Non-technical summary
Systemic inflammation and related disorders, including sepsis, are leading causes of death in hospitalized patients. In most severe cases, systemic inflammation is accompanied by a drop in body temperature (hypothermia). We know that inflammation-associated hypothermia is a brain-mediated response, but mechanisms of this response are unknown. We administered a bacterial product (endotoxin) to rats to cause systemic inflammation and hypothermia. We then used a variety of pharmacological tools to probe whether three different receptors are involved in this hypothermia. We have found that one of the receptors studied, the so-called cannabinoid-1 (CB1) receptor, is crucial for the development of hypothermia. This is the same receptor that is responsible for many effects of marihuana (cannabis). We further show that hypothermia associated with inflammation depends on CB1 receptors located inside the brain. These novel findings suggest that brain CB1 receptors should be studied as potential therapeutic targets in systemic inflammation and sepsis.
Abstract
Abstract
Hypothermia occurs in the most severe cases of systemic inflammation, but the mechanisms involved are poorly understood. This study evaluated whether the hypothermic response to bacterial lipopolysaccharide (LPS) is modulated by the endocannabinoid anandamide (AEA) and its receptors: cannabinoid-1 (CB1), cannabinoid-2 (CB2) and transient receptor potential vanilloid-1 (TRPV1). In rats exposed to an ambient temperature of 22°C, a moderate dose of LPS (25–100 μg kg−1i.v.) induced a fall in body temperature with a nadir at ∼100 min postinjection. This response was not affected by desensitization of intra-abdominal TRPV1 receptors with resiniferatoxin (20 μg kg−1i.p.), by systemic TRPV1 antagonism with capsazepine (40 mg kg−1i.p.), or by systemic CB2 receptor antagonism with SR144528 (1.4 mg kg−1i.p.). However, CB1 receptor antagonism by rimonabant (4.6 mg kg−1i.p.) or SLV319 (15 mg kg−1i.p.) blocked LPS hypothermia. The effect of rimonabant was further studied. Rimonabant blocked LPS hypothermia when administered i.c.v. at a dose (4.6 μg) that was too low to produce systemic effects. The blockade of LPS hypothermia by i.c.v. rimonabant was associated with suppression of the circulating level of tumour necrosis factor-α. In contrast to rimonabant, the i.c.v. administration of AEA (50 μg) enhanced LPS hypothermia. Importantly, i.c.v. AEA did not evoke hypothermia in rats not treated with LPS, thus indicating that AEA modulates LPS-activated pathways in the brain rather than thermoeffector pathways. In conclusion, the present study reveals a novel, critical role of brain CB1 receptors in LPS hypothermia. Brain CB1 receptors may constitute a new therapeutic target in systemic inflammation and sepsis.
Introduction
Thermoregulatory responses are hallmarks of sepsis-related syndromes. Although fever is the most common and better understood response, it is hypothermia that occurs in the most severe cases of sepsis and septic shock (Clemmer et al. 1992; Arons et al. 1999). In the laboratory, this fever-hypothermia dichotomy has been best characterized in a rat model of systemic inflammation induced by bacterial lipopolysaccharide (LPS); for a review, see Romanovsky et al. (2005). In rats exposed to an ambient temperature of 20–25°C, lower doses of LPS cause fever, whereas higher doses induce early (over the first 2 h) hypothermia that may or may not be followed by fever or by late hypothermia (Romanovsky et al. 1996a,b; Steiner et al. 2005). Both fever and hypothermia seem to be regulated thermoregulatory responses brought about by brain-driven changes in thermoeffector activity (Romanovsky et al. 1996b; Almeida et al. 2006a,b;).
The neuroimmune mechanisms that operate the fever–hypothermia switch have been proposed to involve lipid-derived inflammatory mediators, including platelet-activating factor (Ephgrave et al. 1997; Ivanov et al. 2003b), prostaglandins (Engblom et al. 2002; Matsumura & Kobayashi, 2004; Romanovsky et al. 2005; Blatteis, 2006; Spencer et al. 2008), epoxyeicosatrienoic acids (Kozak et al. 2000), and leukotrienes (Paul et al. 1999). A new class of lipid-derived mediators was discovered as a result of the search for endogenous ligands of cannabinoid receptors (reviewed by Freund et al. 2003). This class consists of N-ethanolamides derived from fatty acids, which are commonly referred to as endocannabinoids. Anandamide (arachidonoyl N-ethanolamide, AEA) is the best known member of this class. It works as a full agonist at the cannabinoid-1 (CB1) receptor subtype (Vogel et al. 1993), as a partial agonist at cannabinoid-2 (CB2) receptor subtype (Gonsiorek et al. 2000), and as a full agonist at the transient receptor potential vanilloid-1 (TRPV1) receptor (Smart et al. 2000). The levels of AEA, both in the brain and in the periphery, are elevated during the systemic inflammatory response to LPS (Liu et al. 2003; Fernandez-Solari et al. 2006). Furthermore, the tissue and cell distribution of the AEA receptors is consistent with their possible participation in neuroimmune communication: CB1 receptors are expressed in leukocytes, microgliocytes and neurons (Mailleux & Vanderhaeghen, 1992; Bouaboula et al. 1993; Matsuda et al. 1993; Waksman et al. 1999); CB2 receptors are expressed in leukocytes and microgliocytes, but not in neurons (Munro et al. 1993; Galiegue et al. 1995; Walter et al. 2003); and TRPV1 receptors are expressed primarily in sensory neurons (Tominaga & Caterina, 2004; Dhaka et al. 2006) and, to a lesser extent, in other neural and non-neural cells (reviewed by Romanovsky et al. 2009). The roles of these receptors in LPS fever have been studied (Szekely & Szolcsanyi, 1979; Gourine et al. 2001; Dogan et al. 2004; Iida et al. 2005; Benamar et al. 2007; Fraga et al. 2009), but no study has investigated systematically whether any of these receptors are involved in LPS hypothermia. In the present study, we conducted a series of pharmacological experiments to determine whether LPS-induced hypothermia is affected by blockade of TRPV1, CB1, or CB2 receptors, and whether the receptors involved are located inside or outside of the brain. We then evaluated several potential mechanisms of the pharmacological effects found. Finally, we attempted to clarify the controversial issue of how AEA affects deep body temperature (Tb) under normal conditions (no systemic inflammation) and during systemic inflammation (LPS hypothermia).
Methods
Animals
The experiments were conducted in 294 rats. Two hundred and sixty-five male Long–Evans rats were purchased from Charles River Laboratories (Wilmington, MA, USA) and used in Experiments 1–5 (see below) conducted at St Joseph's Hospital and Medical Center (Phoenix, AZ, USA). Twenty-four male Long–Evans rats and five female Wistar rats were bred, raised, and studied (experiment 5) at the University of Pécs (Pécs, Hungary). The rats weighed 290–400 g at the time of experiments. At both centres, the rats were housed initially in groups; after surgery, they were housed singly. They were housed in a thermally neutral environment (ambient temperature of 28°C) and under a 12:12 h light–dark cycle (lights on at 07.00 h). Standard rat chow and tap water were available ad libitum. At both centres, the rats were trained extensively to stay inside cylindrical confiners made of stainless steel wire. At St Joseph's Hospital, these confiners were also placed inside rat home cages where they enriched the cage space serving as artificial ‘rat holes’. The rats spent some time inside the confiners voluntarily. Rodents are readily adaptable to confinement to an extent that habituated rodents respond to it with neither stress fever (Romanovsky et al. 1998b) nor other signs of stress (Abercrombie & Jacobs, 1987; Hashimoto et al. 1988; Melia et al. 1994; Stamp & Herbert, 1999). The same confiners were used later in the experiments. Each rat was used in an experiment once and killed with sodium pentobarbital (100 mg kg−1i.v. or 400 mg kg−1i.p.) immediately thereafter. All procedures at each centre were conducted under protocols approved by the Animal Care and Use Committee of the respective centre, St Joseph's Hospital or University of Pécs.
Surgical preparation
Four to seven days before an experiment, a rat was implanted with an i.v. catheter or an i.c.v. guide cannula. The procedures were performed under anaesthesia with ketamine–xylazine–acepromazine (55.6, 5.5, and 1.1 mg kg−1i.p.) and antibiotic protection (enrofloxacin, 1.1 mg kg−1s.c.). Typically, no supplemental dose of the anaesthetic cocktail was required. During surgery, the rat was maintained on a board warmed to 37°C.
For i.v. catheterization, a small longitudinal incision was made on the left ventral surface of the neck. The left jugular vein was exposed, freed from its surrounding connective tissue, and ligated. A silicone catheter (ID 0.5 mm, OD 0.9 mm) filled with heparinized (10 U ml−1) saline was passed into the superior vena cava through the jugular vein and secured in place with ligatures. The free end of the catheter was knotted, tunnelled under the skin to the nape, and exteriorized. The skin was sutured. The catheters were flushed daily with heparinized saline.
For i.c.v. cannulation, the rat was fixed to a stereotaxic apparatus (David Kopf, Tujunga, CA, USA). The skin was incised over the sagittal suture; the periosteum was excised; supporting microscrews were driven into the skull; and a steel guide cannula (Plastics One, Roanoke, VA, USA) was implanted. The tip of the cannula was placed 0.5 mm dorsal to the right lateral ventricle using the following stereotaxic coordinates: −0.5 mm from bregma, −1.5 mm from the midline, and 3.5 mm from the skull surface (Paxinos & Watson, 2004). The implanted cannula was attached to the supporting microscrews with acrylic cement.
Experimental set-up
On the day of the experiment, each rat was placed in a confiner to which it had been habituated (see Animals). For measurement of deep Tb, a copper–constantan thermocouple was inserted in the colon, 10 cm beyond the anal sphincter. The thermocouple was fixed to the base of the tail with adhesive tape and plugged into a data logger which conveyed the data to a personal computer. The rat was then transferred to an environmental chamber: a 3940 chamber from Forma Scientific (Marietta, OH, USA) was used in all experiments, except in the part of Experiment 5 conducted at the University of Pécs, for which a Plexiglas chamber was kept inside a temperature-controlled water bath. When present, the i.v. catheter was connected to a PE-50 extension. When the animal had an i.c.v. cannula, an injector needle (Plastics One) was fitted into the cannula and connected to a PE-50 extension; the needle protruded 1 mm beyond the guide cannula to reach the lateral ventricle. The i.v. or i.c.v. extension, filled with the drug of interest or its vehicle, was passed through a port in the chamber wall and connected to a syringe located outside of the chamber. This set-up permits drug administration without disturbing the rat and without causing a marked stress response that often presents a major limitation in thermoregulation experiments (Romanovsky et al. 1998a; Rudaya et al. 2005).
Experimental protocols
Experiment 1
The first experiment was performed to evaluate whether localized desensitization of TRPV1-bearing nerve afferents (or any other resiniferatoxin-sensitive cells) in the abdomen affects LPS-induced hypothermia. Abdominal visceral TRPV1 channels may play roles in thermoregulation (Steiner et al. 2007) and inflammation (Miranda et al. 2007). Although they are not involved in LPS fever (Dogan et al. 2004), they may be involved in LPS hypothermia. Under deep sedation with an acepromazine-enriched ketamine–xylazine–acepromazine cocktail (5.6, 0.6 and 1.2 mg kg−1i.p.), a rat was injected i.p. with resiniferatoxin (20 μg kg−1) or its vehicle. The sedation protocol was chosen based on our earlier study (Dogan et al. 2004) and resulted in a suppressed writhing response to resiniferatoxin. At the dose used, resiniferatoxin (a highly potent TRPV1 agonist) desensitizes TRPV1 receptors in abdominal viscera for at least 19 days, but does not desensitize TRPV1 receptors in other bodily compartments, as has been shown by several studies from our group using an extensive array of tests (Dogan et al. 2004; Steiner et al. 2007). On day 7 following administration of resiniferatoxin or its vehicle, each rat was implanted with an i.v. catheter. The experiment was conducted on day 11. Each rat was transferred to the experimental set-up described above and exposed to an ambient temperature of 22°C. After 4 h of habituation to the experimental conditions, each rat was injected i.v. with a relatively high, hypothermia-inducing dose of LPS (100 μg kg−1) or with saline. Tb was recorded from the beginning of the experiment to at least 240 min after the injection.
As in our previous study (Dogan et al. 2004), the extent of nerve afferent desensitization was assessed by the eye-wiping test (to confirm that desensitization did not reach systemic levels) and the cholecystokinin (CCK)-induced satiety test (to confirm abdominal desensitization). The eye-wiping test was conducted on day 9. It consisted of counting the number of eye-wiping movements for 30 s following corneal application of a chemical irritant (20 μl of 1% NH4OH in saline). The two-measurement satiety test was conducted on day 15 (first measurement) and day 19 (second measurement), each time after 24 h of food deprivation. Each rat was injected i.p. with CCK (6 μg kg−1) on one day and with saline on the other day; the order of injections was randomized. Standard chow was made available 5 min after the injections, and the mass of chow consumed over 30 min was determined. For each rat, the difference in food intake (CCK test minus saline test) was expressed as a percentage of the amount consumed in the saline test.
Experiment 2
In the second experiment we investigated whether LPS-induced hypothermia is altered in rats pretreated systemically with capsazepine, a reasonably selective (Romanovsky et al. 2009) and potent (Gavva et al. 2005) antagonist of the rat TRPV1 receptor in the chemical ligand and heat modes of activation. At an ambient temperature of 22°C, rats were pretreated i.p. with capsazepine (40 mg kg−1) or its vehicle. Ninety minutes later they were injected i.v. with LPS (100 μg kg−1) or saline. Tb was recorded from the beginning of the experiment to at least 240 min after LPS (or saline) administration.
To confirm the effectiveness of the capsazepine pretreatment, we tested whether it blocked capsaicin-induced hypothermia (Dogan et al. 2004). A separate group of rats were pretreated with capsazepine or its vehicle as described above and 90 min later injected i.p. with capsaicin (1 mg kg−1). Their Tb was recorded.
Experiment 3
The third experiment was conducted to evaluate whether LPS-induced hypothermia is altered by systemic pretreatment with rimonabant (an established CB1 receptor antagonist and inverse agonist; Rinaldi-Carmona et al. 1994; Bouaboula et al. 1997) or SR144528 (a CB2 receptor antagonist and inverse agonist; Rinaldi-Carmona et al. 1998; Portier et al. 1999). At an ambient temperature of 22°C, each rat was pretreated i.p. with rimonabant at a dose of 4.6 mg kg−1 (10 μmol kg−1), SR144528 at a dose of 1.4 mg kg−1 (3 μmol kg−1), or their vehicle (1 ml kg−1). The dose of rimonabant used has been shown to produce a maximum inhibition of the classic CB1-mediated in vivo effects, such as cannabinoid-induced analgesia, ring immobility, and barrel rotations (Rinaldi-Carmona et al. 1994). The dose of SR144528 used is known to exhibit a maximum competition for CB2 receptor binding sites in the spleen (Rinaldi-Carmona et al. 1998). Ninety minutes after the pretreatment with an antagonist or vehicle, rats were injected i.v. with LPS (100 μg kg−1) or saline. The rats’Tb was recorded.
Since rimonabant blocked LPS hypothermia (see Results), we sought to test if SLV319, a structurally distinct CB1 receptor antagonist, was also capable of blocking LPS hypothermia. Because SLV319 is 3–4 times less potent than rimonabant (Lange et al. 2004), it was administered i.p. at a dose of 15 mg kg−1. Following the systemic administration of this dose, SLV319 has been shown to occupy >80% of brain CB1 receptors (Need et al. 2006). At an ambient temperature of 22°C, rats received the i.p. pretreatment with SLV319 or its vehicle and 90 min later were injected i.v. with LPS or saline. It should be noted that we performed this test later than all other experiments of this study and had to use a newer lot of LPS (see Drugs). In separate experiments (data not shown), we determined a dose of the newer LPS that produced a hypothermic response comparable in magnitude to the response caused by the 100 μg kg−1 dose of the LPS lot used in all other experiments. This dose was found to be 25 μg kg−1.
Experiment 4
In the fourth experiment we tested whether selective blockade of CB1 receptors in the brain affects LPS-induced hypothermia. Rimonabant was chosen for this experiment because it is the best studied of the two CB1 receptor antagonists employed in the present study. The experiment was conducted at an ambient temperature of 22°C. A low dose of rimonabant (4.6 μg) was administered into the lateral brain ventricle via a pre-implanted i.c.v. cannula. Control rats received an i.p. injection of rimonabant at the same low dose or an i.c.v. injection of the rimonabant vehicle. Ninety minutes after the pretreament (with rimonabant i.c.v., rimonabant i.p., or the vehicle i.c.v.), the rats received an i.v. injection of LPS (100 μg kg−1) or saline. In a subset of the rats, Tb was monitored. In the other subset, blood samples were collected for determination of pro-inflammatory cytokines (viz. tumour necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6), an anti-inflammatory cytokine (viz. IL-10), and hormones of the anti-inflammatory hypothalamo–pituitary–adrenal (HPA) axis (viz. adrenocorticotropic hormone (ACTH) and corticosterone). The blood samples were collected 50 min after the LPS (or saline) administration, a time that corresponds to the onset of the hypothermic response to LPS (Romanovsky et al. 1996b; Steiner et al. 2005). At the time of sample collection, a rat was anaesthetized i.v. with ketamine–xylazine–acepromazine (5.6, 0.6 and 0.1 mg kg−1), its rib cage was opened, and blood was collected from the inferior vena cava. After the blood was collected, the animal was killed with sodium pentobarbital, as described in the Animals section above. Blood plasma was obtained by centrifugation (6000 g, 5 min, 4°C) of blood collected into EDTA-coated vacutainer tubes (Becton-Dickinson, Franklin Lakes, NJ, USA). Blood serum was obtained by allowing blood to clot at room temperature for 20 min and then centrifuging it (8000 g, 10 min, 4°C). Samples were stored at −80°C until assays.
The levels of TNF-α, IL-1β, IL-6 and IL-10 were determined in plasma samples by sandwich enzyme-linked immunosorbent assay (ELISA) using kits from R&D Systems (Minneapolis, MN, USA); the levels of ACTH were determined in plasma samples by sandwich ELISA using a kit from MD Biosciences (St Paul, MN, USA); and the levels of corticosterone were determined in serum samples by competitive ELISA using a kit from Assay Designs (Ann Arbor, MI, USA). Assay ranges (pg ml−1) were: 13–800 for TNF-α; 31–2000 for IL-1β, IL-6, and IL-10; 5–500 for ACTH; and 32–20,000 for corticosterone. Samples were assayed undiluted (for IL-1β, IL-6, IL-10, and ACTH), diluted 1:10 (TNF-α), or diluted 1:100 (corticosterone). In each assay, all samples were run simultaneously, in duplicate.
Experiment 5
The fifth experiment was designed to evaluate whether and how AEA alters Tb under normal conditions and during LPS-induced hypothermia. Because blockade of CB1 receptors within the brain was sufficient to attenuate LPS hypothermia (see Results), AEA was administered i.c.v. in this experiment. The experiment was conducted at 22°C; Tb was recorded. The rats were injected i.v. with LPS (100 μg kg−1) or saline and 50 min later treated i.c.v. with AEA (50 μg). Control rats were treated i.v. with AEA at the same low dose or i.c.v. with the AEA vehicle.
Because there is no agreement in the literature regarding the thermoregulatory effect of i.c.v. AEA (Crawley et al. 1993; Di Marzo et al. 2000; Fraga et al. 2009), and because the experiment described above did not reveal any thermoregulatory effect of i.c.v. AEA at 50 μg in the absence of systemic inflammation (see Results), additional experiments were conducted to test if the Tb of euthermic rats (not treated with LPS) could be changed by AEA administered i.c.v. in a wide dose range (0.07–520 μg) under various experimental conditions. Prostaglandin (PG) E2 (200 ng), a potent fever-inducing agent (Sugimoto et al. 1999), was administered to test the patency of the i.c.v. cannula. These experiments were conducted at two centres (St Joseph's Hospital and the University of Pécs) in male Long–Evans rats from two different colonies and in female Wistar rats. The rats were exposed to an ambient temperature of 15, 22, 25, or 28°C. Some rats were cold-adapted (4°C for 21 days), while others were food-deprived (for 24 h) prior to the experiment. The experimental conditions were chosen based on the facts that the rat strain (Gordon, 1993; Ivanov et al. 2003a) and sex (Mouihate et al. 1998), as well as the ambient temperature during an experiment (Romanovsky et al. 1997; Ivanov et al. 2003b) and the prior thermal experience (e.g. the development of cold adaptation; see Petervari et al. (2003), can affect the direction and magnitude of a Tb response. The feeding status also influences thermoregulatory responses (Szekely, 1979; Steiner et al. 2009b; Krall et al. 2010), and at least some thermoregulatory consequences of the changes in the feeding status are TRPV1 dependent (Kanizsai et al. 2009). Furthermore, the strain (Arnold et al. 2001), sex (Farhang et al. 2009) and feeding status (Matias & Di Marzo, 2007) are determinants of the expression and responsiveness of CB receptors.
Drugs
E. coli 0111:B4 LPS was purchased from Sigma-Aldrich (St Louis, MO, USA). For all experiments except for the test with SLV319 (Experiment 3), we used lot 35H4086, the same lot that was used in our laboratory for years (Romanovsky et al. 1998a; Ivanov & Romanovsky, 2002). In the SLV319 test, we used LPS of a newer lot, 029K4022. The doses of the two LPS preparations used (100 μg kg−1 for 35H4086 and 25 μg kg−1 for 029K4022) were selected to cause a hypothermic response of a similar magnitude. LPS was suspended in saline (100 or 25 μg ml−1) and bolus-injected i.v. at 1 ml kg−1. CCK octapeptide sulfate (Tocris Cookson, Ellisville, MO, USA) was dissolved in saline (6 μg ml−1) and bolus-injected i.v. at 1 ml kg−1. Resiniferatoxin from Euphorbia poisonii (Sigma-Aldrich), capsaicin (Sigma-Aldrich), capsazepine (Tocris Cookson), SR144528 (Cayman Chemical, Ann Arbor, MI, USA), and SLV319 (Cayman Chemical) were dissolved in ethanol–Tween 80–saline (1:1:3 for capsazepine; 1:1:8 for the other drugs). The final concentrations of resiniferatoxin, capsaicin, capsazepine, SR144528 and SLV319 were 0.02, 1, 40, 1.4, and 15 mg ml−1, respectively. These solutions were bolus-injected i.p. at 1 ml kg−1. For i.p. administration, rimonabant (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was dissolved in ethanol–Tween 80–saline (1:1:8) to a final concentration of 4.6 mg ml−1 (Experiment 3) or 0.046 mg ml−1 (Experiment 4), and the resulting solution was bolus-injected at 1 ml kg−1. For i.c.v. administration, rimonabant was dissolved in ethanol–saline (1:1) to a concentration of 2.3 mg ml−1 and microinfused at a rate of 1 μl min−1 for 2 min. AEA (Tocris Cookson) was dissolved in ethanol–saline (1:1) to concentrations of 0.035–260 mg ml−1 and microinfused i.c.v. at 1 μl min−1 for 2 min or i.v. at 50 μl min−1 for 2 min. PGE2 (Cayman) was administered i.c.v. by microinfusing a 100 μg ml−1 solution in ethanol–saline (1:1) at a rate of 1 μl min−1 for 2 min.
Statistical analyses
Statistical comparisons were made using Statistica Advanced 8.0 (StatSoft, Tulsa, OK, USA). Repeated measures ANOVA was employed to evaluate the effects of the pharmacological treatments on Tb, whereas factorial ANOVA was employed to evaluate the effects of treatments on the levels of TNF-α, IL-1β, IL-6, IL-10, ACTH and corticosterone. The ANOVA was followed by a post hoc analysis with Fisher's least significant difference test. Student's t test was employed to evaluate the changes in food intake and in the number of eye wipes in Experiment 1. The level of significance was set at P < 0.05. Data are reported as means ± SEM.
Results
Experiment 1: LPS hypothermia is not affected by desensitization of abdominal TRPV1 receptors
At an ambient temperature (22°C) known to be subneutral for rats in our experimental set-up (Romanovsky et al. 2002), the i.v. administration of a moderately high dose of LPS (100 μg kg−1) to vehicle-pretreated rats caused a typical hypothermic response (a nadir at ∼100 min; P < 0.02), whereas the administration of saline caused no change in Tb (Fig. 1A). The hypothermic response to LPS also occurred in rats desensitized with resiniferatoxin (20 μg kg−1), and it did not differ from the response of vehicle-pretreated rats.
Figure 1. Intra-abdominal TRPV1 desensitization with resiniferatoxin does not affect LPS hypothermia.

A, the Tb responses to i.v. LPS (dose indicated) or saline in rats pretreated i.p. with resiniferatoxin (dose indicated) or its vehicle and exposed to an ambient temperature of 22°C. B, the results of functional tests confirming the extent of resiniferatoxin-induced afferent nerve desensitization. The results of the CCK-induced satiety test (to assess abdominal desensitization) are shown as the relative difference in the amount of food consumed by food-deprived rats during a 30 min period after administration of CCK-8 sulfate (6 μg kg−1i.p.). These results confirm that nerve fibres involved in CCK-mediated satiety were desensitized. The results of the eye-wiping test (to assess systemic desensitization) are shown as the number of eye wipes during a 30 s period after intraocular administration of an irritant. These results show that desensitization did not occur in remote extra-abdominal locations, such as the cornea. Here and in Fig. 2–7, the number of animals in each group is shown in parentheses. *P < 0.05 compared to vehicle pretreatment.
Desensitization of intra-abdominal afferents was verified by testing rats for CCK-induced satiety, a response known to be mediated, at least partly, by abdominal vagal afferent fibres (Smith et al. 1985). CCK decreased the food intake of vehicle-pretreated rats by ∼50%, whereas it had a 5 times smaller effect in resiniferatoxin-desensitized rats (P < 0.04; Fig. 1B). Yet resiniferatoxin pretreatment did not impair the eye-wiping response to a chemical irritant (Fig. 1B), indicating that desensitization did not reach systemic levels.
Experiment 2: LPS hypothermia is not affected by systemic pharmacological blockade of TRPV1 receptors
Vehicle-pretreated controls responded to LPS with statistically significant (P < 0.04) hypothermia (Fig. 2A). Pretreatment with the TRPV1 receptor antagonist, capsazepine (40 mg kg−1i.p.), affected neither the level of Tb in euthermic, saline-injected rats nor the hypothermic response to LPS. However, the same dose of capsazepine was effective (P < 0.04) in blocking the hypothermic response to capsaicin (1 mg kg−1i.p.;Fig. 2B).
Figure 2. TRPV1 receptor antagonism with capsazepine does not affect LPS hypothermia.

A, the Tb responses to i.v. LPS (dose indicated) or saline in rats pretreated i.p. with resiniferatoxin (dose indicated) or its vehicle. B, the results of a functional test confirming the effectiveness of the capsazepine pretreatment based on its ability to block the hypothermic response to the TRPV1 agonist, capsaicin (1 mg kg−1, i.p.). Rats were exposed to an ambient temperature of 22°C during both experiments. The same capsazepine pretreatment that did not affect LPS hypothermia strongly attenuated the hypothermic response to capsaicin.
Experiment 3: blockade of CB1 receptors, but not of CB2 receptors, abolishes LPS-induced hypothermia
Systemic pretreatment with the CB2 receptor antagonist, SR144528 (1.4 mg kg−1i.p.), affected neither the Tb of saline-treated rats nor the hypothermic response to LPS (Fig. 3). The CB1 receptor antagonist, rimonabant (4.6 mg kg−1i.p.), had no effect on the Tb of saline-treated rats, but it abolished the hypothermic response to LPS (P < 0.004). The effect of rimonabant was so pronounced that there was no statistical difference in Tb between rimonabant-pretreated rats injected with LPS and rimonabant-pretreated rats injected with saline.
Figure 3. LPS hypothermia is blocked by a CB1 receptor antagonist (rimonabant), but unaffected by a CB2 receptor antagonist (SR144528).

Effects of i.p. pretreatment with rimonabant, SR144528, or their vehicle on the Tb responses to i.v. LPS or saline in rats exposed to an ambient temperature of 22°C.
The ability of CB1 receptor antagonism to block LPS hypothermia was confirmed using a distinct CB1 receptor antagonist, SLV319. Pretreatment with SLV319 (15 mg kg−1) had no effect on the Tb of saline-treated rats, but it strongly attenuated the hypothermic response to LPS (P < 0.03; Fig. 4).
Figure 4. LPS hypothermia is blocked by SLV319, a CB1 receptor antagonist distinct from rimonabant.

Effects of i.p. pretreatment with SLV319 or its vehicle on the Tb responses to i.v. LPS or saline in rats exposed to an ambient temperature of 22°C. Note that LPS of a different lot was used in this test, and that the dose of LPS was adjusted in order to produce a hypothermic response similar in magnitude to the responses observed in other experiments of this study (see Drugs for details).
Experiment 4: LPS hypothermia is abolished by selective blockade of CB1 receptors in the brain
Figure 5A shows the effect of the i.v. administration of saline or LPS on Tb in rats pretreated i.c.v with either rimonabant at a low dose (4.6 μg) or with its vehicle. LPS lowered the Tb of rats pretreated with vehicle (P < 0.01), but not of rats pretreated with rimonabant. Comparison between LPS-induced effects in the rimonabant- and vehicle-pretreated groups revealed a significant difference (P < 0.04). However, when the same low dose of rimonabant was given i.p., it did not affect LPS hypothermia (Fig. 5B).
Figure 5. Intrabrain rimonabant at a systemically ineffective dose blocks LPS hypothermia.

Effects of pretreatment with a low dose of rimonabant or its vehicle, either i.c.v. (panel A) or i.p. (panel B), on the Tb responses to i.v. LPS or saline.
To investigate whether inhibition of LPS hypothermia by rimonabant might have been associated with stronger activation of the anti-inflammatory HPA axis or with an altered balance between pro- and anti-inflammatory cytokines, we measured the circulating levels of ACTH and corticosterone (HPA hormones), TNF-α, IL-1β and IL-6 (pro-inflammatory cytokines), and IL-10 (an anti-inflammatory cytokine). At the time corresponding to the onset of hypothermia (50 min), vehicle-pretreated rats responded to LPS with significant rises in the levels of ACTH (P < 0.0007) and corticosterone (P < 0.05), as well as in the levels of all cytokines measured (P < 0.003; Fig. 6). Pretreatment with rimonabant (4.6 μg i.c.v.) attenuated the LPS-induced rise in plasma ACTH (P < 0.04), but did not affect the rise in plasma corticosterone. The LPS-induced rise in plasma TNF-α was reduced by rimonabant (P < 0.0002), the rise in IL-1β tended to be attenuated (P < 0.09), and the rises in IL-6 and IL-10 were unaffected.
Figure 6. Intrabrain rimonabant attenuates the LPS-induced rises in circulating ACTH and TNF-α.

Effects of LPS or saline on the circulating levels of ACTH, corticosterone, TNF-α, IL-1β, IL-6 and IL-10 of rats pretreated i.c.v. with a low dose of rimonabant (indicated) or its vehicle. Blood samples were collected 50 min after rats were administered i.v. with LPS (100 μg kg−1) or saline at an ambient temperature of 22°C.
Experiment 5: central administration of AEA does not cause hypothermia, but enhances LPS-induced hypothermia
Figure 7 shows the effect of AEA at a low dose (50 μg) on the Tb of rats injected i.v. with LPS (100 μg kg−1) or saline. The Tb of the saline-injected rats was not affected by the i.c.v. administration of AEA or its vehicle. However, LPS hypothermia was greater in the rats administered i.c.v. with AEA than in the rats administered with its vehicle (P < 0.05). When the same dose of AEA was given i.v., it did not affect LPS hypothermia.
Figure 7. Intrabrain AEA enhances LPS hypothermia.
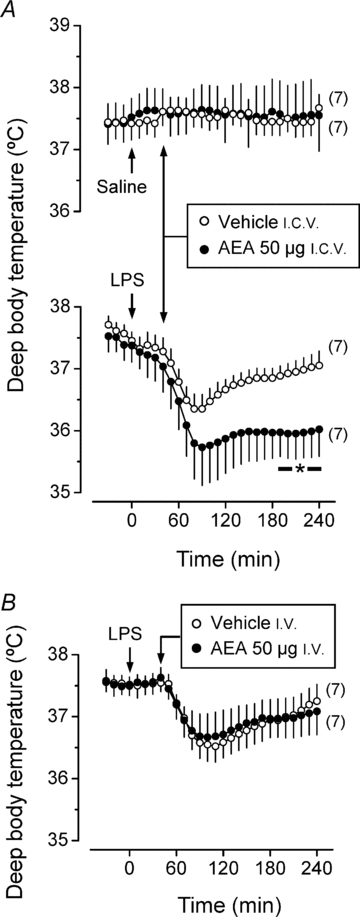
Effects of a low dose of AEA given i.c.v. (panel A) or i.v. (panel B) on the Tb responses to i.v. LPS (100 μg kg−1) or saline. The ambient temperature was 22°C.
The fact that i.c.v. AEA did not affect the Tb of saline-injected rats deserves attention, since there is controversy as to whether the action of AEA in the brain of euthermic rats causes hypothermia, a typical response to exogenous cannabinoid agonists (Crawley et al. 1993; Chaperon & Thiebot, 1999; Di Marzo et al. 2000; Fraga et al. 2009). We addressed this controversy by performing additional experiments, in which we studied thermoregulatory effects of i.c.v. AEA in rats not injected with LPS. AEA was administered over a wide dose range (0.07–520 μg) and under several experimental conditions. We studied two rat strains and sexes (male Long–Evans and female Wistar rats) and four ambient temperatures (15, 22, 25 or 28°C). In some experiments, we also used cold adaptation or food deprivation. Under no circumstance did the i.c.v. administration of AEA decrease (or cause any other change in) the Tb of the rats (Fig. 8). The patency of the i.c.v. cannulas and the overall ability of our methodology to detect a Tb response were verified by administering PGE2 at a relatively low dose (200 ng) and registering a highly significant (P < 0.0001) Tb rise, as compared to the response of rats treated with the vehicle (0 μg AEA; Fig. 8).
Figure 8. Intrabrain AEA does not cause hypothermia.

The figure shows the effects of i.c.v. AEA on the Tb of individual rats under various experimental conditions (indicated). The body temperature responses to i.c.v. PGE2 are shown to confirm the correct placement and patency of the i.c.v. cannulas. Doses of AEA and PGE2 are indicated. Each data point denotes the maximal change in Tb during 0–120 min after the i.c.v. injection.
Discussion
The present study was the first to investigate the potential involvement of TRPV1, CB1 and CB2 receptors in LPS-induced hypothermia. To assess the involvement of the TRPV1 receptor, we studied the effects of TRPV1 desensitization with resiniferatoxin and of the pharmacological antagonism with capsazepine on LPS hypothermia. Resiniferatoxin was our first-choice TRPV1 agonist for desensitization experiments because it is more potent and selective than capsaicin (Szallasi & Blumberg, 1989) and, most importantly, lacks the non-TRPV1-mediated effects of capsaicin, such as attenuation of the first febrile phase (Dogan et al. 2004; Nikami et al. 2008). Capsazepine was our first-choice TRPV1 antagonist because, at moderate doses, it does not exert a thermoregulatory effect of its own in rats (Garami et al. 2010), even though it blocks two modes of TRPV1 receptor activation, i.e. by ligands and heat, with moderate and high potency, respectively (McIntyre et al. 2001; Price et al. 2004; Gavva et al. 2005; Garami et al. 2010). Neither resiniferatoxin desensitization nor capsazepine antagonism altered LPS hypothermia, indicating that TRPV1 receptors are not involved in this response. To confirm this finding, we conducted a supplemental experiment employing AMG0347, a new-generation TRPV1 antagonist that blocks the proton, ligand and heat modes of activation of the rat TRPV1 channel with high potency (Steiner et al. 2007). Like many other TRPV1 antagonists, especially those that are potent blockers of the proton mode of activation (Garami et al. 2010), AMG0347 causes hyperthermia (Steiner et al. 2007; Garami et al. 2010). Indeed, in our supplemental experiment, AMG0347 elevated the basal Tb of rats (Suppl. Fig. 1). However, when these rats were injected with LPS, their deep Tb was decreased to the same level of vehicle-pretreated rats. Because neither TRPV1 desensitization nor pharmacological antagonism (by capsazepine or AMG0347) affects LPS hypothermia, we conclude that the hypothermic response to LPS does not depend on the activation of TRPV1 channels. A convincing way to further support this conclusion would be to study LPS hypothermia in Trpv1 knockout mice. However, unlike rats, mice of several strains do not respond to a non-stressful i.v. injection of LPS with the early (over the first 2 h) hypothermic response (Rudaya et al. 2005). The non-responding strains also include Trpv1+/+ and Trpv1−/− C57BL/6×129 mice from the Amgen colony at Charles River Laboratories (S. P. Wanner, N. R. Gavva & A. A. Romanovsky, unpublished observations).
Our present experiments also show that the hypothermic response to LPS is not affected by CB2 receptor antagonism with SR144528. In line with this finding are reports that CB2 receptor antagonists have no impact on other brain-mediated components of the systemic inflammatory response, e.g. hyperalgesia (Naidu et al. 2010) and fever (Benamar et al. 2007; Fraga et al. 2009). Even though CB2 receptors have been shown to play anti-inflammatory roles in some immune cells (Sacerdote et al. 2000; Germain et al. 2002; Eisenstein et al. 2007), such roles are likely to be limited to localized inflammatory processes (Oka et al. 2005; Naidu et al. 2010).
The most striking finding of the present study was that blockade of CB1 receptors by rimonabant or SLV319 prevented the hypothermic response to LPS. The effect was so strong that the Tb response of rats treated with LPS looked like that of rats treated with saline. Having worked with LPS hypothermia for years (Romanovsky et al. 1996b, 1997, 1998a; Steiner et al. 2004, 2005, 2009a; Rudaya et al. 2005; Almeida et al. 2006a,b; Krall et al. 2010), we have never seen such a complete pharmacological blockade of the robust, highly reproducible hypothermic response of rats to LPS. Rimonabant prevented LPS hypothermia not only when administered systemically at a relatively high dose, but also when administered i.c.v. at a dose that was too low to produce systemic effects. These findings indicate that CB1 receptors in the brain play an essential role in the development of LPS-induced hypothermia. Such a role is unlikely to reflect a direct thermoregulatory (hypothermic) effect because, in our experiments, i.c.v. administration of AEA over a wide dose range and under a variety of experimental conditions failed to produce any hypothermic response in normal (no systemic inflammation) rats. This observation may seem paradoxical, as hypothermia is considered a classic effect of exogenous cannabinoid agonists (Fitton & Pertwee, 1982; Ovadia et al. 1995; Rawls et al. 2002). However, in agreement with our study, i.c.v. AEA failed to cause hypothermia in the studies by Lichyman et al. (1996) and Fraga et al. (2009). In fact, Fraga et al. (2009) have shown that AEA causes fever rather than hypothermia. Only in a study by Porter et al. (2002) did i.c.v. AEA produce a hypothermic effect, possibly by leaking into the systemic circulation and causing hypothermia via an action on peripheral targets. That peripheral administration of AEA causes hypothermia in rats and mice has been shown repeatedly (Crawley et al. 1993; Di Marzo et al. 2000; Fegley et al. 2004; Garami et al. 2011), including in our present study (Suppl. Fig. 2). It has also been recognized that many effects of exogenous cannabinoid agonists (such as the hypothermic effect) do not correspond to intrinsic effects of endogenous AEA (Chaperon & Thiebot, 1999). From this point of view, inhibitors of the AEA-hydrolysing enzyme, fatty acid amide hydrolase, are now being used as a powerful tool to assess the roles of endogenous AEA. It has been shown that inhibition of fatty acid amide hydrolase with URB597 does not result in hypothermia, even when it markedly elevates the brain level of AEA (Fegley et al. 2005).
The present study sheds some light on the physiological and molecular mechanisms of the hypothermic effect of peripheral AEA. In supplemental experiments (Suppl. Fig. 2), we have found that the hypothermic response to i.v. AEA is associated with tail-skin vasodilatation, even though these experiments were conducted at a subneutral ambient temperature, which tends to counteract cutaneous vasodilatatory responses. In the same experiment, we could not block the hypothermic response to i.v. AEA by a combined treatment with CB1 and CB2 receptor antagonists (Suppl. Fig. 2). Cumulatively, these findings suggest that peripheral AEA causes hypothermia, at least in part, by triggering cutaneous vasodilatation via an action on non-CB1, non-CB2 receptors, perhaps on TRPV1 channels. Recently, we have shown the proposed TRPV1 mediation decisively by establishing that Trpv1 knockout mice do not respond with hypothermia to peripheral AEA, whereas control mice develop marked hypothermia (Garami et al. 2011). Furthermore, the vasodilatatory effect of AEA has been shown to be mediated by TRPV1 channels (Zygmunt et al. 1999). Not all literature data can be readily reconciled with the present results. Experiments with inhibitors of the degradation of 2-arachidonyl glycerol suggest that this endogenous cannabinoid can decrease Tb via a CB1-mediated mechanism (Burston et al. 2008; Long et al. 2009). However, it is unclear whether the proposed mechanism involves central or peripheral CB1 receptors, and it is puzzling that the hypothermic response attributed to CB1 receptors still occurred (even though it was attenuated) in mice deficient of the Cnr1 (CB1 receptor) gene (Burston et al. 2008). Clearly, further studies are needed.
While i.c.v. AEA did not cause hypothermia in the present study, it markedly enhanced the hypothermic response to LPS. This finding, together with our observation that a CB1 receptor antagonist (rimonabant) blocks LPS hypothermia, suggests that endocannabinoids act on brain CB1 receptors not within thermoregulatory circuits, but rather on some LPS-activated signalling pathway that ultimately interferes with thermoregulatory circuitry. Consistent with this idea, CB1 receptors have been shown to modulate LPS-induced changes in the hypothalamic concentration of noradrenaline (Villanueva et al. 2009), which, in turn, can drive thermoregulatory responses (Feleder et al. 2007a,b;). Moreover, it is important to note that the blockade of LPS hypothermia by i.c.v. rimonabant in the present study was associated with an attenuated rise in the circulating level of TNF-α, a mediator of LPS hypothermia (Kozak et al. 1995; Leon et al. 1998; Tollner et al. 2000). Recent studies have similarly shown that the LPS-induced rise in circulating TNF-α is strongly attenuated by rimonabant, not only when this CB1 antagonist is administered systemically (Croci et al. 2003), but also when it is administered i.c.v. at systemically ineffective doses (Villanueva et al. 2009; De Laurentiis et al. 2010). It is plausible, therefore, that CB1 receptors in the brain are ‘permissive’ for the LPS-induced rise in circulating TNF-α. Such a permissive role of brain CB1 receptors may explain why inhibitors of degradation or reuptake of endocannabinoids enhance the TNF-α response to LPS (Roche et al. 2008), even though a peripheral action of endocannabinoids (on leukocytes) suppresses TNF-α production (Berdyshev et al. 1997; Cencioni et al. 2010).
Mechanisms by which blockade of brain CB1 receptors suppress the systemic production of TNF-α in response to LPS are unknown, but they are unlikely to involve activation of the anti-inflammatory HPA axis, because rimonabant enhanced neither the LPS-induced rise in plasma ACTH nor the LPS-induced rise in plasma corticosterone in the present study. In fact, the rise in ACTH was attenuated by rimonabant. It is of interest, however, that the vagus nerve (and possibly sympathetic nerves) can convey descending anti-inflammatory signals that suppress the production of TNF-α (Rosas-Ballina et al. 2008). Indeed, surgical vagotomy exaggerates LPS-induced hypothermia (Romanovsky et al. 1997), as well as other systemic effects of LPS, including TNF-α production (Borovikova et al. 2000), while electric stimulation of the peripheral end of the transected vagus attenuates these responses (Borovikova et al. 2000). Furthermore, many areas of the brain (including the hypothalamus) express CB1 receptors (Herkenham et al. 1991; Matsuda et al. 1993; Hirasawa et al. 2004) and project to the dorsal motor nucleus of the vagus (Saper et al. 1976; Chiba & Murata, 1985). Importantly, Derbenev et al. (2004) have provided functional evidence for a connection between CB1 receptors in the brain and vagal output by showing that pharmacological activation of CB1 receptors suppresses excitatory and inhibitory inputs to the motor nucleus of the vagus.
In conclusion, the main novel finding of the present study is that CB1 receptors in the brain are essential for the development of LPS-induced hypothermia, a prominent manifestation of severe systemic inflammation. We also show that brain CB1 receptors are permissive for the rise in the systemic level of TNF-α, an early event thought to contribute to morbidity and mortality in systemic inflammation and sepsis. Hence, brain CB1 receptors should be evaluated as potential therapeutic targets in sepsis and related conditions. The CB1-mediated mechanisms of LPS hypothermia remain speculative, but are likely to involve modulation of TNF-α production in the periphery by a descending pathway, perhaps involving the vagus nerve. In addition, the present study shows that central AEA exaggerates LPS hypothermia, but does not cause hypothermia under normal (without systemic inflammation) conditions. Only when injected peripherally does AEA cause hypothermia under normal conditions, possibly by triggering cutaneous vasodilatation via a TRPV1-mediated action. The present study also provides evidence against an involvement of CB2 and TRPV1 receptors in LPS hypothermia.
Acknowledgments
This study has been supported in part by grant R01NS41233 from the National Institute of Neurological Disorders and Stroke to A.A.R. and grants of the Hungarian Scientific Research Fund (OTKA) 49321 to M.S. and PD-84241 to E.P.
Glossary
Abbreviations
- ACTH
adrenocorticotropic hormone
- AEA
arachidonoyl N-ethanolamide (anandamide)
- CB1 and CB2
cannabinoid-1 and -2 receptors, respectively
- CCK
cholecystokinin
- HPA
hypothalamo–pituitary–adrenal
- IL
interleukin
- LPS
lipopolysaccharide
- PG
prostaglandin
- Tb
body temperature
- TNF
tumour necrosis factor
- TRPV1
transient receptor potential vanilloid-1
Author contributions
A.A.S., A.Y.M., M.D.D., M.C.A., M.S. and A.A.R. designed the study, with the help of S.P., E.P., M.B,. S.P.W. and N.R.G.; A.A.S., A.Y.M., M.D.D., S.P., E.P., M.B., S.P.W., J.E., D.L.O. and M.C.A. conducted the experiments; A.A.S., A.Y.M., M.D.D., S.P., E.P., M.B., J.E., M.C.A., M.S. and A.A.R. analysed the data; and A.A.S. and A.A.R. wrote the manuscript, with the help of A.Y.M., M.D.D., S.P., M.C.A. and M.S.
Authors' present addresses
M. C. Almeida: Universidade Federal do ABC, Santo Andre, SP, Brazil.
S. P. Wanner: Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil.
Supplementary material
Supplemental Figure 1
Supplemental Figure 2
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Abercrombie ED, Jacobs BL. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. II. Adaptation to chronically presented stressful stimuli. J Neurosci. 1987;7:2844–2848. doi: 10.1523/JNEUROSCI.07-09-02844.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida MC, Steiner AA, Branco LG, Romanovsky AA. Cold-seeking behavior as a thermoregulatory strategy in systemic inflammation. Eur J Neurosci. 2006a;23:3359–3367. doi: 10.1111/j.1460-9568.2006.04854.x. [DOI] [PubMed] [Google Scholar]
- Almeida MC, Steiner AA, Branco LG, Romanovsky AA. Neural substrate of cold-seeking behavior in endotoxin shock. PLoS One. 2006b;1:e1. doi: 10.1371/journal.pone.0000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JC, Topple AN, Mallet PE, Hunt GE, McGregor IS. The distribution of cannabinoid-induced Fos expression in rat brain: differences between the Lewis and Wistar strain. Brain Res. 2001;921:240–255. doi: 10.1016/s0006-8993(01)03127-4. [DOI] [PubMed] [Google Scholar]
- Arons MM, Wheeler AP, Bernard GR, Christman BW, Russell JA, Schein R, Summer WR, Steinberg KP, Fulkerson W, Wright P, Dupont WD, Swindell BB. Effects of ibuprofen on the physiology and survival of hypothermic sepsis. Crit Care Med. 1999;27:699–707. doi: 10.1097/00003246-199904000-00020. [DOI] [PubMed] [Google Scholar]
- Benamar K, Yondorf M, Meissler JJ, Geller EB, Tallarida RJ, Eisenstein TK, Adler MW. A novel role of cannabinoids: implication in the fever induced by bacterial lipopolysaccharide. J Pharmacol Exp Ther. 2007;320:1127–1133. doi: 10.1124/jpet.106.113159. [DOI] [PubMed] [Google Scholar]
- Berdyshev EV, Boichot E, Germain N, Allain N, Anger JP, Lagente V. Influence of fatty acid ethanolamides and Δ9-tetrahydrocannabinol on cytokine and arachidonate release by mononuclear cells. Eur J Pharmacol. 1997;330:231–240. doi: 10.1016/s0014-2999(97)01007-8. [DOI] [PubMed] [Google Scholar]
- Blatteis CM. Endotoxic fever: new concepts of its regulation suggest new approaches to its management. Pharmacol Ther. 2006;111:194–223. doi: 10.1016/j.pharmthera.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Perrachon S, Milligan L, Canat X, Rinaldi-Carmona M, Portier M, Barth F, Calandra B, Pecceu F, Lupker J, Maffrand JP, Le Fur G, Casellas P. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1Evidence for a new model of receptor/ligand interactions. J Biol Chem. 1997;272:22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Rinaldi M, Carayon P, Carillon C, Delpech B, Shire D, Le Fur G, Casellas P. Cannabinoid-receptor expression in human leukocytes. Eur J Biochem. 1993;214:173–180. doi: 10.1111/j.1432-1033.1993.tb17910.x. [DOI] [PubMed] [Google Scholar]
- Burston JJ, Sim-Selley LJ, Harloe JP, Mahadevan A, Razdan RK, Selley DE, Wiley JL. N-Arachidonyl maleimide potentiates the pharmacological and biochemical effects of the endocannabinoid 2-arachidonylglycerol through inhibition of monoacylglycerol lipase. J Pharmacol Exp Ther. 2008;327:546–553. doi: 10.1124/jpet.108.141382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cencioni MT, Chiurchiu V, Catanzaro G, Borsellino G, Bernardi G, Battistini L, Maccarrone M. Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly via CB2 receptors. PLoS One. 2010;5:e8688. doi: 10.1371/journal.pone.0008688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaperon F, Thiebot MH. Behavioral effects of cannabinoid agents in animals. Crit Rev Neurobiol. 1999;13:243–281. doi: 10.1615/critrevneurobiol.v13.i3.20. [DOI] [PubMed] [Google Scholar]
- Chiba T, Murata Y. Afferent and efferent connections of the medial preoptic area in the rat: a WGA-HRP study. Brain Res Bull. 1985;14:261–272. doi: 10.1016/0361-9230(85)90091-7. [DOI] [PubMed] [Google Scholar]
- Clemmer TP, Fisher CJ, Jr, Bone RC, Slotman GJ, Metz CA, Thomas FO. Hypothermia in the sepsis syndrome and clinical outcome. Crit Care Med. 1992;20:1395–1401. doi: 10.1097/00003246-199210000-00006. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Corwin RL, Robinson JK, Felder CC, Devane WA, Axelrod J. Anandamide, an endogenous ligand of the cannabinoid receptor, induces hypomotility and hypothermia in vivo in rodents. Pharmacol Biochem Behav. 1993;46:967–972. doi: 10.1016/0091-3057(93)90230-q. [DOI] [PubMed] [Google Scholar]
- Croci T, Landi M, Galzin AM, Marini P. Role of cannabinoid CB1 receptors and tumor necrosis factor-α in the gut and systemic anti-inflammatory activity of SR 141716 (rimonabant) in rodents. Br J Pharmacol. 2003;140:115–122. doi: 10.1038/sj.bjp.0705412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Laurentiis A, Fernandez-Solari J, Mohn C, Burdet B, Zubilete MA, Rettori V. The hypothalamic endocannabinoid system participates in the secretion of oxytocin and tumor necrosis factor-α induced by lipopolysaccharide. J Neuroimmunol. 2010;221:32–41. doi: 10.1016/j.jneuroim.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Derbenev AV, Stuart TC, Smith BN. Cannabinoids suppress synaptic input to neurones of the rat dorsal motor nucleus of the vagus nerve. J Physiol. 2004;559:923–938. doi: 10.1113/jphysiol.2004.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Viswanath V, Patapoutian A. TRP ion channels and temperature sensation. Annu Rev Neurosci. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Breivogel C, Bisogno T, Melck D, Patrick G, Tao Q, Szallasi A, Razdan RK, Martin BR. Neurobehavioral activity in mice of N-vanillyl-arachidonyl-amide. Eur J Pharmacol. 2000;406:363–374. doi: 10.1016/s0014-2999(00)00687-7. [DOI] [PubMed] [Google Scholar]
- Dogan MD, Patel S, Rudaya AY, Steiner AA, Szekely M, Romanovsky AA. Lipopolysaccharide fever is initiated via a capsaicin-sensitive mechanism independent of the subtype-1 vanilloid receptor. Br J Pharmacol. 2004;143:1023–1032. doi: 10.1038/sj.bjp.0705977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein TK, Meissler JJ, Wilson Q, Gaughan JP, Adler MW. Anandamide and Δ9-tetrahydrocannabinol directly inhibit cells of the immune system via CB2 receptors. J Neuroimmunol. 2007;189:17–22. doi: 10.1016/j.jneuroim.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engblom D, Ek M, Saha S, Ericsson-Dahlstrand A, Jakobsson PJ, Blomqvist A. Prostaglandins as inflammatory messengers across the blood-brain barrier. J Mol Med. 2002;80:5–15. doi: 10.1007/s00109-001-0289-z. [DOI] [PubMed] [Google Scholar]
- Ephgrave K, Kremer T, Broadhurst K, Cullen J. The role of platelet-activating factor in conscious, normotensive endotoxemia. J Surg Res. 1997;68:170–174. doi: 10.1006/jsre.1997.5009. [DOI] [PubMed] [Google Scholar]
- Farhang B, Diaz S, Tang SL, Wagner EJ. Sex differences in the cannabinoid regulation of energy homeostasis. Psychoneuroendocrinology. 2009;34(Suppl 1):S237–S246. doi: 10.1016/j.psyneuen.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- Fegley D, Kathuria S, Mercier R, Li C, Goutopoulos A, Makriyannis A, Piomelli D. Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the hydrolysis-resistant inhibitor AM1172. Proc Natl Acad Sci U S A. 2004;101:8756–8761. doi: 10.1073/pnas.0400997101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feleder C, Perlik V, Blatteis CM. Preoptic nitric oxide attenuates endotoxic fever in guinea pigs by inhibiting the POA release of norepinephrine. Am J Physiol Regul Integr Comp Physiol. 2007a;293:R1144–R1151. doi: 10.1152/ajpregu.00068.2007. [DOI] [PubMed] [Google Scholar]
- Feleder C, Perlik V, Blatteis CM. Preoptic norepinephrine mediates the febrile response of guinea pigs to lipopolysaccharide. Am J Physiol Regul Integr Comp Physiol. 2007b;293:R1135–R1143. doi: 10.1152/ajpregu.00067.2007. [DOI] [PubMed] [Google Scholar]
- Fernandez-Solari J, Prestifilippo JP, Bornstein SR, McCann SM, Rettori V. Participation of the endocannabinoid system in the effect of TNF-α on hypothalamic release of gonadotropin-releasing hormone. Ann N Y Acad Sci. 2006;1088:238–250. doi: 10.1196/annals.1366.008. [DOI] [PubMed] [Google Scholar]
- Fitton AG, Pertwee RG. Changes in body temperature and oxygen consumption rate of conscious mice produced by intrahypothalamic and intracerebroventricular injections of Δ9-tetrahydrocannabinol. Br J Pharmacol. 1982;75:409–414. doi: 10.1111/j.1476-5381.1982.tb08802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga D, Zanoni CI, Rae GA, Parada CA, Souza GE. Endogenous cannabinoids induce fever through the activation of CB1 receptors. Br J Pharmacol. 2009;157:1494–1501. doi: 10.1111/j.1476-5381.2009.00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Garami A, Pakai E, Oliveira DL, Steiner AA, Wanner SP, Almeida MC, Lesnikov VA, Gavva NR, Romanovsky AA. Thermoregulatory phenotype of the Trpv1 knockout mouse: thermoeffector dysbalance with hyperkinesis. J Neurosci. 2011;31:1721–1733. doi: 10.1523/JNEUROSCI.4671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garami A, Shimansky YP, Pakai E, Oliveira DL, Gavva NR, Romanovsky AA. Contributions of different modes of TRPV1 activation to TRPV1 antagonist-induced hyperthermia. J Neurosci. 2010;30:1435–1440. doi: 10.1523/JNEUROSCI.5150-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva NR, Tamir R, Qu Y, Klionsky L, Zhang TJ, Immke D, Wang J, Zhu D, Vanderah TW, Porreca F, Doherty EM, Norman MH, Wild KD, Bannon AW, Louis JC, Treanor JJ. AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J Pharmacol Exp Ther. 2005;313:474–484. doi: 10.1124/jpet.104.079855. [DOI] [PubMed] [Google Scholar]
- Germain N, Boichot E, Advenier C, Berdyshev EV, Lagente V. Effect of the cannabinoid receptor ligand, WIN 55,212-2, on superoxide anion and TNF-α production by human mononuclear cells. Int Immunopharmacol. 2002;2:537–543. doi: 10.1016/s1567-5769(01)00200-4. [DOI] [PubMed] [Google Scholar]
- Gonsiorek W, Lunn C, Fan X, Narula S, Lundell D, Hipkin RW. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Mol Pharmacol. 2000;57:1045–1050. [PubMed] [Google Scholar]
- Gordon CJ. Temperature Regulation in Laboratory Rodents. Cambridge, UK: Cambridge University Press; 1993. [Google Scholar]
- Gourine AV, Rudolph K, Korsak AS, Kubatko J, Tesfaigzi J, Kozak W, Kluger MJ. Role of capsaicin-sensitive afferents in fever and cytokine responses during systemic and local inflammation in rats. Neuroimmunomodulation. 2001;9:13–22. doi: 10.1159/000049003. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Suemaru S, Takao T, Sugawara M, Makino S, Ota Z. Corticotropin-releasing hormone and pituitary-adrenocortical responses in chronically stressed rats. Regul Pept. 1988;23:117–126. doi: 10.1016/0167-0115(88)90019-5. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa M, Schwab Y, Natah S, Hillard CJ, Mackie K, Sharkey KA, Pittman QJ. Dendritically released transmitters cooperate via autocrine and retrograde actions to inhibit afferent excitation in rat brain. J Physiol. 2004;559:611–624. doi: 10.1113/jphysiol.2004.066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida T, Shimizu I, Nealen ML, Campbell A, Caterina M. Attenuated fever response in mice lacking TRPV1. Neurosci Lett. 2005;378:28–33. doi: 10.1016/j.neulet.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Kulchitsky VA, Romanovsky AA. Role for the cholecystokinin-A receptor in fever: a study of a mutant rat strain and a pharmacological analysis. J Physiol. 2003a;547:941–949. doi: 10.1113/jphysiol.2002.033183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, Patel S, Kulchitsky VA, Romanovsky AA. Platelet-activating factor: a previously unrecognized mediator of fever. J Physiol. 2003b;553:221–228. doi: 10.1113/jphysiol.2003.055616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, Romanovsky AA. Fever responses of Zucker rats with and without fatty mutation of the leptin receptor. Am J Physiol Regul Integr Comp Physiol. 2002;282:R311–R316. doi: 10.1152/ajpregu.00376.2001. [DOI] [PubMed] [Google Scholar]
- Kanizsai P, Garami A, Solymar M, Szolcsanyi J, Szelenyi Z. Energetics of fasting heterothermia in TRPV1-KO and wild type mice. Physiol Behav. 2009;96:149–154. doi: 10.1016/j.physbeh.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Kozak W, Conn CA, Klir JJ, Wong GH, Kluger MJ. TNF soluble receptor and antiserum against TNF enhance lipopolysaccharide fever in mice. Am J Physiol Regul Integr Comp Physiol. 1995;269:R23–R29. doi: 10.1152/ajpregu.1995.269.1.R23. [DOI] [PubMed] [Google Scholar]
- Kozak W, Kluger MJ, Kozak A, Wachulec M, Dokladny K. Role of cytochrome P-450 in endogenous antipyresis. Am J Physiol Regul Integr Comp Physiol. 2000;279:R455–R460. doi: 10.1152/ajpregu.2000.279.2.R455. [DOI] [PubMed] [Google Scholar]
- Krall CM, Yao X, Hass MA, Feleder C, Steiner AA. Food deprivation alters thermoregulatory responses to lipopolysaccharide by enhancing cryogenic inflammatory signaling via prostaglandin D2. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1512–R1521. doi: 10.1152/ajpregu.00158.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange JH, Coolen HK, van Stuivenberg HH, Dijksman JA, Herremans AH, Ronken E, Keizer HG, Tipker K, McCreary AC, Veerman W, Wals HC, Stork B, Verveer PC, den Hartog AP, de Jong NM, Adolfs TJ, Hoogendoorn J, Kruse CG. Synthesis, biological properties, and molecular modeling investigations of novel 3,4-diarylpyrazolines as potent and selective CB1 cannabinoid receptor antagonists. J Med Chem. 2004;47:627–643. doi: 10.1021/jm031019q. [DOI] [PubMed] [Google Scholar]
- Leon LR, White AA, Kluger MJ. Role of IL-6 and TNF in thermoregulation and survival during sepsis in mice. Am J Physiol Regul Integr Comp Physiol. 1998;275:R269–R277. doi: 10.1152/ajpregu.1998.275.1.R269. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Cook SA, Martin BR. Investigation of brain sites mediating cannabinoid-induced antinociception in rats: evidence supporting periaqueductal gray involvement. J Pharmacol Exp Ther. 1996;276:585–593. [PubMed] [Google Scholar]
- Liu J, Batkai S, Pacher P, Harvey-White J, Wagner JA, Cravatt BF, Gao B, Kunos G. Lipopolysaccharide induces anandamide synthesis in macrophages via CD14/MAPK/phosphoinositide 3-kinase/NF-κB independently of platelet-activating factor. J Biol Chem. 2003;278:45034–45039. doi: 10.1074/jbc.M306062200. [DOI] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavon FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Matias I, Di Marzo V. Endocannabinoids and the control of energy balance. Trends Endocrinol Metab. 2007;18:27–37. doi: 10.1016/j.tem.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Kobayashi S. Signaling the brain in inflammation: the role of endothelial cells. Front Biosci. 2004;9:2819–2826. doi: 10.2741/1439. [DOI] [PubMed] [Google Scholar]
- McIntyre P, McLatchie LM, Chambers A, Phillips E, Clarke M, Savidge J, Toms C, Peacock M, Shah K, Winter J, Weerasakera N, Webb M, Rang HP, Bevan S, James IF. Pharmacological differences between the human and rat vanilloid receptor 1 (VR1) Br J Pharmacol. 2001;132:1084–1094. doi: 10.1038/sj.bjp.0703918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Schroeder R, Bloom FE, Wilson MC. Induction and habituation of immediate early gene expression in rat brain by acute and repeated restraint stress. J Neurosci. 1994;14:5929–5938. doi: 10.1523/JNEUROSCI.14-10-05929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A, Nordstrom E, Mannem A, Smith C, Banerjee B, Sengupta JN. The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience. 2007;148:1021–1032. doi: 10.1016/j.neuroscience.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouihate A, Chen X, Pittman QJ. Interleukin-1β fever in rats: gender difference and estrous cycle influence. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1450–R1454. doi: 10.1152/ajpregu.1998.275.5.R1450. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Kinsey SG, Guo TL, Cravatt BF, Lichtman AH. Regulation of inflammatory pain by inhibition of fatty acid amide hydrolase. J Pharmacol Exp Ther. 2010;334:182–190. doi: 10.1124/jpet.109.164806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Need AB, Davis RJ, Alexander-Chacko JT, Eastwood B, Chernet E, Phebus LA, Sindelar DK, Nomikos GG. The relationship of in vivo central CB1 receptor occupancy to changes in cortical monoamine release and feeding elicited by CB1 receptor antagonists in rats. Psychopharmacology (Berl) 2006;184:26–35. doi: 10.1007/s00213-005-0234-x. [DOI] [PubMed] [Google Scholar]
- Nikami H, Mahmoud ME, Shimizu Y, Shiina T, Hirayama H, Iwami M, Dosoky RM, Ahmed MM, Takewaki T. Capsaicin pretreatment attenuates LPS-induced hypothermia through TRPV1-independent mechanisms in chicken. Life Sci. 2008;82:1191–1195. doi: 10.1016/j.lfs.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Oka S, Yanagimoto S, Ikeda S, Gokoh M, Kishimoto S, Waku K, Ishima Y, Sugiura T. Evidence for the involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in 12-O-tetradecanoylphorbol-13-acetate-induced acute inflammation in mouse ear. J Biol Chem. 2005;280:18488–18497. doi: 10.1074/jbc.M413260200. [DOI] [PubMed] [Google Scholar]
- Ovadia H, Wohlman A, Mechoulam R, Weidenfeld J. Characterization of the hypothermic effect of the synthetic cannabinoid HU-210 in the rat. Relation to the adrenergic system and endogenous pyrogens. Neuropharmacology. 1995;34:175–180. doi: 10.1016/0028-3908(94)00133-d. [DOI] [PubMed] [Google Scholar]
- Paul L, Fraifeld V, Kaplanski J. Evidence supporting involvement of leukotrienes in LPS-induced hypothermia in mice. Am J Physiol Regul Integr Comp Physiol. 1999;276:R52–R58. doi: 10.1152/ajpregu.1999.276.1.R52. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2004. [Google Scholar]
- Petervari E, Koncsecsko-Gaspar M, Balasko M, Szekely M. Increased thermoregulatory responsiveness in cold adapted but not in hyperthyroid hypermetabolic rats. Acta Physiol Hung. 2003;90:1–8. doi: 10.1556/APhysiol.90.2003.1.1. [DOI] [PubMed] [Google Scholar]
- Porter AC, Sauer JM, Knierman MD, Becker GW, Berna MJ, Bao J, Nomikos GG, Carter P, Bymaster FP, Leese AB, Felder CC. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther. 2002;301:1020–1024. doi: 10.1124/jpet.301.3.1020. [DOI] [PubMed] [Google Scholar]
- Portier M, Rinaldi-Carmona M, Pecceu F, Combes T, Poinot-Chazel C, Calandra B, Barth F, le Fur G, Casellas P. SR 144528, an antagonist for the peripheral cannabinoid receptor that behaves as an inverse agonist. J Pharmacol Exp Ther. 1999;288:582–589. [PubMed] [Google Scholar]
- Price TJ, Patwardhan A, Akopian AN, Hargreaves KM, Flores CM. Modulation of trigeminal sensory neuron activity by the dual cannabinoid-vanilloid agonists anandamide, N-arachidonoyl-dopamine and arachidonyl-2-chloroethylamide. Br J Pharmacol. 2004;141:1118–1130. doi: 10.1038/sj.bjp.0705711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Cabassa J, Geller EB, Adler MW. CB1 receptors in the preoptic anterior hypothalamus regulate WIN 55212-2 [(4,5-dihydro-2-methyl-4(4-morpholinylmethyl)-1-(1-naphthalenyl-carbonyl)-6H-pyrrolo[3,2,1ij]quinolin-6-one]-induced hypothermia. J Pharmacol Exp Ther. 2002;301:963–968. doi: 10.1124/jpet.301.3.963. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Neliat G, Caput D, Ferrara P, Soubrie P, Breliere JC, Le Fur G. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, Oustric D, Sarran M, Bouaboula M, Calandra B, Portier M, Shire D, Breliere JC, Le Fur GL. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- Roche M, Kelly JP, O'Driscoll M, Finn DP. Augmentation of endogenous cannabinoid tone modulates lipopolysaccharide-induced alterations in circulating cytokine levels in rats. Immunology. 2008;125:263–271. doi: 10.1111/j.1365-2567.2008.02838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner AA, Turek VF. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci. 2005;10:2193–2216. doi: 10.2741/1690. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, Nakamura K, Burmeister JJ, Nucci TB. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev. 2009;61:228–261. doi: 10.1124/pr.109.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanovsky AA, Ivanov AI, Shimansky YP. Selected contribution: Ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol. 2002;92:2667–2679. doi: 10.1152/japplphysiol.01173.2001. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Kulchitsky VA, Akulich NV, Koulchitsky SV, Simons CT, Sessler DI, Gourine VN. First and second phases of biphasic fever: two sequential stages of the sickness syndrome? Am J Physiol Regul Integr Comp Physiol. 1996a;271:R244–R253. doi: 10.1152/ajpregu.1996.271.1.R244. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Kulchitsky VA, Simons CT, Sugimoto N. Methodology of fever research: why are polyphasic fevers often thought to be biphasic? Am J Physiol Regul Integr Comp Physiol. 1998a;275:R332–R338. doi: 10.1152/ajpregu.1998.275.1.R332. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Shido O, Sakurada S, Sugimoto N, Nagasaka T. Endotoxin shock: thermoregulatory mechanisms. Am J Physiol Regul Integr Comp Physiol. 1996b;270:R693–R703. doi: 10.1152/ajpregu.1996.270.4.R693. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Simons CT, Kulchitsky VA. ‘Biphasic’ fevers often consist of more than two phases. Am J Physiol Regul Integr Comp Physiol. 1998b;275:R323–R331. doi: 10.1152/ajpregu.1998.275.1.R323. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Simons CT, Szekely M, Kulchitsky VA. The vagus nerve in the thermoregulatory response to systemic inflammation. Am J Physiol Regul Integr Comp Physiol. 1997;273:R407–R413. doi: 10.1152/ajpregu.1997.273.1.R407. [DOI] [PubMed] [Google Scholar]
- Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A. 2008;105:11008–11013. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA. Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1244–R1252. doi: 10.1152/ajpregu.00370.2005. [DOI] [PubMed] [Google Scholar]
- Sacerdote P, Massi P, Panerai AE, Parolaro D. In vivo and in vitro treatment with the synthetic cannabinoid CP55,940 decreases the in vitro migration of macrophages in the rat: involvement of both CB1 and CB2 receptors. J Neuroimmunol. 2000;109:155–163. doi: 10.1016/s0165-5728(00)00307-6. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976;117:305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, Chambers JK, Randall AD, Davis JB. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br J Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol Regul Integr Comp Physiol. 1985;249:R638–R641. doi: 10.1152/ajpregu.1985.249.5.R638. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Mouihate A, Galic MA, Pittman QJ. Central and peripheral neuroimmune responses: hyporesponsiveness during pregnancy. J Physiol. 2008;586:399–406. doi: 10.1113/jphysiol.2007.144006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp JA, Herbert J. Multiple immediate-early gene expression during physiological and endocrine adaptation to repeated stress. Neuroscience. 1999;94:1313–1322. doi: 10.1016/s0306-4522(99)00368-1. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Chakravarty S, Robbins JR, Dragic AS, Pan J, Herkenham M, Romanovsky AA. Thermoregulatory responses of rats to conventional preparations of lipopolysaccharide are caused by lipopolysaccharide per se – not by lipoprotein contaminants. Am J Physiol Regul Integr Comp Physiol. 2005;289:R348–R352. doi: 10.1152/ajpregu.00223.2005. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Dogan MD, Ivanov AI, Patel S, Rudaya AY, Jennings DH, Orchinik M, Pace TW, O'Connor KA, Watkins LR, Romanovsky AA. A new function of the leptin receptor: mediation of the recovery from lipopolysaccharide-induced hypothermia. FASEB J. 2004;18:1949–1951. doi: 10.1096/fj.04-2295fje. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Hunter JC, Phipps SM, Nucci TB, Oliveira DL, Roberts JL, Scheck AC, Simmons DL, Romanovsky AA. Cyclooxygenase-1 or -2 – which one mediates lipopolysaccharide-induced hypothermia? Am J Physiol Regul Integr Comp Physiol. 2009a;297:R485–R494. doi: 10.1152/ajpregu.91026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AA, Krall CM, Liu E. A reappraisal on the ability of leptin to induce fever. Physiol Behav. 2009b;97:430–436. doi: 10.1016/j.physbeh.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis JC, Treanor JJ, Gavva NR, Romanovsky AA. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci. 2007;27:7459–7468. doi: 10.1523/JNEUROSCI.1483-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto N, Simons CT, Romanovsky AA. Vagotomy does not affect thermal responsiveness to intrabrain prostaglandin E2 and cholecystokinin octapeptide. Brain Res. 1999;844:157–163. doi: 10.1016/s0006-8993(99)01918-6. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience. 1989;30:515–520. doi: 10.1016/0306-4522(89)90269-8. [DOI] [PubMed] [Google Scholar]
- Szekely M. Nutritional state and endotoxin fever of newborn rabbits. Acta Physiol Acad Sci Hung. 1979;53:279–283. [PubMed] [Google Scholar]
- Szekely M, Szolcsanyi J. Endotoxin fever in capsaicin treated rats. Acta Physiol Acad Sci Hung. 1979;53:469–477. [PubMed] [Google Scholar]
- Tollner B, Roth J, Storr B, Martin D, Voigt K, Zeisberger E. The role of tumor necrosis factor (TNF) in the febrile and metabolic responses of rats to intraperitoneal injection of a high dose of lipopolysaccharide. Pflugers Arch. 2000;440:925–932. doi: 10.1007/s004240000386. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ. Thermosensation and pain. J Neurobiol. 2004;61:3–12. doi: 10.1002/neu.20079. [DOI] [PubMed] [Google Scholar]
- Villanueva A, Yilmaz SM, Millington WR, Cutrera RA, Stouffer DG, Parsons LH, Cheer JF, Feleder C. Central cannabinoid 1 receptor antagonist administration prevents endotoxic hypotension affecting norepinephrine release in the preoptic anterior hypothalamic area. Shock. 2009;32:614–620. doi: 10.1097/SHK.0b013e3181a4fd8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel Z, Barg J, Levy R, Saya D, Heldman E, Mechoulam R. Anandamide, a brain endogenous compound, interacts specifically with cannabinoid receptors and inhibits adenylate cyclase. J Neurochem. 1993;61:352–355. doi: 10.1111/j.1471-4159.1993.tb03576.x. [DOI] [PubMed] [Google Scholar]
- Waksman Y, Olson JM, Carlisle SJ, Cabral GA. The central cannabinoid receptor (CB1) mediates inhibition of nitric oxide production by rat microglial cells. J Pharmacol Exp Ther. 1999;288:1357–1366. [PubMed] [Google Scholar]
- Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, Mackie K, Stella N. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


