & Chorover, 1972; Rinberg et al. 2006a; Bathellier et al. 2008). Mitral cells showed a reduced number of APs generated in each stimulation cycle when the stimulus frequency was increased by either recording from awake animals, often rabbit (which could increase their sniffing rate), or from artificially ventilated doubly-tracheotomized anaesthetized animals (where the stimulus frequency could be changed experimentally). Also the burst onset was phase-shifted to later times (Potter & Chorover, 1976; Chaput & Holley, 1985; Bathellier et al. 2008). Mitral cells began to spike randomly without being phase-locked to the breathing cycle at high sniff frequencies (du Pont, 1987; Bhalla & Bower, 1997; Kay & Laurent, 1999). These observations are strikingly similar to the ones observed in ORNs when the stimulation frequency is increased, suggesting that at least some aspects of ORN firing behaviour and spike patterning shown here are transferred to mitral cells (as is the case in catfish during single odorant exposures; Nikonov & Caprio, 2004, 2007).
Verhagen et al. (2007) monitored odorant-induced Ca2+ transients in olfactory axon nerve terminals in the bulbar glomeruli of awake rats. They found that at low breathing frequencies Ca2+ transients were synchronized with the breathing cycle, but that sniff bouts caused a loss of synchrony and an overall reduction in response amplitude (see also Lecoq et al. 2009), a mechanism described as adaptive filtering. They proposed that an increase in sampling frequency can selectively silence responsive glomeruli in the bulb and serves as a means to subtract a constantly present odour, thus emphasizing responses of newly activated glomeruli. We show here that the primary sensory neurons, the ORNs themselves, function as stimulus frequency-dependent filters and that such stimulus frequency-dependent ORN silencing can occur within one breathing cycle at 5 Hz at high odorant concentrations. In contrast, at low odorant concentrations, when individual ORNs do not reliably respond to every 5 Hz stimulation but are reasonably phase-locked to the stimulus, the integration of all ORNs responses in a specific glomerulus could still lead to reliable coding at the glomerular level, although, depending on the integrative properties/ability of the bulb, the signal might remain small until ORNs fire reliably at every stimulation.
Mice and rats can distinguish odorants within around 200 ms (Uchida & Mainen, 2003; Abraham et al. 2004; Rinberg et al. 2006b). Most of these behavioural experiments were done at high odorant concentrations, which implies that ORN signal transduction contributed only around 25–40 ms of the overall response delay (Fig. 6A and B). The variance of onset of AP generation (Fig. 6G and H) is also very low (2–5 ms) at these concentrations, suggesting that ORNs generated APs in close time proximity to convey to the glomeruli in the bulb, assuming that the inhaled odorant reaches all responsive ORNs at the same time. Lower odorant concentrations substantially increase both the jitter and the delay with which ORNs respond, potentially degrading the animal's ability to discriminate odorants. How and if these response variations contribute to olfactory coding (as suggested by Schaefer & Margrie, 2007) remains to be explored experimentally.
Acknowledgments
We would like to thank Drs A. Gelperin and G. Lowe for critical and constructive reading of the manuscript and Dr D. Reed and F. Duke for help with mouse genotyping. Drs T. Bozza and P. Mombaerts generously made the I7-GFP mouse line available as did Dr K. Touhara for the mOR-EG-GFP mouse line. The work was supported by the Monell Chemical Senses Center, a Morley Kare Fellowship (to J.R.) and a National Institutes of Health grant to J.R. (DC009613). The authors have no conflict of interest.
Glossary
Abbreviations
- AP
action potential
- CNG
cyclic nucleotide-gated
- OR
odorant receptor
- ORN
olfactory receptor neuron
Author contributions
A.S.G. performed the whole epithelial and J.R. the suction pipette experiments. J.R. analysed and interpreted data and wrote the final version of the manuscript. Both authors approved the final version of the manuscript.
References
- Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron. 2004;44:865–876. doi: 10.1016/j.neuron.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Antolin S, Matthews HR. The effect of external sodium concentration on sodium-calcium exchange in frog olfactory receptor cells. J Physiol. 2007;581:495–503. doi: 10.1113/jphysiol.2007.131094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathellier B, Buhl DL, Accolla R, Carleton A. Dynamic ensemble odor coding in the mammalian olfactory bulb: sensory information at different timescales. Neuron. 2008;57:586–598. doi: 10.1016/j.neuron.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Bhalla US, Bower JM. Multiday recordings from olfactory bulb neurons in awake freely moving rats: spatially and temporally organized variability in odorant response properties. J Comput Neurosci. 1997;4:221–256. doi: 10.1023/a:1008819818970. [DOI] [PubMed] [Google Scholar]
- Bhandawat V, Reisert J, Yau KW. Elementary response of olfactory receptor neurons to odorants. Science. 2005;308:1931–1934. doi: 10.1126/science.1109886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Boccaccio A, Lagostena L, Hagen V, Menini A. Fast adaptation in mouse olfactory sensory neurons does not require the activity of phosphodiesterase. J Gen Physiol. 2006;128:171–184. doi: 10.1085/jgp.200609555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza T, Feinstein P, Zheng C, Mombaerts P. Odorant receptor expression defines functional units in the mouse olfactory system. J Neurosci. 2002;22:3033–3043. doi: 10.1523/JNEUROSCI.22-08-03033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Isaacson JS. In vivo whole-cell recording of odor-evoked synaptic transmission in the rat olfactory bulb. J Neurosci. 2003;23:4108–4116. doi: 10.1523/JNEUROSCI.23-10-04108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RM, Verhagen JV, Wesson DW, Pirez N, Wachowiak M. Temporal structure of receptor neuron input to the olfactory bulb imaged in behaving rats. J Neurophysiol. 2009;101:1073–1088. doi: 10.1152/jn.90902.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput MA, Buonviso N, Berthommier F. Temporal patterns in spontaneous and odour-evoked mitral cell discharges recorded in anaesthetized freely breathing animals. Eur J Neurosci. 1992;4:813–822. doi: 10.1111/j.1460-9568.1992.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Chaput MA, Holley A. Responses of olfactory bulb neurons to repeated odor stimulations in awake freely-breathing rabbits. Physiol Behav. 1985;34:249–258. doi: 10.1016/0031-9384(85)90113-1. [DOI] [PubMed] [Google Scholar]
- Cygnar KD, Zhao H. Phosphodiesterase 1C is dispensable for rapid response termination of olfactory sensory neurons. Nat Neurosci. 2009;12:454–462. doi: 10.1038/nn.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Pont JS. Firing patterns of bulbar respiratory neurones during sniffing in the conscious, non-paralyzed rabbit. Brain Res. 1987;414:163–168. doi: 10.1016/0006-8993(87)91340-0. [DOI] [PubMed] [Google Scholar]
- Gesteland RC, Lettvin JY, Pitts WH. Chemical transmission in the nose of the frog. J Physiol. 1965;181:525–559. doi: 10.1113/jphysiol.1965.sp007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell TV, Shepherd GM. Responses of olfactory receptor cells to step pulses of odour at different concentration in the salamander. J Physiol. 1978;282:521–540. doi: 10.1113/jphysiol.1978.sp012479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K, Mataga N, Mori K. Coding of odor molecules by mitral/tufted cells in rabbit olfactory bulb. 1. Aliphatic compounds. J Neurophysiol. 1992;68:1986–2002. doi: 10.1152/jn.1992.68.6.1986. [DOI] [PubMed] [Google Scholar]
- Kaupp UB. Olfactory signalling in vertebrates and insects: differences and commonalities. Nat Rev Neurosci. 2010;11:188–200. doi: 10.1038/nrn2789. [DOI] [PubMed] [Google Scholar]
- Kawai F, Kurahashi T, Kaneko A. Quantitative analysis of Na+ and Ca2+ current contributions on spike initiation in the newt olfactory receptor cell. Jpn J Physiol. 1997;47:367–376. doi: 10.2170/jjphysiol.47.367. [DOI] [PubMed] [Google Scholar]
- Kay LM, Laurent G. Odor- and context-dependent modulation of mitral cell activity in behaving rats. Nat Neurosci. 1999;2:1003–1009. doi: 10.1038/14801. [DOI] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Mainen ZF. The sniff as a unit of olfactory processing. Chem Senses. 2005;31:167–179. doi: 10.1093/chemse/bjj016. [DOI] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Mainen ZF. Rapid and precise control of sniffing during olfactory discrimination in rats. J Neurophysiol. 2007;98:205–213. doi: 10.1152/jn.00071.2007. [DOI] [PubMed] [Google Scholar]
- Kleene SJ. The electrochemical basis of odor transduction in vertebrate olfactory cilia. Chem Senses. 2008;33:839–859. doi: 10.1093/chemse/bjn048. [DOI] [PubMed] [Google Scholar]
- Laing DG. Natural sniffing gives optimum odour perception for humans. Perception. 1983;12:99–117. doi: 10.1068/p120099. [DOI] [PubMed] [Google Scholar]
- Lecoq J, Tiret P, Charpak S. Peripheral adaptation codes for high odor concentration in glomeruli. J Neurosci. 2009;29:3067–3072. doi: 10.1523/JNEUROSCI.6187-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G, Gold GH. The spatial distributions of odorant sensitivity and odorant-induced currents in salamander olfactory receptor cells. J Physiol. 1991;442:147–168. doi: 10.1113/jphysiol.1991.sp018787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma MH, Chen WR, Shepherd GM. Electrophysiological characterization of rat and mouse olfactory receptor neurons from an intact epithelial preparation. J Neurosci Methods. 1999;92:31–40. doi: 10.1016/s0165-0270(99)00089-8. [DOI] [PubMed] [Google Scholar]
- Mainland J, Sobel N. The sniff is part of the olfactory percept. Chem Senses. 2006;31:181–196. doi: 10.1093/chemse/bjj012. [DOI] [PubMed] [Google Scholar]
- Matthews HR. A compact modular flow heater for the superfusion of mammalian cells. J Physiol. 1999;518.P:13P. [Google Scholar]
- Macribes F, Chorover SL. Olfactory bulb units: activity correlated with inhalation cycles and odor quality. Science. 1972;175:84–87. doi: 10.1126/science.175.4017.84. [DOI] [PubMed] [Google Scholar]
- Nikonov AA, Caprio J. Odorant specificity of single olfactory bulb neurons to amino acids in the channel catfish. J Neurophysiol. 2004;92:123–134. doi: 10.1152/jn.00023.2004. [DOI] [PubMed] [Google Scholar]
- Nikonov AA, Caprio J. Responses of olfactory forebrain units to amino acids in the channel catfish. J Neurophysiol. 2007;97:2490–2498. doi: 10.1152/jn.01198.2006. [DOI] [PubMed] [Google Scholar]
- Oka Y, Katada S, Omura M, Suwa M, Yoshihara Y, Touhara K. Odorant receptor map in the mouse olfactory bulb: in vivo sensitivity and specificity of receptor-defined glomeruli. Neuron. 2006;52:857–869. doi: 10.1016/j.neuron.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Potter H, Chorover SL. Response plasticity in hamster olfactory bulb: peripheral and central processes. Brain Res. 1976;116:417–429. doi: 10.1016/0006-8993(76)90490-x. [DOI] [PubMed] [Google Scholar]
- Reisert J. Physiological Laboratory. Cambridge: Cambridge University; 1998. Transduction and adaptation in vertebrate olfactory receptor cells; p. 178. [Google Scholar]
- Reisert J, Matthews HR. Na+-dependent Ca2+ extrusion governs response recovery in frog olfactory receptor cells. J Gen Physiol. 1998;112:529–535. doi: 10.1085/jgp.112.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Adaptation of the odour-induced response in frog olfactory receptor cells. J Physiol. 1999;519:801–813. doi: 10.1111/j.1469-7793.1999.0801n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Response properties of isolated mouse olfactory receptor cells. J Physiol. 2001a;530:113–122. doi: 10.1111/j.1469-7793.2001.0113m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Responses to prolonged odour stimulation in frog olfactory receptor cells. J Physiol. 2001b;534:179–191. doi: 10.1111/j.1469-7793.2001.t01-1-00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Yau KW, Margolis FL. Olfactory marker protein modulates the cAMP kinetics of the odour-induced response in cilia of mouse olfactory receptor neurons. J Physiol. 2007;585:731–740. doi: 10.1113/jphysiol.2007.142471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter D, Zierold K, Schröder WH, Frings S. A depolarizing chloride current contributes to chemoelectrical transduction in olfactory sensory neurons in situ. J Neurosci. 1998;18:6623–6630. doi: 10.1523/JNEUROSCI.18-17-06623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Sparse odor coding in awake behaving mice. J Neurosci. 2006a;26:8857–8865. doi: 10.1523/JNEUROSCI.0884-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Speed-accuracy tradeoff in olfaction. Neuron. 2006b;51:351–358. doi: 10.1016/j.neuron.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Rospars JP, Lansky P, Chaput M, Duchamp-Viret P. Competitive and noncompetitive odorant interactions in the early neural coding of odorant mixtures. J Neurosci. 2008;28:2659–2666. doi: 10.1523/JNEUROSCI.4670-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rospars JP, Lansky P, Duchamp A, Duchamp-Viret P. Relation between stimulus and response in frog olfactory receptor neurons in vivo. Eur J Neurosci. 2003;18:1135–1154. doi: 10.1046/j.1460-9568.2003.02766.x. [DOI] [PubMed] [Google Scholar]
- Schaefer AT, Margrie TW. Spatiotemporal representations in the olfactory system. Trends Neurosci. 2007;30:92–100. doi: 10.1016/j.tins.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TA, Cleland TA. The anatomical logic of smell. Trends Neurosci. 2005;28:620–627. doi: 10.1016/j.tins.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Shibuya T, Shibuya S. Olfactory epithelium: unitary responses in the tortoise. Science. 1963;140:495–496. doi: 10.1126/science.140.3566.495. [DOI] [PubMed] [Google Scholar]
- Sicard G, Holley A. Receptor cell responses to odorants: similarities and differences among odorants. Brain Res. 1984;292:283–296. doi: 10.1016/0006-8993(84)90764-9. [DOI] [PubMed] [Google Scholar]
- Sobel EC, Tank DW. Timing of odor stimulation does not alter patterning of olfactory bulb unit activity in freely breathing rats. J Neurophysiol. 1993;69:1331–1337. doi: 10.1152/jn.1993.69.4.1331. [DOI] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Desmond JE, Glover GH, Goode RL, Sullivan EV, Gabrieli JDE. Sniffing and smelling: separate subsystems in the human olfactory cortex. Nature. 1998;392:282–286. doi: 10.1038/32654. [DOI] [PubMed] [Google Scholar]
- Song Y, Cygnar KD, Sagdullaev B, Valley M, Hirsh S, Stephan A, Reisert J, Zhao H. Olfactory CNG channel desensitization by Ca2+/CaM via the B1b subunit affects response termination but not sensitivity to recurring stimulation. Neuron. 2008;58:374–386. doi: 10.1016/j.neuron.2008.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan AB, Tobochnik S, Reisert J, Zhao H. NCKX4, a calcium regulator, efficiently terminates the olfactory response and moderates the extent of adaptation. Chem Senses. 2010;31:A17. [Google Scholar]
- Tankersley CG, Fitzgerald RS, Kleeberger SR. Differential control of ventilation among inbred strains of mice. Am J Physiol Regul Integr Comp Physiol. 1994;267:R1371–1377. doi: 10.1152/ajpregu.1994.267.5.R1371. [DOI] [PubMed] [Google Scholar]
- Trotier D. Intensity coding in olfactory receptor cells. Semin Cell Biol. 1994;5:47–54. doi: 10.1006/scel.1994.1007. [DOI] [PubMed] [Google Scholar]
- Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci. 2003;6:1224–1229. doi: 10.1038/nn1142. [DOI] [PubMed] [Google Scholar]
- van Drongelen W. Unitary responses of near threshold responses of receptor cells in the olfactory mucosa of the frog. J Physiol. 1978;277:423–435. doi: 10.1113/jphysiol.1978.sp012282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen JV, Wesson DW, Netoff TI, White JA, Wachowiak M. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nat Neurosci. 2007;10:631–639. doi: 10.1038/nn1892. [DOI] [PubMed] [Google Scholar]
- Wesson DW, Carey RM, Verhagen JV, Wachowiak M. Rapid encoding and perception of novel odors in the rat. PLoS Biol. 2008;6:e82. doi: 10.1371/journal.pbio.0060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Mozell MM, Sheehe PR, Hornung DE. A quantitative analysis of sniffing strategies in rats performing odor detection tasks. Physiol Behav. 1987;41:59–69. doi: 10.1016/0031-9384(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Zhao K, Jiang J. Spatial and temporal odorant transport patterns in rat nose: a computational study. Chem Senses. 2008;33:S88. [Google Scholar]


