Non-technical summary
In mammals, an internal timing system in the suprachiasmatic nucleus generates circadian (24 h) rhythms and communicates its circadian signal to other brain areas by means of action potentials where it regulates our daily schedules of physiological and endocrine processes. Several input pathways of the suprachiasmatic nucleus can influence the endogenous timing system and synchronize it with environmental timing cues. We show here that the inhibitory neurotransmitter glycine can modulate the activity of clock neurons and can reset their rhythmic activity depending on the phase of the daily cycle. The knowledge of these synchronizing mechanisms is of importance for understanding the consequences of perturbations of the circadian timing system that could lead to serious health impairments.
Abstract
Abstract
In mammals, the master clock in the suprachiasmatic nucleus (SCN) of the hypothalamus is composed of numerous synchronized oscillating cells that drive daily behavioural and physiological processes. Several entrainment pathways, afferent inputs to the SCN with their neurotransmitter and neuromodulator systems, can reset the circadian system regularly and also modulate neuronal activity within the SCN. In the present study, we investigated the function of the inhibitory neurotransmitter glycine on neuronal activity in the mouse SCN and on resetting of the circadian clock. The effects of glycine on the electrical activity of SCN cells from C57Bl/6 mice were studied either by patch-clamp recordings from acute brain slices or by long-term recordings from organotypic brain slices using multi-microelectrode arrays (MEA). Voltage-clamp recordings confirmed the existence of glycine-induced, chloride-selective currents in SCN neurons. These currents were reversibly suppressed by strychnine, phenylbenzene ω-phosphono-α-amino acid (PMBA) or ginkgolide B, selective blockers of glycine receptors (GlyRs). Long-term recordings of the spontaneous activity of SCN neurons revealed that glycine application induces a phase advance during the subjective day and a phase delay during the early subjective night. Both effects were suppressed by strychnine or by PMBA. These results suggest that glycine is able to modulate circadian activity by acting directly on its specific receptors in SCN neurons.
Introduction
The hypothalamic suprachiasmatic nucleus (SCN) is the primary endogenous oscillator that controls circadian rhythms of numerous behavioural, endocrine and physiological processes (Buijs & Kalsbeek, 2001). The bases for cell-autonomous circadian oscillations are interacting positive and negative transcriptional feedback loops that drive recurrent rhythms in the RNA and protein levels of several clock components (Reppert & Weaver, 2002). Circadian periodicity of the SCN persists in vitro in brain slice preparations or in isolated SCN neurons showing that it is an intrinsic phenomenon that does not depend on inputs from brain structures outside of the SCN. The major role of neural inputs to the SCN is to entrain the intrinsic circadian rhythm of the SCN to prevailing environmental cycles, a critical feature of circadian timing (Brown & Piggins, 2007). Beside the well-characterized retinohypothalamic tract pathway, a number of SCN afferents appears to influence circadian timing. The role of most of these hypothalamic and extra-hypothalamic inputs to the SCN is uncertain. These mechanisms could be responsible for synchronization of inter-neuronal time keeping producing a coherent rhythmic output or could be used for transmitting phase-resetting information to clock cells.
Neurotransmitters probably mediating the synchrony within ventral and dorsal SCN oscillators include the inhibitory transmitter GABA (Albus et al. 2005). Most, if not all, SCN cells express GABA and GABA receptors (Wagner et al. 1997), and exogenous GABA, acting through GABAA receptors, is able to synchronize individual clock cells and to phase-shift neuronal rhythms in cultured SCN neurons (Liu & Reppert, 2000). However, in SCN slices, another report suggests that GABA is possibly not required for synchrony of firing rhythms (Aton et al. 2006).
Similarly to GABA, glycine serves as a neurotransmitter at inhibitory synapses in the central nervous system, where it activates strychnine-sensitive GlyRs which, like GABAA/C receptors, belong to the pentameric nicotinic acetylcholine receptor superfamily (Betz & Laube, 2006). It can also act as co-agonist at the excitatory N-methyl-d-aspartate (NMDA) receptor. Initially, glycinergic neurons were thought to be restricted to spinal cord and brainstem (cf. Lynch, 2009); later the presence of glycinergic axons and somata was confirmed in the forebrain (Rampon et al. 1996), in areas where also GlyRs are abundantly expressed (Sato et al. 1991, 1992). A substantial number of glycine-immunoreactive fibres were observed in the hypothalamus including the anterior and posterior hypothalamus, the lateral hypothalamus, the paraventricular nucleus and the preoptic region (van den Pol & Gorcs, 1988; Rampon et al. 1996). Some glycine-positive fibres enter the suprachiasmatic nucleus, but most of the fibres seem to terminate in the periphery of the nucleus (Mahr, 2008). In the rat brain, electrophysiological studies proved the presence of strychnine-sensitive GlyRs in the SCN which suggests that glycine can act as classical inhibitory neurotransmitter as well as excitatory neuromodulator (Ito et al. 1991). Further evidence for a role of glycine in the SCN comes from organotypic slice cultures which show a circadian release of glycine (Shinohara et al. 1998), and from the observation that high concentrations of glycine can possibly reset the circadian clock in acute brain slices (Prosser et al. 2008). In order to elucidate the role of glycine in the SCN, we performed patch-clamp recordings in acute brain slices and measured additionally long-term neuronal activity in organotypic slices using multimicroelectrode arrays to investigate possible phase-shifting actions of glycine.
Methods
Animals
Acute brain slices were prepared from 3- to 4-week-old wild-type mice (C57Bl/6) and organotypic slices from 2- to 5-day-old animals. Animals were housed under a 12 h light–12 h dark cycle with lights on at 06.00 h and lights off at 18.00 h. All procedures were performed in accordance with The Journal of Physiology standards and advice (Drummond, 2009), with the law for animal experiments issued by the German Government (Tierschutzgesetz) and were approved by the local animal care committee.
Preparation of brain slices
Acute brain slices
C57Bl/6 mice of 3–4 weeks of age were used for recordings from acute brain slices. Animals were deeply anaesthetized with isoflurane (CuraMed Pharma, Karlsruhe, Germany). Following decapitation, the brains were rapidly excised and placed in ice-cold artificial cerebrospinal fluid (aCSF) gassed with 95% oxygen and 5% carbon dioxide. Coronal slices 250 μm thick containing the bilateral SCN were cut on a vibratome. Slices were then incubated at 37°C in a water bath for 1 h before they were transferred to a recording chamber and superfused with aCSF at a rate of 2 ml min−1 at room temperature using a peristaltic pump (Minipuls Gilson, France). The composition of the aCSF was (in mm): NaCl 125, KCl 2.5, NaH2PO4·1.25, CaCl2·2.0, MgCl2·1.0, NaHCO3·26 and d-glucose 25.
Organotypic slices
Animals were rapidly decapitated, and the brain was removed and placed in ice-cold aCSF containing 100 μg ml−1 penicillin–streptomycin. Coronal slices of 250–350 μm thick containing the SCN were cut on a vibratome, and placed on a Millipore culture insert (MilliCell-CM) in a 35 mm culture dish with a small amount of culture medium (ca. 1 ml) consisting of DMEM/F12 medium (Invitrogen, Karlsruhe, Germany) supplemented with 10% fetal calf serum, 2.5 mm glutamax (Invitrogen), 10 mm Hepes (Sigma-Aldrich, München, Germany) and 100 μg ml−1 penicillin–streptomycin (Invitrogen). Medium was exchanged three times per week. The dishes were incubated at 37°C in 5% CO2–95% air for more than 2 weeks. Before recording, the membrane of the culture insert beneath the brain slice was cut to approximately the size of the slice. The slice was inverted with the culture membrane at the top and then placed onto a nitrocellulose-coated MEA (Multichannel Systems, Reutlingen). Organotypic slices were maintained on MEAs for up to 3 weeks under flow-through culture conditions.
Multi-electrode array recordings
Long-term recordings of firing rate from organotypic brain slices were carried out with a MEA-1060 recording system (Multichannel Systems, Reutlingen) as previously described (Tousson & Meissl, 2004). Two types of high-density microelectrode arrays (HD-MEA) with different electrode layouts were used. One type consisted of two fields of 30 electrodes with a diameter of 10 μm and 30 μm spacing. The distance between the two fields was 500 μm. In this case, only one field was covered by the SCN. The second HD-MEA type consisted of one field of 60 electrodes with 10 μm diameter and 40 μm spacing between the electrodes that was covered by one entire SCN. Using this type of HD-MEA, the electrode field had approximately the size of one SCN and was nearly completely covered by the tissue, so that recordings could be obtained from all subdivisions of the SCN simultaneously.
Extracellular voltage signals recorded from the MEA electrodes were amplified ×1200 and sampled at 32 kHz on 60 channels simultaneously. Extracellularly recorded spikes which are usually embedded in biological and thermal noise of about 15 μV peak to peak were detected by using a threshold-based algorithm. Action potentials exceeding a defined voltage threshold were digitized and stored as time-stamped spike cut-outs using the MC_Rack software (Multichannel Systems).
Recording medium was similar to the culture medium with the exception that the Hepes content was elevated to 20 mm and the NaHCO3 levels reduced to 0.56 g l−1. During long-term recordings, medium was exchanged continuously at a flow rate of 20 μl min−1 using a SP 260PZ syringe pump (WPI, Sarasota, USA). The culture chamber, application system and inflow–outflow system were completely sealed to prevent bacterial contamination.
Patch-clamp recordings
For whole-cell patch-clamp recordings of SCN neurons, 250-μm-thick acute brain slices were used. Recordings were performed at room temperature with an EPC-9 patch-clamp amplifier (HEKA Elektronik, Lamprecht, Germany) and Pulse 8.11 software. The patch pipettes were pulled from borosilicate glass tubing (Hilgenberg, Malsfeld, Germany) on a horizontal puller. When filled with internal solution, they had a resistance of 5–10 MΩ. The pipette solution contained (in mm): caesium gluconate 125, CaCl2 1, EGTA 10, MgSO4 4.6, Na-Hepes 10, Na-ATP 4, Na-GTP 0.4, QX-3145 5, Neurobiotin (Vector Laboratories, Burlingame, CA, USA) 15, Alexa-568 (Molecular Probes, Göttingen, Germany) 0.1 (pH 7.3, adjusted with CsOH). Cells were voltage clamped in the whole-cell patch-clamp configuration. Spontaneous spike activity was recorded in some cells in the cell-attached configuration. Neurons from the suprachiasmatic nucleus were selected for recording under visual guidance of differential interference contrast microscopy (Axioscope 2, Zeiss). Input resistances typically were between 600 and 1400 MΩ. Series resistances were 1–2 MΩ and left uncompensated. The cell and pipette capacitances were cancelled. The liquid junction potential of the aCSF with regard to the pipette solution was approximately 15 mV. The holding potential was corrected for the junction potential. The signals were filtered at 1 kHz with an eight-pole Bessel filter built into the EPC-9 amplifier and digitized. The sampling rate was 10 kHz. All experiments were performed during the second part of the day and the early night (Zeitgeber time: ZT 7–15).
After patch-clamp recordings, recorded neurons were filled with Alexa 568 and neurobiotin (Vector Labs). Slices were then fixed for 30 min with 4% paraformaldehyde in 0.1 m phosphate buffer (PB) at room temperature, washed three times with PB and then incubated in 5% (w/v) Chemiblocker (Chemicon, Hofheim, Germany), 0.5% (v/v) Triton X-100 in PB with streptavidin Alexa 568 (1:2000; for 2 h). After incubation, slices were washed in PB and labelled SCN neurons examined with a Zeiss Axioplan 2 microscope.
Drug application
All drugs in the patch-clamp experiments were applied by superfusion through a multibarrel, pressure-driven system (DAD12, ALA Scientific Instruments). The tip of the micromanifold had an inner diameter of 200 μm and was placed within 100 μm of the soma of the recorded cell. Agonists were applied for 5 s at different concentrations. The sequence of the concentrations applied to cells was randomized. All concentrations in patch-clamp experiments refer to the concentration in the micromanifold. The actual concentrations at the cellular level will be lower due to the dilution of the drugs in the chamber which could not be calculated.
ACSF was continuously superfused with the application system at the same rate before and after drug application. This allowed the time interval just before drug application to be used as a control. Between drug applications, the cells were superfused for at least 3 min with aCSF to prevent possible receptor desensitization. The GlyR antagonists strychnine (1 to 10 μm), PMBA (100 μm) or ginkgolide B (10 μm) were applied for 5 s prior to the co-application with 1 mm glycine.
In MEA recordings, drugs were applied by using a Triathlon autosampler (Spark Holland, Emmen, the Netherlands) which was installed into the superfusion system (see Klisch et al. 2009). The autosampler had the advantage that drugs could be applied automatically throughout the 24 h cycle under sterile conditions. The culture chamber of the MEAs had a volume of about 1.5 ml. As the injection volume of the autosampler was 50 μl, drugs in the sample vials were 30-fold concentrated and diluted during the application process in the chamber. The flow rate was 20 μl min−1 during the whole experiment. After application, drugs were slowly washed out by the superfusion medium, so that an effective concentration of glycine was present for approximately 5 h after start of application.
Data analysis and statistics
All normally distributed data are presented as mean ± standard error of the mean. Statistical analysis was performed using Student's t test or ANOVA.
For measurement of the effects of glycine and strychnine on the firing rate in MEA recording, cells were considered as responding if the mean firing rate following application of the drug changed by more than ±20%. Only spontaneously active cells with a discharge rate >0.2 Hz and a stable baseline firing rate in the 15 min period before drug application were counted. Many SCN cells showed a clear trend with an increase or decrease of the firing rate prior to drug application. Responses were excluded from further analysis if they showed a pre-existing trend of the same polarity as the response.
Circadian periodicity before and after drug application was first determined with Lomb & Scargle periodograms (Time Series Analysis Seriel Cosinor; Expert Soft Technology, Esvres, France). The circadian activity rhythm was estimated during the 72 h before drug application, and then, after a pause of 24 h, during the subsequent three cycles. This procedure, which monitors the electrical activity rhythms over many days, should ensure that the observed effects are stable. Only if periods before and after treatment exceeded a power of 600 and featured a single peak between 20 and 28 h were they used for determination of phase shifts. Phase shifts were estimated by fitting a single cosinor model on to the data points to estimate the phases of the electrical activity rhythms (Time Series Analysis Seriel Cosinor; Expert Soft Technology, Esvres, France), by comparing the peak time of the firing rhythm and by using the half-maximal rise time of the activity rhythm as reference point. All these methods to quantify the phase shifts gave consistent results. In acute SCN slices, the peak of neuronal activity occurs around the middle of the subjective day corresponding to circadian time 7 (CT 7; Prosser & Gillette, 1989). This activity peak was used in organotypic slices as a phase reference point and set to CT 7.
Solutions and chemicals
All drugs were purchased from Sigma-Aldrich (München) unless otherwise stated.
Results
Whole-cell patch clamp
We present here evidence that SCN neurons in acute coronal brain slices of mice exhibit a glycine-induced current. This is consistent with the only previous investigation on glycine responses performed in primary cell cultures of the SCN of rats (Ito et al. 1991). In mice, application of glycine at a holding potential of 0 mV generated an outward current in 83% of SCN neurons (113 neurons out of 136); the remaining neurons were glycine insensitive. Effects of glycine were concentration dependent with a threshold concentration at 10 μm (Fig. 1A); above 100 μm glycine elicited a clear response in all glycine-sensitive cells. The concentration–response relationship had a sigmoidal shape and could be fitted using a Hill equation resulting in an EC50 of 780 μm (Fig. 1B). Maximal responses were observed at 10 mm glycine. The accurate concentrations will be lower because of dilution in the superfusion solution. The glycine-induced currents showed a fast activation component, followed by a slow deactivation that began during glycine application (Fig. 1A). The amplitude of glycine-induced currents varied considerably between individual cells. Extracellular recordings of action potentials were performed only in a few cases in the cell-attached mode showing a concentration-dependent suppression of the spontaneous firing activity in most of the glycine-sensitive SCN neurons (5 out of 13) (Fig. 1C). These neurons were mostly located in the ventral to middle part of the SCN; only one cell in the middle SCN was excited by glycine. In addition to glycine, the amino acids taurine and β-alanine induced similar currents in the mouse SCN (Fig. 1D).
Figure 1. Voltage-clamp recording of glycine-induced current in neurons of acute SCN slices.
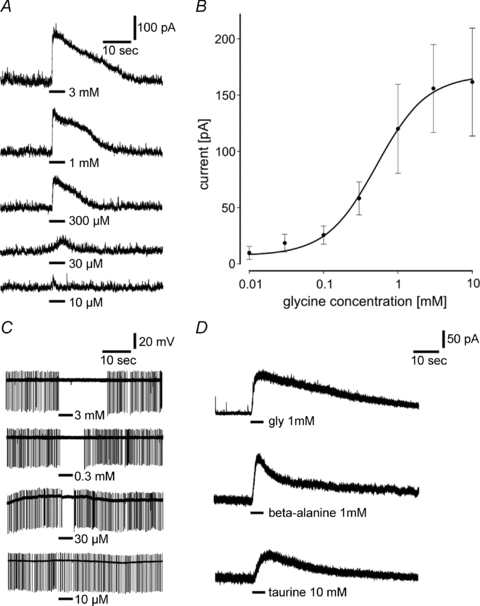
A, locally applied glycine at concentrations between 10 μm and 3 mm for 5 s elicits outward currents at a holding potential of 0 mV. B, dose–response relationship for glycine. Each point represents the average of 4 to 14 SCN neurons. The sigmoidal curve is fitted using the Hill equation showing a maximal current at a concentration of 10 mm and an EC50 of 780 μm. C, extracellular recordings show an inhibition of the spontaneous firing rate in response to glycine application. With increasing concentrations the duration of spike inhibition increases. D, applications of the glycine receptor agonists β-alanine (1 mm) and taurine (10 mm) induce currents with similar characteristics to glycine-induced currents when applied to the same cell (n = 13).
Action of glycine antagonists
Glycine-induced currents could be effectively reduced by 5 μm strychnine, a specific glycine antagonist (Fig. 2A). After washout of the antagonist the response to glycine recovered to its initial value. Strychnine decreased the glycine-induced current by 51.2 ± 7.7% (1 mm glycine, n = 12, Fig. 2C). Higher concentrations of strychnine (25 μm) suppressed the current further to 34.8 ± 6.8% (n = 8, Fig. 2D). This appears to be the maximal effect of strychnine; we never observed a complete suppression of the glycine response which is probably caused by an unspecific action of glycine on GABAA receptors. Indeed, gabazine (5 μm), a blocker of GABAA receptors, reduced the amplitude of the current by 26.2 ± 15.9% (n = 8, Fig. 2E). However, this reduction of the current was in all recordings lower than the effect of glycine. Concomitantly to the suppression of glycine-induced currents, 5 μm strychnine reduced the duration of the inhibition of electrical activity in SCN neurons recorded extracellularly (Fig. 2B). As it is known that higher concentrations of strychnine are also able to inhibit GABAA receptors (Shirasaki et al. 1991), as well as nicotinic acetylcholine receptors (nAChRs) by binding to its α7 subunit (Kuijpers et al. 1994; Matsubayashi et al. 1998) which is expressed in the SCN (Seguela et al. 1993), we used in most experiments concentrations of 5 μm strychnine.
Figure 2. Glycine activates strychnine-sensitive GlyRs in SCN neurons.
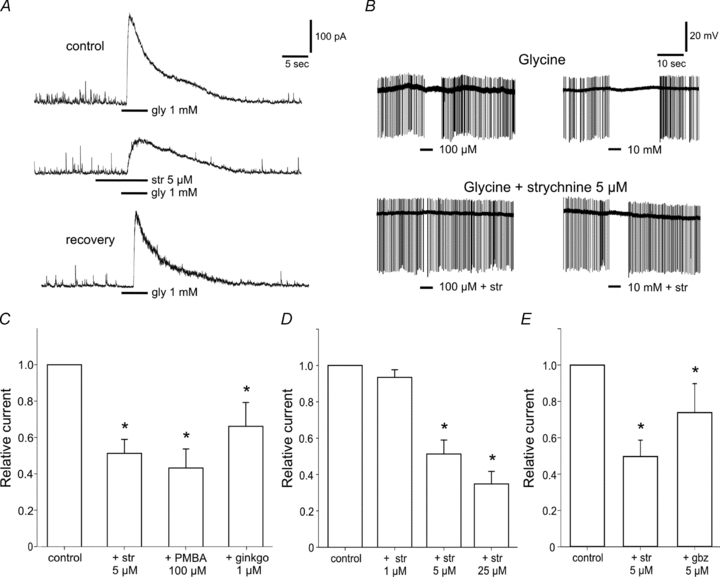
A, typical response to glycine (1 mm) at a holding potential of 0 mV showing an outward current (upper trace) which is reduced by co-application of strychnine (str, 5 μm) (middle trace). The lower trace shows the recovery of the response after wash-out. n = 12. B, extracellular recordings from a SCN neuron in an acute slice. Strychnine (5 μm) reduces the inhibitory effect of glycine on the spike rate (n = 3). C, the GlyR antagonists strychnine (5 μm), PMBA (100 μm) and ginkgolide B (ginkgo, 1 μm) reduce glycine-induced currents by 51, 56 and 34%, respectively (n = 6–12). D, the suppression of the amplitude of the current is enhanced by increasing concentrations of strychnine (1 to 25 μm, n = 8). E, application of gabazine (gbz, 5 μm) together with glycine (1 mm) reduced the current induced by glycine alone to a lesser extent than strychnine (5 μm) (n = 8). *P < 0.05.
Two other antagonists of GlyRs, phenylbenzene ω-phosphono-α-amino acid at 100 μm (PMBA) (Saitoh et al. 1994) and ginkgolide B at 1 μm (Betz & Laube, 2006) caused a similar, statistically significant, reduction of glycine-induced currents (PMBA: 56.8 ± 10.5, n = 9, and ginkgolide B: 33.9 ± 13.1, n = 6) (Fig. 2C).
Ion selectivity and specificity of the glycine-induced current
To discriminate the currents induced by activation of glycine and GABAA receptors, we determined the response characteristics of both currents induced by application of 100 μm GABA and 1 mm glycine (Fig. 3A and B). Usually glycine-induced currents had lower amplitudes than GABA-induced currents (146.7 ± 30.2 pA vs 326.4 ± 64.9 pA, n = 17) and showed different response kinetics. Although activation was fast in both cases, in comparison to GABA currents glycine currents showed a slower and longer lasting deactivation. The current–voltage relationship of glycine currents in SCN neurons was determined by recording the currents produced by 1 mm glycine at different holding potentials ranging from −75 mV to +35 mV. The measured reversal potential was similar for both GABA and glycine currents. The average current–voltage relationship for glycine is shown in Fig. 3B (continuous line, n = 4–12) and depicts a reversal potential at −47.1 mV. This is close to the chloride equilibrium potential, ECl=−51 mV, predicted by the Nernst equation under our recording conditions. The two other lines depict the current–voltage curves for the two cells shown in Fig. 3A for comparison.
Figure 3. Ion selectivity and specificity of the glycine-induced current.
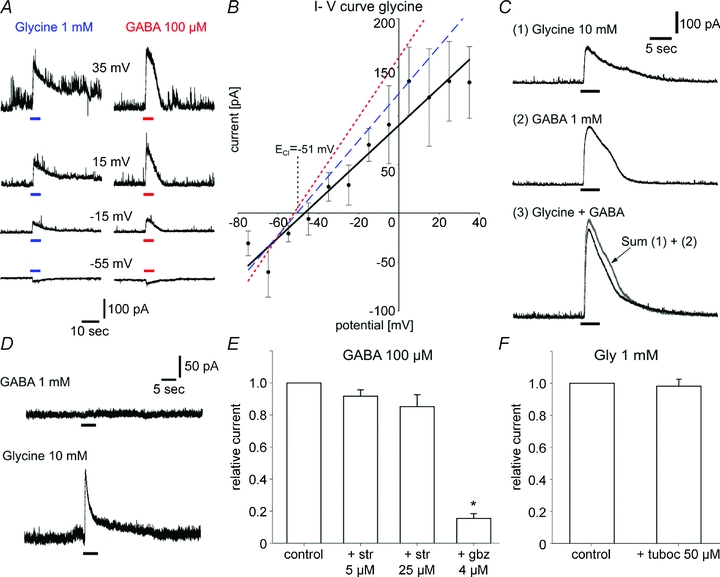
A, currents induced by 1 mm glycine (left traces) and 100 μm GABA (right traces) are recorded at different holding potentials in the same neuron. Note the longer duration of the deactivation phase after glycine application compared to GABA. B, current–voltage relationship for glycine in SCN cells from acute slices (mean values ± SEM of 4 to 12 SCN neurons). The measured reversal potential (−47.1 mV) approaches the equilibrium potential for chloride under these recording conditions which was ECl=−51 mV. The dashed line and dotted line represent the current–voltage curves for glycine-induced and GABA-induced currents in the cell in A, respectively (reversal potentials: glycine, −49.2 mV; GABA, −50.1 mV). C, co-application of saturating concentrations of glycine (10 mm) or GABA (1 mm) induced a current of lower amplitude than the arithmetic sum (3, in grey) of glycine- (1) and GABA- (2) induced currents (n = 12). D, example of a recording from a SCN neuron where glycine (10 mm) induces an outward current at a holding potential of 0 mV (lower trace). The cell is unresponsive to GABA application (1 mm, upper trace). E, strychnine does not significantly decrease the amplitude of the current induced by 100 μm GABA. In contrast, 4 μm gabazine effectively suppresses this current (85%). n = 12. *P < 0.05. F, the nicotinic acetylcholine receptor tubocurarine (50 μm) does not affect glycine-induced currents (n = 10).
When GABA (1 mm) and glycine (10 mm) were co-applied at saturating concentrations, the measured current was smaller than the arithmetic sum of both currents (Fig. 3C, n = 12). The current induced by co-application of GABA and glycine is characterized by a slow deactivation kinetic, which is probably caused by the glycine component, because the deactivation is faster when GABA is applied alone. Three out of seventy-one SCN neurons tested for glycine and for GABA current were unresponsive to GABA application although they were sensitive to glycine (Fig. 3D). Strychnine (5 μm) applied together with GABA had no significant effect on GABA responses (Fig. 3E, n = 12) indicating that the effects of strychnine are caused by a specific blockade of GlyR. In contrast, the GABAA receptor antagonist gabazine (4 μm) successfully suppressed GABA currents. We can also rule out the possibility that glycine acts on nAChRs in the SCN because application of tubocurarine, a blocker of nAChR, had no effect on the amplitude of the glycine-induced current (Fig. 3F, n = 10).
Beside its direct action on strychnine-sensitive GlyRs, glycine can act in isolated SCN neurons of the rat as co-agonist of NMDA receptors (Ito et al. 1991). To examine whether the currents induced by glycine application have a component that is caused by NMDA receptor activation, we co-applied NMDA (100 μm) with glycine (1 μm) at a holding potential of −40 mV in Mg2+-free external solution. Although this glycine concentration should be sufficient to saturate NMDA receptors, application of NMDA and NMDA + glycine induced similar currents (n = 10; data not shown).
Multi-electrode array recordings
Organotypic SCN slice cultures allow in combination with multi-electrode array recordings long-term monitoring of extracellular neuronal activity while keeping an intrinsic network that is very close to the in vivo condition. Advantages of organotypic cultures compared to acute slices are that they can be kept for weeks in culture and electrical activity, i.e. the output signal of the circadian clock, can be monitored for long periods of up to 3 weeks. Almost all electrodes covered by the SCN and showing spike activity exhibited clear oscillations of electrical activity with a circadian rhythm of 23.86 ± 0.37 h (n = 21 experiments with 8–45 cells per experiment). These electrodes usually recorded spontaneous single- and multi-unit activity and showed stable periodicity of the firing rate over several days. Circadian oscillations of spike activity recorded from different electrodes were usually in phase in the same preparation but, in rare cases, the peak of activity in one channel, i.e. from one cell group, was displaced for up to 10 h. Although in some cultures the amplitude of the oscillation could vary, in most of the recordings the mean spike rate, i.e. the amplitude of each cycle, was in a similar range from one day to another and the peak of activity did not decline.
Short-term effects of glycine in MEA recordings
Short-term effects of glycine showed, in MEA recordings, inhibitory as well as excitatory responses on the firing rate (Fig. 4A and C). Strychnine applied alone reduced the spontaneous electrical activity in neurons that were excited by glycine (Fig. 4B), suggesting a counteraction of the effect of glycine on GlyRs. Although both response types, inhibitory and excitatory responses, could be observed throughout the circadian cycle, the proportion of cells that were inhibited by glycine was more prominent at CT 4 than at CT 16 (24 vs. 6%, Fig. 4D). In addition, at CT 4, a subset of neurons responded to glycine with a biphasic change of the firing rate consisting of a short increase of the firing rate followed by a longer decrease (5%). Interestingly, simultaneous recordings from the SCN and one of its targets, the paraventricular hypothalamic nucleus, using high-density MEAs with two recording fields, revealed responses to glycine with opposite direction: excitation in the SCN (Fig. 4E) and inhibition in the paraventricular nucleus (Fig. 4F). This raises the possibility that differences in the response to glycine between clock cells and other hypothalamus cells could depend on the circadian time.
Figure 4. Glycine induced changes in the firing rate of SCN neurons in organotypic cultures.
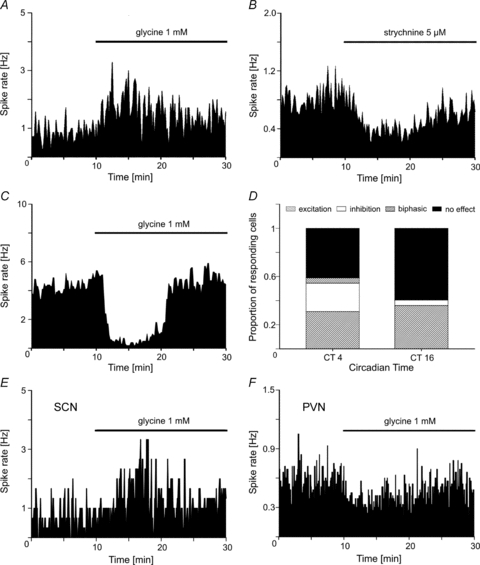
Application of glycine (1 mm) during MEA recordings induces either A, an increase or C, a decrease of the firing rate. The concentrations of the drugs refer to the beginning of the application with the autosampler which is completed after 150 s; then the drug concentration slowly decreases because it is washed out by the superfusion flow. B, in cells that were excited by glycine application, strychnine induces a decrease of the firing rate. D, the proportion of SCN cells responding to glycine with a decrease of their firing rate is more prominent at CT 4 (24%) than at CT 16 (6%). At this time a small subset of SCN neurons (5%) respond to glycine with a biphasic change of their firing rate. n = 58 (CT 4), n = 89 (CT 16). E and F illustrate one experiment where the effects of glycine on the firing rate is opposite in the SCN (E) and in the PVN (F). The bars above the recording traces represent the presence of glycine in the recording chamber.
Glycine phase-shifts the circadian pacemaker
Circadian activity of the firing rate was monitored for at least 3 days, then we bath-applied glycine and recorded the electrical activity for an additional 4 days. Phase shifts of the average neuronal activity of all electrodes showing a rhythm in one MEA were estimated using Cosinor analysis. In parallel, comparison of the peak time of this average activity before and after application, as well as the half-maximal rise time of the firing rate of each cycle, were used to calculate the phase shift in order to confirm the results. To note, phase shifts were calculated for the activity recorded on individual electrodes as well as for the average activity of all electrodes and showed similar results. Application of 1 mm glycine at different times of the circadian cycle evoked clear phase shifts, phase advances as well as phase delays. Glycine applied 3 h before the peak of neuronal activity (CT 4) induced phase advances (1.7 ± 0.2 h, n = 105 cells in 6 explants; Fig. 5B), whereas glycine application at CT 16, shortly before the trough of SCN neuronal activity, induced a phase delay (–1.4 ± 0.2 h, n = 167 cells in 7 explants, Fig. 5C). No clear phase shift was observed in the late night (−0.3 ± 0.2 h, n = 77 cells in 4 explants). The phase–response histograms in Fig. 5D illustrate that the phase-shifting effects of glycine depend on the time at which glycine was applied. Vehicle application using superfusion medium never caused a phase shift of the firing rhythms at CT 4 (Fig. 5A, 0.2 ± 0.1 h, n = 93 cells in 4 experiments) or CT 16 (0.1 ± 0.2 h, n = 64 cells in 3 experiments).
Figure 5. Glycine phase-shifts the circadian rhythm of the firing rate of SCN neurons.
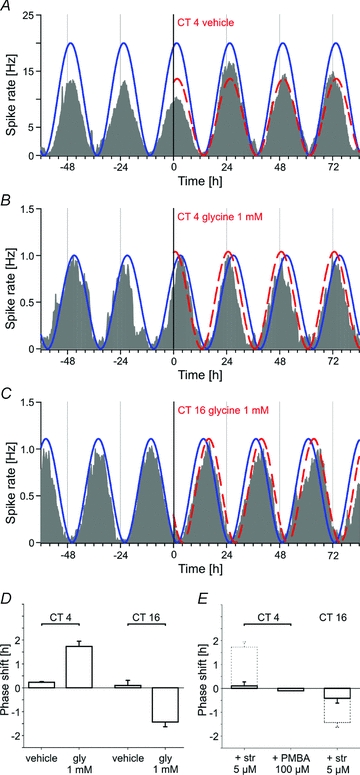
The different shaded traces represent the average activity recorded from all electrodes of a MEA that exhibit rhythmic activity (n = 9–45). In each case, application of vehicle or 1 mm glycine occurs at time = 0 h. The calculated cosine curves are shown as an overlay on the recording traces. The continuous curve indicates the original phase and the dashed line the phase after application. A, example of a vehicle application at CT 4 showing no shift of the circadian oscillation of the firing rate. B, example of a phase advance induced by 1 mm glycine (1.8 h) at the same circadian time. C, example of a phase delay (−1.7 h) following application of 1 mm glycine at CT 16. D, phase–response histograms for vehicle and glycine at CT 4 and CT 16 (n = 64–167 cells in 3–7 explants). E, phase–response histograms showing the effect of co-application of strychnine (5 μm) and glycine (1 mm) at CT 4 and CT 16 (n = 114–130 cells in 5 explants). The histograms with dashed lines illustrate the results obtained with glycine alone. The co-application of PMBA (100 μm) with glycine done in a single experiment, confirms the results obtained with glycine.
Glycine-induced phase shifts are mediated by glycine receptors
To ensure that the observed glycine-induced phase shifts were caused by activation of GlyRs, strychnine (5 μm) was co-applied with glycine at the same time at which glycine alone induced a phase advance or a phase delay (Fig. 5E). Strychnine prevented the phase shifts at CT 4 (0.1 ± 0.2 h, n = 114 cells in 5 explants) as well as at CT 16 (−0.4 ± 0.2, n = 130 cells in 5 explants). The use of another antagonist, PMBA (100 μm) at CT 4, also blocked the glycine-induced phase advance (−0.1 h, n = 21 cells in 1 explant) showing that this effect is mediated by specific GlyRs (Fig. 5E). However, strychnine applied alone without simultaneous glycine application did not change the phase of neural activity (data not shown).
Discussion
The present study addresses the functional role of the inhibitory neurotransmitter glycine in the mouse suprachiasmatic nucleus, an aspect that has not been examined previously. By using a method for continuous long-term recordings from cultured organotypic slices with multi-electrode arrays, we could now establish that glycine is able to phase-shift rhythmic neuronal activity in the master clock by the activation of specific strychnine-sensitive glycine receptors.
Glycine and GABAA receptors belong to the same nicotinic acetylcholine receptor (nAChR) superfamily; both gate ionotropic receptors permeable to chloride ions with similar properties (Betz & Laube, 2006). In the present experiments none of the glycine antagonists could entirely block the glycine-induced current, suggesting that the current is composed of several components triggered by activation of both glycine and GABAA receptors, as has been described in neurons of spinal cord (Jonas et al. 1998) and olfactory bulb (Trombley et al. 1999). Indeed, the GABAA blocker gabazine partially reduced the amplitude of the glycine-induced current. Nevertheless, in the SCN simultaneous application of GABA and glycine at saturating concentrations resulted in responses larger than the maximal response to one of the drugs alone, but equal or smaller than the arithmetic sum of both currents, a phenomenon known from neurons of the rat olfactory bulb (Trombley et al. 1999) and the striatum (Sergeeva, 1998). Moreover, a small proportion of SCN neurons was only sensitive to glycine and not to GABA which suggests that in some neurons glycine activates merely glycine receptors and not GABAA receptors. This is supported by a recent study where the GABAA antagonist gabazine did not block all spontaneous inhibitory postsynaptic currents in the SCN, showing that another inhibitory neurotransmitter, possibly glycine, mediates these inhibitory responses (Klisch et al. 2009).
In MEA recordings from organotypic slice cultures, glycine caused inhibitory as well as excitatory responses of the firing rate. Earlier studies have raised the possibility that the inhibitory neurotransmitter GABA can also function in the mature SCN as an excitatory neurotransmitter depending on the circadian time (Wagner et al. 1997), but these findings have been questioned by others (Gribkoff et al. 1999). Later it was shown that a subset of mature SCN neurons exhibit GABAA receptor-mediated excitations (Choi et al. 2008) and respond with an increase in intracellular calcium concentrations, which is associated with neuronal excitation (Irwin & Allen, 2009). These dual effects of GABA responses might be caused by circadian changes in intracellular chloride concentrations which could be generated either by a change in passive chloride fluxes or by changes in the efficiency of chloride-regulating mechanisms (Wagner et al. 2001).
Intrinsic differences in responsiveness to GABA showing an excitatory action in the dorsal and an inhibitory action in the ventral part of the SCN were also observed by Albus et al. (2005) and the authors hypothesized that GABA transmits phase information between ventral and dorsal oscillators by an excitatory action on dorsal SCN neurons. It deserves further analysis whether excitatory and inhibitory responses to glycine in the SCN could have a similar basis. At least in SCN cell cultures, an increase of intracellular Ca2+ was observed in a small subset of cells after application of glycine (van den Pol et al. 1996b) which denotes excitatory responses. This indicates either that the neuronal network in the SCN is responsible for the glycine-mediated excitation in the SCN, for example when an inhibitory input from structures outside of the SCN is blocked by glycine, or that circadian oscillations of the chloride equilibrium potential cause the switch between excitation and inhibition.
An interaction between excitatory pathways and neuropeptide Y (NPY) signalling arising from the geniculo-hypothalamic tract was also recently proposed where inhibitions may arise from glycinergic trans-synaptic mechanisms (Schmahl & Böhmer, 1997). Interestingly, NPY, like glycine, elicits either a suppression of SCN neuronal discharge when it is bath applied or excitation of the firing rate when it is locally applied (cf. Brown & Piggins, 2007). These responses are either due to presynaptic inhibitory effects on glutamatergic or GABAergic signalling within SCN neurons or to postsynaptic effects on Ca2+ currents (van den Pol et al. 1996a). In the present study we tested this hypothesis and could not find any evidence for an interaction between glycinergic and NPY signalling in the SCN (data not shown), which suggests that glycine-induced phase advance during daytime does not involve NPY-dependent signalling.
Glycine can act as co-agonist of the ionotropic NMDA receptor (Johnson & Ascher, 1987; Kleckner & Dingledine, 1988). In dissociated neurons of the rat SCN, glycine can potentiate NMDA receptor responses by a strychnine-insensitive mechanism, apart from its strychnine-sensitive induction of a chloride current (Ito et al. 1991). However, in acute slices of the mouse hypothalamus, patch-clamp recordings did not show any potentiating effect of glycine on NMDA currents. This could be based on the fact that the maximal concentration of glycine released from the SCN is in a range that is sufficient to saturate its binding site on NMDA receptors (Johnson & Ascher, 1987). Maximum release of glycine occurs at a time that corresponds to the peak time of arginine vasopressin (AVP) release (Shinohara et al. 1998), which is known to occur around CT 6–8 (Kalsbeek et al. 1995). At this time, when the mouse SCN clock, contrary to the hamster SCN (Meijer et al. 1988), is unresponsive to NMDA-mediated phase-shifting effects, applications of exogenous glycine cause phase shifts of the neuronal activity rhythm. This effect is clearly mediated by strychnine-sensitive GlyRs and not by a glycinergic potentiation of NMDA receptors.
Interestingly, in hamster, application of the GABAA receptor agonist muscimol causes a phase advance of locomotor activity rhythm at about the same time as a glycine-induced phase advance in vitro is observed in the mouse SCN (Smith et al. 1989). The action of glycine on the phase of neuronal activity would be then synergistic to the action of GABA. Glycine- and GABA-mediated co-transmission could support the precise regulation of the time course of postsynaptic conductance by the relative amount of both neurotransmitters released from the presynaptic terminal as it was described in the spinal cord (Jonas et al. 1998).
The origin of glycinergic signalling within the SCN could arise from an intrinsic production of glycine by SCN neurons or by a glycinergic innervation from hypothalamic or extrahypothalamic sites. In organotypic brain slices, it was shown that glycine is released from the SCN with a circadian rhythm showing a phase similar to the release of AVP (Shinohara et al. 1998). Other regions of the rodent brain, like the cerebellum, cortex, midbrain and pons also show daily variations in their glycine levels with highest levels during daytime (Ross et al. 1980). Circadian fluctuations in the release of transmitters, especially GABA, seem to be important to synchronize SCN cells that communicate phase-induced changes in responsive neurons to the rest of the nucleus (Liu & Reppert, 2000). In contrast to glycine, data on the temporal profile of GABA release are lacking and data on its content rhythm in the SCN are inconsistent showing either a peak at night (Aguilar-Roblero et al. 1993) or in the morning (Ikeda et al. 1997). Recently an interesting hypothesis has proposed a model where the endogenous release of GABA from efferent SCN nerve terminals mediates the pathway in the dorsal hypothalamus that is responsible for inhibition of pineal melatonin synthesis (Kalsbeek et al. 1999). Thus, the rhythmic release of a neurotransmitter could serve two functions: first, an internal connection and synchronization of neurons within the nucleus and, second, an output signal that drives other rhythmic processes.
Glycine receptors are abundantly expressed in the hypothalamus (Malosio et al. 1991; Kirsch & Betz, 1993) and immunohistochemical studies reveal a substantial number of glycinergic fibres (Rampon et al. 1996). A dense glycinergic innervation of the hypothalamus was confirmed by using a transgenic mouse expressing enhanced green fluorescent protein (EGFP) under the control of glycine transporter 2 (GlyT2) promoter (Zeilhofer et al. 2005). Whereas Zeilhofer et al. (2005) did not describe glycinergic axon terminals in the SCN, a recent immunohistochemical study using the same GlyT2–EGFP mouse model could demonstrate the presence of glycinergic fibres in the SCN, and especially in the tissue surrounding the SCN (Mahr, 2008), which is of importance because many inputs to the SCN come from the structures that directly surround it (Saeb-Parsy et al. 2000). The visualization of pre-terminal EGFP-positive axons entering the hypothalamus along the medial forebrain bundle strongly suggests that glycinergic neurons innervating the forebrain originate from pontine or medullary structures (Zeilhofer et al. 2005), from which especially the pontine structures provide a direct afferent input to the SCN (Krout et al. 2002). Visceral feedback important for regulating the timing of food intake could reach the SCN by this multisynaptic pathway, possibly via the pontine parabrachial nucleus (Krout et al. 2002).
In the early subjective night, around CT 16, when microinjections of NMDA produce phase delays in the hamster SCN (Mintz et al. 1999), Pituitary adenylate cyclase-activating peptide (PACAP) phase-shifts the activity rhythm by potentiating NMDA receptors (Harrington et al. 1999). At the same time glycine has a synergistic effect to NMDA and produces also phase delays mediated by strychnine-sensitive GlyRs. Beside its direct GlyR-mediated effect, glycine could also indirectly potentiate NMDA responses and thus enhance the transmission of photic information to the circadian system.
In this scenario even a weak glycinergic innervation of the SCN, as well as the intrinsic release of glycine from SCN neurons, could lead to a precise fine-tuning of GABA- and NMDA-mediated synchronization and influence phase resetting of the clock.
Acknowledgments
Research was carried out within the scope of the Associated European Laboratory ‘European Laboratory for Circadian Research’, LEA CNRS-ULP-MPG (Lea No. 367), Strasbourg and Frankfurt/M and the CNRS-MPG neurosciences network (GDRE CNRS-MPG). The work was supported by the Max Planck Society, München, the CNRS, Paris, and the University of Strasbourg.
Glossary
Abbreviations
- GlyR
glycine receptor
- MEA
multi-microelectrode arrays
- nAChR
nicotinic acetylcholine receptor
- PMBA
phenylbenzene ω-phosphono-α-amino acid
- SCN
suprachiasmatic nucleus
Author contributions
All experiments were performed in the Max Planck Institute for Brain Research, Frankfurt am Main, Germany. J.M. and A.I. performed and analysed patch-clamp recordings; J.M., D.K. and H.M. performed and analysed multielectrode array recordings. Conception and design of the experiments: all authors; drafting the article or revising it critically for important intellectual content: J.M., E.C., P.P. and H.M. All authors approved the final version.
References
- Aguilar-Roblero R, Verduzco-Carbajal L, Rodriguez C, Mendez-Franco J, Moran J, Perez de la Mora M. Circadian rhythmicity in the GABAergic system in the suprachiasmatic nuclei of the rat. Neurosci Lett. 1993;157:199–202. doi: 10.1016/0304-3940(93)90736-5. [DOI] [PubMed] [Google Scholar]
- Albus H, Vansteensel MJ, Michel S, Block GD, Meijer JH. A GABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock. Curr Biol. 2005;15:886–893. doi: 10.1016/j.cub.2005.03.051. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Huettner JE, Straume M, Herzog ED. GABA and Gi/o differentially control circadian rhythms and synchrony in clock neurons. Proc Natl Acad Sci U S A. 2006;103:19188–19193. doi: 10.1073/pnas.0607466103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz H, Laube B. Glycine receptors: recent insights into their structural organization and functional diversity. J Neurochem. 2006;97:1600–1610. doi: 10.1111/j.1471-4159.2006.03908.x. [DOI] [PubMed] [Google Scholar]
- Brown TM, Piggins HD. Electrophysiology of the suprachiasmatic circadian clock. Prog Neurobiol. 2007;82:229–255. doi: 10.1016/j.pneurobio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci. 2001;2:521–526. doi: 10.1038/35081582. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Lee CJ, Schroeder A, Kim YS, Jung SH, Kim JS, Kim DY, Son EJ, Han HC, Hong SK, Colwell CS, Kim YI. Excitatory actions of GABA in the suprachiasmatic nucleus. J Neurosci. 2008;28:5450–5459. doi: 10.1523/JNEUROSCI.5750-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribkoff VK, Pieschl RL, Wisialowski TA, Park WK, Strecker GJ, de Jeu MTG, Pennartz CMA, Dudek FE. A reexamination of the role of GABA in the mammalian suprachiasmatic nucleus. J Biol Rhythms. 1999;14:126–130. doi: 10.1177/074873099129000515. [DOI] [PubMed] [Google Scholar]
- Harrington ME, Hoque S, Hall A, Golombek D, Biello S. Pituitary adenylate cyclase activating peptide phase shifts circadian rhythms in a manner similar to light. J Neurosci. 1999;19:6637–6642. doi: 10.1523/JNEUROSCI.19-15-06637.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Azuma S, Inoue S. Vitamin B12 enhances GABA content but reduces glutamate content in the rat suprachiasmatic nucleus. Am J Physiol Regul Integr Comp Physiol. 1997;273:R359–R363. doi: 10.1152/ajpregu.1997.273.1.R359. [DOI] [PubMed] [Google Scholar]
- Irwin RP, Allen CN. GABAergic signaling induces divergent neuronal Ca2+ responses in the suprachiasmatic nucleus network. Eur J Neurosci. 2009;30:1462–1475. doi: 10.1111/j.1460-9568.2009.06944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito C, Wakamori M, Akaike N. Dual effect of glycine on isolated rat suprachiasmatic neurons. Am J Physiol Cell Physiol. 1991;260:C213–C218. doi: 10.1152/ajpcell.1991.260.2.C213. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Steinkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Buijs RM, Engelmann M, Wotjak CT, Landgraf R. In vivo measurement of a diurnal variation in vasopressin release in the rat suprachiasmatic nucleus. Brain Res. 1995;682:75–82. doi: 10.1016/0006-8993(95)00324-j. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Cutrera RA, Van Heerikhuize JJ, Van der Vliet J, Buijs RM. GABA release from suprachiasmatic nucleus terminals is necessary for the light-induced inhibition of nocturnal melatonin release in the rat. Neuroscience. 1999;92:453–461. doi: 10.1016/s0306-4522(98)00635-6. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Betz H. Widespread expression of gephyrin, a putative glycine receptor tubulin linker protein, in rat brain. Brain Res. 1993;621:301–310. doi: 10.1016/0006-8993(93)90120-c. [DOI] [PubMed] [Google Scholar]
- Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988;241:835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Klisch C, Inyushkin A, Mordel J, Karnas D, Pévet P, Meissl H. Orexin A modulates neuronal activity of the rodent suprachiasmatic nucleus in vitro. Eur J Neurosci. 2009;30:65–75. doi: 10.1111/j.1460-9568.2009.06794.x. [DOI] [PubMed] [Google Scholar]
- Krout KE, Kawano J, Mettenleiter TC, Loewy AD. CNS inputs to the suprachiasmatic nucleus of the rat. Neuroscience. 2002;110:73–92. doi: 10.1016/s0306-4522(01)00551-6. [DOI] [PubMed] [Google Scholar]
- Kuijpers GAJ, Vergara LA, Calvo S, Yadid G. Inhibitory effect of strychnine on acetylcholine-receptor activation in bovine adrenal-medullary chromaffin cells. Br J Pharmacol. 1994;113:471–478. doi: 10.1111/j.1476-5381.1994.tb17013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Reppert SM. GABA synchronizes clock cells within the suprachiasmatic circadian clock. Neuron. 2000;25:123–128. doi: 10.1016/s0896-6273(00)80876-4. [DOI] [PubMed] [Google Scholar]
- Lynch JW. Native glycine receptor subtypes and their physiological roles. Neuropharmacol. 2009;56:303–309. doi: 10.1016/j.neuropharm.2008.07.034. [DOI] [PubMed] [Google Scholar]
- Mahr S. Fachbereich Biowissenschaften der Universität Frankfurt/M; 2008. Anatomische Grundlagen der Übertragung circadianer Signale zwischen N. suprachiasmaticus und anderen Kerngebieten des Hypothalamus. PhD Thesis. [Google Scholar]
- Malosio ML, Marqueze-Pouey B, Kuhse J, Betz H. Widespread expression of glycine receptor subunit messenger-RNAs in the adult and developing rat brain. EMBO J. 1991;10:2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi H, Alkondon M, Pereira EFR, Swanson KL, Albuquerque EX. Strychnine: a potent competitive antagonist of α−bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal neurons. J Pharmacol Exp Therap. 1998;284:904–913. [PubMed] [Google Scholar]
- Meijer JH, van der Zee EA, Dietz M. Glutamate phase shifts circadian activity rhythms in hamsters. Neurosci Lett. 1988;86:177–183. doi: 10.1016/0304-3940(88)90567-8. [DOI] [PubMed] [Google Scholar]
- Mintz EM, Marvel CL, Gillespie CF, Price KM, Albers HE. Activation of NMDA receptors in the suprachiasmatic nucleus produces light-like phase shifts of the circadian clock in vivo. J Neurosci. 1999;19:5124–5130. doi: 10.1523/JNEUROSCI.19-12-05124.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA, Gillette MU. The mammalian circadian clock in the suprachiasmatic nuclei is reset in vitro by cAMP. J Neurosci. 1989;9:1073–1081. doi: 10.1523/JNEUROSCI.09-03-01073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA, Mangrum CA, Glass JD. Acute ethanol modulates glutamatergic and serotonergic phase shifts of the mouse circadian clock in vitro. Neuroscience. 2008;152:837–848. doi: 10.1016/j.neuroscience.2007.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon C, Luppi PH, Fort P, Peyron C, Jouvet M. Distribution of glycine-immunoreactive cell bodies and fibers in the rat brain. Neuroscience. 1996;75:737–755. doi: 10.1016/0306-4522(96)00278-3. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Ross FH, Sermons AL, Walker CA. Twenty-four hour daily rhythms of glycine in the rodent brain. Life Sci. 1980;26:1019–1022. doi: 10.1016/0024-3205(80)90246-5. [DOI] [PubMed] [Google Scholar]
- Saeb-Parsy K, Lombardelli S, Khan FZ, McDowall K, Au-Yong ITH, Dyball REJ. Neural connections of hypothalamic neuroendocrine nuclei in the rat. J Neuroendocrinol. 2000;12:635–648. doi: 10.1046/j.1365-2826.2000.00503.x. [DOI] [PubMed] [Google Scholar]
- Saitoh T, Ishida M, Maruyama M, Shinozaki H. A novel antagonist, phenylbenzene omega-phosphono-alpha-amino acid, for strychnine-sensitive glycine receptors in the rat spinal cord. Br J Pharmacol. 1994;113:165–170. doi: 10.1111/j.1476-5381.1994.tb16189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Kiyama H, Tohyama M. Regional distribution of cells expressing glycine receptor α-2 subunit messenger-RNA in the rat brain. Brain Res. 1992;590:95–108. doi: 10.1016/0006-8993(92)91085-s. [DOI] [PubMed] [Google Scholar]
- Sato K, Zhang JH, Saika T, Sato M, Tada K, Tohyama M. Localization of glycine receptor α1 subunit mRNA-containing neurons in the rat brain: an analysis using in situ hybridization histochemistry. Neuroscience. 1991;43:381–395. doi: 10.1016/0306-4522(91)90302-5. [DOI] [PubMed] [Google Scholar]
- Schmahl C, Böhmer G. Effects of excitatory amino acids and neuropeptide Y on the discharge activity of suprachiasmatic neurons in rat brain slices. Brain Res. 1997;746:151–163. doi: 10.1016/s0006-8993(96)01220-6. [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineleymiller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain α7: nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeeva OA. Comparison of glycine- and GABA-evoked currents in two types of neurons isolated from the rat striatum. Neurosci Lett. 1998;243:9–12. doi: 10.1016/s0304-3940(98)00072-x. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Honma S, Katsuno Y, Abe H, Honma K. Circadian release of amino acids in the suprachiasmatic nucleus in vitro. Neuroreport. 1998;9:137–140. doi: 10.1097/00001756-199801050-00027. [DOI] [PubMed] [Google Scholar]
- Shirasaki T, Klee MR, Nakaye T, Akaike N. Differential blockade of bicuculline and strychnine on GABA-induced and glycine-induced responses in dissociated rat hippocampal pyramidal cells. Brain Res. 1991;561:77–83. doi: 10.1016/0006-8993(91)90751-g. [DOI] [PubMed] [Google Scholar]
- Smith RD, Inouye ST, Turek FW. Central administration of muscimol phase-shifts the mammalian circadian clock. J Comp Physiol A. 1989;164:805–814. doi: 10.1007/BF00616752. [DOI] [PubMed] [Google Scholar]
- Tousson E, Meissl H. Suprachiasmatic nuclei grafts restore the circadian rhythm in the paraventricular nucleus of the hypothalamus. J Neurosci. 2004;24:2983–2988. doi: 10.1523/JNEUROSCI.5044-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombley PQ, Hill BJ, Horning MS. Interactions between GABA and glycine at inhibitory amino acid receptors on rat olfactory bulb neurons. J Neurophysiol. 1999;82:3417–3422. doi: 10.1152/jn.1999.82.6.3417. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Gorcs T. Glycine and glycine receptor immunoreactivity in brain and spinal cord. J Neurosci. 1988;8:472–492. doi: 10.1523/JNEUROSCI.08-02-00472.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Obrietan K, Chen G, Belousov AB. Neuropeptide Y-mediated long-term depression of excitatory activity in suprachiasmatic nucleus neurons. J Neurosci. 1996a;15:5883–5895. doi: 10.1523/JNEUROSCI.16-18-05883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Strecker GJ, Dudek FE. Excitatory and inhibitory amino acids and synaptic transmission in the suprachiasmatic nucleus. Prog Brain Res. 1996b;111:41–56. doi: 10.1016/s0079-6123(08)60399-4. [DOI] [PubMed] [Google Scholar]
- Wagner S, Castel M, Gainer H, Yarom Y. GABA in the mammalian suprachiasmatic nucleus and its role in diurnal rhythmicity. Nature. 1997;387:598–603. doi: 10.1038/42468. [DOI] [PubMed] [Google Scholar]
- Wagner S, Sagiv N, Yarom Y. GABA-induced current and circadian regulation of chloride in neurones of the rat suprachiasmatic nucleus. J Physiol. 2001;537:853–869. doi: 10.1111/j.1469-7793.2001.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilhofer HU, Studler B, Arabadzisz D, Schweizer C, Ahmadi S, Layh B, Bosl MR, Fritschy JM. Glycinergic neurons expressing enhanced green fluorescent protein in bacterial artificial chromosome transgenic mice. J Comp Neurol. 2005;482:123–141. doi: 10.1002/cne.20349. [DOI] [PubMed] [Google Scholar]


